
1
PETITION TO LIST OLYMPIC PENINSULA
STEELHEAD (Oncorhynchus Mykiss) AS A
THREATENED OR ENDANGERED
SPECIES
Copyright @ John R. McMillan
August 1, 2022
Petitioners
2
NOTICE OF PETITION
August 1, 2022
The Honorable Gina Raimondo
Secretary of Commerce
U.S. Department of Commerce
1401 Constitution Ave. NW
Washington, D.C. 20230
TheSec@doc.gov
via email
The Honorable Janet Coit
Assistant Administrator for Fisheries
NOAA Fisheries
1315 East-West Highway
Silver Spring, MD 20910
janet.coit@noaa.gov
via email
Dear Secretary Raimondo:
Pursuant to Section 4(b) of the Endangered Species Act (16 U.S.C. § 1533(b)), Section 553(e) of
the Administrative Procedures Act (5 U.S.C. § 553(e)), and 50 C.F.R. § 424.14(a), The
Conservation Angler and Wild Fish Conservancy (the “Petitioners”) hereby petition the
Secretary of Commerce, through the National Marine Fisheries Service (“NMFS”), to list the
Olympic Peninsula Steelhead Distinct Population Segment (Oncorhynchus mykiss) as a
threatened or endangered species. Petitioners also request that NMFS designate critical habitat
for Olympic Peninsula steelhead concurrent with the distinct population segment being listed
as threatened or endangered.
The Conservation Angler (TCA) is a Washington-based, nonprofit, public interest organization
that uses scientific expertise and legal advocacy to protect and conserve wild steelhead,
salmon, trout, and char throughout their Pacific range. TCA also operates the Kamchatka
Steelhead Program, an international research program that has advanced scientific knowledge
of wild steelhead for over 25 years.
Wild Fish Conservancy (WFC) is a Washington-based, nonprofit, public interest organization
that uses science, education, and advocacy to promote technically and socially responsible
habitat, hatchery, and harvest management to better sustain wild fish heritage in Washington
State.
3
NMFS has jurisdiction over this petition. This petition sets in motion a specific process, placing
definite response requirements on NMFS. Specifically, NMFS must “make a finding as to
whether the petition presents substantial scientific or commercial information indicating that
the petitioned action may be warranted.” 16 U.S.C. § 1533(b)(3)(A). NMFS must make this
finding “[t]o the maximum extent practicable, within 90 days after receiving the petition.” Id.
Petitioners need not demonstrate that the petitioned action is warranted. Rather, Petitioners
must only present information demonstrating that such action may be warranted. While
Petitioners believe that the best available science demonstrates that listing Olympic Peninsula
steelhead as threatened or endangered is in fact warranted, there can be no reasonable
dispute that the available information indicates that listing the species as either threatened or
endangered may be warranted. As such, NMFS should promptly make a positive initial finding
on the petition and commence a status review as required by 16 U.S.C. § 1533(b)(3)(B) and 50
C.F.R. § 424.14(h)(1)-(2).
Thank you.
THE CONSERVATION ANGLER WILD FISH CONSERVANCY
___________________________________ ____________________________________
David Moskowitz Emma Helverson
Executive Director Executive Director
The Conservation Angler Wild Fish Conservancy
P.O. Box 13121 P.O. Box 402
Portland, OR 97213 Duvall, WA 98019
(971) 235-8953 (425) 788-1167
david@theconservationangler.org emma@wildfishconservancy.org
4
Table of Contents
EXECUTIVE SUMMARY ....................................................................................................................................... 5
LEGAL BACKGROUND ........................................................................................................................................ 10
THE STATUS OF OLYMPIC PENINSULA STEELHEAD ............................................................................................. 13
I. DESCRIPTION ................................................................................................................................................. 13
II. TAXONOMY ................................................................................................................................................... 14
III. HABITAT AND RANGE .................................................................................................................................... 14
IV. LIFE HISTORY .................................................................................................................................................. 24
V. HABITAT REQUIREMENTS .............................................................................................................................. 25
VI. DIET ............................................................................................................................................................... 26
VII. NATURAL MORTALITY ............................................................................................................................... 26
VIII. POPULATION STRUCTURE ......................................................................................................................... 27
IX. PREVIOUS STOCK ASSESSMENTS ................................................................................................................... 28
X. ABUNDANCE AND POPULATION TRENDS ...................................................................................................... 33
XI. PRODUCTIVITY ............................................................................................................................................... 59
XII. DIVERSITY ................................................................................................................................................. 66
XIII. SPATIAL STRUCTURE ................................................................................................................................. 71
ENDANGERED SPECIES ACT LISTING FACTORS .................................................................................................... 72
I. PRESENT OR THREATENED DESTRUCTION, MODIFICATION, OR CURTAILMENT ........................................... 72
OF ITS HABITAT OR RANGE ...................................................................................................................................... 72
II. OVERUTILIZATION FOR COMMERCIAL AND RECREATIONAL PURPOSES ....................................................... 92
III. DISEASE AND PREDATION ............................................................................................................................ 109
IV. INADEQUACY OF EXISTING REGULATORY MECHANISMS ............................................................................ 110
V. OTHER NATURAL OR ANTHROPOGENIC FACTORS ....................................................................................... 130
REQUEST FOR CRITICAL HABITAT DESIGNATION .............................................................................................. 138
REFERENCES .................................................................................................................................................... 139
5
EXECUTIVE SUMMARY
Olympic Peninsula steelhead are at risk of becoming an endangered species within the
foreseeable future. The summer-run component is nearly extinct, and the winter-run
component is declining and losing its life history diversity. The fate of the species now rests on a
depressed and contracted mid- to late-spring component of wild fish whose productivity is
limited or declining depending on the population. The remnants of these runs that historically
numbered in the tens of thousands face declining freshwater and marine habitat conditions,
increasing recreational fishing pressure, and ongoing commercial harvest. Because of these and
other demographic and ecological threats, Olympic Peninsula steelhead are likely to become
endangered within the foreseeable future. Olympic Peninsula steelhead warrant protection
under the Endangered Species Act (ESA).
Abundance
Almost every population of winter steelhead on the Olympic Peninsula is in long-term
decline over their period of record. For example, mean annual run sizes from 1980 – 2017
declined by 37-46% in the Hoh, Queets, and Quinault Rivers (McMillan et al. 2022), resulting in
the Hoh and Queets River populations increasingly failing to meet their escapement goals. The
Quillayute River system population has declined sharply since the 1990s (McMillan et al. 2022).
All populations have continued to decline since 2017 and run sizes have been so small in recent
years that fisheries were closed. Trends appear similar for the smaller populations, with some
not meeting their escapement goals for decades, though the data sets are not always as
extensive. These patterns underscore why the Olympic Peninsula DPS has the second lowest
proportion of populations with increasing trends in Washington State (Cram et al. 2018).
The plight of the winter runs is even more revealing when compared to historical
estimates from circa 1940-1960. Based on those historical estimates (McMillan et al. 2022), the
Quillayute, Queets, Hoh, and Quinault River wild winter steelhead populations have declined by
61%, 69%, 79%, and 81%, respectively, in relation to their most recent five-year mean run size.
Those estimates do not necessarily capture the full extent of decline, however, because they
came after decades of harvest and many years of habitat alterations. When compared to
cannery data in 1923, for instance, the decline in the Queets River increases to 86%. Hence, it is
likely that all the populations are now only a fraction of their former abundance.
The status of the summer-run component is dire. Summer steelhead are not monitored
or managed, but snorkel surveys by Brenkman et al. (2012) and McMillan (2022) suggest
populations in the Quillayute, Hoh, and Quinault Rivers have declined dramatically and are at
critically low levels of abundance, if not already functionally extinct. Unfortunately, almost no
information exists for summer steelhead in the Queets River. The long-term, chronic declining
trends in winter steelhead coupled with the dire plight of summer steelhead puts the Olympic
Peninsula Steelhead DPS at greater risk of extinction than when Busby et al. (1996) conducted
the last status review for the DPS (ESU at the time).
6
Diversity
The diversity of Olympic Peninsula steelhead has been altered in different ways that
could impact their productivity, resilience, and capacity to adapt with changing climate effects.
First, the early-returning component of winter steelhead populations in the Quillayute, Hoh,
and Queets Rivers is severely depleted and consequently, the breadth of run timing is now far
more compressed than it was historically (McMillan et al. 2022). This change in run timing was
not accounted for in the prior status review (Busby et al. 1996), but it could be critical to their
persistence. Steelhead enter and spawn earlier in the winter in warmer, more southerly regions
of their native range (Busby et al. 1996), and as climate impacts progress, winter steelhead on
the Olympic Peninsula will also need to enter and spawn earlier to keep pace with climate
change. Run timing provides one way in which salmonids can adapt to changes in stream flow
and water temperature (Manhard et al. 2017), but that could be impossible for winter
steelhead in the Olympic Peninsula DPS if their original run timing is not restored.
Second, the data on repeat spawners suggest that rates of iteroparity have declined in
most of the four major populations over their period of record. The Queets River population
declined from a high of over 50% down to lows of approximately 10% in recent years, while the
Quillayute River peaked at over 20% in the late-1970s and is now down to around 10%. The
Quinault River population declined from 10-15% to approximately 1-2% in the last couple years.
Repeat spawning rates do not show a declining trend in the Hoh River, but that is likely because
its period of record does not extend back to the late-1970’s to 1980 as it does in the Queets and
Quillayute Rivers.
Third, summer runs are not monitored or managed, but they are caught in fisheries.
Snorkel surveys by Brenkman et al. (2012) and McMillan (2022) indicate summer steelhead are
at critically low levels of abundance in the Quillayute, Hoh, and Quinault Rivers, and they are
exposed to high levels of stray hatchery summer steelhead. There is a strong heritable basis for
the early maturing summer run life history and such allelic variants do not arise independently
via new mutations (Prince et al. 2017; Waples et al. 2022). Given their reduced abundance, it is
likely the populations are already genetically compromised, but they are still producing some
adults. If the declines continue and summer runs go extinct, the genetic basis for the life history
could be lost entirely, leaving behind a massive void in the timing of migration of the overall
steelhead populations in the Quillayute, Hoh, Quinault, and Queets Rivers.
Productivity
Information on productivity is very limited for steelhead in the Olympic Peninsula DPS,
but what data is available suggests that productivity is declining, and recruitment has
increasingly failed to reach replacement levels (Cram et al. 2018). The most recent productivity
estimates (Cram et al. (2018) only account for data up to 2010, so they miss many of the worst
returns on record that have occurred most recently. Considering the increasingly low levels of
productivity in the Quillayute River population, and that peaks and troughs in annual run size in
the Hoh, Queets, and Quinault River populations have generally declined each decade over the
7
period of record (lower peaks, deeper troughs) leading to high levels of depletion in recent
years, it appears the populations are no longer compensating as effectively as they did 20-40
years ago. If a declining productivity trend continues at the rates estimated by McMillan et al.
(2022) and summer runs decline further, which seems conservative given the recent and rapid
declines in run size, winter steelhead run sizes will soon be too small to allow fisheries and
summer steelhead will be extinct.
Spatial structure
While there are no large dams affecting Olympic Peninsula steelhead, there is a
dendritic network of roads and culverts constructed for forest practices, many of which cross
salmon bearing streams and create blockages to upstream migration (Smith 2000). Juvenile
steelhead are often distributed right up to barriers in many small creeks (McMillan and Starr
2008; McMillan et al. 2013), so the blockages have likely truncated their distribution. It is likely
the spatial distribution has been reduced or altered due to the depletion of early-returning
winter steelhead and the very low abundance of summer steelhead. Those components of the
steelhead population spawn in habitats that are otherwise used less or not at all by other life
histories (e.g., Cederholm 1983; McMillan et al. 2007). Hence, changes in run timing of winter
steelhead and depletion of summer steelhead, combined with barriers, have all plausibly
reduced the populations’ spatial distributions.
Harvest impacts
Several winter steelhead populations in the Olympic Peninsula DPS support intensive
recreational and commercial fisheries, and they experience the highest harvest levels of any
steelhead populations in Washington State (Cram et al. 2018). Harvest has had two apparent
effects. First, it has increasingly reduced spawning abundance below management goals. For
instance, due to overharvest, winter steelhead in the Hoh and Queets River have failed to meet
their minimum escapement goals for abundance in 50% of the years since 2003 and 50% of the
years over the past decade, respectively. The Quillayute River system population has generally
met its overall escapement goal, but individually, the Bogachiel and Sol Duc Rivers have failed
to meet their goals in 60% and 70% of the last ten years, respectively. Unlike the larger
populations, many smaller populations no longer experience direct harvest, but nearly all of
them have also failed to meet their escapement goals, including some that have not met their
goals for 20 years or longer.
Second, the strong focus on maximizing harvest of early-timed hatchery winter
steelhead from late-November through early-January has apparently contributed to depletion
of early-returning wild winter steelhead that were formerly very abundant in the 1940’s –
1950’s (McMillan et al. 2022). As a result, the breadth of run timing is now more compressed
than it was historically. These new results challenge the claim by Busby et al. (1996) that
hatchery and wild winter steelhead were temporally segregated due to differences in run
timing. Rather, the hatchery fish have simply replaced the wild fish, underscoring the potential
for a Shifting Baseline (Pauley 1995). This should not be a surprise, however, because according
8
to the traditional ecological knowledge of the Quileute Tribe, the month of January meant the
“time of steelhead running” and February the “time of steelhead spawning” (Frachtenberg
1916).
Hatchery impacts
Hatcheries can exert a range of genetic and ecological impacts on wild salmon and
steelhead (Araki and Schmid 2011), and each year, large numbers of hatchery steelhead are
released into several streams in the Olympic Peninsula DPS with the largest number of fish
being released into the Quinault River (Duda et al. 2018). Unfortunately, hatchery effects are
relatively unstudied, and their impacts are therefore uncertain in the DPS. However, as
mentioned earlier, fisheries focused on maximizing harvest of hatchery steelhead and
interbreeding between hatchery and wild steelhead have both likely contributed to the
depletion of early-timed wild winter steelhead (McMillan et al. 2022). For example, Seamons et
al. (2012) found that after three generations of stocking segregated hatchery winter steelhead
the proportion of wild ancestry smolts and adults declined by 10-20% and up to 80% of
naturally produced winter steelhead were hatchery x wild hybrids. Whatever the relative
contributions of each factor are, it is clear that hatchery and wild steelhead once overlapped
substantially, and the depletion of wild fish coincides with the onset of the hatchery programs.
There are also concerns about overall genetic impacts due to the number of hatchery
steelhead that stray and spawn in nature. Cederholm (1983) noted there was a high degree of
within and between river straying of hatchery steelhead, which could change the long-term
spawning, timing, growth, and survival of wild fish. Similar concerns were raised in the status
review by Busby et al. (1996) because of the “widespread production of hatchery steelhead” in
the DPS. Indeed, a review in 2009 indicated that hatchery steelhead greatly exceeded their
pHOS goals for winter and summer steelhead in the Bogachiel River (WDFW 2022b). And
WDFW suggests that hatchery winter and summer steelhead pose substantial risks to among-
population diversity and fitness of wild steelhead due to introgression that has likely occurred
in the Pysht, Hoko, and Sol Duc Rivers (WDFW 2008).
The record shows that concerns by Cederholm (1983) and others were warranted,
particularly for summer steelhead. As evidenced by snorkel surveys by Brenkman et al. (2012)
and McMillan (2022), a substantial number of hatchery summer steelhead stray into the Hoh
and Quinault Rivers, both of which do not receive any releases of hatchery summer steelhead.
There is also a substantial amount of straying within the Quillayute River system, particularly
into the upper Calawah and Bogachiel Rivers (Brenkman et al. 2012). The high levels of pHOS,
which frequently reach and even exceed 30-50%, combined with very low abundance of wild
summer steelhead, suggest that hatchery impacts could be a significant limiting factor.
Changes to Freshwater and Marine Habitat
The depleted stocks of winter and summer steelhead in the Olympic Peninsula DPS are
threatened with a myriad of environmental challenges, including degraded freshwater habitat,
9
climate impacts, and a changing ocean. Although the largest watersheds in the DPS have their
headwaters in Olympic National Park, a long history of extensive and unsustainable logging has
greatly degraded freshwater habitat outside of the park. Logging and roadbuilding have
increased the frequency of mass wasting events, which cause excessive sedimentation of
stream channels, diminish supplies of large woody debris, impair water quality, increase the
frequency of peak flow events, and reduce habitat connectivity (Smith 2000; East et al. 2017).
Climate change is predicted to degrade freshwater habitat even further, contributing to an
earlier onset of warmer summer water temperatures, more extreme fluctuations in stream
flow, and eventual elimination of all the glaciers that feed the largest, most productive
watersheds (Wade et al. 2013; East et al. 2017; Fountain et al. 2022). Climate impacts will also
alter the productivity potential of the marine environment where adult steelhead spend a
substantial portion of their lives. For example, sea surface temperatures are warming and will
continue to do so, while alterations to ocean currents could alter the extent of upwelling and
there is the potential for increased acidification and changes in the food web (Klingler et al.
2008; Miller et al. 2013; Dalton et al. 2016). Each of these changes, past and future, singular
and cumulative, are expected to negatively affect the survival and productivity of steelhead if
they do not have sufficient abundance, diversity, and spatial structure to adapt and keep pace
with climate change.
Summary
This petition demonstrates that the Olympic Peninsula Steelhead DPS warrants
protection under the ESA. It is divided in two parts. Part One covers the Olympic Peninsula
Steelhead DPS’s description, taxonomy, life history, distribution, and population status. Part
Two describes the current and future threats to the DPS in the context of the ESA’s five listing
factors. Based on the substantial information provided herein, NMFS should list Olympic
Peninsula steelhead as a threatened or endangered species and designate its critical habitat.

10
LEGAL BACKGROUND
The Endangered Species Act
The ESA defines “species” to mean “any subspecies of fish or wildlife or plants, and any
distinct population segment of any species of vertebrate fish or wildlife which interbreeds when
mature.” 16 U.S.C. 1532(16) (Emphasis added).
Olympic Peninsula steelhead are a distinct population segment (DPS) of steelhead
(Busby et al. 1996; 61 Fed Reg 41544 (Aug. 9, 1996)). Genetic data from the Washington
Department of Fish and Wildlife (WDFW) indicate that the Olympic Peninsula steelhead DPS is
“substantially isolated from other regions in western Washington” (Busby et al. 1996). In
addition to genetic differences, Olympic Peninsula steelhead are further characterized by
habitat, climatic, and zoogeographical differences between it and adjacent DPSs, including the
Southwest Washington and Puget Sound DPSs (Busby et al. 1996). NMFS delineates the Olympic
Peninsula steelhead DPS to include populations that occur in river basins to the west of the
Elwha River and south to, but not including, the rivers that flow into Grays Harbor (Busby et al.
1996; 61 Fed Reg 41544 (Aug. 9, 1996)).
Figure 1. Map of the Olympic Peninsula Steelhead Distinct Population Segment (Source: NMFS).
When making a listing determination, NMFS must analyze the status of a species in
conjunction with five statutory listing factors, relying “solely on the best scientific and
commercial data available.” 16 U.S.C. § 1533(b)(1)(A). The five listing factors include:
11
1. The present or threatened destruction, modification, or curtailment of its
habitat or range;
2. Overutilization for commercial, recreational, scientific, or educational
purposes;
3. Disease or predation;
4. The inadequacy of existing regulatory mechanisms; and
5. Other natural or manmade factors that affect its continued existence.
Id. at § 1533(a)(1).
Under the ESA, a species is “endangered” if it “is in danger of extinction throughout all
or a significant portion of its range.” Id. at § 1532(6). A species is “threatened” if it is “likely to
become an endangered species within the foreseeable future throughout all or a significant
portion of its range.” Id. at § 1532(20) (Emphasis added).
The Foreseeable Future and Climate Change
The foreseeable future extends as far into the future as NMFS can reasonably determine
that both future threats and the species’ responses to those threats are likely. 50 C.F.R. §
424.11(d). When analyzing the threats of climate change, it is NMFS policy to “project effects
over the longest possible period for which credible projections are available in order to ensure
the best available science is fully considered” (Tortorici 2016).
NMFS has looked out as far as the end of the 21
st
Century when making listing
determinations (Tortorici 2016). For example, the Ninth Circuit held that it was not arbitrary or
capricious for NMFS to list the ringed seal based on climate change models that projected as far
out as year 2100. Alaska Oil & Gas Ass’n v. Nat’l Marine Fisheries Serv., 722 F. App’x 666, 669
(9th Cir. 2018) quoting Alaska Oil & Gas Ass’n v. Pritzker, 840 F.3d 671, 680 (2016); see also
Alaska Oil & Gas Ass’n v. Pritzker, 840 F.3d 671 (9th Cir. 2016), cert denied 138 S. Ct. 924 (2018)
(upholding NMFS’s decision to list bearded seals as threatened based on climate change models
that predicted that the sea ice the seals depend on for birthing and mating would mostly
disappear by 2095) and Safari Club International v. Salazar (In re Polar Bear Endangered Species
Act Listing and § 4(d) Rule Litigation) 709 F.3d 1 (D.C. Cir. 2013), cert denied 571 U.S. 887 (2013)
(upholding the U.S. Fish & Wildlife Service’s decision to list the polar bear as threatened based,
in part, on projected climate change effects to the species and its habitat 45 years in the
future).
As an example of the feasibility of a 100-year time frame, the Intergovernmental Panel
on Climate Change (“IPCC”) provides climate change projections through 2100 under a range of
plausible emissions scenarios (IPCC 2021). NMFS recognizes the IPCC as a credible information
source. The Service’s guidance on climate change and ESA determinations requires it to “use
climate indicator values projected under the [IPCC’s] Representative Concentration Pathway 8.5
12
when data are available” (Tortorici 2016). For these reasons, the use of 100 years as the
foreseeable future is consistent with NMFS’s climate change policy.
As indicated by NMFS’s policy on climate change and listing decisions (Tortorici 2016),
the best available science standard does not require NMFS to be certain about climate change
and its effects on Olympic Peninsula steelhead.
“While it requires that decisions not be based on mere generalizations or
speculation, the best available science standard does not require that
information be free from uncertainty. For example, to support listing a species
on the basis of climate change related impacts, we must have information
particular to that species to demonstrate that it will be impacted by climate
change, such as through a reduction of suitable habitat within its known range. It
is not necessary, however, to have projections at a particular geographic scale or
to have a complete understanding of the biological reasons for and extent of the
species’ sensitivity to climate change.”
(Tortorici 2016). This petition supports listing Olympic Peninsula steelhead for multiple reasons,
including climate change-related impacts. The best available science, including NMFS’s own
reports, demonstrates that the species is impacted by climate change.
13
PART ONE
THE STATUS OF OLYMPIC PENINSULA STEELHEAD
Threats to a species’ long-term persistence are manifested demographically as risks to its
abundance, population growth rate, spatial structure and connectivity, and genetic and ecological
diversity. These demographic risks thus provide the most direct indices or proxies of extinction risk.
A species at very low levels of abundance and with few populations will be less tolerant to
environmental variation, catastrophic events, genetic processes, demographic stochasticity,
ecological interactions, and other processes (e.g., Meffe and Carroll 1994, Caughley and Gunn
1996). A population growth rate that is unstable or declining over a long period of time indicates
poor resiliency to future environmental change (e.g., Lande 1993, Middleton and Nisbet 1997, Foley
1997). A species that is not widely distributed across a variety of well-connected habitats is at
increased risk of extinction due to environmental perturbations, including catastrophic events
(Schlosser and Angermeier 1995, Hanski and Gilpin 1997, Tilman et al. 1997, Cooper and Mangel
1999). A species that has lost locally adapted genetic and ecological diversity may lack the raw
resources necessary to exploit a wide array of environments and endure short- and long-term
environmental changes (e.g., Groot and Margolis 1991, Wood 1995). Assessing extinction risk of a
species involves evaluating whether risks to its abundance, population growth rate, spatial structure
and or diversity are such that it is at or near an extinction threshold, or likely to become so in the
foreseeable future. As demonstrated by this petition, Olympic Peninsula steelhead are likely to
become an endangered species within the foreseeable future and, therefore, should be listed as a
“threatened” species under the ESA.
I. DESCRIPTION
Olympic Peninsula steelhead are a distinct population segment of steelhead (Busby et
al. 1996; 61 Fed Reg 41544 (Aug. 9, 1996)). Steelhead are the anadromous form of
Oncorhynchus mykiss and may display the most diverse life histories of any salmonid (Kendall et
al. 2015). As with other salmonids, they begin their life cycle in freshwater where they spend 1-
4 years growing as parr, which are relatively drab and natural in coloration to match their
surrounding stream environments (Busby et al. 1996). After reaching a growth threshold in size,
typically between 150 – 200 mm in length, they undergo a complex series of physiological
changes, such as increased levels of NA+/K+-ATPase in the gills, that assist with osmoregulation
and life in saltwater (Busby et al. 1996). As their internal physiology changes, so does their
outward appearance. Their body becomes more fusiform, their scales become more deciduous,
and they take on a silvery appearance (Busby et al. 1996).
Steelhead are known to display three general categories of ocean migrations. There are
typically anadromous fish that spend 1-4 years in the ocean before returning to spawn in
freshwater, attaining sizes of 45 – 1125 mm in length and 0.9 – 20.5 kg in weight (Kendall et al.
2015). There are also half-pounders that only undertake a short, near-shore ocean migration
for a few months attaining a size of 25 – 40 mm in length and a weight of 0.1 – 0.7 kg, but
generally return in an immature state and will undertake a full ocean migration the next year
(Hodge et al. 2014; Kendall et al. 2015). Last, there are individuals that undertake short
14
migrations and attain sizes intermediate to the fully anadromous and half-pounder life histories
but are mature upon return to freshwater and are typically male (Kendall et al. 2015). The latter
are referred to as “estuarine” or “jack” life histories, potentially depending on what part of the
ocean they migrate to (Kendall et al. 2015). While the fully anadromous form is common
throughout the native range of steelhead, far less information exists on the distribution of the
half-pounder life history except for a few rivers where they are abundant in California and
southern Oregon, and almost no information exists on estuarine or jack life histories outside of
the Kamchatka Peninsula, Russia (Kendall et al. 2015).
Adults that return to freshwater to spawn are further delineated into two run types,
including ocean-maturing “winter run” and river-maturing “summer run” life histories (Busby et
al. 1996). Olympic Peninsula steelhead include winter and summer run life histories. Winter
runs enter freshwater sexually mature or close to sexual maturity from late-October through
early-June, while summer runs enter sexually immature from May through October and
sexually mature as they stage in freshwater. The physiological processes responsible for
osmoregulation will reverse to allow the fish to persist in freshwater and they begin to display
signs of secondary sexual characteristics as they approach the spawning season from late-
winter through spring to early-summer. Males become much darker shades of green and red,
while females become less silvery and display various shades of red and light green. Both sexes
may develop fungal infections due to stress, fights, and compromised immune systems.
Individuals that survive the rigors of mating and spawning will once again – if in
sufficient condition and health – restart the smoltification process in preparation for saltwater
entry (Buelow and Moffit 2015). During emigration to the ocean their outward appearance will
also change to become more silvery as they did when they were smolts for the first time.
II. TAXONOMY
Kingdom: Animalia
Phylum: Chordata
Class: Osteichthyes
Order: Salmoniformes
Family: Salmonidae
Genus: Oncorhynchus
Species: Oncorhynchus mykiss
III. HABITAT AND RANGE
The Olympic Peninsula is a large arm of land located in western Washington. It is
bordered by Hood Canal to the east, the Strait of Juan de Fuca to the north, and the Pacific
Ocean to the west. The Olympic Mountains are its natural centerpiece. These coastal
mountains reach 1,200 to 2,400 meters above sea level and currently hold 184 glaciers (Busby
et al. 1996; Reidel et al. 2017). The area receives copious rainfall (McHenry et al. 1996). The
west side of the peninsula receives the most precipitation, ranging from 70 to 100 inches in the
15
lower coastal plains (Dalton et al. 2016) and up to 240 inches in the mountains (McHenry et al.
1996). Olympic National Park protects the only temperate rainforest in the contiguous United
States (Dalton et al. 2016). The park’s foothills are dominated by undisturbed old western
hemlock (Tsuga heterophylla), douglas fir (Pseudotsuga menziesii), and sitka spruce (Picea
sitchensis) (Dalton et al. 2016). Outside of the park, the forest has been extensively logged
(McHenry et al. 1996). Running through this dichotomous landscape of pristine and degraded
habitat are some of the last large, undammed rivers in the Pacific Northwest.
Olympic Peninsula steelhead occur in three distinct water resource inventory areas
(WRIA): WRIAs 19, 20, and 21 (Table 1).
A. Water Resource Inventory Area 19
WRIA 19 begins immediately west of the Elwha River and extends eastward to Cape
Flattery, the northwesternmost point in the lower forty-eight. The area experiences a cool
maritime climate with annual precipitation ranging from 80” to 130” (HSRG 2004). Precipitation
is higher on the west side of WRIA 19 (NOPLE 2015). The Sekiu River basin on the western edge
of WRIA 19 receives 95-120 inches of precipitation per year (NOPLE 2015). The Salt Creek basin
on the eastern edge receives 35-55 inches of precipitation annually (NOPLE 2015).
The forestland has a mix of tree species that vary east to west. In the eastern portion of
WRIA 19, Douglas fir (Psuedotsuga mensiezii) is the dominant species, with red alder (Alnus
rubra), vine maple (Acer cirnicatum), and bigleaf maple (Acer macrophylla) also occurring in the
area (NOPLE 2015). In the western portion of WRIA 19, the forests are dominated by western
hemlock (Tsuga heterophylla) and Sitka spruce (Picea sitchensis) (NOPLE 2015).
Commercial forestry accounts for 76% of land use in WRIA 19 (NOPLE 2015). Private
interests own 56% of the commercial forestland, the Washington Department of Natural
Resources (WDNR) owns 28%, the U.S. Forest Service (USFS) owns 12%, and the remaining 4% is
owned by the county and small landowners (NOPLE 2015). The non-commercial forestland
occurs inside Olympic National Park (11.6%) or is classified as rural, urban, industrial, tribal
reservation, or miscellaneous (12.4%) (NOPLE 2015).
Steelhead are one of the more widely distributed salmonids in WRIA 19 (NOPLE 2015;
Table 1). Winter steelhead occur in several small to medium-sized watersheds, including the
following subbasins listed in order of size: Hoko River (71 sq mi), Lyre River (67.9 sq mi), Pysht
River (46.3 sq mi), Sekiu River (33.2 sq mi), Clallam River (31 sq mi), Salt Creek (19.1 sq mi), East
Twin River (13.6 sq mi), and West Twin River (12.6 sq miles) (NOPLE 2015). The Western Strait
Independents collectively drain 73.3 square miles (NOPLE 2015).
Summer steelhead may occur in WRIA 19 (Table 1). Summer steelhead are thought to
occur in the Lyre River (McHenry et al. 1996; Lyre-Hoko Watershed (WRIA 19) Planning Unit
2008) and may occur in the East and West Twin Rivers, Murdock Creek, Fielding Creek, and
Colville Creek (WRIA 19 Watershed Plan 2008).
16
The Lyre River is the only river in WRIA 19 that originates in an alpine area (approx.
5,500 feet) (NOPLE 2015). All other rivers and streams in WRIA 19 drain from low elevation
foothills ranging 2,000 to 3,500 feet in elevation (NOPLE 2015).
B. Water Resource Inventory Area 20
WRIA 20 begins at Cape Flattery and extends south to, but not including, Kalaloch Creek
(HSRG 2004). The area receives 80” to 240” of rain per year (McHenry et al. 1996). Wind and
heavy rainstorms are common (HSRG 2004). Inside Olympic National Park rests an undisturbed
temperate rainforest, which includes old-growth Sitka spruce, western hemlock, and silver fir
(Abies alba) (HSRG 2004). Outside of the park there is significant habitat disturbance, due in
large part to extensive clearcutting and roadbuilding that occurred during the 1960s-1980s
(HSRG 2004). Commercial forestry is the predominant land use in the lower reaches of WRIA 20
rivers (NPCLE 2020).
Olympic Peninsula steelhead occur in several large and small systems within WRIA 20
(Table 1). The winter and summer-run components of the Olympic Peninsula DPS are found in
the Hoh, Quillayute/Bogachiel, Calawah, and Sol Duc Rivers (Cram et al. 2018). Several other
rivers and streams support the winter run life history only, including the Dickey River, Goodman
Creek, Mosquito Creek, Ozette River, Tsoo-Yess (Sooes) River, and Wa’atch River (Cram et al.
2018).
1. Quillayute River System (Quillayute, Sol Duc, Bogachiel, Calawah, and Dickey Rivers)
The Quillayute River system includes the Quillayute, Sol Duc, Calawah, Bogachiel, and
Dickey Rivers, which collectively drain 628 square miles of land (Klinger et al. 2008). Nearly one-
third of the Quillayute River basin lies within Olympic National Park (32% of the Sol Duc River
basin, 29% of the Bogachiel River basin, and 20% of the Calawah River basin occur inside the
park (Houston and Contor 1984)). The Quillayute River proper, formed by the confluence of the
Bogachiel and Sol Duc Rivers, is only 5.6 miles long (Smith 2000).
a. Sol Duc River
The Sol Duc River originates in the northern Olympic Mountains and generally flows
west and northwest before leaving the boundary of Olympic National Park. After leaving the
park, the river flows south and west through 11.1 miles of the Olympic National Forest, where
more than half of the land is in late successional reserve (Smith 2000). Outside the Olympic
National Forest, the Sol Duc River winds through private and state-owned land. The watershed
receives 90-120 inches of precipitation per year and its upper reaches are influenced by the
rain-on-snow zone.

17
Table 1. Summary of watersheds and steelhead populations, including Watershed Resource Inventory Area (WRIA), whether the
watershed supports(ed) winter and/or summer run steelhead, period of record for monitoring data (usually redd counts) for winter
runs, mean annual run size and range, mean annual escapement and range, trend in annual run size or abundance, mean harvest
rate, percent of last 10-years the population achieved its escapement goal, average number of hatchery smolts released (winter and
summer run), and the population risk score generated by WDFW’s Steelhead at Risk report (Cram et al. 2018). N/A = not available or
applicable. N/A is used to describe “run size” in larger tributaries to Quillayute River and the Clearwater River (tributary of Queets
River) because the tribal fishery occurs almost solely below the tributary mouths and hence, the ultimate destination of the fish is
unknown. Consequently, only escapement can be estimated for those tributaries.
Watershed (Major
tributary)
WRIA
Winter
(W)
and/or
summer
run (S)
Monitoring
period of
record for
winter runs
Mean
(Range)
annual run
size winter
runs
Mean (Range)
annual
escapement
winter runs
Trend in
abundance
2,3,4
Mean
harvest rate
(range)
Percent last
10-years
met
escapement
goal
Average
hatchery
smolts
release 2009-
2013
(Winter,
Summer)
Population
risk score as
of 2013
(Cram et al.
2018)
Hoko River 19 W 1985-2020
N/A
566
(193 – 990)
40% decline
2
N/A
80% 24,000 (W) Low
Pysht River 19 W 1995-2020
N/A
499
(195 – 936)
21% decline
2
14%
9
70% 0 Mod.
Clallam River 19 W 1999-2020
N/A
158
(45 – 284)
27% decline
2
0.7%
8
No goal 2,000 (W) Mod.
Deep Creek 19 W 2010-2020
N/A
95
(47 – 129)
Declining
4
N/A
No goal 0 Insf.
West Twin River 19 W 2010-2020
N/A
60
(21 – 90)
Declining
4
N/A
0% 0 Insf.

