
Clinical Practice Guidelines
Treatment of Acute Hyperkalaemia in Adults
Authors:
Dr Annette Alfonzo
Consultant Nephrologist & Clinical Director, Faculty member of European Resuscitation Council,
Victoria Hospital Kirkcaldy, Fife
Dr Alexander Harrison
Consultant in Nephrology and Intensive Care,
Brighton and Sussex University Hospitals NHS Trust
Dr Richard Baines
Consultant Nephrologist and Head of Renal and Transplant Service,
University Hospitals of Leicester NHS Trust
Dr Ann Chu
Academic Clinical Fellow Renal Medicine and Medical Education, Imperial College London
Mr Simon Mann
Renal Pharmacist, Lancashire Teaching Hospitals NHS Foundation Trust
Mr Murdoch MacRury
Patient representative
Final version: June 2020
Review date: June 2025

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 2
Endorsements
The National Institute for Health and Care Excellence (NICE) has accredited the process used by the Renal
Association to produce its Clinical Practice Guidelines. Accreditation is valid for 5 years from January 2017.
More information on accreditation can be viewed at www.nice.org.uk/accreditation
Method used to arrive at a recommendation
The recommendations for the first draft of this guideline resulted from a collective decision reached by
informal discussion by the authors and, whenever necessary, with input from the Chair of the Clinical
Practice Guidelines Committee. If no agreement had been reached on the appropriate grading of a
recommendation, a vote would have been held and the majority opinion carried. However this was not
necessary for this guideline.
Conflicts of Interest Statement
All authors made declarations of interest in line with the policy in the Renal Association Clinical Practice
Guidelines Development Manual. Further details can be obtained on request from the Renal Association.
Acknowledgements
The authors wish to thank Dr Charlie Tomson, retired Consultant Nephrologist and former Chair of the UK
Renal Association (2010-2012), Professor Ketan Dhatariya, Chair of the Joint British Diabetes Society for In-
Patient Care (JBDS-IP), Dr Clare Crowley, Consultant Pharmacist (Medicines Saftety) at Oxford University
Hospitals and Mr Murdoch MacRury for his insight into the treatment of hyperkalaemia from a patient’s
perspective. We also wish to acknowledge the contributions of the original writing group whose
collaborative efforts produced the 2014 Hyperkalaemia guideline.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 3
Contents
Executive Summary of changes since 2014 Hyperkalaemia Guideline ................................................................5
Guideline development ........................................................................................................................................7
Introduction ..........................................................................................................................................................8
Summary of Clinical Practice Guidelines for Hyperkalaemia ............................................................................ 10
Tables ................................................................................................................................................................. 17
Figures ............................................................................................................................................................... 18
Summary of Audit Measures ............................................................................................................................. 19
Hyperkalaemia in the Community (Guidelines 1.1 – 12.1) ................................................................................ 22
Introduction ................................................................................................................................................... 22
Patient monitoring (Guidelines 1.1-1.2) ....................................................................................................... 26
Management of patients receiving RAASi drugs (Guidelines 2.1-2.7).......................................................... 30
Threshold for treatment of hyperkalaemia (Guideline 3.1) ......................................................................... 33
Indications for hospital assessment (Guidelines 4.1 - 4.2) ........................................................................... 35
Treatment: Dietary interventions (Guideline 5.1) ....................................................................................... 37
Treatment: Sodium bicarbonate (Guideline 6.1) ......................................................................................... 39
Treatment: Diuretics (Guideline 7.1) ........................................................................................................... 41
Treatment: Calcium resonium (Guideline 8.1) ............................................................................................ 42
Treatment: Patiromer (Guidelines 9.1-9.3) ................................................................................................. 44
Treatment: Sodium zirconium cyclosilicate (Guidelines 10.1-10.3) ............................................................ 49
Prevention (Guidelines 11.1-11.3) ................................................................................................................ 53
Treatment Algorithm: Community (Guideline 12.1) .................................................................................... 57
Hyperkalaemia in Hospital ................................................................................................................................. 59
Introduction ................................................................................................................................................... 59
Clinical assessment (Guidelines 13.1-13.2) ................................................................................................... 61
ECG and cardiac monitoring (Guidelines 14.1-14.2) ..................................................................................... 63
Laboratory analysis (Guidelines 15.1-15.3) ................................................................................................... 68
Treatment (Guidelines 16.1-16.6) ................................................................................................................. 73
Blood monitoring (Guidelines 17.1-17.2) .................................................................................................... 101
Treatment of hyperkalaemia in haemodialysis patients (Guidelines 18.1-18.4) ........................................ 105
Referral to specialist services and escalation of care (Guidelines 19.1-19.6) ............................................. 110
Minimum standards for patient transfer (Guidelines 20.1-20.2) ................................................................ 112
Prevention (Guidelines 21.1-21.4) ............................................................................................................... 114

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 4
Treatment Algorithm: Hospital (Guidelines 22.1) ...................................................................................... 118
Hyperkalaemia in Resuscitation ...................................................................................................................... 120
Introduction ................................................................................................................................................. 120
References ................................................................................................................................................... 121
Hyperkalaemic cardiac arrest – Special circumstance (Guidelines 23.1-23.2) ............................................ 121
Audit Measure ............................................................................................................................................. 121
Rationale (Guidelines 23.1) ......................................................................................................................... 121
References ................................................................................................................................................... 122
Resuscitation strategy in dialysis patients (Guidelines 24.1-24.2) ............................................................. 123
Treatment: Calcium chloride (Guidelines 25.1) .......................................................................................... 128
Treatment: Insulin-glucose (Guidelines 25.2)............................................................................................. 129
Treatment: Sodium bicarbonate (Guidelines 25.3) .................................................................................... 132
Treatment: Initiation of dialysis during cardiac arrest (Guidelines 25.4) ................................................... 133
Prevention (Guidelines 26.1-26.2) ............................................................................................................... 140
Lay summary .................................................................................................................................................... 143
Appendices ...................................................................................................................................................... 144
Appendix 1: Oral potassium lowering drugs. .............................................................................................. 145
Appendix 2: Summary of clinical trials of oral potassium lowering drugs ................................................. 146
Appendix 3A: Drug administration and safety - IV calcium preparations .................................................. 147
Appendix 3B: Drug administration and safety –Insulin-glucose infusion .................................................... 149
Appendix 3C: Drug administration and safety - Salbutamol ....................................................................... 151
Appendix 3D: Drug administration and safety – Patiromer ........................................................................ 152
Appendix 3E: Drug administration and safety – Sodium zirconium cyclosilicate........................................ 153
Appendix 3F: Drug administration and safety – Calcium resonium ............................................................ 154
Appendix 4 – Sine wave ECG ....................................................................................................................... 155
Appendix 5 – Hyperkalaemia Algorithm - Community ................................................................................ 156
Appendix 6 – Hyperkalaemia Algorithm – Hospital ..................................................................................... 157
Appendix 7 – Hyperkalaemia Algorithm – Resuscitation ............................................................................ 158
Abbreviations................................................................................................................................................... 159

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 5
Executive Summary of changes since 2014 Hyperkalaemia Guideline
Format of the Guideline
The 2020 Hyperkalaemia guideline has been divided into 3 sections to allow easy navigation:
• Section I: Management of Hyperkalaemia in the Community
• Section II: Management of Hyperkalaemia in Hospital
• Section III: Management of Hyperkalaemia in Resuscitation
New therapies for treating hyperkalaemia
Sodium zirconium cyclosilicate (SZC)
SZC is an oral potassium binder approved by NICE in September 2019 for the following indications:
• life-threatening hyperkalaemia (K
+
≥ 6.5 mmol/l) alongside standard treatment with insulin-glucose
and salbutamol.
• confirmed serum K
+
≥ 6.0 mmol/l in out-patients with CKD Stage 3b-5 (not on dialysis) or heart
failure receiving a sub-optimal dose of RAASi therapy.
Patiromer
Patiromer is an oral potassium binder approved by NICE in February 2020 for the following indications:
• life-threatening hyperkalaemia (K
+
≥ 6.5 mmol/l) alongside standard treatment with insulin-glucose
and salbutamol.
• confirmed serum K
+
≥ 6.0 mmol/l in out-patients with CKD Stage 3b-5 (not on dialysis) or heart
failure receiving a sub-optimal dose of RAASi therapy or who are not taking RAASi because of
hyperkalaemia.
NICE has recommended that therapy with either SZC or Patiromer is started in secondary care and
discontinued if RAASi therapy is stopped.
New recommendations for therapies in treating hyperkalaemia
Insulin-glucose infusion
The 2014 Hyperkalaemia Guideline recommended the use of 10 units of soluble insulin with 25g glucose. In
recent years, there have been multiple published reports of a high incidence of iatrogenic hypoglycaemia.
This has prompted review of this treatment regimen.
The most consistent risk factor for iatrogenic hypoglycaemia is a low pre-treatment blood glucose. Reducing
the dose of insulin alone did not consistently reduce hypoglycaemic episodes. There is more evidence to
support increasing the total glucose load to 50g. The lowest risk of severe hypoglycaemia was associated
with continuous delivery of glucose.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 6
Preserving efficacy is essential in treating hyperkalaemia. There is some evidence to suggest that
conventional dose insulin (10 units) has a greater K
+
-lowering effect than low dose insulin. Analysis of
studies using 10 units insulin also show a trend towards greater efficacy with worsening hyperkalaemia.
These observations require confirmation before a reduction in insulin dose can be recommended.
The 2020 Hyperkalaemia Guideline for moderate or severe hyperkalaemia recommends:
• Give 10 units soluble insulin with 25g glucose
• Give 10% glucose by infusion @ 50ml/hr for 5 hours (25g) to patients with a pre-treatment blood
glucose < 7.0 mmol/l to prevent hypoglycaemia.
• Blood glucose monitoring is required for up to 12 hours after glucose-insulin infusion.
Hyperkalaemic Cardiac Arrest
This is the most serious consequence of hyperkalaemia and yet the most effective treatment, dialysis, is
rarely used. The largest study of hyperkalaemic cardiac arrest demonstrated that survival in patients with
extreme hyperkalaemia (K > 9.0 mmol/l) treated without dialysis was very poor. Over the past three
decades, successful outcomes with all dialysis modalities have been reported during CPR. ECMO enhances
neurological outcome. This approach will be guided by the suitability of the patient and availability of clinical
expertise and equipment.
The 2020 Hyperkalaemia Guideline suggests that dialysis is considered for refractory hyperkalaemic cardiac
arrest and provides a protocol for the initiation of dialysis during resuscitation.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 7
Guideline development
Purpose
This guideline provides an updated version of the original Hyperkalaemia guideline (2014). The main aims
are to provide evidence-based recommendations for the treatment of chronic hyperkalaemia in the
community, acute hyperkalaemia in the hospital setting and to reduce the risk of complications associated
with hyperkalaemia itself and its treatment.
Scope
The original RA Hyperkalaemia Guideline (2014) focussed predominantly the management of hyperkalaemia
in secondary care. The current RA Hyperkalaemia Guideline (2020) provides a comprehensive overview of
the detection and treatment of hyperkalaemia in the community and hospital settings.
Review of Evidence
The literature was reviewed using a multiple database search - PubMed (1960-2020), Ovid MEDLINE (1946-
2020), EMBASE (1974-2020), Science Direct (1995-2020), The Cochrane Library (1995-2020), Web of
Knowledge (2001-2020) for all human studies published in English pertaining to the treatment of
hyperkalaemia in adults. Websites searches included National Institute for Health and Care Excellence
(NICE), Scottish Medicines Consortium (SMC), Healthcare Improvement Scotland, Medicines and Healthcare
products Regulatory Agency (MHRA) and European Medicines Agency (EMA).
The keywords used for literature search were – hyperkalaemia, potassium, treatment,
pseudohyperkalaemia, spurious hyperkalaemia, ECG, point or care, near patient testing, insulin,
hypoglycaemia, salbutamol, calcium, bicarbonate, diet, resonium, patiromer, sodium zirconium cyclosilicate,
dialysis, arrhythmias, resuscitation, and cardiac arrest.
The writing process followed the Renal Association Guideline development manual. The guideline comprises
of a series of guideline statements accompanied by supporting evidence and audit measures. The
recommendations in each guideline statement have been graded using the GRADE system
(www.gradeworkinggroup.org) in evaluating the strength of each recommendation (1 = strong, 2 = weak)
and quality of evidence (A= high, B = moderate, C= low, D = very low). Each guideline statement begins with
a recommendation (Grade 1 evidence) or a suggestion (Grade 2 evidence).

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 8
Introduction
There is no universally accepted definition of hyperkalaemia. This guideline has adopted the European
Resuscitation Council (ERC) Guideline definition with a threshold serum potassium (K+) level of ≥ 5.5
mmol/l, established in 2005
1
and maintained to current date.
2, 3
It is further classified by severity into mild
(5.5-5.9 mmol/l), moderate (6.0-6.4 mmol/l) or severe (≥ 6.5 mmol/l). Hyperkalaemia is a common medical
emergency when it presents acutely. The presence of persistent hyperkalaemia in the community is often
regarded as chronic, usually in the context of drugs that exacerbate the condition.
The incidence of hyperkalaemia in hospital patients ranges from 1.1% and 10%.
4-8
The incidence in the
community varies dependent on the case mix of the population studied. Studies in the general population
report an incidence of hyperkalaemia (K+ >5.5 mmol/l) ranging from 2.3 - 7.2% in patients with an eGFR > 60
ml/min
9, 10
and 2.9 - 40% in patients with an eGFR < 30 ml/min.
11-13
In-hospital mortality is significantly higher in patients with hyperkalaemia (18.1%) compared to those with
hypokalaemia (5.0%) or normokalaemia (3.9%).
8
A U-shaped association between serum potassium and
mortality has been shown in patients with ischaemic heart disease,
14
CKD
11, 15, 16
and in patients receiving
longterm haemodialysis (HD).
17
Patients with severe hyperkalaemia (K+ > 6.5 mmol/l) are most at risk and in
one report, the hospital mortality was 30.7%.
18
The treatment of hyperkalaemia is likely to evolve in the coming years with the availability of novel drugs
and the development of new strategies to improve safety. Clinical decisions on when to treat and how
aggressively to treat require a patient centred approach guided by the clinical setting and rate of change in
serum K+ level. Patients with moderate levels of hyperkalaemia pose the greatest dilemma, especially when
acuity is low, but warrant intervention to avoid deterioration. Severe hyperkalaemia risks arrhythmias and
cardiac arrest, therefore prompt recognition and intervention is required.
References
1. Soar, J., et al., European Resuscitation Council Guidelines for Resuscitation 2005 - Section 7. Cardiac
arrest in special circumstances. Resuscitation, 2005. 67: p. S135-S170.
2. Soar, J., et al., European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac
arrest in special circumstances: Electrolyte abnormalities, poisoning, drowning, accidental
hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution.
Resuscitation, 2010. 81(10): p. 1400-33.
3. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
4. Acker, C.G., et al., Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results
of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med,
1998. 158(8): p. 917-24.
5. Paice, B., et al., Hyperkalaemia in patients in hospital. Br Med J (Clin Res Ed), 1983. 286(6372): p.
1189-92.
6. Moore, M.L. and R.R. Bailey, Hyperkalaemia in patients in hospital. N Z Med J, 1989. 102(878): p.
557-8.
7. Einhorn, L.M., et al., The frequency of hyperkalemia and its significance in chronic kidney disease.
Arch Intern Med, 2009. 169(12): p. 1156-62.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 9
8. Conway, R., et al., Serum potassium levels as an outcome determinant in acute medical admissions.
Clin Med (Lond), 2015. 15(3): p. 239-43.
9. Chang, A.R., et al., Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large
Health System. Hypertension, 2016. 67(6): p. 1181-8.
10. Horne, L., et al., Epidemiology and health outcomes associated with hyperkalemia in a primary care
setting in England. BMC Nephrol, 2019. 20(1): p. 85.
11. Korgaonkar, S., et al., Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study.
Clin J Am Soc Nephrol, 2010. 5(5): p. 762-9.
12. Sarafidis, P.A., et al., Prevalence and factors associated with hyperkalemia in predialysis patients
followed in a low-clearance clinic. Clin J Am Soc Nephrol, 2012. 7(8): p. 1234-41.
13. Turgutalp, K., et al., Community-acquired hyperkalemia in elderly patients: risk factors and clinical
outcomes. Ren Fail, 2016. 38(9): p. 1405-1412.
14. Goyal, A., et al., Serum potassium levels and mortality in acute myocardial infarction. JAMA, 2012.
307(2): p. 157-64.
15. Hayes, J., et al., Association of hypo- and hyperkalemia with disease progression and mortality in
males with chronic kidney disease: the role of race. Nephron Clin Pract, 2012. 120(1): p. c8-16.
16. Collins, A.J., et al., Association of Serum Potassium with All-Cause Mortality in Patients with and
without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am J Nephrol, 2017. 46(3): p. 213-
221.
17. Kovesdy, C.P., et al., Serum and dialysate potassium concentrations and survival in hemodialysis
patients. Clin J Am Soc Nephrol, 2007. 2(5): p. 999-1007.
18. An, J.N., et al., Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care, 2012.
16(6): p. R225.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 10
Summary of Clinical Practice Guidelines for Hyperkalaemia
Section I: Community
Guideline 1.1 – Monitoring of patients at risk of Hyperkalaemia in the community.
We recommend that patients known to have CKD, heart failure and/or diabetes, who are at risk of
hyperkalaemia, undergo regular blood monitoring at a frequency (2-4 times per year) dependent on level of
renal function and degree of proteinuria. (1B)
Guideline 1.2.1 – Monitoring of patients after an episode of mild hyperkalaemia detected in the
community.
We recommend that the serum K+ is repeated within 3 days, or as soon as feasible, if an episode of mild
hyperkalaemia (K+ 5.5 – 5.9 mmol/l) is detected unexpectedly in the community. (1C)
Guideline 1.2.2 – Monitoring of patients after an episode of moderate hyperkalaemia detected in the
community.
We recommend that the serum K+ is repeated within 1 day of an episode of moderate hyperkalaemia (K+ 6.0
– 6.4 mmol/l) when detected in the community. (1C)
Guideline 1.2.3 – Monitoring of patients after an episode of severe hyperkalaemia detected in the
community.
We recommend that patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/l) detected in the community are
admitted for immediate assessment and treatment. (1B)
Guideline 2.1 – Assessment of patients prior to initiation of ACE-I or ARB.
We recommend that urea and electrolytes should be assessed prior to initiation of ACE-I or ARB and these
drugs should be used with caution if the serum K+ is > 5.0 mmol. (1A)
Guideline 2.2 – Assessment of patients prior to initiation of Mineralocorticoid Receptor Antagonists
(MRA).
We suggest that initiation of MRAs should be avoided in patients with a baseline serum K+ > 5.0mmol/l or
eGFR < 30 ml/min. (1B)
Guideline 2.3 – Monitoring of patients after initiation of ACE-I and ARB.
We recommend that urea and electrolytes should be assessed at 1 – 2 weeks after initiation of ACE-I or ARB
and after every dose titration. (1A)
Guideline 2.4 – Monitoring of patients after initiation of MRAs.
We recommend that urea and electrolytes should be assessed at 1 week after initiation of MRA or after dose
up-titration, then monthly for the first 3 months, 3-monthly for the first year and 4-monthly thereafter. (1A)
Guideline 2.5 – Management of hyperkalaemia in patients treated with RAASi drugs.
We suggest increased frequency of monitoring in patients with a serum K+ between 5.5-5.9 mmol/l and
consideration of dose reduction of RAASi drugs (ACE-I, ARB, MRA). (1B)
Guideline 2.6 – Management of hyperkalaemia in patients treated with RAASi drugs during acute illness.
We recommend that RAASi drugs be withheld during acute intercurrent illness (e.g. sepsis, hypovolaemia
and/or AKI) at all severities of hyperkalaemia. (1D)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 11
Guideline 2.7 – Cessation of RAASi drugs in patients with moderate or severe hyperkalaemia.
We recommend cessation of RAASi drugs in patients with serum K ≥ 6 mmol/l who do not meet the criteria
for treatment with patiromer or sodium zirconium cyclosilicate. (1B)
Guideline 3.1 – Threshold for treating Hyperkalaemia in the community.
We recommend that interventions to lower serum potassium be instituted in patients with a serum K+ ≥ 5.5
mmol/l. (1B)
Guideline 4.1 – Indication for assessment in hospital for patients with severe hyperkalaemia detected in
the community.
We recommend urgent hospital assessment for all patients with severe hyperkalaemia (serum K+ ≥ 6.5
mmol/l) detected in the community. (1A)
Guideline 4.2 – Indication for assessment in hospital for patients with mild-moderate hyperkalaemia
detected in the community.
We suggest hospital assessment for acutely unwell patients with mild (serum K+ 5.5 – 5.9 mmol/l) or
moderate hyperkalaemia (serum K+ 6.0 - 6.4 mmol/l), particularly in the presence of an acute kidney injury.
(1B)
Guideline 5.1 – Dietary Intervention for managing Hyperkalaemia in the community.
We recommend that a low potassium diet is instituted for patients with persistent hyperkalaemia with a
serum K+ > 5.5 mmol/l. (1B)
Guideline 6.1 – Sodium bicarbonate for management of Hyperkalaemia in the community
We recommend that sodium bicarbonate is used in CKD patients with a serum bicarbonate level < 22 mmol/l
with or without hyperkalaemia. (1B)
Guideline 7.1 – Use of diuretics for managing Hyperkalaemia in the community
We suggest that loop diuretics may be a useful adjunct for the treatment of chronic hyperkalaemia in
patients who are non-oliguric and volume replete. (2C)
Guideline 8.1 – Calcium resonium for the management of Hyperkalaemia in the community.
We suggest that calcium resonium may be used as a short-term measure to lower serum potassium to a
level of ≤ 5 mmol/l in patients with mild to moderate hyperkalaemia. (2C)
Guideline 9.1 – Patiromer for the management of Hyperkalaemia in the community
We recommend that Patiromer is an option in the management of persistent hyperkalaemia with a
confirmed serum K+ ≥ 6.0 mmol/l in out-patients with CKD Stage 3b-5 (not on dialysis) or heart failure
receiving a sub-optimal dose or not receiving RAASi therapy due to hyperkalaemia. (1A)
Guideline 9.2 – Patiromer for the management of Hyperkalaemia
We recommend that treatment with Patiromer is discontinued if RAASi therapy is stopped. (1A)
Guideline 9.3 – Patiromer for the management of Hyperkalaemia
We recommend that Patiromer is initiated in secondary care only. (1A)
Guideline 10.1 – Sodium Zirconium Cyclosilicate for the management of Hyperkalaemia
We recommend that Sodium Zirconium Cyclosilicate (SZC) is an option in out-patients for the management
of persistent hyperkalaemia with a confirmed serum K+ ≥ 6.0 mmol/l in patients with CKD Stage 3b-5 (not on
dialysis) or heart failure receiving a sub-optimal dose of RAASi therapy. (1A)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 12
Guideline 10.2 – Sodium Zirconium Cyclosilicate for the management of Hyperkalaemia
We recommend that treatment with Sodium Zirconium Cyclosilicate (SZC) in out-patients is discontinued if
RAASi therapy is stopped. (1A)
Guideline 10.3 – Sodium Zirconium Cyclosilicate for the management of Hyperkalaemia
We recommend that Sodium Zirconium Cyclosilicate (SZC) is initiated in secondary care only. (1A)
Guideline 11.1 – Prevention of Hyperkalaemia in the community: monitoring
We recommend monitoring of renal function in patients at risk of hyperkalaemia with known CKD, heart
failure, diabetes and in any patient taking RAASi medication. (1A)
Guideline 11.2 – Prevention of Hyperkalaemia in the community: prescribing
We recommend caution in prescribing trimethoprim to patients with renal impairment or those taking RAASi
drugs. (1A)
Guideline 11.3 – Prevention of Hyperkalaemia in the community: sick day rules
We recommend that healthcare professionals provide advice to patients regarding the risks of AKI and
hyperkalaemia during acute illness and measures to avoid these complications. (1B)
Guideline 12.1 – Treatment Algorithm for Hyperkalaemia in the community
We recommend that the treatment of hyperkalaemia in patients in the community and out-patient setting is
guided by its severity and clinical condition of the patient as summarised in the treatment algorithm. (1B)
Section II: Hospital
Guideline 13.1 – Hyperkalaemia: Clinical Assessment; History and examination
We recommend that all patients presenting with hyperkalaemia undergo a comprehensive medical and drug
history and clinical examination to determine the cause of hyperkalaemia. (1B)
Guideline 13.2 – Hyperkalaemia: Clinical Assessment; NEWS
We recommend that all patients with known or suspected hyperkalaemia undergo urgent clinical assessment
using an early warning scoring system to assess level of acuity. (1C)
Guideline 14.1 – Hyperkalaemia: ECG
We recommend that all hospitalised patients with a serum K + level ≥ 6.0 mmol/L have an urgent 12-lead
ECG (electrocardiogram) performed and assessed for changes of hyperkalaemia. (1B)
Guideline 14.2 – Hyperkalaemia: Cardiac monitoring
We recommend a minimum of continuous 3-lead ECG monitoring for all patients with a serum K+ ≥ 6.5
mmol/L, patients with features of hyperkalaemia on 12-lead ECG, and in patients with a serum K+ 6.0-6.4
mmol/L who are clinically unwell or in whom a rapid rise in serum K+ is anticipated, ideally in a higher-
dependency setting. (1C)
Guideline 15.1 – Hyperkalaemia: Laboratory tests
We recommend that a lithium heparin anti-coagulated specimen is the sample type of choice when rapid
turnaround of urea and electrolytes results is required. (1B)
Guideline 15.2 – Hyperkalaemia: Blood gas analysis
We recommend that in emergencies, K+ level is measured from an arterial or venous blood sample using a
point-of-care blood gas analyser whilst awaiting the results from a formal laboratory measurement. (1B)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 13
Guideline 15.3 – Hyperkalaemia: Pseudo-hyperkalaemia
We recommend that urea and electrolytes are measured using paired lithium heparin and clotted serum
samples from a large vein using gentle traction with prompt laboratory analysis if pseudo-hyperkalaemia is
suspected. (1A)
Guideline 16.1 – Hyperkalaemia: Summary of treatment strategy
We recommend that the treatment of hyperkalaemia in hospital follow a logical 5-step approach. (1B)
Guideline 16.2 – Hyperkalaemia: STEP 1 - Protect the heart; intravenous calcium salts
We recommend that intravenous calcium chloride or calcium gluconate, at an equivalent dose (6.8mmol), is
given to patients with hyperkalaemia in the presence of ECG evidence of hyperkalaemia. (1C)
Guideline 16.3.1 – Hyperkalaemia: STEP 2 - Shift K+ into cells; insulin-glucose infusion
We recommend that insulin-glucose (10 units soluble insulin in 25g glucose) by intravenous infusion is used
to treat severe hyperkalaemia (K+ ≥ 6.5 mmol/l). (1B)
Guideline 16.3.2 – Hyperkalaemia: STEP 2 - Shift K+ into cells; insulin-glucose infusion
We suggest that insulin-glucose (10 units soluble insulin in 25g glucose) by intravenous infusion is used to
treat moderate hyperkalaemia (K+ 6.0 – 6.4 mmol/l). (2C)
Guideline 16.3.3 – Hyperkalaemia: STEP 2 - Shift K+ into cells; avoiding hypoglycaemia
We suggest pre-emptive initiation of an infusion of 10% glucose at 50ml/ hour for 5 hours (25g) following
insulin-glucose treatment in patients with a pre-treatment blood glucose < 7.0 mmol/l to avoid
hypoglycaemia (target blood glucose 4-7 mmol/l). (2D)
Guideline 16.4.1 – Hyperkalaemia: STEP 2 – Shift K+ into cells; Salbutamol
We recommend nebulised salbutamol 10-20 mg is used as adjuvant therapy for severe (K+ ≥ 6.5 mmol/L)
hyperkalaemia. (1B)
Guideline 16.4.2 – Hyperkalaemia: STEP 2 – Shift K+ into cells; Salbutamol
We suggest that nebulised salbutamol 10-20 mg may be used as adjuvant therapy for moderate (K+ 6.0-6.4
mmol/L) hyperkalaemia. (2C)
Guideline 16.4.3 – Hyperkalaemia: STEP 2 – Shift K+ into cells; Salbutamol
We recommend that salbutamol is not used as monotherapy in the treatment of severe hyperkalaemia. (1A)
Guideline 16.5 Hyperkalaemia: STEP2 –Shift K into cells; Sodium bicarbonate
We suggest that intravenous sodium bicarbonate infusion is not used routinely for the acute treatment of
hyperkalaemia. (2C)
Guideline 16.6.1 – Hyperkalaemia: STEP 3 – Remove K+ from body; Potassium binders
We recommend that Sodium Zirconium Cyclosilicate is used as an option in the emergency management of
acute life-threatening hyperkalaemia (serum K+ ≥ 6.5 mmol/l). (1B)
Guideline 16.6.2 – Hyperkalaemia: STEP 3 – Remove K+ from body; Potassium binders
We suggest that Patiromer is an option for the emergency management of acute life-threatening
hyperkalaemia (serum K+ ≥ 6.5 mmol/l). (1C)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 14
Guideline 16.6.3 – Hyperkalaemia: STEP 3 – Remove K+ from body; Cation-exchange resin
We suggest that calcium resonium is not used in the emergency management of severe hyperkalaemia, but
may be considered in patients with moderate hyperkalaemia. (2B)
Guideline 17.1.1 – Hyperkalaemia: STEP 4 - Blood monitoring; serum potassium
We recommend that the serum K+ is monitored closely in all patients with hyperkalaemia to assess efficacy
of treatment and to monitor for rebound hyperkalaemia after the initial response to treatment wanes. (1B)
Guideline 17.1.2 – Hyperkalaemia: STEP 4 - Blood monitoring; serum potassium
We suggest that serum K+ is assessed at least 1, 2, 4, 6 and 24 hours after identification and treatment of
moderate or severe hyperkalaemia. (2C)
Guideline 17.2 – Hyperkalaemia: STEP 4 - Blood monitoring; blood glucose
We recommend that the blood glucose concentration is monitored at regular intervals (0, 15, 30, 60, 90, 120,
180, 240, 360, 480 and 720 minutes) up to 12 hours after administration of insulin-glucose infusion in all
patients with hyperkalaemia. (1C)
Guideline 18.1 - Hyperkalaemia: Treatment in haemodialysis patients
We recommend that haemodialysis patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) receive
dialysis treatment urgently. (1A)
Guideline 18.2 - Hyperkalaemia: Treatment in haemodialysis patients
We recommend that haemodialysis patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) and toxic
ECG changes be treated with intravenous calcium salt to reduce risk of arrhythmias even when dialysis is
immediately available. (1C)
Guideline 18.3 - Hyperkalaemia: Treatment in haemodialysis patients
We recommend that haemodialysis patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) be treated
with standard medical therapies to lower serum potassium if dialysis is not immediately available. (1B)
Guideline 18.4 - Hyperkalaemia: Treatment in haemodialysis patients
We suggest that potassium binders may be considered to reduce the risk of hyperkalaemia during the inter-
dialytic period. (1B)
Guideline 19.1 - Hyperkalaemia: Specialist Referral
We suggest that patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) be referred to their local renal
or critical care team for an urgent opinion, guided by the clinical scenario and its persistence after initial
medical treatment. (2C)
Guideline 19.2 - Hyperkalaemia: Referral to critical care services
We recommend that for patients with severe hyperkalaemia, and where there is no provision of renal
services on site, referral is made to the local critical care team in the first instance, guided by the clinical
scenario and established local policies. (1C)
Guideline 19.3 - Hyperkalaemia: Escalation of care
We recommend that patients are referred to the critical care team by a senior member of the referring team
if escalation of care is required from the outset or if the patient fails to respond to initial treatment. (1B)
Guideline 19.4 - Hyperkalaemia: Treatment facilities - Critical care
We recommend that patients with severe hyperkalaemia and problems with airway, breathing, circulation
and/or conscious level, be referred to the local critical care team in the first instance. (1C)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 15
Guideline 19.5 – Hyperkalaemia: Treatment facilities – Ward, Enhanced Care or Critical Care area
We recommend that stable patients with severe hyperkalaemia be admitted to an area with facilities for
continuous cardiac monitoring which are sufficiently staffed to support clinical monitoring and treatment,
including an acute medical unit, renal unit, coronary care unit, enhanced care area, or critical care unit (HDU
or ICU) depending on local facilities or practice. (1C)
Guideline 19.6 – Hyperkalaemia: RRT in treatment of hyperkalaemia in acutely unwell patients.
We recommend that the decision on timing, suitability and modality for initiation of RRT in patients with life-
threatening hyperkalaemia, either from the outset or resistant to initial medical therapy, is taken urgently by
a nephrologist or critical care specialist. (1C)
Guideline 20.1 - Hyperkalaemia: Transfer to renal services
We suggest that transfer to renal services be considered in clinically stable patients in whom hyperkalaemia
cannot be controlled (i.e. serum K+ < 6.5 mmol/L) using medical measures, particularly in the presence of
advanced or oliguric renal failure (either AKI or CKD). (2C)
Guideline 20.2 - Hyperkalaemia: Minimum standards for safe patient transfer
We suggest that any inter- or intra-hospital patient transfer is coordinated by senior clinicians and follows
national guidelines. (2B)
Guideline 21.1 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend that the need for prescribed medication which can cause hyperkalaemia are reviewed in the
context of the current illness and level of renal function both on and during hospital admission. (1B)
Guideline 21.2 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend a low potassium diet for hospitalised patients with moderate or severe hyperkalaemia. (1C)
Guideline 21.3 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend that community blood monitoring is arranged on discharge for all patients who have
required treatment for hyperkalaemia during hospital admission. (1B)
Guideline 21.4 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend that the risk of recurrence of hyperkalaemia is considered before reinstating previous
medication that may have contributed to the episode. (1B)
Guideline 22.1 – Hyperkalaemia; Algorithm in Hospital
We recommend that hyperkalaemia in hospitalised patients is managed using the treatment algorithm which
provides guidance on the medical therapies and the need for initiation of renal replacement therapy. (1B)
Section III: Resuscitation
Guideline 23.1 – Hyperkalaemia; Cardiac Arrest - special circumstance
We recommend that hyperkalaemia is considered in all patients who have a cardiac arrest, as part of
identifying and treating a reversible cause using the 4 Hs and 4 Ts approach. (1A)
Guideline 24.1 – Hyperkalaemia; Cardiac Arrest – Resuscitation strategy in haemodialysis patients
We recommend that standard ALS practice in cardiac arrest be applied to patients requiring dialysis. (1A)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 16
Guideline 24.2 – Hyperkalaemia; Cardiac Arrest – Defibrillation practice in haemodialysis patients
We recommend disconnection from dialysis equipment prior to defibrillation unless the dialysis machine is
defibrillator-proof. (1C)
Guideline 25.1 – Cardiac Arrest: Treatment - Intravenous calcium
We recommend that intravenous calcium chloride is administered if hyperkalaemia is known or suspected to
be the cause of cardiac arrest. (1C)
Guideline 25.2.1 – Cardiac Arrest: Treatment – Insulin-glucose
We recommend that 10 units soluble insulin and 25g glucose is administered if hyperkalaemia is known or
suspected to be the cause of cardiac arrest. (1B)
Guideline 25.2.2 – Cardiac Arrest: Treatment – Insulin-glucose
We suggest 10% glucose infusion be initiated if the blood glucose is < 7.0 mmol/l at the time of cardiac
arrest. (2C)
Guideline 25.3 – Hyperkalaemia; Cardiac Arrest – Sodium bicarbonate
We suggest that sodium bicarbonate is administered if hyperkalaemia is known or suspected to be the cause
of cardiac arrest. (2C)
Guideline 25.4 – Hyperkalaemia; Cardiac Arrest – Initiation of dialysis during CPR
We suggest that renal replacement therapy with ongoing CPR may be considered for hyperkalaemic cardiac
arrest, if hyperkalaemia is resistant to medical therapy and appropriate staff and facilities are available. (2C)
Guideline 26.1 – Hyperkalaemia; Prevention of Cardiac Arrest in Hyperkalaemia
We recommend that hyperkalaemia is treated urgently in patients with severe hyperkalaemia (K+ ≥ 6.5
mmol/l) and in those with ECG changes suggestive of severe hyperkalaemia. (1C)
Guideline 26.2 – Hyperkalaemia; Prevention of Cardiac Arrest in Hyperkalaemia
We recommend continuous cardiac monitoring for patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/l) in a
setting appropriate for the level of care required. (1C)
Guideline 26.1 – Hyperkalaemia; Algorithm in Cardiac Arrest
We recommend that cardiac arrest attributable to hyperkalaemia is managed using the treatment algorithm
which provides guidance on the medical therapies and the need for initiation of renal replacement therapy
during CPR. (1C)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 17
Tables
Table 1: Risk factors with odds ratio of developing hyperkalaemia in community studies.
Table 2: Prevalence and outcome of hyperkalaemia in patients with eGFR> 60 ml/min in community studies.
Table 3: Prevalence of hyperkalaemia and mortality rate in patients with CKD.
Table 4: Interval for repeat blood monitoring following an episode of hyperkalaemia.
Table 5: Drugs that pose an additive effect on risk of hyperkalaemia in patients receiving RAASi and MRAs.
Table 6: Studies of efficacy of Patiromer in the treatment of hyperkalaemia.
Table 7: Studies of the efficacy of SZC in treatment of hyperkalaemia
Table 8: Drugs implicated in development of hyperkalaemia and exacerbating factors.
Table 9: Factors associated with an increased risk of hyperkalaemia.
Table 10: Mechanism of action of drugs used in treatment of hyperkalaemia.
Table 11: Calcium content of IV calcium salts used in treatment of hyperkalaemia.
Table 12: Incidence and risk factors associated with iatrogenic hypoglycaemia after insulin-glucose infusion
for treatment of hyperkalaemia.
Table 13: Prospective and Retrospective studies of Insulin-glucose therapy.
Table 14: Efficacy and risk of hypoglycaemia with conventional regimen - 10 units Insulin with 25g glucose
(studies without efficacy data excluded)
Table 15: Comparison of studies performed using 5 versus 10 units insulin (studies without efficacy data
excluded).
Table 16: Comparison of studies performed using weight-base Insulin regimen.
Table 17: Studies using 50% glucose in treatment of hyperkalaemia.
Table 18: Risk Factors for Hypoglycaemia following treatment with Insulin-Glucose
Table 19: Efficacy of Nebulised Salbutamol.
Table 20: Studies investigating efficacy of nebulised salbutamol in hyperkalaemia.
Table 21: Proportion of patients taking SZC 10g three times daily achieving restoration of normokalaemia (K
3.5-5.0 mmol/l) during acute phase.
Table 22: Factors associated with an increased risk of hyperkalaemia in HD patients.
Table 23: Dialysate K+ prescription in chronic HD patients.
Table 24: Minimum standards for safe patient transfer.
Table 25: Drugs commonly associate with hyperkalaemia.
Table 26: Incidence and outcome of cardiac arrest in out-patient dialysis units.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 18
Table 27: Timing of cardiac arrest during dialysis in out-patient centres.
Table 28: Outcome of cardiac arrest in patients receiving haemodialysis (HD) in an outpatient dialysis facility
versus all in-hospital cardiac arrests.
Table 29: Special considerations during resuscitation in haemodialysis patients.
Table 30: Outcome of hyperkalaemic cardiac arrest with RRT during CPR.
Table 31: Summary of procedure for initiation of dialysis during CPR.
Figures
Figure 1: Progressive changes in ECG with increasing severity of hyperkalaemia.
Figure 2: ECG in a patient with severe hyperkalaemia (serum K+ 9.1 mmol/l) illustrating peaked T waves (a),
diminished P waves (b) and wide QRS complexes (c).
Figure 3: Arrhythmias in patients with severe hyperkalaemia illustrating bradycardia with wide QRS [K+ 9.6
mmol/L] (a), sine wave with pause [K+ 9.3 mmol/L] (b) and sine wave without pause [K+ 8.4 mmol/L] (c) and
ventricular tachycardia [K+ 9.1 mmol/L] (d).
Figure 4: There are five key steps in the treatment of hyperkalaemia (never walk away without completing all
of these steps).
Figure 5: ECG on admission (a) and following 20ml 10% calcium gluconate IV (b) in a patient with serum K+
9.3 mmol/L who presented with generalised weakness.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 19
Summary of Audit Measures
The Renal Association encourages non-renal specialties to record audit measures for all patients diagnosed
with hyperkalaemia irrespective of whether or not they are referred to renal services. Hospital laboratories
should be capable of providing data to help audit compliance with these guidelines. It is recommended that
the following audit measures be recorded for patients with hyperkalaemia.
1. Frequency of hospital admission for severe hyperkalaemia (serum K+ > 6.5 mmol/l) detected on
routine blood test in the community.
2. Frequency of blood monitoring of patients receiving RAASi drugs in the community.
3. Proportion of patients admitted to hospital with severe hyperkalaemia detected in the community
who subsequently did not warrant emergency treatment on repeat testing.
4. Proportion of patients with moderate hyperkalaemia who have received dietary potassium advice in
the renal out-patient setting.
5. The proportion of out-patients with moderate hyperkalaemia (serum K+ 6.0 - 6.4 mmol/l) treated
with patiromer who achieved a serum K+ ≤ 5.0 mmol/l within 1 week.
6. The proportion of out-patients who achieve maximal dose RAASi therapy whilst taking patiromer.
7. The proportion of out-patients with moderate hyperkalaemia (serum K+ 6.0 - 6.4 mmol/l) treated
with SZC who achieved a serum K+ ≤ 5.0 mmol/l within 48 hours.
8. The proportion of out-patients who achieve maximal dose RAASi therapy whilst taking SZC.
9. Proportion of patients with severe hyperkalaemia (Serum K+ ≥ 6.5 mmol/l) on admission to hospital
who had been provided with ‘Sick Day Rules’ advice.
10. Length of hospital stay and in-hospital mortality of patients admitted with hyperkalaemia.
11. Proportion of patients with a serum K + level ≥ 6.0 mmol/L who had a 12-lead ECG recorded before
and after treatment for hyperkalaemia.
12. The frequency of ECG changes in patients treated with intravenous calcium salts.
13. The proportion of patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/L) treated with insulin-glucose
infusion.
14. The proportion of patients with acute severe hyperkalaemia (serum K+ ≥ 6.5 mmol/l) treated with
Sodium Zirconium Cyclosilicate.
15. The proportion of patients with acute severe hyperkalaemia (serum K+ ≥ 6.5 mmol/l) treated with
Patiromer.
16. The proportion of patients in whom serum K+ was measured at least once within 2 hours of
treatment for severe hyperkalaemia [Audit Standard: 100%].
17. The proportion of patients who have at least one blood glucose test performed within 1 hour of
completion of insulin-glucose infusion [Audit Standard: 100%].
18. The frequency of hypoglycaemia occurring in patients receiving treatment with insulin-glucose for
hyperkalaemia.
19. The incidence of patients requiring emergency dialysis for severe hyperkalaemia.
20. The frequency of hyperkalaemia developing beyond 24 hours of hospital admission.
21. The frequency of prescribed drugs potentially contributing to hyperkalaemia.
22. All cardiac arrests should be audited – hospital participation in the National Cardiac Arrest Audit is
encouraged as part of quality improvement and benchmarking.
23. The proportion of patients treated with intravenous calcium for hyperkalaemic cardiac arrest.
24. The proportion of patients treated with sodium bicarbonate for hyperkalaemic cardiac arrest.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 20
25. The number and outcome of patients with refractory hyperkalaemic cardiac arrest treated with
dialysis initiation during CPR.
Future Research
There are numerous unanswered questions about the treatment of patients with hyperkalaemia. Areas for
future research include:
1. The optimal dose of insulin and glucose to treat acute hyperkalaemia required to minimise
iatrogenic hypoglycaemia without compromising efficacy.
2. The efficacy of potassium binders (patiromer and sodium zirconium cyclosilicate) in combination
with insulin-glucose infusion in the treatment of severe hyperkalaemia in hospitalised patients.
3. The efficacy of potassium binders (patiromer and sodium zirconium cyclosilicate) in the treatment of
moderate hyperkalaemia without the administration of insulin-glucose in hospitalised patients.
4. The efficacy of sodium bicarbonate in the treatment of severe hyperkalaemia in patients with AKI.
Future Developments
The delivery of a specified dose of glucose is dependent on the available preparations. Hyperkalaemia is a
medical emergency, therefore ease of administration is key. The only preparation available that provides
the required amount of glucose (25g) is the 50% solution.
1. Preparations of 10% (250ml) and 20% (125ml) glucose solutions in volumes appropriate for the
treatment of hyperkalaemia.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 21
Section I
Management of Hyperkalaemia
in the Community and Out-patient Clinic

