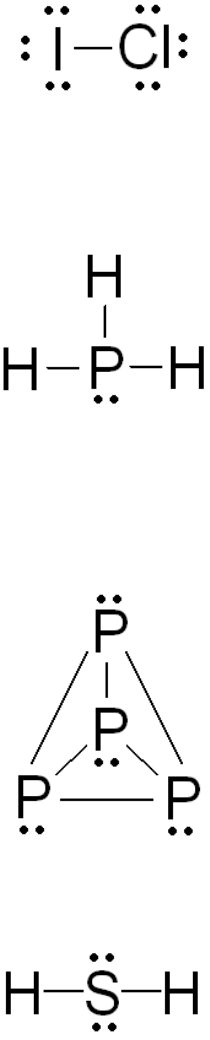
Solution Set 1
9.21 To draw the correct Lewis diagrams, we use the rules on page 272. . .
(a) ICl: Both atoms are halogens (Group 7A) with seven valence electrons, and are inclined
to complete their octet with a single bond. They will bond with each other.
(b) PH
3
: Phosphorus (Group 5A) has five valence electrons, and will complete its octet by
making three bonds. There are three hydrogens, so it makes a single bond with each.
This compounds is the phosphorus-containing analogue to ammonia.
(c) P
4
: Each of the individual phosphorus atoms i s inclined to make three bonds. Each
atom has three neighboring phosphorus atoms, and makes a single bond with each. This
structure is a little tricky to draw on a flat piece of paper, since it makes a sort of
pyramid (a tetrahedron) with the atoms at the points (see page 181).
(d) H
2
S: The sulfur atom (Group 6A) will make two bonds, one with each hydrogen.

(e) N
2
H
4
: Each nitrogen atom (Group 5A) will make three bonds, one single bond with the
other nitrogen and one each with two hydrogens.
(f) HClO
3
: The hydrogen and chlorine atom will each form a single bond with an oxygen
atom, and be terminal. If they form a bond with the same atom, that would be a
complete structure (two bonds for the oxygen), and we would have no place to attach
the other two oxygens. Instead, each must attach to a different oxygen atom and the
remaining oxygen atoms attach to each other in a line of single bonds.
(g) COBr
2
: The carbon atom (Group 4A) will attach with a single bond to each bromine,
and then must double bond to the oxygen.
9.23 To determine what is wrong with these diagrams, we keep in mind the rules on page 272 for
Lewis structures.
(a) A hydrogen, carbon, and nitrogen atom would bring a total of 1+4+5=10 valence elec-
trons to the molecule. The structure given in the book has 3 bonding and 3 lone pairs,
12 valence electrons. That’s too many. We turn a lone pair on the carbon and nitrogen
atom each into another bonding pair, forming a triple bond between the carbon and
nitrogen and reducing us to the correct number of elec trons.

(b) Hydrogen only needs to share one pair of electrons to complete its 1s subshell. These
hydrogens are sharing two pairs. The correct structure would have one bond between
the carbon and hydrogen atoms. Of course, to maintain the carbons atoms’ octets, they
need to have a triple bond between them.
(c) The oxygen atoms each have two lone pairs and share a single bonding pair with tin (Sn).
That’s a total of six electrons, which does not complete the oxygen atoms’ octets. The
tin atom also has an incomplete octet with only two bonds (4 electrons total). Being
a Group 6A element, oxygen typically forms two bonds to complete its octet; being
a Group 4A element, tin typically forms four bonds. We should draw double bonds
between the oxygen atoms and the tin atom instead.
(d) The octet rule for the boron and fluorine atoms appear to be correctly completed in
the original diagram. However, the number of valence electrons is incorrect. The total
number should be 3+3×7 = 24 valence electrons, and the diagram has 26 electrons.
Since we know the fluorine atoms will make a single bond and never make double bonds,
the only solution is to remove the lone pair from the boron atom. This leaves B with
an incomplete octet. The electronegativity difference between boron and fluorine is 2.0,
on the borderline between polar covalent and ionic bonding. We might expect that
boron gives up its three valence electrons to the three fluorine atoms to form an ionic
compound. This, however, is not what really happens. Boron does in fact form polar
covalent bonds with the fluorine atoms, and has an incomplete octet. See page 278,
Section 9.7, for more information about this odd molecule.

(e) The fluorine atom has three lone pairs of e lectrons and is sharing two pairs of electrons
in a double bond. This is a total of ten electrons, which overcompletes the octet. There
should only be a single bond between oxygen and fluorine. The extra bond pair shown
should be a lone pair on the oxygen instead.
(f) The oxygen atom has two lone pairs and is sharing a single pair of electrons in a bond.
This is only six electrons, and does not complete the octet. It needs to form a double
bond with the carbon. The lone pair shown on the carbon becomes the extra bond pair
instead.
(g) The nitrogen atom is sharing three pairs of electrons. This is only six electrons, and does
not complete the atom’s octet. In fact, the molecule should have 5+3×7 = 26 valence
electrons, and the original structure has only 24. We need to add a lone pair to the
nitrogen atom.

9.29 (a) HCO
−
2
: The total number of valence electrons in this molecule should be 1+4+2×6+1=18.
(b) CH
2
NO
−
2
: The total numb er of valence electrons in this molecule should be 4 + 2×1 +
5 + 2×6 + 1 = 24.
9.31 The total number of valence electrons in this molecule should be 1 + 3×5 = 16.

9.33 The total number of valence electrons in this molecule should be 6 + 4 + 5 + 1 = 16.