18
East Twin River 19 W 2010-2020
N/A
57
(31 – 116)
Declining
4
N/A
10% 0 Insf.
Salt Creek 19 W 1995-2020
N/A
116
(32 – 237)
43% decline
2
3.9%
9
0% 0 Mod.
Lyre River 19 W & S
?
N/A N/A N/A N/A N/A
No goal
5,000 (W),
2,000 (S)
Insf.
Sekiu River 19 W
N/A N/A N/A N/A N/A
No goal 12,000 (W) Insf.
Sail River 19 W
N/A N/A N/A N/A N/A
No goal 10,000 (W) Insf.
Tsoo-Yess River 20 W
N/A N/A N/A N/A N/A
No goal 96,000 (W) Insf.
Ozette River 20 W
N/A N/A N/A N/A N/A
No goal 0 Insf.
Quillayute River 20 W & S 1978-2020
1
13,064
(6,456 –
21,615)
9,340
(5,500 – 16,919)
No trend
2,3
28%
(10% - 55%)
90%
243,000 (W),
47,000 (S)
N/A
Dickey R. (trib) 20 W 1978-2020
1
N/A
460
(143 – 1,607)
N/A See Quillayute 100%
0 Low
Sol Duc R. (trib) 20 W & S 1978-2020
1
N/A
3,864
(1,791 – 7,634)
N/A See Quillayute 70%
23,000 (W),
12,000 (S)
NR
Low
Calawah R. (trib) 20 W & S 1978-2020
1
N/A
2,980
(989 – 5,985)
N/A See Quillayute 100%
40,000 (W),
35,000 (S)
Moderate
Bogachiel R. (trib) 20 W & S 1978-2020
1
N/A
1,975
(730 – 4,553)
N/A See Quillayute 60%
180,000 (W) Low
Goodman Creek 20 W 1995-2020
N/A
184
(45 – 374)
54% decline
2
6.8%
7
0% 4,000
NR
High
Mosquito Creek 20 W
N/A N/A N/A N/A N/A N/A
0 Insf.
19
Hoh River 20 W & S 1980-2020
1
4,117
(2,541 – 5,783)
2,726
(1,616 – 4,593)
37% decline
3
33%
(7% - 55%)
60% 68,000 (W) Mod.
Kalaloch Creek 21 W
N/A N/A N/A N/A N/A No goal
0 Insf.
Queets River 21 W & S 1980-2018
1
7,648
(4,200 –
13,309)
4,845
(2,271 – 7,841)
45% decline
3
35%
(10% - 55%)
30% 157,000 (W) Mod.
Clearwater R.
(trib)
21 W & S
?
1980-2018
1
N/A
1,744
(847 – 2,966)
N/A See Queets
50% 0 Low
Quinault River 21 W & S 1978-2020
1
5,883
(2,179 – 9,726)
3,107
(1,366 – 5,774)
44% decline
3
46%
(15% - 65%)
No goal 486,000 (W) Low – Mod
Upper Quinault 21 W & S 1978-2020
1
N/A
1,511
(772 – 2,877)
N/A See Quinalt
1,200 0 Low
Moclips River 21 W 1988-2000
N/A
299
(130 – 560)
27% increase
2
N/A No goal
0 Low
Raft River 21 W
N/A N/A N/A N/A N/A No goal
0 Insf.
Copalis River 21 W
N/A N/A N/A N/A N/A No goal
0 Insf.
? = There are reports of small numbers of unclipped summer steelhead, 1) in the Lyre River, but it has never been determined whether those
were wild fish from the Lyre, strays from another river, or naturalized offspring of hatchery summer steelhead scatter plants in the Lyre River;
and 2) in the Clearwater River, which could be strays from the Queets River or naturalized offspring of hatchery summer steelhead scatter plants
in the Clearwater River.
NR = Hatchery releases have ended on these rivers.
1 = Data range is presumed to be only for winter runs. Data on run size and escapement of summer runs is almost entirely lacking, but redd
counts for winter steelhead may also include some summer steelhead since there is overlap in their spawning distribution.
2 = Source is Cram et al. (2018) Steelhead at Risk Report, with period of record ending in 2013.
20
3 = Source is McMillan et al. (2021).
4 = Source is North Olympic Peninsula Lead Entity (NOPLE) report (2015)
5 = Quillayute River population is not in statistically significant decline over its full period of record, but
it has declined sharply from 1996-2017 at a rate of 5,533 adults/decade (McMillan et al. 2022).
6 = Harvest rate is included in whole population estimate.
7 = Period of record 1995-2009, per Cram et al. 2018
8 = Period of record 1999-2013, per Cram et al. 2018
9 = Period of record 1995-2013, per Cram et al. 2018
b. Bogachiel River
The Bogachiel River is formed by the North and South Forks of the Bogachiel River,
which originate in the Olympic Mountains (Smith 2000). Its upper reaches are in Olympic
National Park, while its middle and lower reaches flow through timber and other agricultural
land (Smith 2000). The river’s most significant tributary is the Calawah River, an important
salmonid tributary itself (Smith 2000). The Bogachiel River’s other important salmonid
tributaries include Murphy, Maxfield, Weeden, Mill, Grader, and Dry Creeks (Smith 2000).
c. Calawah River
The Calawah River is formed by the North and South Forks of the Calawah River, which
originate in the Olympic Mountains (Smith 2000). The South Fork and its largest tributary, the
Sitkum River, provides spawning and rearing habitat for winter and summer steelhead and they
flow through late-successional reserve land inside the Olympic National Forest (Smith 2000).
The Nork Fork Calawah also supports winter and summer steelhead (Smith 2000).
d. Dickey River
The Dickey River is formed by the West, Middle, and East Forks of the Dickey River
(Smith 2000). The Dickey River is a low gradient system, and it has numerous wetlands and
sloughs (Smith 2000). Unlike the Sol Duc, Bogachiel, and Calawah Rivers, the Dickey River does
not originate inside Olympic National Park (Smith 2000). Rather, the river and its tributaries
occupy a heavily logged drainage (Smith 2000).
2. Hoh River
The Hoh River originates deep inside Olympic National Park, on the glacial slopes of Mt.
Olympus, and flows westward for 56 miles before emptying in the Pacific Ocean (Duda et al.
2018). The Hoh River basin drains nearly 299 square miles of land and has an extensive
21
floodplain (WCSSP 2013). The watershed receives 93-240 inches of rain per year (Klinger et al.
2008). The Hoh River has a strong glacial influence (McHenry et al. 1996).
Fifty-seven percent of the watershed is located within Olympic National Park, with the
remaining 43% flowing through state (24.4%), private, and tribal lands (Klinger et al. 2008). The
South Fork of the Hoh River, a major steelhead tributary, joins the mainstem of the Hoh River at
RM 30 (Smith 2000). Other significant salmonid tributaries to the Hoh River include Slide, Falls,
Mt Tom, Jackson, Taft, Snider, East Twin, Canyon, Spruce, Dismal, Pole, Tower, Lindner, Clear,
Willoughby, Elk, Alder, Winfield, Hell Roaring, Lost, Pins, Anderson, Nolan, Braden, and Fossil
Creeks (Smith 2000).
3. Ozette River
The Ozette River originates at the northern end of Ozette Lake and flows 5.3 miles west
to the Pacific Ocean (NPCLE 2020). Coal Creek is the largest tributary to the Ozette River (NPCLE
2020). Multiple tributaries drain into Lake Ozette, including the Big River and Umbrella,
Crooked, Siwash, South, Palmquist, Quinn, Elk, and Lost Net Creeks (NPCLE 2020).
4. Tsoo-Yess (Soose) and Wa’atch Rivers
The Tsoo-Yess and Wa’atch Rivers are short, rain-fed systems that originate in coastal
foothills located in the northeastern corner of WRIA 20 (NPCLE 2020). Both rivers flow through
the Makah Reservation and a small coastal strip of Olympic National Park (NPCLE 2020).
5. Goodman and Mosquito Creeks
Goodman and Mosquito Creeks are located to the north of the Hoh River and south of
the Quillayute River system. Both creeks originate in coastal foothills and flow through state
and private timberland lands and a coastal section of Olympic National Park before emptying
into the Pacific Ocean (NPCLE 2020).
C. Water Resource Inventory Area 21
WRIA 21 begins at Kalaloch Creek in the north and ends at Connor Creek in the south
(QINLE 2011). The area receives heavy rainfall, measuring 120” to 200” per year (HSRG 2004).
Prior to European American settlement, the area was covered by old growth western red cedar
(Thuja plicata), Sitka spruce, Douglas fir, and western hemlock (QINLE 2011). Today, the only
undisturbed forestland in WRIA 21 is in Olympic National Park. WRIA 21 has an extensive
coastal plain and 65 miles of marine shoreline (QINLE 2011).
Within WRIA 21, Olympic Peninsula steelhead occur in the Queets, Clearwater, Quinault,
Raft, Copalis, and Moclips Rivers and Kalaloch Creek (HSRG 2004). The Queets and Quinault
Rivers are large glacially-influenced systems that originate inside Olympic National Park
22
(McHenry et al. 1996, QINLE 2011, WCSSP 2013). The Clearwater, Raft, Copalis, and Moclips
Rivers and Kalaloch Creek are rain-dominant systems (WCSSP 2013).
1. Queets River
The Queets River originates on Humes Glacier on the southeast side of Mt. Olympus.
The river measures 51 miles long, drains 450 square miles of land, and flows at an average rate
of 8,000 cubic feet per second (cfs) in the winter and 1,015 cfs in the summer (McMillan 2006).
Nearly the entire course of the Queets River flows through Olympic National Park, with only the
lower eight miles running through the Quinault Indian Reservation before reaching the Pacific
Ocean (Smith and Caldwell 2001). Its major tributaries include the Clearwater, Sams, and
Salmon Rivers and Matheny and Tshletshy Creeks (Smith and Caldwell 2001). Steelhead spawn
in these tributaries, as well as several smaller ones (e.g., Miller Creek), and in the mainstem of
the Queets River (Smith and Caldwell 2001).
Olympic National Park owns 34 miles of tributary streams, including all of Tsheltchy
Creek and the lower five miles of the Sams River (Smith and Caldwell 2001). The USFS owns
84% of Matheny Creek watershed, 73% of the Sams River watershed, and 30% of the Salmon
River watershed (Smith and Caldwell 2001). The USFS manages these lands as riparian reserves,
late successional reserves, or adaptive management areas (Smith and Caldwell 2001). The
Quinault Tribe owns the lower eight miles of the Queets River and 54% of the Salmon River
drainage (McMillan 2006).
2. Clearwater River
The Clearwater River originates in the foothills of the Olympic Mountains (WCSSP 2013)
and flows 39 miles before emptying into the Queets River several miles upstream of the Pacific
Ocean. WDNR owns 79% of the Clearwater River watershed and roughly 20% is privately owned
(McMillan 2006). Its major tributaries include the Sollecks and Snahapish Rivers and Christmas
and Stequaleho Creeks (Smith and Caldwell 2001). The upper watershed receives 120” to 160”
of rain per year (McHenry et. al 1996).
3. Quinault River
The headwaters of the Quinault River begin in the Mt. Lawson and the Enchanted Valley
watersheds (Smith and Caldwell 2001). The river measures 69 miles long, drains 434 square
miles of land, and flows at an average rate of 6,300 cfs in the winter and 1,080 cfs in the
summer (McMillan 2006). The river feeds into Lake Quinault, which spans 3,729 acres. Below
the lake, the river runs southwesterly through the Quinault Indian Reservation for 33 miles until
it meets the Pacific Ocean (Smith and Caldwell 2001).
Nearly half (47%) of the Quinault River basin occurs within Olympic National Park
(Houston and Contor 1984). The entire North Fork and most of the East Fork occur inside in the
23
park. Roughly one-third (32%) of the basin occurs on the Quinault Indian Reservation, with the
remaining 13% located on USFS land and 4% on private land (Smith and Caldwell 2001).
Winter and summer steelhead occur in the Quinault River (Smith and Caldwell 2001).
Winter steelhead spawn in the mainstem below Lake Quinault, the North Fork of the Quinault
River, and the following creeks: Cook, Elk, Willaby, Falls, Gatton, Zeigler, Kestner, Inner, Slough,
Alder, Big, Fox, Fletcher, Boulder, Ten O’clock, Canoe, Irely, and Bunch Creeks (Smith and
Caldwell 2001). Summer steelhead spawn in the East Fork and North Fork of the Quinault River
(Brenkman et al. 2012).
4. Raft River
The Raft River is located between the Queets and Quinault Rivers (WCSSP 2013). The
basin is 71,824 acres in size and includes the Raft River, North Fork Raft River, Red Creek and
several independent tributaries (Smith and Caldwell 2001). The river originates in the foothills
of the Olympic Mountains and flows through a coastal plain before emptying into the Pacific
Ocean. The Raft River is 11.5 miles long and occurs almost entirely within the Quinault Indian
Reservation (81%). The remaining 19% of the basin is private land (Smith and Caldwell 2001).
5. Moclips River
The Moclips River and its main tributary, the North Fork Moclips River, originate in the
foothills to the south of the Quinault River. Together, the Moclips River and its north fork
measure 17 miles long and flow west to the Pacific Ocean. The Moclips River basin is 53,528
acres in size, most of which is private land (54%) or owned by the Quinault Indian Nation (39%)
(Smith and Caldwell 2001). The state owns the remaining 7% of the basin (Smith and Caldwell
2001). Winter steelhead spawn in the Moclips River mainstem, the North Fork of the Moclips
River, and Wreck Creek (Smith and Caldwell 2001).
6. Copalis River
The Copalis River is a low gradient system located to the south of the Moclips River. The
river originates in the foothills and flows through a coastal plain for a total of 24 miles before
reaching the Pacific Ocean (WSCCP 2013). The Copalis River basin is 36,818 acres in size, nearly
all of which is privately owned (95%), with the remaining 5% owned by the state (Smith and
Caldwell 2001). Winter steelhead spawn in the river’s mainstem (Smith and Caldwell 2001).
7. Kalaloch Creek
The Kalaloch Creek Basin spans 13,649 acres and includes Kalaloch Creek and four
unnamed tributaries. Most of the land is owned by WDNR (41%) or private interests (40%).
Olympic National Park owns 18% of the basin and the Quinault Indian Nation owns 1% (Smith
and Caldwell 2001). Kalaloch Creek supports winter steelhead, which spawn in the lower
mainstem and the West Fork of Kalaloch Creek (Smith and Caldwell 2001).
24
IV. LIFE HISTORY
Steelhead emerge from the gravel from spring through early summer and spend one to
four years in freshwater before migrating to the ocean, where they spend one to four years
before returning to freshwater to spawn (Busby et al. 1996). Steelhead smolts on the Olympic
Peninsula typically migrate to the ocean from April through June, with peak emigration
occurring in mid- to late-May (Busby et al. 1996).
Juvenile steelhead are present off the Washington coast in May and June, with only a
few lingering into July and August (Pearcy et al. 1990). They migrate north and spend their first
summer in the Gulf of Alaska and North Pacific Ocean (Atcheson et al. 2012). Afterwards they
distribute throughout their north Pacific Ocean range (Atcheson et al. 2012). Some will go as far
west as the Kuril Islands (Atcheson et al. 2012).
Steelhead distribution in the Pacific Ocean appears to be driven by temperature and
salinity (Okazaki 1983, Sutherland 1973, Light et al. 1989). The south-north distribution of
steelhead corresponds with a 3° C to 16° C temperature range, with nearly 96% of steelhead
occurring in waters measuring at or below 12° C (Sutherland 1973, as cited in Light et al. 1989).
The northern boundary, which is slightly above the Aleutian Islands, may correspond with
factors other than sea surface temperatures, such as salinity (Light et al. 1989). Steelhead are
generally found within 10 meters of the ocean’s surface (Godfrey et al. 1975, as cited in Light et
al. 1989).
Except for half-pound steelhead stocks, “[i]nformation from tagging studies shows little
or no differences in ocean distribution among stocks, groups, or races.” (Light et al. 1989).
Therefore, Olympic Peninsula steelhead likely follow the same ocean distribution pattern as all
other steelhead, not including half-pounders.
The Olympic Peninsula steelhead DPS includes seven summer-run and twenty-four
winter-run populations (Cram et al. 2018). The summer-run populations return from May
through October (McHenry et al. 1996) and spawn from January through April; however, the
populations remain largely unstudied and, consequently, their status and trends are unknown
(WDFW 1992; Busby et al. 1996; McMillan 2006; Brenkman et al. 2012; Cram et al. 2018).
Historically, a substantial proportion of the winter-run populations returned between
December and January (McMillan et al. 2022). Currently, the winter-run component returns
and spawns between January and May, with a peak in March through April depending on the
population (Busby et al. 1996; McMillan et al. 2007; McMillan et al. 2022).
Steelhead are iteroparous (Busby et al. 1996). Steelhead on their first spawning run are
called “maiden” fish (Light et al. 1989). Maiden fish that survive spawning and return to the
ocean are known as “kelts” (Behnke 2002). Steelhead may return to spawn up to five times
within their lifetimes; however, most steelhead will only spawn once (Busby et al. 1996; Kendall
et al. 2015).
25
V. HABITAT REQUIREMENTS
Steelhead are poikilotherms and evolved to survive within a range of stream and ocean
temperatures. In general, steelhead prefer water temperatures between 10-15°C, but this
varies by life stage and population (McCullough et al. 1999; Hicks 2000). For instance, Fuss
(1998) suggests 5-11°C is optimal for steelhead egg survival and eggs and Rombough (1988)
found alevins that experience water temperatures over 15°C are smaller than individuals that
experience cooler water temperatures. This suggests optimal incubation temperatures are
below 11-12°C (Hicks 2000). The most favorable temperature range for juvenile growth is
between 5-17°C, with the optimal temperatures varying by season (Hicks 2000). Temperatures
over 19°C can limit the occurrence and growth of juvenile steelhead if food is not sufficient,
with lethal temperatures beginning at around 27°C (Hicks 2000). Temperatures of 11.3-12.3°C
are consistently cited as the uppermost constant temperature exposures that will not interfere
with smoltification (Zaugg et al. 1972; Zaugg and Wagner 1973; Zaugg 1981), with detrimental
effects occurring once water temperatures begin to exceed 12.7°C. Adult migration in winter
typically occurs when temperatures are relatively cool, but there is evidence that upstream
migration by adults stops when temperatures reach 20-21°C, which is a concern for summer
steelhead (Hicks 2000). Adult steelhead can begin to die when water temperatures reach 21.6-
23.8°C and generally, steelhead avoid water temperatures of 24°C and above (unless they are
genetically adapted to warmer water temperatures) (Hicks 2000).
Steelhead eggs and juveniles require clean gravel. If fine-grained sediment exceeds 12-
20% of the surface, young steelhead are buried and suffocate (Phillips et al 1975; Reiser and
White 1988; Jensen et al. 2009). Sedimentation in streams can also negatively impact growth
and survival of juvenile salmonids in different ways (Suttle et al. 2004). For example, high levels
of fine sediment can decrease prey, leading to starvation (Murphy et al. 1981).
Large woody debris (LWD) is also an important component of steelhead habitat
(Cederholm et al. 1997; Thompson et al. 2012). LWD stabilizes stream channels, creates pools,
reduces erosion, and provides cover, resting, and feeding areas for juveniles (Bilby and Bisson
1998). Complex floodplain habitat is also important to steelhead survival, as side channels and
other natural features provide feeding habitat, refuge from high water events, and other
benefits (Beechie et al. 1994; Montgomery et al. 1999; Bellmore et al. 2013).
Steelhead adapted to natural hydrographs, and juvenile and adult migrations are timed
to coincide with historic flow regimes that maximize survival (Bjornn and Reiser 1991). Changes
in flow regimes due to climate change and other anthropogenic causes (e.g., water
withdrawals) interfere with these major life history stages, increasing stress and susceptibility
to disease, and, occasionally, leading to direct mortality (e.g., stranding due to low streamflows)
(Bjornn and Reiser 1991; Wade et al. 2013).
26
VI. DIET
A. Freshwater
Steelhead fry consume aquatic invertebrates, including chironomids, mayflies, and
terrestrial macroinvertebrates (Davis 2015). One-year-old juveniles mostly consume insect
larvae and pupae, adult insects, and crustaceans (Davis 2015). By age two, juvenile steelhead
are larger in size and can consume larger and more diverse prey items, such as fish larvae and
small fish (Davis 2015).
B. Marine
Steelhead smolts appear to spend a relatively short period of time feeding in nearshore
waters before dispersing across the vast North Pacific Ocean. In the 1980s, researchers purse
seined the coasts of Washington and Oregon to collect juvenile steelhead and study their
stomach contents (Pearcy et al. 1990). The researchers intercepted juvenile steelhead in May
through August; however, the peak catch occurred in May and June. Their stomach contents
contained a variety of fish (juvenile rockfish, sandlance, brown Irish lord, and greenlings),
euphasids, barnacle larvae, copepods, and other crustraceans (Pearcy et al. 1990). The
researchers estimated that steelhead grow by about 1.1 mm per day between May and early
July.
Steelhead in the open ocean consume a wide variety of prey. Sampling of steelhead
diets in the Gulf of Alaska and Central North Pacific found that the dominant prey were fish and
squid, with the primary species within those categories being Atka mackerel (Pleurogrammus
monopterygius), three-spined stickleback (Gasterosteus aculeatus), lantern fish (Myctophidea spp.),
and the minimal armhook squid (Berryteuthis anonychus) (Atcheson 2010). The study also found
that steelhead consume euphausiids, copepods, amphipods, polychaetes, crustacean larvae, and,
unfortunately, some styrofoam and plastic (Atcheson 2010). Plastic and styrofoam are of particular
concern because it could result in mortality, toxicity, or delayed effects, such as heritable
alterations in gene expression (Myers et al. 2013).
VII. NATURAL MORTALITY
A. Freshwater
Although a tremendous amount of research has been conducted on the survival of adult
steelhead, far less information is available for specific mortality rates for juveniles in freshwater
across a gradient of environments. Mortality at the egg stage can be very high, with survival
from egg to emergence ranging from 18-95% depending on factors such as temperature, intra-
gravel flow, and fine sediment (Shapovalov 1937; Sheppard 1972; Phillips et al. 1975; Bjornn
1978; Biley and Moring 1988). However, Shapovalov and Taft (1954) experimentally concluded
that under ideal conditions, survival to emergence averages about 80-90%. Bjornn (1978) and
27
Phillips et al. (1975) also conducted experiments and found a similar upper limit as Shapovalov
and Taft (1954) when fine sediment was absent from the redd, but survival was only 18% when
70% of the redd consisted of fine sediment.
After emergence, evidence suggests - as with most salmonids - that the mortality rate is
high during the initial weeks to months of life when juvenile fish are small, weak, and fairly
immobile. For example, Burns (1971) estimated June-to-October survival of young-of-the-year
salmonids in a California stream was only 27% (range = 20-29%), while age-1 and older juveniles
survived at a rate of 56% (range = 34-94%). Bjornn (1978) estimated mortality rate of 80-90%
during the first summer of life, with an overall mortality of 94-99% upon completing the first
year of life. A review of these papers and others suggests that mortality rates almost always
exceed 50-60% early in life and thereafter, conditions being equal, survival tends to increase as
fish size and condition increases (Biley and Moring 1988).
Mortality also occurs as smolts migrate to and enter the ocean. For example, Melnychuk
et al. (2007) found that 65-73% mortality of juvenile steelhead occurred in the first month of
smolt migration. Romer et al. (2012) reported that 63-89% of the steelhead smolts they tagged
survived a short freshwater migration to the estuary, but that 50-60% of the fish died in the
estuary despite most fish spending less than one day in the estuary.
B. Marine
Quinn (2005) estimated that approximately half of steelhead life cycle mortality occurs
in the ocean. Typically, smolt to adult survival rates in the ocean is between 2% and 10% (Quinn
2005). Steelhead ocean survival is largely dependent on sea surface temperature during their
first summer in the ocean (Atcheson 2010).
VIII. POPULATION STRUCTURE
Olympic Peninsula steelhead are distinct from other distinct population segments,
including the Southwest Washington and Puget Sound DPSs (Busby et al. 1996). That distinction
still holds true. WDFW acknowledges it is “not yet able to fully evaluate genetic population
structure to aid the process of verifying the 1992 population definitions for Olympia [sic]
Peninsula and SW Washington DPSs” (Cram et al. 2018).
The Olympic Peninsula Steelhead DPS includes summer-run and winter-run populations
(Table 1; Busby et al. 1996). WDFW considers summer steelhead populations in the Sol Duc,
Bogachiel, Calawah, Hoh, Queets, Clearwater, and Quinault Rivers to be distinct from each
other based on geographic isolation (WDF et al. 1993). WDFW considers the following winter
steelhead population to be distinct stocks: Salt Creek/Independents (Salt, Whiskey, Colville, and
Field Creeks), Lyre River, Pysht/Independents (Pysht River, Deep Creek, East Twin River, and
West Twin River), Clallam River, Hoko River, Sekiu River, Sail River, Sooes/Waatch (Sooes and
Waatch Rivers), Ozette River, Sol Duc River, Quillayute/Bogachiel (Quillayute and Bogachiel
Rivers), Calawah River, Dickey River, Goodman Creek, Mosquito Creek, Hoh River, Kalaloch

28
Creek, Queets River, Clearwater River, Quinault/Lake Quinault (Lower Quinault and broodstock
net pet steelhead in Lake Quinault), Quinault River (above Lake Quinault), Raft River, Moclips
River, and the Copalis River (WDF et al. 1993).
IX. PREVIOUS STOCK ASSESSMENTS
There have been several stock assessments for the largest wild winter steelhead
populations in the Olympic Peninsula DPS (Table 2 and Table 3) but assessments for smaller
populations with less consistent data are more limited. Below we list and summarize the
assessments that have been conducted to date. No assessments are available for wild summer
run steelhead because data on those populations is almost entirely lacking (except for data
collected by the National Park Service (Brenkman et al. 2012) and John McMillan (Science
Director, The Conservation Angler: McMillan 2022)).
Table 2. Reports that reviewed the status and trends of wild winter steelhead populations in
the Quillayute, Hoh, Queets, and Quinault Rivers. Only the HSRG 2004 included an evaluation of
summer steelhead.
Reference Status
Nehlsen et al. 1991 Healthy, stable to increasing trend
WDF et al. 1993 Healthy
Busby et al. 1996
Stable to increasing; status review
determined ESA-listing was not warranted
McHenry et al. 1996 Healthy, stable to increasing trend
WDFW 2002 Healthy, stable to increasing trend
HSRG 2004
At risk (summer runs listed as critical, except
for Quinault population which is “at risk”)
Kendall et al. 2017
Declining trend in Hoh and Queets River
populations
Cram et al. 2018
Declining trend in Hoh, Queets, and
Quinault River populations
McMillan et al. 2022
Declining trend in Hoh, Queets, and
Quinault River populations
A. American Fisheries Society (1991)
In 1991, the American Fisheries Society published a list of 214 native, naturally spawning
salmonid stocks that were either at high or medium risk of extinction or were a species of
concern (Nehlsen et al. 1991). This list did not include Olympic Peninsula steelhead (Nehlsen et
al. 1991).
29
B. Washington Department of Fish and Wildlife (1992)
In 1992, the Washington Department of Fisheries (“WDF”), Washington Department of
Wildlife, and the Western Washington Treaty Indian Tribes assessed the status of Olympic
Peninsula winter and summer steelhead stocks (WDF et al. 1993). For WRIA 19 populations,
WDF et al. (1993) identified the Hoko River and Pysht/Independent winter stocks as “healthy.”
WDF et al. (1993) defined “healthy” to mean the stock was “experiencing stable escapement,
survival, and production trends and not displaying a pattern of chronically low abundance.” The
Salt Creek/Independents and Sekiu, Sail, Lyre, and Clallam River populations were listed as
“unknown” (WDF et al. 1993).
For WRIA 20 populations, WDF et al. (1993) identified five winter steelhead stocks as
“healthy” and five as “unknown.” The five healthy stocks included winter steelhead in the
Quillayute/Bogachiel, Dickey, Sol Duc, Calawah, and Hoh Rivers (WDF et al. 1993). WDF et al
(2013) could not determine the status of winter steelhead populations in the Sooes, Wa’atch,
or Ozette Rivers or Goodman, Mosquito, or Kalaloch Creeks (WDF et al. 1993).
For WRIA 21 populations, WDF et al. (1993) determined that four out of five winter
steelhead stocks were healthy, including the Quinault, Moclips, Queets, and Clearwater River
populations. The Departments and the tribes could not determine the status of the Copalis
River population (WDF et al. 1993).
Except for the Queets River summer run population, which WDF et al. (1993) designated
as “healthy,” the departments and tribes could not determine the status of summer steelhead
in the Sol Duc, Bogachiel, Calawah, Hoh, or Clearwater Rivers (WDF et al. 1993).
C. National Marine Fisheries Service (1996)
In 1996, NMFS reviewed the status of Olympic Peninsula steelhead (Busby et al. 1996).
At the time, seven of the twelve winter steelhead stocks that had population trend data were
declining or had relatively flat trends, while the other five were increasing (Busby et al. 1996).
The declining and stable stocks included the following winter steelhead populations: Pysht River
(-5.8%), Hoko River (-7.6%) Quillayute/Bogachiel River (-0.2%), Dickey River (-4.4%), Sol Duc
River (-0.1%), Clearwater River (-0.5%); and Quinault River/Lake Quinault (-2.6%) (Busby et al.
1996). The increasing stocks included the following winter steelhead populations: Calawah
River (1.1%), Hoh River (0.2%), Queets River (0.9%), Upper Quinault River (1.8%), and the
Moclips River (13.6%) (Busby et al. 1996). No population trend data were available for summer
steelhead (Busby et al. 1996).
At the time, most Olympic Peninsula steelhead populations appeared to be self-
sustaining because there were no strong abundance trends (Busby et al. 1996). Additionally,
WDFW provided information indicating that there was “substantial temporal separation
between hatchery and native winter steelhead” (Busby et al. 1996). However, NMFS noted
30
there were “isolated problems” with the population sustainability, including declining trends in
the Pysht/Independents and Quinault River populations (Busby et al. 1996). NMFS also noted
there was a “substantial contribution” of hatchery spawners in the Quinault River population
(Busby et al. 1996). Based on those facts, NMFS determined that Olympic Peninsula steelhead
did not warrant listing at the time (61 Fed Reg. 41541, 41550).
However, the biological review team expressed several concerns regarding Olympic
Peninsula steelhead (61 Fed Reg., at 41550).
“*** [T]he BRT has several concerns about the overall health of this ESU and
about the status of certain stocks within it. The majority of recent abundance
trends are upward (including three of the four largest stocks), although trends in
several stocks are downward. These downward trends may be largely due to
recent climate conditions. There is widespread production of hatchery steelhead
within this ESU, largely derived from a few parent stocks, and this could increase
genetic homogenization of the resource despite management efforts to
minimize introgression of the hatchery gene pool into natural populations.
Estimates of the proportion of hatchery fish on natural spawning grounds range
from 16% to 44%, with the two stocks with the largest abundance of natural
spawners (Queets and Quillayute) having the lowest hatchery proportions.”
(Busby et al. 1996).
D. Washington Department of Fish and Wildlife (2002)
In 2002, WDFW updated its status assessment of Olympic Peninsula steelhead
populations (WDFW 2002). The 2002 assessment downgraded the Quinault River “mixed”
origin winter steelhead stock from “healthy” to “depressed” (WDFW 2002). It changed the
statuses of Queets River summer steelhead and Moclips River winter steelhead from “healthy”
to “unknown” (WDFW 2002). Finally, it changed the Goodman Creek, Pysht/Independents, and
Salt Creek/Independents winter steelhead population statuses from “unknown” to “healthy”
(WDFW 2002).
E. North Olympic Peninsula Lead Entity (2004)
In 2004, a technical review team for the North Olympic Peninsula Lead Entity for Salmon
(“NOPLE”) assessed winter steelhead populations in WRIA 19. The team determined that the
following winter steelhead populations were “depressed”: Sekiu River, Clallam River, West Twin
River, East Twin River, and the Western Strait Independents populations (NOPLE 2015). The
team identified the Hoko River, Pysht River, and the aggregated Salt Creek populations as
“healthy” and the Lyre River population as “unknown” (NOPLE 2015).
31
F. Hatchery Scientific Review Group (2004)
In 2004, the Hatchery Scientific Review Group (HSRG) issued a report on hatchery
operations on the north coast of Washington (HSRG 2004). The report included population
viability ratings from managers of Olympic Peninsula winter and summer steelhead populations
(HSRG 2004). To assess population viability, they considered multiple factors such as age class
structure, spawner escapement, and proportion of hatchery-origin fish in natural spawning
populations (HSRG 2004). Each stock’s viability was rated as “critical,” “at risk,” or “healthy”
(HSRG 2004).
The report did not list any wild Olympic Peninsula steelhead populations as “healthy”
(HSRG 2004). The report identified the following populations’ viability as “critical”: Copalis River
winter steelhead, Goodman Creek winter steelhead, Hoh River summer steelhead, Moclips
River winter steelhead, Mosquito Creek winter steelhead, Kalaloch Creek winter steelhead,
Ozette River winter steelhead, Quillayute River system summer steelhead, and Sooes River
winter steelhead (HSRG 2004). The report rated the following populations as “at risk”: Hoh
River winter steelhead, Hoko River winter steelhead, Queets River winter steelhead, Quillayute
system winter steelhead, Quinault River summer steelhead, and Quinault River winter
steelhead (HSRG 2004). HSRG could not assess the viability of Queets River summer steelhead
(HSRG 2004).
G. Steelhead at Risk Report (2018)
In 2018, WDFW released the Steelhead at Risk Report, which assessed steelhead
populations statewide based on the available, albeit limited, data at the time (Cram et al. 2018).
Importantly, the last year of data the report covered was from 2013 and, therefore, it did not
include data on the significant declines in abundance that occurred from 2014 through 2018.
The authors noted that the “the lack of abundance, productivity, and diversity data was the
most common impediment to conducting wild steelhead status assessments statewide” (Cram
et al. 2018). The authors also explained they had limited productivity data. Cram et al. (2018)
acknowledged “very little is known about temporal and spatial patterns of freshwater
population productivity (smolts per spawner) and smolt to adult return rates (SAR) for Olympic
Peninsula DPS wild steelhead populations, and this is a substantial data gap” (Cram et al. 2018).
However, the authors observed that smolt-to-adult return ratios (SARs) for Olympic Peninsula
steelhead have declined significantly over time (Cram et al. 2018). Additionally, the authors
highlighted that between brood years 2005 and 2010, the population productivity of Olympic
Peninsula steelhead was lower than the productivity of the lower Columbia River, middle
Columbia River, Upper Columbia River, and Snake River steelhead DPSs (Cram et al. 2018).
When possible, Cram et al. (2018) generated a risk score based on short- and long-term
trends in run size, extinction risk, and proportion of time the population met its escapement
goal. Again, the report did not include escapement data for years 2014-2018 and, therefore, it
did not consider significant failures to meet escapement goals during those years. They found
that 11 (73%) of 15 winter steelhead populations displayed a decreasing trend in abundance
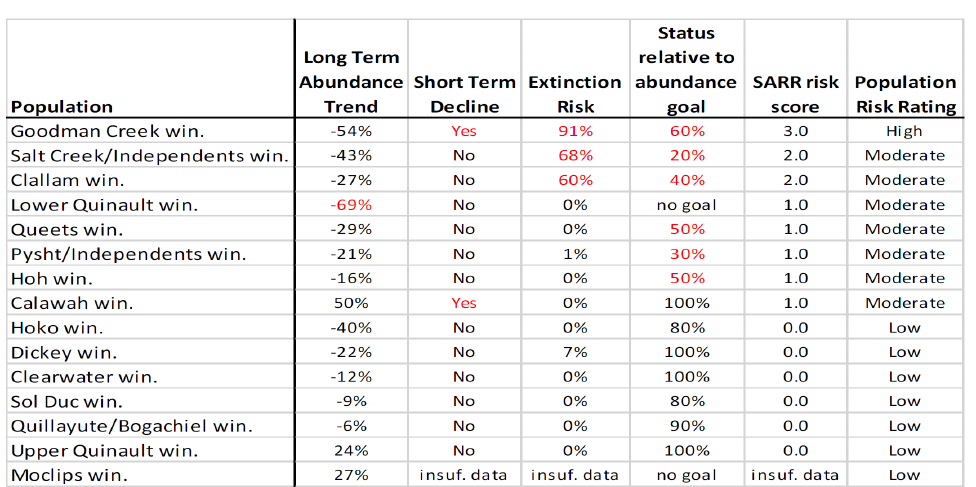
32
(Table 3), but the Lower Quinault River was the only population that met the high-risk standard
for long-term abundance. They also determined Goodman Creek and the Calawah River were
considered as high risk based on declines in short-term abundance, while Goodman Creek, Salt
Creek, and Clallam River populations were identified as having a high risk of extinction (Table
3). Of the 13 populations with defined escapement goals, Cram et al. (2018) identified six
populations at high risk for failure to meet escapement goals, but also noted that 54% of
populations had met escapement goals in at least 70% of last 10 years (2004 – 2013).
Table 3 (from Cram et al. 2018). Risk assessment results and ratings for Olympic Peninsula
Steelhead DPS populations. Red text indicates values that exceeded specific criterion for each
metric. Status relative to abundance goal represents the percentage of years the population
achieved its escapement goal. Abbreviations: win. = winter steelhead; sum. = summer
steelhead; insuf. = insufficient.
In addition to lacking summer steelhead data, the data was insufficient to determine
extinction risks for the following winter steelhead populations: Copalis River, Kalaloch Creek,
Lyre River, Mosquito Creek, Ozette River, Raft River, Sekiu River, Sail River, Sooes River, and
Wa’atch River populations (Cram et al. 2018).
According to the results for all four risk metrics, Cram et al. (2018) determined that one
population was at a high total risk, seven at moderate total risk, seven at low total risk (Table 3)
and 16 at undetermined risk.

33
X. ABUNDANCE AND POPULATION TRENDS
Based on Cram et al. (2018), McMillan et al. (2022), NOPLE (2015), run size and
escapement estimates by WDFW and tribes (co-managers), and an online WDFW data
repository (https://fortress.wa.gov/dfw/score/score/species/steelhead.jsp?species=Steelhead,
Accessed online 3/20/2022), we compiled a list of all the major and small watersheds known to
support wild steelhead by their respective Watershed Resource Inventory Area (WRIA) and
other factors, such as mean annual abundance, in cases where such data was available (Table
1). The populations are predominantly or solely the winter run life history, and the most
abundant populations are found in the Quillayute, Queets, Quinault, and Hoh Rivers, while the
remaining rivers and creeks support much smaller populations (Table 1). Data is almost totally
lacking for summer steelhead, and they are only thought to be present in the four largest
watersheds (Quillayute, Hoh, Queets, and Quinault Rivers). However, snorkel survey data
indicates that summer run populations are at critically low levels and close to extirpation (Table
1). Below we summarize information on the historic and current trends in abundance and
escapement of wild steelhead where such information is available.
A. Historical Abundance Winter and Summer Steelhead
Information on the historic abundance of wild winter steelhead is only available for the
four largest populations of wild winter steelhead in the Olympic Peninsula DPS, which include
the Quillayute, Hoh, Queets, and Quinault River populations (McMillan et al. 2022). There is
also some historical catch information for summer steelhead in each watershed, which we
reference later. We could not find historical data for the other, smaller populations of wild
winter steelhead. McMillan et al. (2022) analyzed old cannery, sport, and tribal catch records to
estimate historic run size and run timing for the Quillayute, Hoh, Queets, and Quinault River
populations. Information on historic releases of hatchery winter and summer steelhead are
available from Duda et al. (2018), which provides a thorough summary of all releases of
hatchery steelhead for watersheds draining from Olympic National Park.
Although there is no formal analysis of historical catch of summer steelhead in the four
largest watersheds known to support them (Quillayute, Hoh, Queets, and Quinault Rivers), we
include recreational and tribal catch data for a few years during the 1950’s when data was
occasionally available. We also include catch data from 1962-1977, which mostly consists of
data from sport record catch cards collected annually by WDFW (Summer steelhead catch data
is in excel file “Summer steelhead catch data for Quillayute_Hoh_Queets_Quinault River
populations” and based on data from .pdf file “Historic winter and summer steelhead tribal
catch by month Hoh, Quinault, Queets, Quillayute 40s-70s”). We ended the “historical” period
in 1977 because that was the first year that hatchery summer steelhead were released into two
tributaries of the Quillayute River system (Calawah and Sol Duc Rivers). It was not possible to
determine the relative proportion of hatchery and wild fish in the catch after 1977 because
hatchery summer steelhead were not outwardly marked with a fin clip until 1985. Regardless,
this data was available to Busby et al. (1996), but it was not evaluated in their status review.
34
WDFW and the treaty tribes (collectively, the “co-managers”) considered steelhead to
be “summer runs” if they were caught from May 1 through October 31, which is slightly
problematic because that suggests they were also potentially catching spawned out winter run
kelts in May and June that were emigrating back to the ocean (McMillan 2006). Kelts can be
distinguished from unspawned summer steelhead upon capture, but it is not known whether
such distinctions were made. Regardless, the overall catch of summer steelhead is quite small
in most years and inconsistent across months, which generally suggests that large numbers of
kelts were not reported in the catch.
We summarized cumulative summer steelhead catch by year and watershed and then
generated a very rough estimate of run size by assuming a harvest rate of 25% and calculating
lower and upper bounds at 15% and 35% harvest rates. We do not assume the estimates of
summer run are robust owing to the simple calculation. Nonetheless, we provide them because
they offer some insight into how catch could translate to abundance.
Below, we summarize what is known about the historical winter steelhead and summer
steelhead catch and run sizes. Overall, the estimates suggest the largest watersheds historically
contained abundant runs of winter and summer steelhead, with fresh steelhead likely entering
the rivers each month of the year (McMillan 2006).

35
Table 4 (modified from McMillan et al. 2022). Comparison of historical mean annual wild winter steelhead abundance estimates
based on cannery record data, expansion of historical commercial and recreational catch data, historical commercial fishery catch
per unit effort (CPUE), and accessible stream kilometers (SKM) of habitat, an ensemble historical (circa 1948 - 1960) mean estimate,
the contemporary (circa 1980 - 2017) mean estimate, and the percent decline of each population in relative to the ensemble
historical estimate. Percent decline is the difference between the ensemble historical abundance estimate and the contemporary
mean abundance. The percent decline reported for the most recent five-year period in McMillan et al. (2021) refers to 2013-2017
(except for Quinault which referred to 2009-2013). Since the estimate in McMillan et al. (2021) does not capture the most recent run
sizes, we updated the estimate so that the comparison between the ensemble estimate and the most recent five-year period for
each population refers to 2016-2020.
Population
Cannery
records
Historical
catch
Historical
CPUE
Accessible
habitat
(SKM)
Ensemble
historical
abundance
estimate
1
Contemporary
mean
abundance
Percent
decline
Most recent
5-yr mean
abundance
(~ 2016-2020)
Percent
decline for
most recent
5-yr
Quillayute 22,567 23,391 19,571 21,843 13,595 38 % 8,528 61 %
Hoh 15,923 14,160 10,431 13,505 4,206 69 % 2,880 79 %
Queets 32,659 19,875 13,553 12,144 15,191 7,648 50 % 4,658 69 %
Quinault 13,743 22,226 14,723 16,897 6,181 63 % 3,370 80 %
1
Ensemble historical estimate does not include cannery record estimate for Queets in 1923
36
1. WRIA 20 Populations
a. Hoh River
Using historical catch data, McMillan et al. (2022) estimated the average historical run
size from 1948 through 1960 to be 15,923 winter steelhead (lower and upper bounds for
historic mean: 9,023, 24,901) (Table 4). With that approach, the lowest and highest single-year
estimate for the Hoh River population was 7,118 and 24,684 winter steelhead in 1948 and
1956, respectively. Using historical catch per unit effort (CPUE) and total accessible habitat
produced average historical run size estimates of 14,160 and 10,431 steelhead, respectively
(Table 4). The average ensemble estimate for annual run size for all three approaches was
13,505 steelhead (Table 4).
Estimates of historic run size prior to the modern monitoring period are not available for
summer run steelhead. Because they were not a target species less data is available on historic
catch. We could only find two years of harvest data prior to 1962 (i.e., tribal catch in 1954 and
1957: data is in excel file “Summer steelhead catch data for Quillayute_Hoh_Queets_Quinault
River populations” and based on data from .pdf file “Historic winter and summer steelhead
tribal catch by month Hoh, Quinault, Queets, Quillayute 40s-70s”), after which sport catch
record card data is available in addition to a few years of tribal catch up to 1977. We consider
this timeframe to be the “historical” period because it is prior to the onset of releases of
hatchery summer steelhead that began in 1977. Mean catch of summer steelhead for the
period beginning in 1954 and ending in 1977 was 118 fish and ranged from a low of 38 fish to a
high of 274 fish (Table 5).
Assuming a 25% harvest rate and using bounds of 15% and 35% harvest rates, we
estimated a mean run size of 472 summer steelhead (Lower bound = 337, Upper bound =
1,179) with a low run size of 152 fish and a high of 1,096 (Table 6). The mean of the top-five run
sizes is 884 summer steelhead (Lower bound = 631, Upper bound = 1,473).
Although there is uncertainty associated with our estimations, wild summer steelhead
were formerly abundant in the Hoh River. Further, it is likely that the run sizes were larger if the
harvest rates are lower than we assume and not all the catch was reported, both of which are
plausible. Last, the earliest data point we found was from 1954, which is decades after the
onset of commercial and recreational fisheries and industrial logging practices. Therefore, it is
almost certain that wild summer steelhead were more abundant than we report here.
b. Quillayute System
Using historical catch, McMillan et al. (2022) estimated that the average historical
abundance for winter steelhead in the Quillayute River from 1948-1960 was 22,567 steelhead
(lower and upper bounds for the historical mean: 16,733, 31,591) (Table 4). Annual variability
within the historical period ranged from the lowest and highest single year estimates in the
Quillayute River of 6,702 and 34,757 winter steelhead in 1948 and 1951, respectively. Using
37
historical CPUE and total accessible habitat produced average historical run size estimates of
23,391 and 19,571 steelhead, respectively (Table 4). The average ensemble estimate for annual
run size for all three approaches was 21,843 steelhead.
Estimates of historic run size are not available for summer run steelhead. Because they
were not a target species less data is available on historic catch. However, there is evidence
that summer steelhead were present in each of the three largest tributaries, including the
Bogachiel, Sol Duc, and Calawah Rivers (McMillan 2006). We summarized all the catch data for
the tributaries as part of the Quillayute River system (Data is in excel file “Summer steelhead
catch data for Quillayute_Hoh_Queets_Quinault River populations” and based on data from
.pdf file “Historic winter and summer steelhead tribal catch by month Hoh, Quinault, Queets,
Quillayute 40s-70s”). Mean catch of summer steelhead in 1946 and from 1962-1977 was only
67 fish with annual catch ranging from 12 to 309 fish (Table 5), suggesting not many fishers
focused on catching summer steelhead in the Quillayute system.
Assuming a 25% harvest rate and using bounds of 15% and 35% harvest rates, we
estimated a mean run size of 268 summer steelhead (Lower bound = 191, Upper bound = 670)
with a low run size of 48 fish and a high of 848 (Table 6). The mean of the top-five run sizes is
478 summer steelhead (Lower bound = 342, Upper bound = 797). If the population was
harvested with the range of rates we proposed, then wild summer steelhead were once quite
abundant in the Quillayute River system.
2. WRIA 21 Populations
a. Quinault River
Using historical catch, McMillan et al. (2022) estimated the average historical
abundance for winter steelhead in the Quinault River from 1948-1960 was 13,743 steelhead
(lower and upper bounds for the historical mean: 9,345, 20,258) (Table 4). Annual variability
within the historical period ranged from the lowest and highest single year estimates in the
Quinault River of 7,475 and 30,332 winter steelhead in 1956 and 1952, respectively. Using
historical CPUE and total accessible habitat produced average historical run size estimates of
22,226 and 14,723 steelhead, respectively (Table 4). The average ensemble estimate for annual
run size for all three approaches was 16,897 steelhead.
We summarized all the catch data on wild summer steelhead for the Quinault River
system for 1954-1955, 1957, and 1962-1977 (Table 5, Raw data is in excel file “Summer
steelhead catch data for Quillayute_Hoh_Queets_Quinault River populations” and based on
data from .pdf file “Historic winter and summer steelhead tribal catch by month Hoh, Quinault,
Queets, Quillayute 40s-70s”). Mean catch of summer steelhead in 1954-55, 1957, and from
1962-1977 was 162 fish with annual catch ranging from 12 to 447 fish, and peak catch being
reported in 1954 (Table 5).
38
Assuming a 25% harvest rate and using bounds of 15% and 35% harvest rates, we
estimated a mean run size of 649 summer steelhead (Lower bound = 464, Upper bound =
1,624) with a low run size of 48 fish and a high of 1,788 (Table 6). The mean of the top-five run
sizes is 1,130 summer steelhead (Lower bound = 807, Upper bound = 1,884). This suggests
summer steelhead were very abundant in the Quinault River dating back to 1954. Further, as
with other populations, catch records were very low in several years, suggesting some of our
estimates are likely much lower than the true total run size.

39
Table 5. Catch of wild sumer steelhead in Quillayute, Hoh, Queets, and Quinault Rivers prior to onset of hatchery summer steelhead
releases in Quillayute system in 1977, with 1946-1957 representing tribal gillnet catch and 1962-1977 representing almost solely
recreational catch. Tribal catch data was only reported and included here for 1972 and 1975-1978 in Quillayute River system, 1975-
1976 in Hoh River, 1974-1977 in Queets River, and 1974-1976 in Quinault River. Bolded values represent peak catch for the period
of record for each population. Data is in excel file “Summer steelhead catch data for Quillayute_Hoh_Queets_Quinault River
populations” and based on data from .pdf file “Historic winter and summer steelhead tribal catch by month Hoh, Quinault, queets,
quillayute 40s-70s”),
Population 1946 1954 1955 1957 1962 1963 1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977
Quillayute
River
15 N/A N/A N/A 12 36 39 30 37 78 105 81 84 116 309 66 175 143 136 51
Hoh River N/A 133 N/A 39 59 106 126 112 160 137 106 105 125 142 274 169 188 66 92 72
Queets
River
N/A 373 29 136 111 143 230 220 266 225 299 239 217 337 345 267 315 385 171 124
Quinault
River
N/A 447 15 49 165 157 208 119 206 151 238 279 221 180 241 289 236 140 357 12

40
Table 6. Mean estimated run size of wild summer steelhead based on recreational and tribal catch data in Table 7, where we
estimated run size with an assumed 25% harvest rate and we estimated upper and lower bounds using 15% and 35% harvest rates,
respectively, for the top five run sizes for each population. Data from 1946-1957 is from tribal gillnet catch records. Data from 1962-
1977 almost solely consists of recreational catch, with tribal catch only being reported for 1972 and 1975-1978 in Quillayute River
system, 1975-1976 in Hoh River, 1974-1977 in Queets River, and 1974-1976 in Quinault River. Bolded values represent peak catch
for the period of record for each population. Calculations for each year’s run size, if not clear to the reader in this petition, can be
accessed in the excel file “Summer steelhead catch data for Quillayute_Hoh_Queets_Quinault River populations”
Population 1946 1954 1955 1957 1962 1963 1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977
Quillayute
River
60
N/A
N/A
N/A
48
144
156
120
148
312
420
(300-
700)
324
(231-
540)
336
(240-
560)
464
(331-
773)
848
(606–
1,413)
264
308
104
296
204
Hoh River
N/A
532
N/A
156
236
424
504
448
640
(457-
1,067)
548
(391-
913)
424
420
500
568
(406-
947)
1,096
(783-
1,827)
676
(483-
1,127)
520
152
360
288
Queets
River
N/A
1,492
(1,066-
2,487)
116
544
444
572
920
880
1,064
(760-
1,773)
900
1,196
(854-
1,993)
956
868
1,348
(963-
2,247)
1,380
(986-
2,300)
788
676
212
380
496
Quinault
River
N/A
1,788
(1,277-
2,980)
60
196
660
628
832
476
824
604
952
(680-
1,587)
1,116
(797-
1,860)
884
(631-
1,473)
720
964
(689-
1,607)
484
596
296
212
48
41
b. Queets River
Unlike the other populations, there are two historic data periods for Queets winter
steelhead: one from a cannery record in 1923 and another from tribal catch records from 1948-
1960. In 1923, 1,500 cases of wild winter steelhead were packed at the Queets River cannery,
with each case containing 48 cans at 1 lb per can, or 48 lb per case (McMillan et al. 2022). This
is equivalent to a total weight of canned winter steelhead of 72,000 lb. Based on a wastage rate
of 0.40 and a mean weight of 9.8 lb per Queets River winter steelhead, the number of wild
winter steelhead processed in 1923 is equivalent to 12,245 fish. Assuming a cannery
exploitation rate equivalent to the median contemporary exploitation rate (0.38), the total
1923 Queets return of wild winter steelhead is estimated to be 32,223 fish, with upper and
lower bounds of 43,732 and 27,829 fish, based on 75% and 25% quartiles of contemporary
exploitation rates (0.44, 0.28), respectively (McMillan et al. 2022).
Using historical catch, McMillan et al. (2022) estimated the average historical
abundance for winter steelhead in the Queets River from 1948-1960 was 19,875 steelhead
(lower and upper bounds for the historical mean: 13,025, 32,878) (Table 4). Annual variability
within the historical period ranged from the lowest and highest single year estimates in the
Queets River of 6,191 and 52,200 winter steelhead in 1960 and 1954, respectively. Using
historical CPUE and total accessible habitat produced average historical run size estimates of
13,553 and 12,144 steelhead, respectively (Table 4). During the peak estimate year in the
Queets River, which was the largest estimated run size amongst all years and populations, over
14,000 winter steelhead were harvested, including over 5,200 winter steelhead harvested in
the month of December alone. The average ensemble estimate for annual run size for all three
approaches was 15,191 steelhead.
We summarized catch data on wild summer steelhead for the Queets River system for
1954-1955, 1957, and 1962-1977 (Table 5: Raw data is in excel file “Summer steelhead catch
data for Quillayute_Hoh_Queets_Quinault River populations” and based on data from .pdf file
“Historic winter and summer steelhead tribal catch by month Hoh, Quinault, Queets, Quillayute
40s-70s”). Mean catch of summer steelhead in 1954-55, 1957, and from 1962-1977 was 200
fish with annual catch ranging from 29 to 373 fish, with peak catch being reported in 1954
(Table 5).
Assuming a 25% harvest rate and using bounds of 15% and 35% harvest rates, we
estimated a mean run size of 802 summer steelhead (Lower bound = 573, Upper bound =
2,004) with a low run size of 116 fish and a high of 1,492 (Table 6). The mean of the top-five run
sizes is 1,296 summer steelhead (Lower bound = 926, Upper bound = 2,160). Based on the catch
data wild summer steelhead were formerly abundant in the Queets River.
3. WRIA 19 Populations
As mentioned previously, we could not find quantifiable information on historic
abundance for populations in WRIA 19. However, there is likely some historic catch information
42
from WDFW punch-cards, and anecdotal references suggest that “the largest steelhead trout
populations were found in the Lyre, Pysht, and Hoko rivers. The Clallam and Sekiu rivers, as well
as Deep Creek also supported significant steelhead populations” (NOPLE 2015). Additionally,
each stream was fished for winter steelhead by Native American tribes that lived in the area,
including the Lower Elwha Klallam Tribe and the Makah Tribe.
B. Current Abundance and Population Trends (circa 1980-2020)
Data on run size and escapement is not available for all populations in the Olympic
Peninsula DPS (Table 1). The Steelhead at Risk Report for Washington State by Cram et al.
(2018) identified 31 populations of wild steelhead (24 winter run populations and 7 summer
run populations) in the DPS, which included delineating larger sub-basins within each major
watershed (e.g., counted Sol Duc, Bogachiel, Calawah, and Dickey River (tributaries in
Quillayute River watershed) as individual populations). We did not delineate the Upper and
Lower Quinault River into sub-populations per Cram et al. (2018). We otherwise delineated
populations the same as Cram et al. (2018). We identified 22 distinct watersheds, with the
Quillayute River system consisting of four sub-basins and the Queets River containing the
Clearwater River, resulting in 26 populations of winter steelhead (Table 1). The Hoh, Calawah,
Queets, and Quinault Rivers are known to support wild populations of summer steelhead,
though data is very limited because they are not monitored. There is also evidence of summer
steelhead in the Sol Duc, Bogachiel, and Clearwater Rivers (Table 1). This brings the total
number of populations to 30 for the entire DPS.
Cram et al. (2018) evaluated current abundance and population trends for winter
steelhead where data was available. Cram et al. (2018) reported that sufficient escapement
abundance data were available for 48% (15 of 31) of populations (Figure 2). We use Cram et al.
(2018), a report by NOPLE (2015), and a publication by McMillan et al. (2022) to summarize
existing information on the run size, escapement, and trends in run size (or escapement if only
escapement data is available). We also rely on McMillan et al. (2022) to compare contemporary
abundance (circa 1980 – 2018) to historical abundance (circa 1948-1960: Table 4) to illustrate
that contemporary populations, most of which are in decline (Cram et al. 2018, McMillan et al.
2022), had already experienced a high level of depletion before the onset of the modern
monitoring programs that began in the late-1970’s through the early-1980s (Table 4).