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 22
Hyperkalaemia in the Community (Guidelines 1.1 – 12.1)
Introduction
Hyperkalaemia is commonly detected in the community and the patient groups most at risk are those with
CKD, diabetes mellitus and heart failure. Hyperkalaemia may also occur in the context of an AKI triggered by
acute illness, initiation or titration of RAASi medications, or worsening of heart failure.
1
Hyperkalaemia
develops in approximately 10% of out-patients within one year after initiation of RAASi drugs, thereby
limiting treatment in the patients who receive the greatest benefit from this therapy.
2
The management of patients with heart failure is challenging given the high prevalence of renal impairment
and increased risk of hyperkalaemia. In clinical trials of RAASi monotherapy, the incidence of hyperkalaemia
ranges from 3 – 7%.
3
The overall incidence of hyperkalaemia was generally higher in clinical trials involving
aldosterone antagonists.
3
Combination therapy of RAASi and aldosterone antagonist increases the risk of
hyperkalaemia and hospitalisation.
4
Mortality in patients with heart failure is significantly increased with
worsening severity of hyperkalaemia: serum K+ levels between 4.8 – 5.0 mmol/l (HR 1.34), 5.1 – 5.5 mmol/l
(HR 1.60) and 5.6 – 7.4 mmol/l (HR 3.31).
5
Table 1: Risk factors with odds ratio of developing hyperkalaemia in community studies.
Several risk factors contribute to community-acquired hyperkalaemia as shown in Table 1. The presence of
multiple co-morbidities or other risk factors further increase the risk of hyperkalaemia.
6-9
RAASi drugs are
frequently implicated in AKI and hyperkalaemia, but there are conflicting reports in the literature.
10, 11
Given
the potential risk of AKI, ‘sick day rules’ guidance recommending the cessation of RAASi drugs during acute
illness has been proposed by some groups including NICE, but this remains controversial.
1, 10, 12, 13, 14
Risk Factor for
Hyperkalaemia
Odds Ratio
[Turgutalp] [6]
Odds Ratio
[Sarafidis] [7]
Odds Ratio
[Nakhoul] [8]
Odds Ratio
[Horne] [9]
Renal Failure
5.55
2.06
( eGFR < 15)
1.25
(per 5ml/min
decrease)
1.04
Diabetes
1.53
0.95
Heart Failure
0.95
≥2 Co-morbidities
2.22
Serum bicarbonate <
25
1.30
ARB
2.68
1.85
1.4
15.89
ACE-I
2.24
1.85
1.4
13.63
Spironolactone
2.53
2.10
7.77
NSAIDS
2.68
Beta blocker
2.14
1.06
Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 23
Study
Country
Setting
N=
eGFR
ml/min
Definition
of HyperK
mmol/l
Prevalence
HyperK
%
Mortality risk
with HK
Liamis
2013
15
Netherlands
General
population
(age > 55)
5179
>60
≥6.0
0.3
#
OR 2.08
Chang
2016
16
USA
Health
care
system –
HBP
(age ≥ 18)
155,695
>60
>5
10.8
NA
>5.5
2.3
Hughes-
Austin
2017
17
USA
Multi-
ethnic
general
population
(age ≥65)
9651
>60
≥5.0
2.8
+
HR 1.41
Horne
2019
9
UK
General
population
(age ≥ 18)
195,178
>60
5.0 – 5.4
91.2
∞
2.51
5.5 – 6.0
7.2
∞
3.83
>6
1.6
∞
12.57
Table 2: Prevalence and outcome of Hyperkalaemia in patients with eGFR> 60 ml/min in community
studies.
#
OR- Odds Ratio;
+
HR- Hazard Ratio;
∞
All-cause mortality; HBP – hypertensive; NA – not available
The reported incidence of hyperkalaemia in the general population is variable depending on the specific
patient group, study design, level of renal function and definition of hyperkalaemia.
6, 9, 15-19
The prevalence of
hyperkalaemia in patients with an eGFR > 60 ml/min is shown in Table 2. In a large UK primary care study,
the overall incidence rate of a hyperkalaemic event was 2.9 per 100 person years.
9
In this study, the use of
RAASi was strongly associated with hyperkalaemia with an odds ratio of 13.6 - 15.9.
Hyperkalaemia is more common in patients with CKD and the incidence increases with declining renal
function. Sarafadis et al found that over 30% of patients experienced hyperkalaemia (K+ > 5.5 mmol/l) in the
pre-dialysis setting (eGFR < 15 ml/min).
7
A summary of the prevalence of hyperkalaemia in patients with
CKD is shown in Table 3.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 24
Study
Country
Setting
N=
eGFR
ml/min
Definition
of HyperK
mmol/l
Prevalence
HyperK
%
Mortality by
K
+
level
Korgaonkar
2010
20
USA
Renal Clinic
820
25.4
≥5.5
7.9
+
HR 1.57
Sarafidis
2012
7
UK
Low
Clearance
clinic
238
14.5
5.0 – 5.4
22.7
NA
5.5 – 5.9
23.1
NA
≥6.0
8.4
NA
Nakhoul
2015
8
USA
CKD
Registry
(USA)
36,359
47
5.0 – 5.4
11
#
OR 1.12
>5.5
3.3
#
OR 1.65
Turgutalp
2016
6
Turkey
Elderly
population
(age > 65)
40,092
23-35
≥5.5
2.9
AUC values by
age
p< 0.001
Luo
2016
19
USA
Health care
system
(age ≥ 18)
55,266
< 60
5.0 – 5.4
14.9
*IRR 1.01
5.5 – 5.9
3.9
*IRR 1.11
≥6.0
1.1
*IRR 3.08
Furuland
2018
18
UK
Health care
database
191,964
50.9
5.0 – 5.4
45.1
*IRR 1.1
5.5 – 5.9
15.9
*IRR 1.60
≥6.0
4.9
*IRR 2.88
Table 3: Prevalence of hyperkalaemia and mortality rate in patients with CKD.
NA – not available;
+
HR – Hazard Ratio;
#
OR - Odds Ratio; AUC- Area Under Curve; *IRR- Incident rate ratio
Hyperkalaemia is associated with increased hospitalisation, prolongation of hospital stay and increased
mortality. Horne et al showed the incidence rates for all-cause hospitalisation in adults was 14.1 per 100
person years.
9
Turgutalp et al demonstrated a higher incidence of hospitalisation for hyperkalaemia in the
elderly population: age 65-74 years (46%), age 75-84 years (44%) and ≥ 85 years (74%).
6
Mortality increases
with worsening severity of hyperkalaemia in the general population and in patients with CKD.
8, 9, 18, 19
This chapter focuses on the detection, treatment and prevention of hyperkalaemia in the community. It will
address the management of patients receiving RAASi drugs, indications for hospital admission and the use of
novel oral potassium lowering drugs.
References
1. Clark, A.L., et al., Change in renal function associated with drug treatment in heart failure: national
guidance. Heart, 2019. 105(12): p. 904-910.
2. Palmer, B.F. and D.J. Clegg, Diagnosis and treatment of hyperkalemia. Cleve Clin J Med, 2017. 84(12):
p. 934-942.
3. Tromp, J. and P. van der Meer, Hyperkalaemia: aetiology, epidemiology, and clinical significance. Eur
Heart J Suppl, 2019. 21(Suppl A): p. A6-A11.
4. Juurlink, D.N., et al., Rates of hyperkalemia after publication of the randomized aldactone evaluation
study. New England Journal of Medicine, 2004. 351(6): p. 543-551.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 25
5. Aldahl, M., et al., Associations of serum potassium levels with mortality in chronic heart failure
patients. Eur Heart J, 2017. 38(38): p. 2890-2896.
6. Turgutalp, K., et al., Community-acquired hyperkalemia in elderly patients: risk factors and clinical
outcomes. Ren Fail, 2016. 38(9): p. 1405-1412.
7. Sarafidis, P.A., et al., Prevalence and factors associated with hyperkalemia in predialysis patients
followed in a low-clearance clinic. Clin J Am Soc Nephrol, 2012. 7(8): p. 1234-41.
8. Nakhoul, G.N., et al., Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney
Disease. Am J Nephrol, 2015. 41(6): p. 456-63.
9. Horne, L., et al., Epidemiology and health outcomes associated with hyperkalemia in a primary care
setting in England. BMC Nephrol, 2019. 20(1): p. 85.
10. Whiting, P., et al., What are the risks and benefits of temporarily discontinuing medications to
prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open, 2017. 7(4): p.
e012674.
11. Mansfield, K.E., et al., Prescription of renin-angiotensin system blockers and risk of acute kidney
injury: a population-based cohort study. BMJ Open, 2016. 6(12): p. e012690.
12. Doerfler, R.M., et al., Usability Testing of a Sick-Day Protocol in CKD. Clin J Am Soc Nephrol, 2019.
14(4): p. 583-585.
13. Martindale, A.M., et al., Understanding the implementation of 'sick day guidance' to prevent acute
kidney injury across a primary care setting in England: a qualitative evaluation. Bmj Open, 2017.
7(11).
14. National Institute for Health and Care Excellence. Acute kidney Injury: prevention, detection and
management. Clinical guideline [CG169]. London, 2013.
15. Liamis, G., et al., Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med,
2013. 126(3): p. 256-63.
16. Chang, A.R., et al., Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large
Health System. Hypertension, 2016. 67(6): p. 1181-8.
17. Hughes-Austin, J.M., et al., The Relation of Serum Potassium Concentration with Cardiovascular
Events and Mortality in Community-Living Individuals. Clinical Journal of the American Society of
Nephrology, 2017. 12(2): p. 245-252.
18. Furuland, H., et al., Serum potassium as a predictor of adverse clinical outcomes in patients with
chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC
Nephrol, 2018. 19(1): p. 211.
19. Luo, J., et al., Association between Serum Potassium and Outcomes in Patients with Reduced Kidney
Function. Clin J Am Soc Nephrol, 2016. 11(1): p. 90-100.
20. Korgaonkar, S., et al., Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study.
Clin J Am Soc Nephrol, 2010. 5(5): p. 762-9.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 26
Patient monitoring (Guidelines 1.1-1.2)
Guideline 1.1 – Monitoring of patients at risk of Hyperkalaemia in the community.
We recommend that patients known to have CKD, heart failure and/or diabetes, who are at risk of
hyperkalaemia, undergo regular blood monitoring at a frequency (2-4 times per year) dependent on level of
renal function and degree of proteinuria. (1B)
Guideline 1.2.1 – Monitoring of patients after an episode of mild hyperkalaemia detected in the
community.
We recommend that the serum K+ is repeated within 3 days, or as soon as feasible, if an episode of mild
hyperkalaemia (K+ 5.5 – 5.9 mmol/l) is detected unexpectedly in the community. (1C)
Guideline 1.2.2 – Monitoring of patients after an episode of moderate hyperkalaemia detected in the
community.
We recommend that the serum K+ is repeated within 1 day of an episode of moderate hyperkalaemia (K+ 6.0
– 6.4 mmol/l) when detected in the community. (1C)
Guideline 1.2.3 – Monitoring of patients after an episode of severe hyperkalaemia detected in the
community.
We recommend that patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/l) detected in the community are
admitted for immediate assessment and treatment. (1B)
Audit measure
1. Frequency of hospital admission for severe hyperkalaemia (serum K+ > 6.5 mmol/l) detected on
routine blood test in the community.
Rationale (Guideline 1.1 – 1.2)
Patients with CKD are at risk of hyperkalaemia and progression of their underlying kidney disease, therefore
require regular blood monitoring in the community. The NICE CKD Guideline suggests that the frequency of
monitoring should be tailored to the level of renal function, rate of decline in renal function and degree of
proteinuria.
1
Patients with CKD 1-3 require monitoring at least 1-2 times per year and patients with CKD 4-5
require monitoring at least 2-4 times per year. More frequent monitoring is indicated during acute illness
and following an episode of AKI or hyperkalaemia.
Several observational studies have reported the frequency of blood monitoring in patients with CKD in
relation to detection of hyperkalaemic events. Chang et al showed that the proportion of patients who had
a serum K+ level performed over a 3 year period was 0 tests/ year (20%), <2 tests/ year (58%), 2-3 tests/ year
(16%) and ≥4 tests/ year (6%).
2
In patients with an eGFR < 30ml/min who had ≥4 tests per year,
hyperkalaemia was found in 30%.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 27
Luo et al reported the frequency of blood monitoring stratified by level of renal function and level of serum
K+.
3
In patients with an eGFR < 30 ml/min, the mean frequency of tests per year was 1.69 ± 1.35 (serum K+
5.5 – 5.9 mmol/l) and 1.37 ± 0.98 (serum K+ ≥ 6 mmol/l) respectively. In patients with an eGFR 50-59 ml/min,
the mean frequency of tests per year was 1.34 ± 0.92 (serum K+ 5.5 – 5.9 mmol/l) and 1.21 ± 0.73 (serum K+
≥ 6 mmol/l) respectively. Similar to Chang et al, detection of hyperkalaemia increases with frequency of
testing. Overall, the frequency of monitoring in these studies was generally 1-2 times per year, with more
frequent testing in patients with an eGFR < 30 ml/min.
The interval between hyperkalaemic episodes was reported in a large retrospective cohort of patients with
CKD.
4
This study utilised data from primary care records for approximately 7% of the UK population over a
mean follow-up of 4.9 years. Patients experiencing at least one episode of hyperkalaemia was stratified in
three groups: serum K+ 5.0 – 5.4 mmol/l (45.2%), 5.5 – 5.9 mmol/l (15.9%) and ≥ 6.0 mmol/l (4.9%). The
time interval to a recurrent episode of hyperkalaemia progressively shortened in each severity group. The
interval between the first to second episodes in patients with serum K+ 5.5 – 5.9 mmol/l was 0.84 years and
reduced to 0.59 years between the second and third episode and 0.48 years between the third and fourth
episode. The interval between recurrent episodes was shorter in patients with serum K+ ≥ 6 mmol/l (0.65,
0.41 and 0.30 years respectively).
This collective data would suggest that monitoring serum K+ at least twice per year in patients at risk of
hyperkalaemia is a reasonable approach. The frequency of monitoring should be increased to at least four
times per year in patients with an eGFR < 30 ml/min and in patients with a serum K+ ≥ 6 mmol/l given the
high risk of recurrence.
The interval for blood monitoring after a hyperkalaemic event is less well documented. Horne et al
demonstrated that only 5.8% of patients had a repeat serum K+ performed within 14 days of the
hyperkalaemic event, but a large number of patients had a serum K+ < 5.5 mmol/l which may have been
perceived to be non-urgent.
5
A repeat level occurred more commonly in patients with K+ > 6.0 mmol/l
(55.3%) compared with those with a serum K+ 5.6 – 6.0 mmol/l (23.4%) or serum K+ 5.0 – 5.5 mmol/l (3.9%).
In patients with a serum K+ > 6.0 mmol/l at the index event, 36.8% had an elevated K+ level on re-testing.
‘Think Kidneys’ have provided practical guidance on repeat testing after a hyperkalaemic episode.
6
The
timing is guided by the level of hyperkalaemia and clinical context. In patients with mild hyperkalaemia (K+
5.5 – 5.9 mmol/l), a repeat test is recommended within 3 days if the result was unexpected or as soon as
feasible if the patient is clinically stable. In patients with moderate hyperkalaemia (K+ 6.0 – 6.4 mmol/l), a
repeat test is recommended within 1 working day if detected on a routine check in a stable patient, but
referral to hospital should be considered if clinically unwell or if an AKI is present. Patients with severe
hyperkalaemia (K+ ≥ 6.5 mmol/l) warrant urgent referral to hospital for immediate assessment and
The more often you test, the more often you will detect hyperkalaemia,
especially in patients at risk.
Patients with CKD 4-5 have a high risk of hyperkalaemia and warrant
regular testing.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 28
treatment if required. The recommended interval for repeat testing after a hyperkalaemic episode is
summarised in Table 4.
Severity of
Hyperkalaemia
Clinically well
(no AKI)
Unexpected result
Clinically unwell or AKI
MILD
K
+
5.5 – 5.9 mmol/l
Repeat within 14
days
Repeat within 3 days
#
Consider if hospital referral is
indicated
Assess for cause (drugs, diet) and address in community
MODERATE
K
+
6.0 – 6.4 mmol/l
Repeat within 1
working day*
Repeat within 24
hours
Refer to hospital
Assess for cause (drugs, diet) and address community or hospital
SEVERE
K
+
≥ 6.5 mmol/l
Refer to hospital for immediate assessment and treatment
Assess for cause and address during hospital admission
Table 4: Interval for repeat blood monitoring following an episode of hyperkalaemia.
#
Need for hospital referral will be guided by clinical circumstance and risk of further deterioration.
*Routine bloods tests unavailable at weekends and out of hours from community.
(Modified from Think Kidneys Guideline)
6
There is increasing use of point of care testing (POCT) in the hospital setting for rapid potassium
measurement, but achieving rapid blood analysis in the community can be challenging. POCT has been
validated in several studies in the hospital setting within the Emergency Department and Critical Care.
7-12
POCT has also been shown to improve early recognition of hyperkalaemia in patients with CKD presenting to
the Emergency Department.
13
Use of POCT in the pre-hospital setting is less well reported.
14,15
A study of
the utilisation and validation of POCT devices by community paramedics demonstrated good correlation with
laboratory measurement.
14
Technology is rapidly developing with the use of medical biosensors and Smart
phone technology potentially making POCT easily accessible for patients.
16
References
1. National Institute for Health and Care Excellence: Chronic kidney disease. Scenario: Management of
chronic kidney disease. Last revised in May 2020. www.cks.nice.org.uk/chronic-kidney-
disease#!scenarioRecommendation:1.
2. Chang, A.R., et al., Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large
Health System. Hypertension, 2016. 67(6): p. 1181-8.
3. Luo, J., et al., Association between Serum Potassium and Outcomes in Patients with Reduced Kidney
Function. Clin J Am Soc Nephrol, 2016. 11(1): p. 90-100.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 29
4. Furuland, H., et al., Serum potassium as a predictor of adverse clinical outcomes in patients with
chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC
Nephrol, 2018. 19(1): p. 211.
5. Horne, L., et al., Epidemiology and health outcomes associated with hyperkalemia in a primary care
setting in England. BMC Nephrol, 2019. 20(1): p. 85.
6. 'Think Kidneys' - Changes in kidney function and serum potassium during ACE/ARB/diuretic
treatment in primary care. A position statement from Think Kidneys, the Renal Association and the
British Society for Heart Failure; 2017. www.thinkkidneys.nhs.uk
7. Mirzazadeh, M., et al., Point-of-care testing of electrolytes and calcium using blood gas analysers: it
is time we trusted the results. Emerg Med J, 2016. 33(3): p. 181-6.
8. Dashevsky, M., et al., Agreement Between Serum Assays Performed in ED Point-of-Care and Hospital
Central Laboratories. West J Emerg Med, 2017. 18(3): p. 403-409.
9. Florkowski, C., et al., Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM) -
does it leverage any advantage in clinical decision making? Crit Rev Clin Lab Sci, 2017. 54(7-8): p.
471-494.
10. Allardet-Servent, J., et al., Point-of-Care Versus Central Laboratory Measurements of Hemoglobin,
Hematocrit, Glucose, Bicarbonate and Electrolytes: A Prospective Observational Study in Critically Ill
Patients. PLoS One, 2017. 12(1): p. e0169593.
11. Chacko, B., et al., Electrolytes assessed by point-of-care testing - Are the values comparable with
results obtained from the central laboratory? Indian J Crit Care Med, 2011. 15(1): p. 24-9.
12. Gibbons, M., et al., How closely do blood gas electrolytes and haemoglobin agree with serum values
in adult emergency department patients: An observational study. Emerg Med Australas, 2019. 31(2):
p. 241-246.
13. You, J.S., et al., Evaluating the utility of rapid point-of-care potassium testing for the early
identification of hyperkalemia in patients with chronic kidney disease in the emergency department.
Yonsei Med J, 2014. 55(5): p. 1348-53.
14. Blanchard, I.E., et al., Community paramedic point of care testing: validity and usability of two
commercially available devices. BMC Emerg Med, 2019. 19(1): p. 30.
15. Tortella, B.J., et al. Precision, Accuracy, and Managed Care Implications of a Hand-Held Whole Blood
Analyzer in the Prehospital Setting. Am J Clin Pathol, 1996. 106:p.124-127.
16. Liu, J., et al., Point-of-care testing based on smartphone: The current state-of-the-art (2017-2018).
Biosens Bioelectron, 2019. 132: p. 17-37.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 30
Management of patients receiving RAASi drugs (Guidelines 2.1-2.7)
Guideline 2.1 – Assessment of patients prior to initiation of ACE-I or ARB.
We recommend that urea and electrolytes should be assessed prior to initiation of ACE-I or ARB and these
drugs should be used with caution if the serum K+ is > 5.0 mmol. (1A)
Guideline 2.2 – Assessment of patients prior to initiation of Mineralocorticoid Receptor Antagonists
(MRA).
We suggest that initiation of MRAs should be avoided in patients with a baseline serum K+ > 5.0mmol/l or
eGFR < 30 ml/min. (1B)
Guideline 2.3 – Monitoring of patients after initiation of ACE-I and ARB.
We recommend that urea and electrolytes should be assessed at 1 – 2 weeks after initiation of ACE-I or ARB
and after every dose titration. (1A)
Guideline 2.4 – Monitoring of patients after initiation of MRAs.
We recommend that urea and electrolytes should be assessed at 1 week after initiation of MRA or after dose
up-titration, then monthly for the first 3 months, 3-monthly for the first year and 4-monthly thereafter. (1A)
Guideline 2.5 – Management of hyperkalaemia in patients treated with RAASi drugs.
We suggest increased frequency of monitoring in patients with a serum K+ between 5.5-5.9 mmol/l and
consideration of dose reduction of RAASi drugs (ACE-I, ARB, MRA). (1B)
Guideline 2.6 – Management of hyperkalaemia in patients treated with RAASi drugs during acute illness.
We recommend that RAASi drugs be withheld during acute intercurrent illness (e.g. sepsis, hypovolaemia
and/or AKI) at all severities of hyperkalaemia. (1D)
Guideline 2.7 – Cessation of RAASi drugs in patients with moderate or severe hyperkalaemia.
We recommend cessation of RAASi drugs in patients with serum K ≥ 6 mmol/l who do not meet the criteria
for treatment with patiromer or sodium zirconium cyclosilicate. (1B)
Audit measure
1. Frequency of blood monitoring of patients receiving RAASi drugs in the community.
Rationale (Guidelines Hyperkalaemia 2.1 – 2.7)
Patients with CKD, heart failure and diabetes are particularly at risk of hyperkalaemia and these conditions
often co-exist. RAASi drugs have become the standard of care to slow progression of CKD and in the
management of patients with diabetes and heart failure. However, hyperkalaemia frequently limits use or
titration of RAASi drugs. Epstein et al conducted a large study (> 7 million of electronic patient records) to
determine the impact of hyperkalaemia on the optimal versus real-world treatment with RAASi.
1
In patients
for whom RAASi was recommended by treatment guidelines for cardiorenal disease, >50% were prescribed
lower than recommended dose and 14-16% discontinued RAASi therapy.
1

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 31
Sub-optimal treatment for patients with heart failure and renal disease also affects patient outcome.
Mortality rates has been shown to be higher in patients who receive sub-maximal dosing (8%) and in those
who have discontinued RAASi (11%) compared to those who received maximal dosing (4%).
2
Similarly,
Ouwerkerk et al demonstrated increased hospitalisation and increased mortality in patients with heart
failure with reduced ejection fraction (HFrEF) who receive less than half of the recommended doses of ACE-I
or ARB (HR 1.72) and beta blockers (HR 1.70) compared to patients who reached optimal doses.
3
The balance
between optimising treatment and compromising renal function poses a significant clinical dilemma.
Monitoring of serum K+ in patients receiving RAASi drugs reduces the risk of adverse events. Raebel et al
demonstrated that patients with diabetes who underwent potassium monitoring during the first year of
treatment with RAASi drugs were less likely to experience hyperkalaemia-associated adverse events
(hospitalisation, Emergency Department attendance or death) with an adjusted relative risk of 0.50 (0.37,
0.66) compared to those who were not monitored.
4
The sub-set of patients with CKD had an adjusted
relative risk of 0.29 (0.18, 0.46). Park et al conducted an observational study of hospitalised patients newly
started on an ARB and demonstrated that the highest incidence of hyperkalaemia occurred on the first day
and 52.4% of hyperkalaemic events occurred within the first week of initiation.
5
In this study, hyperkalaemia
also occurred earlier in patients with reduced GFR, higher baseline K+ level (patients with K+ >5.5 mmol/l
were excluded) and in patients with diabetes.
The KDIGO Guideline (2012) recommends measuring serum K+ level within 1 week of starting RAASi drugs
and after every dose increment in patients with reduced renal function.
6
The NICE Guideline for CKD (2014)
recommends measuring serum K+ level before starting, within 1 to 2 weeks of initiation of RAASi therapy
and after every dose increment.
7
The NICE CKD Guideline (2014) also recommends that RAASi drugs should be withdrawn if the serum K+ is ≥
6 mmol/l.
7
However, NICE has recently approved the use of potassium binders (patiromer and sodium
zirconium cyclosilicate) in selected patients with CKD 3b-5 (not on dialysis) or heart failure who have
confirmed persistent hyperkalaemia with a serum K+ ≥ 6 mmol/l and are not receiving an optimal dose of
RAASi.[8][9] RAASi should be withdrawn in all patients with serum K+ is ≥ 6 mmol/l who do not meet the
criteria for these novel potassium binders.
The Renal Association and the British Society for Heart Failure (2019) have recently collaborated to provide
consensus recommendation for the use of RAASi in patients with heart failure with reduced left ventricular
ejection fraction (HFrEF).
10
Monitoring of renal function is mandatory during initiation and titration of RAASi
treatment. In the context of acute illness (sepsis, hypovolaemia and/or AKI), withdrawal of RAASi was
advised at all severities of hyperkalaemia. However, in the context of decompensated heart failure, the
continuation/ reduction of RAASi therapy was permitted in patients with mild or moderate hyperkalaemia,
Assess urea & electrolytes prior to initiation of RAASi drugs.
Monitor urea & electrolytes at 1 week after initiation and after each dose titration.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 32
but withdrawn if serum K+ ≥ 6.5 mmol/l. RAASi re-introduction was recommended after recovery and when
K+ was < 5.5 mmol/l. Patients receiving multiple RAASi drugs and/or MRA should re-start one drug at a time.
Mineralocorticoid receptor antagonists (MRAs) have significantly improved heart failure management, but
their use alone or in combination with RAASi, may exacerbate hyperkalaemia. The European Society of
Cardiology
11
and American Heart Association (AHA)/ American College of Cardiology (ACC)
12
guidelines
provide guidance on initiation, monitoring and response to treating hyperkalaemia in patients receiving
MRAs. The parameters for initiation of MRA are a serum K+ < 5.0 mmol/l and an eGFR > 30ml/min. Close
monitoring of urea and electrolytes (U&Es) is required following initiation at 1, 4, 8 and 12 weeks.
Thereafter, monitoring is required every 3 months during first year, then every 3-4 months from second year
onwards. The approach to managing hyperkalaemia in patients with heart failure on MRA is shown in the
text box below. In patients without heart failure, drug cessation is recommended if serum K+ ≥ 6.0 mmol/l.
Adherence to guideline recommendations appears to be poor. In a large population-based study of new
users of RAASi drugs, < 33% of patients had a K+ measurement within 30 days of drug initiation and only 76%
had at least one measurement within the first year of treatment.
13
In another study, Chang et al reported
that 20% of patients had no serum K+ monitoring within 3 years of initiation of antihypertensive medication
that affect potassium levels.
14
Combined treatment of RAASi and an aldosterone antagonist increase the risk
of hyperkalaemia, but Sinnott et al reported <33% of patients taking a RAASi had biochemical monitoring
within two weeks of initiation of an aldosterone antagonist.
15
This highlights the gap in knowledge and
clinical practice.
References
1. Epstein, M., Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone
inhibition: the widening gap between mandated treatment guidelines and the real-world clinical
arena. Kidney Int Suppl (2011), 2016. 6(1): p. 20-28.
2. Epstein, M., et al., Evaluation of the treatment gap between clinical guidelines and the utilization of
renin-angiotensin-aldosterone system inhibitors. Am J Manag Care, 2015. 21(11 Suppl): p. S212-20.
3. Ouwerkerk, W., et al., Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-
blockers in patients with heart failure: a prospective European study. Eur Heart J, 2017. 38(24): p.
1883-1890.
4. Raebel, M.A., et al., Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J
Gen Intern Med, 2010. 25(4): p. 326-33.
5. Park, I.W., et al., Onset time of hyperkalaemia after angiotensin receptor blocker initiation: when
should we start serum potassium monitoring? J Clin Pharm Ther, 2014. 39(1): p. 61-8.
Strategies for Managing Hyperkalaemia in patients with Heart failure on MRA
• K
+
5.5-5.9 mmol/l: Reduce dose by half and monitor U&Es
• K
+
> 6.0 mmol/l: Start potassium binder (Patiromer or SZC) and monitor U&Es

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 33
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Working Group. KDIGO 2012 Clinical
Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl
2013; 3: 1-150.
7. National Institute for Health and Care Excellence: Chronic Kidney Disease in adults - assessment and
management. Clinical Guideline 182; July 2014. www.nice.org.uk/guideline/cg182/
8. National Institute for Health and Carel Excellence - Sodium Zirconium Cycosilicate for treating
hyperkalaemia. Technology appraisal guidance [TA599]. September 2019.
www.nice.org/guidance/TA599.
9. National Institute for Health and Care Excellence - Patiromer for treating hyperkalaemia. Technology
appraisal guidance. [TA623]. February 2020. www.nice.org.uk/guidance/TA623.
10. Clark, A.L., et al., Change in renal function associated with drug treatment in heart failure: national
guidance. Heart, 2019. 105(12): p. 904-910.
11. Ponikowski, P., et al., 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart
failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the
European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure
Association (HFA) of the ESC. Eur Heart J, 2016. 37(27): p. 2129-2200.
12. Yancy, C.W., et al., 2013 ACCF/AHA guideline for the management of heart failure: a report of the
American College of Cardiology Foundation/American Heart Association Task Force on Practice
Guidelines. J Am Coll Cardiol, 2013. 62(16): p. e147-239.
13. Bandak, G., et al., Hyperkalemia After Initiating Renin-Angiotensin System Blockade: The Stockholm
Creatinine Measurements (SCREAM) Project. J Am Heart Assoc, 2017. 6(7).
14. Chang, A.R., et al., Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large
Health System. Hypertension, 2016. 67(6): p. 1181-8.
15. Sinnott, S.J., et al., Biochemical monitoring after initiation of aldosterone antagonist therapy in users
of renin-angiotensin system blockers: a UK primary care cohort study. BMJ Open, 2017. 7(11): p.
e018153.
Threshold for treatment of hyperkalaemia (Guideline 3.1)
Guideline 3.1 – Threshold for treating Hyperkalaemia in the community.
We recommend that interventions to lower serum potassium be instituted in patients with a serum K+ ≥ 5.5
mmol/l. (1B)
Rationale (Guideline 3.1)
The detection of hyperkalaemia in the community is frequently the result of blood monitoring in relation to
the prescription of RAASi medication. Outwith this context, most observational studies have based diagnosis
of hyperkalaemia on a single blood test. Pseudo-hyperkalaemia may occur in the community after long
transit time to the laboratory, therefore unexpected results should be repeated.
Existing National guidelines recommend initiation of strategies to manage hyperkalaemia when the serum K+
rises to ≥ 5.5 mmol/l.
1,2
Data from several studies performed in the general population and in patients with
CKD show an increased mortality risk in patients with a serum K+ ≥ 5.5 mmol/l.
3-9
Mortality risk increases

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 34
further when the serum K+ exceeds 6 mmol/l, therefore measures should be taken to avoid a further rise in
serum K+ level.
Drugs are frequently implicated in the development of hyperkalaemia in the community. Serum K+ levels
increase by 0.4 – 0.6 mmol/l during RAASi treatment in patients with diabetic and non-diabetic kidney
disease and approximately 1 – 1.7% of patients develop hyperkalaemia.
1
Although RAASi and non-selective
beta-blockers can increase K+ levels, consider the degree of hyperkalaemia and the indication for use before
reducing or withholding the drug.
Stop other medications known to exacerbate hyperkalaemia (e.g. oral potassium supplements, NSAIDs,
trimethoprim). Other strategies for lowering serum K+ in the community include dietary interventions
(Guideline 5.1), treating metabolic acidosis (Guideline 6.1) and controlling hyperglycaemia. Re-introduction
of medications that influence K+ levels requires slow titration and close monitoring.
References
1. National Institute for Health and Care Excellence: Chronic Kidney Diseases in adults - assessment and
management. Clinical Guideline 182; July 2014. www.nice.org.uk/guideline/cg182/
2. Clark, A.L., et al., Change in renal function associated with drug treatment in heart failure: national
guidance. Heart, 2019. 105(12): p. 904-910.
3. Korgaonkar, S., et al., Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study.
Clin J Am Soc Nephrol, 2010. 5(5): p. 762-9.
4. Turgutalp, K., et al., Community-acquired hyperkalemia in elderly patients: risk factors and clinical
outcomes. Ren Fail, 2016. 38(9): p. 1405-1412.
5. Hughes-Austin, J.M., et al., The Relation of Serum Potassium Concentration with Cardiovascular
Events and Mortality in Community-Living Individuals. Clinical Journal of the American Society of
Nephrology, 2017. 12(2): p. 245-252.
6. Horne, L., et al., Epidemiology and health outcomes associated with hyperkalemia in a primary care
setting in England. BMC Nephrol, 2019. 20(1): p. 85.
7. Nakhoul, G.N., et al., Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney
Disease. Am J Nephrol, 2015. 41(6): p. 456-63.
8. Collins, A.J., et al., United States Renal Data System public health surveillance of chronic kidney
disease and end-stage renal disease. Kidney Int Suppl (2011), 2015. 5(1): p. 2-7.
9. Furuland, H., et al., Serum potassium as a predictor of adverse clinical outcomes in patients with
chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC
Nephrol, 2018. 19(1): p. 211.
Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 35
Indications for hospital assessment (Guidelines 4.1 - 4.2)
Guideline 4.1 – Indication for assessment in hospital for patients with severe hyperkalaemia detected in
the community.
We recommend urgent hospital assessment for all patients with severe hyperkalaemia (serum K+ ≥ 6.5
mmol/l) detected in the community. (1A)
Guideline 4.2 – Indication for assessment in hospital for patients with mild-moderate hyperkalaemia
detected in the community.
We suggest hospital assessment for acutely unwell patients with mild (serum K+ 5.5 – 5.9 mmol/l) or
moderate hyperkalaemia (serum K+ 6.0 - 6.4 mmol/l), particularly in the presence of an acute kidney injury.
(1B)
Audit Measures
Proportion of patients admitted to hospital with severe hyperkalaemia detected in the community who
subsequently did not warrant emergency treatment on repeat testing.
Rationale (Guidelines Hyperkalaemia 4.1 – 4.2)
There is substantial variability in clinical practice related to referral for hospital assessment for
hyperkalaemia, which may be partly explained by incidental findings in clinically well patients. The detection
of hyperkalaemia in the community is rising with the increasing use of RAASi drugs for multiple clinical
indications and the necessity for regular biochemical surveillance. Drug up-titration or co-administration of
another drug that affects K+ level, as shown in Table 5, can precipitate severe hyperkalaemia.
1
Acute illness
is another common antecedent to acute kidney injury and hyperkalaemia.
Table 5: Drugs that pose an additive effect on risk of hyperkalaemia in patients receiving RAASi and
MRAs.
The risk of adverse events increases with worsening hyperkalaemia. Severe hyperkalaemia is an
independent predictor of all-cause and in-hospital mortality as well as hospitalisation.
2
Therefore, defined
Drugs that potentiate risk of hyperkalaemia in patients
receiving RAASi and/or MRAs
• Trimethoprim/co-trimoxazole
• Potassium supplements
• Potassium sparing diuretics
• Salt substitutes (lo-salt)
• NSAID
• Non-selective beta-blockers
• Digoxin toxicity

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 36
thresholds for triggering intervention in the community and for prompting referral to hospital could improve
patient outcome.
There is little evidence for a specific threshold for hospital admission for management of hyperkalaemia.
Charytan et al undertook a small study (n=23) to assess the practice relating to hospitalisation of patients for
hyperkalaemia.
3
In this study, 11 patients with hyperkalaemia at hospital admission were compared with 12
hyperkalaemic patients managed as an outpatient. The study lacked power to determine the relative safety
of location of treatment. Horne et al demonstrated the impact of hyperkalaemia on mortality and healthcare
utilisation, including hospital admission, in the UK general population, but did not provide a distinct
threshold warranting hospital admission.
4
However, this study noted a significantly higher incidence rate of
all-cause hospitalisation for patients with a serum K+ > 6.0 mmol/l of 28.93/ 100 person-years compared
with patients with a serum K+ 5.0-5.5 mmol/l of 13.86/ 100 person-years.
The position statement for ‘Think Kidneys’, a collaboration of The Renal Association and British Society for
Heart Failure, recommend hospital admission in all patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/l)
and in patients with moderate hyperkalaemia (K+ 6.0-6.4 mmol/l) who are acutely unwell or have an AKI.
6
Hospital admission should be considered in patients with mild hyperkalaemia (K+ 5.5-5.9 mmol/l) if acutely
unwell or have an AKI.
An important consideration in the management of an episode of hyperkalaemia is the balance between the
immediate risk versus the impact of cessation of RAASi drugs in patients for whom these drugs are crucial in
controlling symptoms and improving survival. Minimising the duration of cessation of treatment and clear
communication after hospital discharge is essential. Involvement of specialist services, renal and heart
failure teams, may facilitate safer re-introduction of treatment.
References
1. Ben Salem, C., et al., Drug-induced hyperkalemia. Drug Saf, 2014. 37(9): p. 677-92.
2. Rosano, G.M.C., et al., Expert consensus document on the management of hyperkalaemia in patients
with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors:
coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of
Cardiology. Eur Heart J Cardiovasc Pharmacother, 2018. 4(3): p. 180-188.
3. Charytan, D. and D.S. Goldfarb, Indications for hospitalization of patients with hyperkalemia. Arch
Intern Med, 2000. 160(11): p. 1605-11.
4. Horne, L., et al., Epidemiology and health outcomes associated with hyperkalemia in a primary care
setting in England. BMC Nephrol, 2019. 20(1): p. 85.
5. Clark, A.L., et al., Change in renal function associated with drug treatment in heart failure: national
guidance. Heart, 2019. 105(12): p. 904-910.
6. 'Think Kidneys' - Changes in kidney function and serum potassium during ACE/ARB/diuretic
treatment in primary care. A position statement from Think Kidneys, the Renal Association and the
British Society for Heart Failure; 2017. www.thinkkidneys.nhs.uk.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 37
Treatment: Dietary interventions (Guideline 5.1)
Guideline 5.1 – Dietary Intervention for managing Hyperkalaemia in the community.
We recommend that a low potassium diet is instituted for patients with persistent hyperkalaemia with a
serum K+ > 5.5 mmol/l. (1B)
Audit measures
1. Proportion of patients with moderate hyperkalaemia who have received dietary potassium advice in
the renal out-patient setting.
Rationale (Guideline 5.1)
Dietary potassium comes from a wide range of foods including fruit and vegetables, meat and meat
products, cereals, drinks, milk and milk products. Fruit and vegetables accounts for approximately 33%
dietary potassium intake.
1
In adults without kidney disease, the WHO recommends an average dietary K+
intake of approximately 3.9g/ day (100mmol).
2
In the USA, the Institute of Medicine, Food and Nutrition
Board recommend a higher daily K+ intake of 4.7g/ day (120mmol/l).[3] In patients with kidney disease, the
National Kidney Foundation (NKF) suggests an unrestricted potassium intake in patients with CKD 1-5 (non-
dialysis) unless the serum K+ is elevated.
4
In advanced CKD and in ESRD, hyperkalaemia may result if the
dietary input of potassium exceeds the output, therefore prevention of hyperkalaemia requires
management of dietary K+ load.
5
A low K+ diet is defined as a dietary intake of 2-3g/day (51-77 mmol/day)
5
. In patients with CKD with
persistent hyperkalaemia (serum K+ ≥ 5.5mmol/l), a dietary K+ restriction of < 3g/ day (< 77 mmol/l)
6
or 1
mmol/kg/IBW
7
is recommended. In reality, a step-wise reduction in potassium intake is usually undertaken.
Excessive dietary restrictions can result in a poorer diet which may risk development of cardiovascular
disease
8
and contribute to malnutrition, particularly in advanced CKD. The development of constipation is
also counterproductive as this will reduce K+ excretion by the gut. Therefore, a balanced intake of fresh
fruit, vegetables and fibre is the ultimate goal.
A renal dietitian is skilled to provide individualised advice to patients with Stage 4-5 CKD managed by
specialist renal services taking into account other factors including diabetic status, cultural needs and patient
preferences. Assessment of biochemical trends would ensure that the need for ongoing dietary restrictions
is reviewed. The Renal Association Guideline on Undernutrition in Chronic Kidney Disease also suggests
assessment by a specialist renal dietitian when patients begin education about RRT and within one month of
dialysis initiation.
9
Although dietary intervention has become standard practice, this has not been demonstrated in a
randomised controlled trial and would potentially be technically difficult to achieve. Three studies reported
on dietary intervention for managing hyperkalaemia.
10,11,12
Ahuja et al reported a retrospective analysis of patients attending a renal clinic (n=119) to determine the
predictors for development of hyperkalaemia in patients on ACE-I.
6
Overall, 46/119 patients (38.6%) of
patients developed hyperkalaemia (mean serum K+ 5.68 mmol/l). Hyperkalaemia resolved in 20/46 patients
(43%) with a low K+ diet alone (<2g/day; 51 mmol/l) and in 11/46 patients (24%) with dietary advice and

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 38
dose reduction of ACE-I. Hyperkalaemia persisted in the remaining 15/46 patients (33%) despite dietary
advice and reduction of ACE-I necessitating discontinuation of ACE-I.
Bushinsky et al studied the difference in serum K+ in hyperkalaemic patients (K+ 5.5-6.2 mmol/l) with CKD 2-
4 on a random diet versus controlled K+ diet during the 72-hour run-in phase of a treatment trial (n=25).
7
Patients received stable doses of RAASi medication. The study demonstrated a wide inter-individual variation
in serum K+ on a random diet. Variation decreased significantly after 24 hours on a low K+ diet (2.4g or 60
mmol/ day). The study concluded that this observation may have implications for the interpretation of
clinical trials assessing directional change of serum K+ with a pharmaceutical intervention.
Maclaughlin et al prospectively investigated the prevalence of hyperkalaemia in a population of CKD patients
(n=356) attending low clearance clinics who underwent regular nutritional assessment and dietary
education.
8
All patients were pre-dialysis with an eGFR ranging from 8 – 20 ml/min. The prevalence of
hyperkalaemia (serum K+ > 5.5 mmol/l) was 26.5% before the dietetic program in 2011 and 10.5% after it
was instituted in 2014. The prevalence of hyperkalaemia also reduced in patients with K+ > 6.0 mmol/l from
8.4% to 2.5% during the same intervals. A dietary education program delivered by specialist renal dietitians
can be very effective in reducing the prevalence of hyperkalaemia.
In patients receiving renal replacement therapy, the KDOQI guidelines suggest a potassium intake of
approximately 2.7-3.1 g/day (69-79 mmol/day) in haemodialysis patients and 3-4g/day (77-102 mmol/day)
in peritoneal dialysis patients.
3
Individualised adjustments are guided by the serum K+ level.
References
1. National Diet and Nutirtion Survey (NDNS) rolling program for 2014 to 2015 and 2015 to 2016
(results from Years 7 and 8 combined). www.gov.uk/government/statistics/ndns-results-from-years-
7-and-8-combined.
2. World Health Organisation (WHO). Guideline: Potassium Intake for Adults and Children; WHO:
Geneva, Switzerland, 2012.
3. Water, potassium, sodium, chloride, and sulfate. Washington, DC: National Academy of Sciences,
Institute of Medicine, Food and Nutrition Board, 2004.
4. K/DOQI, National Kidney Foundation Clinical Practice Guidelines for nutrition in chronic renal failure.
Am K Kidney Disease 2000; 35: S1-S140.
5. Cupisti, A., et al., Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with
Decreased Kidney Function. Nutrients, 2018. 10(3).
6. Kalantar-Zadeh, K. and D. Fouque, Nutritional Management of Chronic Kidney Disease. N Engl J Med,
2017. 377(18): p. 1765-1776.
7. Fouque, D., et al., EBPG guideline on nutrition. Nephrol Dial Transplant, 2007. 22 Suppl 2: p. ii45-87.
8. Khoueiry, G., et al., Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J Ren
Nutr, 2011. 21(6): p. 438-47.
9. Renal Association Clinical Practice Guideline - Undernutrition in Chronic Kidney Disease. June 2019.
www.renal.org/wp-content/uploads/2019/06/FINAL-Nutirion-guideline-June-2019.pdf.
10. Ahuja, T.S., et al., Predictors of the development of hyperkalemia in patients using angiotensin-
converting enzyme inhibitors. Am J Nephrol, 2000. 20(4): p. 268-72.
11. Bushinsky, D.A., et al. Wide range in variation in serum potassium in hyperkalaemic patients with
CKD, response to a fixed 60 mEq potassium diet. J Am Soc Nephrol 2015; 26: SA-PO924.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 39
12. MacLaughlin, H., et al,. Pro-active individualised dietary modification reduces the prevalence of
hyperkalaemia in patients with advanced CKD. British Renal Society Conference Abstract 2015.
www.britishrenal.org/ukkw2018-2/2015-abstracts.
Treatment: Sodium bicarbonate (Guideline 6.1)
Guideline 6.1 – Sodium bicarbonate for management of Hyperkalaemia in the community
We recommend that sodium bicarbonate is used in CKD patients with a serum bicarbonate level < 22 mmol/l
with or without hyperkalaemia. (1B)
Rationale (Guideline 6.1)
Epidemiological studies show a prevalence of metabolic acidosis of 15-19% in patients with CKD stages 3-5.
1
The prevalence increases with severity of kidney disease with metabolic acidosis found in 30-50% of patients
with eGFR < 30 ml/min.
2,3
Furthermore, serum bicarbonate levels steadily decrease with age > 60 years.
4
Despite its prevalence, there is variability in clinical practice for treatment of mild acidosis in patients with
CKD attending renal services and it is not routinely assessed or treated in primary care.
The benefit of treating chronic acidosis goes beyond the management of hyperkalaemia. Metabolic acidosis
is also associated with muscle wasting, bone disease and increased mortality in patients with CKD.
5
Additionally, there is growing evidence that metabolic acidosis contributes to the progression of CKD.
1,6,7
Goraya et al demonstrated that an increase in serum bicarbonate by 4 - 6.8 mmol/l was associated with a
reduction in decline in eGFR by 4 ml/min over 6 to 24 months compared with control patients.
1
The mechanism for potassium lowering is the transcellular shift of K+ into cells following alkalinisation of the
serum. Despite this theoretical benefit, few studies have shown any benefit of sodium bicarbonate in the
treatment of acute or chronic hyperkalaemia. In two long-term studies (i.e. > 2 months), alkali therapy has
been shown to be associated with a significant net decrease in the serum K+ by approximately 0.7 mmol/l,
but no significant change was shown in short term studies (≤ 7 days).
6,8
The BiCARB Trial evaluated the benefits and adverse effects of sodium bicarbonate in older patients with
CKD for a period of up to 2 years. This was a double-blind placebo-controlled RCT which includes 380
community-based patients in the UK aged ≥ 60 years with an eGFR < 30 ml/min and serum bicarbonate < 22
mmol/l.
9
In contrast to other longterm studies, this study found no significant reduction in serum K+ level.
The BiCARB trial also reported no improvement in physical function or renal function and a higher rate of
adverse events compared with placebo.
In the pre-dialysis setting, Sarafidis et al performed a prospective study to examine the factors influencing K+
metabolism in patients attending a low clearance clinic (mean eGFR 14.5 ± 4.8 mmol/l).
10
This study
demonstrated that patients with K+ ≥ 5.5 mmol/l had significantly higher urea, lower eGFR and lower serum
bicarbonate levels. This sub-group also had a higher usage of sodium bicarbonate than in patients without
hyperkalaemia (65.3% versus 45.4%, p=0.008).
10
The potential detrimental effect of sodium load with sodium bicarbonate replacement is an important
consideration, particularly in patients at risk of fluid overload. Dubey et al showed that patients with CKD 3

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 40
and 4 with co-existing diabetes, hypertension and coronary artery disease had a trend towards worsening
hypertension and oedema necessitating a greater use of diuretics.
7
Similar findings have been reported in
other studies with alkali replacement in CKD patients necessitating discontinuation of sodium bicarbonate
due to hypertension and oedema although these studies did not focus on management of hyperkalaemia.
8,
11,12
A meta-analysis of all published RCTs investigating the effect of oral bicarbonate therapy in adults with CKD
showed a slightly higher eGFR and serum bicarbonate levels in patients treated with oral replacement
compared with placebo and this positive effect was attenuated in studies reporting outcomes at one year.
13
This study did not assess potassium levels.
There remains a paucity of evidence from clinical trials on the efficacy and safety of bicarbonate therapy,
therefore many existing guidelines are based on the sparse evidence and expert consensus opinion. KDOQI
guidelines recommend the maintenance of serum bicarbonate level ≥ 22 mmol/l to reduce metabolic
complications.
14
The 2007 Cochrane Review of alkali therapy in CKD found insufficient evidence of benefit.
15
The NICE CKD Guideline 2014 suggests that oral sodium bicarbonate should be considered in patients with
CKD 4 or 5 with a serum bicarbonate < 20 mmol/l.
16
The National Kidney Foundation ‘Best Practices In
Managing Hyperkalaemia in CKD’ suggests the use of oral sodium bicarbonate for chronic hyperkalaemia.
17
References
1. Goraya, N. and D.E. Wesson, Clinical evidence that treatment of metabolic acidosis slows the
progression of chronic kidney disease. Curr Opin Nephrol Hypertens, 2019. 28(3): p. 267-277.
2. Moranne, O., et al., Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol, 2009.
20(1): p. 164-71.
3. Shah, S.N., et al., Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am
J Kidney Dis, 2009. 54(2): p. 270-7.
4. Frassetto, L.A. and C.Y. Hsu, Metabolic acidosis and progression of chronic kidney disease. J Am Soc
Nephrol, 2009. 20(9): p. 1869-70.
5. Kraut, J.A. and N.E. Madias, Metabolic Acidosis of CKD: An Update. Am J Kidney Dis, 2016. 67(2): p.
307-17.
6. de Brito-Ashurst, I., et al., Bicarbonate supplementation slows progression of CKD and improves
nutritional status. J Am Soc Nephrol, 2009. 20(9): p. 2075-84.
7. Dubey, A.K., et al., Correction of metabolic acidosis improves muscle mass and renal function in
chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant, 2018.
8. Mathur, R.P., et al., Effects of correction of metabolic acidosis on blood urea and bone metabolism in
patients with mild to moderate chronic kidney disease: a prospective randomized single blind
controlled trial. Ren Fail, 2006. 28(1): p. 1-5.
9. Witham, M. et al on behalf of the BiCARB study group, Clinical and cost-effectiveness of oral sodium
bicarbonate therapy for older patients with chronic kidney disease and low-grade acidosis (BiCARB):
a pragmatic randomised, double-blind, placebo-controlled trial. BMC Med 2020; 18: p. 91-117.
10. Sarafidis, P.A., et al., Prevalence and factors associated with hyperkalemia in predialysis patients
followed in a low-clearance clinic. Clin J Am Soc Nephrol, 2012. 7(8): p. 1234-41.
11. Abramowitz, M.K., et al., Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc
Nephrol, 2013. 8(5): p. 714-20.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 41
12. Jeong, J., S.K. Kwon, and H.Y. Kim, Effect of bicarbonate supplementation on renal function and
nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press, 2014.
12(2): p. 80-7.
13. Hu, M.K., M.D. Witham, and R.L. Soiza, Oral Bicarbonate Therapy in Non-Haemodialysis Dependent
Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomised Controlled
Trials. J Clin Med, 2019. 8(2).
14. National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease:
evaluation, classification, and stratification. American Journal of Kidney Diseases: the official journal
of the National Kidney Foundation. 2002; 39(Suppl 1):S1-266.
15. Roderick, P., et al., Correction of chronic metabolic acidosis for chronic kidney disease patients.
Cochrane Database Syst Rev, 2007(1): p. CD001890.
16. National Institute for Health and Care Excellence: Chronic kidney disease in adults - assessment and
management. Clinical Guideline 182; July 2014. www.nice.org.uk/guideline/cg182/
17. Rabinowitz, L. National Kidney Foundation. Best Practices in Managing Hyperkalaemia in Chronic
Kidney Disease. 2016.
Treatment: Diuretics (Guideline 7.1)
Guideline 7.1 – Use of diuretics for managing Hyperkalaemia in the community
We suggest that loop diuretics may be a useful adjunct for the treatment of chronic hyperkalaemia in
patients who are non-oliguric and volume replete. (2C)
Rationale (Guideline 7.1)
In patients with preserved renal function, the kidneys are the primary route of potassium elimination. Loop
and thiazide diuretics enhance K+ excretion by increasing flow and delivery of sodium to the collecting ducts
and may be useful in treating mild to moderate hyperkalaemia in patients with adequate renal function.
1, 2
Loop diuretics (e.g. furosemide, bumetanide) are the most effective class that promote urinary K+ excretion
and remain effective in patients with moderate renal impairment.
1,3
On the other hand, thiazide diuretics
are effective in patients with an eGFR > 30ml/min.
1
Diuretics should be avoided in patients who are
hypovolaemic or oliguric.
Patients with heart failure are susceptible to both hyperkalaemia and volume overload. RAASi therapy is
frequently used in this setting and loop diuretics are a useful adjunct in controlling chronic hyperkalaemia
whilst treating congestion.
4,5
Decompensated heart failure in the presence of mild-moderate hyperkalaemia
often necessitates a reduction or cessation of cardioprotective medication which may worsen heart failure.
The joint guideline from the Renal Association and British Society of Heart Failure (2019) recommends
consideration of combination therapy with a loop and thiazide diuretic in patients with decompensated
heart failure (HFrEF) and mild-moderate hyperkalaemia.
4
This combination potentiates diuresis and should
theoretically enhance K+ excretion.
Diuretic therapy has a place in the management of chronic hyperkalaemia in patients who are
normovolaemic or hypervolaemic. There is little evidence to support its use in acute hyperkalaemia.
6
A

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 42
multi-modal approach including diuretics, treatment of metabolic acidosis and dietary potassium restriction
may allow the continuation of cardioprotective medications in patients with mild hyperkalaemia.
References
1. Palmer, B.F. and D.J. Clegg, Diagnosis and treatment of hyperkalemia. Cleve Clin J Med, 2017. 84(12):
p. 934-942.
2. Nyirenda, M.J., et al., Hyperkalaemia. BMJ, 2009. 339: p. b4114.
3. Sterns, R.H., et al., Ion-exchange resins for the treatment of hyperkalemia: are they safe and
effective? J Am Soc Nephrol, 2010. 21(5): p. 733-5.
4. Clark, A.L., et al., Change in renal function associated with drug treatment in heart failure: national
guidance. Heart, 2019. 105(12): p. 904-910.
5. Rosano, G.M.C., et al., Expert consensus document on the management of hyperkalaemia in patients
with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors:
coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of
Cardiology. Eur Heart J Cardiovasc Pharmacother, 2018. 4(3): p. 180-188.
6. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015(10).
Treatment: Calcium resonium (Guideline 8.1)
Guideline 8.1 – Calcium resonium for the management of Hyperkalaemia in the community.
We suggest that calcium resonium may be used as a short-term measure to lower serum potassium to a
level of ≤ 5 mmol/l in patients with mild to moderate hyperkalaemia. (2C)
Rationale (Guideline 8.1)
Calcium polystyrene sulphonate (CPS, Calcium resonium) and sodium polystyrene sulphonate (SPS,
Kayexalate) are cation exchange resins that work in the lower GI tract to enhance the elimination of K+ in the
faeces. Each gram of resin has a theoretical in vitro exchange capacity of approximately 1.3 – 2 mmol of K+,
but in vivo, it will be less.
1
Resins cause constipation, therefore laxatives are given to accelerate resin transit
and to increase K+ excretion in stools.
2
Lactulose is an osmotic laxative and is commonly used in the UK.
Macrogol 3350 (Laxido®, Movicol®) should be avoided as it contains potassium (46.6mg or 5.4mmol/l per
sachet).
CPS is approved for use in Europe and SPS approved for use in the USA. SPS was approved by the Food and
Drug Administration (FDA) in 1958 on the basis of two small uncontrolled case series undertaken in the
1950’s.
3
This approval preceded the Kefauver-Harris Drug Amendment (1962) and the European Union
EC/65/65 directive (1965) requiring drug manufacturers to prove the effectiveness and safety of their drug.
4
Following multiple reports of colonic necrosis and other serious gastrointestinal adverse events (perforation,

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 43
bleeding), the FDA applied safety recommendations in 2009. The FDA also advised against the concomitant
administration of sorbitol, but serious complications have also been reported without the use of sorbitol.
5
There are 3 RCTs including SPS as an intervention. Gruy-Kapral (1998) reported a placebo-controlled
randomised study of SPS in normokalaemic patients with ESRD on HD (n=6) and failed to show any
significant reduction in serum K+.
6
The size, design and insufficient baseline data renders this study weak.
Nasir et al (2014) performed a RCT to compare the efficacy and safety of CPS and SPS in CKD patients (n=97)
with hyperkalaemia.
7
Although both drugs lowered serum K+, the study lacked adequate statistical analysis
to substantiate the claim of equal efficacy and there was no control arm (i.e. placebo group). Of note, fewer
side effects were reported with CPS than SPS. Lepage et al (2015) conducted a single centre double-blind
RCT (n=33) in outpatients with CKD and mild hyperkalaemia (K+ 5.0-5.9 mmol/l) comparing efficacy of SPS
30g daily to placebo for 7 days.
4
This study reported an absolute reduction of serum K+ level of 1.25 mmol/l
(p<0.001), but the proportion of patients who achieved normokalaemia did not reach statistical significance
(p=0.07). This trial lacked intermediate efficacy time points. None of these studies met the inclusion criteria
for the Cochrane Review (2015).
8
The evidence for use of SPS is otherwise sparse. Chernin et al (2012) conducted a retrospective study (n=14)
to assess the efficacy of SPS in CKD patients receiving RAASi medication, and observed a reduction in serum
K+ from 6.4 ± 0.3 mmol/l to 4.6 ± 0.6 mmol/l ( p< 0.01) over a median follow up of 14.5 months. The size,
lack of a control group and other confounding factors rendered this study difficult to interpret. Fordjour et al
(2014) conducted a prospective chart review of treatment of hyperkalaemia in hospitalised patients and
found that SPS was included in 95% of treatment regimens with K+ reduction ranging from 0.7 – 1.1 mmol/l.
Effectiveness was deemed to be similar among patients with CKD and those receiving dialysis. Combination
regimens yielded the greatest K+ reduction. Batterink et al (2015) conducted a retrospective study (n=138)
and reported that SPS reduces serum K+ by 0.14 mmol/l more than control, but concluded that this level of
treatment effect may not be clinically important.
9
The evidence for use of CPS is equally sparse. Chaaban et al (2013) conducted a retrospective study (n=70) to
assess the effectiveness of calcium resonium in controlling hyperkalaemia in HD patients.
1
This study
showed poor efficacy attributed to lack of adherence to the drug and dietary restrictions as well as poor
tolerability. Yu et al (2017) reported a retrospective analysis of 247 CKD patients in an out-patient setting
treated with low dose CPS (8.0 ± 3.6 g/day) over a variable duration from > 3 months to beyond 1 year.
10
Baseline eGFR was 30 ± 15 ml/min and serum K+ was ≥ 5.0 mmol/l. Serum K+ decreased significantly from
5.8 ± 0.3 mmol/l to 4.9 ± 0.7 mmol/l (p<0.001) with CPS treatment without any serious adverse effects over
a long period.
CPS and SPS have been used widely for decades for the non-emergency treatment of hyperkalaemia despite
the lack of robust randomised clinical trials to document efficacy or safety. Tolerability and the risk of severe
gastrointestinal adverse effects limit their longterm use. The availability of novel potassium binders
(patiromer and sodium zirconium cyclosilicate) with a stronger evidence base for efficacy and more
favourable side-effect profiles may replace CPS and SPS in clinical practice in the future. However at present,
UK guidance from NICE restricts their use to specific circumstances and the Scottish Medicines Consortium
(SMC) have not approved either drug. Therefore these alternatives to calcium resonium are not currently
available to many patients.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 44
References
1. Chaaban, A., et al., Potassium binders in hemodialysis patients: a friend or foe? Ren Fail, 2013. 35(2):
p. 185-8.
2. Emmett, M., et al., Effect of 3 Laxatives and a Cation-Exchange Resin on Fecal Sodium and Potassium
Excretion. Gastroenterology, 1995. 108(3): p. 752-760.
3. Parks, M. and D. Grady, Sodium Polystyrene Sulfonate for Hyperkalemia. JAMA Intern Med, 2019.
4. Lepage, L., et al., Randomized Clinical Trial of Sodium Polystyrene Sulfonate for the Treatment of Mild
Hyperkalemia in CKD. Clin J Am Soc Nephrol, 2015. 10(12): p. 2136-42.
5. Harel, Z., et al., Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use:
a systematic review. Am J Med, 2013. 126(3): p. 264 e9-24.
6. Gruy-Kapral, C., et al., Effect of single dose resin-cathartic therapy on serum potassium concentration
in patients with end-stage renal disease. J Am Soc Nephrol, 1998. 9(10): p. 1924-30.
7. Nasir, K. and A. Ahmad, Treatment of hyperkalemia in patients with chronic kidney disease: a
comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll
Abbottabad, 2014. 26(4): p. 455-8.
8. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
9. Batterink, J., et al., Effectiveness of Sodium Polystyrene Sulfonate for Short-Term Treatment of
Hyperkalemia. Can J Hosp Pharm, 2015. 68(4): p. 296-303.
10. Yu, M.Y., et al., Long-term efficacy of oral calcium polystyrene sulfonate for hyperkalemia in CKD
patients. PLoS One, 2017. 12(3): p. e0173542.
Treatment: Patiromer (Guidelines 9.1-9.3)
Guideline 9.1 – Patiromer for the management of Hyperkalaemia in the community
We recommend that Patiromer is an option in the management of persistent hyperkalaemia with a
confirmed serum K+ ≥ 6.0 mmol/l in out-patients with CKD Stage 3b-5 (not on dialysis) or heart failure
receiving a sub-optimal dose or not receiving RAASi therapy due to hyperkalaemia. (1A)
Guideline 9.2 – Patiromer for the management of Hyperkalaemia
We recommend that treatment with Patiromer is discontinued if RAASi therapy is stopped. (1A)
Guideline 9.3 – Patiromer for the management of Hyperkalaemia
We recommend that Patiromer is initiated in secondary care only. (1A)
Audit measures
1. The proportion of out-patients with moderate hyperkalaemia (serum K+ 6.0 - 6.4 mmol/l) treated
with patiromer who achieved a serum K+ ≤ 5.0 mmol/l within 1 week.
2. The proportion of out-patients who achieve maximal dose RAASi therapy whilst taking patiromer.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 45
Rationale (Guideline 9.1 – 9.3)
Patiromer is a non-absorbed, sodium free, K+-binding polymer.
1
Calcium is used, rather than sodium, as the
counter ion for K+ exchange. This avoids the potential for excessive sodium absorption and volume overload.
The drug is active throughout the gastrointestinal tract but mostly in the colon. The onset of action is slow at
4-7 hours.
1
Patiromer has the potential to bind to some co-administered oral medication (e.g. metformin,
levothyroxine and ciprofloxacin), therefore administration needs to be separated from other oral
medications by ≥3 hours.
1
To date, the standard approach to treating chronic hyperkalaemia has been a dose reduction or cessation of
cardioprotective medication along with the institution of a low K+ diet. Dietary K+-restriction was
implemented in patiromer trials and K+-lowering drugs are unlikely to replace a low K+ diet although may
allow a less restrictive intake.
The definition of hyperkalaemia used in the patiromer trials to guide treatment differed from the Renal
Association (2014)
2
and European Resuscitation Council (2015)
3
guidelines. In the patiromer trials, mild
hyperkalaemia was defined as serum K+ 5.1 - 5.4 mmol/l and moderate to severe hyperkalaemia as serum K+
5.5 - 6.4 mmol/l.
4-10
Early studies included 3 Phase I clinical pharmacology studies and 12 single dose drug-
drug interaction studies as summarised in Table 6.
The PEARL-HF [11] and PEARL-HF extension
9
studies are the only patiromer trials with all participants having
a diagnosis of chronic heart failure. Fewer patients in OPAL-HK (42%)
5
and AMETHYST-DN (35%)
6
had heart
failure (Appendix 2). In the PEARL-HF study, almost half of the patients treated with patiromer developed
hypokalaemia (K+ < 4 mmol/l) which also infers a higher risk of mortality in heart failure. However, in the
PEARL-HF extension study, spironolactone could be optimised with a lower starting dose of patiromer whilst
reducing the incidence of hypokalaemia.
In the OPAL-HK trial, 76% of patients with HF achieved serum K+ levels within the target range with
patiromer. Hypokalaemia (K+ <3.5 mmol/l) occurred in 3% of patients. During the withdrawal phase,
hyperkalaemia (K+ ≥5.5 mmol/l) recurred in 52% of patients compared with 8% in patients who remained on
patiromer. By the end of the 8-week period, 100% in the patiromer group remained on RAASi compared with
only 55% in the placebo group.
Patients with CKD were well represented in the clinical trials – Bushinsky (100%), OPAL-HK (100%), PEARL-HF
extension study (100%), AMETHYST-DN (87%) and Tourmaline (76%). Notably, the original PEARL-HF
involving MRA titration, included few patients with CKD (27%) and the study duration may have been too
short (4 weeks) to detect a worsening of renal function. In contrast, worsening of renal function was found
in 9.2% of participants of the AMETHYST-DN trial (52 weeks)
6
and 13% of participants in the PEARL-HF
extension study (8 weeks)
9
. In both of these studies, spironolactone was implicated in some cases. In
AMETHYST-DN, this was the most frequently reported adverse event and was the most common cause for
discontinuation. Despite its duration, this study also failed to show any clinically significant reduction in
albuminuria.
A meta-analysis of the patiromer clinical trials showed that the mean reduction in serum K+ at Day 3 was
0.36 mmol/l and at 4 weeks was 0.70 mmol/l.
12
Overall, 93% of patients could continue, start or titrate RAASi
therapy during the maintenance phase of the studies.
12