43
Figure 2 (from Cram et al. 2018). Abundance data for the Olympic Peninsula Steelhead DPS
populations available from years 1980 to 2013. The thick blue line represents the total
escapement (for the population or a portion of it, based on data availability). The thin grey line
represents total run size (escapement plus reported sport and tribal harvest plus, for Hoh River
population only, estimated non-tribal and tribal bycatch mortality). The dashed grey line is the
escapement goal. The light grey number is the percent change in abundance (increase or
decrease) over the time period with data.
In terms of numbers of populations, the Olympic Peninsula DPS is predominantly
composed of small populations of wild steelhead in small coastal creeks and streams (Table 1).
Data sets for most of those populations are less consistent compared to the larger, major
populations, which contribute the greatest proportion of fish to the cumulative total number of
wild steelhead in the DPS. We provide a synthesis of the location of each watershed (by WRIA),
the type of steelhead life history that is present, the period of record of monitoring, mean and
range for run size and escapement, and the status of the population trend – as either declining
or increasing, and the rate of decline (based on Cram et al. 2018, NOPLE 2015, and McMillan et
al. 2022). As we outline below, the data indicates that most populations of wild winter
steelhead are in decline, and comparatively, Cram et al. (2018) estimated the Olympic

44
Peninsula DPS had the second lowest proportion of populations with increasing trends (20%)
among all the steelhead population segments in Washington State (Figure 3).
Figure 3 (From Cram et al. 2018). Washington steelhead population escapement abundance
trends in each DPS in time periods from 1980-2013. N represents in the number of populations
in each DPS that had suitable abundance data for trend analysis and the percentage indicates
the proportion of populations that have increased in abundance since 1980.
Run size declines in wild winter steelhead have generally continued since the report by
Cram et al. (2018), with most populations reaching their lowest level of abundance in the past
several years (Figures 4-8). To account for the most recent data, we updated the most recent
five-year estimates by McMillan et al. (2022) to 2016-2020 instead of 2013-2017. Using that
adjustment, we estimate the Quillayute, Queets, Hoh, and Quinault River wild winter steelhead
populations have declined by 61%, 69%, 79%, and 81%, respectively, when comparing the most
recent five-year average run size to the historic estimates from the 1950s (Table 4). The
estimated decline for the Queets winter steelhead population increases to 86% when
compared to the 1923 cannery estimate in McMillan et al. (2022). Given the older cannery
record from the Queets River, declines are likely even greater than estimated in McMillan et al.
(2022) because their estimates only dated back to the 1950’s, which is after the initial boom in
logging and the operation of commercial salmon canneries at the mouths of the Quillayute,
Hoh, Queets, and Quinault Rivers in the early-1900’s.

45
The long-term, chronic declining trends coupled with a recent sharp downturn not
documented in Cram et al. (2018) puts the Olympic Peninsula Steelhead DPS at greater risk of
extinction than when Busby et al. (1996) conducted the last federal status review for the DPS
(ESU at the time). Further, as we outline below, the populations of wild summer steelhead are
almost all at critically low levels of abundance, something that was also not reported by Busby
et al. (1996), and could be facing extirpation in the near term if some are not already
functionally extinct.
1. Winter Steelhead
The four largest winter steelhead populations on the Olympic Peninsula have been on a
forty-year-long decline.
Figure 4 (From McMillan et al. 2022). Contemporary trends (circa 1980 – 2017) in wild winter
Steelhead abundance in the a) Quillayute, b) Hoh, c) Queets, and d) Quinault Rivers. Black lines
represent the best fit linear regression models.
a. WRIA 20 Populations
i. Hoh River
The Hoh River has one of the largest populations of wild winter steelhead in the Olympic
Peninsula DPS (Table 1). From 1980-2020 the mean annual winter steelhead run size of Hoh
River winter steelhead was 4,117 fish with annual run sizes ranging from 2,541 – 5,783 fish
(Table 1). The population was in a significant declining trend from 1980-2013 (Figure 2) and

46
from 1980-2017 (Figure 4), and it has experienced most of its lowest returns on record in the
past decade (Figure 5). Cram et al. (2018) estimated a 16% decline from 1978-2013, while
McMillan et al. (2022) estimated a decline of 37% from 1980-2017 (Table 1), which is equivalent
to a loss of 513 (95% CI: 320, 710) adults per decade (linear regression on log-transformed
abundance, F
1, 36
= 29.01, P < 0.001: McMillan et al. 2022). The decline has not reversed since
the analysis by McMillan et al. (2022) (Figure 5).
The Hoh River population of winter steelhead has failed to meet its escapement goal in
5 of the past 11 years (2010-2020), and it just barely made its escapement goal in the past two
years (Figure 5).
Figure 5. Annual run size and escapement of wild winter steelhead in the Hoh River from 1980-
2021 in relation to the escapement goal of 2,400 fish. Years when escapement falls below the
escapement goal indicate that the fishery did not meet its spawner target goal.
Compared to the historic period (circa 1948-1960), mean annual abundance in the
contemporary period (1980-2017) has declined 69% from its mean annual abundance during
the historical period (circa 1948-1960) (Table 4). The decline jumps to 79% when comparing the
mean historical abundance to the most recent five-year average run size (Table 4).
We believe this should be considered a minimum level of depletion because the
approach assumes all caught fish were sold and reported in the catch. This is unlikely, as Wilcox
(1898) indicated for the Quinault River in 1895: “…quite a large number of salmon are taken by
Indians for their winter supply of food, and a small amount … was sold to buyers…” It could
therefore be anticipated that a considerable number of steelhead caught on the Queets River in

47
1923 were used by Native Americans for their own winter food supply rather than sold to the
cannery (McMillan 2006). In addition, the historical estimates in McMillan et al. (2022) were
based on fish populations that had already experienced decades of habitat changes and the
onset of canneries and commercial fisheries.
If this downward trajectory continues, run sizes will begin to consistently come in below
the escapement goals, eliminating the potential for fisheries and potentially leading to a level
where they can no longer effectively compensate (e.g., Ward et al. 2000; Atlas et al. 2015).
ii. Quillayute River System
The Quillayute River supports the largest population of wild winter steelhead in the
Olympic Peninsula DPS. Its mean annual run size from 1978-2020 was 13,064 steelhead (range
= 6,456 – 21,615) (Table 1). The Quillayute River system is largely comprised of three large
tributaries, including the Sol Duc, Calawah, and Bogachiel Rivers, and one smaller tributary, the
Dickey River (Table 1). We summarize all of these populations as part of the “Quillayute River”
system for purposes of simplicity and because each of these populations is showing relatively
similar patterns in annual abundance (Figure 2).
Unlike the Hoh, Queets, and Quinault Rivers, there was not a simple linear trend for wild
winter steelhead run size in the Quillayute River (Figure 4). This is because winter steelhead
returns increased from 8,761 fish in 1980 to a peak of 21,615 fish in 1996. Since the peak in
1996, annual returns of winter steelhead have declined at a rate of 5,533 fish per decade
(Figure 4). Run sizes for more recent years, which were not included in McMillan et al. (2022),
suggest the recent declining trend has continued (Figure 6a). As a result, winter steelhead run
sizes in recent years have been the lowest on record. Although the Quillayute River population
has fared slightly better than the others, the co-managers implemented restrictive regulations
in the 2020 and 2021 seasons due to poor returns, raising concerns about the long-term
sustainability of the population and the fisheries it supports.
a.

48
b.
c.
d.
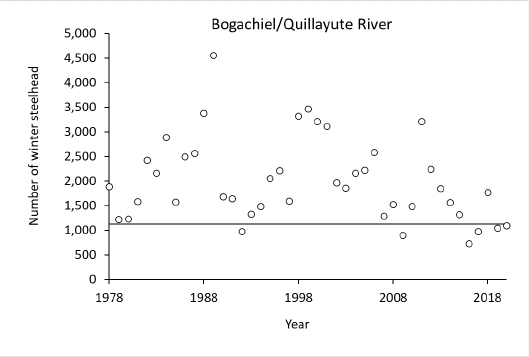
49
e.
Figure 6. Annual run size and escapement of wild winter steelhead in a) entire Quillayute River
watershed system, and annual escapement of wild winter steelhead in the four major individual
tributaries, which include the b) Dickey River, c) Sol Duc River, d) Calawah River, and e)
Bogachiel and Quillayute River proper. Black circles represent run size while open circles
represent escapement. The escapement goals for individual rivers within the Quillayute River
system are as follows: Calawah River (1,740 fish), Sol Duc River (2,910 fish), Bogachiel and
Quillayute River proper (1,127 fish), and Dickey River (123 fish), resulting in a system-wide goal
of 5,900 fish (Duda et al. 2018). Years when escapement falls below the escapement goal
indicate that the fishery did not meet its spawner target goal.
Mean annual abundance of wild winter steelhead in the Quillayute River from 1978-
2017 has declined by 38% compared to historic mean annual abundance from 1948-1960 (Table
4). The decline increases to 61% when compared to the mean annual abundance for the most
recent five-year period (Table 4). If the population continues to decline at its current rate
(1996-2020), run sizes in the coming decade to decades could – on average – come in lower
than the escapement goal, effectively eliminating the potential for future fisheries while
simultaneously increasing the risk of extinction.
iii. Other Populations
There are four other small populations of wild winter steelhead in WRIA 20, including
the Tsoo-Yess, Wa’atch, and Ozette Rivers, which are located north of the Quillayute River, and
Goodman Creek and Mosquito Creeks, which are located between the Quillayute and Hoh
Rivers.
Goodman Creek is the only stream for which we could find monitoring data on
escapement (Table 1). From 1995-2020 the mean annual escapement was 184 wild steelhead
(range of 45 – 374) and consequently, it has not met its escapement goal of 206 fish once in the
past decade ending in 2020 (Table 1). In fact, Goodman Creek has met its escapement goal only
once since 2004. Cram et al. (2018) estimated the average annual escapement has declined by
50
54% over its period of record, and the decline has continued through 2020. Data on run size
and escapement was otherwise not available.
b. WRIA 21 Populations
i. Quinault River
From 1978 to 2020 the mean annual winter steelhead run size was 5,883 fish (range =
2,179 – 9,726) in the Quinault River (Table 1). Unlike the Hoh and Quillayute Rivers, the
Quinault River is managed in two sections, including: (1) the lower part below the lake, which is
owned and managed by the Quinault Indian Nation, and (2) the upper section above the lake,
which is owned mostly by the Olympic National Park and its fish managed by WDFW (Figure 2,
from Cram et al. 2018). There is no escapement goal for the lower Quinault River, but there is
an escapement goal of 1,200 fish for the upper section (Cram et al. 2018).
There is a significant declining trend in Quinault River wild winter steelhead (Table 1).
Cram et al. (2018) estimated a decline of 69% for escapement of wild steelhead in the Lower
Quinault River and an increase of 24% in escapement for the Upper Quinault River (Figure 2).
McMillan et al. (2022) grouped the upper and lower river because run size cannot be estimated
for each component of the population separately, and they estimated a 44% decline (Table 1).
The McMillan et al. (2022) estimate equates to a loss of 1,052 (590 - 1,510) adults per decade
(Figure 4; F
1, 32
= 21.20, P < 0.001: McMillan et al. 2022). We have access to the full data set
from 1978-2020 (included in our data package) and the declining trend has continued, with the
smallest run sizes on record occurring in the most recent five-year period (Figure 7). The mean
escapement has been 3,107 fish with a range of 1,366 to 5,774 fish (Table 1). While it is unclear
whether the population would be meeting a biologically defensible escapement goal for the
whole watershed, the declining trend is concerning and more restrictive fishery practices,
including early closures, were implemented in 2020 and 2021 to limit impacts on the small run
sizes.

51
Figure 7. Annual run size and escapement of wild winter steelhead in the Quinault River from
1978-2021. There is no escapement goal for the entire Quinault population, as it is delineated
into Upper (above the lake) and Lower (below the lake) populations. Figure 2 illustrates the
trend by Cram et al. (2018) for the Upper and Lower Quinault populations.
Overall, McMillan et al. (2022) estimated that the contemporary mean annual
abundance of wild winter steelhead in the Quinault River from 1980-2013 declined by 63%
when compared to historic mean annual abundance from 1948-1960 (Table 4). The decline
increases to 80% when compared to the mean annual abundance for the most recent five-year
period (Table 4). If the decline rate of 1,052 adults per decade continues, Quinault River winter
steelhead will decline from their recent five-year average of 3,370 fish down to 2,318 fish
during the following decade. This concern is particularly heightened now because the dramatic
declines in 2019 and 2020 highlight how quickly the population could further deplete (Figure 7).
ii. Queets River
From 1980-2018 the mean annual winter steelhead run size was 7,648 fish (range =
4,200 – 13,309) in the Queets River (Table 1). Like the Hoh and Quinault Rivers, there is a
significant declining trend for winter steelhead in the Queets River from 1980-2017 (Figure 4),
which includes a 12% decline in the escapement trend for its major tributary, the Clearwater
River, from 1980-2013 (Figure 2). The extent of decline is greater in McMillan et al. (2022) (45%
decline) because Cram et al. (2018) (29% decline) only used data up until 2013, while McMillan
et al. (2022) included data up until 2018. The run size continued to decline after 2013 (Figure 4).
The decline reported by McMillan et al. (2022) equates to a loss of 1,220 (640, 1,820) adults per
decade (F
1, 36
= 19.56, P < 0.001).

52
Unlike the Hoh River population, the co-managers have not reached an agreement on
an escapement goal for the Queets River population, which has affected management for
several decades. The Queets Tribe uses a goal of 2,600 fish, while WDFW uses a goal of 4,200
fish (Cram et al. 2018). We show escapement in relation to the latter (Figure 8a) because it is
the only goal for which we can find scientific support (Gibbons et al. 1985). The Clearwater
River is a major tributary of the Queets watershed that has a separate escapement goal of
1,450 fish (Cram et al. 2018). Clearwater steelhead run size and escapement is included in the
overall data for the Queets River population, but Figure 8b depicts the escapement of wild
winter steelhead for just the Clearwater River. As a result of the sharp declines in run size in
recent years (Figure 8a), the Queets River fishery (as with the Quinault River) was closed to
protect small run sizes.
a.
b.
Figure 8. Annual run size and escapement of wild winter steelhead in the a), Queets River from
1980-2020 and b) escapement of wild winter steelhead in the Clearwater River, a major
53
tributary. Black circles represent run size, open circles represent escapement. In the Queets
River panel, the bold line depicts the 4,200 fish escapement goal (Escapement goal 1) that is
used by WDFW and is recognized by the National Park Service (a substantial portion of the
Queets River watershed lies within the Olympic National Park) and the 2,500 fish escapement
goal used by the tribe (Escapement goal 2). The bold line in the Clearwater River panel refers to
WDFW’s escapement goal of 1,450 fish. Years when escapement falls below the escapement
goal indicate that the fishery did not meet its spawner target goal.
The mean annual abundance of wild Queets River winter steelhead from 1980-2017 has
declined by 50% when compared to historic mean annual abundance from 1948-1960 (Table 4).
The decline increases to 69% when compared to the mean annual abundance for the most
recent five-year period (Table 4). However, as we mentioned previously, the declines could be
more substantial. For example, McMillan et al. (2022) analyzed one year of cannery data in
1923, when more than nominal cans of steelhead were reported. The run size estimate is
32,659 wild steelhead (Table 4), which would represent a decline of 86% compared to the most
recent five-year average run size. If the decline rate of 1,220 adults per decade continues, wild
winter steelhead abundance in the Queets River will decline from its recent five-year average of
4,658 fish down to an average of 3,438 fish during the following decade. Like the Hoh River, this
suggests that in just one decade run sizes would be coming in under the 4,200 fish escapement
goal set by WDFW and would be encroaching on the 2,400 fish goal set by the tribe.
Unfortunately, run sizes in recent years have declined rapidly, resulting in fishery
closures and suggesting that the population is on a chronic path that could lead to extinction
unless the 40-year trend is reversed.
iii. Other populations
There are four smaller populations of wild winter steelhead in WRIA 21, including
Kalaloch Creek, Moclips River, Raft River, and the Copalis River populations (Table 1). Among
those populations, we could find escapement data only for the Moclips River, which spans a
relatively short period that is not up to date. From 1988-2000, the mean escapement in the
Moclips River was 299 steelhead (range = 130 – 560), and based on that data set, Cram et al.
(2018) reported a 27% increasing trend in abundance (Table 1 and Figure 2). Given the limited
period of record and overall missing data, it is difficult to draw conclusions about the status of
these populations.
c. WRIA 19 Populations
There are ten small streams in WRIA 19 that support populations of wild winter
steelhead (Table 1). The Hoko River has the largest population with a mean annual escapement
of 566 fish (range = 193 – 990). The Hoko River has met an escapement goal of 400 fish in 80%
of the last ten years (Table 1). Based on the period of record, however, the Hoko River
population declined by 40% from 1985-2013 (Table 1) and that decline has not subsided in

54
recent years (WDFW Score website, Accessed 3/20/2022:
https://fortress.wa.gov/dfw/score/score/species/population_details.jsp?stockId=6357).
The Pysht River (and tributaries) and the Clallam River support the second and third
largest populations, respectively, and both populations are in long-term decline (Cram et al.
2018). Cram et al. (2018) estimate that winter steelhead abundance declined by 21% in the
Pysht River from 1995-2013 and 27% in the Clallam River from 1999-2013 (Table 1). More
recent data indicates these declines have continued through 2020 in the Pysht River (WDFW
Score website, Accessed 3/20/2022:
https://fortress.wa.gov/dfw/score/score/species/population_details.jsp?stockId=6343) and the
Clallam River populations (WDFW Score website, Accessed 3/20/2022:
https://fortress.wa.gov/dfw/score/score/species/population_details.jsp?stockId=6350).
Consequently, the Pysht River population has only made its escapement goal in 70% of the past
ten years (Table 1).
Salt Creek supports the fourth largest population in WRIA 19. The population has only
met its escapement goal once in the past decade (Table 1) and once overall since 2004. Cram et
al. (2018) estimated wild steelhead abundance declined by 43% from 1995-2013, and more
recent data suggests the decline has not reversed but may have stabilized (WDFW Score
website, Accessed 3/20/2022:
https://fortress.wa.gov/dfw/score/score/species/population_details.jsp?stockId=6329).
Escapement estimates are also available for populations in Deep Creek and the East and
West Twin Rivers from circa 1995 through 2020 (WDFW Score website, Accessed 3/20/2022:
https://fortress.wa.gov/dfw/score/score/species/population_details.jsp?stockId=6343). Using
this data set, a regional recovery document suggests that all three populations of wild
steelhead were in decline and there is a high level of concern about the depressed status of the
stocks (NOPLE 2015). Of particular concern, wild winter steelhead have only met the
escapement goal of 86 steelhead once in the past decade (Table 1) and in two of the past
twenty years. Even worse, the population in West Twin River has missed its escapement goal of
103 fish every year in the past decade (Table 1) and every year dating back to 2001. We could
not find any estimates of escapement for the Sekiu, Sail, and Lyre Rivers (Table 1).
2. Summer Steelhead
The co-managers do not monitor any populations of wild summer steelhead on the
Olympic Peninsula, nor do they have any established escapement goals for these populations.
The only contemporary data on abundance and distribution of adult summer steelhead comes
from snorkel surveys conducted by Olympic National Park (e.g., Brenkman et al. 2012), which
manages summer steelhead habitat and fisheries in the park, and McMillan (2022) (Table 7).
Those snorkel surveys were conducted in the Sol Duc River, Calawah River, SF Calawah River, NF
Calawah River, Sitkum River, Bogachiel River, SF Hoh River, and the EF and NF Quinault Rivers.
Snorkel surveys were generally conducted annually from September through October and,
occasionally early November, to ensure summer steelhead had time to migrate through the

55
watershed and reach the upper most habitats where they stage for several months before
spawning from winter through spring.
Table 7. Counts of summer steelhead via snorkel surveys in the Calawah, Sitkum, SF Calawah,
NF Calawah, SF Hoh, Sol Duc, EF Quinault, and NF Quinault Rivers, including year(s) of surveys,
mean abundance, and mean proportion of hatchery steelhead (Figures 9 – 11). Data from
McMillan (2022) is based on snorkel surveys conducted in early September through early
November, while data from Sam Brenkman et al. is based on snorkel surveys conducted in late
summer (Brenkman et al. 2012). Standard Deviation and range is provided for McMillan (2022),
while only range is provided for Brenkman et al. (2012).
Study and population Year (s)
Mean abundance
(SD and range)
Mean proportion
hatchery steelhead
(SD range)
McMillan 2022
Calawah River system
1
2002
89 (wild),
214 (hatchery)
N/A
Sitkum River
2002 – 2006,
2009 – 2021
53 (25.2, 19-105) 3% (3%, 0% – 10%)
SF Calawah River 2003 – 2006
18 (5.6, 10 – 23) 7% (6%, 0% – 13%)
NF Calawah River
2000 – 2006,
2009 – 2021
4 (4.1, 0 – 14) 33% (35%, 0% – 100%)
SF Hoh River
2000 – 2006,
2009 – 2014,
2016 – 2019
7 (3.9, 1 – 16) 41% (12%, 20% – 67%)
Brenkman et al. 2012
Bogachiel River 2005 – 2010 15 (8 – 26) 13% (0% – 23%)
Sol Duc River 2005 – 2010 6 (0 – 15) N/A
SF Hoh River 2005 – 2010 8 (4 – 12) 40% (14% – 76%)
EF Quinault River 2005 – 2010 17 (4 – 35) 16% (0% – 38%)
NF Quinault River
2005 – 2010
3 (0 – 8)
43% (0% – 100%)

56
1
Calawah River survey data from 2002 is a census of the entire watershed accessible to adult summer steelhead in
September and October.
Below we summarize the results of the surveys and when possible, compare them to
our historic estimates. The findings generally suggest that wild summer steelhead now only
exist at exceptionally low abundance (Brenkman et al. 2012; McMillan 2022) and are headed
towards extirpation if their status and trends are not rapidly improved.
a. WRIA 20 Populations
i. Hoh River
Snorkel surveys on the Hoh River have been conducted by the National Park Service
(Brenkman et al. 2012) and McMillan (2022). These surveys focused on several long sections
where summer steelhead were observed staging during previous years. The results are not a
census, but rather, an estimate of peak abundance in those important habitats.
The snorkel surveys in the SF Hoh River by Brenkman et al. (2012) sometimes covered a
longer section of stream than McMillan (2022), though not always, and in most years their
surveys overlapped spatially. Unfortunately, both sets of surveys indicate a very low abundance
of wild summer steelhead. Brenkman et al. (2012) data cover the period from 2005 – 2010
while surveys by McMillan (2022) ranged from 1999-2006, 2009-2014, and 2016-2019.
Brenkman et al (2012) reported a mean abundance of eight wild summer steelhead with peak
annual peak counts of 12 and 16 fish (Table 7). The longer time series by McMillan (2022)
suggests summer steelhead abundance has remained low, with a mean abundance of seven
wild summer steelhead (Figure 9). The lowest abundance estimates have almost all occurred
from 2016 to 2020 (McMillan 2022).
57
Figure 9 (From McMillan 2022). Box-whisker plot displaying mean (+) annual abundance of wild
and hatchery summer steelhead counted during snorkel surveys from late-summer through
early-fall in the SF Hoh River (1999-2006, 2009-2014, 2016-2019). Internal rectangle indicates
95% confidence interval for the mean. Tukey whiskers extend to data points that are less than
1.5 interquartile range away from 1
st
/3
rd
quartile.
Both surveyors also reported high levels of hatchery summer steelhead, including more
hatchery than wild adults in some years (Table 7). For example, the mean abundance of four
hatchery steelhead was not significantly lower than the mean estimate of seven wild steelhead
(Figure 9). Hatchery summer steelhead are not released into the Hoh River, but hatchery
summer steelhead could have strayed from their release locations in the Bogachiel or
Wynoochee Rivers, eventually ending up in the headwaters of the SF Hoh River.
Unfortunately, there is almost no information on summer run abundance in the
mainstem Hoh River, which likely supports most of the population. Nonetheless, there is also
little evidence to suggest that mainstem component is faring any better, even if more
abundant. Overall, the data indicate that SF Hoh River wild summer steelhead exist at low levels
of abundance. Considering our rough historic estimates of population size based on
recreational catch – a mean run size of 472 steelhead with a high of 1,096 fish (Table 6) – it is
likely that the SF Hoh River sub-component is critically depleted, and that the entire population
could be threatened with extinction.
ii. Quillayute System
Data on abundance of wild summer steelhead is limited to snorkel surveys conducted in
the Sol Duc, Bogachiel, Calawah, NF Calawah, SF Calawah, and Sitkum Rivers (Table 7). Surveys
by Brenkman et al. (2012) found a mean abundance of 15 wild summer steelhead in the upper
Bogachiel River from 2005 – 2010 and only six wild summer steelhead in the upper Sol Duc
River (Table 4). In some years no wild summer steelhead were observed in the Sol Duc River
surveys conducted by Brenkman et al. (2012).
McMillan (2022) conducted snorkel surveys in the NF Calawah River, Sitkum River, and
SF Calawah River (only 2003 – 2006) from circa 2000 – 2006 and from 2009 – 2021. These
locatoins are thought to be the predominant staging and spawning areas for summer steelhead
(McMillan 2006). Data is also available from a snorkel census in 2002 that covered 85% of all
habitats available to adult steelhead during the summer (e.g., McMillan et al. 2013). The diver
counted adult steelhead during the survey but did not publish the data in McMillan et al. 2013.
During the 2002 census, a total of 89 wild summer steelhead and 214 hatchery summer
runs were counted (Table 7). Snorkel surveys in the Sitkum River and NF Calawah River
produced a mean annual abundance of 53 and five wild summer steelhead, respectively (Figure
10a and 10b). Peak abundance in the Sitkum River snorkel counts was 105 wild summer runs in
2005, but its lowest estimates have dropped to 19 fish (Table 7) and counts were less than 30
adults in 2020 and 2021 (McMillan 2022). Surveys of sections of the upper SF Calawah from

58
2003-2006 suggest it also has summer steelhead staging and spawning habitat, though overall
abundance appears lower than in the Sitkum (McMillan 2022). Counts of wild summer
steelhead in the NF Calawah River have generally ranged between 0 – 10 fish (Figure 10b), with
the two peaks occurring in 2001 and 2002 (McMillan 2022). A drought in 2002 dewatered all of
the lower NF Calawah except for the lower-most 1km of stream, which killed thousands of
salmonids including all the adult steelhead, and since then, abundance of summer runs has
been at very low levels (McMillan 2022). In fact, zero adult steelhead were counted in the NF
Calawah from 2014-2021 except for 2015, when a single adult was observed (McMillan 2022).
The snorkel survey data has not been adjusted to account for potential sources of error in the
diver counts, and there is not a whole basin estimate for summer steelhead abundance. With
those caveats, the current counts still suggest the Quillayute River system population is severely
depleted in comparison to the historical catch estimates.
a.
b.
59
Figures 10 a-b (From McMillan 2022). Box-whisker plot displaying mean (+) annual abundance
of wild and hatchery summer steelhead counted during snorkel surveys from late-summer
through early-fall in the, a) Sitkum River (2003-2006, 2009-2019), and b) NF Calawah River
(2000-2006, 2009-2021). Internal rectangle indicates 95% confidence interval for the mean.
Tukey whiskers extend to data points that are less than 1.5 interquartile range away from
1
st
/3
rd
quartile.
b. WRIA 21 Populations
i. Quinault River
The only data we could find on the abundance of summer steelhead in the Quinault
River is from Brenkman et al. (2012). They conducted snorkel surveys in the EF and NF Quinault
Rivers from 2005 – 2010. The mean annual abundance of wild summer steelhead during those
surveys was 17 and three wild steelhead in the EF and NF Quinault Rivers, respectively (Table
7). The surveys suggest the Quinault River population of wild summer steelhead has
dramatically declined from the historic estimates we calculated based on recreational catch
from the 1950s through 1976, which show a mean run size of 649 summer steelhead with a
peak run size of 1,788 fish (Table 6). Although the current run size is unknown, it appears the
population of summer steelhead in the Quinault River is less than 1% of its historic abundance
and is close to extirpation.
ii. Queets River
The are no snorkel survey counts to determine the population trend of summer
steelhead in the Queets or Clearwater Rivers. McMillan (2006) estimated that, based on
available catch data, the run size of wild summer steelhead returning to the Queets and
Clearwater Rivers combined was no more than 100 fish. If the population is close to 100 fish,
that would suggest the population is now only a fraction of the historic estimate we calculated
based on catch, where the mean population size was 802 summer steelhead and the peak was
1,492 fish (Table 6). This uncertainty creates a great deal of concern for the status and
trajectory of the population moving forward.
XI. PRODUCTIVITY
In comparison to estimates of abundance, productivity data is very limited for the
Olympic Peninsula Steelhead DPS. Cram et al. (2018) reported on nine productivity data sets in
the Olympic Peninsula DPS from circa 1980-2013, eight of which focused on spawner-per-
spawner production (Figure 11) and one that focused on smolt-to-adult return survival (Figure
11). Hall et al. (2016) reported on the productivity of adults to juveniles in the Twin Rivers. We
reviewed the co-managers’ annual data sets and could only find productivity information for
three major populations, including 1) recruits-per-spawner data for the Quillayute River (1978-
2015), 2) Hoh River (2000-2016) populations of wild winter steelhead, and 3) a raw estimate of
number of adult returns per wild/natural winter steelhead smolt in the Queets River (1982-

60
2013). Cram et al. (2018) did not list which populations were included in their productivity
estimates, but it would seem likely that our data sets overlap.
Figure 11 (From Cram et al. 2018). For each DPS, population growth rates represented as the
log of the numbers of natural-origin adult spawners produced per spawner for all populations
with suitable data for brood years 1970 to 2010. The thick black line represents the average
value for each DPS, while the thin lines represent data from individual populations within
each DPS. The y-axis numbers in parentheses for the Puget Sound figure are the non-
transformed values for reference. A log productivity of 0, shown by the red lines, corresponds
to an untransformed productivity of 1 spawner per spawner (replacement). Data are available
for multiple populations in each DPS that occur in Washington, including: 7 populations in the
Puget Sound DPS; 8 in the Olympic Peninsula DPS; 5 in the Southwest Washington DPS, 8 in
the Lower Columbia River (LCR) DPS, 1 in the Mid-Colubmia River (MCR) DPS, 4 in the Upper
Columbia River (UCR) DPS, and 3 in the Snake River Basin (SRB) DPS.
There appears to be a slight decline in spawner-to-spawner recruitment from 1978-2010
for wild winter steelhead in the Olympic Peninsula DPS, and after the mid-1990’s, the
populations increasingly fail to replace themselves (Figure 11, Cram et al. 2018). Smolt-to-adult
return declined significantly over time for the single wild winter steelhead population evaluated
by Cram et al. (2018) (Figure 12).

61
Figure 12 (from Cram et al. 2018). For each DPS, smolt survival, estimated as smolt-to-adult
return (SAR) rates of natural-origin steelhead spawners from smolt outmigration year 1975 to
2011 (16 populations represented: 2 populations and 1 sub-population in Puget Sound; 4 sub-
populations and 1 population in Olympic Peninsula; 1 population each in Southwest
Washington, MCR, and UCR DPSs; 3 populations in the LCR DPS; and 2 populations in the SRB
DPS). The thick black line represents the average SAR value for each DPS, and the thin grey lines
represent data from individual populations therein.
Cram et al. (2018) indicated recruits-per-spawner productivity was not available for any
populations in the DPS. However, the co-manager data sets we analyzed referred to “recruits
per spawner” estimates for the Quillayute and Hoh Rivers. Perhaps this is just a minor
difference in terminology. We include data on recruits per spawner and extend the productivity
period of record for the Quillayute River population to 1978-2015 and the Hoh River population
to 1990-2016, both of which suggest a continued declining trend (Figure 13a).

62
a.
b.
Figure 13. Estimates of wild steelhead productivity, including a.) recruits per spawner for wild
winter steelhead in Quillayute (1978-2015 brood years) and Hoh Rivers (1990-2016 brood
years), and b.) smolt to adult return data for wild winter steelhead in Queets River from 1982-
2013. Dashed line in Panel a.) represents replacement of 1 recruit/spawner, while dashed in
Panel b.) represents mean smolt to adult return rate for Queets wild steelhead during period of
record, which is 0.15.
Of particular concern, productivity has been below replacement (recruits per spawner =
1.0) in four of the past ten years in both the Quillayute and the Hoh River populations, and the
Hoh has been below 1.0 in ten (50%) of the past twenty years (Figure 13a). There is no clear

63
trend in the Queets River population smolt-to-adult return data (Figure 13b), largely because of
two peaks in survival that seem abnormally high. Perhaps the co-managers adjust their
estimates or there were errors in the data sets that we had access to.
Cram et al. (2018) also reported that variation in freshwater per-capita productivity
among populations in the Olympic Peninsula DPS declined as abundance increased, as did Hall
et al. (2016) for the Twin Rivers. Indeed, the estimates of recruits per spawner for the
Quillayute (Figure 14a) and Hoh River populations (Figure 14b) reveal a similar pattern. As Cram
et al. (2018) described, the relationships suggest there are potential density dependent effects
on productivity (Walters et al. 2013; ISAB 2015). This could suggest habitat is limiting (Cram et
al. 2018). However, density-dependence effects on growth can be highest at low densities
(Lobon-Cervia 2007), and there are other factors to consider before we can conclusively
determine that productivity is being solely limited by habitat potential and that the populations
have the compensatory capacity to rebuild from their current low levels of abundance.
Figure 14a. Estimates of recruits per spawner for wild steelhead populations in the Quillayute
River.

64
Figure 14b. Estimates of recruits per spawner for wild steelhead populations in the Hoh River
(1990-2016).
The first factor to consider is the lack of data pointing to specific causes of productivity
declines. While there is suggestive evidence of density dependence in the Quillayute and Hoh
River populations based on the adult-to-adult recruits per spawner estimates, the factors
limiting these populations are unknown due to a lack of data on juveniles and smolts. There has
not been a systematic, long-term decline in marine survival on the Washington Coast (Kendall
et al. 2017; Welch et al. 2018), so it is possible that fishery, hatchery, and habitat effects have
combined to reduce the productivity of the populations. For example, the historic run timing of
wild winter steelhead has changed dramatically in the Quillayute, Hoh, and Queets River
populations with early-entering wild fish being replaced by hatchery fish (Figure 15; McMillan
et al. 2022), resulting in compressed migration timing that could increase density-dependent
effects by reducing spatial and temporal distribution and diversity of adults and juveniles.
Several studies on Atlantic salmon (Einum et al. 2006, 2008; Teichert et al. 2011; Finstad
et al. 2013) indicate that contraction in spatial distribution can lead to heightened density
dependence even without changes in habitat conditions. In this vein, declines in abundance
could have also resulted in reduced spatial distribution of spawners (Isaak and Thurow 2006),
which in turn could contribute to depensation (Atlas et al. 2015). There is also evidence that
diversity in run timing can help stagger the use of habitat by newly emerged offspring,
essentially increasing the productivity of the existing habitat (Gharett et al. 2013).
Additionally, the frequency of repeat spawners – which can be the most productive
individuals (Christie et al. 2018) – has apparently declined in the Queets and the Hoh River
populations, and they currently occur at low levels in the Quillayute River and are almost non-
existent in the Quinault River (Figure 16). Because life history diversity is critical to the
productivity and resilience of steelhead (Moore et al. 2014), it is plausible that productivity will

65
continue to decline because the original fabric of diversity has been greatly reduced. Although
this is postulation, the dearth of information outside the adult-to-adult data does not provide
the65 necessary insight to determine how and when population productivity is being regulated.
Figure 15 (from McMillan et al. 2022). Historical (circa 1955–1963) and contemporary (2000 -
2017) migration timing estimates based on CPUE of wild and hatchery winter steelhead (left
panels) in the (A–B) Quillayute, (C–D) Hoh, and (E–F) Queets rivers, and comparison of
cumulative run timing with estimates of dates at which 25% (q25), 50% (q50), and 75% (q75) of
the run had passed for historical and contemporary wild winter steelhead (right panels). Dark
gray lines and triangles represent contemporary hatchery returns; black lines and black circles
represent historical wild returns; and light gray lines and light gray circles represent
66
contemporary wild returns. Run timing estimation in the (G) Quinault River was limited to the
historical period because contemporary CPUE data were not available.
The second factor to consider is that productivity estimates for the major populations of
wild winter steelhead in Cram et al. (2018) ended in 2010 and the co-manager data sets we
accessed (ending in 2015) do not account for the recent declines in run size in the Quillayute,
Hoh, and Queets River populations. We also could not find estimates of productivity for the
Quinault River population (though we assume the co-managers do have estimates of
productivity). It will take several years for the productivity estimates to be updated so that we
can better understand the compensatory dynamics that have occurred over the past decade.
Petitioners are concerned that the populations are not compensating as effectively now
as they did 20-40 years ago for several reasons. For example, there are increasingly low levels
of productivity in the Quillayute River population (Figure 14a). Additionally, the peaks and
troughs in annual run size for the Hoh, Queets, and Quinault River populations have generally
declined each decade over the period of record (i.e., lower peaks, deeper troughs). These
factors have led to high levels of depletion in recent years (Figures 5-8),
The third factor to consider is that there is no data on the productivity of summer
steelhead stocks, nearly all of which are completely unstudied. This is a very large data gap.
Considering the declines in wild winter steelhead abundance and productivity, there is a high
level of risk that summer steelhead are facing an even more dire situation.
The fourth factor to consider is that there is tremendous uncertainty about productivity
for numerous smaller populations of wild winter steelhead that inhabit independent tributaries.
While these populations are indeed smaller, they collectively represent an important
component of diversity that spatially covers a much larger extent of the DPS than the four
largest, major populations. An HSRG (2004) report claims productivity is limiting for 15 ½ out of
16 wild steelhead populations (the Quinault River winter steelhead received two ratings), and
they associated the reduced productivity with degraded and “inadequate” habitat conditions.
And a report by NOPLE (2015) indicates the number of smolts produced in the following WRIA
19 populations had declined: Salt Creek (1995-2006), East Twin River (2001-2005); West Twin
River (2001-2005); Deep Creek (1998-2005). NOPLE (2015) did not provide smolt production
numbers for the Pysht, Clallam, or Hoko River populations.
In sum, the general assumption, based on fish data and habitat evaluations, is that wild
steelhead productivity is in long-term decline for most populations in the Olympic Peninsula
DPS. That trend, combined with depleted abundance and diversity, puts them at greater risk of
extinction in the coming decades.
XII. DIVERSITY
A robust population should maintain both genotypic and phenotypic diversity and have
distributions that are spatially and temporally diverse. The array of life history diversity of a
67
population is analogous to a “Portfolio” in the financial realm, where having different life
histories is like having a more diverse financial portfolio (Schindler et al. 2010). The diversity
helps buffer individuals and populations from environmental effects, the cumulative effects of
which improve the performance and resilience of populations with stronger and more diverse
portfolios (Schindler et al. 2010). Moore et al. (2014) applied this to steelhead populations in
British Columbia and found that life history diversity helped dampen fluctuations in population
abundance and increased their resilience to environmental factors.
Overall, there is very little data on the genetic diversity of wild steelhead on the Olympic
Peninsula or on the genetic relationships between the several apparently demographically
independent populations. However, there is evidence that life history diversity has been
reduced in several ways that could compromise their ability to remain productive and resilient
in the face of a changing climate. Important elements of steelhead life-history include: the
frequency and age-specific proportions of repeat spawners; proportions of smolt ages of each
sex; proportions of spawner ages of each sex; size-specific fecundity; and timing of migrations
to spawning habitat.
Repeat spawning is particularly important to the resilience of steelhead populations
(Hard et al. 2015; Gayeski et al. 2016). For example, available historical (Withler 1966) and
contemporary data from Kamchatka (Pavlov et al. 2001) indicate that the proportion of repeat
spawners in the annual returns of the area’s steelhead population historically exceeded 20-30%
of total spawners. This is also true for the Queets River, which had relatively high levels of
repeat spawners in the 1980s (Figure 16). Such relatively high levels of repeat spawning
suggests that this is a life-history characteristic to compensate for high and/or variable juvenile
marine survival rates. When this is the case, the proportion of repeat spawners can determine
whether a population can sustain levels of recruits per spawner above 1.0 (see Gayeski et al.
2016, pp. 92-93, and Table 1).