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 46
STUDY
N=
Study
Duration
Mean
Baseline
K
+
(mmol/l)
Study
Groups
CHANGE IN SERUM K
+
by
PATIROMER DOSE
(dose in g twice daily)
4.2g
8.4g
12.6g
15g
16.8g
PEARL – HF
Pitt 2011
12
Phase II trial
104
4 weeks
4.69
Patiromer
N= 55
-0.22
4.65
Placebo
N= 49
+0.2
3
OPAL-HK
Weir 2015
6
Phase III trial
243
Phase 1
Treatment
4 weeks
5.3
Mild HK
5.0-5.4
N= 92
-0.65
5.7
Mod-Sev
HK
5.5-6.4
N= 151
-1.23
107
Phase 2
Withdrawal
8 weeks
4.49
Patiromer
N=55
0
Daily dose on entry: 12.8g (mild) and 21.4g
(mod)
After first 4 weeks, dose increase was
allowed only for the first occurrence of K ≥
5.1 mmol/l
4.45
Placebo
N=52
+ 0.72
AMETHYST-
DN
Bakris 2015
7
Phase II trial
306
52 weeks
5.3
Mild HK
5.0-5.5
N=222
-0.35
-0.51
-0.55
Mod HK
5.6-5.9
N=84
-0.87
-0.97
-0.92
Bushinsky
2015
8
Prospective
25
48 hours
5.93
All
7hrs: -
0.21
20hrs:
-0.52
48hrs:
-0.75
TOURMALIN
E
Pergola
2017
9
Randomised
Open label
112
4 weeks
5.34
With
Food
N=55
-0.65
median daily dose was 8.4g (8.4, 12.6)
5.44
Without
Food
N=57
-0.62
median daily dose was 8.4g (8.4, 14.1)
PEARL-HF
extension
study
Pitt 2018
10
Open-label
63
8 weeks
4.78
All
-0.13
Table 6: Studies of efficacy of Patiromer in the treatment of hyperkalaemia.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 47
Patiromer was approved for the treatment of chronic hyperkalaemia in the USA in 2015 and in the EU in
2017. The major caveat is that twice daily patiromer dosing was utilized in most trials, whereas the FDA-
approved dose is once daily. This modification stems from concern over the potential for drug interaction
between patiromer and other co-administered medications as discussed above.
NICE has approved the use of patiromer in the treatment of hyperkalaemia for the above indications (text
box).
13
The key evidence for clinical effectiveness was derived from the OPAL-HK study which demonstrated
a reduction in serum K+ by a mean of 1.01 mmol/l after 4 weeks (Phase 1).
5
The mean serum K+ was 0.72
mmol/l higher in patients who were withdrawn compared with those who remained on patiromer (Phase 2).
However, the study cohort did not include patients with clinically significant hyperkalaemia. The patiromer
trials also did not demonstrate an improvement in quality of life or survival in patients with chronic
hyperkalaemia.
The Scottish Medicines Consortium (SMC) did not approve patiromer for the treatment of hyperkalaemia in
NHS Scotland.
14
They concluded that there were several weaknesses and uncertainties with the economic
analysis of the OPAL-HK study given its short duration and endpoints that do not readily correlate with long-
term CKD or cardiovascular outcomes.
Serum K+ should be monitored as clinically indicated.
1
A reasonable approach would be weekly for the first
month and after every dose titration, then monthly thereafter. A rebound in serum K+ occurs on cessation of
patiromer, therefore withdrawal should be undertaken cautiously. The serum K+ may rise as early as two
days after cessation of patiromer, especially if RAASi therapy is continued,
1
therefore monitor serum K+
within one week after drug cessation.
NICE has approved the use of Patiromer in the treatment of chronic hyperkalaemia in
patients with:
• CKD Stage 3b-5 OR Heart Failure
AND
• Serum K
+
confirmed to be ≥ 6.0 mmol/l
AND
• Receiving a sub-optimal dose or not taking RAASi due to hyperkalaemia
AND
• Not on dialysis
Patiromer should be initiated in secondary care.
Stop Patiromer if RAASi therapy is discontinued.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 48
References
1. Vifor Fresenius Medical Care Renal Pharma UK. Veltassa (Patiromer): Annex 1 - Summary of Product
Characteristics. www.ema.europa.eu/en/documents/product-information/veltassa
2. Alfonzo, A., et al. Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. UK Renal
Association. March 2014. www.renal.org/wp-content/uploads/2017/06/hyperkalaemia-guideline-1.
3. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
4. Pitt, B., et al., The PEARL-HF (Multicenter, Randomized, Double-blind, Placebo-Controlled, Parallel-
Group, Multiple-Dose to Evaluate the Effects of RLY5016 in Heart Failure Patients) Trial. Journal of
Cardiac Failure, 2010. 16(11): p. 912.
5. Weir, M.R., et al., Patiromer in patients with kidney disease and hyperkalemia receiving RAAS
inhibitors. N Engl J Med, 2015. 372(3): p. 211-21.
6. Bakris, G.L., et al., Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and
Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA, 2015. 314(2): p. 151-
61.
7. Bushinsky, D.A., et al., Patiromer induces rapid and sustained potassium lowering in patients with
chronic kidney disease and hyperkalemia. Kidney Int, 2015. 88(6): p. 1427-1433.
8. Pergola, P.E., et al., Patiromer Lowers Serum Potassium When Taken without Food: Comparison to
Dosing with Food from an Open-Label, Randomized, Parallel Group Hyperkalemia Study. Am J
Nephrol, 2017. 46(4): p. 323-332.
9. Pitt, B., et al., Evaluation of an individualized dose titration regimen of patiromer to prevent
hyperkalaemia in patients with heart failure and chronic kidney disease. ESC Heart Fail, 2018. 5(3): p.
257-266.
10. Veltassa (Patiromer):European Medicines Agency - Assessment report. 2017. Procedure No.
EMEA/H/C/004180/0000. www.ema.europa.eu/en/documents/assessment-report/veltassa-epar-
public-assessment-report_en.
11. Pitt, B., et al., Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a
double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur
Heart J, 2011. 32(7): p. 820-8.
12. Meaney, C.J., et al., Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium
Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia. Pharmacotherapy, 2017.
37(4): p. 401-411.
13. National Institute for Health and Care Excellence - Patiromer for treating hyperkalaemia. Technology
appraisal guidance [TA623]. February 2020. www.nice.org.uk/guidance/TA623.
14. Scottish Medicines Consortium - Patiromer for the treatment of hyperkalaemia in adults. July 2018.
www.scottishmedicines.org.uk/media/3651/patiromer-sorbitex-calcium-veltassa-final-july-2018-for-
website.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 49
Treatment: Sodium zirconium cyclosilicate (Guidelines 10.1-10.3)
Guideline 10.1 – Sodium Zirconium Cyclosilicate for the management of Hyperkalaemia
We recommend that Sodium Zirconium Cyclosilicate (SZC) is an option in out-patients for the management
of persistent hyperkalaemia with a confirmed serum K+ ≥ 6.0 mmol/l in patients with CKD Stage 3b-5 (not on
dialysis) or heart failure receiving a sub-optimal dose of RAASi therapy. (1A)
Guideline 10.2 – Sodium Zirconium Cyclosilicate for the management of Hyperkalaemia
We recommend that treatment with Sodium Zirconium Cyclosilicate (SZC) in out-patients is discontinued if
RAASi therapy is stopped. (1A)
Guideline 10.3 – Sodium Zirconium Cyclosilicate for the management of Hyperkalaemia
We recommend that Sodium Zirconium Cyclosilicate (SZC) is initiated in secondary care only. (1A)
Audit measures
1. The proportion of out-patients with moderate hyperkalaemia (serum K+ 6.0 - 6.4 mmol/l) treated
with SZC who achieved a serum K+ ≤ 5.0 mmol/l within 48 hours.
2. The proportion of out-patients who achieve maximal dose RAASi therapy whilst taking SZC.
Rationale (Guideline 10.1 – 10.3)
Sodium Zirconium Cyclosilicate (SZC) is a non-absorbed potassium binder that preferentially exchanges H+
and Na+ for K+ and ammonium ions throughout the entire gastrointestinal tract.
1
SZC selectively entraps
monovalent cations (i.e. K+ and ammonium) compared with divalent cations (Ca2+ and Mg2+). Therefore,
unlike patiromer, SZC does not affect Mg2+ levels. SZC binding of ammonium ions increases serum
bicarbonate levels, which is favourable in the context of hyperkalaemia. In-vitro studies have shown that the
K+-binding capacity of SZC is up to 9 times greater than that of sodium polystyrene sulphonate (SPS).
2
The
K+-exchange capacity of SZC is also > 25 times more selective for K+ over Ca2+ or Mg2+ compared with SPS.
3
A comparison of the mechanism of action of all of the oral potassium binders is shown in Appendix 1.
SZC is generally well tolerated. The most common adverse effects are oedema (5.7%) and hypokalaemia
(4.1%). SZC exchanges Na+ for K+, accounting for the potential risk of worsening oedema, hypertension and
heart failure. Product information and administration is described in Appendix 3E.
Three randomised controlled trials and one open label clinical trial have been reported. The first was a
double-blind RCT to investigate the safety and efficacy of SZC across a range of doses over a 2-day period.
4
A
dose-dependent reduction in serum K+ was demonstrated. The primary endpoint of rate of decline of serum
K+ was achieved at the approved dose of 10g three times daily. This was followed by two multi-national
Phase III RCT trials (ZS-003, ZS-004) to evaluate the efficacy and safety of SZC over a longer duration.
5,6
The
most recent study, ZS-005, is an open-label study to assess the efficacy of SZC with longterm use (52 weeks).
7
Patients with CKD, heart failure, diabetes mellitus and receiving RAASi medication were included in these
studies. The studies were conducted in stable out-patients and excluded patients on dialysis, with life-

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 50
threatening hyperkalaemia or diabetic ketoacidosis. There was also no restriction on dietary K+ intake in all
of SZC trials.
The key clinical trials for SZC in the treatment of hyperkalaemia are summarised in Table 7. These studies
were designed to determine the efficacy of SZC in controlling hyperkalaemia over a 48-hr induction phase,
followed by sustained control during a maintenance phase of variable duration – 14 days (ZS-003), 28 days
(ZS-004) and 52 weeks (ZS-005). The proportion of patients with CKD, diabetes, heart failure and taking
RAASi drugs were similar in these studies (Appendix 2).
These clinical trials have demonstrated the efficacy of SZC. The onset of action of SZC is within 1 hour after
ingestion and there is a close correlation between the initial serum K+ level and the size of the treatment
effect.
1
The median time to normalisation of serum K+ was 2.2 hours.
6
SZC lowers serum K+ by 1.1 mmol/l
within 48 hours.
6
The ZS-003 and ZS-004 clinical trials also demonstrated a greater K+-lowering effect with
increasing severity of hyperkalaemia.
5,6
In patients with a serum K+ > 6.0 mmol/l, SZC lowers serum K+ by
1.5 mmol/l within 48 hours.
6
In the longterm study conducted over 12 months, 87% of patients were able to
continue RAASi or increase the dose and only 11% discontinued RAASi therapy.
7
STUDY
Study
Design
N =
Study
duration
Dose of SZC
(x3/day)
Renal
function eGFR
(ml/min)
Mean
Baseline K
+
(mmol/l)
K
+
Change
(mmol/l)
ZS-002
4
Ash
2015
Phase II RCT
90
48 hrs
Placebo
0.3g
3g
10g
58.1 ± 26.5
56.5 ± 24.0
57.1 ±22.1
51.6 ± 22.3
5.1 ± 0.4
5.2 ± 0.3
5.0 ± 0.3
5.1 ± 0.4
- 0.26 ± 0.4
- 0.39 ± 0.4
- 0.42 ± 0.4
- 0.92 ± 0.5
ZS-003
5
Packman
2015
Phase III RCT
753
Stage 1
48 hrs
Induction
(randomised)
Placebo
1.25g
2.5g
5g
10g
5.3
5.4
5.4
5.3
5.3
- 0.25 (0.19-0.32)
0.30
- 0.46 (0.39-0.53)
- 0.54 (0.47-0.62)
- 0.73 (0.65-0.82)
Stage 2
Days
3-14
Maintenance
(randomised)
Placebo
SZC 5g
3.5 – 4.9
+ 0.47%/ hr
+ 0.09%/ hr
Placebo
SZC 10g
+ 1.04%/ hr
+ 0.14%/ hr
ZS-004
6
HARMONIZE
Kosiborod
2014
Phase III RCT
258
Stage 1
48 hrs
Induction
(open label)
10g
46.3 ± 30.5
5.6 ± 0.4
- 1.1 (1.0-1.1)
Stage 2
28 days
Maintenance
(randomised)
Placebo
5g
10g
15g
48.0 ± 28.8
48.0 ± 30.7
44.7 ± 30.7
44.9 ± 29.5
4.6 ± 0.4
4.5 ± 0.4
4.4 ± 0.4
4.5 ± 0.4
- 0.4 (0.3-0.6)
- 0.8 (0.6-0.9)
- 1.1 (0.9-1.3)
- 1.2 (1.0-1.4)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 51
STUDY
Study
Design
N =
Study
duration
Dose of SZC
(x3/day)
Renal
function eGFR
(ml/min)
Mean
Baseline K
+
(mmol/l)
K
+
Change
(mmol/l)
ZS-004E
1
Extension of
ZS-004
123
11 mths
Maintenance
(open label)
10g once daily
46.3 ± 30.5
4.6
88% of patients
achieved K < 5.1
mmol/l
ZS-005
7
Spinowitz
2019
Phase III
Open-label
Prospective
(single arm)
751
24-72 hrs
Acute Phase
10g
< 60: 73.5%
≥ 60: 25.3%
5.6
- 0.8
12 mths
Extended
Phase
5g once daily
titrated to
10 or 15g/ day
OR 5g alt days
5.6
- 1.0
HARMONIZE-
GLOBAL
8
Zannad
2020
Phase III RCT
267
48 hrs
Correction
Phase
10g tds
5.7 ± 0.5
-1.28
28 days
Maintenance
(randomised)
Placebo
5g
10g
3.5 – 5.0
Geometric LSM
5.32 (5.16, 5.49)
4.81 (4.69, 04.94)
4.38 (4.27, 4.50)
Table 7: Studies of the efficacy of SZC in treatment of Hyperkalaemia
SZC – Sodium zirconium cyclosilicate; hrs – hours; mths – months; LSM – least squares mean
A meta-analysis of the SZC trials have shown that it lowers serum K+ by 0.17 mmol/l at 1 hour and 0.67
mmol/l at 48 hours after administration.
9
In a subgroup analysis of patients with a baseline serum K+
ranging from 6.1 – 7.2 mmol/l, SZC lowered serum K+ by a mean of 0.4 mmol/l at 1 hour after administration
of 10g dose.
10
In the recently published HARMONIZE-Global study, significantly more patients achieved
normokalaemia with SZC 5mg (58.6%) and SZC 10mg (77.3%) compared with placebo (24%).
8
Approval for SZC was delayed due to concerns about the manufacturing processes, but was granted in the
EU in March 2018, followed by the FDA in May 2018. Although the marketing authorisation states SZC may
be used for the ‘treatment of hyperkalaemia in adults’, the submission to NICE was limited to patients with
CKD and/ or heart failure.
NICE assessed the clinical and cost effectiveness of SZC based on the ZS-004 and ZS-005 clinical trials.
11
A few
short-falls were identified in the SZC trials including the definition of hyperkalaemia (K+ ≥ 5.1 mmol/l) which
was lower than the Renal Association and European Resuscitation Council guidelines (K+ ≥ 5.5 mmol/l). The
clinical trials did not compare the efficacy of SZC versus dietary restriction or any other active K+-lowering
drug. There was also no evidence that SZC improves quality of life or extends life. However, given the strong
evidence for use of RAASi drugs in patients with CKD and heart failure, the cost-effectiveness analysis
suggests that the use of SZC in facilitating patients staying on RAASi drugs is a good use of NHS resources.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 52
NICE has approved SZC in the treatment of persistent hyperkalaemia for the above indications (text box).
11
Safety and efficacy has been shown up to 52 weeks of therapy, but the duration of treatment in clinical
practice will likely be lifelong unless RAASi is discontinued. SZC will complement, rather than replace, a low-
K+ diet. SZC may allow less strict dietary restrictions, thereby improving quality of life for patients.
The aim is to achieve the minimum effective dose of SZC to prevent recurrence of hyperkalaemia. The
recommended starting dose is 5g once daily, with up-titration to a maximum dose of 10g once daily or
down-titration to 5g alternate days if required (Appendix 3E).
1
Dose titration or cessation will be led by
secondary care. In real-world practice, blood monitoring will shared with primary care, therefore clear
guidance or protocols will be necessary. Based on the ZS-005 trial conducted over 12 months, blood
monitoring should be performed weekly for the first month, then monthly thereafter.
7
Serum K+ should also
be assessed one week after drug cessation.
7
The role for SZC in the treatment of hyperkalaemia is likely to evolve as clinical experience is gained and as
further evidence becomes available.
References
1. Astra Zeneca. Lokelma (sodium zirconium cyclosilicate) for oral suspension: Summary of Product
Characteristics. 2018. www.ema.europa.eu/ema/
2. Yang, A., et al., In Vitro Ion Exchange Capacity and Selectivity of Zs-9, a Novel, Selective Cation Trap
for the Treatment of Hyperkalemia. American Journal of Kidney Diseases, 2014. 63(5): p. A115-A115.
3. Stavros, F., et al., Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One,
2014. 9(12): p. e114686.
4. Ash, S.R., et al., A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney
disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int, 2015. 88(2):
p. 404-11.
5. Packham, D.K., et al., Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med, 2015. 372(3): p.
222-31.
NICE has approved the use of SZC in the treatment of persistent hyperkalaemia in the
out-patient setting under these circumstances:
• Patients with CKD Stage 3b-5 OR Heart Failure
AND
• Serum K
+
confirmed to be ≥ 6.0 mmol/l
AND
• Patient is receiving a sub-optimal dose of RAASi due to hyperkalaemia
AND
• Not on dialysis
Initiation of SZC is restricted to secondary care.
Stop SZC if RAASi therapy is discontinued.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 53
6. Kosiborod, M., et al., Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days
among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA, 2014.
312(21): p. 2223-33.
7. Spinowitz, B.S., et al., Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-
Month Phase 3 Study. Clin J Am Soc Nephrol, 2019. 14(6): p. 798-809.
8. Zannad, F., et al., Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the
randomized, placebo-controlled HARMONIZE-Global study. ESC Heart Fail, 2020. 7(1): p. 54-64.
9. Meaney, C.J., et al., Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium
Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia. Pharmacotherapy, 2017.
37(4): p. 401-411.
10. Bianchi, S. and G. Regolisti, Pivotal clinical trials, meta-analyses and current guidelines in the
treatment of hyperkalemia. Nephrol Dial Transplant, 2019. 34(Suppl 3): p. iii51-iii61.
11. National Institute for Health and Care Excellence - Sodium Zirconium Cyclosilicate for treating
hyperkalaemia. Technology appraisal guidance [TA599]. September 2019.
www.nice.org/guidance/TA599.
Prevention (Guidelines 11.1-11.3)
Guideline 11.1 – Prevention of Hyperkalaemia in the community: monitoring
We recommend monitoring of renal function in patients at risk of hyperkalaemia with known CKD, heart
failure, diabetes and in any patient taking RAASi medication. (1A)
Guideline 11.2 – Prevention of Hyperkalaemia in the community: prescribing
We recommend caution in prescribing trimethoprim to patients with renal impairment or those taking RAASi
drugs. (1A)
Guideline 11.3 – Prevention of Hyperkalaemia in the community: sick day rules
We recommend that healthcare professionals provide advice to patients regarding the risks of AKI and
hyperkalaemia during acute illness and measures to avoid these complications. (1B)
Audit measures
1. Proportion of patients with severe hyperkalaemia (Serum K+ ≥ 6.5 mmol/l) on admission to hospital
who had been provided with ‘Sick Day Rules’ advice.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 54
Rationale (Guideline 11.1 - 11.3)
Hyperkalaemia is an anticipated complication in patients with a history of CKD, heart failure or diabetes
mellitus. Patients requiring RAASi drugs for other indications, e.g. spironolactone for decompensated liver
disease, also require surveillance for hyperkalaemia. Blood monitoring is discussed in Guidelines 1.1-1.2.
Good communication between primary and secondary care regarding monitoring and drug titration is
essential.
Drug prescribing in the community and out-patient setting is a major factor for the development of
hyperkalaemia. The elderly are very susceptible to hyperkalaemia and polypharmacy is a common problem.
Increased awareness of drugs that can cause hyperkalaemia and monitoring patients at risk may reduce
morbidity, hospital admissions and mortality.
Drugs commonly implicated in hyperkalaemia are shown below in Table 8.
Table 8: Drugs implicated in development of hyperkalaemia and exacerbating factors.
The Medicines and Healthcare products Regulatory Agency (MHRA) issued a safety alert, initially in June
2014, regarding the concomitant use of ACEi or ARB with MRAs (i.e. spironolactone or eplerenone) given the
increased risk of severe hyperkalaemia particularly in patients with advanced renal impairment.
1
The MHRA
recommend caution in co-prescription of these drugs with regular monitoring of serum biochemistry and
discontinuation if hyperkalaemia develops.
NICE Clinical Guideline on ‘CKD in adults: assessment and management’ states that RAASi should not be
routinely started in patients with a serum K+ level ≥ 5.0 mmol/l and should be discontinued if serum K+ is ≥
6.0 mmol/l.
2
The NICE Clinical Guideline on ‘Chronic Heart Failure in adults: assessment and management’
states that serum K+ should be monitored before and after starting a RAASi or changing the dose, but does
not specify the K+ level at which RAASi should be avoided or discontinued.
3
Given the potential benefits of
RAASi therapy in patients with CKD and heart failure, NICE has recently approved the use of SZC and
patiromer to facilitate continuing RAASi therapy in selected patients.
4,5
Trimethoprim is a first-line antibiotic, most commonly prescribed for simple urinary tract infections (UTI). It
can be prescribed alone or in combination with sulfamethoxazole (co-trimoxazole). The mechanism by
which trimethoprim causes hyperkalaemia is by reducing renal K+ excretion through competitive inhibition
RAASi (ACE Inhibitors, Angiotensin II Receptor
Blockers, Mineralocorticoid Receptor Antagonists)
Potassium supplements
Potassium-sparing diuretics
Trimethoprim/ Co-trimoxazole
NSAIDs
Non-selective beta-blockers
RISK OF HYPERKALMAEMIA
INCREASED IN:
Renal Impairment
Diabetes Mellitus
Elderly
Use of > 1 RAASi drug
Combining any of these
groups of drugs

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 55
of epithelial sodium channels in the distal nephron.
6
An increase in serum K+ level of 0.36 – 1.21 mmol/l or
higher can occur within 3-10 days of treatment.
7
Treatment with RAASi or NSAIDs exacerbates
hyperkalaemia.
6
There have been multiple reports confirming the risk of hyperkalaemia and AKI in patients treated with
trimethoprim.
8-10
Antoniou et al reported a 7-fold increased risk of hospital admission for hyperkalaemia in
elderly patients (age ≥ 66 years) taking trimethoprim- sulfamethoxazole compared with other antibiotics for
UTI.
8
In a large UK cohort study (n=1,191, 905) of older adults (age ≥ 65 years), Crellin et al demonstrated an
increased risk of developing hyperkalaemia (OR 2.27) and AKI (OR 1.72) within 14 days of trimethoprim
prescription compared with amoxicillin.
9
The risk of hyperkalaemia has been shown in patients receiving
high-dose
11, 12
and low-dose
10
trimethoprim.
Nutritional intake is another important factor in preventing hyperkalaemia, particularly in patients with CKD.
In patients with advanced CKD, the ability to adapt to an increased potassium intake diminishes and
becomes almost negligible in ESRD, making these patients very susceptible to hyperkalaemia.
13
A low-K+
diet is usually instituted when the serum K+ is consistently ≥ 5.5 mmol/l and has been discussed in Guideline
5.1.
The bowel compensates for the reduction in renal K+ loss as renal function declines. The capacity for the
bowel to secrete K+ is inversely related to residual renal function and becomes the main route of K+
excretion in patients with ESRD.
13,14
Therefore, constipation predisposes to hyperkalaemia in patients with
renal impairment.
The ‘Sick day rules’ provides information to patients taking drugs known to cause AKI and hyperkalaemia
(e.g. RAASi, NSAIDs) advising temporary discontinuation of these medications during acute illness,
particularly in the context of volume depletion (e.g. diarrhoea and/or vomiting, fevers/ rigors). The use of
this strategy is controversial. The NICE ‘Clinical Guideline on AKI’ advocates use of sick day guidance.
15
On
the other hand, ‘Think Kidneys’ urges caution as the evidence-base for this guidance is weak, discontinuation
of cardio-protective medication could exacerbate underlying condition and patients may not restart
medication on recovery or achieve previous dosage.
16
The ‘Think Kidneys’ Programme Board recommends
that it is reasonable to provide sick day guidance to patients at high risk of AKI based on an individual risk
assessment, but a more systematic roll-out of the ‘Sick day rules’ should be undertaken in the context of a
formal evaluation.
In clinical practice, many patients admitted to hospital with an AKI at initial presentation are receiving one or
more drugs that can exacerbate hyperkalaemia. It is standard practice to withhold these until renal
Trimethoprim
• Use trimethoprim with caution in patients with severe renal impairment (eGFR <
30 ml/min)
• Avoid trimethoprim in patients receiving RAASi drugs (high risk of AKI and
hyperkalaemia)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 56
recovery. The Sick Day rules moves the timeline to discontinuation earlier in patients at risk of AKI and if
applied appropriately, may reduce the risk of severe hyperkalaemia during acute illness.
References
1. Medicines and Healthcare products Regulatory Agency -Spironolactone and renin-angiotensin
system drugs in heart failure: risk of potentially fatal hyperkalaemia - February 2016.
2. National Institute for Health and Care Excellence: Chronic kidney disease in adults - assessment and
management. Clinical Guideline 182; July 2014. www.nice.org.uk/guideline/cg182/
3. National Institute for Health and Care Excellence: Chronic heart failure in adults: diagnosis and
management. Clinical Guideline 106; September 2018. www.nice.org.uk/guideline/ng106
4. National Institute for Health and Care Excellence. Sodium zirconium cyclosilicate for treating
hyperkalaemia. Technology appraisal guidance [TA599]. September 2019. www.nice.org.uk/TA599.
5. National Institute for Health and Care Excellence - Patiromer for treating hyperkalaemia. Technology
appraisal guidance [TA623]. February 2020. www.nice.org.uk/guidance/TA623.
6. Ben Salem, C., et al., Drug-induced hyperkalemia. Drug Saf, 2014. 37(9): p. 677-92.
7. Perazella, M.A., Trimethoprim-induced hyperkalaemia - Clinical data, mechanism, prevention and
management. Drug Safety, 2000. 22(3): p. 227-236.
8. Antoniou, T., et al., Trimethoprim-Sulfamethoxazole-Induced Hyperkalemia in Patients Receiving
Inhibitors of the Renin-Angiotensin System A Population-Based Study. Archives of Internal Medicine,
2010. 170(12): p. 1045-1049.
9. Crellin, E., et al., Trimethoprim use for urinary tract infection and risk of adverse outcomes in older
patients: cohort study. BMJ, 2018. 360: p. k341.
10. Higashioka, K., et al., Renal Insufficiency in Concert with Renin-angiotensin-aldosterone Inhibition Is a
Major Risk Factor for Hyperkalemia Associated with Low-dose Trimethoprim-sulfamethoxazole in
Adults. Intern Med, 2016. 55(5): p. 467-71.
11. Gentry, C.A. and A.T. Nguyen, An evaluation of hyperkalemia and serum creatinine elevation
associated with different dosage levels of outpatient trimethoprim-sulfamethoxazole with and
without concomitant medications. Ann Pharmacother, 2013. 47(12): p. 1618-26.
12. Nguyen, A.T., C.A. Gentry, and R.Z. Furrh, A comparison of adverse drug reactions between high- and
standard-dose trimethoprim-sulfamethoxazole in the ambulatory setting. Curr Drug Saf, 2013. 8(2):
p. 114-9.
13. Cupisti, A., et al., Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with
Decreased Kidney Function. Nutrients, 2018. 10(3).
14. Ahmed, J. and L.S. Weisberg, Hyperkalemia in dialysis patients. Seminars in Dialysis, 2001. 14(5): p.
348-356.
15. National Institute for Health and Care Excellence: Acute Kidney Injury. Clinical Guideline QS76;
December 2014. www.nice.org.uk/guideline/qs76
16. 'Think Kidneys' - "Sick day" guidance in patients at risk of Acute Kidney Injury: a Position Statement
from the Think Kidneys Board. January 2018.www.thinkkidneys.nhs.uk/aki

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 57
Treatment Algorithm: Community (Guideline 12.1)
Guideline 12.1 – Treatment Algorithm for Hyperkalaemia in the community
We recommend that the treatment of hyperkalaemia in patients in the community and out-patient setting is
guided by its severity and clinical condition of the patient as summarised in the treatment algorithm. (1B)
Rationale (Guideline 12.1)
Hyperkalaemia is commonly detected in the community and the approach to monitoring and treatment is
variable. An algorithm has been designed to assist clinicians in the out-patient and primary care settings as
shown in Appendix 5.
Patients with a serum K+ < 5.5 mmol/l do not require any specific treatment. Patients with persistent mild
hyperkalaemia (K+ 5.5 – 5.9 mmol/l) warrant a review of medication (e.g. RAASi) and dietary K+ intake.
Treatment of metabolic acidosis (serum bicarbonate < 22 mmol/l) and initiation of diuretics may be helpful
in chronic hyperkalaemia.
Patients with persistent moderate hyperkalaemia (K+ 6.0 – 0.4 mmol/l) who are not acutely unwell require
similar considerations – medication review, treatment of metabolic acidosis and a low K+ diet. However,
some patients may be candidates for a potassium binder (Patiromer or SZC) if they meet the NICE criteria as
discussed in Guidelines 9 and 10.
Patients with moderate hyperkalaemia who are acutely unwell and those with severe hyperkalaemia (K+ ≥
6.5 mmol/l) warrant referral to hospital for urgent assessment. RAASi drugs should be withheld until
recovery.
Blood monitoring is essential after a hyperkalaemic event and the urgency is guided by the severity.
Recommended intervals for blood monitoring are discussed in Guideline 1.1-1.2. Recurrence of
hyperkalaemia is common, particularly in patients with CKD, therefore it is important to consider
preventative measures.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 58
Section 2
Management of Hyperkalaemia
in Hospital

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 59
Hyperkalaemia in Hospital
Introduction
Hyperkalaemia is a potentially life-threatening medical emergency. The incidence in hospitalised patients
ranges from 1 – 10%.
1-5
It is associated with increased hospitalisation and mortality.
6-8
Despite this, there is
limited evidence to guide treatment. This may account for the observed variability in the treatment of
patients with hyperkalaemia, even within the same hospital.
9
Therefore, guidance on the treatment of
hyperkalaemia based on the current evidence is needed.
The most serious consequences of hyperkalaemia are arrhythmias and cardiac arrest. The risk of these
events increases with K+ level ≥ 6.5 mmol/L and even small elevations in K+ above this concentration can
lead to rapid progression from peaked T waves to ventricular fibrillation or asystole.
9
The longer a patient
has a high K+ level, the greater the risk of sudden deterioration.
10
Urgent treatment can avoid life-
threatening complications.
11
The threshold for emergency treatment varies, but most guidelines recommend that emergency treatment
should be given if the serum K+ is ≥ 6.5 mmol/L with or without ECG changes.
11-13,14
It is also accepted that
emergency treatment should be initiated before serum biochemistry is known if hyperkalaemia is suspected
on clinical grounds or in the presence of ECG changes.
11
The evidence base for drug treatment in hospitalised patients is limited. Indeed, the Cochrane review for
treatment of acute hyperkalaemia in adults included only 7 studies.
13
Intravenous calcium salts (gluconate
and chloride) are life-saving, but there are no clinical trials to prove efficacy.
13
Insulin-glucose infusion is the
most effective treatment to lower serum K+, but the conventional treatment regimen was based on small
historical studies mostly in dialysis patients. Beta-agonists appear to be effective in lowering serum K+, but
some patients are unresponsive. Sodium bicarbonate was frequently used in clinical practice, but there is
little favourable evidence of its efficacy in treating acute hyperkalaemia.
13
Over the past 5 years, there has been some progress in the treatment of hyperkalaemia relating to
management in hospitalised patients. Several retrospective studies have been conducted to investigate the
incidence and causes of iatrogenic hypoglycaemia following insulin-glucose administration.
15-19
Other studies
have compared conventional versus low dose insulin regimens.
20-22
The use of variable dosing regimens of
insulin and glucose in these reports is a confounding factor. Ultimately, iatrogenic hypoglycaemia appears to
be multifactorial with a low pre-treatment blood glucose being a consistent risk factor. Another important
development is the availability of novel potassium binders, patiromer and sodium zirconium cyclosilicate,
which have both been approved by NICE for the treatment of life-threatening hyperkalaemia although their
efficacy in the acute setting has not yet been reported.
23,24
The management of acute hyperkalaemia in hospital requires a systematic and consistent approach. This
section of the guideline reviews clinical assessment, ECG and laboratory tests, the 5-step approach to
treatment, timely specialist referral, escalation of care and prevention in hospitalised patients.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 60
References
1. Moore, M.L. and R.R. Bailey, Hyperkalaemia in patients in hospital. N Z Med J, 1989. 102(878): p.
557-8.
2. Paice, B., et al., Hyperkalaemia in patients in hospital. Br Med J (Clin Res Ed), 1983. 286(6372): p.
1189-92.
3. Shemer, J., et al., Incidence of Hyperkalemia in Hospitalized-Patients. Israel Journal of Medical
Sciences, 1983. 19(7): p. 659-661.
4. Conway, R., et al., Serum potassium levels as an outcome determinant in acute medical admissions.
Clin Med (Lond), 2015. 15(3): p. 239-43.
5. Fleet, J.L., et al., Validity of the International Classification of Diseases 10th revision code for
hyperkalaemia in elderly patients at presentation to an emergency department and at hospital
admission. Bmj Open, 2012. 2(6).
6. Palaka, E., et al., Associations between serum potassium and adverse clinical outcomes: A systematic
literature review. Int J Clin Pract, 2020. 74(1): p. e13421.
7. Collins, A.J., et al., Association of Serum Potassium with All-Cause Mortality in Patients with and
without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am J Nephrol, 2017. 46(3): p. 213-
221.
8. Thongprayoon, C., et al., Admission Serum Potassium Levels in Hospitalized Patients and One-Year
Mortality. Medicines (Basel), 2019. 7(1).
9. Acker, C.G., et al., Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results
of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med,
1998. 158(8): p. 917-24.
10. Sood, M.M. and R.P. Pauly, A case of severe hyperkalemia: fast, safe and effective treatment is
required. Journal of Critical Care, 2008. 23(3): p. 431-433.
11. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
12. Alfonzo, A.V.M., et al., Potassium disorders - clinical spectrum and emergency management.
Resuscitation, 2006. 70(1): p. 10-25.
13. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
14. Guidelines and Audit Implementation Network (GAIN) -Guidelines for the Treatment of
Hyperkalaemia in Adults. August 2014. www.rqia.org.uk
15. Schafers, S., et al., Incidence of hypoglycemia following insulin-based acute stabilization of
hyperkalemia treatment. Journal of Hospital Medicine, 2012. 7(3): p. 239-242.
16. Apel, J., S. Reutrakul, and D. Baldwin, Hypoglycemia in the treatment of hyperkalemia with insulin in
patients with end-stage renal disease. Clin Kidney J, 2014. 7(3): p. 248-50.
17. Coca, A., et al., Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients
with impaired renal function. PLoS One, 2017. 12(2): p. e0172961.
18. Scott, N.L., et al., Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the
emergency department. Am J Emerg Med, 2019. 37(2): p. 209-213.
19. Boughton, C.K., et al., Preventing Hypoglycemia Following Treatment of Hyperkalemia in Hospitalized
Patients. J Hosp Med, 2019. 14(5): p. 284-287.
20. Pierce, D.A., G. Russell, and J.L. Pirkle, Jr., Incidence of Hypoglycemia in Patients With Low eGFR
Treated With Insulin and Dextrose for Hyperkalemia. Ann Pharmacother, 2015. 49(12): p. 1322-6.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 61
21. LaRue, H.A., G.D. Peksa, and S.C. Shah, A Comparison of Insulin Doses for the Treatment of
Hyperkalemia in Patients with Renal Insufficiency. Pharmacotherapy, 2017. 37(12): p. 1516-1522.
22. Garcia, J., et al., Reduced Versus Conventional Dose Insulin for Hyperkalemia Treatment. J Pharm
Pract, 2018: p. 897190018799220.
23. National Institute for Health and Care Excellence - Sodium Zirconium Cyclosilicate for treating
hyperkalaemia. Technology appraisal guidance [TA599]. September 2019.
www.nice.org/guidance/TA599.
24. National Institute for Health and Care Excellence - Patiromer for treating hyperkalaemia. Technology
appraisal guidance [TA623]. February 2020. www.nice.org.uk/guidance/TA623
Clinical assessment (Guidelines 13.1-13.2)
Guideline 13.1 – Hyperkalaemia: Clinical Assessment; History and examination
We recommend that all patients presenting with hyperkalaemia undergo a comprehensive medical and drug
history and clinical examination to determine the cause of hyperkalaemia. (1B)
Guideline 13.2 – Hyperkalaemia: Clinical Assessment; NEWS
We recommend that all patients with known or suspected hyperkalaemia undergo urgent clinical assessment
using an early warning scoring system to assess level of acuity. (1C)
Audit measures
1. Length of hospital stay and in-hospital mortality of patients admitted with hyperkalaemia.
Rationale (Guideline 13.1-13.2)
A careful medical history may identify risk factors for hyperkalaemia as shown in Table 9. It is important to
elicit any history of pre-existing kidney disease and any factors which may contribute to an acute kidney
injury (e.g. diarrhoea & vomiting, infection, medications). Apply a high index of suspicion of hyperkalaemia
in patients groups at risk, e.g. patients with end-stage renal failure, diabetes, heart failure or liver failure.
Access to electronic patient records and historical biochemical results can help establish baseline renal
function.
Symptoms are often non-specific and may be overshadowed by the acute illness whilst other patients are
asymptomatic.
1
Muscle weakness and/ or paraesthesiae may occur in severe cases.
1-4
The medication
history is an important part of determining the aetiology of hyperkalaemia. Ask about current medication,
recent changes and use of over the counter medications.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 62
Table 9: Factors associated with an increased risk of hyperkalaemia.
The most significant consequences of hyperkalaemia are arrhythmias and cardiac arrest, therefore early
recognition, cardiac monitoring and prompt treatment are essential. Early identification of hyperkalaemia,
with or without adverse clinical signs, enables specific interventions, specialist referral (if required) and
appropriate escalation of care.
The ABCDE approach is an established method for rapid systematic assessment of the acutely ill patient and
allows problems, including hyperkalaemia, to be identified and treated promptly.
5
The National Early
Warning Score (NEWS) was developed by the Royal College of Physicians.
6
NEWS uses several vital signs to
detect abnormalities and identify acute ill patients.
7
Baseline assessment and serial monitoring is essential
in identifying patients who are deteriorating and the NEWS can facilitate timely decisions for escalation of
care.
These standardised methods of patient assessment and monitoring improve patient safety and facilitates
clear communication about acutely unwell patients.
References
1. Alfonzo, A.V., et al., Potassium disorders--clinical spectrum and emergency management.
Resuscitation, 2006. 70(1): p. 10-25.
2. Sanson, G., et al., Tetraparesis and failure of pacemaker capture induced by severe hyperkalemia:
case report and systematic review of available literature. J Emerg Med, 2015. 48(5): p. 555-61 e3.
3. Quaresma, F. and M. Bentes Jesus, Tetraparesia: an unusual presentation of disseminated
tuberculosis. BMJ Case Rep, 2017. 2017.
4. Marques, J.S. and A.N. Diogo, Dead man walking: an extreme case of sinusoidal wave pattern in
severe hyperkalemia. J Am Coll Cardiol, 2012. 59(23): p. 2118.
5. Thim, T., et al., Initial assessment and treatment with the Airway, Breathing, Circulation, Disability,
Exposure (ABCDE) approach. Int J Gen Med, 2012. 5: p. 117-21.
6. National Early Warning Score (NEW) 2. Royal College of Physicians. December 2017.
www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2.
Risk factors for Hyperkalaemia:
• Acute Kidney Injury
• Dialysis dependency (haemodialysis or peritoneal dialysis)
• Chronic Kidney Disease Stages 4 & 5 (CKD, eGFR < 30 ml/min/1.73m
2
)
• Drugs (renin-angiotensin-aldosterone inhibitors, NSAIDs, trimethoprim)
• Cardiac failure
• Diabetes mellitus (renin-angiotensin drugs, diabetic keto-acidosis)
• Liver disease (spironolactone, hepato-renal failure)
• Addison’s Disease (primary adrenal insufficiency)
• Hyporeninaemic hypoaldosteronism (Type IV renal tubular acidosis)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 63
7. National Institute for Health and Care Excellence. Acutely Ill Adults in hospital: recognising and
responding to deterioration. Clinical guideline 50. July 2007 (updated April 2019).
www.nice.org.uk/guidance/cg50.
ECG and cardiac monitoring (Guidelines 14.1-14.2)
Guideline 14.1 – Hyperkalaemia: ECG
We recommend that all hospitalised patients with a serum K + level ≥ 6.0 mmol/L have an urgent 12-lead
ECG (electrocardiogram) performed and assessed for changes of hyperkalaemia. (1B)
Guideline 14.2 – Hyperkalaemia: Cardiac monitoring
We recommend a minimum of continuous 3-lead ECG monitoring for all patients with a serum K+ ≥ 6.5
mmol/L, patients with features of hyperkalaemia on 12-lead ECG, and in patients with a serum K+ 6.0-6.4
mmol/L who are clinically unwell or in whom a rapid rise in serum K+ is anticipated, ideally in a higher-
dependency setting. (1C)
Audit measures
1. Proportion of patients with a serum K + level ≥ 6.0 mmol/L who had a 12-lead ECG recorded before
and after treatment for hyperkalaemia.
Rationale (Guideline 14.1 – 14.2)
The ECG is used to assess cardiac toxicity and risk of arrhythmias in patients with known or suspected
hyperkalaemia and may be the most readily available diagnostic tool. In terms of clinical significance, the
type of ECG changes present is an important factor in predicting the outcome of patients with severe
hyperkalaemia.
1
ECG abnormalities may reflect the severity and rate of rise of serum K+.
2
The ECG changes associated with hyperkalaemia are attributable to the physiological effect of a raised serum
K+ on myocardial cells. The atrial myocardium is more sensitive than the ventricular myocardium to the
effects of hyperkalaemia and the specialised tissue (sinoatrial node and bundle of His) is the least sensitive.
3
Hyperkalaemia is associated with depression of conduction between adjacent cardiac myocytes, manifesting
in prolongation of the PR interval and QRS duration. The P wave amplitude is diminished in the early stages
as T wave amplitude increases. Suppression of sinoatrial function results in bradycardia or standstill.
Suppression of atrioventricular (AV) conduction will give rise to varying degrees of AV block.
The ECG changes may be progressive with worsening severity as shown in Figure 1, but these changes do not
always occur sequentially and multiple changes may occur concurrently.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 64
Figure 1: Progressive changes in ECG with increasing severity of hyperkalaemia.
The most commonly recognised ECG sign is peaked T waves, but on its own is rarely a sign of life-threatening
hyperkalaemia.
2
A normal T wave usually has an amplitude of < 5mm in the precordial leads and < 10mm in
the limb leads. The normal shape is asymmetric with a slow upstroke and a rapid down stroke. Peaked T
waves have a high amplitude, narrow base, sharp pointy apex and are generally symmetrical.
4,5
Early studies
reported the frequency of peaked T waves was 36% in hospitalised patients with hyperkalaemia.
6
Similarly,
Freeman et al reported peaked T waves at presentation in 35% of patients with a serum K+ > 6.0 mmol/l.
7
Durfey et al also reported peaked T waves in 30% of patients with a serum K+ ≥ 6.5 mmol/l.
1
Given that
peaked T waves occur in approximately one third of patients with moderate to severe hyperkalaemia, early
recognition can prompt early intervention and prevent deterioration.
The typical ECG features of hyperkalaemia are shown below in Figure 2.
The reported utility of the ECG is variable. Some reports suggest that 50-64% of patients with a serum K+ ≥
6.5 mmol/L show no ECG changes consistent with hyperkalaemia.
6-8
In contrast, Durfey et al analysed the
incidence of hyperkalaemic ECG changes by severity: K+ 6.5 – 6.9 mmol/l (66%), K+ 7.0 – 7.4 mmol/l (70%),
K+ 7.5 – 7.9 mmol/l (74%), K+ 8.0 – 8.4 mmol/l (100%) and K+ 8.5 mmol/l (100%).
1
This would suggest that
5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 9.5 10.0
Serum potassium (mmol/L)
Figure 2: ECG in a patient with
severe hyperkalaemia (serum K+
9.1 mmol/l) illustrating peaked T
waves (a), diminished P waves
(b) and wide QRS complexes (c).

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 65
the ECG becomes more reliable with increasing severity, although there are reports of a normal ECG in
extreme hyperkalaemia.
When the diagnosis of hyperkalaemia can be established based on the ECG, treatment can be initiated even
before serum biochemistry is available and this strategy was applied in 16% of patients in one series.
7
Durfey
et al reported that the presence of a historical ECG for comparison did not affect the frequency of detection
of ECG abnormalities suggestive of hyperkalaemia.
1
The ECG can be used to risk stratify in patients with severe hyperkalaemia. Durfey et al examined the ECG
performed within 1 hour of K+ measurement in patients with severe hyperkalaemia (serum K+ ≥ 6.5
mmol/l).
1
Adverse events occurred in 15% of patients within the first 6 hours including symptomatic
bradycardia (11.7%), ventricular tachycardia (1.1%), cardiac arrest (1.1%), and death (2.1%). All occurred
before IV calcium was administered and all but one occurred before any K+-lowering treatment was
initiated. All patients with an adverse event had a preceding ECG demonstrating at least one hyperkalaemic
abnormality. Similarly, An et al demonstrated a higher in-hospital mortality in patients with serum K+ ≥ 6.5
mmol/l with typical ECG findings of hyperkalaemia compared with those with no ECG changes.
Although the ECG is useful in assessing patients with hyperkalaemia, there are some shortfalls. Firstly, the
value of the ECG is dependent on the skill of the interpreter. Physician interpretation of the ECG in
hyperkalaemia is variable.
9,10
Rafique et al reported a mean sensitivity of 0.19 (± 0.16) and specificity 0.97 (±
0.04).
9
This suggests that the ECG can be used to rule in a diagnosis of hyperkalaemia, but not to rule it out.
Secondly, the ECG may be normal even in the presence of severe hyperkalaemia.
11-15
Thirdly, the ECG
appearance may be atypical with a pseudo-STEMI pattern or Brugada phenocopy.
2,16-18
Finally, the first
presentation with severe hyperkalaemia may be ventricular fibrillation or asystole.
19
Patients with pacemaker devices are not protected from the cardiac effects of hyperkalaemia. It can affect
the function of both temporary and permanent pacemakers, particularly when the serum K+ exceeds 7.0
mmol/l.[20, 21] Hyperkalaemia causes three important clinical abnormalities in patients with pacemakers:
1. widening of the paced QRS complex
2. increased atrial and ventricular pacing thresholds that may cause failure to capture 3) increased
latency manifested by a greater delay from pacemaker stimulus to onset of depolarization.
ECG features are present in approximately 66% of patients with
severe hyperkalaemia (K
+
≥ 6.5 mmol/l).
The ECG may be normal even in severe hyperkalaemia.
Consider the clinical picture alongside ECG – severity of
hyperkalaemia, rate of rise and level of acuity of patient.
Seek senior help if in doubt.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 66
Continuous ECG monitoring will enable early recognition and prompt treatment of life-threatening
arrhythmias in patients with hyperkalaemia. Hyperkalaemia causes arrhythmias by causing hyperpolarisation
of cells, making them less able to depolarise when necessary.
22
Arrhythmias can occur at any time in the
patient’s presentation without prior toxic ECG changes.
23
All arrhythmias have been reported in patients with
hyperkalaemia, including atrial fibrillation,
24
bradycardia,
25-32
and ventricular tachycardia.
33,34
Some typical
arrhythmias are shown in Figure 3.
Figure 3: Arrhythmias in patients with severe hyperkalaemia illustrating bradycardia with wide QRS [K+
9.6 mmol/L] (a), sine wave with pause [K+ 9.3 mmol/L] (b), sine wave without pause [K+ 8.4 mmol/L] (c),
and ventricular tachycardia [K+ 9.1 mmol/L] (d).
ECG signs most closely correlated with adverse events are QRS prolongation, bradycardia (HR < 50), and/or
junctional rhythms.
1
Bradycardia and/or complete heart block associated with severe hyperkalaemia may be
resistant to conventional treatment with atropine and even temporary pacing may be ineffective and induce
arrhythmias.
23,28
Negatively chronotropic drugs (e.g. beta blockers) exacerbate bradycardia in hyperkalaemic
patients.
29-32
The BRASH (Bradycardia, renal failure, atrioventricular node blockers, shock and hyperkalaemia)
syndrome is a recently coined term to describe refractory bradycardia and haemodynamic instability in the
context of hyperkalaemia and in patients receiving rate controlling drugs.
29-31
External pacing may be useful
whilst treatment for hyperkalaemia is initiated. Although bradycardia is documented to be a potential
adverse effect of IV calcium salts, IV calcium can increase the heart rate in patients with hyperkalaemia-
induced bradycardia.
27,35,36
a
b
c
d

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 67
References
1. Durfey, N., et al., Severe Hyperkalemia: Can the Electrocardiogram Risk Stratify for Short-term
Adverse Events? West J Emerg Med, 2017. 18(5): p. 963-971.
2. Littmann, L. and M.A. Gibbs, Electrocardiographic manifestations of severe hyperkalemia. J
Electrocardiol, 2018. 51(5): p. 814-817.
3. El-Sherif, N. and G. Turitto, Electrolyte disorders and arrhythmogenesis. Cardiol J, 2011. 18(3): p. 233-
45.
4. Somers, M.P., et al., The prominent T wave: electrocardiographic differential diagnosis. Am J Emerg
Med, 2002. 20(3): p. 243-51.
5. Wrenn, K.D., C.M. Slovis, and B.S. Slovis, The ability of physicians to predict hyperkalemia from the
ECG. Ann Emerg Med, 1991. 20(11): p. 1229-32.
6. Acker, C.G., et al., Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results
of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med,
1998. 158(8): p. 917-24.
7. Freeman, K., et al., Effects of presentation and electrocardiogram on time to treatment of
hyperkalemia. Acad Emerg Med, 2008. 15(3): p. 239-49.
8. Fordjour, K.N., T. Walton, and J.J. Doran, Management of hyperkalemia in hospitalized patients. Am J
Med Sci, 2014. 347(2): p. 93-100.
9. Rafique, Z., et al., Can physicians detect hyperkalemia based on the electrocardiogram? Am J Emerg
Med, 2019.
10. Montague, B.T., J.R. Ouellette, and G.K. Buller, Retrospective review of the frequency of ECG changes
in hyperkalemia. Clin J Am Soc Nephrol, 2008. 3(2): p. 324-30.
11. Szerlip, H.M., J. Weiss, and I. Singer, Profound hyperkalemia without electrocardiographic
manifestations. Am J Kidney Dis, 1986. 7(6): p. 461-5.
12. Aslam, S., E.A. Friedman, and O. Ifudu, Electrocardiography is unreliable in detecting potentially
lethal hyperkalaemia in haemodialysis patients. Nephrol Dial Transplant, 2002. 17(9): p. 1639-42.
13. Ryuge, A., et al., Warning: The ECG May Be Normal in Severe Hyperkalemia. Intern Med, 2017.
56(16): p. 2243-2244.
14. Varga, C., et al., ECG alterations suggestive of hyperkalemia in normokalemic versus hyperkalemic
patients. BMC Emerg Med, 2019. 19(1): p. 33.
15. Khattak, H.K., et al., Recurrent life-threatening hyperkalemia without typical electrocardiographic
changes. J Electrocardiol, 2014. 47(1): p. 95-7.
16. Heckle, M., M. Agarwal, and S. Alsafwah, ST Elevations in the Setting of Hyperkalemia. JAMA Intern
Med, 2018. 178(1): p. 133-134.
17. Xu, G., et al., Relation of the Brugada Phenocopy to Hyperkalemia (from the International Registry on
Brugada Phenocopy). American Journal of Cardiology, 2018. 121(6): p. 715-717.
18. McNeill, H. and C. Isles, Electrocardiographic recognition of life-threatening hyperkalaemia: the
hyperkalaemic Brugada sign. JRSM Open, 2019. 10(9): p. 2054270419834243.
19. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
20. Barold, S.S. and B. Herweg, The effect of hyperkalaemia on cardiac rhythm devices. Europace, 2014.
16(4): p. 467-76.
21. Barold, S.S., F. Leonelli, and B. Herweg, Hyperkalemia during cardiac pacing. Pacing Clin
Electrophysiol, 2007. 30(1): p. 1-3.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 68
22. Putcha, N. and M. Allon, Management of hyperkalemia in dialysis patients. Semin Dial, 2007. 20(5):
p. 431-9.
23. Alfonzo, A.V., et al., Potassium disorders--clinical spectrum and emergency management.
Resuscitation, 2006. 70(1): p. 10-25.
24. Lisowska, A., et al., The incidence and prognostic significance of cardiac arrhythmias and conduction
abnormalities in patients with acute coronary syndromes and renal dysfunction. Kardiol Pol, 2011.
69(12): p. 1242-7.
25. Walter, R.B. and E.B. Bachli, Near-fatal arrhythmia caused by hyperkalaemia. Heart, 2002. 88(6): p.
578.
26. Kim, N.H., S.K. Oh, and J.W. Jeong, Hyperkalaemia induced complete atrioventricular block with a
narrow QRS complex. Heart, 2005. 91(1): p. e5.
27. Noble, K. and C. Isles, Hyperkalaemia causing profound bradycardia. Heart, 2006. 92(8): p. 1063.
28. Slade, T.J., J. Grover, and J. Benger, Atropine-resistant bradycardia due to hyperkalaemia. Emerg
Med J, 2008. 25(9): p. 611-2.
29. Diribe, N. and J. Le, Trimethoprim/Sulfamethoxazole-Induced Bradycardia, Renal Failure, AV-Node
Blockers, Shock and Hyperkalemia Syndrome. Clin Pract Cases Emerg Med, 2019. 3(3): p. 282-285.
30. Sattar, Y., et al., Bradycardia, Renal Failure, Atrioventricular-nodal Blocker, Shock, and Hyperkalemia
Syndrome Diagnosis and Literature Review. Cureus, 2020. 12(2): p. e6985.
31. Prabhu, V., et al., Bradycardia, Renal Failure, Atrioventricular Nodal Blockade, Shock, and
Hyperkalemia (BRASH) Syndrome as a Presentation of Coronavirus Disease 2019. Cureus, 2020.
12(4): p. e7816.
32. Simmons, T. and E. Blazar, Synergistic Bradycardia from Beta Blockers, Hyperkalemia, and Renal
Failure. J Emerg Med, 2019. 57(2): p. e41-e44.
33. Grimm, W., P. Alter, and B. Maisch, Image of the month. Cardiac arrest due to severe hyperkalemia.
Herz, 2004. 29(3): p. 353.
34. Glober, N., B.D. Burns, and C.R. Tainter, Rapid Electrocardiogram Evolution in a Dialysis Patient. J
Emerg Med, 2016. 50(3): p. 497-500.
35. Al Aseri, Z., Calcium salt administration for circulatory shock due to severe hyperkalemia. Saudi J
Anaesth, 2019. 13(3): p. 240-242.
36. McKellar G., et al. Hyperkalaemia: causes, electrocardiographic changes and management. J R Coll
Physicians Edinb 2006; 36: 5-11.
Laboratory analysis (Guidelines 15.1-15.3)
Guideline 15.1 – Hyperkalaemia: Laboratory tests
We recommend that a lithium heparin anti-coagulated specimen is the sample type of choice when rapid
turnaround of urea and electrolytes results is required. (1B)
Rationale (Guideline 15.1)
The treatment of hyperkalaemia requires timely access to accurate serum K+ measurements. Potassium
measurement can be undertaken in the laboratory or at the point of care using a variety of techniques.
Laboratory measurements of K+ focus on those in blood plasma or serum. This provides an advantage over
Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 69
whole blood measurements from blood gas analysers because haemolysis can be identified by visual
inspection after centrifugation or by spectrophotometric analysis of the specimen for the presence of
haemoglobin.
The impact of in-vitro haemolysis of blood samples is a variable increase in K+ concentrations leading to
misclassification of normokalaemic patients as hyperkalaemic, and hypokalaemic patients as
normokalaemic.
1
The use of hospital pneumatic tube systems for delivering samples to the central
laboratory reduces result turnaround time, but may contribute to a degree of haemolysis due to the impact
of speed, air pressure and vibration in transit.
2
Automated assessment of haemolysis using the haemolysis
index has standardised the process for identification of haemolysed samples.
3
The choice of specimen sent to the laboratory will depend on the tests requested and the urgency. Routine
samples for measurement of urea and electrolytes are usually requested in a clotted serum sample. In
emergencies where hyperkalaemia is suspected, specimens collected in a lithium heparin tube can be
analysed more rapidly as there is no requirement to wait for the sample to clot before centrifugation.
Laboratories may differ in their requirements for other tests and different reference intervals may also
apply.
References
1. Hartland, A.J. and R.H. Neary, Serum potassium is unreliable as an estimate of in vivo plasma
potassium. Clinical Chemistry, 1999. 45(7): p. 1091-1092.
2. Kapoula, G.V., P.I. Kontou, and P.G. Bagos, The impact of pneumatic tube system on routine
laboratory parameters: a systematic review and meta-analysis. Clinical Chemistry and Laboratory
Medicine, 2017. 55(12): p. 1834-1844.
3. Goyal, T. and C.L. Schmotzer, Validation of Hemolysis Index Thresholds Optimizes Detection of
Clinically Significant Hemolysis. American Journal of Clinical Pathology, 2015. 143(4): p. 579-583.
Guideline 15.2 – Hyperkalaemia: Blood gas analysis
We recommend that in emergencies, K+ level is measured from an arterial or venous blood sample using a
point-of-care blood gas analyser whilst awaiting the results from a formal laboratory measurement. (1B)
Rationale (Guideline 15.2)
Blood gas analysers (BGA) are increasingly available at the point-of-care with analytical repertoires that
include electrolyte measurements. This method provides rapid results, can shorten time to clinical
intervention and reduce cost.
1
Despite these advantages, there is frequently doubt about the validity of
point-of-care methods compared with central laboratory tests. Haemolysis is an important confounding
factor in the measurement of K+, especially when using whole blood specimens via BGA. A greater
concordance has been reported between BGA and the laboratory results when the K+ concentration is
Send Lithium Heparin tube for urgent analysis of K
+
level.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 70
greater than 3 mmol/L.
2
A larger blood sample (i.e. more than 1mL) can reduce the extent of haemolysis and
improve accuracy.
3
BGA potassium measurement has been compared with central laboratory venous analysis in many clinical
settings with variable recommendations.
1. During cardiac arrest, blood analysis is time-sensitive and rapid correction of an electrolyte disorder
could help achieve return of spontaneous circulation. One study in cardiac arrest reported that the
limits of agreement between ABG analysis and the central laboratory was wide and recommended
caution.
4
However, other studies have demonstrated that ABG analysis enhances resuscitation.
5-7
Ahn et al reported that all cases of life-threatening hyperkalaemia was detected using ABG analysis
with a sensitivity of 85% and specificity of 97%.
5
2. In the ICU, several studies have demonstrated good agreement between K+ values measured using
BGA analyser and the central laboratory allowing timely clinical decisions in critically ill patients.
1, 8-10
3. In the emergency department (ED), early identification of electrolyte disturbances has the potential
benefits of ensuring prompt treatment, appropriate triage, safe patient transfer and appropriate
ward placement. Several studies have validated the use of BGA analyser in measuring serum K+ in
the ED.
11-15
Point of care testing in the ED can also reduce length of stay and improve patient flow.
15
Local laboratory medicine specialists should ensure that the all methods used for measurement of potassium
are fit for purpose and that the methods are appropriately quality controlled and quality assessed. Point of
care testing systems and processes, used for the measurement of potassium, should follow best practice as
identified by the MHRA (Medicines and Healthcare Regulatory Agency, 2010).
16
Local risk assessments of the
relative value and safety of point of care versus laboratory delivery of potassium measurements should form
part of the development process.
References
1. Hohmann, C., et al., Determination of Electrolytes in Critical Illness Patients at Different pH Ranges:
Whom Shall We Believe, the Blood Gas Analysis or the Laboratory Autoanalyzer? Crit Care Res Pract,
2019. 2019: p. 9838706.
2. Chacko, B., et al., Electrolytes assessed by point-of-care testing - Are the values comparable with
results obtained from the central laboratory? Indian J Crit Care Med, 2011. 15(1): p. 24-9.
3. Hawkins, R., Measurement of whole-blood potassium--is it clinically safe? Clin Chem, 2003. 49(12): p.
2105-6.
4. Johnston, H.L. and R. Murphy, Agreement between an arterial blood gas analyser and a venous blood
analyser in the measurement of potassium in patients in cardiac arrest. Emerg Med J, 2005. 22(4): p.
269-71.
Use a point of care blood gas analyser to provide rapid and reliable K
+
level when an urgent result is required.
Send a formal laboratory sample, but initiate treatment if indicated
based on BGA result.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 71
5. Ahn, S., et al., Potassium values in cardiac arrest patients measured with a point-of-care blood gas
analyzer. Resuscitation, 2011. 82(12): p. e25-6.
6. Shin, J., et al., Initial blood pH during cardiopulmonary resuscitation in out-of-hospital cardiac arrest
patients: a multicenter observational registry-based study. Crit Care, 2017. 21(1): p. 322.
7. Kim, Y.J., Y.J. Lee, and S.M. Ryoo, Role of Blood Gas Analysis During Cardiopulmonary Resuscitation in
Out-of-hospital Cardiac Arrest Patients (vol 95, e3960, 2016). Medicine, 2016. 95(34).
8. Jain, A., I. Subhan, and M. Joshi, Comparison of the point-of-care blood gas analyzer versus the
laboratory auto-analyzer for the measurement of electrolytes. Int J Emerg Med, 2009. 2(2): p. 117-
20.
9. Jose, R.J. and J. Preller, Near-patient testing of potassium levels using arterial blood gas analysers:
can we trust these results? Emerg Med J, 2008. 25(8): p. 510-3.
10. Allardet-Servent, J., et al., Point-of-Care Versus Central Laboratory Measurements of Hemoglobin,
Hematocrit, Glucose, Bicarbonate and Electrolytes: A Prospective Observational Study in Critically Ill
Patients. PLoS One, 2017. 12(1): p. e0169593.
11. Dashevsky, M., et al., Agreement Between Serum Assays Performed in ED Point-of-Care and Hospital
Central Laboratories. West J Emerg Med, 2017. 18(3): p. 403-409.
12. Acikgoz, S.B., et al., Agreement of serum potassium measured by blood gas and biochemistry
analyzer in patients with moderate to severe hyperkalemia. Am J Emerg Med, 2016. 34(5): p. 794-7.
13. Zhang, J.B., J. Lin, and X.D. Zhao, Analysis of bias in measurements of potassium, sodium and
hemoglobin by an emergency department-based blood gas analyzer relative to hospital laboratory
autoanalyzer results. PLoS One, 2015. 10(4): p. e0122383.
14. Uysal, E., et al., How reliable are electrolyte and metabolite results measured by a blood gas analyzer
in the ED? Am J Emerg Med, 2016. 34(3): p. 419-24.
15. Kankaanpaa, M., et al., Comparison of the use of comprehensive point-of-care test panel to
conventional laboratory process in emergency department. BMC Emerg Med, 2018. 18(1): p. 43.
16. Medicines and Healthcare Regulatory Agency. Device Bulletin, Management and Use of IVD point of
care test devices. DB2010(02). Feb 2010.
Guideline 15.3 – Hyperkalaemia: Pseudo-hyperkalaemia
We recommend that urea and electrolytes are measured using paired lithium heparin and clotted serum
samples from a large vein using gentle traction with prompt laboratory analysis if pseudo-hyperkalaemia is
suspected. (1A)
Rationale (Guideline 15.3)
Ideally, the laboratory measurement will reflect the K+ concentration in the extra-cellular fluid in vivo.
Pseudo-hyperkalaemia, first reported in 1955, describes the finding of a raised serum (clotted blood) K+
value concurrently with a normal plasma (non-clotted blood) K+ value.
1
The clotting process releases K+ from
cells and platelets increasing the serum K+ concentration by an average of 0.4 mmol/L.
Pseudo-hyperkalaemia can be excluded by performing simultaneous measurements of plasma K+ in a lithium
heparin anti-coagulated specimen and in a clotted sampled.
2
Pseudo-hyperkalaemia is detected when the
serum K+ level exceeds that of the plasma by more than 0.4 mmol/L. Consider pseudo-hyperkalaemia in the
context of normal renal function, normal ECG and in patients with haematological disorders.
3

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 72
The most common cause of pseudo-hyperkalaemia is a prolonged transit time to the laboratory or poor
storage conditions. Other causes of pseudo-hyperkalaemia include difficult venepuncture, a high platelet
count, haemolysis, erythrocytosis, prolonged storage time of clotted samples, or cold storage conditions.
When using evacuated tubes for blood collection, if the order of draw is wrong, the sample can be
contaminated with potassium EDTA (for full blood count).
3, 4
Another common cause of contamination is
sampling from the arm into which potassium-containing fluids are being infused. An inverse relationship
between ambient temperature and potassium concentration has been reported with higher K+ values in the
winter months and has been termed ‘seasonal’ pseudo-hyperkalaemia.
5
Laboratories have developed standard protocols to reduce the risks of pseudo-hyperkalaemia and pseudo-
normokalaemia. Labelling the time of collection on specimens, reducing transit times, and optimising
storage conditions (i.e. avoiding wide fluctuations in temperature) for specimens from primary care are
important strategies. These measures may in turn reduce out-of-hours calls to deputising services and
admissions to acute medicine units for the investigation of hyperkalaemia.
The importance of recognition of pseudo-hyperkalaemia is the avoidance of unnecessary treatment which
could cause harm.
6
References
1. Hartmann, R.C., J.V. Auditore, and D.P. Jackson, Studies on Thrombocytosis .1. Hyperkalemia Due to
Release of Potassium from Platelets during Coagulation. Journal of Clinical Investigation, 1958. 37(5):
p. 699-707.
2. Teh, M.M., et al., When is a high potassium not a high potassium? J R Soc Med, 2003. 96(7): p. 354-5.
3. Smellie, W.S., Spurious hyperkalaemia. BMJ, 2007. 334(7595): p. 693-5.
4. Sharratt, C.L., et al., EDTA sample contamination is common and often undetected, putting patients
at unnecessary risk of harm. International Journal of Clinical Practice, 2009. 63(8): p. 1259-1262.
5. Rampul, A., et al., Big data analysis reveals the existence of seasonal pseudohyperkalaemia even in
temperate climates. Clin Chim Acta, 2019. 497: p. 110-113.
6. Rivera De Rosales, A., et al., Pseudohyperkalemia: Look Before You Treat. Saudi Journal of Kidney
Diseases and Transplantation, 2017. 28(2): p. 410-414.
If pseudo-hyperkalaemia is suspected, send paired blood samples in a clotted
tube (serum) and a lithium heparin tube (plasma).
Send FBC to exclude a haematological disorder.
Pseudo-hyperkalaemia is present if:
[Serum K
+
] – [Plasma K
+
] > 0.4 mmol/l

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 73
Treatment (Guidelines 16.1-16.6)
Guideline 16.1 – Hyperkalaemia: Summary of treatment strategy
We recommend that the treatment of hyperkalaemia in hospital follow a logical 5-step approach. (1B)
Rationale (Guideline 16.1)
The treatment of hyperkalaemia currently varies considerably. A systematic approach, as shown in Figure 4,
takes into account clinical priorities, may reduce variability, enhance patient outcome and reduce adverse
events related to hyperkalaemia and its treatment.
1
Figure 4: There are five key steps in the treatment of hyperkalaemia (never walk away without completing
all of these steps).
This process begins with an assessment of the risk of arrhythmias, followed by action to reduce the serum K+
concentration by shifting K+ back into cells and removing it from the body. Treatment efficacy is assessed by
monitoring the serum K+. Hypoglycaemia is a serious adverse effect of insulin-glucose, therefore frequent
blood glucose monitoring is essential. Treatment is not complete until the cause is identified and steps
taken to prevent recurrence. The hyperkalaemia treatment algorithm for hospitalised patients outlines this
sequential approach (Guideline 22.1). Drug therapies with mechanism of action and interventions for
treating hyperkalaemia are shown in Table 10.
STEP 1
Protect the heart
IV Calcium Gluconate or Chloride
STEP 2
Shift potassium into cells
Insulin-Glucose
Salbutamol
STEP 3
Remove potassium from the body
Sodium Zirconium Cyclosilicate
Cation-exchange resins
Step 1: Protect the heart
Step 5: Prevent recurrence
Step 4: Monitor K
+
and glucose
Step 3: Remove K
+
from body
Step 2: Shift K
+
into cells