68
Figure 16. Proportion of repeat spawning individuals (repeats/total run size) by year for wild
winter steelhead populations in the Quillayute (1978-2021), Hoh (1993-2020), Queets (1980-
2019), and Quinault Rivers (1991-2020).
A. Summer Steelhead
The summer run life history represents an important component of the overall diversity
of the Olympic Peninsula Steelhead DPS. There is a heritable basis to the summer run life
history in steelhead (Prince et al. 2017; Fraike et al. 2021) that allows them to enter streams in
a highly immature state and stage for several months to up to a year in freshwater before
spawning. Entering in summer when temperatures are warmer also helps allow them ascend
waterfalls that winter runs cannot, providing them with access to habitats to headwater
habitats that would otherwise go unused. Thus, losing the summer life history component could
reduce, and likely already has, the spatial distribution of Olympic Peninsula steelhead. The
extinction of summer steelhead would also create large gaps in migration timing, hindering
each population’s ability to adapt to and keep pace with changing selective pressures
associated with climate change.
As noted by McMillan (2006), the situation for summer steelhead on the Olympic
Peninsula is dire:
“It is apparent that the Olympic Peninsula summer steelhead populations
examined are at the edge of extirpation. The Quinault population may be the
most dire, with estimated returns of less than 50 fish for the entire watershed
whose spawning destinations are further reduced in their split between the
69
North and East forks – potentially less than 25 fish destined for each. The
Clearwater population of the Queets system, and the Sol Duc and Bogachiel
populations of the Quileute system may be similarly low, with only 2-3 dozen fish
returning to each. In fact, the Quinault, Clearwater, Sol Duc, and Bogachiel
populations may already be functionally extinct.”
Snorkel data from Brenkman et al. (2012) and McMillan (2022) suggests the situation is
even worse now compared to McMillan (2006).
Considering the allelic variants for early migration have not arisen independently via
new mutations (Prince et al. 2017) and the life history is at critically low levels of abundance, it
is likely the genetic integrity of the populations is impaired. Consequently, there is great
concern wild summer steelhead populations are close to extinction and their demise could lead
to irreparable loss of the genetic basis for premature migration in the Quillayute, Hoh, Queets,
and Quinault River populations.
B. Early Returning Winter Steelhead
There is strong evidence that the migration timing of wild winter steelhead has changed
significantly from its historic norm (McMillan et al. 2022). Migration and run timing are strongly
heritable in salmonids such as steelhead (Carlson and Seamons 2008; Abadıa-Cardoso et al.
2013) and differences in migration and spawn timing represent adaptive responses that
maximize their reproductive fitness to local environmental conditions (Brannon et al. 2004).
However, there is concern that indirect and direct hatchery effects, combined with habitat
degradation, have selected against the early entering component (November – January) of the
wild winter steelhead (Bahls 2001; McMillan 2006). Fisheries can induce directional selection in
the timing of migration in salmon (Quinn et al. 2007), and in turn, shorten breeding seasons,
reduce phenotypic diversity, and lower population productivity (Tillotson and Quinn 2018).
Recently, McMillan et al. (2022) published a study that evaluated potential changes in
run timing of wild winter steelhead in the Quillayute, Hoh, and Queets Rivers. The author’s
comparison of tribal CPUE data for wild winter steelhead between circa 1948-1960 (historical
period) and 1980-2017 (contemporary period) revealed large changes in run timing (Figure 15,
McMillan et al. 2022). Overall, wild winter steelhead now enter later, and as a result, their
overall period of entry is compressed compared to the historical period (Figure 15, McMillan et
al. 2022).
Reconstruction of run timing provided evidence that wild winter steelhead runs began
significantly earlier in all three populations during the historical period (Figure 15). For example,
the percentage of wild winter steelhead migrating before January 1
st
(pJan1) during the
contemporary period was between 18% (14%-23%) and 43% (35%-50%) less than the historical
period in the Quillayute and Hoh Rivers, respectively (McMillan et al. 2022). The reduction of
earlier returning wild winter steelhead also corresponds with a significant shift in the date at
which half of the population had passed (q50) in the Quillayute and Hoh Rivers (Figure 15b and
70
16d). Estimated q50 occurred 25 (16-33 days) and 61 (47-71 days) days later during the
contemporary period in the Quillayute and Hoh Rivers, respectively. The magnitude of the shift
in q50 in the Queets River was similar to other populations, occurring 54 (-23-70 days) days
later during the contemporary period. Importantly, the timing of contemporary hatchery
steelhead returns directly overlaps with the historical early returning wild fish, which are now
greatly depleted (Figure 15).
The later entry timing during the contemporary period is causing run compression
(Figure 15). As a result, the number of days elapsed between when 25% and 75% of the run had
passed historically is now 16 (6-29 days), 26 (11-53 days), and 22 days (-4-83 days) shorter in
the Quillayute, Hoh, and Queets Rivers, respectively (McMillan et al. 2022).
McMillan et al. (2022) could not explicitly determine cause and effect, but they did offer
two hypotheses. First, spawning and rearing habitat used by early entering individuals may
have been sufficiently degraded to reduce the productivity of those fish (discussed in McMillan
et al. 2022). Second, declines in earlier migrating wild winter steelhead are also associated with
the introduction of hatchery winter steelhead (Cederholm and TU 1984; Bahls 2001).
Most hatchery programs on the western side of the Olympic Peninsula selectively bred
adults to return from November through early January, under the assumption that wild
steelhead were not abundant during that period (Crawford 1979; Cram et al. 2018). The
assumption was erroneous, however, because the return of the hatchery steelhead directly
overlaps with wild run timing in the historical period (Figure 15). This would have created
opportunities for interbreeding for wild steelhead that also spawned early. For example,
Seamons et al. (2012) found that a segregated winter steelhead hatchery program using the
early returning Chambers Creek stock failed to prevent interbreeding with wild winter
steelhead in Forks Creek, Washington. After only three generations of hatchery stocking, the
proportion of wild ancestry smolts and adults declined by 10-20% and up to 80% of naturally
produced winter steelhead were hatchery x wild hybrids (Seamons et al. 2012).
Further, the production and timing of hatchery winter steelhead set the stage for mixed-
stock in-river fisheries in which recreational and commercial fisheries targeting hatchery winter
steelhead also subjected co-mingling earlier-returning wild winter steelhead to high and
potentially unsustainable exploitation rates (Cederholm and TU 1984; Naish et al. 2007; Cram et
al. 2018). Contemporary fishing effort is highest from December through mid-January,
corresponding with the timing of hatchery winter steelhead returns and thereby providing the
potential for fisheries-induced directional selection against earlier migrating wild winter
steelhead (Quinn et al. 2007; Tillotson and Quinn 2018). Given the heritable component
underlying timing of migration, its plausible that reduced survival due to interbreeding with
hatchery fish combined with a variable fishing intensity that peaks from late-November through
early-January are the two most important factors responsible for the declines in early returning
wild steelhead. Regardless of the exact causes, unless action is taken to restore and protect
diversity, the fate of wild Olympic Peninsula steelhead rests on late-returning winter steelhead
71
that may not be able to keep pace with changes in spring and summer streamflow and
temperatures.
XIII. SPATIAL STRUCTURE
Ensuring that populations are well represented across diverse habitats helps to maintain
and enhance genetic and life history variability and population resilience (McElhany et al. 2000).
Additionally, ensuring wide geographic distribution across diverse climate and geographic
regions helps to minimize risk from catastrophes (e.g., droughts, floods, etc.; McElhany et al.
2000).
The Steelhead at Risk Report authors evaluated spatial structure of steelhead in the
Olympic Peninsula DPS using only previously documented or historical classifications (Cram et
al. 2018). The region is fortunate in that no habitat has been lost to large dams or barriers
(Cram et al. 2018). However, much of the landscape is covered with a dendritic network of
roads that were constructed for forest practices, many of which cross salmon-bearing streams
and have culverts (or lack culverts) that prevent upstream access (Smith 2000). We could not
determine how much habitat has been blocked by culverts and roads, but it is likely extensive
(Smith 2000). Considering that juvenile steelhead are distributed up to barriers in many small
creeks (McMillan and Starr 2008; McMillan et al. 2013), it is likely that numerous roads and
impassable culverts have truncated their spatial distribution.
It is also possible that distribution has been altered due to changes in run timing
(McMillan et al. 2022) due to spatial structure of spawning adults (McMillan et al. 2007). Early
returning steelhead often spawn in smaller streams or stream sections within a watershed
(Cederholm 1983; McMillan et al. 2007), perhaps because they are only accessible during
higher flows of early winter and such places may offer more secure spawning habitat during the
high flows of winter. Reduced abundance of those fish could alter the overall distribution of
steelhead. Similarly, the depletion of summer steelhead has potentially changed how and when
different life histories use parts of the watershed. While any changes in spatial distribution due
to the aforementioned effects are speculative, the lack of data and the importance of spatial
structure underscore the uncertainty associated with steelhead in this DPS.
Last, spatial structure of a DPS is also related to the patterns and frequency of natural
demographic exchange between apparently demographically independent populations. This
affects the extent to which the DPS and its component populations represent or exhibit
characteristics of metapopulations and/or exhibit source-sink dynamics and over what
temporal periods. This requires, among other things, knowledge of the genetic relationships
between populations, which appears to be largely lacking on Olympic Peninsula steelhead.
72
PART TWO
ENDANGERED SPECIES ACT LISTING FACTORS
I. PRESENT OR THREATENED DESTRUCTION, MODIFICATION, OR CURTAILMENT
OF ITS HABITAT OR RANGE
Olympic Peninsula steelhead habitat is threatened both inside and outside of Olympic
National Park (Klinger et al. 2008). Outside the park, steelhead habitat is degraded from
decades of destructive logging operations (McHenry et al. 1996). Timber harvesting has altered
hydrographs, increased water temperatures, and accelerated erosion. Extensive road building is
causing erosion and delivering fine sediments to streams. A widespread distribution of culverts
block access to spawning and rearing habitat and impede the downstream delivery of wood and
sediment (Smith 2000). These impacts continue to limit steelhead productivity despite
improved forest practices that were adopted in the 1990s.
“*** [T]he majority of streams on the Olympic Peninsula will not recover for well
over a century or possibly longer. The condition and land-use history on the
Olympic Peninsula are representative of the Pacific Northwest. The implications
for salmonid habitat, population, and resource value are not encouraging.
Degraded habitats will continue to produce at levels below their full capacity,
while other habitats will decline in quality.”
(McHenry et al. 1996). Climate change will only worsen these impacts on steelhead and their
habitat (Wade et al. 2013; Frissell et al. 2014).
Inside Olympic National Park, climate change is more than a threat – it is reality. Glaciers
that provide cold summer flows are shrinking and some have disappeared. By 2070, they may
all be gone (Fountain et al. 2022) and late spring snowpack is expected to decrease by 40-60%
(see Figure 17). Summer streamflows are decreasing, and peak winter streamflows are
increasing. Most models project drier summers, which will decrease summer and early fall
streamflows, especially in rain-dominant basins.

73
Figure 17. Trends in April Snowpack in the Western United States, 1955–2020, from:
https://www.epa.gov/climate-indicators/climate-change-indicators-snowpack.
Summer steelhead, which are nearly extinct, are particularly vulnerable to these habitat
changes (e.g., low summer flows during adult migration). Winter steelhead, which are declining,
are also vulnerable to these changes. These impacts will increase throughout the century.
A. Overview of Habitat Conditions on the Olympic Peninsula
1. WRIA 20 Habitat
Outside of Olympic National Park, steelhead habitat in WRIA 20 suffers from extensive
habitat degradation (Smith 2005, Klinger et al. 2008). A 2005 study conducted for the
Washington State Conservation Commission rated salmonid habitat in WRIA 20 as “poor-fair”
(Smith 2005). The study rated the following habitat attributes as poor: side-channel floodplain,
sediment quantity, sediment quality, bank/streambed/channel stability, instream large woody
debris (“LWD”), riparian, and water temperature (Smith 2005). The following attributes were
rated as fair: road density and hydro maturity high flows (Smith 2005). The only attribute rated
as “good” was pool habitat (Smith 2005).
74
All rivers in WRIA 20 where Olympic Peninsula steelhead occur have degraded habitat,
including but not limited to the following examples:
• The Sol Duc River has excessive sedimentation from landslides and high road densities,
poor LWD recruitment and riparian conditions, loss of wetland and off-channel habitat,
low and warm summer streamflows, loss of fog drip, blockages in tributaries, and loss of
cover and winter refuge habitat provided by debris jams (Smith 2000).
• The Bogachiel River has poor riparian and LWD conditions, an aggraded mainstem that
worsens as the river moves downstream, collapsing banks in the lower mainstem, fines
from exposed clay layers, and warm summer water temperatures (Smith 2000).
• The Calawah River experiences extensive landslides, high road densities, historic fire
and subsequent salvage logging impacts, excessive sedimentation, poor levels of LWD,
incised floodplains in the North Fork Calawah and South Fork Calawah Rivers (as well as
several tributaries), and low and warm summer streamflows (Smith 2000).
• The Hoh River suffers from significant habitat degradation outside of Olympic National
Park. Debris flows in the basin have been “common and devastating, resulting in
scoured, incised channels with few spawning gravels and LWD” (Smith 2000). There are
numerous areas with poor LWD and riparian conditions, passage blockages (in
tributaries), degraded water quality, few floodplain complexes, and fog drip loss due to
large conifer removal (Smith 2000). The Hoh River is also experiencing higher magnitude
flood events and lower summer flows (Piety et al. 2004; East et al. 2017; NIFC 2020)
• The Dickey River experiences sedimentation from roads and logging operations. The
Dickey River has poor riparian habitat, extensive substrate embeddedness, low and
warm summer flow, increased distribution of predators (e.g., northern pikeminnow),
passage blockages, reduced fog drip due to the removal of old conifers, altered
wetlands, decreasing LWD, and degraded channel and floodplain conditions in several
tributaries (Smith 2000). Low streamflows and high water temperatures are believed to
limit steelhead production in the river (Klinger et al. 2008).
• The Ozette River Basin has low levels of LWD, poor riparian conditions, warm water
temperatures, poor hydrologic maturity, invasive plant species, and a lack of marine-
derived nutrients (Smith 2000). The Ozette River basin has particularly high road
densities, which contribute to high sedimentation rates, a major limiting factor for the
area (Smith 2000; Klinger et al. 2008).
• The Wa’atch and Tsoo-yes Rivers suffer from numerous blockages throughout their
basins, lack LWD, and experience substantial sedimentation, mass wasting, high water
temperatures, and lack marine-derived nutrients (Smith 2000; Klinger et al. 2008).
75
• Goodman and Mosquito Creeks suffer from sedimentation, poor riparian conditions,
and, in the middle reach of Goodman Creek, low levels of LWD (Smith 2000).
2. WRIA 21 Habitat
Outside of Olympic National Park, steelhead habitat in WRIA 21 is degraded (QINLE
2011). Throughout most of the 20
th
century, the forest outside of the park was widely
harvested for timber, causing significant effects on the quantity and quality of salmon habitat
(Cederholm and Salo 1979; Cederholm et al. 1978; Smith and Caldwell 2001; McMillan 2006).
There is excessive sedimentation in the more intensely logged areas, especially in the
Clearwater subbasin (Cederholm et al. 1980; Klinger et al. 2008). Generally, water temperatures
and side-channel floodplain habitat are in poor condition (Smith 2005; Klinger et al. 2008).
Instream LWD, pool habitat, and riparian habitat are in fair condition (Smith 2005). Off-channel
habitat is limited in the Clearwater, Sams, and Salmon Rivers and Matheny Creek (Klinger et al.
2008). A glacier that fed the Quinault River melted away in 2011, contributing to lower summer
flows (Ahearn 2015). On the Queets River, peak and low flows are intensifying (QINLE 2011).
3. WRIA 19 Habitat
Steelhead habitat in WRIA 19 is degraded from decades of poor logging practices
(McHenry et al. 1996). Approximately 95% of the watershed’s old growth forest has been
converted into tree farms, and landslides triggered by logging operations have been common
(McHenry et al. 1996). For example, between 1950 and 1995, at least 330 logging-related
landslides occurred in the Hoko River basin alone (McHenry et al. 1996). Mass wasting events
have also occurred in the Clallam, Sekiu, and Pysht River basins (McHenry et al. 1996).
The basins in WRIA 19 suffer from floodplain development and alterations, loss of LWD,
estuary and nearshore alterations at stream mouths, degraded water quality, warm summer
stream temperatures, passage barriers, and conversion of riparian forests to non-forest uses
(McHenry et al. 1996; NOPLE 2015). Smith (2005) rated the following habitat attributes as being
in “poor” condition: side-channel floodplain habitat, sediment quantity, sediment quality,
bank/streambed/channel stability, instream LWD, riparian habitat, and hydro maturity high
flows. Water temperature varies from “poor” to “good” (McHenry et al. 1996, Smith 2005).
B. FOREST PRACTICES
Forestry has been, and continues to be, the primary land use outside of the park
(McHenry et al. 1998; Smith and Cadwell 2001; Copass 2016). Despite improvements to logging
practices on federal, state, and private land, the habitat of Olympic Peninsula steelhead
continues to suffer the consequences of past and ongoing logging operations throughout WRIA
19, 20, and 21 (McHenry et al. 1998; Smith 2000; NIFC 2020).
76
1. Timber Harvest on Federal and State Lands
a. Olympic National Forest
The Olympic National Forest spans 628,115 acres and surrounds most of Olympic
National Park. Logging in Olympic National Forest began in the 1920s and grew more intense in
the latter half of the 20th century (Halofsky et al. 2011). Extensive road building occurred
during the 1950s through the 1980s (Halofksy et al. 2011). Today, there are approximately
3,500 km of roads in the Olympic National Forest, which is roughly the driving distance between
Forks, Washington and Milwaukee, Wisconsin (Halosfky et al. 2011). Between the 1960s and
1990s, approximately half of the forest’s suitable land base was harvested (Halofksy et al.
2011). Prior to the adoption of the Northwest Forest Plan in 1994, timber operations involved
harvesting riparian trees, clearcutting, and broadcast burning (Halofksy et al. 2011). Stream
clearing and splash damming removed large wood from numerous stream channels (Halofksy et
al. 2011).
The adoption of the 1994 Northwest Forest Plan ushered in a new era of management,
which incorporates ecosystem management principles and specific protections for aquatic
ecosystems (Halofsky et al. 2011). However, despite improved forest practices, the effects of
logging continue to impair steelhead habitat in the Olympic National Forest. Logging roads and
associated channel crossings are still major issues for fish habitat quality (Halofsky et al. 2011).
The USFWS describes the sediment input from roads in the Olympic National Forest as
“chronic” (USFWS 2020). Although conifers have regenerated along some waterways, “many
riparian corridors have few conifers to provide large wood to streams” (Halofksy et. al 2011).
b. State Lands
The State of Washington began to overhaul its forest practice rules in the 1990s to
benefit salmonids and other species such as the marbled murrelet (Brachyramphus
marmoratus) and northern spotted owl (Strix occidentalis caurina). Prior to 1998, Washington’s
forestry rules lacked certain measures to protect salmonid habitat such as requiring timber
harvesters to leave trees in riparian zones for LWD recruitment (McHenry et al. 1998).
However, even with these improvements, the benefits to salmonid habitat are not clear.
“The effectiveness of currently implemented forest practices for minimizing
impacts remains uncertain. For example, incorrectly applied or inadequately
designed riparian management zones and incorrect stream typing classifications
are known problems that impair habitat protection strategies (Hansen 2001).
These practices result in loss of large woody material, fish passage impacts,
altered hydrology, water quality impacts, mass wasting (landslides), and elevated
stream temperatures (Naiman et al. 1998).”
(Cram et al. 2018).
77
The Olympic Experimental State Forest
The Olympic Experimental State Forest (OESF) was designated in 1992 and covers
approximately 270,000 acres of land (WDNR 2016). The OESF includes parcels of state-owned
trust land interspersed with private, federal, and tribal lands (Minkova et al. 2021). WDNR’s
goal is to manage the state trust lands to generate sustainable revenue for counties,
universities, and other trust beneficiaries, while also maintaining ecological values (WNDR
2016). Nearly half of the forest is young, with trees ranging between 20 and 39 years of age
(WDNR 2016).
Several major rivers occur in the OESF, including the Hoh, Queets, Quillayute,
Clearwater, Hoko, and Pysht Rivers (Minkova et al. 2021). WDNR does not manage streams in
the OESF to meet desired future conditions for salmonids (WDNR 2018). Instead, it seeks to
maintain or aid the restoration of riparian functions, water quantity, and water quality (WDNR
2018).
It is unclear whether the forest practices in OESF are significantly improving habitat for
steelhead. For example, Pollock et al. (2004) found that “within the OESF, a majority of streams
do not meet WDOE water quality standards for temperature, and that stream temperatures in
harvested basins are often (but not always) higher and more variable than stream
temperatures in unharvested basins” (Pollock et al. 2004). Additional study by Pollock et al.
(2009) found that the impact of harvest activities could not be fully mitigated by riparian
buffers alone. However, two studies by WDNR did not find similar water temperature
exceedances within OESF (Martens et al. 2019; Devine et al. 2022).
2. Logging Effects on Steelhead Habitat
Logging operations harm steelhead habitat by increasing water temperatures and
sedimentation in streams, removing large woody debris, reducing streamflows, and decreasing
habitat connectivity.
a. Temperature
Logging operations increase water temperatures in several ways: by removing upland
and riparian vegetation, which increases thermal radiation; decreasing groundwater inflow;
increasing sedimentation rates, which widen and shallowing river channels; and reducing large
woody debris recruitment (Cederholm and Salo 1979; Lynch et al. 1984; Frissell et al. 2014).
High water temperatures harm salmonids by causing direct mortality and metabolic distress,
altering migration and breeding behavior, increasing susceptibility to disease, and creating
thermal barriers that block access to habitat (Hicks 1999).
Several studies indicate that logging operations on the Olympic Peninsula increase
stream temperatures. Hatten and Conrad (1995) studied managed (i.e., logged) and
unmanaged stream reaches on tributaries to the Hoh, Queets, and Bogachiel Rivers and
78
Kalaloch Creek. They found that unharvested sites exceeded the state’s 16° C temperature
standard at an average of 1.8 days over a 39-day monitoring period (Hatten and Conrad 1995).
In contrast, the managed sites exceeded 16° C an average of 18.3 days during the same period
(Hatten and Conrad 1995). Because the harvested sites were representative of low elevation
harvesting sites, Hatten and Conrad (1995) assumed that most managed stream segments in
the western Olympic Peninsula did not meet water quality standards for temperature. Those
standards are intended to protect salmonids.
Although riparian buffer standards have been implemented on federal and state
forestland, research conducted by Hatten and Conrad (1995) and Pollack et al. (2009) indicate
that buffers alone may not be sufficient to improve stream temperatures. For example, Hatten
and Conrad (1995) found that the most important variable affecting stream temperature is the
total amount of land logged in a basin. However, Devine et al. (2022) did not find similar water
temperature exceedances in OESF as Pollock et al (2009), which may indicate that increased
shade in riparian zones is benefiting stream temperature.
Logging has had significant impacts in WRIA 19 basins, which have mostly been
converted into tree farms (McHenry et al. 1996). For example, water temperatures as high as
69° F and 73.4° F have been recorded in the Hoko River (McHenry et al. 1996).
b. Hydrologic Flows
Timber harvest affects streamflow by increasing permeable surface areas, transporting
water directly to streams, removing vegetation that stores water, and altering snow retention
and melt rates (Davis and Schroeder 2009, as cited in WCSSP 2013). These impacts change the
timing and magnitude of peak and low streamflows (Davis and Schroeder 2009, as cited in
WCSSP 2013). Peak flows can scour eggs and flush juveniles out of rearing habitat (Davis and
Schroeder 2009, as cited in WCSSP 2013). Low flows can alter flow-related migration cues,
disconnect habit, and increase crowding, competition, and vulnerability to predation (Davis and
Schroeder 2009, as cited in WCSSP 2013).
A study on the Hoh River showed that logging significantly impacts peak and mean daily
streamflows at watershed, subbasin, and basin level in descending order (Achet 1997).
“If about 27% of the basin was harvested (extracting 46. 9 million m
3
of timber)
the impact on peak would increase by 44 to 51% at basin, 34 to 41% at sub-basin
and 55 to 141% at watershed level in medium and high hydrologic conditions. By
reducing the timber harvest by 26%, the corresponding reductions in peak at the
basin level, sub-basin level and watershed level were 9 to 10%, 7 to 9% and 16 to
18% respectively. Regarding mean daily flow, harvesting 27% of the basin would
result in increase in mean daily flow by 30 to 47% at the basin level, 22 to 36 % at
the at the sub-basin level and 77 to 137% at the watershed level in medium and
high hydrologic conditions. When the timber harvest was 34.6 million m3 the
79
corresponding reduction in impact on mean daily flow at basin, sub-basin and
watershed level would be 7 to 10%, 5 to 8% and 14 to 20%” (Achet 1997).
c. Sediment
Logging operations increase sedimentation through debris flows and surface runoff
from logging roads, which expand channel widths and reduce pool depth (Furniss et al. 1991;
Jones et al. 2000). Increased sedimentation affects salmonids throughout their freshwater life
stages by suffocating incubating eggs and fry, reducing macroinvertebrate composition/prey,
causing respiratory failure, reducing or blocking access to habitat, and warming stream
temperatures by lowering water depth (McHenry et al. 1996, USFWS 2020).
Sedimentation from timber harvest significantly impairs aquatic habitat on the Olympic
Peninsula. Logging operations have caused landslides throughout the area. The most severe
damage has occurred on the Quinault River, Queets River, Clearwater River, Hoh River, Calawah
River, Sol Duc River, Hoko River, Seiku River, Sooes River, and Deep Creek basins (McHenry et
al. 1996; Cederholm et al. 1980; WSCC 2001; USFWS 2020).
Landslides have been particularly destructive in the Hoh River basin (Logan et al. 1991).
For example, in the winter of 1988-1989, 105 landslides occurred on Huelsdonk Ridge above
the Hoh River, mobilizing an estimated 83,000 cubic yards of sediment that deposited in the
South Fork and mainstem Hoh River (Logan et al. 1991). Only two of the 105 landslides
occurred in entirely old growth stands (Logan et al. 1991).
Sedimentation from logging roads impacts all watersheds where Olympic Peninsula
steelhead occur. The Hoh River basin suffers from excessive sedimentation caused by logging
roads (USFWS 2020). The sub-basins with the largest percentage of sediment over background
levels include the South Fork of the Hoh River (156%), Owl Creek (286%) and Winfield Creek
(210%) (Marsh 2012).
d. Large Woody Debris
Logging removes wood from riparian, upland, and headwater riparian areas that could
be recruited to streams (Klinger et al. 2008). The loss of wood increases the magnitude of peak
flow events, gravel scour, and bank erosion. It also decreases channel sinuosity and stability as
well as the quantity of pools and off-channel features (East et al. 2017). As a result, steelhead in
streams with low levels of LWD have less cover, food, off-channel habitat, and pools for rearing
and refuge. Depletion of LWD has long-term consequences for stream productivity.
Outside of Olympic National Park, nearly all rivers and streams suffer from the loss of
LWD (Smith 2000; Piety et al. 2004). Based on GIS imagery, East et al. (2017) found that the
Quinault River has less LWD than the Hoh and Queets Rivers. The Quinault River had 1.9% LWD
cover by area compared to 2.5% on the Queets River and 2.7% on the Hoh River (East et al.
2017).
80
e. Habitat Connectivity
Logging roads and culverts decrease access to on-channel and off-channel habitat (Davis
and Schroeder 2009, as cited in WCSSP 2013). As a result, there is diminished spawning,
rearing, and foraging habitat; increased competition caused by crowding; and less access to
refuges during high flow events (Davis and Schroeder 2009, as cited in WCSSP 2013).
Undersized culverts also compromise habitat quality for varying downstream distances due to
their disruption of the downstream transport of water, wood, and sediment.
f. Roads
Outside of Olympic National Park, most areas in WRIAs 19-21 are not properly
functioning for salmonid habitat due to road density (NIFC 2020). According to NMFS,
watersheds with road densities greater than three miles per square mile of watershed are not
properly functioning for salmonid habitat (NMFS 1996). Based on this criterion, nearly all
forestland outside of the park is not properly functioning for salmon habitat (NIFC 2020). The
West Fork Dickey River watershed has the highest road density (5.5 miles per square mile)
(NIFC 2020). Other high-density watersheds include Elk Creek-Calawah, Crooked Creek, the East
Fork Dickey, the Lower Bogachiel River and Big River watersheds (NIFC 2020).
Roads threaten several of the larger watersheds as well. For example, road density
remains high in the Queets River watershed on both federal and private land (USFWS 2020).
Road density in the Clearwater drainage is 3.2 miles per square mile in the upper section and
3.7 miles per square mile in the lower section (USFWS 2020). There are approximately 2.5 miles
of road per square mile in the Queets River floodplain outside of the Olympic National Forest
(USFWS 2020).
Roads cause excessive sedimentation on the lower Hoh River (USFWS 2020). Private
landowners and the Washington Department of Natural Resources have extensively logged the
Hoh River watershed and logging roads cause excessive sedimentation (USFW 2020).
Despite lower road densities, the Quinault River is also impacted by roads (USFWS
2020). Logging roads built during the early 1900s spread throughout the basin and streambank
hardening is degrading habitat conditions (USFWS 2020).
Roads have also triggered mass wasting events. For example, the USFS (2012) found
that 49% of mass wasting events in the Sitkum and South Fork Calawah watershed were caused
by roads.
C. CLIMATE CHANGE
The evidence of climate change is irrefutable. According to the IPCC, “[i]t is unequivocal
that human influence has warmed the atmosphere, ocean and land” and that “[w]idespread
and rapid changes in the atmosphere, ocean, cryosphere and biosphere have occurred” (IPCC

81
2021). Each of the last four decades have been successively warmer than the last (IPCC 2021).
Under status quo emissions levels, average global temperatures are expected to rise 1.6° C
above 1850-1900 levels between 2021 and 2041. Under the very high GHG emissions scenario
(SSP5-8.5), global temperature will likely increase by 2°C between 2041 and 2060 (IPCC 2021).
Climate change will affect the climate and hydrology of the Pacific Northwest. The
region is projected to lose snowpack and glacier mass and incur frequent and extreme
hydrological conditions (Mantua et al. 2010; Halofsky et al. 2011). Most models indicate that
winters will be wetter and summers will be warmer and drier (Mote and Salathé 2010; Elsner et
al. 2010; Kunkel et al. 2013). Summer droughts will be more frequent and severe (CIG 2009;
Mantua et al. 2010; Halofsky et. al 2011).
The western Olympic Peninsula is anticipated to warm, although it may warm slightly
less than other areas of the Pacific Northwest due to the moderating effect of the Pacific Ocean
(Halofsky et al. 2011; Dalton et al. 2016). Annual precipitation is projected to increase during
the winter and spring and decrease during the summer (Dalton et al 2016). Snowpack is
projected to decline and streamflows are projected to increase in the winter and decrease in
the summer (Dalton et al. 2016). The Olympic Peninsula is also expected to experience sea level
rise, stronger and more frequent storms, increased erosion, warmer water temperatures, more
low and high flow events, and increased glacial melt (Miller et al. 2013).
Climate change will also significantly alter the marine environment. Under the RCP8.5
scenario, models indicate that more multi-year warming events will occur in the northeast
Pacific Ocean (marine heatwaves, MHW), which may cause profound effects on salmonid
habitat (Joh and Di Lorenzo 2017; Oliver et al. 2019; Cheung and Frolicher 2020).
“Widespread and extreme negative impacts on marine life and fisheries
associated with the 2014-2015 marine heat wave are well documented. If the
projected increases in the area, magnitude and frequency of extreme warm
events are realized, and they are superimposed upon a systematic
anthropogenic warming trend; this combination would likely cause profound
negative impacts on marine life and fisheries all along the west coast of North
America, particularly those in the Gulf of Alaska in the second half the 21st
century.”
(Joh and Di Lorenzo 2017).
These changes will impact Olympic Peninsula steelhead, especially the summer-run
component, which is nearly extinct. Climate change is expected to cause widespread declines in
the quantity and quality of habitat for Olympic Peninsula steelhead (Halofsky et al. 2011). It is
unknown whether these fish will adapt quickly enough to these changes.
“It remains an open question whether present-day salmonid fish populations on
the Olympic Peninsula can adapt (either through phenological, phenotypic, or

82
evolutionary responses) at rates required to deal with the combination of
anthropogenic climate change and other habitat and ecosystem changes that
will come in the next century (Crozier et al. 2008).” (Halfosky et al. 2011).
1. Projected Climate and Hydrological Changes
a. Air Temperature
The Pacific Northwest has not experienced the same magnitude of warming since the
period between glacial and interglacial periods (Dalton et al. 2016). Between 1900 and 2014,
the average air temperature in Washington rose by approximately 1.5°F (USFWS 2020; Mote
and Salathe 2010). This warming trend will continue.
“Regardless of the scenario, warming is projected to continue throughout the
21
st
century in the Pacific Northwest. For the 2050s (2041 to 2070) relative to
1950-1999, temperature is projected to rise +5.8°F (range: +3.1 to +8.5°F) for a
high greenhouse gas scenario (RCP8.5). Much greater warming is possible after
mid-century under the more aggressive scenarios (RCP 6.0 and 8.5) ***.”
1
Models using less aggressive emission scenarios also project increased warming over the course
of the 21
st
Century. For example, Elsner et al. (2010) used data from the IPCC Fourth
Assessment Report to project that air temperatures will increase 0.3 C per decade in the Pacific
Northwest. However, even this lower rate of warming could produce “profound changes in the
hydrology and environment of the Northwest” (Mote and Salathe 2010).
Modeling shows that summer air temperatures in the Pacific Northwest will rise
significantly. For example, multi-model averages of the A1B scenario indicate that average June
through August air temperatures will increase at the following rates over the century: 1.7° C
(0.43° C to 3.4° C) by the 2020s; 2.7° C (1.3° C to 5.1° C) by the 2040s; and 4.7° C (2.7° C to 8.1°
C) by the 2080s (Mantua et al. 2010). Multi-model averages of the B1 scenario project June
through August temperature to increase as follows: 1.2° C (0.18° C to 2.4° C) by the 2020s; 1.8°
C (0.2° C to 3.7° C) by the 2040s; and 2.9° C (1.3° C to 5.1° C) by the 2080s (Mantua et al. 2010).
Air temperatures on the Olympic Peninsula are projected to increase (USFWS 2020).
Under the business-as-usual emissions scenario, air temperatures in the Olympic National
Forest are projected to increase by 2° F to 5° F (approximately 1° C to 2.5° C) between 2016 and
2045 relative to late 20
th
Century temperatures (USFWS 2020). Air temperatures in Olympic
National Park are projected to increase by 2.93° F between 2016 and 2045 and by 5.85° F
between 2046 to 2075 (LCD 2022).
1
https://cig.uw.edu/learn/climate-change/
83
b. Glaciers & Snowpack
Climate change is melting the Olympic Peninsula’s glaciers (USFWS 2020) and reducing
snowpack (Figure 17). Between 1980 and 2015, glaciers on the Olympic Peninsula decreased by
34% (Riedel et al. 2015). During that period, thirty-five glaciers and 16 perennial snowfields
disappeared (Fountain et al. 2022), including the Anderson Glacier, which contributed to
streamflow to the Quinault River (Dalton et al. 2016; NIFC 2020). Other glaciers that provide
water to the Quinault, Queets, Hoh, and Bogachiel Rivers (McHenry et al. 1996) are receding at
rates higher than previously recorded (NIFC 2020). Between 1981 and 2015, the glaciers that
feed into the Hoh River decreased by 40% (NIFC 2020). Under the RCP 8.5 scenario, Fountain et
al. (2022) estimates that glaciers will largely disappear from the Olympic Peninsula by 2070.
Climate change is expected to reduce late spring snowpack on the Olympic Peninsula
(Halofsky et al. 2011). Parts of the Olympic National Forest historically maintained snowpack
until April 1
st
(USFWS 2020). By mid-century, most spring snowpack will only exist at high
elevations in Olympic National Park, which will also experience “dramatic reductions” in spring
snowpack (Elsner et al. 2010; USFWS 2020).
c. Precipitation
The majority of climate models project that spring and summer rain will decrease on the
Olympic Peninsula, with up to a 24% decrease in summer rainfall (USFWS 2020). At least one
model, however, shows slightly wetter summers (LCD 2022).
The majority of climate models project that fall and winter rain will increase on the
Olympic Peninsula (USFWS 2020). Precipitation during the months of December through
February is likely to increase by 4.5% to 5% on average and depending on location (Halofksy et
al. 2011). Across the Pacific Northwest, spring precipitation has already increased by about 2%-
5% per decade over the past century, a trend that will likely continue (Abatzoglou et al. 2014). A
multi-mean model using a high emissions scenario projects a 6.5% increase in spring
precipitation by 2041-2070 (Dalton et al. 2016).
The Pacific Northwest will also experience wetter storm events (Miller et al. 2013).
Some models indicate that storms will be wetter and stronger on the Olympic Peninsula
(Halofsky et al. 2011).
d. Streamflow
Climate change is altering the hydrology of watersheds on the Olympic Peninsula.
Winter peak flows are getting higher, and summer low flows are getting lower (NIFC 2020).
These conditions are predicted to become worse for the major steelhead producing watersheds
in the coming decades. As illustrated in Table 8, these changes are occurring throughout the
DPS and are likely to have significant effects on winter and summer steelhead. For example, as
we discussed for steelhead, the lower 9km of the NF Calawah River (tributary of Calawah River

84
in Quillayute River system) went completely dry in 2002 killing thousands of salmonids,
including all adult and juvenile steelhead (McMillan et al. 2013; McMillan 2002). The Calawah
River is a lower-elevation, rain-fed tributary and is susceptible to drought conditions (Smith
2000). Considering low summer flows are likely to become worse in the future (Wade et al.
2013), streams like the NF Calawah may become uninhabitable during the summer months.
This also helps explain why the Calawah River has experienced a large change in summer low
flows relative to other watersheds (Table 8).
Table 8: Changes in low and high streamflow in Calawah, Quinault, Hoh, and Hoko Rivers from
1976-2019. (Source: NIFC 2020)
Watershed
Low flow
decrease
High flow
increase
Calawah River -48% +5%
Quinault River -20% +7%
Hoh River -15% +18.4%
Hoko River -13% +17.8%
Summer streamflows will continue to decrease on the Olympic Peninsula (Mantua et al
2010; Wade et al. 2013; Beechie et al. 2013; Dalton et al. 2016). Using A1B and B1 warming
scenarios, Mantua et al. (2010) found that annual summer low flows on the Olympic Peninsula
will be approximately 5% to 45% lower by mid-century. According to Dalton et al. (2016),
average summer flows are projected to decline by 30% across the Quillayute, Hoh, Queets, and
Quinault River basins. Summer flows in headwater areas will likely become more ephemeral or
stop during the summer months, and the duration of low flow periods will increase significantly
in all but the most rain-dominant basins (i.e., Hoh and Queets River basins) (Halofsky et al.
2011). Increased winter flooding may exacerbate summer low flow conditions by increasing
porous sediment deposits (Halofsky et al. 2011).
Winter flooding is increasing and will continue to do so throughout the 21
st
century
(NIFC 2020, Mantua et al. 2010). Peak flows are commonly at or above flood stage on the
Bogachiel and Calawah Rivers (NIFC 2020). Average winter flows are projected to increase by at
least 30% over the majority of stream reaches in the Quillayute, Hoh, Queets, and Quinault
River basins (Dalton et al. 2016). Winter flow in stream reaches on the Quinault and Quillayute
Rivers are projected to increase by 40% (Dalton et al. 2016). Late fall and early winter flooding
is also projected to increase (Halofsky et al. 2011).
It is important to note that these summer and winter streamflow projections are based
on moderate (A1B) and low (B1) emission scenarios established by the IPCC. These scenarios

85
project similar amounts of emissions through the mid-21
st
century as the A2 scenario, which is a
high emissions scenario (Halofsky et al. 2011). The A2 scenario, however, projects higher
emissions during the latter half of the century (Halofsky et al. 2011).
Figure 18. Current and projected (year 2040) reductions in summer streamflow and increases in
winter streamflow in the Sol Duc, Dickey, Quillayute, Calawah, Bogachiel, Hoh, Queets, and
Quinault Rivers (Reeves et al. 2018).
e. Water Temperature
Water temperatures on the Olympic Peninsula are projected warm. Increasing stream
temperatures pose higher risks to the quality and quantity of steelhead habitat because
steelhead typically however, it is unlikely that they will exceed the thermal tolerances of

86
steelhead (USFS 2020; Miller et al. 2013; Mantua et al. 2010). Using A1B and B1 emission
scenarios, Mantua et al. (2010) projected that water temperatures will warm by 1° to 2° C (
Fig. 19. Current and projected 2040 summer water temperatures in the Sol Duc, Dickey,
Quillayute, Calawah, Bogachiel, Hoh, Queets, and Quinault Rivers (Reeves et al. 2018).
87
f. Other Water Quality Issues
Increased winter precipitation will likely increase runoff and landslides from logging
operations, which would increase sediment pollution in Olympic Peninsula rivers and streams
(Klinger et al. 2008; Halofsky et al. 2011).
2. Effect of Freshwater Habitat Changes on Olympic Peninsula Steelhead
Climate change will adversely modify freshwater salmonid habitat on the Olympic
Peninsula. Because of their longer freshwater residency, steelhead are more sensitive to these
freshwater habitat changes than other salmonids (Halofsky et al. 2011).
a. Low Summer and Early Fall Streamflow
Olympic Peninsula steelhead have high exposure to extreme low flows (Wade et al.
2013). Lower flows will decrease habitat availability, elevate water temperatures, and induce
thermal stress on salmonids (Crozier and Zabel 2006; Wade et al. 2013; Dalton et al. 2016;
Ohlberger et al. 2018). Reduced summer and fall streamflows in rain-dominated basins may
adversely affect summer steelhead migration (Holafsky et. al. 2011). For example, there will be
less cold water and fewer holding pools for migrating steelhead, which may be particularly
stressful for summer steelhead and lower their reproductive success (Dalton et al. 2016). Low
streamflows are already a limiting factor on the Calawah River (Phinney et al. 1975), particularly
the NF Calawah (McMillan 2002), and summer streamflows are showing a decreasing trend on
many Olympic Peninsula Rivers, such as the Hoh River (NIFC 2020). Climate change will
exacerbate these effects (NIFC 2020).
b. High Late Fall and Winter Streamflow
Olympic Peninsula steelhead have high exposure to increased winter streamflows
(Wade et al. 2013). Average winter flows (January – April) are expected to increase by at least
30% by 2040 (Dalton et al. 2016). Average winter flows are projected to increase by 31-40% in
the Hoh, Queets, and Quinault Rivers (Dalton et al. 2016). Increased winter flows could reduce
the survival of developing eggs, embryos, and juveniles (Dalton et al. 2016) This is a higher risk
in confined streams, which are more susceptible to scour (Halofsky et al. 2011). Summer
steelhead juveniles that emerge in winter in high gradient streams are at greater risk of
displacement (Dalton et al. 2016). Peak flows could also reduce the availability of slow-water
habitat for juveniles (Mantua et al. 2010; Halofsky et al. 2011), which may reduce parr-smolt
survival rates (Halofsky et al. 2011). Increased runoff will also likely increase sedimentation in
steelhead habitat (East et al. 2017).
c. Water Temperature
Based on models that use A1B and B1 scenarios, water temperatures are unlikely to
exceed lethal limits for steelhead, but they would increase to levels that reduce growth and
88
increase the risk of predation (Dalton et al. 2016). For example, temperatures at or above 15° C
can inhibit the smolt transformation process (Miller et al. 2013). As explained in the water
quality section, many stream reaches on the Olympic Peninsula already exceed 16° C and
warmer spring stream temperatures could impede smolting and force the populations to adapt
to smolt during an earlier time.
It is also possible that warmer temperatures could expand growing seasons and increase
food web productivity, which would benefit steelhead (Halofsky et al. 2011). However, this
benefit could be offset by increased flooding, lower summer flows, increase predation risks
(Dalton et al. 2016). Additional food availability may be also offset by increased competition
(Dalton et al. 2016).
3. Projected Changes in Marine Environment
Climate change is altering nearshore and offshore habitat of Olympic Peninsula
steelhead (Klinger et al. 2008; Miller et al. 2013). These changes include warming sea surface
temperatures and potential alterations in upwelling, hypoxia, and acidification (USFWS 2020).
The scope and intensity of these impacts are uncertain (Miller et al. 2013). For example, it is
possible that climate change could decrease upwelling, which would lower productivity (Klinger
et al. 2008). It could also increase upwelling, although warmer ocean temperatures could limit
productivity benefits (Miller et al. 2013). Increased sea surface temperatures will drive the
southern boundary of steelhead northward, potentially contracting the range of the species
(Abdul-Aziz et al. 2011). These changes will negatively affect salmon and steelhead survival
rates (e.g., Kilduff et al. 2015).
a. Sea Surface Temperature
Sea surface temperatures are projected to increase in the Pacific Northwest (Mote and
Salathe 2010; Miller et al. 2013; USFWS 2020). Mote and Salathe (2010) projected that ocean
surface temperatures will increase by approximately 1.2° C by 2050 relative to the 1970-1999
average temperature. Other models project that surface temperatures may increase by 1.2° C
to 3° C by mid to late century (USFWS 2020). These projected increases are substantially
outside 20
th
century variability (Mote and Salathe 2010). Marine heatwaves such as the 2014-
2015 “Blob” are also likely to reoccur more frequently (Oliver et al. 2019; USFWS 2020) as the
ocean heat content increases (Cheung and Frölicher 2020; von Schuckmann et al. 2020).

89
Fig. 20. Sea Surface Temperature Anomaly from August 1 - 31, 2019 (Image source:
https://earthobservatory.nasa.gov/images/145602/marine-heat-wave-returns-to-the-
northeast-pacific)
b. Upwelling
Climate change may affect upwelling, although there is much uncertainty about this
possibility. For example, models have produced mixed results on whether upwelling favorable
winds will change (Miller et al. 2013). There is some evidence indicating that upwelling patterns
may become shorter and more intense (USFWS 2020). Increasing surface temperatures may
influence the timing and magnitude of upwelling as well (Miller et al. 2013).
c. Acidification
The Pacific Northwest is vulnerable to ocean acidification (Miller et al. 2013). Increased
acidification threatens key species in the food web, including zooplankton, pteropods, crabs,
and krill (Busch et al. 2013; Mathis et al. 2015; Dalton et al. 2016).
90
d. Anoxic and Hypoxic Events
Anoxic and hypoxic events have occurred off the Washington coast (Klinger et al. 2018;
USFWS 2020;). As sea surface temperatures warm, dissolved oxygen levels are expected to
decrease (Miller et al. 2013).
4. Effect of Changed Marine Conditions on Olympic Peninsula Steelhead
NMFS anticipates that climate change will continue to limit ocean productivity for
salmonids (Ford 2022).
“Historically, ocean conditions cycled between periods of high and low
productivity. However, global climate change is likely to disrupt this pattern, in
general, leading to a preponderance of low productivity years, with an unknown
temporal distribution (Crozier et al. 2019). Recent (2015–19) ensemble ocean
indicator rankings include four of the worst seven years in the past 20, meaning
that an entire salmon or steelhead generation could have been subjected to
poor ocean productivity conditions.” (Ford 2022).
Warming will also cause steelhead ocean habitat to progressively decrease throughout
the century (Abdul-Aziz et al. 2011). Summer marine habitat is anticipated to contract 8-10% by
the 2020s, 15%-19% by the 2040s, and 24%-43 by the 2080s (Abdul-Aziz et al. 2011).
Although some forage fish may benefit from climate change, forage fish abundance is
likely to decrease (USFWS 2020), resulting in less prey for steelhead.
C. WATER QUALITY
Limited water quality monitoring makes it difficult to identify the status and trends of
water quality on the Olympic Peninsula (Klinger et al. 2008). However, the limited data that
does exist shows that there are many river and stream segments where Olympic Peninsula
steelhead occur that do not meet water quality standards (Klinger et al. 2008; WDOE 2016).
These water quality standards are intended to protect salmonids.
In WRIA 19, thirteen rivers and streams have segments that do not meet state
temperature standards and need Total Maximum Daily Loads (TMDLs). These waterbodies
include: Deep Creek, Hoko River, Little Hoko River, Clallam River, Sekiu River, Sekiu River (North
Fork), Sekiu River (South Fork), Pysht River, Pysht River (South Fork), Salt Creek, Lyre River, and
the West Twin River (WDOE 2016). Deep, Salt, and Bear Creeks are also on the 303(d) list for
dissolved oxygen (WDOE 2016).
Dozens of rivers and stream segments in WRIA 20 do not meet water temperature
standards (WDOE 2016). The following rivers and streams have at least one river segment that
91
does not meet temperature standards and are in need of a TMDL: Hoh River, Coal Creek,
Crooked Creek, Ozette River, Umbrella Creek, Willoughby Creek, Owl Creek, Split Creek, Maple
Creek, Anderson Creek, Line Creek, Alder Creek, Winfield Creek, Nolan Creek, Bogachiel River,
Coal Creek, Dickey River (mainstem, East Fork, Middle Fork, and West Fork) Elk Creek, Sol Duc
River, SF Calawah River, Sitkum River, Sooes River, Trout Creek, Big River, Fisher Creek
(McQuarry Creek), Ozette River, and Lake Creek (WDOE 2016). Monitoring by the Hoh Tribe
between 2006 and 2015 revealed that all but one major salmonid tributary to the Hoh River
had summer water temperatures that exceeded state water quality standards (NIFC 2020).
In addition to temperature exceedances, several rivers and tributaries in WRIA 20 have
at least one stream segment that does not meet pH or dissolved oxygen standards and need
TMDLs for these parameters (WDOE 2016). The following rivers and stream are on the 303(d)
list for pH: Coal Creek, Crooked Creek, Ozette River, Sol Duc River, Big River, and Palmquist
Creek (WDOE 2016). The following rivers and streams are on the 303(d) list for dissolved
oxygen: Big River, Coal Creek, Ozette River, Siwash Creek, South Creek, Umbrella Creek, Lake
Creek, and Bear Creek (WDOE 2016).
There is less water quality data for WRIA 21. Kalaloch Creek, Matheny Creek, and the
Sams River are on the 303(d) list for temperature (WDOE 2016).
D. ANTHROPOGENIC MIGRATION BARRIERS
When summarizing habitat conditions for Olympic Peninsula steelhead, Busby et al.
(1996) assumed that “minor blockages (such as impassable culverts) are likely throughout the
region." Today, we know that at least 1,214 human-made barriers exist in WRIA 19-21, many of
which are completely impassable (WDFW 2020). According to WDFW, there are 272, 470, and
473 barriers in WRIAs 19, 20, and 21, respectively (WDFW 2020). Although progress has been
made on removing and repairing culverts and other human-caused barriers, there are still
passage issues on many streams throughout the range of Olympic Peninsula steelhead.
The Quinault Indian Nation, Quileute Indian Nation, Hoh Tribe, Makah Nation, and other
western Washington tribes won a major legal victory for salmonids that requires the State of
Washington to repair or replace state-owned culverts throughout the Olympic Peninsula and
elsewhere. United States v. Washington, 853 F.3d 946 (9th Cir. 2017) aff 138 S.Ct. 1832 (2018).
However, the Washington State Department of Transportation has less than 12% of the funding
it needs to complete these actions by the court-imposed deadline (NIFC 2020). If the state
continues at its current rate, it will not complete its work until 2034 (NIFC 2020).
State and private landowners have made progress repairing culverts on state and large
private forest roads under the Road Maintenance and Abandonment Plan (NIFC 2020).
Although the plan was supposed to be completed in 2021, approximately 85% of the work is
done in the coastal region (NIFC 2020). However, there are hundreds of non-RMAP culverts on
private, county, and federal land throughout WRIAs 19, 20, and 21 (NIFC 2020). Roughly half of
the non-RMAP culverts are not passable (NIFC 2020).
92
II. OVERUTILIZATION FOR COMMERCIAL AND RECREATIONAL PURPOSES
Wild winter steelhead populations in the Olympic Peninsula DPS have a long history of
supporting intensive recreational and commercial fisheries until the last few years when
fisheries were altered and eventually closed due to conservation concern over unprecedented
low run sizes (WDFW blog post: https://wdfw.medium.com/changes-to-the-coastal-steelhead-
season-67131dd05ba7). The chronic declines in run size combined with altered run-timing
(McMillan et al. 2022), low levels of repeat spawning, and now, dramatic freshwater and
marine effects linked to climate change, suggest that the four major populations of wild winter
steelhead are being overutilized in fisheries. Cram et al. (2018) was able to estimate harvest
rates for 33% of the populations in the Olympic Peninsula DPS up until 2013 and found a mean
annual harvest rate of 25.6%, which was the highest harvest rate among all steelhead DPSs in
Washington State. We updated the harvest estimates for the Quillayute, Hoh, Queets, and
Quinault Rivers through 2020 (Table 1) and discuss those results for each population specifically
in the Harvest Impacts section.
These harvest fisheries put Olympic Peninsula steelhead at risk. WDFW’s Steelhead at
Risk report did not evaluate “population-specific impacts” within the DPS because the “harvest
rates are difficult to interpret without accompanying demographic analyses, since risks posed
by harvest depend on a population’s productivity” (Cram et al. 20198). WDFW acknowledges
there are substantial data gaps regarding the productivity of Olympic Peninsula steelhead
(Cram et al. 2018). Nonetheless, WDFW and the Treaty tribes manage Olympic Peninsula
steelhead under a maximum sustainable harvest regime that does not include the level of detail
necessary to responsibly manage harvest or maintain the persistence of the species (Gibbons et
al. 1985; Burge et al. 2006). And summer steelhead are not managed or monitored but are
subject to indirect harvest in fisheries.
A. Escapement Goals
The framework for the modern winter steelhead fishery was established following the
1974 “Boldt Decision” (U.S. v. Washington, 384 F.Supp. 312 (1974)), which reaffirmed the
tribe’s Treaty rights to fish (Clark 1985). Shortly after the Boldt Decision was issued, WDFW
conducted a study based on a series of snorkel surveys to estimate steelhead parr production in
populations across western Washington, including the Quillayute River and its tributaries,
which they then used to generate models and estimate a range of escapement goals under
varying assumptions (Gibbons et al. 1985). While the effort was laudable at the time, there are
several limitations, some of which include sample size and representation (e.g., the juvenile
snorkel surveys only occurred in a few rivers), survey counts that were not adjusted for diver
error (likely resulting in underestimates), and failure to account for the depletion of early-timed
steelhead (McMillan et al. 2022) or the effects of spatial distribution (Finstad et al. 2013; Atlas
et al. 2015). Further, the escapement goals do not account for potential mining of certain life
histories in species that display a high level of life history diversity, which in turn can reduce
93
productivity and resilience (Ricker 1963). Last, there has not been any effort to evaluate or
develop escapement goals or management targets for populations of summer steelhead.
Despite these limitations, the range of escapement goals estimated for each population
of winter steelhead were not unreasonable for the time. In fact, the highest estimates could
prove to be sufficiently conservative if the major questions are addressed through a sensitivity
analysis and aspects of diversity, such as run timing and iteroparity, were accounted for.
Unfortunately, WDFW and the co-managers disagreed over the escapement goal science and
ultimately, the parr production models were rejected in favor of Maximum Sustained Harvest
(MSH) and as a result, the agreed upon escapement goals were either among the lowest or
were lower than the lowest estimates in Gibbons et al. (1985). The disagreement continues to
this day, and as such, there is no agreed upon escapement goal for the Queets River or the
Lower Quinault River (Cram et al. 2018). This is a great concern given the declines of wild winter
steelhead specifically in those populations, but also overall because wild steelhead in the
Olympic Peninsula DPS experience the highest harvest rates of any steelhead populations in
Washington (Cram et al. 2018) and likely the world. The high harvest rates and declining
population trends raise serious questions about whether the management targets are sufficient
to sustain the winter steelhead populations and the fisheries they provide into an uncertain
future.
A previous assessment of winter steelhead management in western Washington argued
that a strong reliance on an MSH approach jeopardizes the health and resilience of a diverse
species such as steelhead (Burge et al. 2006):
“It has become abundantly clear that MSH theory and harvest models have not
provided adequate protection for wild steelhead in the 20th and 21st centuries
because they are too simplistic and allow high harvest rates that are
unsustainable. These models do not annually or temporally account or plan for
environmental variation, management error, the role of genetic and life history
diversity in stock resilience and productivity, or the rebuilding of depleted
tributary or seasonal runs. Rather, the models are rigid numbers-based
equations and provide management tools that were developed to provide
maximum harvests from a population without adequate considerations for long
term stock health or the sustainability of annual fisheries.”
Limited information and poor data quality on escapement levels can contribute to
population decline (Knudsen 2000). A 2018 report from the National Park Service noted that
some escapement estimates of salmonids on the Olympic Peninsula “are currently derived from
surveys that occur in limited index reaches or are based on one or a few surveys during the
peak of spawning” (Duda et al. 2018). The authors also noted there is a need “to fully
understand the extent of bycatch and incidental mortality of Pacific salmonids in recreational
and commercial fisheries in Olympic Peninsula Rivers” (Duda et al. 2018).
94
Given what we have outlined above, current escapement goals for the four major
Olympic Peninsula populations are likely far too low and should be revised based on
consideration of all available data. To ensure adequate escapement, the escapement goals
should be revised to incorporate precautionary estimates of parr and smolt densities required
to secure the resilience of each population given current and expected future variability in early
marine survival. Estimates should be based on spawner age-composition and repeat spawner
frequency targets with the primary objective of attaining minimum egg deposition sufficient to
attain target mean parr or smolt densities in rearing habitats (see Gayeski et al. 2016 for some
likely target parr density values). And the goals should begin to account for life history diversity
that underpins the evolutionary strengths of steelhead.
In addition, any harvest – whether tribal commercial, ceremonial, subsistence, or
recreational – should be reduced far below current comanager estimates of maximum
sustainable yield/harvest (MSY). Recent declines in productivity indicate that MSY harvest rates
are likely incompatible with population persistence or recovery. Petitioners obtained
population data sets from WDFW for the Hoh River and Quillayute River winter steelhead
populations (WDFW 2022d) These data sets contained run reconstructions that provided time
series of spawners and maiden recruits spanning more than 25 years. Gayeski conducted a
preliminary Bayesian estimation of the Ricker spawner-recruit model with time-varying
productivity parameter (alpha) and constant capacity parameter (beta) on both data sets. The
analyses were conducted in the program Stan. A copy of this estimation is included with this
petition (See Appx. A).
Gayeski’s estimates are shown in Figures 21 and 22. Both populations display a strong
declining trend in alpha and the unfished equilibrium abundance (EQ). The Hoh River
population displays a steady decline since 2003; the Quileute River population shows a steady
decline beginning in 2007. The average value of alpha for the Hoh River population for the
recent period of decline (2003 to 2013) is 2.51; the average value of EQ is 2,297. The average
value of alpha for the Quillayute River population for the recent period of decline (2007 to
2015) is 2.16; the average value of EQ is 9,058. For comparison, the peak values of alpha and EQ
for the Hoh River population during the period spanned by the data (1987 to 2013) occurred at
the beginning of the time series, where alpha averaged between 3.6 and 3.7, and EQ between
3,200 and 3,300. Peak values for the Quillayute River population during the period spanned by
the data (1978 to 2015) occurred in the early 1990s, where alpha averaged between 4.2 and
4.5, and EQ averaged between 17,000 and 18,000. These are biologically very significant and
worrying declines. Further, values of both parameters in the past three years of record for each
population are significantly lower than the recent average values.

95
Figure 21. Time series of time-varying Ricker productivity parameter alpha and unfished
equilibrium abundance for fixed beta parameter from a Bayesian stock recruit analysis of Hoh
winter-run steelhead.
1.5
1.7
1.9
2.1
2.3
2.5
2.7
2.9
3.1
3.3
3.5
3.7
3.9
1500
1600
1700
1800
1900
2000
2100
2200
2300
2400
2500
2600
2700
2800
2900
3000
3100
3200
3300
3400
3500
1986 1990 1994 1998 2002 2006 2010 2014
Equilibrium Spawner Abundance
Brood Year
Hoh winter run steelhead BYs 1987 to 2013, left axis = EQ;
right axis = Alpha
time-varying Ricker alpha with constant Beta = 2523.
EQ Alpha

96
Figure 22. Time series of time-varying Ricker productivity parameter alpha and unfished
equilibrium abundance for fixed beta parameter from a Bayesian stock recruit analysis of
Quileute winter steelhead.
It is very probable that the other two large winter steelhead populations (i.e., Queets
and Quinault River populations) and numerous smaller populations have experienced similar
declines. These declines are cause for alarm.
B. Harvest Impacts
Wild winter steelhead populations in the Olympic Peninsula DPS are fished for and
harvested in recreational and tribal fisheries at the highest levels of any steelhead populations
in Washington State (Cram et al. 2018). Harvest can affect abundance, diversity, productivity,
and spatial structure of salmon and steelhead populations, which in turn interact to shape the
short- and long-term viability of the exploited populations (McElhaney et al. 2000). There are
two main sources of harvest, including impacts from tribal gillnets and from recreational catch
and release fishing.
Tribal commercial harvest of wild steelhead annually occurs in the Quinault, Queets,
Hoh, and Quillayute River systems, and up until 2016, recreational anglers were permitted to
kill wild steelhead on eight rivers on the Olympic Peninsula (Cram et al. 2018). Tribal harvest
includes all fish that were reported, including commercial and subsistence catch.
1.5
1.7
1.9
2.1
2.3
2.5
2.7
2.9
3.1
3.3
3.5
3.7
3.9
4.1
4.3
4.5
6500
7000
7500
8000
8500
9000
9500
10000
10500
11000
11500
12000
12500
13000
13500
14000
14500
15000
15500
16000
16500
17000
17500
18000
18500
1978 1982 1986 1990 1994 1998 2002 2006 2010 2014
Equilibrium Spawner Abundance
Brood Year
Quileute winter run steelhead BYs 1978 to 2015, left axis = EQ; right
axis = Alpha time-varying Ricker alpha with constant Beta = 11985.
EQ Alpha
97
The recreational fishery is now catch and release only for wild steelhead, and although
catch and release generally has low mortality rates (2-10%: Hooton 1986; Barnhardt and Taylor
1996; Nelson et al. 2005), recreational fishing pressure and success has increased to the point
where anglers are now catching and releasing the entire escapement, on average, more than
one time (Bentley 2017; WDFW 2022c). Prior to 2016, WDFW determined recreational harvest
using punch cards. Now that the fishery has shifted to catch and release, WDFW relies on creel
surveys to estimate angler encounter rates with wild winter steelhead and estimate a 10%
mortality to fish that are caught and released (Bentley 2017; Cram et al. 2018).
It is likely that harvest rates in the Quillayute, Hoh, Queets, and Quinault Rivers are
underestimates. For example, we could not find estimates of gillnet dropout rates for wild
winter steelhead in the tribal commercial fisheries. Injuries sustained by fish that escape gillnets
can dramatically reduce their reproductive success if they survive to spawn (Baker et al. 2013).
It is unknown how many redds are counted each year that were constructed by fish with lower
fitness due to such injuries.
In addition, on average, every wild winter steelhead that escaped to spawn in the Hoh
River in 2014 was caught and released, on average, 1.4 times by anglers (Bentley 2017). WDFW
has several years of raw creel survey data, but the Hoh River in 2014 was the only stream and
year for which they expanded those counts to estimate total encounter rates. The raw creel
data suggests high encounter rates in other streams, with possibly even higher encounter rates
in the Bogachiel and Sol Duc Rivers (WDFW 2022c, see Fishing Regulations section below).
Although the 10% mortality rate that WDFW applies to recreational encounters is relatively
high and conservative, those estimates are based on a fish being caught one time (Hooton
1987; Hooton 2001; Nelson et al. 2005; Twardek et al. 2018). The mortality rate for fish caught
multiple times is unknown, but fish that enter early and remain in the system longer have a
higher frequency of being caught more than once (Hooton 1987; Hooton 2001). Further,
emerging research on Atlantic salmon indicates there can be significant sub-lethal impacts that
can alter the migration and reduce the reduce fitness of adults (up to ~ 20-25%) that are caught
and released (Richard et al. 2013; Bouchard et al. 2021; Papatheodoulou et al. 2021).
Potential effects from net dropouts and high recreational encounter rates, combined
with the high harvest levels, raise concerns whether current estimates of harvest fully account
for all impacts.
Below we review what is currently known about harvest rates for the four major wild
winter steelhead in the Olympic Peninsula DPS and discuss harvest in relation escapement
goals, how it has most likely contributed to changes in run timing, and how it is potentially
affecting size and age and iteroparity.
We also summarize harvest records for summer steelhead and, when possible, we
distinguish between wild and hatchery fish, but that is a challenge with the catch data. See
(WDFW 2022e). From 1978-1985 returning adults were not marked with an adipose fin clip, and

98
thus, only total catch of summer steelhead is reported for tribal and recreational fisheries.
Thereafter, recreational catch consistently delineates hatchery and wild summer steelhead
from 1985-2016, while the tribal catch records do not – except for the Quillayute River system
from 2001-2020. Retention of wild summer steelhead by recreational anglers was banned by
WDFW and the National Park Service in 1992, and consequently, from 1993 onward all
recreational fisheries for wild summer runs were catch and release. Even though contemporary
catch of wild summer steelhead is not high by tribes and anglers must employ catch and
release, impacts could be more deleterious than they seem given the very depleted status of
the stocks and the high levels of hatchery fish (see Hatchery Impacts section below).
1. Quillayute River System
Annual harvest rates of wild winter steelhead from 1978-2020 ranged from 10% to 55%
in the Quillayute River system (Figure 23a), with a mean annual harvest rate of 28%, which is
the lowest harvest rate of the four major populations (Table 1). Compared to the mean annual
run size of 13,064 wild winter steelhead, the mean harvest rate equates to 3,724 fish per year.
Total wild winter steelhead harvested per year ranged from 1,166 to 7,561 fish. Harvest rates
have generally declined the past few years (Figure 23a) in response to smaller run sizes and
shortened fishing seasons. The proportion of hatchery to wild winter steelhead in the
commercial fishery was 2.1:1, and the proportion of hatchery to wild winter steelhead in the
recreational fishery was 3.3:1 (Duda et al. 2018). The mean annual harvest rate of hatchery
winter steelhead was 64% (range 32% – 86% of total run size; SD 12%) (Duda et al. 2018).

99
Figure 23. Annual harvest rates of wild winter steelhead populations in the a) Quillayute, b)
Hoh, c) Queets, and d) Quinault Rivers. Based on co-manager data sets covering period circa
1980-2020.
Harvest rates of wild summer steelhead from 1978-2020 were not available because co-
managers do not monitor summer runs. We do, however, have catch data for summer
steelhead, though wild and hatchery fish could not be distinguished prior to 1986, the year the
first adults returned with adipose fin-clips. Hatchery summer runs were first planted in the
Calawah and Sol Duc Rivers in 1977 and were stopped in the Sol Duc in 2012 (Figure 24), and
according to the hatchery records we reviewed, the Quillayute River watershed is the only
system to have received direct plants of hatchery summer steelhead (Duda et al. 2018).
Figure 24. Number of hatchery summer steelhead smolts released into the Sol Duc and Calawah
Rivers, which are tributaries to the Quillayute River watershed.
We separated the Quillayute River catch data into recreational (1978-2016) and tribal
Treaty catch (1978-2020) because the recreational catch distinguishes between hatchery and
wild fish in all years possible while we could only find that delineation for tribal catch from
2001-2020. As mentioned previously, historical catch of wild summer steelhead was very low
and inconsistent in the Quillayute prior to hatchery releases in 1977, but from 1978 to 1985
there was a spike in harvest of adult summer steelhead, many of which were likely hatchery
(Figure 25). After fin-clipped hatchery adults started to return in 1986, the catch of wild fish
declined and remained low while the harvest of hatchery fish increased (Figure 25). Retention
of wild summer steelhead by recreational anglers was banned by WDFW and the National Park
Service in 1992, and consequently, from 1993 onward all recreational fisheries for wild summer
runs were catch and release. However, WDFW records show some wild summer steelhead

100
harvest after 1992, and we are not sure why, though it is possible the data represents fish that
were illegally harvested (Figure 25). Regardless, based on the punch card data, 2006 was the
last year a wild summer steelhead was reported as harvested by recreational anglers in the
Quillayute River system (Figure 25). Mean annual recreational harvest from 1986-2006 was 54
wild summer steelhead (range = 0 – 239) and from 1986-2016 mean annual recreational
harvest was 673 hatchery summer steelhead (range = 119 – 1,974).
Figure 25. Number of summer steelhead harvested by recreational anglers from 1962-2016 in
the four largest populations in the Olympic Peninsula DPS. The black line denotes wild
steelhead with an adipose fin. The grey line denotes unknown steelhead because harvest
occurred during a period when hatchery fish were introduced, but not outwardly marked, so
wild and hatchery could not be distinguished. The dashed black line denotes hatchery
steelhead. Retention of wild summer steelhead by recreational anglers was banned from 1993
onward by WDFW and the National Park Service.
We found two sets of tribal catch data with slightly different annual catch numbers. The
first ranges from 1978-2016 and does not distinguish between hatchery and wild summer
steelhead. The second data set ranges from 2001-2020 and, although it does not contain catch
records for all years, it does delineate hatchery and wild steelhead in years when catch records
were available (Figure 26 and Figure 27). The data sets are compared in Figure 27, which shows
the data from the 1978-2016 and 2001-2020.

101
Overall, the tribal harvest of summer steelhead in the Quillayute River is generally
higher than other populations, except for the Quinault River (Figure 26). Mean annual tribal
catch from 1978-2016 was 341 summer steelhead of unknown origin (range = 43 – 1,120), while
mean annual tribal catch from 2001-2020 was 107 wild summer steelhead (range = 24 - 244)
and 308 hatchery summer steelhead (range = 28 – 839). Given the lack of monitoring and
escapement goals, there should be concern because the reported harvest of wild steelhead is
high (Figure 24) compared to the snorkel counts of staging adults (Table 7) from circa 2000-
2010.
Figure 26. Number of summer steelhead harvested by tribal fishers in Quillayute, Hoh, Queets,
and Quinault Rivers, with occasional data available for some populations from 1946-1975 and
generally consistent annual catch data available from 1976-2017. Data were provided by
WDFW and did not include information on which fish were hatchery or which were wild. Based
on releases of hatchery smolts in Quillayute River system in 1977, 1978 would be the first year
of potential adult returns. So, data thereafter is conflated by presence of hatchery fish, which
could represent a substantial component of the catch – even in watersheds outside the
Quillayute River as evidenced by sport catch data in Hoh and Queets Rivers.

102
Figure 27. Number of summer steelhead harvested in Quillayute River by tribal fishers, which
includes two disparate data sets: data set 1, which only provides total numbers of summer
steelhead harvested and does not delineate between hatchery and wild; and data set 2, which
delineates total harvest by hatchery and wild. We provide both data sets because they
contained different harvest numbers.
2. Hoh River
The mean annual harvest rate from 1980-2020 was 35% for Hoh River wild winter
steelhead (Table 1), with a range of 7% to 55% per year (Figure 23b). This equates to a harvest
of 1,361 wild winter steelhead per year, with a range of 261 – 2,800, compared to a mean
annual run size of 4,118 steelhead. Harvest rates have generally declined the past few years
(Figure 23b) in conjunction with declining run sizes and shortened fishing seasons. The ratio of
hatchery to wild winter steelhead in the commercial fishery was 2:1, and it was 3.4:1 in the
recreational fishery. (Duda et al. 2018).
As with the other populations, we delineate the recreational harvest of summer
steelhead in the Hoh River population into three periods, including: (1) the historical period
discussed in a previous section dating from circa-1950s through 1977; (2) the post hatchery
release period when wild and hatchery steelhead could not be distinguished (1978-1985); and
(3) the period from 1985 to the present when wild and hatchery fish could be distinguished
(Figure 25). Although the Hoh River did not receive hatchery releases, the harvest of summer
steelhead sharply peaked from 1978-1985 like the Quillayute River did. Since 1985, the harvest
of wild summer steelhead declined to zero in 2007, while the harvest of hatchery summer runs
has remained relatively high (Figure 25). Mean annual recreational catch from 1978-1985 was
459 steelhead (range = 96 - 711). The last year a wild summer steelhead was reported as
harvested by recreational anglers was 2006, the same as the Quillayute River system, though
103
retention of wild summer runs was banned by managers in 1993. Mean recreational harvest
from 1986-2006 was 52 wild summer steelhead (range = 0 - 257) and, interestingly, 204
hatchery summer steelhead (range = 33 - 433). Given the consistent hatchery catch by
recreational anglers over the period of record in the Hoh River, the spike in catch from 1978-
1985 was potentially associated with hatchery steelhead straying from the Quillayute River.
Tribal harvest of summer steelhead has been variable since 1978 and was not
delineated by hatchery and wild fish (Figure 26). The mean annual tribal catch from 1978-2016
was 207 summer steelhead of unknown origin, with a peak of 937 fish and a low of 10. Overall,
the reported catch levels, as with the Quillayute River system, seem high compared to the very
low abundance of wild summer steelhead reported in snorkel surveys in the SF Hoh River (Table
4). This is even more concerning due to the lack of monitoring and escapement and fishery
goals.
3. Queets River
The mean annual harvest rate from 1978-2020 was 35% for Queets River wild winter
steelhead (Table 1), with a range of 10% to 55% per year (Figure 23c). This equates to a harvest
of 2,672 wild winter steelhead per year, with a range of 575 – 6,291, compared to a mean
annual run size of 7,384 steelhead. There could be a slight declining trend in harvest rate over
the period of record (Figure 23c). The ratio of hatchery to wild winter steelhead in the
commercial fishery was 0.7:1 and 4.7:1 in the recreational fishery. (Duda et al. 2018). Since
1976, the mean annual commercial harvest of hatchery steelhead has been 1,472 fish (range
298 – 3,308; SD 766) (Duda et al. 2018).
Recreational catch of wild summer steelhead in the Queets River was highest during the
historical period, and then showed a similar peak in catch as the Quillayute and Hoh Rivers from
1978-1985, followed by a sharp decline in catch thereafter until recreational harvest of wild
summer runs was eliminated in 1993 though some summer steelhead were reported as
harvested from 2001-2004 (Figure 25). Like the Hoh River, the Queets River does not receive
hatchery summer steelhead smolts, and yet, they have been caught each year by anglers since
hatchery fish were first marked (Figure 25). Mean annual recreational catch from 1978-1985
was 201 steelhead (range = 100 - 310), compared to 53 wild summer steelhead (range = 0 - 153)
from 1986-1992 and 2001-2004 and 30 hatchery summer steelhead (range = 8 - 81) from 1986-
2016.
Tribal catch is not delineated by hatchery and wild. Zero catch was reported in 1984 and
only two fish were reported in 1985. Except for these years, relatively low levels of catch have
been reported (Figure 26). Mean annual tribal catch from 1978-2016 was 102 summer
steelhead (range = 2 – 469). Unfortunately, there is almost no data on the abundance of wild
summer steelhead in the Queets River, so it is very difficult to draw inferences about potential
harvest impacts. Nonetheless, wild summer steelhead were formerly quite abundant as we
described earlier in the Historic Abundance section, and it is likely they are now highly
depleted. Consequently, we have grave concern about this population because it is not
104
monitored, there are no escapement goals, and there has been no effort to evaluate status and
trends to inform management and conservation.
4. Quinault River
Annual harvest rates of wild winter steelhead from 1978-2020 ranged from 15% to 65%
in the Quinault River (Figure 23d), with a mean of 46% (Table 1). That is the highest mean
harvest rate among the four largest populations in the DPS (Table 1). It equates to 2,832 fish
per year, with a range of 814 – 6,356, compared to a mean annual run size of 5,968 wild winter
steelhead. Since 1977, there has been a mean annual commercial harvest of 7,089 hatchery
winter steelhead (SD = 3,956) with a range of 1,068 – 15,979 fish, and a mean annual
recreational harvest of 692 hatchery winter steelhead (SD = 418) and a range of 61 – 1,656 fish
(Duda et al. 2018). The ratio of hatchery to wild winter steelhead in the commercial fishery was
2.9:1 and in the recreational fishery it was 3.0:1 (Duda et al. 2018).
The pattern and extent of annual recreational catch of summer steelhead in the
Quinault River differs from other watersheds in that it did not experience the peak in catch
during the period when hatchery fish were unmarked and overall, catch remained very low
until some hatchery steelhead started to show up in the catch in the late-1990’s (Figure 25).
Overall, mean annual recreational catch from 1978-1985 was 15 steelhead (range = 8 - 34),
compared to 8 wild summer steelhead (range = 0 - 18) from 1986-1992 and 2001-2002 and 15
hatchery summer steelhead (range = 0 - 44) from 1986-2016.
Tribal catch of summer steelhead from 1976-2017 is higher than all other populations
(Figure 26). The mean annual catch was 753 steelhead, with a range of 191 to 2,345 fish.
However, given the very low snorkel counts of wild adult summer steelhead in the upper EF and
NF Quinault Rivers (see Abundance section), it is unclear whether true wild summer runs are
being harvested at very high rates or whether some of the fish are winter run kelts. It is also
unclear whether hatchery summer runs are contributing. A combination of all three factors
could possibly explain the very high catch levels reported by the tribe.
We delineated catch by month. The highest mean catch was in May, which thereafter
declined through September until catch slightly increased in October (Figure 28). May is an
important month of spawning for Olympic Peninsula steelhead (Busby et al. 1996), so some (or
many) of those fish could be kelts. Kelts are likely being captured in June, considering spawn
timing in other nearby populations (e.g., McMillan et al. 2007). Perhaps some of the fish are
also hatchery summer steelhead from releases in streams further south in Grays Harbor, such
as the Wynoochee River. Whatever the case, it seems imperative to determine the life histories
and source of the fish, because snorkel surveys in the upper watershed suggest the population
is greatly depleted and potentially close to extinction.
105
C. Recent failures to Meet Escapement Goals
A report by Cram et al. (2018) evaluated the number of years the harvested wild winter
steelhead populations met their escapement goals from 2004-2013 (Table 3). We updated
those estimates to cover the most recent ten-year period (2011-2020) for all populations and
tributary populations (Table 1). There is an overall problem with many populations failing to
meet their escapement goals, particularly in recent years (Table 1). For example, among the
wild steelhead populations that experience harvest, the Hoh River has only met its escapement
goal of 2,400 fish in six of the past ten years (Table 1) and only 50% of the years dating back to
2003 (Figure 5).
The Queets River population has only met its WDFW and National Park Service
escapement goal of 4,200 fish in 30% of the last ten years (Table 1), and run sizes are now
coming in below the escapement goal (Figure 8a).
The Clearwater River, the major tributary of the Queets, has met its escapement goal of
1,450 fish only 50% of the years in the past decade (Table 1), with recent escapements being
the lowest during the period of record (Figure 8b).
There is no escapement goal for the entire Quinault River population, but its run sizes
have collapsed to the point where it certainly would be below any reasonable estimate for
spawner goals (Figure 7).
The Quillayute River system overall has remained the strongest among the populations,
meeting its escapement goal of 5,900 fish for the entire watershed in nine out of the past ten
years (Table 1) and only missing the goal in two of the past twenty years (Figure 6a). Among its
major tributaries, the Dickey (Figure 6b) and Calawah Rivers (Figure 6d) have met their
escapement goal each of the past ten years (Table 1), and they are the only two tributaries that
do not have a long history of receiving releases of hatchery winter steelhead. In contrast, the
Bogachiel/Quillayute (Figure 6e) and Sol Duc Rivers (Figure 6c) have only met their escapement
goal in 60% and 70% of the last ten years, respectively (Table 1).
As mentioned in the abundance and trends section, nearly all the smaller, independent
populations of wild winter steelhead have failed to meet their escapement goals during much
of – if not all – the past decade (Table 1). Most concerning, some of the populations have not
achieved their escapement goals for extensive periods of time dating back 20 years or more.
D. Demographic, Genetic, and Ecological Risks Caused by Harvest
Harvest poses demographic and genetic risk to wild salmon and steelhead (ISAB 2005;
Hard et al. 2008) and can alter attributes including, but not limited to, size and age at maturity,
migration timing, spatial distribution, and life history diversity, which, in turn, interact to shape
the productivity and resilience of wild salmon and steelhead populations (McElhaney et al.
2000; ISAB 2005; Hard et al. 2008). Given the high exploitation rates on the four largest
106
populations of Olympic Peninsula wild winter steelhead, it is possible that chronic, high levels of
harvest have imposed a variety of impacts. Unfortunately, research on such topics is almost
entirely lacking for the DPS, except for evidence of change in run timing of wild winter
steelhead (McMillan et al. 2022).
Cram et al. (2018) noted the risk of fishery selection on run timing since the number of
fishing days per week for treaty fisheries is highest early in the season, when the fishery targets
greater harvest of hatchery adults. Historical recreational fisheries were structured similarly,
with a focus on harvesting early returning hatchery adults that were intermixed with wild
adults. The variable historical and contemporary fishing rates indeed appear to have
contributed to the substantial depletion of early returning wild steelhead (Figure 15). As a
result, run timing is more compressed than it was historically.
The results challenge the findings in Busby et al. (1996), which described hatchery and
wild winter steelhead as being temporally segregated because of differences in run timing. It
also underscores the importance of the Shifting Baseline Syndrome, where managers and
scientists come to accept the current level of abundance and diversity as the norm (Pauly
1995). For example, in the Quileute Tribal language, the month of January is defined as the
“time of steelhead running” and February is defined as the “time of steelhead spawning”
(Frachtenberg 1916). Thus, based on quantitative data and traditional ecological knowledge, it
is clear there has been a large change in run timing that has not been accounted for or
addressed in previous reviews of the Olympic Peninsula DPS (e.g., Busby et al. 1996).
The change in run timing could impact the population in several ways. Per McMillan et
al. (2022):
“Changes in run timing can shorten breeding seasons, reduce phenotypic
diversity, and lower population productivity (Tillotson and Quinn 2018). More
protracted anadromous fish migrations, like those that we estimated to occur
historically for wild Winter Steelhead, can allow fish to temporally stagger the
use of spawning habitat (Gharrett et al. 2013), thereby reducing density-
dependent effects on juvenile survival and increasing local habitat capacity
(Chandler and Bjornn 1988).
Migration timing is also often associated with the spatial structure of breeding
locations in anadromous fish populations (e.g., Everest 1973; Stewart et al. 2002;
Beacham et al. 2012). On the [Olympic Peninsula], McMillan et al. (2007)
observed spatial correlations with spawn timing in the Quillayute River, where
spawning higher in the stream network in smaller stream channels occurred
about a month earlier than in the lowermost mainstem spawning reaches.
Previous observations by Cederholm (1984) also found that earlier returning
Queets River wild Winter Steelhead were more likely to spawn in smaller
tributaries, while mainstem spawning tended to occur several weeks later in the
season. Importantly, Cederholm (1984) observed this occurrence in low
107
elevation tributary streams, so the pattern of earlier spawn timing in tributaries
is not necessarily limited to higher elevation headwaters as might be inferred
just from the results of McMillan et al. (2007), but rather it appears to be a
pattern associated with stream size.”
In addition, migration timing underpins a population’s adaptive capacity to keep pace
with shifting climatic conditions, such as changing streamflow and temperature regimes
(Manhard et al. 2017; Reed et al. 2011; Austin et al. 2020). For example, winter steelhead tend
to migrate and spawn earlier in the season in warmer streams in more southerly habitats
(Busby et al. 1996), presumably because the higher streamflows provide better access and
earlier spawning is needed to ensure offspring emerge before the onset of summer baseflows.
Climate change models predict water temperature regimes on the Olympic Peninsula (see
Climate Change section) will become more similar to those in more southerly climates (Wade et
al. 2013). This suggests that early migrating life histories will become increasingly important to
population resilience. However, rebuilding earlier-timed wild winter steelhead migrations will
be challenging without addressing the current fishery structure and hatchery practices (see
Hatchery section below).
Harvest could also be impacting diversity though selection on size and age and selection
against iteroparity. Harvest can alter size and age in salmonids (Hard et al. 2008), and Cram et
al. (2018) reported that scale samples indicated gillnetting was selectively capturing older adult
wild steelhead, while recreational fisheries were capturing more younger fish (Cram et al.
2018). It is unknown how these effects have manifested over the decades, but it is possible that
both fisheries have truncated the overall distribution of size and age in the populations
experiencing high levels of harvest.
Steelhead are iteroparous, and individuals that repeat spawn are significantly more
productive on their second attempt than their first and forgo early reproduction to devote
additional energy to continued survival (Christie et al. 2018). Repeat spawning fish may also
come back larger and with more eggs (Christie et al. 2018; Gayeski, personal observation on
recaptured tagged steelhead in the Sopichnaya River, Kamchatka 2005). In this context, kelts
migrating back to the ocean after spawning their first time are only likely to contribute to
reproduction if they are allowed to spawn a second time. Harvesting those individuals is
analogous to removing the most productive life history from the next generation of spawners.
Kelts can appear in the system beginning as early as late-January, but they are most common
after the peak of spawning from April through May. During that time kelts are harvested in
gillnet fisheries and captured by recreational anglers in catch and release fisheries.
Although the steelhead fishery is closed in May, there have traditionally been
recreational and tribal Spring/Summer Chinook Salmon fisheries in each of the rivers that
begins in April and runs through May and into June. Those fisheries could have significant
impacts on kelts. McMillan (2006) hypothesized a portion of the relatively high numbers of wild
steelhead harvested by Treaty fisheries in May are likely kelts – up to 1,800 fish in May in some
years – despite being considered “summer runs” by co-managers. This seems possible,
108
especially in the Quinault River where the catch numbers of steelhead in May are particularly
high (Figure 28). There is also an intensive sport fishery in the Quillayute and Sol Duc Rivers for
hatchery spring/summer Chinook, although it is unclear how many kelts are caught, it could be
substantial because most anglers are using bait. And while sport fisheries for spring Chinook
salmon are now closed on the Hoh and Queets Rivers, historically they were also open to
recreational angling at different periods. Because kelts start to feed after spawning, they are
also prone to being caught by recreational anglers and, considering the low lipid levels and poor
condition of kelts (Penney and Moffitt 2014), any further expenditure of energy – such as being
hooked and caught – could result in delayed mortality.
In addition, as noted by Gayeski et al. 2016, steelhead kelts (if unstressed by post-
spawning capture in river fisheries) have higher survival rates to repeat spawning in the
following one or two years than smolts that must survive several years in the marine
environment in order to become maiden spawners. Thus, repeat spawners provide a
considerable boost to the overall productivity and juvenile capacity of the population than
smolts that survive to spawn for the first time. This is particularly critical to the recovery of
depressed populations.
Ultimately, the sharp declines in iteroparity in the Queets River population since 1980
and low levels of iteroparity in other wild winter steelhead populations (Figure 16) have likely
contributed to the chronic declines over the past twenty years or more. The depleted early
returning wild steelhead combined with potential changes in size and age and reductions in
iteroparity raise serious questions about the sustainability of the fishery model and highlights
the need to incorporate run timing and repeat spawning into steelhead management and
fisheries.

109
Figure 28. Proportion of repeat spawning individuals (repeats/total run size) by year for wild
winter steelhead populations in the Quillayute (1978-2021), Hoh (1993-2020), Queets (1980-
2019), and Quinault Rivers (1991-2020).
III. DISEASE AND PREDATION
A. Disease
The M genogroup of the infectious hematopoietic necrosis virus (IHNV), which is
harmful to steelhead, has been previously detected in seven hatchery locations where wild
Olympic Peninsula steelhead occur (Breyta et al. 2013). Between 2007 and 2011, there were
two distinct waves of the virus that occurred in multiple watersheds, including the Hoh, Queets,
Quinault, and Quillayute Rivers (Breyta et al. 2013). Most fish detected with the virus were
hatchery fish, however, wild fish are less commonly sampled (Breyta et al. 2013). The virus was
detected in wild fish in the Hoh and Quinault Rivers (Breyta et al. 2013). According to Breyta et
al. (2013), “it is not clear what the future burden of IHNV in coastal steelhead trout might be.”
B. Predation
There is an increased distribution of predators in the Dickey River, likely because of
warming water temperatures (Smith 2000). It is likely that predation risks will increase in
Olympic Peninsula rivers as summer streamflows decrease and water temperatures increase
(Dalton et al. 2016).