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 74
Table 10: Mechanism of action of drugs used in treatment of hyperkalaemia.
References
1. Alfonzo, A.V., et al., Potassium disorders--clinical spectrum and emergency management.
Resuscitation, 2006. 70(1): p. 10-25.
Guideline 16.2 – Hyperkalaemia: STEP 1 - Protect the heart; intravenous calcium salts
We recommend that intravenous calcium chloride or calcium gluconate, at an equivalent dose (6.8mmol), is
given to patients with hyperkalaemia in the presence of ECG evidence of hyperkalaemia. (1C)
Audit Measures
1. The frequency of ECG changes in patients treated with intravenous calcium salts.
Rationale (Guideline 16.2)
The use of intravenous (IV) calcium in the treatment of hyperkalaemia is well established in clinical practice
but is based on sparse evidence. The toxic effects of potassium on the heart and antagonism by calcium was
first demonstrated in an animal model in 1883
1
and later confirmed in 1939.
2
Much of the evidence to
support its use is based on case reports and anecdotal experience, but there is little doubt of the importance
of IV calcium in the emergency treatment of hyperkalaemia.
3-5
The electrophysiological effect of K+ on the heart is dependent on its extracellular concentration, direction
of change (hypokalaemia or hyperkalaemia) and rate of change. The effect of K+ on the resting membrane
potential of cardiac myocytes is modulated by the simultaneous calcium concentration such that an elevated
calcium concentration decreases the depolarisation effect of an elevated K+ level.
6
IV calcium antagonises the cardiac membrane excitability provoked by excess potassium, thereby protecting
the heart against arrhythmias. It is effective within 3 minutes at improving adverse ECG appearances (e.g.
narrowing of the QRS complex).
4, 7-9
The dose should be repeated if there is no effect within 5-10 minutes.
The duration of action is only 30-60 minutes, so further doses may be necessary if hyperkalaemia remains
uncontrolled. As IV calcium does not lower serum K+, other interventions are urgently required.
Table 11: Calcium content of IV calcium salts used in treatment of hyperkalaemia.
10 ml 10% Calcium Chloride = 6.8 mmol Ca
2+
10 ml 10% Calcium Gluconate = 2.26 mmol Ca
2+

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 75
The choice of calcium salt (chloride or gluconate) is guided by practicalities such as availability, local practice
and the clinical condition of the patient. There are some important differences between the two available
solutions. Both preparations, calcium chloride
10
and calcium gluconate
11
, are available in the form of 10ml of
10% solution but calcium chloride contains 3 times more calcium than calcium gluconate as shown in Table
11. The bioavailability of both preparations appear to be similar with a study in patients with poor liver
function (pre-transplant) demonstrating that calcium gluconate does not require hepatic activation to
become effective.
12
Calcium gluconate is less irritant to the skin than calcium chloride, but both salts can
cause tissue necrosis. Calcium chloride has been recommended in the setting of haemodynamic instability,
including cardiac arrest.
13
Adverse effects reported from the use of IV calcium salts include:
8,9
• tissue necrosis if extravasation occurs
• hypotension, peripheral vasodilation, hot flushes and/or chalky taste (mainly after too rapid
infusion)
• bradycardia, arrhythmias (frequency unknown)
Report all adverse events via the Yellow Card system.
Historical evidence suggests that the administration of intravenous calcium may potentiate digoxin toxicity,
but this is limited to case reports
14,15,16
. In contrast, no dysrhythmias or increased mortality was
demonstrated in a retrospective study over a 17-year period in which 23/ 161 patients identified with
digoxin toxicity received IV calcium
17
, but some methodological concerns in this paper has been
highlighted.[18] In instances where digoxin toxicity was unrecognised at presentation, no adverse event after
IV calcium administration was reported.
19,20
In clinical practice, there have been several pitfalls in the administration of IV calcium:
1. A single dose of 10ml 10% calcium gluconate is often administered irrespective of the response
which may have been inadequate.
2. The 12-lead ECG is frequently not repeated after administration to assess response. Look for a
narrowing of the QRS complex (Figure 5), reduction in T wave amplitude (Figure 5), increase in heart
rate if bradycardic or reversal of arrhythmia.
3. IV calcium can cause bradycardia, therefore there may be reluctance to administer if the patient’s
heart rate is already slow. IV Calcium remains indicated and may be live-saving in hyperkalaemia-
induced bradycardia.
21
4. The relatively short duration of action of IV calcium (30-60 minutes) may not be considered in
patients with prolonged hyperkalaemia. Repeat ECG and consider a further dose if patient remains
hyperkalaemic.
5. IV calcium may not be deemed necessary when emergency dialysis is planned or being initiated for
severe hyperkalaemia, but this remains essential.
There is general agreement that IV calcium salts should be used in the presence of:
• life-threatening ECG changes (absent P waves, wide QRS, sine-wave pattern)
3,13

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 76
• cardiac arrhythmias
13
• cardiac arrest
13,22
There is no consensus about using IV calcium in the following circumstances:
• isolated peaked T waves
• normal ECG
Figure 5: ECG on admission (a) and following 20ml 10% calcium gluconate IV (b) in a patient with serum K+
9.3 mmol/L who presented with generalised weakness.
In summary, IV calcium has been widely recommended for the treatment and prophylaxis of arrhythmias in
patients with hyperkalaemia. The use of IV calcium buys time for other interventions to take effect. Given
that 1) the ECG is the best tool for assessing cardiac toxicity, 2) the effect of IV calcium is assessed by an
improvement in ECG appearance, and 3) IV calcium is not without risk, it seems reasonable to reserve IV

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 77
calcium for patients with ECG changes of hyperkalaemia. When 10% calcium gluconate is used, sequential
doses of 10ml solution are often required whereas a single dose of calcium chloride is more likely to be
effective. Therefore, we recommend an equivalent dosage of calcium chloride or gluconate (6.8 mmol) for
initial therapy.
References
1. Ringer, S., A further Contribution regarding the influence of the different Constituents of the Blood on
the Contraction of the Heart. J Physiol, 1883. 4(1): p. 29-42 3.
2. Winkler, A.W., H.E. Hoff, and P.K. Smith, Factors affecting the toxicity of potassium. American Journal
of Physiology, 1939. 127(3): p. 430-436.
3. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
4. Long, B., J.R. Warix, and A. Koyfman, Controversies in Management of Hyperkalemia. J Emerg Med,
2018. 55(2): p. 192-205.
5. Rafique, Z., et al., Can physicians detect hyperkalemia based on the electrocardiogram? Am J Emerg
Med, 2019.
6. Hoffman, B.F. and E.E. Suckling, Effect of Several Cations on Transmembrane Potentials of Cardiac
Muscle. American Journal of Physiology, 1956. 186(2): p. 317-324.
7. Chamberlain, M.J., Emergency Treatment of Hyperkalaemia. Lancet, 1964. 1(7331): p. 464-7.
8. Ettinger, P.O., T.J. Regan, and H.A. Oldewurtel, Hyperkalemia, cardiac conduction, and the
electrocardiogram: a review. Am Heart J, 1974. 88(3): p. 360-71.
9. Weisberg, L.S., Management of severe hyperkalemia. Crit Care Med, 2008. 36(12): p. 3246-51.
10. Calcium Chloride Intravenous Infusion, 10% w/v: Summary of Product Information.
www.medicines.org.uk/emc/product/4126/smpc.
11. Calcium Gluconate Injection: Summary of Product Information.
www.medicines.org.uk/emc/product/6264/smpc.
12. Martin, T.J., et al., Ionization and hemodynamic effects of calcium chloride and calcium gluconate in
the absence of hepatic function. Anesthesiology, 1990. 73(1): p. 62-5.
13. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
14. Bower, J.O. and H.A.K. Mengle, The additive effect of calcium and digitalis - A warning, with report of
two deaths. Journal of the American Medical Association, 1936. 106: p. 1151-1153.
15. Shrager, M.W., Digitalis Intoxication - a Review and Report of 40 Cases, with Emphasis on Etiology.
Archives of Internal Medicine, 1957. 100(6): p. 881-893.
16. Kne, T., et al. Fatality from calcium chloride in a chronic digoxin toxic patient. J Toxicol Clin Toxicol
1997; 5:505.
17. Levine, M., H. Nikkanen, and D.J. Pallin, The effects of intravenous calcium in patients with digoxin
toxicity. J Emerg Med, 2011. 40(1): p. 41-6.
18. Gupta, A., et al., Digoxin and Calcium: The Verdict Is Still Out. Journal of Emergency Medicine, 2010.
39(1): p. 102-102.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 78
19. Van Deusen, S.K., R.H. Birkhahn, and T.J. Gaeta, Treatment of hyperkalemia in a patient with
unrecognized digitalis toxicity. Journal of Toxicology-Clinical Toxicology, 2003. 41(4): p. 373-376.
20. Fenton, F., A.J. Smally, and J. Laut, Hyperkalemia and digoxin toxicity in a patient with kidney failure.
Ann Emerg Med, 1996. 28(4): p. 440-1.
21. Al Aseri, Z., Calcium salt administration for circulatory shock due to severe hyperkalemia. Saudi J
Anaesth, 2019. 13(3): p. 240-242.
22. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia during
cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest.
Resuscitation, 2016. 98: p. 105-11.
Guideline 16.3.1 – Hyperkalaemia: STEP 2 - Shift K+ into cells; insulin-glucose infusion
We recommend that insulin-glucose (10 units soluble insulin in 25g glucose) by intravenous infusion is used
to treat severe hyperkalaemia (K+ ≥ 6.5 mmol/l). (1B)
Guideline 16.3.2 – Hyperkalaemia: STEP 2 - Shift K+ into cells; insulin-glucose infusion
We suggest that insulin-glucose (10 units soluble insulin in 25g glucose) by intravenous infusion is used to
treat moderate hyperkalaemia (K+ 6.0 – 6.4 mmol/l). (2C)
Guideline 16.3.3 – Hyperkalaemia: STEP 2 - Shift K+ into cells; avoiding hypoglycaemia
We suggest pre-emptive initiation of an infusion of 10% glucose at 50ml/ hour for 5 hours (25g) following
insulin-glucose treatment in patients with a pre-treatment blood glucose < 7.0 mmol/l to avoid
hypoglycaemia (target blood glucose 4-7 mmol/l). (2D)
Audit measure
1. The proportion of patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/L) treated with insulin-glucose
infusion.
Rationale (Guidelines 16.3.1 – 16.3.4)
Insulin is the most reliable drug for shifting K+ into cells in patients with hyperkalaemia.[1] Insulin shifts K+
into cells by activating Na+-K+ ATPase and recruiting intracellular pump components into the plasma
membrane. Insulin binding to specific membrane receptors results in extrusion of Na+ and cellular uptake of
K+. This effect is independent of its hypoglycaemic action.
Following insulin-glucose infusion, serum K+ level starts to fall within 15 minutes
2,3
, with the peak reduction
(ranging from 0.65-1.0 mmol/l) occurring between 30-60 minutes.
2-6
The reduction in serum K+ may be
sustained for up to 2 hours after administration following which there is usually a gradual rebound. The main
risk of insulin-glucose therapy is hypoglycaemia. Insulin sensitivity varies from patient to patient and is
affected by diabetic status and level of renal function.
The efficacy of insulin-glucose is increased if given in combination with salbutamol. The peak K+ lowering
effect with combination therapy at 60 minutes is 1.5 mmol/L with intravenous beta-agonist therapy and 1.2
mmol/L with nebulised beta-agonist therapy.[7] Co-administration of salbutamol also appears to reduce the
risk of insulin-induced hypoglycaemia.
8
Hyperkalaemia may occur in the context of diabetic emergencies, in particular, diabetic ketoacidosis (DKA).
In this setting, the primary problem is the redistribution of K+ out of cells although the total body K+ may be

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 79
reduced. The K+ level falls as hyperglycaemia is controlled with fluids and insulin administration. Follow the
DKA treatment protocol and monitor the serum K+ and blood glucose level closely.
9
The evidence guiding treatment recommendations for the insulin-glucose regimen has been analysed by
assessing the:
• Incidence of iatrogenic hypoglycaemia
• Dose of insulin for optimal efficacy
• Dose of insulin to reduce the risk of hypoglycaemia
• Dose of glucose to reduce the risk of hypoglycaemia
• Patient-related factors increasing the risk of hypoglycaemia
Iatrogenic Hypoglycaemia after Insulin-glucose therapy
Hypoglycaemia is the most serious complication of treatment with insulin-glucose for acute hyperkalaemia.
Over the last decade, several observational studies, using variable treatment regimens, have highlighted this
risk as shown in Table 12.
10-14,15
In one of these studies, Boughton et al used the UK Renal Association
Hyperkalaemia Guideline (2014) protocol, however 10 units insulin was given in 100ml 20% glucose (~ 20g),
slightly lower than the guideline recommendation of 125 ml 20% glucose (25g). This may have influenced
hypoglycaemic events.
14

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 80
Study
Country
N=
Baseline Blood
glucose
(BG)
Time
Interval
to Hypo
(hrs)
Hypo
(%)
*Severe
Hypo
(%)
Risk factors for
Hypo
#
Schafers
2012
10
US
219
8.6 (No hypo group)
6.7 (Hypo group)
3.0
8.7
2.3
Low pre-treatment
blood glucose
Renal impairment
#
Apel
2014
11
US
(ESRD)
221
9.0 (no hypo group)
5.8 (hypo group)
2.0
13
5.9
Low pre-treatment
blood glucose
No history of
diabetes
#
Estep
2015
14
US
86
8.3 (No hypo group
had BG 2.4 mmol/l
higher than hypo
group)
1.45
17.4
3.5
Low pre-treatment
blood glucose
Coca
2017
12
Spain
164
8.5 (No hypo group)
6.2 (Hypo group)
3.5
6.1
1.2
Low pre-treatment
blood glucose
#
Scott
2019
13
US
(ED)
409
7.3 (Hypo occurred
in 34% of patients
with baseline BG <
5.6)
NA
17
8
Low pre-treatment
blood glucose
Lower glucose dose
Higher insulin dose
Boughton
2019
14
UK
662
8.7 (No hypo group)
5.8 (Hypo group)
NA
17.5
7.1
Low pre-treatment
blood glucose
Older age
Low body weight
Table 12: Incidence and risk factors associated with iatrogenic hypoglycaemia after insulin-glucose infusion
for treatment of hyperkalaemia.
*Definition of Severe hypoglycaemia: glucose < 3.0 mmol/l [Boughton]; glucose < 2.8 mmol/l [Apel, Scott]; glucose < 2.2 mmol/l
[Schafers, Coca, Estep]
#
Studies without a standardised Insulin-glucose protocol
ESRD – end-stage renal disease; ED – Emergency Department
These studies demonstrate that the most consistent factor contributing to hypoglycaemia after insulin-
glucose treatment is a low pre-treatment blood glucose level (< 7 mmol/l). This is an important
consideration in designing a safe and effective treatment protocol.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 81
Insulin and Glucose dose: Efficacy
The evidence-base for efficacy of insulin-glucose in the treatment of acute hyperkalaemia is heterogenous
consisting of variable study designs, insulin doses, glucose doses, method of administration (bolus or
infusion), and study populations as shown in Table 13.
Early prospective studies
2,3,5,6,16,17
and one more recent study
18
, were performed predominantly in stable
haemodialysis patients and included small patient cohorts. Few prospective studies included patients with
acute kidney injury.
4,8,19
Retrospective studies reported over the past decade have attempted to address the optimal regimen to
reduce the risk of hypoglycaemia without compromising efficacy.
12,20-25
Some studies have considered
reduced insulin dose (5 units)
20,22,23
, higher glucose dose (50g)
10,12,21,22,24
, body weight
21,25
, glycaemic status
26
and level of renal function
20,26
to tailor treatment regimens. Importantly, assessment of efficacy is
dependent on the timing of blood monitoring after treatment. Given the retrospective design of these
studies, the timing of blood monitoring was variable with K+ measurements ranging between 1 to 4 hours
after administration of insulin-glucose.
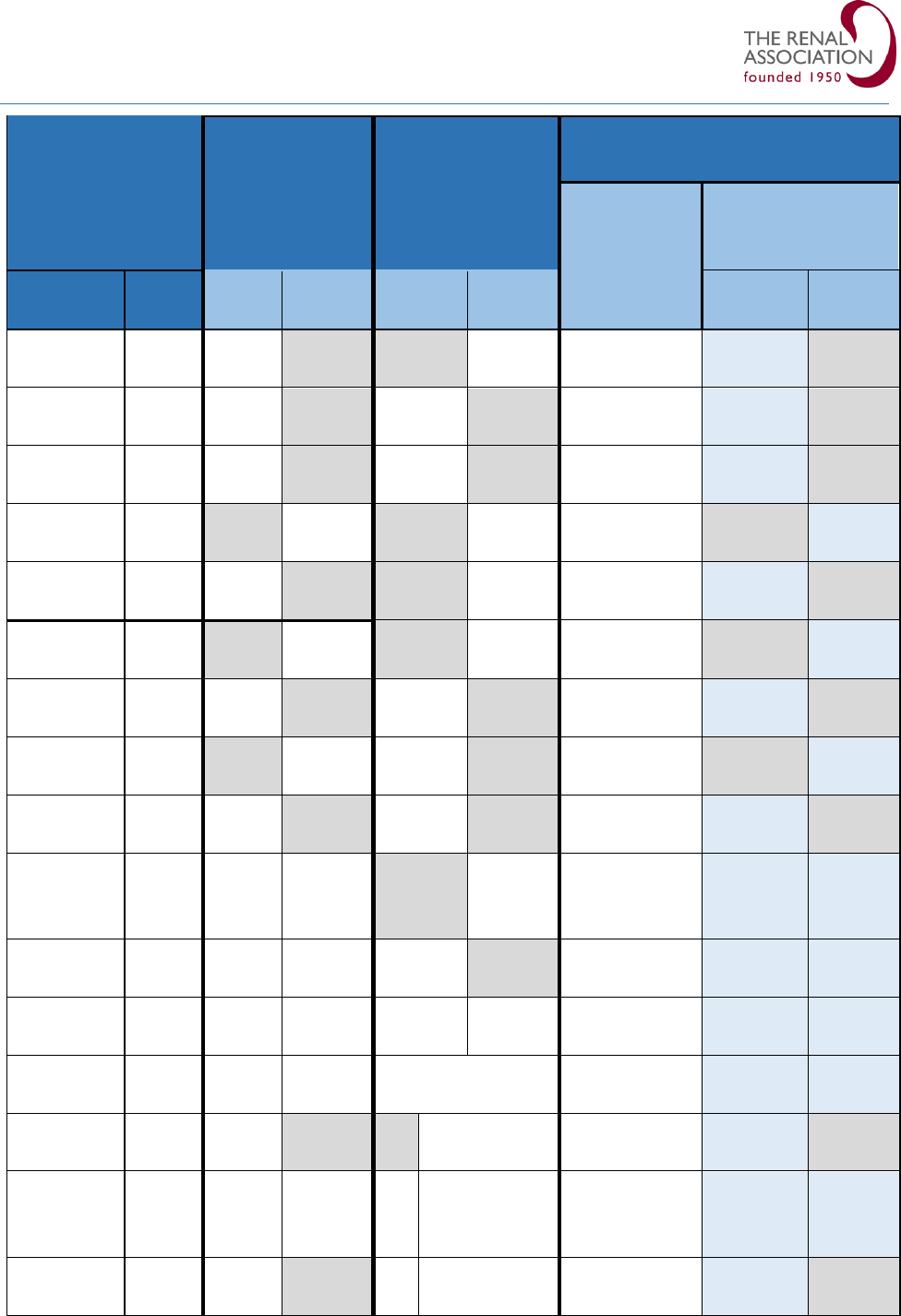
Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 82
Efficacy
Insulin
Dose
Glucose
Dose (g)
Potassium Level
Mean
Baseline K
+
(mmol/L)
Serum K
+
Reduction
(mmol/L)
Study
n=
10
Units
Other
Units
25g
Other
Dose
10
Units
Other
Units
Lens
1989
4
10
10
40
6.7
1.0
Allon
1990
2
12
10
25
5.48
0.65
Ljutic
1993
5
9
10
25
6.33
0.76
Allon
1996
3
8
0.5 U/
kg/min
60
4.28
0.85
Duranay
1996
16
20
10
30
6.71
0.99
Kim
1996
6
8
0.5 U/
kg/min
40
6.3
0.7
Ngugi
1997
8
70
10
25
6.9
1.14
Mahajan
2001
17
30
12
25
6.59
0.83
Mushtaq
2006
23
15
10
25
6.5
0.8
Chothia
2014
18
10
10
0
50
6.01 [10
units]
6.23 [0 units]
0.83
0.50
Pierce
2015
20
149
10
5
25
6.3
1.08
1.1
Wheeler
2016
21
132
10
0.1
U/kg
50
6.1
#
NI
#
NI
La Rue
2017
22
675
10
5
25 + 25 ± 25
6.4
1.0
1.0
Coca
2017
12
164
10
50
6.85
1.18
Garcia
2018
23
401
10
5
25
0, 12.5, 50
6.15 [10
units]
6.24 [5 units]
0.90
0.81
Farina
2018
24
240
10
25
50
6.5 [25g]
6.3 [50g]
1.0 [25g]
1.1 [50g]
Table 13: Prospective and Retrospective studies of Insulin-glucose therapy.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 83
Insulin dose – Conventional Regimen: Insulin 10 units in 25g glucose
The majority of the prospective studies used a dose of 10 units of soluble insulin as shown in Table 14.
2, 4, 5, 8,
16, 18,23,19
The most commonly used dose of glucose was 25g.
2, 5, 8, 23,19
The mean baseline serum K+ ranged from
5.48 – 6.9 mmol/l in patients treated with this conventional regimen. The efficacy demonstrated in these
studies showed a reduction in serum K+ ranging from 0.65 – 1.14 mmol/l as shown below in Table 14. This
evidence formed the basis of the historical recommendation to treat acute hyperkalaemia with insulin 10 units
and glucose 25g. Recent observational studies have shown similar efficacy (0.9-1.08 mmol/l) with this
regimen.
20, 23, 24
Table 14: Efficacy and risk of hypoglycaemia with conventional regimen - 10 units Insulin with 25g glucose
(studies without efficacy data excluded)
DM – Diabetes Mellitus. BM – Blood glucose. *all patients non-diabetic.
Insulin dose – 5 versus 10 units
Three retrospective studies, consisting of large cohorts of up to 675 patients, have compared the efficacy
and hypoglycaemic risk with regimens using 5 versus 10 units of insulin in patients with renal impairment as
shown in Table 15.
20,22,23
The K+-lowering achieved using 10 units insulin was 0.9 – 1.08 mmol/l compared
with 0.81 – 1.1 mmol/l using 5 units insulin.
Interpretation of the data in these studies is confounded by the low proportion of patients receiving 5 units
insulin in two of the studies
22,23
, the use of variable glucose regimens which may have influenced the
incidence of hypoglycaemia, and the use of concomitant K+-lowering drugs which may have influenced
STUDY
Insulin
dose
(units)
Glucose
Dose
(g)
Baseline
K
+
(mmol/l)
K
+
lowering
(mmol/l)
DM
(%)
Baseline
blood
glucose
(mmol/l)
Hypo
(BM ≤ 3.9)
(%)
Allon 1990
(n=12)
2
10
25
5.48
0.65
0
4.8
*75
Ljutic 1993
(n=9)
5
10
25
6.33
0.77
NA
NA
11
Ngugi 1997
(n=70)
8
10
25
6.9
1.14
NA
NA
20
Mushtaq
2006
(n=15)
23
10
25
6.5
0.8
NA
7.5
0
Pierce 2015
(n=149)
20
10
25
6.3
1.08
55
NA
16.7
Garcia 2018
(n=401)
23
10
25
6.15
0.9
36.2
8.7
10.7
Farnia 2018
(n=240)
24
10
25
6.5
1.0
26.7
7.0
15.8

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 84
efficacy. Albuterol, a beta-agonist, was used in more patients treated with 5 units insulin in two of the
reports
22,23
and was not stated in the other report.
20
Table 15: Comparison of studies performed using 5 versus 10 units insulin (studies without efficacy data
excluded).
Proportion of patients treated with 5 units insulin: Pierce: 48% [20]; La Rue: 20% [22]; Garcia: 23% [23]
Dose of glucose: Pierce: 25g [20]; La Rue: 25g + 25g at 1hr ± 25g at 3hrs if blood glucose < 3.9 mmol/l (poor adherence to this
protocol) [22]; Garcia: 0-50g (25g used in 68% of patients treated with 5 units insulin and 82% of patients treated with 10 units
insulin; 50g used in 19.5% treated with 5units insulin and 11% treated with 10 units insulin) [23]
*Definition of Severe hypoglycaemia: Pierce: glucose < 2.8 mmol/l [20]; La Rue: glucose < 2.2 mmol/l [22]
# p = 0.03
NA – not available; DM- Diabetes mellitus; Hypo – hypoglycaemia; BM – blood glucose.
Dose-dependent effect of Insulin on K+-lowering
There is some evidence of a dose-dependent effect of insulin on lowering K+ level. Garcia et al reported a
post-hoc analysis of patients with a serum K+ ≥ 6.0 mmol/l and showed a trend towards higher K+-lowering
in patients treated with 10 units insulin compared with those treated with 5 units insulin. (difference -0.238
mmol/l; p=0.018).
23
This observation has been shown more definitively in a recent large study (n=700).
Moussavi et al reported significantly greater K+-lowering in patients treated with 10 units insulin (1.11 ± 0.8
mmol/l, p=0.008) compared with those treated with < 10 units insulin (0.94 ± 0.71 mmol/l).
27
Impact of severity of hyperkalaemia on efficacy
It is unclear if the severity of hyperkalaemia affects the degree of K+-lowering with insulin. The efficacy
reported in studies conducted using 10 units of insulin (Table 13) was compared between patients with a
mean K+ ≥ 6.5 mmol/l (n=6) vs those with a mean K+ < 6.5 mmol/l (n=6). Interestingly, this showed a trend
towards higher K+-lowering in studies with more severe hyperkalaemia (mean reduction 1.02 mmol/l vs 0.87
mmol/l; difference -0.15 mmol/l). The extent of this correlation may be affected by the narrow range in K+
level as no studies exceeded a mean K+ level of > 7.0 mmol/l, however this observation suggests that the
higher the serum K+, the greater the efficacy of insulin.
STUDY
Insulin
dose
(units)
Albuterol
Use
(%)
Baseline
K
+
(mmol/l)
K
+
lowering
(mmol/l)
DM
(%)
Baseline
blood
glucose
(mmol/l)
Hypo
(BM ≤
3.9)
(%)
*Severe
Hypo
(%)
Pierce
2015
20
(n =149)
10
NA
6.3
1.08
55
NA
16.7
8.9
5
NA
6.3
1.1
46
NA
19.7
7.0
La Rue
2017
22
(n=675)
10
30.3
6.4
1.0
49.1
7.6
28.6
6.8
5
36.8
6.4
1.0
42.9
6.9
19.5
3.0
Garcia
2018
23
(n=401)
10
15.2
6.15
0.9
36.2
8.8
10.7
NA
5
#
25
6.24
0.81
29.4
7.6
8.7
NA

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 85
Insulin and Glucose dose: Strategies to Reduce the Risk of Hypoglycaemia
Factors influencing the risk of hypoglycaemia are the dose of insulin, dose of glucose, and patient-related
factors.
Insulin dose – Conventional regimen
The studies conducted prior to 2010, using a regimen of 10 units of insulin with 25g glucose, showed a wide
variation in incidence rate of hypoglycaemia ranging from 11 – 20% in two studies,
5,8
no episodes in one
study
19
and as high as 75% in a study including only patients without diabetes.
2
Similarly, over the past
decade, the incidence of hypoglycaemia reported using this regimen ranged from 5 - 28%.
10, 11, 14, 20, 23, 24, 26
Insulin dose – Low dose regimen
Studies assessing the hypoglycaemic risk using regimens of 5 vs 10 units of insulin were confounded by the
proportion of patients who received 5 units insulin (range 20 – 48%) and the variable glucose dosing (Table
15).
20, 22, 23
Pierce et al, the only study with almost equal study arms, showed no significant difference in
incidence of hypoglycaemia between conventional vs low-dose insulin treatment groups (16.7% vs 19.7%,
p=0.79).
McNicholas et al conducted as a 2-part audit comparing the incidence of hypoglycaemia in patients treated
with 5 vs 10 units insulin with 25g glucose based on level of renal function.
26
Adherence to the treatment
protocol improved in Audit 2 with a higher number of patients with CKD/ESRD receiving 5 units insulin
resulting in a reduction in hypoglycaemic events from 28% to 11% with no severe episodes.
Insulin dose – Weight-based regimen
Tailoring insulin dose to body weight is another potential strategy as shown in Table 16.
3, 6, 21, 25
Two small
early studies in HD patients reported no hypoglycaemic events.
3,6
More recently, Wheeler et al
demonstrated a significant reduction in hypoglycaemic events with a weight based regimen.
21
However this
study only reported the lowest serum K+ level achieved in the 12 hours following treatment, making it
difficult to assess efficacy. Brown et al found a marginally significant difference in hypoglycaemic rates
(6.67% vs 5.8%, p=0.05) in favour of the weight-based cohort.
25
In clinical practice, a weight-based regimen
would be difficult to safely and reliably implement in a medical emergency.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 86
Table 16: Comparison of studies performed using weight-base Insulin regimen.
*Severe hypoglycaemia – glucose < 2.8 mmol/l.
**
p=0.05 NA – not available.
#
NI – not included as study reported the lowest serum K
+
level achieved in the 12 hours following treatment.
Glucose dose
Studies assessing the effect of glucose dose (25g vs 50g) on hypoglycaemic risk are shown in Table 17. Farnia
et al reported a trend towards a lower incidence of hypoglycaemia at 60 minutes in patients treated with 50g
glucose.
24
Sub-group analysis of patients with a baseline blood glucose < 6.1 mmol/l and those without
diabetes showed a significant reduction in hypoglycaemic events when treated with 50g glucose. Coca et al
delivered an infusion of 50g glucose with 10 units insulin over 240 minutes and showed a low hypoglycaemic
rate at 6.1% with this strategy.
12
STUDY
Insulin
dose
(units)
Glucose
Dose
(g)
Baseline
K
+
(mmol/l)
K
+
lowering
(mmol/l)
DM
(%)
Baseline
blood
glucose
(mmol/l)
Hypo
(BM ≤
3.9)
(%)
*Severe
Hypo
(%)
Allon
1996
3
(n=8)
5
mU/kg/
min
60
4.28
0.85
0
4.8
0
0
Kim
1996
6
(n=8)
5
mU/kg/
min
40
6.3
0.7
NA
NA
0
0
Wheeler
2016
21
(n=132)
0.1U/kg
50
6.1
#
NI
NA
8.2
12.1
NA
10
50
#
NI
NA
9.2
27.3
NA
Brown
2018
25
(n=264)
0.1U/kg
(8.3)
24
6.1
0.6
52
9.0
6.7
2.6
8.7
26
6.2
0.6
45
8.5
**
5.8
10.1

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 87
Table 17: Studies using 50% glucose in treatment of hyperkalaemia.
*p< 0.5; **p=0.11
#
NI – not included as study reported the lowest serum K
+
level achieved in the 12 hours following treatment.
Glucose without Insulin
Theoretically, administering glucose alone should stimulate insulin release and reduce the risk of
hypoglycaemia and some studies have shown K+-lowering of 0.2-0.6 mmol/l with this approach.
18, 28
Chothia
et al showed a reduction in serum K+ was 0.83 mmol/l (insulin-glucose group) compared with 0.5 mmol/l
(glucose-only group).
18
However, endogenous insulin levels are unlikely to rise to the necessary therapeutic
level to cause a rapid, reliable and clinically useful degree of K+ shift into cells.
1,29
This approach also risks a
paradoxical worsening of hyperkalaemia by causing a shift of K+ out of cells.
30-32
This strategy is not
recommended.
Other risk factors
Several risk factors have been identified that may contribute to hypoglycaemia after insulin-glucose
treatment. Patient-related factors are listed below in Table 18. Insulin has a longer half-life in patients with
renal failure making them more at risk of hypoglycaemia.
33-35
The reported incidence of hypoglycaemia in
patients with ESRD is up to 33%.
10, 20, 21
Treatment-related factors include the dose of insulin and dose of
glucose used.
STUDY
Glucose
dose
(units)
Insulin
Dose
(units)
Baseline
K
+
(mmol/l)
K
+
lowering
(mmol/l)
DM
(%)
Baseline
blood
glucose
(mmol/l)
Hypo
(BM ≤
3.9)
(%)
*Severe
Hypo
(%)
Chothia
2014
18
(n =10)
50
0
6.23
0.50
NA
5.1
0
NA
50
10
6.01
0.83
NA
5.6
20
NA
Wheeler
2016
21
(n=132)
50
0.1 U/kg
6.1
#
NI
NA
8.2
12.1
NA
50
10
#
NI
NA
9.2
*27.3
NA
Coca
2017
12
(n=164)
-
-
-
-
-
-
-
-
50
10
6.85
1.18
8.3
6.1
1.2
Garcia
2018
23
(n=401)
0
5 (2%)
6.24
0.81
29
7.6
8.7
25
5 (16%)
50
5 (5%)
Garcia
2018
23
(n=401)
0
10 (4%)
6.15
0.9
36
8.8
10.7
25
10 (63%)
50
10 (9%)
Farnia
2018
24
(n=240)
25
10 (50%)
6.5
1.0
27
7.0
**15.8
50
10 (50%)
6.3
1.1
27
5.9
8.3

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 88
Table 18: Risk Factors for Hypoglycaemia following treatment with Insulin-Glucose
Most of the patient-related factors are not modifiable with the exception of the baseline blood glucose.
Based on the available studies, a pre-treatment blood glucose < 7 mmol/l appears to be the threshold for
identifying patients potentially at risk of hypoglycaemia.
10-14, 18, 21-25
. Both treatment-related factors are
modifiable.
Tailoring the treatment protocol to address one or more of these risk factors will increase complexity and
likely affect adherence as seen in two reports.
22, 26
However, a single protocol to fit all will continue to risk
hypoglycaemia.
Summary
Historical evidence has guided the development of previous guidelines with the conventional regimen of 10
units soluble insulin with 25g glucose being standard practice for decades.
36, 37, 38
The incidence of
hypoglycaemia following this treatment regimen remains high, therefore recent studies have been
comprehensively reviewed to determine if a change in practice is warranted. Unfortunately, the available
evidence is limited by small cohort sizes in the early prospective studies, retrospective design in recent
studies and use of multiple insulin-glucose treatment regimens.
The risk of hypoglycaemia is increased in patients without diabetes and in patients with a pre-treatment
blood glucose < 7 mmol/l. In a sub-group of patients without diabetes, hypoglycaemia developed in 7.9%
within 1 hour.
24
Reducing the dose of insulin alone is insufficient to reduce hypoglycaemic events which
remains at 8.7-19.7% with low-dose insulin, although it does appear to reduce the incidence of severe
hypoglycaemia in some studies. There appears to be more evidence that increasing the dose of glucose more
consistently reduces hypoglycaemic events with the larger studies (with efficacy data) reporting rates of 6.1-
8.3%.
12,24
The method of administration of glucose may be important. LaRue et al attempted sequential doses of 25g
glucose (i.e. second dose after 1 hour and third dose after 3 hours if the blood glucose < 3.9 mmol/l), but
Potential risk Factors for Iatrogenic Hypoglycaemia
Patient-related:
Low pre-treatment blood glucose
Renal impairment (AKI, CKD 4-5, ESRD)
Low body weight
Older age
Non-diabetic status (no prior history and no diabetic medication)
Treatment-related:
High Insulin dose regimen ( ≥ 10 units soluble insulin)
Low Glucose dose regimen (≤ 25g glucose)