110
IV. INADEQUACY OF EXISTING REGULATORY MECHANISMS
A. FEDERAL
1. National Forest Management Act & Northwest Forest Plan
The National Forest Management Act (NFMA) requires the U.S. Forest Service (USFS) to
manage fish and wildlife habitat in the Olympic National Forest to “maintain viable populations
of existing native and desired non-native vertebrate species.” 36 C.F.R. § 219.19. In 1990, the
USFS adopted a Land and Resource Management Plan (“LRMP") for the Olympic National Forest
(USFS 1990). The USFS claimed that implementation of the plan would increase fish production
potential by more than 10% by the end of first 10 years of implementation (USFS 1990). It also
claimed that it would correspond with 1,200,000 additional anadromous smolts and that
anadromous fish production would increase by 25% above then current levels due to decreased
sedimentation and habitat enhancement projects (USFS 1990).
In 1998, the LRMP was amended to include management changes consistent with the
Northwest Forest Plan (1994) (Hoffman 1998). The NFP includes an Aquatic Conservation
Strategy (ACS), which establishes measures intended to restore and maintain ecological
processes of aquatic and riparian habitat (Thomas et al. 1993; Reeves et al. 2006). Among other
things, the ACS requires the USFS to “maintain and restore the sediment regime under which
aquatic ecosystems evolved” (USDA 1994). To date, the ACS has not maintained or restored the
sediment regime under which Olympic Peninsula steelhead evolved, and Petitioners did not
locate evidence to suggest that the LRMP and the improvements made by the ACS have
increased anadromous fish production by 25% or more over 1990 levels.
The ACS also established riparian reserves, which place primary emphasis on protecting
fish habitat. The size of the buffers depends on whether the streams are designated as fish- or
non-fish-bearing. Riparian buffers on fish-bearing streams are two site-potential tree heights (>
200 years old) or 300 ft, whichever is greater. Riparian buffers for non-fish bearing streams are
one site-potential tree height or 100 ft, whichever is greater (Wilhere and Quinn 2018). The
buffers are intended to help ensure that species meet certain viability standards (Wilhere and
Quinn 2018). When developing the ACS, the USFS assumed that if a species has at least an 80%
likelihood of a stable, well-distributed population over 100 years, it is viable (Wilhere and Quinn
2018).
The USFS is also guided by an Olympic National Forest Strategic Plan, which “integrates
aquatics, wildlife, silviculture, and fire, helping to identify priority areas for management
activities such as habitat restoration, road decommissioning, forest thinning, and fuel reduction
treatments” (Halofsky et al. 2011). It also has a Road Management Strategy, which it
developed, in part, to meet ACS standards (Halofsky et al. 2011). The road strategy sets
priorities for road maintenance, upgrading, and decommissioning based on several factors,
including aquatic risk and high-value watershed goals (Halofsky et al. 2011).
111
The NFP established the Aquatic and Riparian Ecosystem Monitoring Program (AREMP)
to monitor whether implementation of the ACS is improving watershed conditions (Gaines et al.
2022). Unfortunately, the program has been hindered by insufficient funding and changes to
monitoring protocols, which make it challenging to measure the impacts of the ACS on aquatic
resources (Gaines et al. 2022).
Roads
The ACS has not been effective in reducing road density or improving other road-related
factors that affect fish (Frissell et al. 2014). According to Frissell et al. “[t]he magnitude of
existing road impacts on watersheds and streams in the [NFP] may equal or exceed the effect of
all other activities combined” (Frissell 2014).
Roads present major risks to fish and aquatic resources in the Olympic National Forest.
In 2020, the USFWS stated that “forest roads in the Olympic National Forest have been a
chronic source of sediments for decades” (USFWS 2020). Although the USFS has
decommissioned 435 miles of roads in the Olympic National Forest since 1990 (Halofsky et al.
2011), hundreds of road miles still present significant risks to aquatic resources (USFS 2015).
Fifty-one percent (1,032 miles) of all roads in the Olympic National Forest present high aquatic
risks (USFS 2015). Thirty-three percent (651 miles) of the roads in the national forest are rated
as presenting medium aquatic risks (USFS 2015). As a result, only 17% (338 miles) of roads in
Olympic National Forest present low aquatic risk (USFS 2015). Nearly one-third of the Olympic
National Forest’s roads are proposed for decommissioning (Halofsky et al 2011).
In addition to falling behind on road decommissioning, the Olympic National Forest has
not received the funding necessary to bring all of the roads up to current standards (USFWS
2020). Additionally, some roads that have been previously maintained need additional work
(USFWS 2020).
Riparian Revegetation
Although there has been some revegetation in riparian corridors in the Olympic National
Forest, many corridors continue to have few conifers because of historic logging practices
(Halofsky et al. 2011). Reestablishing conifers in these corridors would help restore sources of
large woody debris (Halofsky et al. 2011). However, these projects require costly, long-term
commitments and, therefore, they have not been a high priority for forest managers (Halofsky
et al. 2011).
Additionally, treatments in riparian areas can disturb soils and decrease effectiveness in
retaining sediment and nutrients (Frissell et al. 2014). Thinning in riparian areas can diminish
summer flows because of increased water demand by regrowth of vegetation (Frissell et al.
2014).
112
Climate Change
It is unclear if the USFS has reviewed the ACS to determine whether any science-based
changes to the strategy are necessary in response to climate change (Frissell 2014). Current
forest plans are not flexible enough to adapt to climate change challenges (Gaines et al. 2022).
To strengthen watershed resiliency to climate change, the ACS could be revised to include
additional steps to reduce non-climatic stressors (Gaines et al. 2022). For example, reducing the
impact of roads would foster greater ecosystem resiliency to climate change (Gaines et al.
2022). To date, the ACS has not been updated to specify steps to reduce non-climate-related
stressors on watersheds as a method to increase resiliency to climate change.
2. Endangered Species Act
Several populations of Olympic Peninsula steelhead occur in areas where ESA-related
conservation and recovery efforts are underway for other species, including bull trout (USFWS
2020). As a result, Olympic Peninsula steelhead may benefit from the habitat protections
afforded to bull trout (USFWS 2020). The status of Olympic Peninsula steelhead, however,
demonstrates that these benefits are not sufficient to halt or reverse their decline. Olympic
Peninsula steelhead need their own protection under the ESA.
a. Critical Habitat for Bull Trout
In 2005, the USFWS designated a series of waterbodies as critical habitat for coastal
populations of bull trout. 70 Fed. Reg. 56212, 56304-56306 (Sept. 26, 2005). In 2010, the
USFWS updated the designations for 32 critical habitat units (CHUs), including the Olympic
Peninsula Unit (75 Fed. Reg. 63898 (Oct. 18, 2010)). The USFWS designated 121 waterbodies as
critical habitat in the Olympic Peninsula Unit, excluding certain geographic areas covered by the
Washington State Forest Practices Plan (HCP) or covered by tribal plans. Id., at 63968-69369.
Critical habitat for bull trout overlaps with multiple areas where Olympic Peninsula
steelhead occur (75 Fed. Reg. 63875-63978) Specifically, Olympic Peninsula steelhead occur in
the following critical habitat for bull trout: Clearwater River, Copalis River, Hoh River, Kalaloch
Creek, Moclips River, Mosquito Creek, Quinault River, Queets River, Raft River, Salmon River,
South Fork Hoh River, and Tshletshy Creek. As a result, Olympic Peninsula steelhead in these
rivers and streams may benefit from critical habitat protection afforded to bull trout.
b. Biological Opinion on Forest Management Activities on the Olympic National
Forest
On June 24, 2020, the USFWS issued a biological opinion addressing Forest Management
Activities on the Olympic National Forest (USFWS 2020). The biological opinion evaluates the
potential effects of management actions on ESA-listed bull trout, Northern Spotted Owl, and
Marbled Murrelet. It requires certain conservation measures for projects that occur within bull
trout core area watersheds or in designated critical habitat for bull trout. Several populations of
113
Olympic Peninsula steelhead occur in bull trout core area watersheds and critical habitat and
therefore, may indirectly benefit from these conservation measures. These measures include
the following: steps to prevent erosion and enable large wood recruitment; limits on
commercial thinning and road maintenance activities; prohibitions on new culvert installations
or culvert replacements; no-cut buffers restrictions; and road standards (USFWS 2020).
However, despite these conservation measures, the USFWS anticipates that adverse
impacts will continue to occur in bull trout habitat (USFWS 2020). Olympic Peninsula steelhead
that occur in the same areas as bull trout will likely incur some of these impacts as well. For
example, new and temporary road construction, existing road reconstruction, road repairs, log
hauling, road grading/blading, and drainage maintenance is anticipated to cause indirect
adverse effects to bull trout. These effects include increased fine sedimentation, altered
watershed hydrology, reduced water quality, and increased substrate embeddedness (USFWS
2020). The USFWS expects that the Queets and Quinault Core Areas, which overlap with the
habitat of Olympic Peninsula steelhead, will continue to be depressed by poor water quality
(e.g., up to 408 tons of sediment per year in the Queets River) increased substrate
embeddedness, and altered flow regimes caused by logging operations (USFWS 2020).
c. Habitat Conservation Plans
Olympic Peninsula steelhead may benefit from the Washington Department of Natural
Resources Trust Lands Habitat Conservation Plan (DNR HCP) and the Forest Practices Habitat
Conservation Plan (FPHCP). However, these plans have not been fully implemented and there
are conflicting data on whether forest practices are improving aquatic resources. These plans
are discussed in the state forest management section.
3. Clean Water Act
a. Overview
The Clean Water Act (CWA), 33 U.S.C. §§ 1251-1387, is the principal federal law
regulating water quality in U.S. surface waters. The CWA seeks “to restore and maintain the
chemical, physical, and biological integrity of the Nation’s waters.” 33 U.S.C. § 1251(a). The Act
establishes a goal to eliminate all discharges of pollutants into navigable waters by 1985. 33
U.S.C. §§ 1251(a)(1). It also sets an interim goal of achieving, wherever attainable, “water
quality which provides for the protection and propagation of fish, shellfish, and wildlife and
provides for recreation in and on the water” by July 1, 1983. Id., at 1251(a)(2).
The U.S. Environmental Protection Agency (EPA) is the primary federal agency
responsible for administering the CWA. EPA implements water pollution control programs,
establishes pollution control technology standards for industries, and reviews water quality
standards set by states. The U.S. Army Corps of Engineers (Corps) regulates the discharge of
certain materials (e.g., fill) in the waters of the United States.
114
The CWA requires states to establish water quality standards to protect public health
and welfare, enhance water quality, and serve the purposes of the Act. States follow a two-step
process when setting water quality standards. First, they establish “designated uses” for
individual waterbodies, such as the protection and propagation of fish. Next, they set allowable
levels of pollutants to protect those uses. 40 C.F.R. §§ 131.10, 131.11. Washington has EPA-
approved water quality standards; however, it is not meeting them in many rivers and streams
where Olympic Peninsula steelhead occur (WDOE 2016; NIFWC 2020).
The CWA requires states to list “impaired waters,” which include segments of
waterbodies that do not meet water quality standards. The states must develop Total
Maximum Daily Loads (TMDLs) that set the maximum amounts of pollutants that may enter
impaired waters without violating water quality standards. The state may distribute those
amounts or “loads” to various sources of point and nonpoint sources of pollution. In effect, a
TMDL operates as a pollution budget for an impaired waterbody. Nonpoint source pollution
drives water quality exceedances in the rivers and streams where Olympic Peninsula steelhead
occur.
Washington has not developed, and EPA has not approved, any TMDLs for any
waterbodies where Olympic Peninsula steelhead occur.
b. Nonpoint Source Pollution
Nonpoint source pollution is the “leading cause of water pollution across the nation and
in Washington.” Wash. Dep’t of Ecology, Publ’n No. 040-010-009, Enforcement Report on Policy
and Trends, 49 (2004). Agriculture causes most nonpoint source pollution; however, that
industry is exempt from certain CWA regulations.
A limited number of logging-related activities are subject to the CWA. 40 C.F.R. §
1227.27. The EPA’s silvicultural rule defines silvicultural point sources to include “any
discernable, confined and discrete conveyance related to *** log sorting *** or log storage
facilities which are operated in connection with silvicultural activities and from which pollutants
are discharged in waters of the United States.” Id. § 1227.27(b)(1). The rule defines log sorting
and log storage facilities to mean “facilities whose discharge results from the holding of
unprocessed wood, for example, logs or roundwood with bark or after removal of bark held in
self-contained bodies of water (mill ponds or log ponds) or stored on land where water is
applied intentionally on the logs (wet decking). Id. § 1227.27(b)(3).
The silviculture rule does not apply to major sources of water pollution caused by
logging operations. Among other activities, the rule exempts harvesting operations, surface
drainage, and road construction and maintenance from which there is natural runoff. 40 C.F.R.
at § 1227(b)(1). In 2013, the U.S. Supreme Court upheld the EPA’s decision not to regulate
stormwater runoff from logging roads. Nw. Envtl. Def. Ctr. v. Decker, 133 S. Ct 1326, 1338
(2013). The following year, Congress amended the CWA to effectively prohibit the EPA from
requiring NPDES permits for discharges resulting from several silviculture-related activities,
115
including surface drainage, road construction, and road maintenance. 33 U.S.C. § 1342(l).
Therefore, the Clean Water Act fails to adequately protect Olympic Peninsula steelhead.
Many river and stream miles occupied by Olympic Peninsula steelhead will experience
water quality impacts caused by logging operations for the foreseeable future (McHenry et al.
1996). The synergistic effect of timber harvesting and heavier rainfall caused by climate change
will impair water quality in rivers and streams where Olympic Peninsula steelhead occur.
4. National Environmental Policy Act
The National Environmental Policy Act (NEPA), 42 U.S.C. §§4321 et seq., requires federal
agencies to identify and evaluate the impacts of “major Federal actions significantly affecting
the quality of the human environment.” Thus, the U.S. Forest Service, U.S. National Park
Service, Bureau of Indian Affairs, and other federal agencies whose major actions may impact
Olympic Peninsula steelhead are subject to NEPA. Major federal actions include those actions
“subject to federal control and responsibility.” 40 C.F.R. §1508.1(q). To determine whether the
impacts of a proposed action are “significant” federal agencies must assess the “potentially
affected environment and degree of the effects.” Id. at § 1501.3(b). Federal agencies must
consider the short-term and long-term effects, beneficial and adverse effects, effects on public
health and safety, and effects that would violate laws that protect the environment. Id.
Federal agencies must prepare environmental impact statements (EISs) for proposed
actions with significant impacts. 42 U.S.C. § 4332. An EIS must assess the following topics: (1)
the environmental impacts of the proposal; (2) unavoidable adverse effects; (3) alternatives to
the proposed action; (4) the relationship between the short-term uses of the environment and
maintenance of long-term productivity; and (5) any irretrievable commitment of resources
involved if the proposed action is implemented. Id. An EIS must also provide a detailed
statement regarding the scope of its assessment, which must cover the following subjects: (1)
connected or similar actions; (2) a reasonable number of alternatives to the proposed action,
including no action alternatives and other reasonable alternatives, as well as mitigation
measures; and (3) effects. 40 C.F.R. §§ 1501.9, 1502.14. Federal agencies must release draft
EISs for comments from other agencies and the public. 42 U.S.C. § 4332(2)(C).
After undertaking all these analyses and procedures, NEPA does not require federal
agencies to select the alternatives with the least impacts on the environment; it simply requires
federal agencies to take a “hard look” at them. Robertson v. Methow Valley Citizens Council,
490 U.S. 332, 350 (1989). Therefore, the USFS and other federal agencies whose actions may
impact Olympic Peninsula steelhead are not required to choose the alternatives that best
protects the species. If they follow the procedural requirements of NEPA, agencies may select
any alternatives, including those that harm Olympic Peninsula steelhead. Therefore, NEPA is not
sufficient to protect Olympic Peninsula steelhead. The DPS requires protection under the ESA.

116
5. Governmental Failure to Adequately Address Climate Change
Climate change impacts in freshwater and marine habitats pose significant threats to
the survival of Pacific salmonids. A critical feature of the contemporary and future threats of
climate change is the failure of the US government to address these threats through the
adoption of “green” energy policies and actions to drastically reduce reliance on fossil fuel
energy generation. These actions are necessary to begin a rapid reduction in the amounts of
greenhouse gases that are annually released into the atmosphere.
It is widely recognized in the peer-reviewed scientific literature that the concentration
of atmospheric carbon dioxide (CO2) is the primary driver of climate warming. The
concentration of CO2 in the atmosphere is currently 412.5 ppm [NOAA Climate.gov
https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-
carbon-dioxide (accessed June 1, 2022)]. This level is the major contributor to the current global
energy imbalance. In 2020, von Schuckmann et al. estimated the current global energy
imbalance to be 0.87 +/0.12 watts-per-square meter (W m
-2
) of the earth’s surface. “The
amount of CO2 in the atmosphere would need to be reduced from 410 to 353 ppm to increase
heat radiation to space by 0.87Wm
-2
, bringing Earth back towards energy balance” (von
Schuckmann et al, 2020, p. 2014).
Considering both current policy and the fragmented political climate in the US today,
there is a high probability that such a timely reduction in atmospheric CO2 will not occur in time
to avoid significant and continued (and perhaps even acceleration of) global warming within the
next few decades. This further emphasizes the realism of making climate change predictions
based on the IPCC’s “business-as-usual”, RCP8.5 scenario, which Cheung and Frölicher (2020)
note leads to global atmospheric surface warming … of 3.2 C by 2081 – 2100 relative to
preindustrial” (Cheung and Frölicher (2020). Further, as explained by von Schuckmann et al.
(2020), the current large global energy imbalance coupled to the imbalance that has existed for
the past several decades means that considerable warming of the atmosphere as a result of the
legacy of heat already stored in marine waters is already locked in for several decades. This
would still be true even if the current imbalance were to disappear tomorrow. Therefore, the
current failure to adopt green energy policies that would rapidly reduce the global energy
imbalance should be understood as a fundamental component of the failure of existing
regulatory mechanisms to protect salmon and steelhead and the many components of
salmonid ecosystems on which they depend.
6. National Park Service Organic Act and Other Regulatory Mechanisms
The habitat in Olympic National Park has been protected for over 100 years, and it is in
relatively pristine condition (Halofsky et al. 2011). In 1988, Congress designated 95 percent of
the park as the Olympic Wilderness. The Olympic Wilderness, which was renamed the Daniel J.
Evans Wilderness, covers 1,370 square-miles, including 48 miles on the Washington coastline.
117
a. National Park Service Organic Act
Portions of several Olympic Peninsula steelhead populations spawn and rear inside the
Olympic National Park, which provides relatively pristine aquatic habitat conditions compared
to areas outside of the park. The park’s enabling legislation and the National Park Service
Organic Act of 1916 (Organic Act) protect these resources. Specifically, the Organic Act requires
the NPS “to conserve the scenery and the natural and historic objects and wildlife therein and
to provide for the enjoyment of the same in such manner and by such means as will leave them
unimpaired for the enjoyment of future generations.” 16 U.S.C. § 1.
b. Fishing Regulations
Olympic Peninsula National Park has its own fisheries management program. The
program has three goals: (1) “manage aquatic resources as an important part of the park
ecosystem;” (2) “preserve and restore native fishes and their habitats;” and (3) “provide
recreational fishing opportunities for the enjoyment of park visitors, consistent with the first
two objectives.” Except for saltwater areas within park, a Washington State fishing license is not
required to fish in Olympic National Park. 36 C.F.R. § 2.3(b). However, a Washington State catch
record card is required to fish for steelhead.
All wild steelhead must be released in Olympic National Park. Except during limited
periods in select areas, bait fishing is generally prohibited in the park in order to reduce catch
and release mortality on steelhead. Studies show that catch-and-release fishing mortality is less
than 2% when fish are caught on flies and less than 5-7% overall with most gear types, except
for bait, which can have much higher rates of mortality – for both juvenile and adult steelhead
– and when fish are caught in elevated water temperatures (Warner and Johnson 1978; Hooton
1987; Pauley and Thomas 1993; Schisler and Bergersen 1996; Taylor and Barnhart 1997;
Hooton 2001; Nelson et al. 2005; Twardek et al. 2018). However, as mentioned earlier, the
regulations do not account for potential sublethal impacts that can reduce fitness of individuals
that survive catch and release (up to ~ 20-25% reduction in fitness: Richard et al. 2013;
Bouchard et al. 2021; Papatheodoulou et al. 2021).
The National Park Service has taken emergency actions to protect vulnerable steelhead.
For example, in February 2021, the NPS closed recreational fishing for steelhead on the Queets
River inside Olympic National Park in response to record low returns (LaBossiere 2021).
c. Roads & Structures
Historically, maintenance and repair of Olympic National Park roads that are adjacent to
the Sol Duc, Hoh, Queets, and Quinault Rivers have caused major impacts on fish and aquatic
life (Halofsky et al. 2011). Today, the National Park Service takes steps to reduce these impacts.
For example, when feasible, the National Park Service relocates roads and other facilities from
floodplains to other areas (Halofsky et al. 2011). It also limits construction of new facilities
within floodplains to protect fish habitat (Halofsky et al. 2011).
118
B. STATE
1. Washington Department of Fish and Wildlife
WDFW management of Olympic Peninsula steelhead is guided by the Statewide
Steelhead Management Plan, Hatchery and Fishery Reform Policy (C-3624), and harvest
management plans with the Quinault, Quileute, Hoh, and Makah Tribes (WDFW 2008, WDFW
2021, Hoh Tribe and WDFW 2020; Quileute Tribe 2020; Quinault Dept. of Fisheries 2021). The
continued decline of Olympic Peninsula steelhead with these plans in place shows they are
insufficient to adequately manage these populations. Further, the Washington Fish and Wildlife
Commission recently replaced its previous hatchery policy, C-3619, with its new hatchery
policy, C-3624, which further weakens the standards governing the allowable proportion of
hatchery-origin spawners (pHOS) on spawning grounds. The adoption of C-3624 represents a
step backwards in addressing the harms posed by hatcheries.
a. Statewide Steelhead Management Plan (2008)
The 2008 Statewide Steelhead Management Plan describes strategies intended to help
restore and maintain the abundance, distribution, diversity and long-term productivity of the
state’s wild steelhead and their habitats to assure healthy stocks (WDFW 2008). To achieve this
goal, the plan establishes a series of policies to guide natural production, habitat protection and
restoration, fishery management, artificial production, regulatory compliance, monitoring and
evaluation, adaptive management, research, and education (WDFW 2008).
The plan provides that “[s]teelhead management shall place the highest priority on the
protection of wild steelhead stocks to maintain and restore stocks to healthy levels.” (WDFW
2008). To achieve this goal, in part, the policy calls for providing sufficient wild steelhead
spawners, which requires setting escapement objectives (WDFW 2008). The plan acknowledges
that setting informed escapement objectives requires an understanding of stock population
dynamics, habitat conditions, and stock status (WDFW 2008). WDFW maintains a list, which
was last updated in 2002, that describes the status of each stock as “unknown,” “depressed,”
“critical,” or “healthy.” If a stock’s status is unknown, WDFW is supposed to “apply a
precautionary strategy by implementing low risk fishery and hatchery management regimes.”
(WDFW 2008). If the status is “depressed,” “critical,” or ESA-listed, WDFW should promote a
trend of increasing wild fish numbers. (WDFW 2008). If the status is “healthy,” WDFW should
maintain wild steelhead escapement objectives at or above MSH levels (WDFW 2008).
Regardless of whether the nearly two-decades-old “healthy” status designations still
apply to certain Olympic Peninsula steelhead stocks - which they do not (e.g., McMillan et al.
2022) - recent (2010-2021) escapement numbers indicate that WDFW is not maintaining
escapement levels at or above MSH levels. For example, WDFW did not maintain Queets River
wild winter steelhead escapement at or above MSH levels during the last eight out of nine years
119
and have often failed to do so in the Hoh River (WDFW 2022a). Further, they do not monitor or
manage wild summer steelhead.
Additionally, the Statewide Steelhead Management plan requires WDFW fish and
wildlife managers to establish a network of wild stock gene banks across the state. These gene
banks are intended to be located where steelhead were “largely protected from the effects of
hatchery programs”. This policy came about from a 2004 review of Washington hatchery
facilities by the Hatchery Scientific Research Group, recognizing the need to protect populations
from the threat of hatchery effects (HSRG, 2004).
The plan calls for establishing one wild stock gene bank for each major population group
(“MPG”) within a larger conservation unit, such as a DPS (WDFW 2008). The plan defines
“MPG” to mean:
“[a] group of populations within a larger conservation unit such as a DPS or ESU
that share genetic, life-history, or ecological characteristics that are sufficiently
distinct from those of other groups of populations to make conservation or
recovery of the group essential for the conservation or recovery of the larger
conservation unit.”
(WDFW 2008). This definition implies that more than one MPG should occur within a
conservation unit, as an MPG is a subcomponent of a DPS or similar conservation unit.
Fourteen years after the plan was adopted, there is still only one wild steelhead gene
bank on the Olympic Peninsula: the Sol Duc Wild Steelhead Gene Bank, which WDFW
established in 2013. As a result, since the Snider Creek hatchery was closed in accordance with
the Sol Duc gene bank designation. Although this has benefitted Sol Duc steelhead, WDFW
should be establishing additional gene banks on the Olympic Peninsula.
Considering the differences between the WRIA 19-21 populations, WDFW should have
established more gene banks. There are characteristics that make these groups of populations
sufficiently distinct from the group of populations represented by the Sol Duc gene bank
designation. For example, WRIA 19 populations ecologically differ from WRIA 20 populations,
which includes the Sol Duc population. WRIA 19 populations occur in rain-dominant basins that
discharge into the Strait of Juan de Fuca. WRIA 20 populations occur rain-dominant basins –
several of which are glacially influenced – that discharge into the Pacific Ocean. The WRIA 21
populations also have characteristics that may make them sufficiently distinct from WRIA 20
populations. Despite these differences, WDFW has not announced plans to designate additional
gene banks for any other major population groups within the Olympic Peninsula DPS. Although
Cram et al. (2018) recommended establishing additional gene banks in other DPSs, it did not
recommend establishing more gene banks within the Olympic Peninsula DPS. Based on Cram et
al. (2018), it does not appear that WDFW intends to establish additional gene banks for the
Olympic Peninsula steelhead DPS.
120
b. Hatchery and Harvest Reform Policy
In the years following NMFS’s 1996 status assessment of Olympic Peninsula steelhead
(Busby et al. 1996), WDFW developed two hatchery reform policies. The original Hatchery and
Fishery Reform Policy (C-3619) included several guidelines that, had they been implemented,
could have reduced hatchery threats to Olympic Peninsula steelhead. Recently, WDFW adopted
a new policy that rolls back these guidelines. As a result, WDFW’s hatchery policies have failed
to protect Olympic Peninsula steelhead.
On November 6, 2009, the Washington Fish & Wildlife Commission (WFWC) adopted a
Hatchery and Fishery Reform Policy that focused on the “scientific and systematic redesign of
hatchery programs to recover wild salmon and steelhead and support sustainable fisheries.”
(Policy C-3619). The policy directed WDFW to follow eleven policy guidelines (Policy C-3619).
The first three guidelines were the following instructions:
1. Use the principles, standards, and recommendations of the HSRG to
guide WDFW’s hatcheries, and to enable adaptive management based on
a structured monitoring, evaluation, and research program.
2. Prioritize improved broodstock management to reduce the genetic and
ecological impacts of hatchery fish.
3. Develop watershed-specific action plans that implement hatchery reform
as part of a comprehensive strategy for meeting conservation and
harvest goals. (C-3619).
The 2009 policy was reviewed through the State Environmental Policy Act and within
that review committed WDFW to follow a “phased approach” where subsequent reform efforts
would be developed and implemented for each hatchery facility, known as Hatchery Action
Implementation Plans (WDFW, 2009). To the best of our knowledge these plans were never
developed, submitted for state environmental review, or implemented.
In 2018, the Commission suspended these three guidelines for salmon species and
directed WDFW to review the hatchery and fishery reform policy and the department’s
performance of its strategies. Subsequently, WDFW produced two reports: (1) A Review of
Hatchery Reform Science in Washington State (Anderson et al. 2020), including independent
review by the Washington Academy of Natural Sciences; and, (2) WDFW Hatchery and Fishery
Reform Policy Implementation Assessment: Final Report, 2009-2019 (Murdoch and Marston
2020).
i. Review of Hatchery Reform Science in Washington State (Anderson et al. 2020)
As directed by the FWC through the C-3619 review process, WDFW and the Washington
State Academy of Sciences conducted a Review of Hatchery Reform Science in Washington
121
State to identify advances in hatchery reform science that have occurred since C-3619 was
adopted in 2009. This thorough WDFW-produced and independently reviewed report provides
the following key conclusions:
1. “The HSRG principles of reducing pHOS and increasing pNOB to achieve fitness
gains in wild populations are well-founded and should be fundamental goals in
any hatchery reform management action.
2. Excessive hatchery program size requires more careful scrutiny and scientific
justification because it affects virtually every aspect of hatchery risks.
3. Hatcheries have potential for large magnitude ecological impacts on natural
populations that are not well understood, not typically evaluated and not
measured.
4. Hatchery risks include fishery risks, ecological risks and genetic risks. Fisheries
targeting abundant hatchery runs can unintentionally increase mortality of co-
mingled natural populations.
5. Research on ecological [HxW] interactions lags far behind the attention devoted
to genetic risks of hatcheries. Importantly, research suggests the potential for
ecological interactions in marine environments shared between multiple
hatchery and natural populations, yet very little is known about the likelihood or
magnitude of population-scale ecological impacts of hatcheries.”
6. Studies comparing the number of offspring produced by hatchery-origin fish and
natural-origin fish when both groups spawn in the wild (relative reproductive
success, RRS) have demonstrated a general pattern of lower reproductive
success of hatchery-origin fish.
7. In WDFW’s hatchery system, a focus on efficiency and maximizing abundance
prevents widespread implementation of risk reduction measures.
8. We recommend a more rigorous, consistent and intentional evaluation of
cumulative hatchery effects across multiple hatchery programs operating within
a geographic region.”
9. WDFW invests considerable effort into population monitoring, yet this
information does not often achieve its potential as a hatchery evaluation tool
because analysis, reporting, and synthesis are typically underfunded.
Furthermore, for many hatchery programs, the absence of a clear framework for
application of monitoring data in decision making precludes clearly articulated
risk tolerance thresholds.”
(Anderson et al. 2020).
ii. WDFW Hatchery and Fishery Reform Policy Implementation Assessment (Murdoch
and Marston 2020)
As directed by the WFWC through the C-3619 review process, WDFW also conducted a
Hatchery and Fishery Reform Policy Implementation Assessment (2020) to evaluate the policy’s
effectiveness at protecting wild salmon and steelhead populations (Murdoch and Marston
122
2020). However, for reasons described in the report beginning on page 3, WDFW found that the
monitoring data necessary to answer that question were unavailable or inadequate (Murdoch
and Marston 2020). Consequently, WDFW re-focused the assessment to evaluate whether and
to what extent the agency had implemented the fishery and hatchery reform actions mandated
in the 2009 policy C-3619. Among the many findings in this report, the following WDFW
conclusions are particularly relevant to this petition: A lack of funding was a common reason
that prevented implementation of some guidelines; a lack of comprehensive statewide
monitoring and evaluation program are areas of special concern; and defining program success
and collecting and analyzing data to adaptively manage our programs are critical missing
components.
WDFW’s review also found that the department had made little to no progress
implementing seven HSRG recommendations for the Bogachiel Hatchery summer and winter
steelhead programs, as required under the first guideline of the 2009 Hatchery and Fishery
Reform Policy (Murdoch and Marston 2020). Those recommendations included steps intended
to set clear goals for conservation, ensure hatcheries are meeting management goals, and
minimize risks to natural populations (Murdoch and Marston 2020). As discussed in the
hatchery section of this petition, the winter and summer steelhead programs at the Bogachiel
Hatchery did not meet pHOS goals in 2009 (WDFW 2022b). Data for other years are not
available on WDFW’s online database (WDFW 2022b).
On April 9, 2021, the Commission adopted a revised Hatchery Policy (C-3624),
superseding the previous policy (C-3619) (WFWC 2021). The revised policy abandons
commitments to follow HSRG guidelines, and it sets forth new direction for WDFW to follow
when managing salmon and steelhead hatcheries. Among other things, the policy directs
WDFW to prepare Hatchery Management Plans for every WDFW salmon and steelhead
hatchery program (WFWC 2021). These HMPs must be based on the best available science
regarding the risks of hatchery production on wild salmon and steelhead. However, the HMP
provisions must “*** reflect a balance between minimizing genetic and ecological risks to
coincident wild populations and providing for the ecological and societal benefits of hatchery
propagated salmon and steelhead ***.” (WFWC 2021).
The Washington Fish and Wildlife Commission replaced C-3619 with C-3624 without
State Environmental Policy Act review, which is the subject of ongoing litigation. Wild Fish
Conservancy et al. v. Washington Dep’t. of Fish & Wildlife et al., King County Superior Court
Docket No. 21-2-13546-0 SEA.
Even after adoption, the new C-3624 hatchery policy is behind schedule on creating the
evaluation protocols for the yet-to-be-developed hatchery management plans. Despite the
known, ongoing harms that the steelhead hatchery programs are causing to the Olympic
Peninsula Steelhead DPS, no changes to relevant hatchery management plans have been
proposed. Additionally, previous phased state environmental reviews are being abandoned,
starting the decade-long process all over again. It is unclear if, or when, WDFW will release a
HMP for its Bogachiel steelhead hatchery operations.
123
c. Harvest Management Plans
The Quinault, Hoh, Quileute, and Makah Tribes have treaty rights to receive shares of
salmon and steelhead as determined in U.S. v. Washington, 384 F. Supp. 312, aff’d 520 F.2d 676
(9
th
Cir. 1975), cert. denied 423 U.S. 1086. Each year, WDFW and the tribes prepare harvest
management plans that set guidelines for tribal fisheries, seek to promote wild steelhead
conservation, establish monitoring protocols (e.g., sample catches for size, sex, and age), and
commit the parties to working together to evaluate fishery mortalities that occur in tribal and
non-tribal fisheries (e.g., catch and release mortality, net drop out, and marine mammal
predation) (Hoh Tribe and WDFW 2020; Quileute Tribe 2020; Quinault Dept. of Fisheries 2021).
Despite the monitoring protocols in harvest management plans, it is unclear how many
steelhead are harvested each year in tribal fisheries, as indicated in Cram et al. (2018).
“Methods used to estimate treaty harvest and non-retention mortality are not
well-documented and do not currently contain estimates of uncertainty. This is a
problem particularly in areas where harvest rates are relatively high (e.g.,
Olympic Peninsula, Grays Harbor, upstream of Bonneville Dam on the Columbia
River). Work with tribal co-managers is needed to document methods used to
estimate treaty harvest, to test assumptions related to estimations, and to
report estimates of uncertainty.”
(Cram et al. 2018). Cram et al. (2018) did not explain why previous work with tribal co-
managers has failed to fill this data gap.
d. Monitoring
WDFW does not sufficiently monitor Olympic Peninsula winter steelhead, and it does
not monitor Olympic Peninsula summer steelhead at all. The monitoring recommendations in
Cram et al. (2018) acknowledge that there are significant data gaps. For example, Cram et al.
(2018) recommends that WDFW “initiate” monitoring of wild summer-run steelhead in the
Olympic Peninsula DPS. Cram et al. (2018) also recommends initiating robust population-scale
monitoring in one of more Olympic Peninsula steelhead populations and expanding life cycle
monitoring of the DPS.
Likewise, WDFW Hatchery and Fishery Reform Policy Implementation Assessment
(Murdoch and Marston 2020) found that a lack of comprehensive statewide monitoring and
evaluation program are areas of special concern. Defining program success and collecting and
analyzing data to adaptively manage WDFW hatchery programs are critical missing components
despite previous WDFW plans that required this data to be collected.
124
2. Washington Forest Practices Act
The Washington Forest Practices Act (FPA) regulates timber harvest on state and private
lands. Wash. Rev. Code §§ 76.09.010-.935. Among other goals, the FPA seeks to “recognize
both the public and private interest in the profitable growing and harvesting of timber,” and
“provide for regulation of forest practices so as to avoid unnecessary duplication in such rules,”
and “achieve compliance with all applicable requirements of federal and state law with respect
to nonpoint sources of water pollution from forest practices.” Wash. Rev. Code §
76.09.010(2)(c), (e), (g). The Act established a Forest Practices Board charged with adopting
forest practice regulations, and it established a permit system operated by DNR that covers
certain forest practices. Id. §§ 76.090.030, 76.09.050, 76.09.020(7).
a. The State Trust Lands Habitat Conservation Plan
WDNR manages state trust forestlands under the State Trust Lands Habitat
Conservation Plan (the “DNR HCP”) (WDNR 1997). The OESF is one of nine planning units under
the DNR HCP (WDNR 1997). The DNR HCP includes a Riparian Forest Restoration Strategy that
aims to restore salmonid habitat (WDNR 1997). Under that strategy, riparian buffers differ in
width based on particular stream needs and disturbance history (WDNR 2016). Generally, the
average riparian buffers, which are measured horizontally from the outer edge of the 100-year
floodplain, are 150 feet on type 1 and 2 streams and 100 feet on type 3 and 4 streams (WDRN
2016). These buffers are intended to minimize disturbance to unstable banks and adjacent
hillslopes and maintain key biological and physical functions. This approach is not designed to
achieve a desired future condition for salmonid habitat, but rather to “maintain or aid
restoration of riparian functions important to salmonid habitat” (WNDR 2016). In addition to
riparian buffers, the HCP includes other habitat protections, including road building
requirements and wetland protections (WDNR 2016).
Improved management standards have not increased large woody debris in streams in
the OESF. Martens et al. (2019) found that large woody debris was either stable at reduced
levels or declining, and that using passive restoration alone is unlikely to increase salmonid
productivity.
“Our results add to the current scientific literature that has found passive
restoration of salmonid habitat in the Pacific Northwest is a slow process, which
could take an additional 12–70 years for riparian forests and over 50 years for
instream wood to accumulate (McHenry et al. 1998; Connolly and Hall 1999;
Kaylor et al. 2017).”
(Marten et al. 2019).
The most recent monitoring report for the OESF also found that the majority of LWD is
in decay, and that historic logging practices continue to interrupt the supply of new LWD to
streams (Devine et al. 2022).
125
Some research indicates that riparian buffers alone will not be sufficient to meet water
quality standards for temperature in the OESF (Pollack et al. 2009). A 2009 study collected
water temperature data from 42 subbasins on DNR lands, including 22 readings on tributaries
to the mainstem of the Hoh River, 10 on the South Fork of the Hoh River, nine on the
Clearwater River, and one small tributary to the Bogachiel River (Pollock et al. 2009). The study
found that 17 out of 40 streams had at least one seven-day Average Daily Maximum (7DADM)
temperature that exceeded 16.0°C, and thus did not meet water quality standards for
temperature in core salmon rearing and spawning habitat (Pollock et al. 2009). One of six
subbasins with 25-50% harvest, nine of eighteen subbasins with 50-75% harvest, and seven of
nine subbasins with >75% harvest had 7DADM exceedances (Pollock et al. 2009). None of the
temperature exceedances occurred in unharvested basins (Pollock et al. 2009).
Pollock et al. (2009) explained that several mechanisms related to logging could be
responsible for increased stream temperatures, including widening and shallowing of stream
channels, widening above-channel canopy openings, decreasing large woody debris and
alluvium, and increasing debris flow frequency (Pollock et al. 2009). If these mechanisms are
causing these changes, the authors concluded that “*** reestablishment of riparian forests
alone will not be sufficient to return stream temperature regimes to natural conditions”
(Pollock et al. 2009).
Other researchers have found different temperature results. For example, Martens et al.
(2019) found that 7-day average water temperatures for DNR-managed watersheds were below
the 16°C temperature standard. Martens et al. (2019) found that summer stream temperatures
have decreased and riparian canopy cover has increased (Martens et al. 2019). Devine et al.
(2022) reported that the average 7-day maximum water temperatures on DNR managed lands
was 14.4°C, with only 16 exceedances of 16°C out of 329 observations of the 7-day maximum.
Devine et al (2022) explained that the cool maritime climate and increased shading influenced
water temperatures.
b. Washington State Forest Practices Habitat Conservation Plan
The Washington State Forest Practices HCP (“FPHCP”) applies to private lands on the
Olympic Peninsula. Although the FPHCP includes habitat protections that benefit aquatic
species, NMFS and USFWS noted that forestry activities could still potentially adversely affect
aquatic habitat by increasing temperature pollution and sedimentation and decreasing large
wood recruitment (NMFS and FWS 2006). Additionally, legal questions have been raised
regarding whether the HCP’s Clean Water Act assurances violate Washington’s antidegradation
policy and undermine the TMDL program (Steifel 2013). Federal agencies, including NMFS, have
also raised concerns about water temperature, riparian function, and Clean Water Act
assurances.
There are ongoing issues with water typing classifications as well. Water typing is
fundamental to effectively protect riparian buffers from forest practices, and it can be used in
other management contexts such as fish population assessments and harvest management.
126
Within the context of forest practices, the FPHCP riparian buffer width rules are based on a
water type classification system intended to identify Type F (fish-habitat) stream reaches and
Type N (non fish-habitat) reaches. The state, however, has been operating under Interim Water
Type Rules (WAC 222-16-031) since 1999, which inappropriately allows streams to be classified
as Type N based on the results of a single point-in-time electrofishing survey.
NMFS and USFWS have warned WDNR that its water typing practices are not consistent
with the FPHCP. In 2015, NMFS and USFWS sent a letter to Washington’s Commission of Public
Lands, describing WDNR’s failure to follow the FPHCP.
“Since the HCP was signed, too many water type determinations that result in
permanent modifications continue not to use physical stream characteristics or
models specified by the FPHCP. Water typing decisions based on a
demonstration of “fish absence” are not consistent with the permanent
approaches agreed to in the FPHC.”
(Letter from Kim Kratz, Assistant Regional Administrator, NMFS, and Eric V. Rickerson, State
Supervisor, USFWS, to Peter Goldmark, Commissioner of Public Lands, DNR (July 2, 2015) (copy
provided). As a result of WDNR’s water typing practices, NMFS and USFWS do not believe fish
habitat has been correctly identified in a “substantial number of instances” Id. Therefore, the
Services requested that WDNR consider its water type modifications as temporary rather than
permanent until “water typing is better aligned with the original HCP requirements.” Id.
In addition to water typing concerns, NMFS and USFWS expressed concerns about
WDNR’s compliance reporting. Id. Specifically, the Services stated that “sample sizes are
decreasing and reported patterns are disconcerting.” Id. It is unclear if these compliance
monitoring issues have been resolved to the Services’ satisfaction.
WDNR is still behind schedule on fulfilling its commitment to “rapidly and iteratively
improv[e] the water typing map and model” (NMFS and USFWS 2015). Although WDNR has
committed to develop and adopt permanent water type rules that more accurately identify fish
habitat as defined in WAC 222-16-010, including recoverable fish habitat, this still has not
occurred despite being 16 years into the 50-year habitat conservation plan (WDNR, 2019).
2. Water Pollution Control Act
Washington has established water quality standards for surface waters throughout the
state. The purposes of these standards are to protect public health and public enjoyment of
Washington’s surface waters and to “protect[] *** fish, shellfish, and wildlife ***” pursuant to
the Washington Pollution Control Act. WAC 173-201A-010(1).
The WPCA prohibits the Washington Department of Ecology (WDOE) from establishing a
permit system for nonpoint source pollution caused by forest practices. RCW § 90.48.420(3). It
also prohibits WDOE from fining or penalizing nonpoint sources of pollution so long as the

127
polluters follow forest practice rules. Id. WDOE is allowed to review forest practice rules, and
WDOE and DNR are required to agree that the rules will allow water quality standards to be
met. RCW § 90.48.420(3).
At least one author has suggested that the incidental take permit (ITP) associated with
the FPHCP may violate Washington’s antidegradation policy, which seeks to “restore and
maintain the highest possible quality of the surface waters of Washington.” (Stiefel 2013). The
policy provides that “[n]o degradation may be allowed that would interfere with, or become
injurious to, existing or designated uses ***.” Wash. Admin. Code 173-201A-200. Because the
ITP authorizes activities that impair fish and aquatic life uses, the ITP expressly violates
Washington’s antidegradation policy (Stiefel 2013).
3. State Environmental Policy Act
The State Environmental Policy Act (SEPA) requires state and county agencies to identify
environmental impacts of proposed projects before committing to a particular course of action.
RCW Ch. 43.21C. Under SEPA, state and county agencies must evaluate these impacts, consider
alternatives and mitigation measures, and involve the public in decision making. Like NEPA,
SEPA is a procedural law; it does not require agencies to select the alternative that causes the
least harm to the environment.
4. Fishing regulations
The Olympic Peninsula DPS supports the most popular and intensive recreational
fisheries for wild winter steelhead in Washington State (Bentley 2017; Cram et al. 2018), largely
because most rivers in Puget Sound are now closed to steelheading in winter (Burge et al.
2006). With little other opportunity, many fishing guides have relocated to the Olympic
Peninsula and as such, each year thousands of anglers travel to fish famed rivers like the Sol
Duc, Bogachiel, Hoh, Queets, and Quinault Rivers from November through April, which has
resulted in very high encounter rates with wild steelhead (e.g., Bentley 2017). Some other
smaller populations, including but not limited to the Hoko River, Clallam River, and Sekiu River,
also support fisheries, though not to nearly the same extent as the largest populations. Further,
nearly all the rivers and streams – both large and small – in the DPS are open to recreational
fishing during summer (June – October) when anglers may encounter summer steelhead.
Below we discuss how fishery regulations and assumptions by WDFW have potentially helped
and hindered the productivity and resilience of wild steelhead.
WDFW has taken steps with fishery regulations to reduce impacts on wild steelhead,
including eliminating retention of wild summer steelhead (we could not determine the date
when retention was eliminated by WDFW and National Park) and eliminating retention of wild
winter steelhead in 2015. Further, in response to the sharp declines in run size the past few
years WDFW has shortened or closed fishing seasons for wild winter steelhead and reduced
angler effectiveness by eliminating fishing from boats and banning the use of bait
(https://wdfw.medium.com/frequently-asked-questions-march-2022-coastal-steelhead-
128
closure-364cfa62826f). Each of these actions may have slowed the decline of steelhead, but
these actions, as a whole, have not seemed to reverse the plight of wild steelhead.
However, a lack of prior due diligence and regulatory action by WDFW has also likely
contributed to the recent declines and closures. The largest watersheds on the Olympic
Peninsula experience high levels of angling pressure that has resulted in very high encounter
rates with wild winter steelhead (Bentley 2017). Bentley (2017) evaluated the creel data
collected by WDFW to estimate total angler effort and total number of wild steelhead caught
and released in the Hoh River in 2014-2015. That analysis indicated WDFW’s prior method
(referred to hereafter as the “old expansion method”) for expanding creel surveys
underestimated the total angler effort and catch by a large amount (Bentley 2017). For
example, using the old expansion method WDFW estimated 2,580 wild steelhead were caught
and released by recreational anglers in 2014-2015, but that number jumped to 4,580 steelhead
using the improved model by Bentley (2017). That equates to a 1.77x increase. Total
escapement that year was 3,171 steelhead, meaning that every steelhead that escaped to
spawn was caught, on average, 1.4 times by anglers (Table 9).
Table 9. Estimated number of wild winter steelhead caught and released (CnR) by recreational
anglers in 2014-2015 in relation to escapement goal using the old expansion method by WDFW
(old method) and using the new model developed by WDFW (Bentley et al. 2017) in the Hoh
River and Quillayute River system excluding the Dickey River, which does not get creel surveys.
Bentley et al. (2017) only applied their model to the Hoh River in 2014-2015 and based on that
analysis, the old method underestimated catch by 1.77 times. We applied that multiplier to
estimates of catch using the old method in the Bogachiel, Calawah, and Sol Duc Rivers (summed
as the “Quillayute system”) for the 2014-2015 season to generate a rough estimate of potential
recreational catch using assumptions in Bentley et al. (2017).
Population Escapement
Old Method
Total wild
steelhead CnR
Bentley et al.
(2017)
1.77 multiplier
Percent of escapement
CnR
Old
method
1.77
multiplier
Hoh 3171 2580 4580 0.8 1.4
Quillayute system 7914 5277 9340 0.7 1.2
Calawah 3081 576 1019 0.2 0.3
Bogachiel 1315 1893 3350 1.4 2.5

129
Sol Duc 3229 2808 4970 0.9 1.5
Bentley (2017) did not evaluate any other years or streams but did suggest re-evaluating
the prior year’s assumptions using the new method. Unfortunately, WDFW has not done that
so far, and therefore, they do not have a rigorous estimate of total encounter rates outside of
the single year analyzed by Bentley (2017). To help better understand the level of encounters in
the neighboring Quillayute River system, we applied the 1.77x correction to the old expansion
totals generated by WDFW for the Bogachiel, Calawah, and Sol Duc Rivers, and then summed
those for the entire system (Table 9). To be clear, Bentley (2017) did not come up with a
“correction factor” that can be applied to other populations. Rather, we did this only as a
means of roughly gaging how much WDFW has underestimated total numbers of fish caught
and released. The encounter rates rose sharply when we applied an expansion, but even the old
method suggested very high encounter rates in the Bogachiel and Sol Duc Rivers, which are
more easily accessed than the Calawah River (Table 9). If those estimates are in the general
ballpark, then encounter rates in the Bogachiel and Sol Duc River are, like the Hoh River, very
high.
It is difficult to estimate what effects such encounter rates could have. WDFW assumes
a fairly conservative 10% mortality rate with each encounter, which is higher than the typical 3-
5% rate found in many studies (Hooton 1987; Taylor and Barnhart 1997; Hooton 2001; Nelson
et al. 2005; Twardek et al. 2018). Nonetheless, they don’t account for fish being caught multiple
times, and they do not account for potential sublethal impacts associated with catch and
release. As discussed earlier, emerging research on Atlantic salmon indicates there can be
significant sub-lethal impacts that can alter the migration and reduce the reduce fitness of adult
females (up to ~ 20-25%) that are caught and released (Richard et al. 2013; Bouchard et al.
2021; Papatheodoulou et al. 2021). If sublethal impacts are similar in steelhead, then those
impacts, combined with the high encounter rates could be far more detrimental to productivity
than a simple assumption of a 10% mortality.
There are two other areas of notable concern. First, the fishery for summer steelhead is
not monitored and while WDFW has enacted emergency regulations for other stocks, including
closures, to protect depleted run sizes of wild winter steelhead (WDFW emergency regulations,
accessed online: https://wdfw.wa.gov/news/state-announces-full-closure-coastal-steelhead-
fishing-support-conservation-following) and summer coho salmon (WDFW 2021 emergency
regulations, accessed online:
https://wdfw.wa.gov/sites/default/files/about/regulations/filings/2021/2021_nof_statewide_r
ec_ces_final_7_1_2021.pdf), they have done nothing to help conserve wild summer steelhead.
Although WDFW does not monitor summer steelhead, they have been aware of the snorkel
counts in Brenkman et al. (2012) for well over a decade. Even low encounter rates by
recreational anglers could have impacts on the struggling populations, particularly because
mortality increases when water temperatures are warmer and sublethal impacts are not
accounted for. For instance, Barnhardt and Taylor (1996) found mortality greatly increased
when water temperatures reached and exceeded 21°C. While it is rare for the Hoh and Queets

130
Rivers to reach 21°C during summer, such temperatures are fairly common in sections of the
Calawah (John McMillan, The Conservation Angler, unpublished instantaneous water
temperature data 2000-2006; 2009-2021) and Sol Duc Rivers (Sol Duc stream and temperature
gage: https://apps.ecology.wa.gov/ContinuousFlowAndWQ/StationDetails?sta=20A070).
Second, it is unclear how recreational angling is impacting juvenile steelhead. Juvenile
steelhead are found throughout a variety of main-stem and tributary habitats (McHenry et al.
1996; Smith 2000; McMillan and Starr 2008; McMillan et al. 2013), most of which are open to
angling during the summer months. It is possible that many juvenile steelhead are captured
each season by anglers, though at this time we are nor sure to what extent and how such
encounters could impact survival. Perhaps more worrisome is the potential for high encounter
rates on older, larger steelhead parr and steelhead smolts that are found in the main-stem
rivers during the winter steelhead season – from January – April. If juveniles are killed during
summer there is potential for compensation by other individuals, but if smolts are killed during
their journey downstream, there is almost no chance for compensation. Personal fishing
experiences and observations (John McMillan, The Conservation Angler) indicate many juvenile
steelhead are captured on steelhead gear in winter, and smolts are commonly captured during
the spring when they migrate to the ocean. Because they are non-target fish and the gear is for
larger fish, lethal hookings appear more common and smolts are often handled without care.
Given the estimated smolt to adult survival of Olympic Peninsula steelhead, a river like the Hoh
may only be producing 30,000-40,000 smolts per year. Anglers reported catching and releasing
over 4,500 adults in the Hoh River in 2014-2015 (Bentley 2017). Anglers likely encounter more
smolts than adults, perhaps substantially so because they are feeding. Understanding these
impacts, although challenging, seems important because of the sheer number of anglers and
their potential for catching and releasing large numbers of juvenile steelhead. At this point,
given the declines in the populations, all smolts are important to productivity and resilience.
V. OTHER NATURAL OR ANTHROPOGENIC FACTORS
A. Hatcheries
In 1996, NMFS acknowledged the genetic risk that hatcheries pose to wild steelhead on
the Olympic Peninsula (Busby et al. 1996). At the time, around 40,000 summer steelhead,
primarily out-of-DPS Skamania stock, were annually released in the Quillayute River Basin
(Crawford 1979; WDF et al. 1993); 840,000 winter steelhead, primarily from
Bogachiel/Chambers/Cook Creek stock, were annually planted; (WDF et al. 1993; WDFW
1994a); and winter steelhead of mixed origin were being released into the Quinault River
(Busby et al. 1996). The proportion of natural spawning winter steelhead composed of
hatchery-origin recruits (pHOS) ranged from 16% in Quillayute River to 44% in Quinault River
(Busby et al. 1996).
In the twenty-five years following the 1996 Status Review, additional studies have
discussed the genetic and ecological harm hatchery steelhead programs cause to wild steelhead
populations (e.g., Goodman 2005, Araki et al. 2008; Araki et al. 2009; Araki and Schmid 2010;
131
Chilcote 2003; Naish et al. 2007; Chilcote et al. 2011; Kostow et al. 2003; Kostow and Zhou
2006; Kostow 2009). Considering these risks, state, and federal agencies, as well as the
Hatchery Scientific Review Group, have raised concerns about the effects of steelhead hatchery
production on the Olympic Peninsula (HSRG 2004, 2015; USFWS 2009; Duda et al. 2018; Cram
et al. 2018). And research by McMillan et al. (2022) indicates hatchery fish, both directly and
indirectly, have likely contributed to the depletion of early returning wild winter steelhead.
Below we summarize what is known about the extent of hatchery operations in the DPS,
straying of hatchery steelhead, and potential ecological and genetic impacts posed by hatchery
fish.
1. Hatchery Operations on the Olympic Peninsula
Hatchery steelhead have been released into Olympic Peninsula rivers for over a century
(Duda et al. 2018). Between 1933 and 2014, a total of 9.2 million winter steelhead and 1.6
million summer steelhead were released into the Quillayute River system (Duda et al. 2018).
Between 1958 and 2014, a total of 4.1 million winter steelhead and 0.9 million summer
steelhead were released into the Hoh River (Duda at al. 2018). And, between 1962 and 2010, a
total of 3.01 million juvenile winter-run steelhead were planted in WRIA 19 streams (NOPLE
2015).
The largest releases have occurred in WRIA 21, primarily on the Quinault and Queets
Rivers (Table 1). From 1915 to 2014, 26.2 million winter steelhead were released into the
Quinault River, and from 1974 to 2014, 5.2 million winter steelhead were released into the
Queets (Duda et al. 2018). The Quinault River program continues to release close to half-a-
million steelhead every year: 395,606 (2015), 452,482 (2016), 487,190 (2017), and 475,488
(2018) (QDF and WDFW 2021).
As of 2013, eighteen hatchery release operations (on-site and off-site) were ongoing on
the following rivers: Calawah River (summer: Figure 24, and winter steelhead), Clallam River,
Goodman Creek, Hoh River, Hoko River, Lower Quinault River, Lyre River, Pysht/Independents,
Queets River, Quillayute/Bogachiel River, Sail River, Sekiu River, Sooes/Waatch Rivers, Sol Duc
Rivers (terminated all hatchery steelhead releases after 2013), and Upper Quinault River (Table
1; Cram et al. 2018). Between 2000 and 2008, these programs released an average of 1,383,024
hatchery steelhead per year (Cram et al. 2018.) As a result of some off-site release program
reductions that occurred between 2009 and 2013, the average annual release has reduced to
1,072,781 (Cram et al. 2018). Population-specific data from Cram et al. (2018) are provided in
Table 1.
These programs are operated for harvest augmentation and mostly use early returning
Chambers Creek winter stock and early returning Skamania summer stock, neither of which are
native to the Olympic Peninsula DPS (Cram et al. 2018). It is unclear if the Quinault programs
are segregated (Cram et al. 2018). In 2013, an integrated hatchery program using early
returning wild broodstock was initiated on the Bogachiel River, and summer and winter
132
steelhead hatchery releases were terminated on the Sol Duc River (Table 1 and Figure 24; Cram
et al. 2018)
a. Straying
Hatchery steelhead released within, and outside of, the Olympic Peninsula DPS
boundaries are known to stray into the DPS’s river and streams (Cederholm 1993; Houston and
Contor 1984; Phelps et al. 1997; McMillan 2006; USFWS 2009; Brenkman et al. 2012; Kassler et
al. 2011; Cram et al. 2018). WDFW acknowledges this threat to the genetic integrity of Olympic
Peninsula steelhead (Cram et al. 2018). As a result of past and current hatchery practices, the
Sitkum River summer steelhead population may be the only population of Olympic Peninsula
steelhead that has escaped large levels of hatchery straying (Table 7). Almost no information is
available for straying of hatchery winter steelhead, if only because it is difficult to discern which
populations the fish came from, and because in places like the Queets River, the hatchery
steelhead are not marked. The lack of marking for hatchery winter steelhead in the Queets, and
one of the hatchery stocks in the Quinault River, is of great concern because it is possible those
fish and their feral offspring are masking (Quinones et al. 2013; Willmes et al. 2018) potentially
greater declines in wild winter steelhead than is currently thought. Not marking hatchery fish
also undermines state selective fishing regulations that are intended to protect wild fish from
harvest while reducing pHOS by encouraging harvest of hatchery fish.
Snorkel survey data suggests there is a substantial amount of straying by hatchery
summer steelhead into watersheds that do not have any releases of hatchery summer
steelhead (Table 7). For example, surveys by Brenkman et al. (2012) from 2005-2010 found a
mean percent origin hatchery adult of 40% in the SF Hoh River, 16% in the EF Quinault River,
and 43% in the NF Quinault River (Table 7). In some years, there were so few wild summer
steelhead and so many hatchery summer runs that the annual proportion of hatchery adults
ranged up to 76% and 100% in the SF Hoh and NF Quinault Rivers (Table 7). McMillan (2022)
reported similar results as Brenkman et al. (2012) in the SF Hoh River, although over a longer
period (Figure 9), with a mean percent hatchery steelhead of 41% and an annual peak of 67%
(Table 7). Although it is possible some hatchery steelhead moved downstream in the Quillayute
River system and may not have spawned in the upper watershed, the surveys were purposely
conducted late in the season to minimize that behavior (McMillan 2022). Regardless, if we
translate the proportion of hatchery adults in late summer and early fall into pHOS, the
estimates easily exceed the HSRSG’s recommended pHOS limits of 5%-10% for segregated
programs (WCSSP 2013; HSRG 2015.
Snorkel survey data also suggests hatchery steelhead are straying within the Quillayute
River system outside of the release location in the lower Calawah River. Brenkman et al. (2012)
reported a mean proportion of hatchery steelhead of 13% in the upper Bogachiel River (Table
7), while McMillan (2022) reported a mean of 33% in the NF Calawah River with an annual
range of 0% up to 100% (Table 7). In contrast, relatively few hatchery steelhead have been
documented in the Sitkum River and upper SF Calawah River, where most of the spawning is
believed to occur (Table 7). The most likely explanation is the series of numerous waterfalls that
133
must be ascended to reach the upper Sitkum and SF Calawah Rivers (McMillan 2006; McMillan
2022). Most steelhead ascend the falls from late summer through early fall after the first
freshets in October (McMillan 2022). It is likely that many summer steelhead are not capable of
ascending the falls and others are not able to capably time their passage with the appropriate
weather window.
As of 2013, weirs and other adult traps intended to remove hatchery steelhead were
absent on multiple Olympic Peninsula rivers (Cram et al. 2018). Specifically, as of 2013, there
were no weirs or traps on the Calawah River, Goodman Creek, Hoh River, Lyre River,
Pysht/Independents, Sail River, or Sekiu River (Cram et al. 2018). It is unclear if weirs or traps
were in place on the lower or upper sections of the Quinault River in 2013 (Cram et al. 2018); it
is unclear if they are there now.
Even where weirs and adult traps are in place, they may not sufficiently protect wild
populations (Seamons et al. 2012). They may not be complete barriers and they do not prevent
hatchery fish from spawning elsewhere in a watershed (Quinn 1993; Dittman et al. 2010).
Overall, straying hatchery summer steelhead combined with low abundance of wild
summer steelhead would appear to represent a great risk to the genetic integrity and diversity
of the wild populations, particularly in the Hoh, Quinault, and NF Calawah Rivers. Further, there
is almost no information on how many of those hatchery summer steelhead remain in the
system and spawn with wild winter runs. The uncertainty and potential for strong negative
hatchery impacts underscores the need for an ESA listing for the Olympic Peninsula steelhead
DPS.
b. Genetic and Ecological Risks
Concerns regarding interbreeding between hatchery and wild steelhead on the Olympic
Peninsula have been raised for nearly thirty years, if not longer. In the early 1980’s, Cederholm
(1983) raised concerns about the large number of hatchery smolts released in the Quinault,
Queets, Hoh, and Quillayute Rivers. Cederholm (1983) noted there was a high degree of within
and between river straying and that there may be interbreeding with wild stocks, which could
change the “long-term, spawning, timing, growth and survival of wild fish.” (Cederholm 1983).
The record shows Cederholm was correct, and in fact, subsequent snorkel surveys (mentioned
in previous section) indicate this is also a significant problem for wild summer steelhead.
NMFS expressed similar concerns in 1996 (Busby et al. 1996). In its stock assessment,
the BRT mentioned there was “widespread production of hatchery steelhead” within the DPS,
which presented genetic risks despite management efforts to minimize genetic introgression
(Busby et al. 1996).
In 2004, the Hatchery Scientific Review Group (HSRG) raised several concerns regarding
interbreeding on the Olympic Peninsula (HSRG 2004). Specifically, the HSRG identified
interbreeding between wild and hatchery steelhead as a concern for Quillayute River winter

134
and summer steelhead populations and the Hoh River winter steelhead population, and it
recommended the termination of multiple off-site release programs (HSRG 2004). Several of
those off-site release programs have been terminated to avoid genetic and ecological risks. For
example, the Quinault Tribe discontinued transfers Quinault NFH winter steelhead to the Hoh
River and Cook Creek. (https://www.fws.gov/Quinaultnfh/Hatchery.cfm).
The Washington Coast Sustainable Salmon Plan (2013) indicates that previous
proportions of hatchery fish spawning with natural origin spawners likely exceeds the HSRG’s
recommended pHOS limits of 5%-10% for segregated programs (WCSSP 2013; HSRG 2017). The
report includes the following estimated percentages of natural winter steelhead spawners by
river system: Sooes/Waatch: 50-74%; Ozette: 75-94%; Quil/Bogie: 75-94%; Dickey: 95-100%; Sol
Duc: 75-94%; Calawah: 75-94%; Goodman Creek: 50-74%; Mosquito Creek: 75-94%; Hoh River:
75-94%; Klalaloch Creek 95-100%; Queets River: 75-94%; Clearwater River 95-100%; Raft River:
75-94%; Quinault/Lake Quinault (75-94%); Quinault River (50-74%); Moclips River (95-100%);
and Copalis: (95-100%) (WCSSP 2013). Based on these estimates, genetic risk would be
increased on all systems except Kalaloch Creek and the Dickey, Moclips, and Copalis Rivers.
In 2008, WDFW reported that introgression resulting from the release of Chambers
Creek winter steelhead may have occurred in the Pysht/Independent, Hoko River, and Sol Duc
River populations (WDFW 2008). The report describes Chambers Creek winter and Skamania
River summer steelhead as “pos[ing] substantial risk to both the among-population diversity
and the fitness of natural steelhead populations” (WDFW 2008). Because of introgression, the
report estimated that the Pysht/Independents, Hoko, and Sol Duc winter steelhead populations
had “high” reductions in diversity (WDFW 2008).
“A limited amount of information was available to evaluate changes in spatial
structure and diversity in the Olympic Peninsula region. Most notably, we
compared the 1993 genetic characteristics of the Pysht Winter and Hoko Winter
steelhead populations with samples collected in 1973. The analysis indicated a
5.5-14.5% gene flow (modal value of 9.5%) from hatchery-origin, Chambers type
stock to the Hoko natural population; a 12-75% gene flow (modal value of
26.5%) to the Pysht natural population; and a 2.5-6% (modal value of 4%) to the
Sol Duc winter natural population (see Chapter 4, Artificial Production).”
(WDFW 2008). WDFW did not review gene flow samples from other wild steelhead populations
for the 2008 report. Fortunately, in 2012, WDFW stopped releasing hatchery winter and
summer steelhead in the Sol Duc River (Cram et al. 2018), but to date, we are not aware of data
on potential hatchery effects before and after cessation of hatchery releases.
In 2009, WDFW reported pHOS measurements related to the operation of its hatchery
program on the Bogachiel River (See
https://fortress.wa.gov/dfw/score/score/hatcheries/hatchery_details.jsp?hatchery=Bogachiel%
20Hatchery). WDFW did not meet its 5% and 10% pHOS goals for winter and summer
135
steelhead, respectively. WDFW reported a 9% pHOS rate for winter steelhead and a 23% pHOS
rate for summer steelhead (WDFW Cons. 2021).
Ecological impacts of hatchery programs may occur through a variety of pathways, and
the effects – ranging from predation to competition for food and space – have been reviewed
extensively (Einum and Fleming 2001; Weber and Fausch 2003; Kostow 2009; Tatara and
Berejikian 2012; Rand et al. 2012). Given the high pHOS levels in many of the largest
populations, and the ability of those fish to produce some feral offspring and interbreed with
wild fish, there is heightened potential for those feral and hybrid juveniles to compete with wild
steelhead for limited food and habitat, and negatively influence declining and depleted
populations of wild steelhead.
Outside of freshwater, there is a growing body of evidence that large-scale releases of
hatchery pink and chum salmon are negatively impacting growth and survival of other
salmonids in the North Pacific (Levin et al. 2001; Ruggerone et al. 2010; Ruggerone and Connors
2015; Ruggerone and Irvine 2018). A recent presentation on the subject found hatchery pink
salmon negatively impacted the growth and survival of steelhead in the Thompson River and
Chinook salmon in Puget Sound and the Columbia River (Ruggerone et al. 2021). It is therefore
possible that wild steelhead in the Olympic Peninsula DPS, particularly populations with older
fish that migrate further into the North Pacific, are also being impacted by competition with
pink and chum salmon.
c. Hatchery Fishery Structure and Erosion of Adaptive Capacity
As we described earlier, Cram et al. (2018) noted the risk of fishery selection on run
timing in wild winter steelhead since the number of fishing days per week for treaty fisheries is
highest early in the season to target greater harvest on hatchery adults. However, it should be
noted that the recreational fisheries operated in the same manner for decades before hatchery
steelhead were marked, and as such, both wild and hatchery steelhead were harvested at high
rates (McMillan 2006). Further, while it is now catch and release only for wild winter steelhead,
individuals that enter earlier in the season and remain in the system for longer periods are
more likely to be caught multiple times, and such effects are unknown (Hooton and Lirette
1986). Owing to this combination of factors, and others, it appears the hatchery fishery
structure has contributed to the depletion of early returning wild steelhead and as a result, run
timing is more compressed than it was historically (McMillan et al. 2022).
This is a concern because run timing is highly heritable in salmonids and it represents a
mechanism through which fish can adapt to changes in stream flow and water temperature
(Manhard et al. 2017; Tillotson and Quinn 2018). As outlined in this document previously,
stream flows and water temperatures are predicted to become flashier and warmer,
respectively (Wade et al. 2013). Winter steelhead enter and spawn earlier in the winter and
spring in more southerly areas of their native range (Busby et al. 1996), which suggests wild
steelhead on the Olympic Peninsula will need to rebuild the front end of their run timing to
keep pace with climate change.
136
Environmental conditions on the Olympic Peninsula have changed in the past forty years
and they will continue to shift in relation to climate effects. For example, early winter peak
flows are expected to become more intense and summer streamflows and temperatures will
occur earlier and for longer durations (Wade et al. 2013). To take advantage of higher
streamflows earlier in the winter and to ensure they get their juveniles out of the gravel before
the onset of summer base flows and peak temperatures, steelhead will need to increasingly
enter and spawn earlier. This will not be possible if the current hatchery management and
fishery framework remains in place, because those impacts are essentially blocking the
potential for adaptations in migration timing. Hence, the population could become increasingly
less productive if the fishery and hatchery practices continue to select for later entering and
spawning adults.
Of course, run timing is only one trait that is likely to be under selection as climate
effects unfold. Genetics are a complex field and numerous other traits will also likely need to
change.
“Many traits appear to have responded to recent climate change, apparently
without genetic adaptation. However, to keep pace with climate change, genetic
adaptation may be necessary in the long-run; thus maintaining genetic diversity
within DPSs and species as a whole is a high priority for salmon conservation.”
Crozier et al. (2019). See also (Pitman et al. 2020).
Although steelhead are highly adaptable, they have their limits (Wade et al. 2013) and it
is uncertain whether Olympic Peninsula steelhead can adapt quickly enough to climate change
(Halofesky et al. 2011). This concern is particularly heightened for early returning wild winter
steelhead and wild summer runs, the latter of which may no longer have the necessary genetic
and phenotypic diversity to sustain fitness and productivity in the coming years and decades.
B. Ocean Conditions
Correlations exist between recurring, decadal-scale variability (including the Pacific
Decadal Oscillation and the El Nino Southern Oscillation) and salmonid abundance in the Pacific
Northwest (Stout et al. 2012). When ocean conditions are unfavorable for salmonids, their
abundance declines. Historically, salmonids have persisted through shifts in ocean productivity.
However, it is uncertain whether salmonids can withstand these shifts in marine conditions on
top of climate change, habitat loss, and other increasing threats.
C. Loss of Salmon Nutrients
The loss of marine-derived nutrients from other salmonids in Olympic Peninsula rivers
and streams is likely limiting steelhead productivity (Minkova et al. 2021; Bilby et al. 1996,
1998; NOPLE 2015; McMillan 2006; Halofsky et al. 2011). For example, McMillan (2006)
137
estimated that the combined loss of pink, chum, and coho in the Quillayute River system has
resulted in 87,411 - 201,761 fewer salmon as compared to early 20th century numbers, a
significant reduction in marine nutrients. As a result of losing these nutrients, streams are less
able to produce steelhead smolts that spend an average of two years instream before
outmigrating (McMillan 2006). The same is likely true for all other Olympic Peninsula rivers that
historically supported significant numbers of salmonids.
EFFECTIVENESS OF CONSERVATION ACTIVITIES BY STATES AND OTHER PARTIES
State, local, and tribal governments, as well as nonprofit organizations, have invested
significant resources in recovering steelhead habitat on the Olympic Peninsula. For example,
the Wild Salmon Center, Western Rivers Conservancy, The Nature Conservancy, and WDNR
have purchased thousands of acres of habitat along the Hoh River to protect migration, rearing,
and spawning habitat (McMillan 2006; McMillan and Starr 2008). This is in addition to many
other projects to restore habitat, which can be reviewed on the Washington Governor’s Salmon
Recovery Office website: https://srp.rco.wa.gov/projectmap?mlayer=projects.
WDNR administers a Family Forest Fish Passage Program that assists private landowners
with fish passage improvements. Over seventeen years, the program has completed 67 projects
on the Olympic Peninsula (WDNR 2020). WDNR also administers a River and Open Space
Program that purchases habitat for conservation purposes (WDNR 2020). Since the program’s
inception, it has funded 16 conservation easements that protect 1,043 acres, although it is
unclear if any of those easements occur within Olympic Peninsula steelhead habitat.
Since 1999, the Pacific Coast Salmon Recovery Fund has distributed $12.6 million in
funding to habitat projects throughout Washington’s coast. This federal spending leveraged an
additional $33 million in federal and state funding for these projects.
Although habitat has likely improved in the limited areas where habitat restoration has
occurred, the results – as measured by increased abundance, productivity, spatial distribution,
and diversity – are unclear. For example, Bilby et al. (2022) reported that habitat restoration in
Deep Creek watershed produced mixed results in response to habitat restoration. Additionally,
Bilby et al. (2022) indicates that land use practices continue to interfere with restoration
efforts.
“Historical and ongoing land-use practices (road construction, logging) continue
to influence the effectiveness of the restoration activities. Mass-wasting events
and avulsions can dramatically change or negate the effects of restoration.
Equilibration of the systems will take decades or longer to occur. Riparian
recovery in particular will take centuries.”
(Bilby et al 2022).
138
To make restoration projects effective, there need to be enough fish to utilize restored
habitat. As McMillan (2006) explained, “habitat purchases made to recreate functioning salmon
and steelhead ecosystems are rendered ineffective without sufficient numbers of the key
species that drive them.”
REQUEST FOR CRITICAL HABITAT DESIGNATION
The Petitioners request the designation of critical habitat for Olympic Peninsula
steelhead concurrent with listing. Critical habitat should encompass all known and potential
freshwater spawning and rearing areas, migratory routes, estuarine habitats, riparian habitats
and buffers, and essential near-shore ocean habitats.
Acknowledgments
The following individuals prepared this petition:
John McMillan, Science Director, The Conservation Angler
Rob Kirschner, Legal and Policy Director, The Conservation Angler
Nick Gayeski, Fisheries Scientist, Wild Fish Conservancy
Conrad Gowell, Biologist, Wild Fish Conservancy

139
REFERENCES
The following references include links to the publications. Petitioners have also submitted
copies of open source/open access publications. Petitioners have also provided copies of data
sets referred to in this petition via a shared Google Drive.
Abad´ıa-Cardoso, A., E. C. Anderson, D. E. Pearse, and J. C. Garza. 2013. Large-scale parentage
analysis reveals reproductive patterns and heritability of spawn timing in a hatchery population
of steelhead (Oncorhynchus mykiss). Molecular Ecology 22:4733–4746.
Abatzoglou, J. T., Barbero, R., Wolf, J. W., & Holden, Z. A. 2014. Tracking Interannual
Streamflow Variability with Drought Indices in the U.S. Pacific Northwest, Journal of
Hydrometeorology, 15(5), 1900-1912. Retrieved Jun 10, 2022,
from https://journals.ametsoc.org/view/journals/hydr/15/5/jhm-d-13-0167_1.xml
Abdul-Aziz, O.I., Mantua N.J, and Myers, K.W. 2011. Potential climate change impacts on
thermal habitats of Pacific salmon (Oncorhynchus spp.) in the North Pacific Ocean and adjacent
seas. Can. J. Fish. Aquatic Sci. 68:1660-1680.
Achet, S. 1997. An Integrated Approach to Modeling the Impact of Timber Harvest on
Streamflow: A GIS Based Hydrologic Model. Ph.D. dissertation. Seattle, WA: University of
Washington.
Ahearn, A. (December 2, 2015). "Facing Rising Waters, A Native Tribe Takes Its Plea To Paris
Climate Talks". NPR. https://www.npr.org/2015/12/01/455745765/facing-rising-waters-a-
native-tribe-takes-its-plea-to-paris-climate-talks
Anderson, J.H., K.I. Warheit, B.E. Craig, T.R. Seamons, and A.H. Haukenes. 2020. A review of
hatchery reform science in Washington State. Washington Department of Fish and Wildlife.
Final report to the Washington Fish and Wildlife Commission, January 23, 2020.
Araki, H., Berejikian, B.A., Ford, M.J. and Blouin, M.S. 2008. Fitness of hatchery-reared
salmonids in the wild. Evolutionary Applications, 1: 342-355. https://doi.org/10.1111/j.1752-
4571.2008.00026.x
Araki, H., Cooper, B. and M.S. Blouin. 2009. Carry-over effect of captive breeding reduces
reproductive fitness of wild-born descendants in the wild. Biology letters 5:621-624,
doi:10.1098/rsbl.2009.0315.
Araki, H., and C. Schmid. 2010. Is hatchery stocking a help or harm?: Evidence, limitations and
future directions in ecological and genetic surveys. Aquaculture, 308, S2-S11.

140
Atcheson, M.E. 2010. Interannual variation in steelhead trout (Oncorhynchus mykiss) diet,
growth, and consumption in North Pacific marine ecosystems. Masters of Science Thesis,
University of Washington.
Atcheson, M.E., K.W. Myers, D.A. Beauchamp, and N.J. Mantua. 2012. Bioenergetic response by
steelhead to variation in diet, thermal habitat, and climate in the North Pacific Ocean.
Transactions of the American Fisheries Society 141:4 1081–1096.
Atlas, W. I., T. W. Buehrens, D. J. F. McCubbing, R. Bison, and J. W. Moore. 2015. Implications of
spatial contraction for density dependence and conservation in a depressed population of
anadromous fish. Canadian Journal of Fisheries and Aquatic Sciences 72:1682–1693.
Austin, C. S., T. E. Essington, and T. P. Quinn. 2020. In a warming river, wild Chinook Salmon
spawn later but hatchery-origin conspecifics do not. Canadian Journal of Fisheries and Aquatic
Sciences 78:68–77.
Bahls, P. 2001. How healthy are healthy stocks? Case studies of three salmon and steelhead
stocks in Oregon and Washington, including population status, threats, and monitoring
recommendations. David Evans and Associates Inc, Portland, Oregon.
Baker MR, Swanson P, and G Young. 2013. Injuries from Non-Retention in Gillnet Fisheries
Suppress Reproductive Maturation in Escaped Fish. PLoS ONE 8(7):e69615.
doi:10.1371/journal.pone.0069615
Beacham, T. D., C. G. Wallace, K. D. Le, and M. Beere. 2012. Population structure and run timing
of steelhead in the Skeena River, British Columbia. North American Journal of Fisheries
Management 32:262–275.
Beechie TJ, Beamer E, Wasserman L. 1994. Estimating coho salmon rearing habitat and smolt
production losses in a large river basin, and implications for habitat restoration. North American
Journal of Fisheries Management 14: 797–811.
Beechie T, Imake H, Greene J, Wade A, Wu H, Pess G, Roni P, Kimball J, Stanford J, Kiffney P,
Mantua N. Restoring Salmon Habitat for a Changing Climate. River Research and Applications.
2013. 29: 939-960 DOI: 10.1002/rra.2590.
Behnke, R.J. 2002. Trout and Salmon of North America. The Free Press, New York, NY.
Bellmore, J.R., C.V. Baxter, K. Martens, and P.J. Connolly. 2013. The floodplain food web
mosaic: a study of its importance to salmon and steelhead with implications for their recovery.
Ecol. Soc. Am., 23 (2013), pp. 189-207, 10.1890/12-0806.1
Bentley, K. 2017. Evaluation of creel survey methodology for steelhead fisheries on the
Quillayute and Hoh rivers. Washington Department of Fish and Wildlife. Olympia, Washington.
FPT 17-03.

141
Beschta, R.L., R.E. Bilby, G.W. Brown, L.B. Holtby, and T.D. Hofstra. 1987. Stream Temperature
and Aquatic Habitat: Fisheries and Forestry Interactions. In Streamside Management: Forestry
and Fishery Interactions, Salo EO, Cundy TW (eds). Institute of Forest Resources, University of
Washington: Seattle, WA; 191–232.
Bilby, R.E. and P.A. Bisson. 1998. Function and Distribution of Large Woody Debris. In: Naiman,
R.J.; Bilby, R.E. (Eds.). River ecology and management. Springer-Verlag, New York: 324-346.
Bilby, R.E., B.R. Fransen, and P.A. Bisson. 1996. Incorporation of nitrogen and carbon from
spawning coho salmon into the trophlc system of small streams: evidence from stable isotopes.
Canadian Journal of Fisheries and Aquatic Sciences 53: 164- 173.
Bilby, R., A. Johnson, J. R. Foltz, A. L. Puls. 2022. Management implications from Pacific
Northwest intensively monitored watersheds. Pacific Northwest Aquatic Monitoring
Partnership. 99 pages. https://www.pnamp.org/document/15207
Bjornn, T.C. 1978. Survival, production, and yield of trout and chinook salmon in the Lemhi
River, Idaho. Univ. Idaho, Coll. For., Wildl. Range Sei. Bull. 27. 57 pp.
Bjornn, T.C. and D.W. Reiser. 1991. Habitat Requirements of Salmonids in Streams. Pages 83-
138 in W. R. Meehan, editor. Influences of forest and rangeland management on salmonid
fishes and their habitats. American Fisheries Society Special Publication 19, Bethesda,
Maryland.
Bouchard, R., Wellband, K., Lecomte, L., Bernatchez, J., and J. April. 2021. Captive-breeding and
catch-and-release’s effects on the reproductive success of Atlantic salmon (Salmo salar L.).
PLoS-ONE, Preprint, Accessed online at:
https://www.biorxiv.org/content/10.1101/2021.04.06.438651v1
Brannon, E. L., M. S. Powell, T. P. Quinn, and A. Talbot. 2004. Population structure of Columbia
River basin Chinook Salmon and steelhead trout. Reviews in Fisheries Science 12:2-3, 99–232.
Brenkman, S.J., J.R. Boetsch, and P.K. Kennedy. 2012. Monitoring riverine fish communities in
the North Coast and Cascades Network: 2010 annual report. Natural Resource Technical Report
NPS/NCCN/NRTR – 2012/530. National Park Service, Fort Collins, Colorado.
Breyta R., A. Jones, B. Stewart, R. Brunson, J. Thomas, J. Kerwin, J. Bertolini, S. Mumford, C.
Patterson, G. Kurath. 2013. Emergence of MD type infectious hematopoietic necrosis virus in
Washington State coastal steelhead trout. Dis. Aquat. Org 104: 179-195 doi: 10.3354/dao02596
Brosofske, K. D., J. Chen, R. J. Naiman and J. F. Franklin. 1997. "Harvesting effects on
microclimatic gradients from small streams to uplands in western Washington." Ecological
Applications 7(4): 1188-1200.

142
Buelow, J. and C.M. Moffitt, C.M. 2015. Physiological indices of seawater readiness in
postspawning steelhead kelts. Ecology of Freshwater Fish 24: 112-
122. https://doi.org/10.1111/eff.12130
Burge, R.T., N.T. Mantua, J.W. Berryman, and L.B. Doyle. 2006. The Status of Wild Steelhead
and Their Management in Western Washington: Strategies for Conservation and Recreation.
Wild Steelhead Coalition (Kirkland, WA).
Burns, J.W. 1971. The carrying capacity for juvenile salmonids in some northern California
streams. Calif. Fish Game 57:44-57.
Busby, P.J., T.C. Wainwright, G.J. Bryant, L.J. Lierheimer, R.S. Waples, F.W. Waknitz, and I.V.
Lagomarsino. 1996. Status Review of West Coast Steelhead from Washington, Idaho, Oregon,
and California. NOAA Technical Memorandum NMFS-NWFSC-27. August 1996, Seattle, WA and
Long Beach, CA.
Busch DS, Harvey CJ, McElhany P. 2013. Potential impacts of ocean acidification on the Puget
Sound food web. ICES J Mar Sci. 70(4):823–33. https://doi.org/10.1093/icesjms/fst061
Carlson, S. M., and T. R. Seamons. 2008. A review of quantitative genetic components of fitness
in salmonids: implications for adaptation to future change. Evolutionary Applications 1:222–
238.
Caughley, G, and A. Gunn. 1996. Conservation biology in theory and practice / Graeme
Caughley, Anne Gunn Blackwell Science Cambridge, U.S. ; Carlton, Vic.
Cederholm, C.J., L.C. Lestelle, B.G. Edie, D.J. Martin, J.V. Tagart, and E.O. Salo. 1978. The effects
of landslide siltation on the salmon and trout resources of Stequaleho Creek and the main
Clearwater River, Jefferson County, Washington, 1972-1975, Final report - Part II. University of
Washington College of Fisheries, Seattle, Washington.
Cederholm, C. J., and E. 0. Salo. 1979. The effects of logging road landslide siltation on the
salmon and trout spawning gravels of Stequaleho Creek and the Clearwater River basin,
Jefferson County, Washington, 1972-1978. FRI-UW-7915. Fisheries Research Institute,
University of Washington, Seattle. 99 p.
Cederholm, C. J., L. M. Reid, and E. 0. Salo. 1980. Cumulative effects of logging road sediment
on salmonid populations in the Clearwater River, Jefferson County, Washington. In Proceedings
from the conference Salmon Spawning Gravel: A Renewable Resource in the Pacific Northwest?
College of Fisheries, University of Washington, Seattle.

143
Cederholm, C.J. 1983. Clearwater River Wild Steelhead Spawning Timing. In Proceedings of the
Olympic Wild Fish Conference, edited by Jim W. Walton and Doug B. Houston, 257-268. Port
Angeles, WA: Peninsula College Fisheries Technology Program.
Cederholm, C.J. and The Forks Chapter Northwest Steelhead and Salmon Council of Trout
Unlimited. 1983. “The Sol Duc River “Native” Winter-Run Steelhead Project.” In Proceedings of
the Olympic Wild Fish Conference, edited by Jim M. Walton and Doug B. Houston, 243-254. Port
Angeles, WA: Peninsula College Fisheries Technology Program.
Cederholm, C. J., R. E. Bilby, P. A. Bisson, T. W. Bumstead, B. R. Fransen, W. J. Scarlett, and J.W.
Ward. 1997. Response of juvenile coho salmon and steelhead to placement of large woody
debris in a coastal Washington stream. North American Journal of Fisheries Management
17(4):947– 963.
Chandler, G. L., and T. C. Bjornn. 1988. Abundance, growth, and interactions of juvenile
steelhead relative to time of emergence. Transactions of the American Fisheries Society
117:432–443.
Christie, M. R., McNickle, G. G., French, R. A., and M.S. Blouin. 2018. Life history variation is
maintained by fitness trade-offs and negative frequency-dependent selection. Proceedings of
the National Academy of Sciences of the United States of America, 115(17): 4441–4446.
https://doi.org/10.1073/pnas.1801779115
Cheung, W.W.L., and T.L. Frölicher. 2020. Marine heatwaves exacerbate climate change
impacts for fisheries in the northeast Pacific. Sci Rep 10, 6678. https://doi.org/10.1038/s41598-
020-63650-z
Chilcote, M. W. 2003. Relationship between natural productivity and the frequency of wild fish
in mixed spawning populations of wild and hatchery steelhead (Oncorhynchus mykiss).
Canadian Journal of Fisheries and Aquatic Sciences, 60(9), 1057-1067.
Chilcote, M., Goodson, K., and M.R. Falcy. 2011. Reduced recruitment performance in natural
populations of anadromous salmonids associated with hatchery-reared fish. Canadian Journal
of Fisheries and Aquatic Sciences. 68(3): 511-522. https://doi.org/10.1139/F10-168
Clark, W. G. 1985. Fishing in a sea of court orders: Puget Sound salmon management 10 years
after the Boldt decision. North American Journal of Fisheries Management 5:417–434.
Climate Impacts Group (CIG). 2009. The Washington Climate Change Impacts Assessment, M.
McGuire Elsner, J. Littell, and L Whitely Binder (eds). Center for Science in the Earth System,
Joint Institute for the Study of the Atmosphere and Oceans, University of Washington, Seattle,
Washington. Available at: http://www.cses.washington.edu/db/pdf/wacciareport681.pdf

144
Connolly, P. J. and J.D. Hall. 1999. Biomass of coastal cutthroat trout in unlogged and
previously clear-cut basins in the central coast range of Oregon. T. Am. Fish. Soc., 128, 890–899.
Cooper AB, and M. Mangel M. 1999. The dangers of ignoring metapopulation structure for the
conservation of salmonids. Fishery Bulletin (NOAA) 97: 213–226.
Copass C., N. Antonova, S. Clary. 2016. Landsat-based monitoring of landscape dynamics in
Olympic National Park: 1985-2010. Natural Resource Data Series NPS/NCCN/NRDS—
2016/1053. National Park Service, Fort Collins, Colorado.
Coutant, C. Perspectives on Temperature in the Pacific Northwest's Fresh Waters. United
States. https://doi.org/10.2172/9042
Cram, J., N. Kendal, A. Marshall, T. Buehrens., T. Seamons, B. Leland, K. Ryding, and E.
Neatherlin. 2018. Steelhead Risk Report: Assessment of Washington’s Steelhead Populations.
Crawford, B. A. 1979. The origin and history of the trout brood stocks of the Washington
Department of Game. Washington State Game Department, Fishery Research Report, Olympia.
Crozier, L.G. and R.W. Zabel. 2006. Climate impacts at multiple scales: evidence for differential
population responses in juvenile Chinook salmon. Journal of Animal Ecology, 75: 1100-
1109. https://doi.org/10.1111/j.1365-2656.2006.01130.x
Crozier, L.G., Hendry, A.P., Lawson, P.W., Quinn, T.P., Mantua, N.J., Battin, J., Shaw, R.G. and
Huey, R.B. 2008. Potential responses to climate change in organisms with complex life histories:
evolution and plasticity in Pacific salmon. Evolutionary Applications, 1: 252-
270. https://doi.org/10.1111/j.1752-4571.2008.00033.x
Crozier L.G., McClure MM, Beechie T, Bograd SJ, Boughton DA, Carr M, et al. (2019) Climate
vulnerability assessment for Pacific salmon and steelhead in the California Current Large Marine
Ecosystem. PLoS One 14(7): e0217711. https://doi.org/10.1371/journal.pone.0217711)
Dalton, M.M, L. Benda, M. Case, S. Chisholm Hatfield, N. Cohn, M. Conlin, J. Lawler, P. Mote, D.
Sharp, G. Reeves, P. Ruggiero, and K. Serafin. 2016. Climate Change Vulnerability Assessment
for the Treaty of Olympia Tribes: A Report to the Quinault Indian Nation, Hoh Tribe, and
Quileute Tribe. Oregon Climate Change Research Institute, Corvallis, OR.
Davis, L., and Schroeder, J. 2009. Internal science assessment of Washington’s coastal rivers.
Seattle: The Nature Conservancy of Washington.
Davis, S.R. 2015. Diet Preferences of Juvenile Steelhead (Oncorhynchus Mykiss): Comparison
Between Three Hood Canal Rivers. Master’s Thesis, The Evergreen State College, Olympia, WA.

145
Devine, W.D., Minkova, T., Martens, K.D., Keck, J., Foster, A.D. 2022. Status and trends
monitoring of riparian and aquatic habitat in the Olympic Experimental State Forest: 2013-2020
results. Washington State Department of Natural Resources, Forest Resources Division,
Olympia, WA.
Dittman AH, May D, Larsen DA, Moser ML, Johnston M, and D. Fast. 2010. Homing and
spawning site selection by supplemented hatchery- and natural-origin Yakima River spring
Chinook salmon. Trans Am Fish Soc 139:1014–1028.
Duda, J. J., S. J. Brenkman, and P. Crain. 2018. Ch. 4.2.1: Pacific Salmon-Natural Resource
Condition Assessment Olympic National Park. Pages 123–167 in R. McCaffery and K. Jenkins,
editors. Natural resource condition assessment: Olympic National Park. National Park Service,
Natural Resource Report NPS/OLYM/NRR 2018/1826, Fort Collins, Colorado.
East, A. E., Jenkins, K. J., Happe, P. J., Bountry, J. A., Beechie, T. J., Mastin, M. C., Sankey, J. B.,
and Randle, T. J. 2017. Channel-planform evolution in four rivers of Olympic National Park,
Washington, USA: the roles of physical drivers and trophic cascades. Earth Surf. Process.
Landforms, 42: 1011– 1032. doi: 10.1002/esp.4048.
Einum, S., and Fleming, I.A. 2001. Implications of stocking: ecological interactions between wild
and released salmonids. Nordic J. Freshw. Res. 75: 56–70.
Einum S, Sundt-Hansen L, and KH Nislow. 2006. The partitioning of density-dependent
dispersal, growth and survival throughout ontogeny in a highly fecund organism. Oikos
113:489–496
Einum, S., K. H. Nislow, J. D. Reynolds, and W. J. Sutherland. 2008. Predicting population
responses to restoration of breeding habitat in Atlantic Salmon. Journal of Applied Ecology
45:930–938.
Elsner, M.M., L. Cuo, N. Viosin, J.S. Deems, A.F. Hamlet, J.A. Vano, D.P. Lettenmaier. 2010.
Implications of 21
st
Century Climate Change for the Hydrology of Washington State. Climatic
Change 102(1): 225-260.
Everest, F. H. 1973. Ecology and management of summer steelhead in the Rogue River. Oregon
State Game Commission, Fishery Research Report 7, Corvallis.
Finstad, A. G., L.M. Sættem, and S. Einum. 2013. Historical abundance and spatial distributions
of spawners determine juvenile habitat accessibility in salmon: implications for the population
dynamics and management targets. Canadian Journal of Fisheries and Aquatic Sciences
70:1339–1345.
Foley, P. 1997. Extinction models for local populations. In I. A. Hanski and M. E. Gilpin (eds.),
Metapopulation biology: ecology genetics and evolution, p. 215-246. Academic Press, San
Diego, CA.

146
Ford, M. J., editor. 2022. Biological Viability Assessment Update for Pacific Salmon and
Steelhead Listed Under the Endangered Species Act: Pacific Northwest. U.S. Department of
Commerce, NOAA Technical Memorandum NMFS-NWFSC-171.
Fountain, A. G., Gray, C., Glenn, B., Menounos, B., Pflug, J., & Riedel, J. L. 2022. Glaciers of the
Olympic Mountains, Washington—The past and future 100 years. Journal of Geophysical
Research: Earth Surface, 127, e2022JF006670. https://doi.
Frachtenberg, L.J. 1916. Eschatology of the Quileute Indians: LaPush, Washington. (Field
notebooks, manuscript No. E0 (W3a5). [Freeman No. 3177] in American Philosophical Society
Library, Philadelphia.
Fraik, AK, McMillan JR, Liermann M, Bennett T, McHenry ML, McKinney GJ, Wells AH, Winans G,
Kelley JL, Pess GR, and Nichols KM. 2021. The Impacts of Dam Construction and Removal on
the Genetics of Recovering Steelhead (Oncorhynchus mykiss) Populations across the Elwha
River Watershed. Genes 12, no. 1: 89. https://doi.org/10.3390/genes12010089
Frissell, C.A., …. Conservation of Aquatic and Fishery Resources in the Pacific Northwest:
Implications of New Science for the Aquatic Conservation Strategy of the Northwest Forest
Plan. F
Furniss, M.J., T.D. Roelofs, and C.S. Yee. 1991. Road Construction and Maintenance in Influences
of Forest and Rangeland Management on Salmonid Fishes and Their Habitats, Meehan, W.R.,
Editor. American Fisheries Society Special Publication 19 (1991): 297-323.
Gayeski, N., Pess, G., and Beechie, T. 2016. A life-table model estimation of the parr capacity of
a late 19th century Puget Sound steelhead population. FACETS. 1(): 83-
104. https://doi.org/10.1139/facets-2015-0010
Gharrett, A. J., J. Joyce, and W. W. Smoker. 2013. Fine-scale temporal adaptation within a
salmonid population: mechanism and consequences. Molecular Ecology 22:4457–4469.
Gibbons, R.G., P.K.J. Hahn, and T.H. Johnson. 1985. Methodology for Determining MSH
Steelhead Spawning Escapement Requirements. Washington State Game Department. Fisheries
Management Division.
Goodman, D. 2005. Selection equilibrium for hatchery and wild spawning fitness in integrated
breeding programs. Can. J. Fish. Aquat. Sci. 62: 374–389
Groot, C., and L. Margolis, eds. 1991. Pacific salmon life histories. Vancouver, BC: University of
British Columbia Press. 564 p
Hall, J., Roni, P., Bennett, T., McMillan, J., Hanson, K., Moses, R., McHenry, M., Pess, G. and
Ehinger, W. 2016. Life History Diversity of Steelhead in Two Coastal Washington Watersheds.

147
Transactions of the American Fisheries Society, 145: 990-
1005. https://doi.org/10.1080/00028487.2016.1194893
Halofsky, Jessica E.; Peterson, David L.; O’Halloran, Kathy A.; Hawkins Hoffman, Catherine, eds.
2011. Adapting to climate change at Olympic National Forest and Olympic National Park. Gen.
Tech. Rep. PNW-GTR-844. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific
Northwest Research Station. 130 p.
Hanski, I.A., and Gilpin, M.E. (Editors). 1997. Metapopulation biology: ecology, genetics, and
evolution. Academic Press.
Hard, J.J., Gross, M.R., Heino, M., Hilborn, R., Kope, R.G., Law, R. and Reynolds, J.D. 2008.
Evolutionary consequences of fishing and their implications for salmon. Evolutionary
Applications, 1: 388-408. https://doi.org/10.1111/j.1752-4571.2008.00020.x
Hard, J.J., J.M. Myers, E.J. Connor, R.A. Hayman, R.G. Kope, G. Lucchetti, A.R. Marshall, G.R.
Pess, and B.E. Thompson. 2015. Viability criteria for steelhead within the Puget Sound distinct
population segment. U.S. Dept. Commer., NOAA Tech. Memo. NMFS-NWFSC-129.
Hatchery Scientific Review Group (HSRG). 2004. North Coast Hatchery Reform
Recommendations.
Hatchery Science Review Group (HSRG). 2015. A report on the application of up-to-date science
in the management of salmon and steelhead hatcheries in the Pacific Northwest.
www.hatcheryreform.us.
Re
Hatten, J.R. and R.H. Conrad. 1995. A comparison of summer stream temperatures in
unmanaged and managed sub-basins of Washington's western Olympic peninsula. Olympia,
Northwest Indian Fisheries Commission, Olympia, WA.
Hicks, M. 1999. Evaluating criteria for the protection of aquatic life in Washington’s surface
water quality standards. Olympia, Washington State Department of Ecology: 1-48.
Hicks, M. 2000. Preliminary Review Draft Discussion Paper Evaluating Standards for Protecting
Aquatic Life In Washington’s Surface Water Quality Standards Temperature Criteria.
Washington State Department of Ecology, Water Quality Program, Watershed Management
Section. Olympia, Washington.
Hodge, B. W., M. A. Wilzbach, and W. G. Duffy. 2014. Potential fitness benefits of the half-
pounder life history in Klamath River steelhead. Transactions of the American Fisheries Society
143:864–875.
Hoh Tribe and WDFW. 2020. Harvest Management Plan for Steelhead Returning to the Hoh
River in the Winter of 2020-2021.