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 89
non-adherence to the protocol resulted in a lower dose of glucose administered (34 - 39g).
22
Coca et al
administered 50g glucose with insulin over a 4-hour infusion and reported a low rate of hypoglycaemia
(6.1%) and the lowest rate of severe hypoglycaemia (1.2%), but this strategy delays assessment of efficacy.
12
Another approach is the initiation of a continuous infusion of 10% glucose at 50ml/hr following initial
treatment with 25g glucose.
30,39
If the infusion is given over 5 hours (25g), this would deliver a total glucose
dose of 50g. This method allows continuous delivery of glucose throughout the risk period for
hypoglycaemia, titration of the infusion guided by blood glucose level and avoids the transient
hyperglycaemia after a 50g glucose dosing.
Achieving a lower hypoglycaemic rate without compromising efficacy is the ultimate goal. Although most
studies have shown that reducing the dose of insulin does not appear to compromise efficacy, two reports
have highlighted a dose-dependent effect with 10 units insulin showing greater efficacy than 5 units
insulin.
23, 27
There also appears to be a trend for greater efficacy with increasing severity of hyperkalaemia in
patients treated with 10 units insulin.
These observations raise potential concern for the treatment of patients with potentially life-threatening
hyperkalaemia. The standard multi-modal approach to treating hyperkalaemia may not be feasible in critical
illness and in cardiac arrest, leaving insulin-glucose as the main therapeutic option. On balance, the risk of
sub-optimal K+-lowering treatment appears to out-weigh the risk of hypoglycaemia in the setting of life-
threatening hyperkalaemia. Further study is required before a reduction in insulin dosage to 5 units can be
recommended.
The KDIGO statement (2019) on the management of acute hyperkalaemia acknowledges that there is limited
evidence, but has recommended the administration of 5 units in place of 10 units insulin.
40
This document
also advocates other treatment options without a clear evidence base including sodium bicarbonate (if
acidosis present) and diuretics. The implications of this change in dose of insulin to patients in whom a multi-
modal treatment approach may not be feasible (i.e. peri-arrest or cardiac arrest) does not appear to have
been considered.
The current Renal Association guideline (2020) recommends the use of 10 units soluble insulin with 25g
glucose, followed by an infusion of 10% glucose at 50ml/hour for 5 hours (25g) in patients with a pre-
treatment blood glucose < 7 mmol/l. This approach is also likely to benefit patients without diabetes and
those with low body weight. Blood glucose monitoring is discussed in Guideline 17.2 and the treatment of
hypoglycaemia should follow existing guidelines.
41
Administration will depend on the concentration of glucose solution chosen (Appendix 3B). The use of 50%
glucose has reduced in recent years in view of the potential risk of extravasation injury.
40
Although 20%
glucose is readily available in most hospitals, the administration of 25g requires 125ml of solution. This
poses a challenge for administration as 20% glucose is generally available in 100ml bottles (i.e. 20g). As the
concentration of glucose solution reduces, the volume required to achieve 25g increases (i.e. 50% = 50ml,
20% = 125ml, 10% = 250ml). Volume overload is a potential concern in patients with renal failure. The
choice of solution may be influenced by local availability, ease of administration and the volume status of the
patient.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 90
Further research is required with well-designed prospective randomised studies to confirm the optimal
insulin and glucose dosing regimen to maintain efficacy whilst avoiding hypoglycaemia. A further area for
study is the use of potassium binders in place of insulin-glucose therapy in moderate hyperkalaemia in the
hospital setting.
References
1. Weisberg, L.S., Management of severe hyperkalemia. Crit Care Med, 2008. 36(12): p. 3246-51.
2. Allon, M. and C. Copkney, Albuterol and insulin for treatment of hyperkalemia in hemodialysis
patients. Kidney International, 1990. 38(5): p. 869-872.
3. Allon, M. and N. Shanklin, Effect of bicarbonate administration on plasma potassium in dialysis
patients: Interactions with insulin and albuterol. American Journal of Kidney Diseases, 1996. 28(4): p.
508-514.
4. Lens, X.M., et al., Treatment of Hyperkalemia in Renal-Failure - Salbutamol V Insulin. Nephrology
Dialysis Transplantation, 1989. 4(3): p. 228-232.
5. Ljutic, D. and Z. Rumboldt, Should glucose be administered before, with, or after insulin, in the
management of hyperkalemia? Ren Fail, 1993. 15(1): p. 73-6.
6. Kim, H.J., Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia
in end-stage renal disease patients. Nephron, 1996. 72(3): p. 476-482.
7. Ahee, P. and A.V. Crowe, The management of hyperkalaemia in the emergency department. J Accid
Emerg Med, 2000. 17(3): p. 188-91.
8. Ngugi, N.N., S.O. McLigeyo, and J.K. Kayima, Treatment of hyperkalaemia by altering the transcellular
gradient in patients with renal failure: Effect of various therapeutic approaches. East African Medical
Journal, 1997. 74(8): p. 503-509.
9. Savage, M.W., et al., Joint British Diabetes Societies guideline for the management of diabetic
ketoacidosis. Diabetic Medicine, 2011. 28(5): p. 508-515.
10. Schafers, S., et al., Incidence of hypoglycemia following insulin-based acute stabilization of
hyperkalemia treatment. Journal of Hospital Medicine, 2012. 7(3): p. 239-242.
11. Apel, J., S. Reutrakul, and D. Baldwin, Hypoglycemia in the treatment of hyperkalemia with insulin in
patients with end-stage renal disease. Clin Kidney J, 2014. 7(3): p. 248-50.
12. Coca, A., et al., Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients
with impaired renal function. PLoS One, 2017. 12(2): p. e0172961.
Insulin-glucose protocol for treatment of hyperkalaemia
▪ Check blood glucose prior to insulin administration.
▪ Give 10 units soluble Insulin with 25 g glucose.
▪ Give 10% glucose by infusion at 50ml/hr (25g) for 5 hours in
patients with a pre-treatment blood glucose < 7.0 mmol/l.
➢ target blood glucose: 4.0 – 7.0 mmol/l
➢ titrate rate of infusion if required
▪ Monitor serum K
+
and blood glucose (see treatment algorithm).
▪ Anticipate and treat hypoglycaemia promptly.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 91
13. Scott, N.L., et al., Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the
emergency department. Am J Emerg Med, 2019. 37(2): p. 209-213.
14. Boughton, C.K., et al., Preventing Hypoglycemia Following Treatment of Hyperkalemia in Hospitalized
Patients. J Hosp Med, 2019. 14(5): p. 284-287.
15. Estep, P., et al. Evaluation of hypoglycaemia incidence and risk factors in patients treated with IV
Insulin Aspart for Hyperkalaemia. Endocrinol Diabetes Res 2014; Vol:1 (1). doi.10.4172/2470-
7570.1000103.
16. Duranay, M., et al., Comparison of aminophylline and insulin infusions in treatment of hyperkalemia
in patients with end-stage renal disease. Nephron, 1996. 73(1): p. 105.
17. Mahajan, S.K., M. Mangla, and K. Kishore, Comparison of aminophylline and insulin-dextrose
infusions in acute therapy of hyperkalemia in end-stage renal disease patients. J Assoc Physicians
India, 2001. 49: p. 1082-5.
18. Chothia, M.Y., et al., Bolus administration of intravenous glucose in the treatment of hyperkalemia: a
randomized controlled trial. Nephron Physiol, 2014. 126(1): p. 1-8.
19. Mushtaq, M.A., et al. Treatment of hyperkalaemia with salbutamol and insulin. Pak J Med Sci, 2006;
22: p. 176-179.
20. Pierce, D.A., G. Russell, and J.L. Pirkle, Jr., Incidence of Hypoglycemia in Patients With Low eGFR
Treated With Insulin and Dextrose for Hyperkalemia. Ann Pharmacother, 2015. 49(12): p. 1322-6.
21. Wheeler, D.T., et al., Weight-based insulin dosing for acute hyperkalemia results in less
hypoglycemia. J Hosp Med, 2016. 11(5): p. 355-7.
22. LaRue, H.A., G.D. Peksa, and S.C. Shah, A Comparison of Insulin Doses for the Treatment of
Hyperkalemia in Patients with Renal Insufficiency. Pharmacotherapy, 2017. 37(12): p. 1516-1522.
23. Garcia, J., et al., Reduced Versus Conventional Dose Insulin for Hyperkalemia Treatment. J Pharm
Pract, 2018: p. 897190018799220.
24. Farina, N. and C. Anderson, Impact of dextrose dose on hypoglycemia development following
treatment of hyperkalemia. Ther Adv Drug Saf, 2018. 9(6): p. 323-329.
25. Brown, K., et al., Assessing the Impact of an Order Panel Utilizing Weight-Based Insulin and
Standardized Monitoring of Blood Glucose for Patients With Hyperkalemia. American Journal of
Medical Quality, 2018. 33(6): p. 598-603.
26. McNicholas, B.A., et al., Treatment of Hyperkalemia With a Low-Dose Insulin Protocol Is Effective and
Results in Reduced Hypoglycemia. Kidney Int Rep, 2018. 3(2): p. 328-336.
27. Moussavi, K., et al., Comparison of IV Insulin Dosing Strategies for Hyperkalemia in the Emergency
Department. Crit Care Explor, 2020. 2(4): p. e0092.
28. Muto, S., et al., Effect of oral glucose administration on serum potassium concentration in
hemodialysis patients. Am J Kidney Dis, 2005. 46(4): p. 697-705.
29. Kamel, K.S. and C. Wei, Controversial issues in the treatment of hyperkalaemia. Nephrology Dialysis
Transplantation, 2003. 18(11): p. 2215-2218.
30. Allon, M., Hyperkalemia in end-stage renal disease: mechanisms and management. J Am Soc
Nephrol, 1995. 6(4): p. 1134-42.
31. Goldfarb, S., et al., Acute hyperkalemia induced by hyperglycemia: hormonal mechanisms. Ann Intern
Med, 1976. 84(4): p. 426-32.
32. Conte, G., et al., Acute increase in plasma osmolality as a cause of hyperkalemia in patients with
renal failure. Kidney Int, 1990. 38(2): p. 301-7.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 92
33. Bilotta, F., et al., Insulin infusion therapy in critical care patients: regular insulin vs short-acting
insulin. A prospective, crossover, randomized, multicenter blind study. J Crit Care, 2015. 30(2): p. 437
e1-6.
34. DeFronzo, R.A., et al., Insulin resistance in uremia. J Clin Invest, 1981. 67(2): p. 563-8.
35. Iglesias, P. and J.J. Diez, Insulin therapy in renal disease. Diabetes Obes Metab, 2008. 10(10): p. 811-
23.
36. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015 Section 4. Cardiac
arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
37. Vanden Hoek, T.L., et al., Part 12: cardiac arrest in special situations: 2010 American Heart
Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.
Circulation, 2010. 122(18 Suppl 3): p. S829-61.
38. The UK Renal Association. Clinical Practice Guidelines: Treatment of Acute Hyperkalaemia in Adults.
March 2014. renal.org/wp-content/uploads/2017/06/hyperkalaemia-guideline-1.
39. Mount DB. Treatment and prevention of hyperkalaemia. In: Basow DS, ed. UpToDate. Waltham,
MA: UpToDate; 2011.
40. Clase, C.M., et al., Potassium homeostasis and management of dyskalemia in kidney diseases:
conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference.
Kidney Int, 2019.
41. Joint British Diabetes Societies for Inpatient Care - The Hospital Management of Hypoglycaemia in
Adults with Diabetes Melliitus, 3rd Edition. April 2018.
Guideline 16.4.1 – Hyperkalaemia: STEP 2 – Shift K+ into cells; Salbutamol
We recommend nebulised salbutamol 10-20 mg is used as adjuvant therapy for severe (K+ ≥ 6.5 mmol/L)
hyperkalaemia. (1B)
Guideline 16.4.2 – Hyperkalaemia: STEP 2 – Shift K+ into cells; Salbutamol
We suggest that nebulised salbutamol 10-20 mg may be used as adjuvant therapy for moderate (K+ 6.0-6.4
mmol/L) hyperkalaemia. (2C)
Guideline 16.4.3 – Hyperkalaemia: STEP 2 – Shift K+ into cells; Salbutamol
We recommend that salbutamol is not used as monotherapy in the treatment of severe hyperkalaemia. (1A)
Rationale (Guideline 16.4.1 – 16.4.3)
Salbutamol is a beta-2 adrenoceptor agonist and promotes the intracellular shift of K+ by activation of the
Na-K+ ATPase pump.
1
Salbutamol and other beta-agonists are equally effective given intravenously or by
nebuliser.
2-4
The nebulised route is easier to administer and causes fewer side-effects, such as tremor,
palpitations and headache.
5
There are no studies to assess the safety of salbutamol in patients with cardiac
disease, therefore a lower dose and cardiac monitoring is recommended.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 93
Dose
Efficacy
10mg
decreases serum K
+
by 0.53 - 0.88 mmol/l
20mg
decreases serum K
+
by 0.66 - 0.98 mmol/l
Table 19: Efficacy of Nebulised Salbutamol.
The effect of salbutamol is dose-dependent as shown above in Table 19. The onset of action is within 30
minutes and duration of action is for at least 2 hours as shown below in Table 20.
2, 3, 6-11
The peak effect of
10mg nebulised salbutamol is seen at 120 minutes and at 90 minutes for the 20mg nebulised dose.
4
The
degree of potassium lowering is variable and 20-40% of patients have a decline in serum K+ < 0.5 mmol/L.
12
The combination of salbutamol with insulin-glucose is more effective than either treatment alone.
9, 13
The
peak K+ lowering effect with combination therapy at 60 minutes was 1.5 mmol/L with intravenous beta-
agonist therapy
13
and 1.2 mmol/L with nebulised beta-agonist therapy
9
. Mild hyperglycaemia (2-3 mmol/L
increase) has also been reported and this may partly protect against insulin-induced hypoglycaemia.
STUDY
N
Dose of
Salbutamol
Mean
initial K
+
(mmol/L)
Peak
reduction
in K
+
(mmol/L)
Time of
max action
Duration
of Effect
(min)
Allon
6
1989
10
10 mg
5.93
0.62
90
>120
Allon
7
1996
8
10 mg
4.29
0.53
60
>60
Liou
2
1994
17
10 mg
5.8
0.88
90
>60
Montoliu
11
1990
10
15 mg
6.5
0.9
30
>360
Kim
8
1997
9
15 mg
5.99
0.57
60
> 60
Allon
6
1989
10
20 mg
5.81
0.98
90
>120
Allon
9
1990
12
20 mg
5.56
0.66
60
>60
McClure
3
1994
11
2.5/ 5 mg*
5.9
0.61
30
>300
Mandelberg
10
1999
17
1200g
(via MDS-I)
5.5
0.4
60
ns
Table 20: Studies investigating efficacy of nebulised salbutamol in hyperkalaemia.
*children (aged 5-18 years)
ns – not stated

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 94
Salbutamol may be ineffective in some patients with hyperkalaemia. Non-selective beta-blockers may
prevent the hypokalaemic response to salbutamol.
14
Up to 40% of patients with end stage renal disease do
not respond to salbutamol and the mechanism for this resistance is unknown.
6, 9
Given its variable efficacy,
salbutamol should therefore not be used as monotherapy for treatment of hyperkalaemia.
4
References
1. Moratinos, J. and M. Reverte, Effects of catecholamines on plasma potassium: the role of alpha- and
beta-adrenoceptors. Fundam Clin Pharmacol, 1993. 7(3-4): p. 143-53.
2. Liou, H.H., et al., Hypokalemic effects of intravenous infusion or nebulization of salbutamol in
patients with chronic renal failure: comparative study. Am J Kidney Dis, 1994. 23(2): p. 266-71.
3. McClure, R.J., V.K. Prasad, and J.T. Brocklebank, Treatment of hyperkalaemia using intravenous and
nebulised salbutamol. Arch Dis Child, 1994. 70(2): p. 126-8.
4. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
5. Effa, E. and A. Webster, Pharmacological interventions for the management of acute hyperkalaemia
in adults. Nephrology (Carlton), 2017. 22(1): p. 5-6.
6. Allon, M., R. Dunlay, and C. Copkney, Nebulized albuterol for acute hyperkalemia in patients on
hemodialysis. Ann Intern Med, 1989. 110(6): p. 426-9.
7. Allon, M. and N. Shanklin, Effect of bicarbonate administration on plasma potassium in dialysis
patients: Interactions with insulin and albuterol. American Journal of Kidney Diseases, 1996. 28(4): p.
508-514.
8. Kim, H.J., Acute therapy for hyperkalemia with the combined regimen of bicarbonate and beta(2)-
adrenergic agonist (salbutamol) in chronic renal failure patients. J Korean Med Sci, 1997. 12(2): p.
111-6.
9. Allon, M. and C. Copkney, Albuterol and insulin for treatment of hyperkalemia in hemodialysis
patients. Kidney International, 1990. 38(5): p. 869-872.
10. Mandelberg, A., et al., Salbutamol metered-dose inhaler with spacer for hyperkalemia: how fast?
How safe? Chest, 1999. 115(3): p. 617-22.
11. Montoliu, J., et al., Treatment of Hyperkalemia in Renal-Failure with Salbutamol Inhalation. Journal
of Internal Medicine, 1990. 228(1): p. 35-37.
12. Kamel, K.S. and C. Wei, Controversial issues in the treatment of hyperkalaemia. Nephrology Dialysis
Transplantation, 2003. 18(11): p. 2215-2218.
13. Lens, X.M., et al., Treatment of Hyperkalemia in Renal-Failure - Salbutamol V Insulin. Nephrology
Dialysis Transplantation, 1989. 4(3): p. 228-232.
14. Ahmed, J. and L.S. Weisberg, Hyperkalemia in dialysis patients. Seminars in Dialysis, 2001. 14(5): p.
348-356.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 95
Guideline 16.5 Hyperkalaemia: STEP2 –Shift K into cells; Sodium bicarbonate
We suggest that intravenous sodium bicarbonate infusion is not used routinely for the acute treatment of
hyperkalaemia. (2C)
Rationale (Guideline 16.5)
There is currently insufficient evidence to support the routine use of intravenous sodium bicarbonate for the
acute treatment of hyperkalaemia. Almost all of the available evidence comes from studies performed in
stable chronic haemodialysis patients. When compared with other K+-lowering regimens, sodium
bicarbonate monotherapy failed to lower serum K+ acutely.
1-5
Although, some studies have suggested
bicarbonate may increase the efficacy of other therapies, such as insulin-glucose
4, 6
and salbutamol
5
, others
have not demonstrated any additional benefit from bicarbonate administration when added to insulin-
glucose
1
or salbutamol
1, 6
. The combination of all three treatments was the most effective strategy in one
study.
6
Prolonged administration of sodium bicarbonate may lower K+, but at the expense of a sodium load.
3
A
randomised controlled trial conducted by Jaber et al assessed the effect of using hypertonic sodium
bicarbonate (4.2%) in critically ill patients with severe metabolic acidosis (pH < 7.2).
7
There was no
difference in the primary outcome (composite of death from any cause by day 28 or 1 organ failure at day 7),
however the bicarbonate group had significantly lower K+ levels and a lower requirement for renal
replacement therapy. A recent retrospective study of the use of bicarbonate infusion in patients with sepsis
reported improved survival in the sub-group of patients with severe acidosis associated with AKI stage 2 or
3.
8
There is no evidence to suggest that sodium bicarbonate is more effective at lowering serum K+ as the
severity of metabolic acidosis increases. Changes in serum K+ did not correlate with basal values of plasma
bicarbonate or blood pH.
3,9
There is also no evidence to suggest that sodium bicarbonate is more effective in
patients as the severity of hyperkalaemia increases.
3
Nevertheless, a recent report advocates the
administration of hypertonic sodium bicarbonate (100-250ml 8.4% solution) in patients with metabolic
acidosis (pH < 7.2) or in patients in whom intravenous calcium is deemed to be contraindicated (e.g.
hypercalcaemia).
10
Overall, the available evidence is limited and may not reflect the clinical response in patients with
hyperkalaemia in the context of acute kidney injury. The use of sodium bicarbonate comes with the risk of
sodium and fluid overload and the risks may outweigh any potential (unproven) benefits in this patient
group. The use of sodium bicarbonate in hyperkalaemic cardiac arrest is discussed in Guideline 24.3.
References:
1. Allon, M. and N. Shanklin, Effect of bicarbonate administration on plasma potassium in dialysis
patients: Interactions with insulin and albuterol. American Journal of Kidney Diseases, 1996. 28(4): p.
508-514.
2. Blumberg, A., et al., Effect of Various Therapeutic Approaches on Plasma Potassium and Major
Regulating Factors in Terminal Renal-Failure. American Journal of Medicine, 1988. 85(4): p. 507-512.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 96
3. Blumberg, A., P. Weidmann, and P. Ferrari, Effect of Prolonged Bicarbonate Administration on
Plasma Potassium in Terminal Renal-Failure. Kidney International, 1992. 41(2): p. 369-374.
4. Kim, H.J., Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia
in end-stage renal disease patients. Nephron, 1996. 72(3): p. 476-482.
5. Kim, H.J., et al., The acute therapy of hyperkalemia with the combined regimen of bicarbonate and
beta(2) agonist (salbutamol) to ESRD patients. Kidney International, 1997. 51(3): p. 957-957.
6. Ngugi, N.N., S.O. McLigeyo, and J.K. Kayima, Treatment of hyperkalaemia by altering the transcellular
gradient in patients with renal failure: Effect of various therapeutic approaches. East African Medical
Journal, 1997. 74(8): p. 503-509.
7. Jaber, S., et al., Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the
intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. The
Lancet, 2018. 392(10141): p. 31-40.
8. Zhang, Z., et al., Effectiveness of sodium bicarbonate infusion on mortality in septic patients with
metabolic acidosis. Intensive Care Med, 2018. 44(11): p. 1888-1895.
9. Gutierrez, R., et al., Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium
concentration in patients with end-stage renal disease. Miner Electrolyte Metab, 1991. 17(5): p. 297-
302.
10. Depret, F., et al., Management of hyperkalemia in the acutely ill patient. Ann Intensive Care, 2019.
9(1): p. 32.
Guideline 16.6.1 – Hyperkalaemia: STEP 3 – Remove K+ from body; Potassium binders
We recommend that Sodium Zirconium Cyclosilicate is used as an option in the emergency management of
acute life-threatening hyperkalaemia (serum K+ ≥ 6.5 mmol/l). (1B)
Audit Measures
1. The proportion of patients with acute severe hyperkalaemia (serum K+ ≥ 6.5 mmol/l) treated with
Sodium Zirconium Cyclosilicate.
Rationale (Guideline 16.6.1)
Until recently, there had been no new advances in treatment of acute hyperkalaemia for decades. Sodium
Zirconium Cyclosilicate (SZC) is a non-absorbed potassium binder that preferentially exchanges H+ and Na+
for K+ and ammonium ions throughout the entire gastrointestinal tract.
1
The K+-binding capacity of SZC is up
to 9 times greater than that of SPS.
2
The SZC clinical trials have been discussed in detail in Guidelines 10.1-10.3 and include three RCTs [3-5] and
one open label clinical trial.[6] Major limitations are that all studies were performed in the stable out-
patient setting and the threshold for treatment was lower than standard practice with few patients having a
serum K+ ≥ 6.0 mmol/l.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 97
Table 21: Proportion of patients taking SZC 10g three times daily achieving restoration of normokalaemia
(K 3.5-5.0 mmol/l) during acute phase.
SZC provides a potential option for treating severe acute hyperkalaemia for several reasons. It has a rapid
onset of action within 1 hour.
1
The median time to normalisation of serum K+ is 2.2 hours and SZC lowers
serum K+ by 1.1 mmol/l within 48 hours.
5
The ZS-003 and ZS-004 trials also demonstrated a greater K+-
lowering effect with increasing severity of hyperkalaemia.
4, 5
In patients with a serum K+ > 6.0 mmol/l, SZC
lowers serum K+ by 1.5 mmol/l within 48 hours. The efficacy of SZC over the first 24-72 hours (Table 21),
demonstrates that 66% of patients achieved normokalaemia within 24 hours.
5, 6
NICE has approved SZC as an option in the treatment of acute life-threatening hyperkalaemia alongside
standard care in hospitalised patients.
7
Given the lack of evidence of SZC in the acute setting, NICE assessed
its efficacy in patients with clinically relevant hyperkalaemia. The number of patients with a serum K+ of 5.5-
5.9 mmol/l was 38.8% in ZS-004 and 45% in ZS-005. The number of patients with K+ ≥ 6.0mmol/l was 15.1%
in ZS-004 and 16.8% in ZS-005.
7
A post-hoc analysis of the sub-group of patients with K+ ≥ 6.0 mmol/l in the
ZS-004 and ZS-005 studies showed that most patients treated with SZC achieved a serum K+ between 4.0-6.0
mmol/l.
7
The treatment threshold for ‘life-threatening’ hyperkalaemia is a serum K+ ≥ 6.5 mmol/l. SZC 10g three
times daily can be used for up to 72 hours (correction phase), but if hyperkalaemia is not controlled by this
time, it should be discontinued. NICE concluded that randomised evidence demonstrating improved survival
was not needed in the context of treating life-threatening hyperkalaemia in emergency circumstances.
7
The
cost-effectiveness analysis suggested that SZC for acute hyperkalaemia is a good use of NHS resources.
Following the correction phase, the pharmaceutical company marketing authorisation suggests maintenance
therapy with SZC. The starting dose of 5g daily may be up-titrated to a maximum dose of 10g daily or down-
titrated to 5g alternate days with the aim of preventing recurrence.
1
However, maintenance treatment is
not consistent with current clinical practice and there is no evidence for this in the acute setting at present.
Treatment with SZC beyond the first 72 hours (correction phase) will be guided by clinical circumstances.
Further research in the acute setting is required to demonstrate the need for maintenance therapy.
Acute Phase
ZS-003 [4]
ZS-004 [5]
ZS-005 [6]
24 hours
66%
66%
48 hours
86.4%
88%
75%
72 hours
78%
NICE has approved the use of SZC as an option in the treatment acute life-threatening
hyperkalaemia alongside standard care in hospitalised patients.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 98
The ENERGIZE trial is an ongoing Phase 2 (NCT03337477), multi-centre randomised, double-blind placebo
controlled study to evaluate SZC as an adjunct to insulin-glucose in treatment of acute hyperkalaemia for
normalisation of serum K+.
8
The primary outcome is the mean absolute change in serum K+ from baseline
until 4 hours after starting SZC/ placebo. The study will include patients with a serum K+ ≥ 5.8 mmol/l,
although this is below the current treatment threshold (K+ ≥ 6.0 mmol/l) for insulin-glucose. Patients will be
randomised to receive SZC 10g three times daily over 10 hours (at 0, 4, and 10 hrs) or placebo during the
treatment period of 24 hours alongside standard care with insulin (0.1 units/kg) and glucose 25g.
Further study is warranted to determine whether SZC could be used as an alternative to insulin-glucose in
patients with moderate hyperkalaemia. The main advantage of this strategy would be reducing the risk of
hypoglycaemia in patients who are at a lower risk of arrhythmias.
References:
1. Astra Zeneca. Lokelma (sodium zirconium cyclosilicate) for oral suspension: Summary of Product
Characteristics. 2018. www.ema.europa.eu/ema/
2. Yang, A., et al., In Vitro Ion Exchange Capacity and Selectivity of Zs-9, a Novel, Selective Cation Trap
for the Treatment of Hyperkalemia. American Journal of Kidney Diseases, 2014. 63(5): p. A115-A115.
3. Ash, S.R., et al., A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney
disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int, 2015. 88(2):
p. 404-11.
4. Packham, D.K., et al., Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med, 2015. 372(3): p.
222-31.
5. Kosiborod, M., et al., Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days
among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA, 2014.
312(21): p. 2223-33.
6. Spinowitz, B.S., et al., Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-
Month Phase 3 Study. Clin J Am Soc Nephrol, 2019. 14(6): p. 798-809.
7. National Institute of Clinical Excellence - Sodium Zirconium Cyclosilicate for treating hyperkalaemia.
Technology appraisal guidance [TA599]. September 2019. www.nice.org/guidance/TA599.
8. Clinical Trials.gov. A Study to Evaluate a Potassium Normalization Treatment Regimen Including
Sodium Zirconium Cyclosilicate (ZS) Among Patients With S-K > 5.8 (ENERGIZE). NCT03337477.
November 2017. www.clinicaltrials.gov/ct2/show/NCT03337477.
Guideline 16.6.2 – Hyperkalaemia: STEP 3 – Remove K+ from body; Potassium binders
We suggest that Patiromer is an option for the emergency management of acute life-threatening
hyperkalaemia (serum K+ ≥ 6.5 mmol/l). (1C)
Audit Measures
1. The proportion of patients with acute severe hyperkalaemia (serum K+ ≥ 6.5 mmol/l) treated with
Patiromer.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 99
Rationale (Guideline 16.6.2)
Patiromer is a novel potassium binder that has been discussed in detail in Guidelines 9.1-9.3. A role for
patiromer in the acute setting has not been investigated as all major clinical trials were performed in stable
out-patients.
1-6
The onset of action is slow (4-7 hours),
7
therefore its contribution to rapid control of serum
potassium (within the first 4 hours) may be limited in the acute setting. Patiromer also has the potential to
bind to some co-administered drugs, therefore it cannot be taken within 3 hours of other medications.
2
However, patiromer is generally better tolerated than calcium resonium.
The key evidence for clinical effectiveness was derived from the OPAL-HK study which demonstrated a
reduction in serum K+ by a mean of 1.01 mmol/l after 4 weeks (Phase 1).
1
The mean serum K+ was 0.72
mmol/l higher in patients who were withdrawn compared with those who remained on patiromer (Phase 2).
The patiromer studies initiated treatment at a lower threshold compared with routine medical practice.
Given that most patients did not have clinically significant hyperkalaemia, the efficacy of patiromer in the
context of life-threatening hyperkalaemia is not known.
The first study to assess the efficacy of patiromer in the acute setting was a pilot study within an Emergency
Department.
8
This was a single-centre randomised open-label study of patients with ESRD with a serum K+ ≥
6.0 mmol/l. Patients were randomised to receive standard care (n=15) or standard care plus a single dose of
patiromer 25.2g (n=15). Patients treated with patiromer showed a significantly lower serum K+ at 2 hours
(6.51 mmol/l vs 5.9 mmol/l, p=0.009), but there was no difference in serum K+ at 6 hours (6.32 mmol/l vs
5.81 mmol/l, p=0.155) compared with standard care.
NICE has approved patiromer for treating patients with life-threatening hyperkalaemia alongside standard
medical therapy including insulin and glucose.
9
Patiromer should not be used instead of standard treatment,
but could replace calcium resonium. Although the dosing regimens in clinical trials were twice daily, the FDA
and NICE have approved patiromer for single daily dosing only in view of the potential risk of drug
interactions.
References
1. Weir, M.R., et al., Patiromer in patients with kidney disease and hyperkalemia receiving RAAS
inhibitors. N Engl J Med, 2015. 372(3): p. 211-21.
2. Pitt, B., et al., Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a
double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur
Heart J, 2011. 32(7): p. 820-8.
3. Bakris, G.L., et al., Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and
Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA, 2015. 314(2): p. 151-
61.
NICE has approved the use of patiromer as an option in the treatment acute life-
threatening hyperkalaemia alongside standard care in hospitalised patients.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 100
4. Bushinsky, D.A., et al., Patiromer induces rapid and sustained potassium lowering in patients with
chronic kidney disease and hyperkalemia. Kidney Int, 2015. 88(6): p. 1427-1433.
5. Pergola, P.E., et al., Patiromer Lowers Serum Potassium When Taken without Food: Comparison to
Dosing with Food from an Open-Label, Randomized, Parallel Group Hyperkalemia Study. Am J
Nephrol, 2017. 46(4): p. 323-332.
6. Pitt, B., et al., Evaluation of an individualized dose titration regimen of patiromer to prevent
hyperkalaemia in patients with heart failure and chronic kidney disease. ESC Heart Fail, 2018. 5(3): p.
257-266.
7. Vifor Fresenius Medical Care Renal Pharma UK. Veltassa (Patiromer): Annex 1 - Summary of Product
Characteristics. www.ema.europa.eu/en/documents/product-information/veltassa-epar-product-
information/en.pdf
8. Rafique, Z., et al., Patiromer for Treatment of Hyperkalemia in the Emergency Department: A Pilot
Study. Acad Emerg Med, 2020. 27(1): p. 54-60.
9. National Institute for Health and Care Excellence - Patiromer for treating hyperkalaemia. Technology
appraisal guidance [TA623]. February 2020. www.nice.org.uk/guidance/TA623.
Guideline 16.6.3 – Hyperkalaemia: STEP 3 – Remove K+ from body; Cation-exchange resin
We suggest that calcium resonium is not used in the emergency management of severe hyperkalaemia, but
may be considered in patients with moderate hyperkalaemia. (2B)
Rationale (Guideline 16.6.3)
Cation-exchange resins, sodium polystyrene sulfonate (SPS) or calcium polystyrene sulfonate (CPS) are cross-
linked polymers with negatively charged structural units which entraps K+ in the distal colon in exchange for
Ca2+. The most common resin used in hospitals in the UK is CPS, Calcium resonium®.
1
The onset of action is
slow (> 4 hours) and efficacy is unpredictable excluding its use in emergencies. It is also poorly tolerated due
to taste and constipation. The most serious adverse effect of resins is intestinal necrosis.
2,3
Evidence in support for the use of cation-exchange resins in the treatment of hyperkalaemia is very limited
and these drugs were approved before evidence-based practice was established.
4, 5
It is unclear whether the
resins have a K+-lowering effect in isolation or whether this is caused by the induction of diarrhoea by
cathartics. Multiple doses are required over several days, with the effect on lowering the serum K+ noted
over 1 to 5 days.
6, 7
The Cochrane Review for the acute management of hyperkalaemia included no studies
on resins.[8] Resins play no role in the emergency management of severe hyperkalaemia.
8, 9
The use of resins
in chronic hyperkalaemia is discussed in Guideline 8.1.
Resins may be considered in patients with moderate hyperkalaemia in the acute setting (with no or mild ECG
changes) where slower reduction in serum K+ may not compromise the patient.
9
Nasir et al demonstrated
equal efficacy of SPS and CPS in lowering serum K+ over a 3-day period in patients with CKD.
10
Resins may
also be used in combination with other strategies (i.e. insulin-glucose, low K+ diet), as an alternative to
treating patients who are poor candidates for dialysis, or when dialysis is delayed.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 101
References
1. Calcium resonium. Summary of Product Information.
www.medicines.org.uk/emc/product/1439/smpc.
2. Harel, Z., et al., Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use:
a systematic review. Am J Med, 2013. 126(3): p. 264 e9-24.
3. McGowan, C.E., et al., Intestinal Necrosis due to Sodium Polystyrene Sulfonate (Kayexalate) in
Sorbitol. Southern Medical Journal, 2009. 102(5): p. 493-497.
4. Parks, M. and D. Grady, Sodium Polystyrene Sulfonate for Hyperkalemia. JAMA Intern Med, 2019.
5. Sterns, R.H., et al., Ion-exchange resins for the treatment of hyperkalemia: are they safe and
effective? J Am Soc Nephrol, 2010. 21(5): p. 733-5.
6. Flinn, R.B., J.P. Merrill, and W.R. Welzant, Treatment of the oliguric patient with a new sodium-
exchange resin and sorbitol; a preliminary report. N Engl J Med, 1961. 264: p. 111-5.
7. Scherr, L., et al., Management of hyperkalemia with a cation-exchange resin. N Engl J Med, 1961.
264: p. 115-9.
8. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
9. Kamel, K.S. and M. Schreiber, Asking the question again: are cation exchange resins effective for the
treatment of hyperkalemia? Nephrol Dial Transplant, 2012. 27(12): p. 4294-7.
10. Nasir, K. and A. Ahmad, Treatment of hyperkalemia in patients with chronic kidney disease: a
comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll
Abbottabad, 2014. 26(4): p. 455-8.
Blood monitoring (Guidelines 17.1-17.2)
Guideline 17.1.1 – Hyperkalaemia: STEP 4 - Blood monitoring; serum potassium
We recommend that the serum K+ is monitored closely in all patients with hyperkalaemia to assess efficacy
of treatment and to monitor for rebound hyperkalaemia after the initial response to treatment wanes. (1B)
Guideline 17.1.2 – Hyperkalaemia: STEP 4 - Blood monitoring; serum potassium
We suggest that serum K+ is assessed at least 1, 2, 4, 6 and 24 hours after identification and treatment of
moderate or severe hyperkalaemia. (2C)
Audit measures
1. The proportion of patients in whom serum K+ was measured at least once within 2 hours of
treatment for severe hyperkalaemia [Audit Standard: 100%].
Rationale (Guidelines 17.1.1 – 17.1.2)
The timing for assessing response to treatment is guided by the onset of action and duration of action of K+-
lowering drugs. Insulin-glucose infusion and nebulised salbutamol are the most effective treatments in
reducing serum K+ levels in current practice. The time to peak effect with insulin-glucose ranges from 30-60
minutes
1-5
and for nebulised salbutamol from 30-90 minutes.
1, 2, 5-9
Therefore, the combined effect of these
drugs can be assessed between 30-90 minutes after treatment. Their effects last for up to 4-6 hours.
10
The