148
Hooton, R.S. and M.G. Lirette. 1986. Telemetric study of winter steelhead, Gold River, 1982-83.
B.C. Fish. Br., Fish. Manage. Rep. No. 86: 30p.
Hooton, R. S., 1987. Catch and release as a management strategy for steelhead in British
Columbia, in Barnhart, R., Roelofs, T., (Eds.), Proceedings of Catch and Release Fishing – A
Decade of Experience. Sept 30 – Oct 1, 1987. Humboldt State University, Arcata, California.
Hooton, R.S., 2001. Facts and issues associated with restricting terminal gear types in the
management of sustainable steelhead sport fisheries in British Columbia. Report for the
Ministry of Lands, Forestry, and Natural Resources, pp 28.
Houston, D.B. and R.J. Contor. 1984. Anadromous fish in Olympic National Park: status and
management considerations. In J.M. Walton and D.B. Houston (eds.). Proceedings of the
Olympic Wild Fish Conference (March 23-25, 1983). Fisheries Technology Program, Peninsula
College, Port Angeles, WA. 97-111.
IPCC. 2021. 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science
Basis. Contribution of Working Group I to the Sixth Assessment Report of the
Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S. L.
Connors, C. P an, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E.
Lonnoy, J.B.R. Matthews, T. K. Maycock, T. Waterfield, O. Yelek i, R. Yu and B. Zhou (eds.)].
Cambridge University Press. In Press.
Independent Scientific Advisory Board (ISAB). 2005. Report on Harvest Management of
Columbia Basin Salmon and Steelhead. Document ISAB 2005-4. Report to the Northwest Power
and Conservation Council. Portland, OR. http://www.nwcouncil.org/library/isab/2005-
4/isab2005_4.pdf
Independent Scientific Advisory Board (ISAB). 2015. Density dependence and its implications for
fish management and restoration programs in the Columbia River. Northwest Power and
Conservation Council, Portland, Oregon. ISAB Report 2015-1. [Online.] Available from
www.nwcouncil.org/fw/isab/ isab2015-1
Isaak, D. J., and R. F. Thurow. 2006. Network-scale spatial and temporal variation in Chinook
Salmon (Oncorhynchus tshawytscha) redd distributions: patterns inferred from spatially
continuous replicate surveys. Canadian Journal of Fisheries and Aquatic Sciences 63:285–296.
Jensen, D.W., Steel, E.A., Fullerton, A.H., and Pess, G.R. 2009. Impact of fine sediment on egg-
to-fry survival of pacific salmon: a meta-analysis of published studies. Rev. Fish. Sci. 17(3): 348–
359. doi:10.1080/10641260902716954.
Joh, Y., E. Di Lorenzo. 2017. Increasing coupling between NPGO and PDO leads to prolonged
marine heatwaves in the Northeast Pacific. Geophys. Res. Lett., 44, 11,663-11,671.
https://doi.org/10.1002/2017GL075930

149
Jones, J.A., F.J. Swanson, B.C. Wemple and K.U. Snyder. 2000. Effects of roads on hydrology,
geomorphology, and disturbance patches in stream networks. Conservation Biology 14: 76-85.
Kassler, T.W., S. Brenkman, J. Gilbertson, M. Gross, D. Low, A. Spidle. 2011. Genetic Analysis of
Steelhead (Oncorhynchus mykiss), Coho (O. kisutch), and Chinook (O. tshawytscha) from
Washington’s Olympic Peninsula with an Emphasis on the Hoh River.
DOI:10.13140/2.1.1669.1527
Kaylor MJ, Warren DR, and PM Kiffney. 2017. Long-term effects of riparian forest harvest on
light in Pacific Northwest (USA) streams. Freshw Sci 36(1) https://doi.org/10.1086/690624
Kendall, N.W., McMillan, J.R., Sloat, M.R., Buehrens, T.W., Quinn, T.P., Pess, G.R., Kuzishchin,
K.V., McClure, M.M., and R.W. Zabel. 2015. Anadromy and residency in steelhead and rainbow
trout (Oncorhynchus mykiss): a review of the processes and patterns. Canadian Journal of
Fisheries and Aquatic Sciences. 72(3): 319-342. https://doi.org/10.1139/cjfas-2014-0192
Kendall, N. W., G. W. Marston, and M. M. Klungle. 2017. Declining patterns of Pacific Northwest
steelhead trout (Oncorhynchus mykiss) adult abundance and smolt survival in the ocean.
Canadian Journal of Fisheries and Aquatic Sciences 74:1275–1290.
Kilduff, D. P., E. Di Lorenzo, L. W. Botsford, and S. L. H. Teo. 2015. Changing central Pacific El
Niños reduce stability of North American salmon survival rates. Proceedings of the national
Academy of Science 112(35): 10962 – 10966.
Klinger, T., R.M. Gregg, K. Herrmann, K. Hoffman, J. Kershner, J. Coyle, and D. Fluharty.
2008. Assessment of Coastal Water Resources and Watershed Conditions at Olympic National
Park, Washington. Natural Resource Technical Report NPS/NRPC/WRD/NRTR—2008/068.
National Park Service, Fort Collins, Colorado.
Knudsen, E. 2000. Managing Pacific salmon escapements: The gaps between theory and reality.
In Sustainable Fisheries Management: Pacific Salmon. Edited by E. Knudsen, C. Steward, D.
MacDonald, J. Williams and D. Reiser, 237-72. Lewis Publishers, New York, NY.
Kostow, K., A. Marshall, and S.R. Phelps. 2003. Natural Spawning Hatchery Steelhead Contribute
to Smolt Production but Experience Low Reproductive Success. Transactions of the American
Fisheries Society 132: 780- 790.
Kostow, K., and S. Zhou. 2006. The effect of an introduced summer steelhead hatchery stock on
the productivity of a wild winter steelhead population. Transactions of the American Fisheries
19 Society. 135(8): 825-841. 20

150
Kostow, K. 2009. Factors that contribute to the ecological risks of salmon and steelhead
hatchery programs and some mitigating strategies. Rev. Fish. Biol. Fish. (2009) 19:9-31.
https://doi.org/10.1007/s11160-008-9087-9
Kunkel, K. E., L. E. Stevens, S. E. Stevens, L. Sun, E. Janssen, D. Wuebbles, K. T. Redmond, and J.
G. Dobson, 2013: Regional Climate Trends and Scenarios for the U.S. National Climate
Assessment: Part 6. Climate of the Northwest U.S. NOAA Technical Report NESDIS 142-6. 75 pp.
LaBossiere, P. 2021. Fishing: Olympic National Park to shut down fishing on West End rivers.
Peninsula Daily News, January 12, 2021. Accessed online at:
https://www.peninsuladailynews.com/sports/fishing-olympic-national-park-to-shut-down-
fishing-on-west-end-rivers/
Lande, R. 1993. Risks of population extinction from demographic and environmental
stochasticity, and random catastrophes. American Naturalist 142:911-927.
Landscape Climate Dashboard (LCD). 2022. Climate projections for federally and tribally
protected lands of the West: Olympic National Park.
Levin, P. S., Zabel, R. W. and J.G. Williams. 2001. The road to extinction is paved with good
intentions: Negative association of fish hatcheries with threatened salmon. Proc. R. Soc. Lond.
Ser. B Biol. Sci. 268, 1153–1158.
Letter from Kim Kratz, Assistant Regional Administrator, NMFS, and Eric V. Rickerson, State
Supervisor, USFWS, to Peter Goldmark, Commissioner of Public Lands, DNR (July 2, 2015) (copy
provided).
Li, H. W., G. A. Lamberti, T. N. Pearsons, C. K. Tait, J. L. Li and J. C. Buckhouse. 1994.
"Cumulative effects of riparian disturbances along high desert trout streams of the John Day
Basin, Oregon." Trans. Am. Fish. Soc. 123(4): 627-640.
Light, J. T., C. K. Harris, and R. L. Burgner. 1989. Ocean distribution and migration of steelhead
(Oncorhynchus mykiss, formerly Salmo gairdneri). (Document submitted to the International
North Pacific Fisheries Commission.) 50 pp. FRI-UW-8912. Fisheries Research Institute,
University of Washington, Seattle.
Lobon-Cervia, J. 2007. Density-dependent growth in stream-living Brown Trout Salmo trutta L.
Functional Ecology, 21: 117-124. https://doi.org/10.1111/j.1365-2435.2006.01204.x
Logan, R.L., Kaler, K.L., and Biglow, P.K. 1991. Prediction of sediment yield from tributary basins
along Huelsdonk Ridge, Hoh River, Washington. Washington Division of Geology and Earth
Resources Open-file Report 91-7.

151
Lynch, J. A., G. B. Rishel, and E. S. Corbett. 1984. Thermal alteration of streams draining clearcut
watersheds: quantification and biological implications. Hydrobiologia 111:161–169.
Lyre-Hoko Watershed (WRIA 19) Planning Unit. 2008. WRIA 19 Watershed Plan for Lyre and
Hoko Rivers. Funded by Washington Department of Ecology Grant no. G050013. Accessed
online: http://www.clallam.net/assets/applets/WRIA_19_DRAFT_PLAN_11-3-08a.pdf
Manhard, C. V., J. E. Joyce, and A. J. Gharrett. 2017. Evolution of phenology in a salmonid
population: a potential adaptive response to climate change. Canadian Journal of Fisheries and
Aquatic Sciences 74:1519–1527.
Mantua, N.J and R.C. Francis. 2004. Natural climate insurance for Pacific northwest salmon and
salmon fisheries: finding our way through the entangled bank. American Fisheries Society
Symposium. 43:127–140
Mantua, N., I. Tohver, and A. Hamlet. 2010. Climate change impacts on streamflow extremes
and summertime stream temperature and their possible consequences for freshwater salmon
habitat in Washington State. Climatic Change 102:187-223.
Marsh, W. 2012. Hoh River Salmon Recovery Restoration Priorities for the Middle Hoh Basin:
Oxbow Canyon to Olympic National Park (RM 17-30).
Martens, K.D., W.D. Devine, T.V. Minkova, A.D. Foster. 2019. Stream Conditions after 18 Years
of Passive Riparian Restoration in Small Fish-bearing Watersheds. Environmental Management
63:673-690. https://doi.org/10.1007/s00267-019-01146-x.
Mathis, J.T., J.N. Cross, W. Evans, and S.C. Doney. 2015. Ocean acidification in the surface
waters of the Pacific-Arctic boundary regions. Oceanography 28(2):122–135,
http://dx.doi.org/10.5670/oceanog.2015.36.
McCullough, D. 1999. A Review and Synthesis of Effects of Alterations to the Water
Temperature Regime on Freshwater Life Stages of Salmonids, with Special Reference to
Chinook Salmon. Columbia Intertribal Fisheries Commission, Portland, OR. Prepared for the U.S.
Environmental Protection Agency Region 10. Published as EPA 910-R-99-010.
McElhany, P, M.H. Ruckelshaus, M.J. Ford, T.C. Wainwright, E.P. Bjorkstedt. 2000. Viable
salmonid populations and the recovery of evolutionarily significant units. U.S. Dept. Commer.,
NOAA Tech. Memo. NMFS-NWFSC-42, 156 p.
McHenry, M.J., J.A. Lichatowich J., and R. Kowalski-Hagaman. 1996. Status of Pacific Salmon and
their Habitats on the Olympic Peninsula, Washington. Report to the Lower Elwha Klallam Tribe,
Port Angeles, WA.

152
McHenry M.L., E. Shott, R.H. Conrad, G.B. Grette. 1998. Changes in the quantity and
characteristics of large woody debris in streams of the Olympic Peninsula, Washington, U.S.A.
(1982-1993). Can. J. Fish. Aquat. Sci. 55: 1395-1407.
McMillan B. 2006. Historic Steelhead Abundance: Washington Northwest Coast and Puget
Sound. Wild Salmon Center. Portland, OR.
McMillan, J. R., S. L. Katz, and G. R. Pess. 2007. Observational evidence of spatial and temporal
structure in a sympatric anadromous (winter steelhead) and resident Oncorhynchus mykiss
mating system on the Olympic Peninsula, Washington State. Transactions of the American
Fisheries Society 136:736-748.
McMillan J.R and J.C. Starr. 2008. Identification and prioritization of salmon tributaries for
conservation in the Hoh River Basin, Washington State. Wild Salmon Center. Portland, Oregon.
McMillan, J. R., M. C. Liermann, J. Starr, G. R. Pess, and X. Augerot. 2013. Using a stream
network census of fish and habitat to assess models of juvenile salmonid distribution.
Transactions of the American Fisheries Society 142:942–956.
McMillan, J. 2021. Unpublished data for snorkel surveys in NF Calawah River, SF Calawah River,
Calawah River, Sitkum River, and SF Hoh River from 2000-2006; 2009-2021.
McMillan, J.R. 2022. Abundance of wild and hatchery adult summer steelhead in two large
watersheds on the west-side of the Olympic Peninsula. Annual Report TCA-1 for The
Conservation Angler, 18p.
McMillan, J.R., Sloat, M.R., Liermann, M. and Pess, G. 2022. Historical Records Reveal Changes
to the Migration Timing and Abundance of Winter Steelhead in Olympic Peninsula Rivers,
Washington State, USA. North Am J Fish Manage, 42: 3-
23. https://doi.org/10.1002/nafm.10722
Meffe, G.K. and C.R. Carroll. 1994. Principles of conservation biology. Sinauer Associates,
Sunderland, Massachusetts.
Melnychuk, M.C., Welch, D.W., Walters, D.J., and Christensen, V. 2007. Riverine and early ocean
migration and mortality patterns of juvenile steelhead trout (Oncorhynchus mykiss) from the
Cheakamus River, British Columbia. Hydrobiologia, 582: 55-65. doi:10.1007/s10750-006-0541-1.
Middleton, D.A.J., A.R. Veitch, and R.M. Nisbet. 1995. The effect of an upper hit to population
size on persistence time. Theor. Popul. Biol. 48:277-305.
Miller, I.M., Shishido, C., Antrim, L, and Bowlby, C.E. 2013. Climate Change and the Olympic
Coast National Marine Sanctuary: Interpreting Potential Futures. Marine Sanctuaries

153
Conservation Series ONMS-13-01. U.S. Department of Commerce, National Oceanic and
Atmospheric Administration, Office of National Marine Sanctuaries, Silver Spring, MD. 238 pp.
Minkova, T.V., M.P. Hicks, K.D. Martens. 2021. Chapter 12: Ecoregion 7.1.8 Coast Range:
Olympic Experimental State Forest, Washington in Ryan, Douglas F., ed. 2021. Biological
responses to stream nutrients: a synthesis of science from experimental forests and ranges.
Gen. Tech. Rep. PNW-GTR-981. Portland, OR: U.S. Department of Agriculture, Forest Service,
Pacific Northwest Research Station. 522 p.
Moore, J.W., J.D. Yeakel, D. Peard, J. Lough, and M. Beere. 2014. Life-history diversity and its
importance to population stability and persistence of a migratory fish: steelhead in two large
North American watersheds. Journal of Animal Ecology 83(5): 1035–1046.
Montgomery, D.R., Beamer, E.M., Pess, G.R., and T.P. Quinn. 1999. Channel type and salmonid
spawning distribution and abundance. Canadian Journal of Fisheries and Aquatic Sciences 56,
377–387.
Mote, P. W., and E.P. Salathé. 2010. Future climate in the Pacific Northwest. Climatic
Change 102(1-2): 29-50, doi: 10.1007/s10584-010-9848-z.
Murdoch A., and G. Marston. 2020. WDFW Hatchery and Fishery Reform Policy Implementation
Assessment Final Report, 2009-2019. WDFW, Olympia, WA.
Murphy, M.L., Hawkins, C.P. and Anderson, N.H. 1981. Effects of Canopy Modification and
Accumulated Sediment on Stream Communities. Transactions of the American Fisheries
Society, 110: 469-478. https://doi.org/10.1577/1548-8659(1981)110<469:EOCMAA>2.0.CO;2
Myers, K.W., Davis, N.D., Walker, R.V., and M.E. Atcheson. 2013. Potential mechanisms of
ocean mortality of juvenile salmon and steelhead due to ingestion of plastic marine debris.
North Pacific Anadromous Fish Commission, Technical Report No. 9: 169-170.
Naish, K. A., J. E. Taylor III, P. S. Levin, T. P. Quinn, J. R. Winston, D. Huppert, and R. Hilborn.
2007. An evaluation of the effects of conservation and fishery enhancement hatcheries on wild
populations of salmon. Advances in Marine Biology 53:61–194.
Nehlsen. W., J. E. Williams, and J.A. Licatowich. 1991. Pacific Salmon at the crossroads: Stocks at
risk from California, Oregon, and Washington. Fisheries 16(2):4-21.
Nelson, T.C., Rosenau, M.L. and Johnston, N.T. 2005. Behavior and Survival of Wild and
Hatchery-Origin Winter Steelhead Spawners Caught and Released in a Recreational Fishery.
North American Journal of Fisheries Management, 25: 931-943. https://doi.org/10.1577/M04-
192.1

154
National Marine Fisheries Service (NMFS). 1996. Coastal Salmon Conservation: Working
Guidance for Comprehensive Salmon Restoration Initiatives on the Pacific Coast. Washington,
D.C.
National Marine Fisheries Service and U.S. Fish and Wildlife Service. 2006. Endangered Species
Act Section 7 Consultation Biological Opinion and Section 10 Statement of Findings and
Magnuson-Stevens Fishery and Conservation and Management Act Essential Fish Habitat
Consultation for Washington State Forest Habitat Conservation Plan. National Marine Fisheries
Services, Northwest Region.
North Olympic Peninsula Lead Entity for Salmon (NOPLE). 2015. Water Resource Inventory Area
19 (Lyre-Hoko) Salmonid Restoration Plan. Clallam County, WA.
North Pacific Coast Lead Entity. 2020. North Pacific Coast (WRIA 20) Salmon Restoration
Strategy. University of Washington, Olympic Natural Resources Center, Forks, WA.
Northwest Indian Fisheries Commission Member Tribes (NIFC). 2020. 2020 State of Our
Watersheds: A Report by the Treaty Tribes in Western Washington.
Ohlberger, J. T.W. Buehrens, S.J. Brenkman, P. Crain, T.P. Quinn, R. Hilborn. 2018. Effects of
past and projected river discharge variability on freshwater production in anadromous fish.
Freshwater Biology 63: 331-340.
Oliver ECJ, Burrows MT, Donat MG, Sen Gupta A, Alexander LV, Perkins-Kirkpatrick SE,
Benthuysen JA, Hobday AJ, Holbrook NJ, Moore PJ, Thomsen MS, Wernberg T and Smale DA.
2019. Projected Marine Heatwaves in the 21st Century and the Potential for Ecological
Impact. Front. Mar. Sci. 6:734. doi: 10.3389/fmars.2019.00734
Papatheodoulou, M., Závorka, L., Koeck, B., Metcalfe, N.B., and SS. Killen. 2021. Simulated pre-
spawning catch and release of wild Atlantic salmon (Salmo salar) results in faster fungal spread
and opposing effects on female and male proxies of fecundity. Canadian Journal of Fisheries
and Aquatic Sciences. 79(2): 267-276. https://doi.org/10.1139/cjfas-2021-0089
Pauly, D. 1995. Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology
and Evolution 10:430.
Pauly, G.B. and G.L. Thomas. 1993. Mortality of anadromous coastal Cutthroat Trout caught
with artificial lures and natural bait. N. Am. J. Fish. Manage. 13, 337–345.
Pavlov, D.S., Savvaitova, K.A., and Kuzishchin, K.V. 2001. Theoretical aspects of the problem of
the distribution pattern and formation of life-history strategy of mikizha (Parasalmo mykiss
(Walbaum), Salmonidae, Salmoniformes) on the Kamchatka peninsula. J. Ichthyol. 379: 344–
346.

155
Pearcy, W.G., R.D. Brodeur, J.P. Fisher. 1990. Distribution and Biology of Juvenile Cutthroat
Trout Oncorhychus clarki and Steelhead O. mykiss in Coastal Waters off Oregon and
Washington. Fishery Bulletin 88, 697-711.
Penney, Z. L., and C. M. Moffitt. 2014. Fatty acid profiles of white muscle and liver tissue in
stream-maturing steelhead during early migration and kelt emigration. Journal of Fish Biology
86:105-120.
Phelps, S.R., S.A. Leider, P.L. Hulett, B.M. Baker, and T. Johnson. 1997. Genetic analyses of
Washington Steelhead: preliminary results incorporating 36 new collections from 1995 and
1996. Unpublished WDFW Progress Report. Available upon request from WDFW Fish Program,
Conservation Biology Unit, Olympia, WA.
Phillips, R.W., Lantz, R.L., Claire, E.W., and Moring, J.R. 1975. Some effects of gravel mixtures on
emergence of coho salmon and steelhead trout fry. Transactions of the American Fisheries
Society 104: 461-466.
Phinney, D. and Bucknell. 1975. A catalog of Washington streams and salmon utilization. Vol. 2
Coastal Region. Washington Department of Fisheries, Olympia, Washington.
Piety, L.A., J.A. Bountry, T.J. Randle, E.W. Lyon. 2004. Summary Report for Geomorphic Assessment
of Hoh River in Washington State: River Mile 17 to 40 Between Oxbow Canyon and Mount Tom
Creek. US Department of Interior, Bureau of Reclamation, Denver, CO.
Pitman, K.J., Moore, J.W., Sloat, M.R., Beaudreau, A.H., Bidlack, A.L., Brenner, R.E., Hood, E.W.,
Pess, G.R., Mantua, N.J., Milner, A.M., Radic, V., Reeves, G.H., Schindler, D.E., Whited, D.C..
2020. Glacier Retreat and Pacific Salmon, BioScience, Volume 70, Issue 3, March 2020, Pages
220–236, https://doi.org/10.1093/biosci/biaa015
Pollock M.M., S. Baker, R. Bigley, W. Scarlett. 2004. Summer Stream Temperatures in the Olympic
Experimental State Forest, Washington. Washington State Department of Natural Resources,
Olympia, WA.
Pollock MM, Beechie TJ, Liermann M, Bigley RE. 2009. Stream temperature relationships to forest
harvest in western Washington. Journal of the American Water Resources Association 45(1): 141–
156.
Prince, D. J., O’Rourke, S. M., Thompson, T. Q., Ali, O. A., Lyman, H. S., Saglam, I. K., Hotaling,
T.J., Spidle, A.P., and M.R. Miller. 2017. The evolutionary basis of premature migration in Pacific
salmon highlights the utility of genomics for informing conservation. Science Advances, 3(8),
e1603198. https://doi.org/10.1126/sciadv.1603198

156
Quinault Department of Fisheries and Washington Department of Fish and Wildlife (ODFW and
WDFW). 2021. Stock Status and Harvest Management Plan for Winter-Run Steelhead Returning
to the Quinault River System in the 2020-2021 Season.
Quinault Indian Nation Lead Entity (QINLE). 2011. WRIA 21 Queets/Quinault Salmon Habitat
Recovery Strategy. Prepared by WRIA 21 lead entity. Tahola, Washington.
Quinn, T.P. 1993. A review of homing and straying of wild and hatchery-produced salmon. Fish
Res 18:29–44.
Quinn, T. P. 2005. The behavior and ecology of Pacific salmon and trout. American Fisheries
Society, Bethesda, Maryland.
Quinn, T. P., S. Hodson, L. Flynn, R. Hilborn, and D. E. Rogers. 2007. Directional selection by
fisheries and the timing of Sockeye Salmon (Oncorhynchus nerka) migrations. Ecological
Applications 17:731–739.
Rand PS, Berejikian B, Bidlack A, Bottom D, Gardner J, Kaeriyama M, Lincoln R, Nagata M,
Pearsons TN, Schmidt M, Smoker W, Weitkamp L, and LA Zhivotovsky. 2012. Ecological
interactions between wild and hatchery salmon and key recommendations for research and
management actions in selected regions of the North Pacific. Environ Biol Fish. doi:10.1007/
s10641-012-9988-2
Reed, T. E., D. E. Schindler, M. J. Hague, D. A. Patterson, E. Meir, R. S. Waples, and S. G. Hinch.
2011. Time to evolve? Potential evolutionary responses of Fraser River Sockeye Salmon to
climate change and effects on persistence. PLoS (Public Library of Science) ONE [online serial]
6(6):e20380.
Reeves, G.H. 2006. The Aquatic Conservation Strategy of the Northwest Forest Plan: an
assessment after ten years. In: Haynes, R.W.; Bormann, B.T.; Lee, D.C.; Martin, J.R., tech. eds.
2006. Northwest Forest Plan—the first 10 years (1994– 2003): synthesis of monitoring and
research results. Gen. Tech. Rep. PNW-GTR-651. Portland, OR: U.S. Department of Agriculture,
Forest Service, Pacific Northwest Research Station: 181–217. Chapter 9.
Reeves, G.H., D.H. Olson, S.M Wondzell, PA. Bisson, S. Gordon, S.A. Miller, J.W. Long, and M.J.
Furniss. Chapter 7: The Aquatic Conservation Strategy of the Northwest Forest Plan – A Review
of the Relevant Science After 23 Years. In Spies, T.A..; Stine. P.A.; Gravenmier R.; Long, J.W.;
Reilly, M.J., tech cords. 2018. Synthesis of Science to Inform Land Management Within the
Northwest Forst Plan area. Gen. Tech. Rep. PNW-GTR-966. Portland, OR: U.S. Department of
Agriculture, Forest Service, Pacific Northwest Research Station: 461-624.
Reiser, D.W., and R.G. White. 1988. Effects of two sediment size classes on survival of steelhead
and chinook salmon eggs. North American Journal of Fisheries Management 8: 432-437.

157
Richard, A., Dionne, M., Wang, J. Bernatchez., L. 2013. Does catch and release affect the mating
system and individual reproductive success of wild Atlantic salmon (Salmo salar L.). Mol. Ecol.
22, 187-200.
Ricker, W. E. 1963. Big Effects from Small Causes: Two Examples from Fish Population
Dynamics. Journal of the Fisheries Research Board of Canada. 20(2): 257-
264. https://doi.org/10.1139/f63-022
Riedel, J., Wilson, S., Baccus, W., Larrabee, M., Fudge, T., and Fountain, A. 2015. Glacier status
and contribution to streamflow in the Olympic Mountains, Washington, USA. Journal of
Glaciology, 61(225), 8–16. https://doi.org/10.3189/2015JoG14J138
Rombough, P.J. 1988. Growth, aerobic metabolism, and dissolved oxygen requirements of
embryos and alevins of steelhead, Salmo gairdneri. Can. J. Zool. 66:651-660.
Romer JD, Leblanc CA, Clements S, Ferguson JA, Kent ML, Noakes D, and C.B. Schreck. 2012.
Survival and behavior of juvenile steelhead trout (Oncorhynchus mykiss) in two estuaries in
Oregon, USA. Environ Biol Fish. doi:10.1007/ s10641-012-0080-8
Ruggerone, G. et al. 2021. Record-setting Abundances of Pink Salmon Impact Pacific Salmon
and other Marine Species, including Southern Resident Killer Whales. American Fisheries
Society Conference. Accessed online at:
https://www.idahoafs.org/committees/presentations/2.6FWB_GregRuggerone.pdf
Ruggerone, G. T., R. M. Peterman, B. Dorner, and K. W. Myers. 2010. Magnitude and trends in
abundance of hatchery and wild Pink, Chum, and Sockeye salmon in the North Pacific Ocean.
Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science. Marine and
Coastal Fisheries: Dynamics, Management, and Ecosystem Science 2:306–328.
Ruggerone, G.T., and B.M. Connors. 2015. Productivity and life history of sockeye salmon in
relation to competition with pink and sockeye salmon in the North Pacific Ocean. Canadian
Journal of Fisheries and Aquatic Sciences 72: 818-833.
Ruggerone, G.T., and J.R. Irvine. 2018. Numbers and biomass of natural- and hatchery-origin
pink, chum, and sockeye salmon in the North Pacific Ocean, 1925-2015. Marine and Coastal
Fisheries: Dynamics, Management, and Ecosystem Science 10: 152-168.
Schindler DE, Hilborn R, Chasco B, Boatright, CP, Quinn, TP, Rogers, LA, and MS Webster. 2010.
Population diversity and the portfolio effect in an exploited species. Nature 465: 609–12.
Schisler, G.J. and E.P. Bergersen. 1996. Post release Hooking Mortality of Rainbow Trout Caught
on Scented Artificial Baits. North American Journal of Fisheries Management, 16: 570-
578. https://doi.org/10.1577/1548-8675(1996)016<0570:PHMORT>2.3.CO;2

158
Schlosser IJ, and P.L. Angermeier. 1995. Spatial variation in demographic processes in lotic
fishes: Conceptual models, empirical evidence, and implications for conservation. American
Fisheries Society Symposium 17: 360–370
Seamons, T.R., L. Hauser, K.A. Naish, and T.P. Quinn. 2012. Can interbreeding of wild and
artificially propagated animals be prevented by using broodstock selected for a divergent life
history? Evolutionary Applications, 5(7), 705-709. https://doi.org/10.1111/j.1752-
4571.2012.00247.x
Shapovalov, L. 1937. Experiments in hatching steelhead eggs in gravel. Calif. Fish Game 23:208-
214.
Shapovalov, L., and A.C. Taft. 1954. The life histories of the steelhead rainbow trout (Salmo
gairdneri gairdneri) and silver salmon (Oncorhynchus kisutch) with special reference to Waddell
Creek, California, and recommendations regarding their management. California Department of
Fish and Game. Fish. Bulletin No. 98. 575 pp.
Sheppard, D. 1972. The present status of the steelhead trout stocks along the Pacific coast.
Pages 519-556 in D.H. Rosenberg, ed. A review of the oceanography and renewable resources
of the northern Gulf of Alaska. Univ. Alaska, Inst. Mar. Sei., IMS Rep. R72-23, Sea Grant Rep. 73-
3.
Smith, C.J. 2000. Salmon and steelhead habitat limiting factors in the north Washington coastal
streams of WRIA 20. Washington State Conservation Commission. Lacey, WA.
Smith, C.J. 2005. Salmon Habitat Limiting Factors in Washington State. Washington State
Conservation Commission. Olympia, WA.
Smith, C.J., and J. Caldwell. 2001. Salmon and steelhead habitat limiting factors in the
Washington coastal streams of WRIA 21. Prepared for the Washington State Conservation
Commission. Lacey, WA.
Stewart, D. C., G. W. Smith, and A. F. Youngson. 2002. Tributary specific variation in timing of
return of adult Atlantic Salmon (Salmo salar) to fresh water has a genetic component. Canadian
Journal of Fisheries and Aquatic Sciences 59:276–281.
Stiefel, O. 2013. All carrot and no stick: why Washington's Clean Water Act assurances violate
state and federal water quality laws. Washington Law Review, vol. 88, no. 2, June 2013, pp. 683
Stout, H.A., P.W. Lawson, D.L. Bottom, T.D. Cooney, M.J. Ford, C.E. Jordan, R.G. Kope, L.M.
Kruzic, G.R. Pess, G.H. Reeves, M.D. Scheuerell, T.C. Wainwright, R.S. Waples,
E. Ward, L.A. Weitkamp, J.G. Williams, and T.H. Williams. 2012. Scientific conclusions of the
status review for Oregon coast coho salmon (Oncorhynchus kisutch). U.S. Dept. Commer., NOAA
Tech. Memo. NMFS-NWFSC-118, 242 p.

159
Suttle, K.B., Power, M.E., Levine, J.M. and McNeely, C. 2004. How fine sediment in riverbeds
impairs growth and survival of juvenile salmonids. Ecological Applications, 14: 969-974.
Tatara CP, and BA Berejikian. 2012. Mechanisms influencing competition between hatchery and
wild juvenile anadromous Pacific salmonids in fresh water and their relative competitive
abilities. Environ Biol Fish. doi:10.1007/s10641-011-9906-z
Taylor, G., and R.A. Barnhart. 1997. Mortality of angler caught and released summer steelhead.
Steelhead Trout Catch Report. California Cooperative Fishery Research Unit and Humboldt State
University pp. 1–31.
Teichert, M. A. K., A. Foldvik, T. Forseth, O. Ugedal, S. Einum, A. G. Finstad, R. D. Hedger, and E.
Bellier. 2011. Effects of spawning distribution on juvenile Atlantic Salmon (Salmo salar) density
and growth. Canadian Journal of Fisheries and Aquatic Sciences 68:43–50.
Thomas, J.W.; Raphael, M.G.; Anthony, R.G.; Forsman, E.D.; Gunderson, A.G.; Holthausen, R.S.;
Marcot, B.G.; Reeves, G.H.; Sedell, J.R.; Solis, D.M. 1993. Viability assessments and management
considerations for species associated with late successional and old-growth forests of the
Pacific Northwest: the report of the Scientific Analysis Team. Portland, OR: U.S. Department of
Agriculture, National Forest System, Forest Service Research. 530 p.
Thompson, L.C., Voss, J.L., Larsen, R.E., Tietje, W.D., Cooper, R.A. and Moyle, P.B. 2012.
Southern Steelhead, Hard Woody Debris, and Temperature in a California Central Coast
Watershed. Transactions of the American Fisheries Society, 141: 275-
284. https://doi.org/10.1080/00028487.2012.662200
Tillotson, M. D., and T. P. Quinn. 2018. Selection on the timing of migration and breeding: a
neglected aspect of fishing-induced evolution and trait change. Fish and Fisheries 19:170–181.
Tilman, D., Lehman, C.L., and Kareiva, P. 1997. Population dynamics in spatial habitats. In
Spatial ecology: the role of space in population dynamics and interspecific interactions. Edited
by D. Tilman and P. Kareiva. Princeton University Press. pp. 3-20.
Tortorici, C. 2016. Guidance for Treatment of Climate Change in NMFS Endangered Species Act
Decisions. National Marine Fisheries Service Procedural Instruction 02-110-18. Silver Springs,
MD.
Twardek, W.M., T.O. Gagne, L.K. Elmer, S.J. Cooke, M.C. Beere, A.J. Danylcuck. 2018.
Consequences of catch-and-release angling on the physiology, behaviour and survival of wild
steelhead Oncorhynchus mykiss in the Bulkley River, British Columbia. Fisheries Research, 206:
235-246. https://doi.org/10.1016/j.fishres.2018.05.019.
U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior, Bureau of Land
Management [USDA and USDI]. 1994b. Environmental assessment for the implementation of

160
interim strategies (PACFISH) for managing anadromous fish-producing watersheds in eastern
Oregon and Washington, Idaho, and portions of California. Washington, DC. [Pages unknown].
U.S. Fish and Wildlife Service (USFWS). 2009. Quilcene, Quinault, and Makah National Fish
Hatcheries: Assessments and Recommendations. Final Report, May 2009. Hatchery Review
Team, Pacific Region. U.S. Fish and Wildlife Service, Portland, Oregon.
U.S. Fish and Wildlife Service (USFWS). 2020. Biological Opinion for Programmatic Forest
Management Activities on the Olympic National Forest June 15, 2020 to June 15, 2030. USFW
Reference: 13410-2009-F-0388-R001. Lacey, WA.
Von Schuckmann et al. 2020. Heat stored in the Earth system: where does the energy go? Earth
Syst. Sci. Data, 12, 2013–2041. https://doi.org/10.5194/essd-12-2013-2020
Wade, A.A., T.J. Beechie, E. Fleishman, N.J. Mantua, H. Wu, J.S. Kimball, D.M. Stoms, and J.A.
Stanford. 2013. Steelhead vulnerability to climate change in the Pacific Northwest. Journal of
Applied Ecology 50:1093–1104.
Walters, A.W., Copeland, T. and Venditti, D.A. 2013. The density dilemma: limitations on
juvenile production in threatened salmon populations. Ecol Freshw Fish, 22: 508-
519. https://doi.org/10.1111/eff.12046
Waples, R.S., Ford, M.J., Nichols, K., Kardos, M., Myers, J., Thompson, T.Q., Anderson, E.C.,
Koch, I.J., McKinney, G., Miller, M.R., Naish, K., Narum, S.R., O’Malley, K.G., Pearse, D.E., Pess,
G.R., Quinn, T.P., Seamons, T.R., Spidle, A., Warheit, K.I., and S.C. Willis. 2022. Implications of
Large-Effect Loci for Conservation: A Review and Case Study with Pacific Salmon, Journal of
Heredity, Volume 113, Issue 2, March 2022, Pages 121–
144, https://doi.org/10.1093/jhered/esab069
Ward, B.R. 2000. Declivity in steelhead (Oncorhynchus mykiss) recruitment at the Keogh River
over the past decade. Canadian Journal of Fisheries and Aquatic Sciences. 57 (2): 298-
306. https://doi.org/10.1139/f99-243
Warner, K. and P.R. Johnson. 1978. Mortality of landlocked Atlantic Salmon (Salmo salar)
hooked on flies and worms in a river nursery area. Trans. Am. Fish. Soc. 107, 772–775.
Washington Coast Sustainable Salmon Partnership (WCSSP). 2013. Washington Coast
Sustainable Salmon Plan.
Washington Department of Fish and Wildlife (WFDW). 1992. Washington State salmon and
steelhead stock inventory. Appendix one: North Puget Sound Vol., Hood Canal, and Strait of
Juan de Fuca Stocks Vol., South Puget Sound Vol.; Appendix two: Coastal Stocks; and Appendix
three: Columbia River Stocks. Washington Department of Fish and Wildlife. June, 1993.
Olympia, Washington.

161
Washington Department of Fish and Wildlife (WDFW). 1994a. Hatchery release data for
steelhead, 1982 to 1994. Electronic data submitted to the ESA Administrative Record for coastal
steelhead, August 1994. (Available from Washington Department of Fish and Wildlife, 600
Capitol Way N, Olympia, WA 98501-1091.)
Washington Department of Fisheries, Washington Department of Wildlife, and Western
Washington Treaty Indian Tribes. 1993. 1992 Washington State Salmon and Steelhead Stock
Inventory. Washington State Washington Department of Fisheries, Olympia, WA.
Washington Department of Fish and Wildlife and Western Washington Treaty Tribes. 2002.
Salmon and steelhead inventory (SaSI). WDFW, Olympia.
Washington Department of Fish and Wildlife. 2008. Statewide Steelhead Management Plan:
Statewide Policies, Strategies, and Actions. Olympia, WA.
Washington Department of Fish and Wildlife. 2020. Washington State Fish Passage.
https://geodataservices.wdfw.wa.gov/hp/fishpassage/index.html (last viewed on April 2, 2022).
Washington Department of Fish and Wildlife. 2022a. WDFW-Salmonid Stock Inventory
Population Escapement. https://data.wa.gov/Natural-Resources-Environment/WDFW-
Salmonid-Stock-Inventory-Population-Escapemen/fgyz-n3uk/data (last viewed on April 2,
2022).
Washington Department of Fish and Wildlife. 2022b. Bogachiel Hatchery.
https://fortress.wa.gov/dfw/score/score/hatcheries/hatchery_details.jsp?hatchery=Bogachiel%
20Hatchery
Washington Department of Fish and Wildlife. 2022c. Creel survey data for Quillayute River
system 2014-2015. Available at https://wdfw.wa.gov/fishing/reports/creel/steelhead/2014-
2015 (last visited on June 22, 2022).
Washington Department of Fish and Wildlife. 2022d. Quillayute Winter Steelhead Data. Copy
provided in submitted materials.
Washington Department of Fish and Wildlife. 2022e. Unredacted Quillayute River Summer
Steelhead Data. Available at
https://app.box.com/s/2twbwbmvc7f22xatl8fqh2leyv2fey1p/file/913529149812
Washington Department of Natural Resources. 2016. Olympic Experimental State Forest
Habitat Conservation Planning (HCP) Unit Forest Land Plan. Washington Department of Natural
Resources, Olympia, WA.

162
Washington Department of Ecology. 2004. Enforcement Report on Policy and Trends.
Publication Number 04-01-009.
Washington Department of Ecology. 2016. Current Water Quality Assessment 303(d)/305(b)
List. Available at
https://apps.ecology.wa.gov/ApprovedWQA/ApprovedPages/ApprovedSearch.aspx (last
viewed on April 2, 2022).
Washington Fish and Wildlife Commission. 2009. Washington Department of Fish and Wildlife
Hatchery and Fishery Reform. Policy Number: C-3619. Olympia, WA.
Washington Fish and Wildlife Commission. 2021. Anadromous Salmon and Steelhead Hatchery
Policy. Policy Number: C-3624. Olympia, WA.
Weber, E.D. and K.D. Fausch. 2003. Interactions between hatchery and wild salmonids in
streams: differences in biology and evidence for competition. Canadian Journal of Fisheries and
Aquatic Sciences. 60 (8): 1018-1036. https://doi.org/10.1139/f03-087
Welch, D.W., Porter, A.D., and E.L. Rechisky. 2018. The coast-wide collapse in marine survival
of west coast Chinook and steelhead: slow-moving catastrophe or deeper failure? PLoS-ONE,
Pre-print version accessed online: doi: https://doi.org/10.1101/476408
Wilhere, G., and T. Quinn. 2018. How Wide is Wide Enough?: Science, Values, and Law in
Riparian Habitat Conservation, 58 Nat. Resources J. 279. Available at:
https://digitalrepository.unm.edu/nrj/vol58/iss2/13
Willmes, M., Hobbs, J.A., Sturrock, A.M., Bess, Z., Lewis, L.S., Glessner, J.J.G., Johnson, R.C.,
Kurth, R., and J. Kindopp. 2018. Fishery collapse, recovery, and the cryptic decline of wild
salmon on a major California river. Canadian Journal of Fisheries and Aquatic Sciences. 75(11):
1836-1848. https://doi.org/10.1139/cjfas-2017-0273
Withler, I. L. 1966. Variability in life history characteristics of steelhead trout (Salmo gairdneri)
along the Pacific coast of North America. Journal of the Fisheries Research Board of Canada
23:365-392.
Wood, C.C. 1995. Life history variation and population structure in sockeye salmon. American
Fisheries Society Symposium. 17: 195-216.
WRIA 19 Watershed Plan. 2008. WRIA 19 Watershed Plan for Lyre and Hoko Rivers. Prepared by
the Lyre-Hoko Watershed (WRIA 19) Planning Unit. Funded by Washington Department of
Ecology Grant no. G050013. Accessed online:
http://www.clallam.net/assets/applets/WRIA_19_DRAFT_PLAN_11-3-08a.pdf

163
Zasoski, R., H. Dawson, and L. Lestelle. 1977. Impacts of forest management practices on the
aquatic environment - Phase III. Quinault Indian Nation final report to the Office of Water
Research and Technology.
Zaugg, W.S., B.L. Adams, and L.R. Mclain. 1972. Steelhead migration: potential temperature
effects as indicated by gill adenosine triphosphatase activities. Science 176:415-416.
Zaugg, W.S., and H.H. Wagner. 1973. Gill ATPase Activity Related to Parr-Smolt Transformation
and Migration n Steelhead Trout (Salmo gairdneri): Influence of Photoperiod and Temperature.
Comp. Biochem. Physiol. 45B:955-965.
Zaugg, W.S. 1981. Relationships between smolt indices and migration in controlled and natural
environments. Salmon and Trout Migratory Behavior Symposium, E.L. Brannon and E.O. Salo,
editors. June, 1981.
164
APPENDIX A
Summary of Bayesian analysis of Hoh and Quillayute wild winter-run steelhead spawner-recruit
analyses from which Figures 21 and 22 were created
Nick Gayeski
Wild Fish Conservancy
June 17, 2022
Spawner and recruit data for each river were provided in the two attached Excel files received
by Wild Fish Conservancy (WFC) from WDFW in May 2022. See Gayeski Bayesian Analysis data
folder provided in submitted materials.
Sheet “Recruits Per Spawner 1” of the file “Quilayutte_Winter_Steelhead_data.xlsx, columns C
and G (rows 6 to 43) contained spawner and recruit data for brood years 1978 to 2015
calculated by WDFW staff and was taken at face value. The corresponding data file used in the
Stan analysis is named “QuilBY78-15.txt”.
The data set for Hoh wild winter run steelhead received by WFC (“Hoh Stlhd broodyear return
by age.xlsx”) did not include recruits calculated from the cohort analysis (worksheet “Cohort
Analysis”) so I calculated maiden recruits from the cohort analysis data (Gayeski 2022). This is
contained in workheet “R and S” of the file “Hoh Stlhd broodyear return by
age_NG_June_2022.xlsx” columns BI and BJ (rows 6 to 32). The corresponding data file used in
the Stan analysis is named “HohBY87-13.txt”.
The text files “Printfit_Hoh steelhead_RickerRW May 9 2022.txt” and “Printfit_Quil
steelhead_RickerRW May 9 2022.txt” contain the summary output of the Bayes analyses
conducted in program Stan (Gayeski 2022a)
The Ricker random walk in alpha model treats the natural logarithm of the Ricker alpha as
undergoing a random walk with the capacity parameter beta treated as a constant (estimated
from the data as part of the fitting of the model). In fitting each of the two data sets, broad
uniform prior distributions were placed on beta (between 1000 and 20000), sigmaw, the
standard error of the annual random walk (between 0 and 2.0), and sigma, the population-and-
time (brood year)-specific process error of the R/S relationship. A normal prior in natural log
space was placed on log alpha (mean = 1.5078, standard deviation = 0.3486), which confines
alpha to the interval [1.8, 11].
Four chains of 10000 replicates each were run, the first 5000 samples of which were treated as
“warmup” and the last 5000 retained as samples from the posterior distribution of the
parameters, for a total of 20,000 posterior samples of each parameter. Both models converged
rapidly. Trace plots of log alpha, beta, sigmaw, and sigma showed coherent sampling of the
parameter posteriors, and Rhat values were all equal to 1.00+ indicating convergence.