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 102
onset of action of the potassium binders vary. Sodium zirconium cyclosilicate (SZC) works within 1 hour and
the median time to normalisation of serum K+ is 2.2 hours.
11
Patiromer works within 4-7 hours.
12
The aim of treatment is to achieve rapid control with a serum K+ < 6.0 mmol/L within 2 hours of initiation of
treatment. The peak efficacy of three of the K+-lowering drugs can be assess at 1-2 hours. Therefore,
measure serum K+ at 1 and 2 hours after initial treatment to determine if the K+ level has decreased
sufficiently.
Further monitoring at 4 and 6 hours is required to assess for any rebound in serum K+ as the effects of
insulin-glucose and salbutamol wears off.
13-17
The use of potassium binders may lower this rebound
phenomenon and may provide better control of hyperkalaemia beyond initial acute treatment. Measure the
serum K+ at 24 hours to ensure that control of hyperkalaemia has been maintained.
References
1. Allon, M. and C. Copkney, Albuterol and insulin for treatment of hyperkalemia in hemodialysis
patients. Kidney International, 1990. 38(5): p. 869-872.
2. Allon, M. and N. Shanklin, Effect of bicarbonate administration on plasma potassium in dialysis
patients: Interactions with insulin and albuterol. American Journal of Kidney Diseases, 1996. 28(4): p.
508-514.
3. Lens, X.M., et al., Treatment of Hyperkalemia in Renal-Failure - Salbutamol V Insulin. Nephrology
Dialysis Transplantation, 1989. 4(3): p. 228-232.
4. Ljutic, D. and Z. Rumboldt, Should glucose be administered before, with, or after insulin, in the
management of hyperkalemia? Ren Fail, 1993. 15(1): p. 73-6.
5. Kim, H.J., Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia
in end-stage renal disease patients. Nephron, 1996. 72(3): p. 476-482.
6. Allon, M., R. Dunlay, and C. Copkney, Nebulized albuterol for acute hyperkalemia in patients on
hemodialysis. Ann Intern Med, 1989. 110(6): p. 426-9.
7. Liou, H.H., et al., Hypokalemic effects of intravenous infusion or nebulization of salbutamol in
patients with chronic renal failure: comparative study. Am J Kidney Dis, 1994. 23(2): p. 266-71.
8. Montoliu, J., et al., Treatment of Hyperkalemia in Renal-Failure with Salbutamol Inhalation. Journal
of Internal Medicine, 1990. 228(1): p. 35-37.
9. McClure, R.J., V.K. Prasad, and J.T. Brocklebank, Treatment of hyperkalaemia using intravenous and
nebulised salbutamol. Arch Dis Child, 1994. 70(2): p. 126-8.
10. Ahee, P. and A.V. Crowe, The management of hyperkalaemia in the emergency department. J Accid
Emerg Med, 2000. 17(3): p. 188-91.
11. Kosiborod, M., et al., Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days
among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA, 2014.
312(21): p. 2223-33.
12. Vifor Fresenius Medical Care Renal Pharma UK. Veltassa (Patiromer): Annex 1 - Summary of Product
Characteristics. www.ema.europa.eu/en/documents/product-information/veltassa-epar-product-
information/en/pdf.
13. Ahmed, J. and L.S. Weisberg, Hyperkalemia in dialysis patients. Seminars in Dialysis, 2001. 14(5): p.
348-356.
14. Elliott, M.J., et al., Management of patients with acute hyperkalemia. CMAJ, 2010. 182(15): p. 1631-
5.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 103
15. Weisberg, L.S., Management of severe hyperkalemia. Crit Care Med, 2008. 36(12): p. 3246-51.
16. Sterns, R.H., et al., Ion-exchange resins for the treatment of hyperkalemia: are they safe and
effective? J Am Soc Nephrol, 2010. 21(5): p. 733-5.
17. Harel, Z. and K.S. Kamel, Optimal Dose and Method of Administration of Intravenous Insulin in the
Management of Emergency Hyperkalemia: A Systematic Review. PLoS One, 2016. 11(5): p.
e0154963.
Guideline 17.2 – Hyperkalaemia: STEP 4 - Blood monitoring; blood glucose
We recommend that the blood glucose concentration is monitored at regular intervals (0, 15, 30, 60, 90, 120,
180, 240, 360, 480 and 720 minutes) up to 12 hours after administration of insulin-glucose infusion in all
patients with hyperkalaemia. (1C)
Audit measure
1. The proportion of patients who have at least one blood glucose test performed within 1 hour of
completion of insulin-glucose infusion [Audit Standard: 100%].
2. The frequency of hypoglycaemia occurring in patients receiving treatment with insulin-glucose for
hyperkalaemia.
Rationale (Guideline 17.2)
Hypoglycaemia, defined as a blood glucose of < 4.0 mmol/L, is the most common adverse event following
insulin-glucose infusion for the treatment of hyperkalaemia.
1-5
Even mild hypoglycaemia is associated with
an increased risk of mortality in hospitalised patients.[6] Severe hypoglycaemia, is defined as a blood glucose
of < 2.8 mmol/L.
2, 4
The clinical manifestations of hypoglycaemia tend to be progressive, but the early signs are not always
detected. Mild hypoglycaemia often presents with sweating, palpitations, tremor and hunger. Severe
hypoglycaemia results in more serious symptoms including confusion, coma or even death.
7
The impact of
hypoglycaemia is independent of diabetic status and adverse outcomes have been shown in patients with
diabetes mellitus or without diabetes.
7, 8
One mechanism by which hypoglycaemia may be detrimental is by
reducing myocardial blood flow in patients with diabetes and as well as in healthy adults.
9, 10
Iatrogenic hypoglycaemia is a significant patient safety concern, therefore should be anticipated with regular
blood glucose monitoring. Risk factors for hypoglycaemia are shown in Table 12; Guideline 16.3. The
incidence of hypoglycaemia appears to be lower in patients treated with 50g vs 25g glucose as initial
treatment as discussed in Guideline 16.3.
11
The majority of hypoglycaemic events occur between 2.0 to 3.5 hours after insulin-glucose infusion (Table
12; Guideline 16.3).
1, 4, 5, 12
Apel et al demonstrated that 75% of hypoglycaemic episodes occurred within 3
hours of insulin administration (median 2 hours) and persist for a median of 2 hours.
4
However, in patients
without diabetes, hypoglycaemia may occur within 1 hour.
11
The risk of hypoglycaemia persists for as late as
6 hours after administration of IV insulin.
1, 4, 5, 12, 13
The Joint British Diabetes Societies for inpatient care has
advised blood glucose monitoring for 12 hours after insulin-glucose infusion for treatment of hyperkalaemia:
at least 3 times in the first hour followed by at least 6 further measurements over the subsequent 11 hours.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 104
Recent studies suggest that variables related to treatment (dose of insulin, dose of glucose) and the baseline
clinical parameters (e.g. pre-treatment glucose) have a greater influence on the rate of hypoglycaemia than
non-modifiable baseline patient characteristics.
2, 5
Therefore, this is a potentially preventable adverse
event. The current guideline recommendation provides an increased glucose dose to the patients
anticipated to be at the greatest risk of hypoglycaemia (i.e. pre-treatment blood glucose < 7.0 mmol/l). This
criteria will likely overlap with other patient groups at risk including patients with low body weight, advanced
age or renal failure. Frequent blood glucose monitoring remains the cornerstone of preventing
hypoglycaemia.
References
1. Schafers, S., et al., Incidence of hypoglycemia following insulin-based acute stabilization of
hyperkalemia treatment. Journal of Hospital Medicine, 2012. 7(3): p. 239-242.
2. Scott, N.L., et al., Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the
emergency department. Am J Emerg Med, 2019. 37(2): p. 209-213.
3. Boughton, C.K., et al., Preventing Hypoglycemia Following Treatment of Hyperkalemia in Hospitalized
Patients. J Hosp Med, 2019. 14(5): p. 284-287.
4. Apel, J., S. Reutrakul, and D. Baldwin, Hypoglycemia in the treatment of hyperkalemia with insulin in
patients with end-stage renal disease. Clin Kidney J, 2014. 7(3): p. 248-50.
5. Coca, A., et al., Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients
with impaired renal function. PLoS One, 2017. 12(2): p. e0172961.
6. Bruno, A., et al., Normal glucose values are associated with a lower risk of mortality in hospitalized
patients. Diabetes Care, 2008. 31(11): p. 2209-10.
7. Bonds, D.E., et al., Severe hypoglycemia symptoms, antecedent behaviors, immediate consequences
and association with glycemia medication usage: Secondary analysis of the ACCORD clinical trial
data. BMC Endocr Disord, 2012. 12: p. 5.
8. Wei, M., et al., Low fasting plasma glucose level as a predictor of cardiovascular disease and all-
cause mortality. Circulation, 2000. 101(17): p. 2047-52.
9. Rana, O., et al., Acute hypoglycemia decreases myocardial blood flow reserve in patients with type 1
diabetes mellitus and in healthy humans. Circulation, 2011. 124(14): p. 1548-56.
10. Quah, J., et al., Insulin-induced hypoglycaemia and the detection of myocardial injury using an
ultrasensitive troponin assay. Int J Cardiol, 2016. 215: p. 446-8.
11. Farina, N. and C. Anderson, Impact of dextrose dose on hypoglycemia development following
treatment of hyperkalemia. Ther Adv Drug Saf, 2018. 9(6): p. 323-329.
12. Pierce, D.A., G. Russell, and J.L. Pirkle, Jr., Incidence of Hypoglycemia in Patients With Low eGFR
Treated With Insulin and Dextrose for Hyperkalemia. Ann Pharmacother, 2015. 49(12): p. 1322-6.
13. Wheeler, D.T., et al., Weight-based insulin dosing for acute hyperkalemia results in less
hypoglycemia. J Hosp Med, 2016. 11(5): p. 355-7.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 105
Treatment of hyperkalaemia in haemodialysis patients (Guidelines 18.1-18.4)
Guideline 18.1 - Hyperkalaemia: Treatment in haemodialysis patients
We recommend that haemodialysis patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) receive
dialysis treatment urgently. (1A)
Guideline 18.2 - Hyperkalaemia: Treatment in haemodialysis patients
We recommend that haemodialysis patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) and toxic
ECG changes be treated with intravenous calcium salt to reduce risk of arrhythmias even when dialysis is
immediately available. (1C)
Guideline 18.3 - Hyperkalaemia: Treatment in haemodialysis patients
We recommend that haemodialysis patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) be treated
with standard medical therapies to lower serum potassium if dialysis is not immediately available. (1B)
Guideline 18.4 - Hyperkalaemia: Treatment in haemodialysis patients
We suggest that potassium binders may be considered to reduce the risk of hyperkalaemia during the inter-
dialytic period. (1B)
Audit measures
1. The incidence of patients requiring emergency dialysis for severe hyperkalaemia.
Rationale (Guidelines 18.1 – 18.4)
Haemodialysis (HD) patients have a high risk of hyperkalaemia. Hyperkalaemia has been found to be a
significant factor contributing to mortality in dialysis patients
1-6
and was shown to be responsible for 3-5% of
deaths.
1, 4
Factors contributing to hyperkalaemia in HD patients are summarised in Table 22. The risk of
hyperkalaemia in longterm HD patients is influenced by dietary K+ intake, frequency and duration of dialysis,
blood glucose level and constipation.
7-9
The most common time for hyperkalaemic events in HD patients is immediately after the 3-day weekend
break (i.e. Mondays for patients dialysed on Mon/Wed/Fri or Tuesdays for patients dialysed on
Tue/Thu/Sat).
10, 11
The long inter-dialytic break also correlates with hospitalisation
12, 13
and mortality in HD
patients.
10, 13-15
The PORTEND (Potassium and Cardiac Rhythm Trends in MainENance HemoDialysis)
observational study in the USA showed an incidence of pre-dialysis hyperkalaemia (K+ > 5.0 mmol/l) after
the long inter-dialytic interval of 37% in patients dialysing on a dialysate K+ concentration of ≤ 2mmol/l and
21% in patients dialysing on a dialysate K+ ≥ 3mmol/l.
16
The UK Renal Association Clinical Practice Guideline
on Haemodialysis (2019) recommends an optimal pre-dialysis serum K+ in the range of 4.0 – 6.0 mmol/l.
17

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 106
Table 22: Factors associated with an increased risk of hyperkalaemia in HD patients.
Dialysis: Dialysis is the definitive treatment for hyperkalaemia in patients receiving longterm HD. In one
report, hyperkalaemia was the reason for emergency dialysis 24% of the time in their maintenance HD
program.
18
Although a degree of ‘tolerance’ to hyperkalaemia has been postulated in HD patients,
19
there
remains a risk of arrhythmias, cardiac arrest or sudden death in HD patients with severe hyperkalaemia.
Each HD session removes approximately 70 – 100 mmol K+.
20
The dialysate K+ concentration determines
the rate of K+ removal. Serum K+ concentration typically falls by 1 mmol/l during the first hour of dialysis
when the gradient between the serum and dialysate K+ is highest, then by 1 mmol/l over the next 2 hours.
20
The serum K+ reaches a steady state during the last hour of the treatment. The choice of dialysate fluid is
guided by the severity of hyperkalaemia as shown below in Table 23. The use of a 1 mmol/l K+ dialysate
fluid is potentially associated with an increased risk of arrhythmias,
21
therefore telemetry and close
monitoring of K+ level is essential. An alternative approach is the use of sequential dialysis sessions.
22
Pre-dialysis serum K
+
(mmol/l)
DIALYSATE K
+
CONCENTRATION (mmol/l)
CHRONIC HAEMODIALYSIS
4.5 – 5.5
2 or 3
(based on individual trend)
5.6 – 8.0
2
>8.0
1
(telemetry + 30min K
+
checks; switch to 2 mmol K
+
when serum K
+
< 7)
Table 23: Dialysate K+ prescription in chronic HD patients.[22]
Factors contributing to hyperkalaemia in Haemodialysis patients:
• Duration since last dialysis session
• Dietary K
+
intake
• Dialysate K
+
concentration
• Problems with dialysis access - poor blood flow, recirculation
• Poor dialysis adequacy
• Medication
• Diabetic status – glycaemic control
• Constipation
• Compliance – poor attendance, shortened treatment time

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 107
Bridging to dialysis initiation: Intravenous calcium reduces the risk of arrhythmias (Guideline 16.2),
therefore is warranted in patients with severe hyperkalaemia and toxic ECG changes even if dialysis can be
established quickly. More frequently, dialysis may not be immediately available and temporising measures
will also be necessary. Drugs used in lowering the serum K+ may have a variable effective in HD patients.
Fortunately, the K+-lowering effect of insulin is preserved in patients with renal failure,
23
but these patients
are more prone to hypoglycaemia. Although some studies have suggested a lower dose of insulin in treating
patients with poor renal function,
24, 25
a pre-treatment blood glucose < 7 mmol/l was the most consistent risk
factor for hypoglycaemia (Guideline 16.3). The effectiveness of salbutamol may be reduced, with studies
demonstrating up to 40% of patients with ESRD appear to be resistant to the hypokalemic effect of
salbutamol, even those who are not receiving beta-blockers.
26, 27
Medical therapies provide a bridge to
dialysis initiation, but a gradual rebound in hyperkalaemia should be anticipated.
The use of drugs to shift K+ from the extra- to intracellular space reduces serum K+ without reducing the
total body K+. This transcellular shift is thought to reduce the amount of K+ available in the serum to be
removed during HD. Driver et al conducted a retrospective study (n=479) in patients presenting to the
Emergency Department with hyperkalaemia who subsequently underwent HD.
28
Shifting medication was
administered in 50% of patients. Recurrent hyperkalaemia within 24 hours occurred in 27% of patients who
received shifting drugs versus 18% in those who did not. Repeat HD within 24 hours was required in 30% of
patients who received shifting drugs and 25% in those who did not. The authors concluded that transcellular
K+ shifting before emergent dialysis is not associated with recurrent hyperkalaemia or need for multiple HD
sessions, however it is noteworthy that the median time from drug administration to start of HD was 4.2
hours (2.5-8.4 hours) and the effect of drugs may have worn off.
Prevention: Until recently, the options for preventing hyperkalaemia in HD patients has been limited to
dietary K+ restriction and low K+ dialysate solutions. Novel potassium binders may provide an additional
strategy.
29
Patiromer and SZC may help to control serum K+ levels in patients treated with less frequent HD.
Selection of patients for this strategy is important as poor adherence to medication and diet could have
potentially serious consequences.
Patiromer: Three studies have investigated the use of patiromer in HD patients.
30-32
Kovesky et al
demonstrated that patiromer reduced serum K+ by an average of 0.5 mmol/l and in HD patients with severe
hyperkalaemia (K+ ≥ 6.5 mmol/l), the reduction was in the order of 1.0 mmol/l.[31] The relative proportion
of patients with severe hyperkalaemia in this study was reduced by approximately 50%. Similarly, Chatoth et
al showed a reduction in the number of hyperkalaemic events and hospitalisation in HD patients treated
with patiromer.
32
Bushingsky et al performed an inpatient study of only 6 HD patients showing a significant
decrease in serum K+.
30
Given that patiromer uses Ca2+ as the counter exchange cation for K+, there is a
potential risk of increased vascular calcification. Phosphate binders were generally withheld during
patiromer trials, therefore these factors may have implications for longterm management in dialysis
patients.
Inform the Renal Team immediately if a dialysis patient presents with
hyperkalaemia as medical treatments will only temporarily control K
+
level.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 108
The TWOPLUS-HD trial [NCT03740048] is an ongoing pilot trial of twice versus thrice weekly HD in patients
with incident ESRD to investigate whether patiromer can have a dialysis-sparing effect.
33
Patients
randomised to twice-weekly regimen will receive a loop diuretic and sodium bicarbonate with the addition
of patiromer if they develop hyperkalaemia during the 6 week study period.
SZC: The DIALIZE trial was a Phase IIIb RCT (NCT03303521) designed to evaluate SZC in controlling
hyperkalaemia in haemodialysis (HD) patients.
34
This is the first randomised, double-blind, placebo
controlled trial to assess a potassium binder in HD patients. The primary end-point was the proportion of
patients who maintained pre-dialysis serum K+ of 4.0 – 5.0 mmol/l during at least 3 long interdialytic periods
over the 4-week evaluation period that followed dose titration. The study demonstrated a significant
reduction in pre-dialysis hyperkalaemia at the highest risk period (41.2% vs 1.0% in the placebo arm) and a
reduction in need for emergency treatment for hyperkalaemia (2.1% vs 5.1% in placebo arm).
References
1. Shibata, M., T. Kishi, and H. Iwata, Clinical study of complications in dialyzed diabetics. Tohoku J Exp
Med, 1983. 141 Suppl: p. 417-25.
2. Lowrie, E.G. and N.L. Lew, Death risk in hemodialysis patients: the predictive value of commonly
measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis,
1990. 15(5): p. 458-82.
3. Kovesdy, C.P., et al., Serum and dialysate potassium concentrations and survival in hemodialysis
patients. Clin J Am Soc Nephrol, 2007. 2(5): p. 999-1007.
4. Morduchowicz, G., et al., Causes of Death in Patients with End-Stage Renal-Disease Treated by
Dialysis in a Center in Israel. Israel Journal of Medical Sciences, 1992. 28(11): p. 776-779.
5. Unruh, M.L., et al., Skipped treatments, markers of nutritional nonadherence, and survival among
incident hemodialysis patients. American Journal of Kidney Diseases, 2005. 46(6): p. 1107-1116.
6. Iseki, K., et al., Impact of the initial levels of laboratory variables on survival in chronic dialysis
patients. Am J Kidney Dis, 1996. 28(4): p. 541-8.
7. Rossignol, P., et al., Hyperkalaemia prevalence, recurrence and management in chronic
haemodialysis: a prospective multicentre French regional registry 2-year survey. Nephrol Dial
Transplant, 2017. 32(12): p. 2112-2118.
8. Sumida, K., K. Yamagata, and C.P. Kovesdy, Constipation in CKD. Kidney Int Rep, 2020. 5(2): p. 121-
134.
9. Genovesi, S., et al., Sudden cardiac death in dialysis patients: different causes and management
strategies. Nephrol Dial Transplant, 2019.
10. Bleyer, A.J., G.B. Russell, and S.G. Satko, Sudden and cardiac death rates in hemodialysis patients.
Kidney International, 1999. 55(4): p. 1553-1559.
11. Yusuf, A.A., et al., Serum Potassium Levels and Mortality in Hemodialysis Patients: A Retrospective
Cohort Study. Am J Nephrol, 2016. 44(3): p. 179-86.
12. Fotheringham, J., et al., The mortality and hospitalization rates associated with the long interdialytic
gap in thrice-weekly hemodialysis patients. Kidney Int, 2015. 88(3): p. 569-75.
13. Foley, R.N., et al., Long interdialytic interval and mortality among patients receiving hemodialysis. N
Engl J Med, 2011. 365(12): p. 1099-107.
14. Karnik, J.A., et al., Cardiac arrest and sudden death in dialysis units. Kidney Int, 2001. 60(1): p. 350-7.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 109
15. Zhang, H., et al., Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule
affects day-of-week mortality. Kidney Int, 2012. 81(11): p. 1108-15.
16. Potassium and Cardiac Rhythm Trends in MaintENance HemoDialysis: A multicenter, Prospective,
Observational Study (PORTEND). ClinicalTrials.gov; NCT02609841.
17. Ashby, D., et al, UK Renal Association Clinical Practice Guideline - Haemodialysis. July 2019.
www.renal.org/wp-content/uploads/2019/10/FINAL-HD-Guideline.pdf.
18. Sacchetti, A., et al., ED hemodialysis for treatment of renal failure emergencies. Am J Emerg Med,
1999. 17(3): p. 305-7.
19. Frohnert, P.P., et al., Statistical investigation of correlations between serum potassium levels and
electrocardiographic findings in patients on intermittent hemodialysis therapy. Circulation, 1970.
41(4): p. 667-76.
20. Bansal, S. and P.E. Pergola, Current Management of Hyperkalemia in Patients on Dialysis. Kidney Int
Rep, 2020. 5(6): p. 779-789.
21. Pun, P.H., et al., Modifiable risk factors associated with sudden cardiac arrest within hemodialysis
clinics. Kidney Int, 2011. 79(2): p. 218-27.
22. Pirklbauer, M., Hemodialysis treatment in patients with severe electrolyte disorders: Management
of hyperkalemia and hyponatremia. Hemodial Int, 2020.
23. Alvestrand, A., et al., Insulin-mediated potassium uptake is normal in uremic and healthy subjects.
Am J Physiol, 1984. 246(2 Pt 1): p. E174-80.
24. Pierce, D.A., G. Russell, and J.L. Pirkle, Jr., Incidence of Hypoglycemia in Patients With Low eGFR
Treated With Insulin and Dextrose for Hyperkalemia. Ann Pharmacother, 2015. 49(12): p. 1322-6.
25. McNicholas, B.A., et al., Treatment of Hyperkalemia With a Low-Dose Insulin Protocol Is Effective and
Results in Reduced Hypoglycemia. Kidney Int Rep, 2018. 3(2): p. 328-336.
26. Allon, M., R. Dunlay, and C. Copkney, Nebulized albuterol for acute hyperkalemia in patients on
hemodialysis. Ann Intern Med, 1989. 110(6): p. 426-9.
27. Allon, M. and C. Copkney, Albuterol and insulin for treatment of hyperkalemia in hemodialysis
patients. Kidney International, 1990. 38(5): p. 869-872.
28. Driver, B.E., et al., Is Transcellular Potassium Shifting With Insulin, Albuterol, or Sodium Bicarbonate
in Emergency Department Patients With Hyperkalemia Associated With Recurrent Hyperkalemia
After Dialysis? J Emerg Med, 2018. 55(1): p. 15-22 e3.
29. Palmer, B.F., Potassium Binders for Hyperkalemia in Chronic Kidney Disease-Diet, Renin-Angiotensin-
Aldosterone System Inhibitor Therapy, and Hemodialysis. Mayo Clin Proc, 2020. 95(2): p. 339-354.
30. Bushinsky, D.A., et al., Patiromer Decreases Serum Potassium and Phosphate Levels in Patients on
Hemodialysis. Am J Nephrol, 2016. 44(5): p. 404-410.
31. Kovesdy, C.P., et al., Real-World Evaluation of Patiromer for the Treatment of Hyperkalemia in
Hemodialysis Patients. Kidney Int Rep, 2019. 4(2): p. 301-309.
32. Chatoth, D., et al., Real-World Outcomes of Hyperkalemia Management with Patiromer in End-Stage
Renal Disease Patients Undergoing Hemodialysis in the United States. Nephrology Dialysis
Transplantation, 2017. 32: p. 657-+.
33. A pilot trial of twice-weekly versus thrice weekly haemodialysis in patients with incident end-stage
kidney disease (The TWOPLUS-HD). ClinicalTrials.gov - NCT03740048.
34. Fishbane, S., et al., A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium
Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia. J Am Soc Nephrol,
2019.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 110
Referral to specialist services and escalation of care (Guidelines 19.1-19.6)
Guideline 19.1 - Hyperkalaemia: Specialist Referral
We suggest that patients with severe hyperkalaemia (serum K+ 6.5 mmol/L) be referred to their local renal
or critical care team for an urgent opinion, guided by the clinical scenario and its persistence after initial
medical treatment. (2C)
Guideline 19.2 - Hyperkalaemia: Referral to critical care services
We recommend that for patients with severe hyperkalaemia, and where there is no provision of renal
services on site, referral is made to the local critical care team in the first instance, guided by the clinical
scenario and established local policies. (1C)
Guideline 19.3 - Hyperkalaemia: Escalation of care
We recommend that patients are referred to the critical care team by a senior member of the referring team
if escalation of care is required from the outset or if the patient fails to respond to initial treatment. (1B)
Guideline 19.4 - Hyperkalaemia: Treatment facilities - Critical care
We recommend that patients with severe hyperkalaemia and problems with airway, breathing, circulation
and/or conscious level, be referred to the local critical care team in the first instance. (1C)
Guideline 19.5 – Hyperkalaemia: Treatment facilities – Ward, Enhanced Care or Critical Care area
We recommend that stable patients with severe hyperkalaemia be admitted to an area with facilities for
continuous cardiac monitoring which are sufficiently staffed to support clinical monitoring and treatment,
including an acute medical unit, renal unit, coronary care unit, enhanced care area, or critical care unit (HDU
or ICU) depending on local facilities or practice. (1C)
Guideline 19.6 – Hyperkalaemia: RRT in treatment of hyperkalaemia in acutely unwell patients.
We recommend that the decision on timing, suitability and modality for initiation of RRT in patients with life-
threatening hyperkalaemia, either from the outset or resistant to initial medical therapy, is taken urgently by
a nephrologist or critical care specialist. (1C)
Rationale (Guidelines 19.1 – 19.6)
Hyperkalaemia may be present on hospital admission or develop during the course of admission due to
acute illness or alterations in medications. It may be feasible to manage most cases of mild to moderate
hyperkalaemia on a non-renal ward. In many of these cases, hyperkalaemia resolves after treating the
precipitant (e.g. discontinuing a RAASi drug).
Patients with moderate hyperkalaemia who are at risk of further rise (e.g. oliguria, rhabdomyolysis) and
those with severe hyperkalaemia should be assessed by a senior clinician (i.e. registrar or consultant grade).
Referral to the renal or critical care team should be guided by the cause of hyperkalaemia, level of acuity,

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 111
response to initial medical treatment and availability of services locally.
1
Further considerations to guide
escalation of care are the likelihood of survival (e.g. reversible illness), extent of comorbidity, accurate
assessment of pre-morbid functional status, and the patient’s wishes. The management plan, ceiling of care
(i.e. ward, HDU or ICU) and resuscitation status should be documented early.
Placement is guided by the level of care required. The need for basic or advanced organ support, including
dialysis, defines the appropriate clinical area. Patients with severe hyperkalaemia require continuous cardiac
monitoring and need to be triaged to an area with these facilities.
2
Enhanced care areas have been
developed in some regions to provide a level of care between high dependency and ward level.
3
Patients
requiring acute RRT (e.g. haemodialysis or haemofiltration) meet the criteria for Level 2 care which can be
delivered in a renal or critical care unit. Patients receiving a minimum of two organ support (e.g. renal and
cardiovascular or respiratory support) meet the criteria for Level 3 care.
4
Severe hyperkalaemia can cause abrupt cardiac arrest, sometimes without warning ECG changes. It is a key
indication for emergency RRT.
5
Where a decision has been taken to treat with RRT, it should be performed
with due regard for potential deterioration.
1,3,6
The provision of RRT in renal units and ICUs varies across the
country with respect to the timing of initiation, prescribed dose, and modality of RRT available.
7
Conventional intermittent haemodialysis (IHD) is thought to be the most effective method for K+ removal,
but continuous venovenous haemofiltration (CVVH) and continuous veno-venous haemodiafiltration
(CVVHDF) are more frequently available in ICUs in the UK.
8
Nearly 90% of UK ICUs have facilities for RRT.
9
Traditionally, it has been thought that CVVH is not as efficient as IHD at removing K+ and therefore was not
generally recommended as the first line extracorporeal therapy in hyperkalaemic patients. However, CVVH
and CVVHDF are acceptable RRT techniques for management of hyperkalaemia, albeit with a slower initial
reduction in serum K+ than with IHD, but followed by sustained correction of electrolyte abnormalities.
10
Potassium removal with IHD decreases after 2 hours and rebound occurs after dialysis is stopped.
10
The main advantages of continuous methods are their potential benefits in haemodynamically unstable
patients, lower risk of rebound hyperkalaemia (given the continuous nature and kinetics of solute removal),
ability to tailor K+ removal according to serum K+ measurements and, importantly, the wide availability in
ICUs.
3
References
1. National Institute for Health and Carel Excellence. Acute kidney injury: prevention, detection and
management. Clinical guideline [CG169]. August 2013. www.nice.org.uk/guidance/cg/169.
2. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
3. The Faculty of Intensive Care Medicine - Enhanced Care: Guidance on Service Development in Acute
Care. November 2019.
www.ficm.ac.uk/sites/default/files/enhanced_care_guidance_for_open_consultation-
nov_2019_v2.pdf.
4. The Faculty of Intensive Care Medicine. Guidelines for Provision of Intensive Care Services (GPICS-
Edition 2). June 2019. www.ficm.ac.uk/sites/default/files/gpics-v2.
5. Gemmell, L., R. Docking, and E. Black, Renal replacement therapy in critical care. Bja Education, 2017.
17(3): p. 88-93.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 112
6. Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney
Injury. Kidney Int Suppl 2012; 2(1). doi:10.1038/kisup.2012.
7. Fayad, A.I., D.G. Buamscha, and A. Ciapponi, Intensity of continuous renal replacement therapy for
acute kidney injury. Cochrane Database of Systematic Reviews, 2016(10).
8. Jones, S.L. and M.A.J. Devonald, How acute kidney injury is investigated and managed in UK intensive
care units - a survey of current practice. Nephrology Dialysis Transplantation, 2013. 28(5): p. 1186-
1190.
9. Gatward, J.J., et al., Renal replacement therapy for acute renal failure: a survey of practice in adult
intensive care units in the United Kingdom. Anaesthesia, 2008. 63(9): p. 959-966.
10. Depret, F., et al., Management of hyperkalemia in the acutely ill patient. Ann Intensive Care, 2019.
9(1): p. 32.
Minimum standards for patient transfer (Guidelines 20.1-20.2)
Guideline 20.1 - Hyperkalaemia: Transfer to renal services
We suggest that transfer to renal services be considered in clinically stable patients in whom
hyperkalaemia cannot be controlled (i.e. serum K
+
< 6.5 mmol/L) using medical measures, particularly
in the presence of advanced or oliguric renal failure (either AKI or CKD). (2C)
Guideline 20.2 - Hyperkalaemia: Minimum standards for safe patient transfer
We suggest that any inter- or intra-hospital patient transfer is coordinated by senior clinicians and
follows national guidelines. (2B)
Rationale (Guidelines 20.1 – 20.2)
The most important aspect of patient transfer is ensuring safety. There are three key steps in
optimising patient transfer - firstly, to decide if transfer is absolutely necessary; secondly, to optimise
the patient prior to transfer; and thirdly, to coordinate and perform the transfer itself.
1
The decision to transfer the patient with hyperkalaemia will be guided by the availability of renal
services locally. Intra-hospital patient transfer from a ward or emergency department to a high
dependency area, renal unit or ICU within the referring hospital is less complicated, but still requires
good communication and coordination. Cardiac monitoring and resuscitation equipment are essential
for the transfer of patients with hyperkalaemia, either within or between hospitals.
Inter-hospital transfer to the nearest renal unit or ICU may be required for definitive management.
This decision must be made by the responsible consultant, in conjunction with consultant colleagues
from relevant specialities in both the referring and receiving hospitals.
2
The timing and urgency of
transfer will be decided by the nephrologist and/or intensivist. The decision to accept a transferred
patient should be made by a consultant in the receiving unit.
Pre-transfer stabilisation is essential for all patients.
1,3
Following appropriate medical therapy for
hyperkalaemia, the response to treatment should be assessed with repeat observations,
biochemistry, blood glucose and ECG prior to transfer. We suggest that a patient should not, in
general, be transferred between hospitals if the serum K
+
is 6.5 mmol/L, though other factors (in

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 113
particular, the location of intensive care and dialysis facilities) will occasionally over-ride this
consideration. Critical care review is essential for patients with any concern regarding oxygenation,
ventilation or haemodynamic instability.
Table 24: Minimum standards for safe patient transfer.
Adapted from Dunn (2007)
3
, FICM guidelines (2019),
1
and ICS guidelines (2011),
4
NICE (2017)
5
The organisation of the patient transfer itself requires a coordinated approach and liaison with the
receiving team to ensure that they are prepared for the patient’s arrival.
2
The use of a transfer
checklist, protocols and skilled staff reduce mortality.
4
The clinical risk of the transfer and the level of
competence required by escorting staff will be guided by the patient’s condition.
Every hospital should have suitable arrangements in place for providing patient transfer including
trained personnel, equipment, and drugs to treat the specific problem.
4
Hospitals should form
transfer networks to co-ordinate and manage clinically indicated transfers.
2
Record keeping is a legal
requirement for all patient transfers.
1
Clear records should be maintained at all stages of transfer
including the patient’s condition, reason for transfer, names of referring and accepting consultants,
clinical status prior to transfer, during transfer and on arrival. Arrangements should be in place for
the return of staff and equipment after transfer. The procedure for safe patient transfer is
summarised in Table 27.
Prompt clinical re-assessment by the receiving medical team is required following transfer, including
observations, bloods and ECG. The K
+
-lowering effect of medical treatment for hyperkalaemia is
temporary (< 6 hours), therefore repeat bloods to assess for rebound hyperkalaemia is important
(Guideline 18.1). The potential for hypoglycaemia up to 6 hours after administration of insulin-
glucose should be considered and blood glucose checked on arrival.
Summary of requirements for safe patient transfer:
1. Decision regarding need for patient transfer
2. Review of investigations and treatment and ensure clear management plan
3. Pre-transfer assessment and stabilisation
4. Good communication between referring team, critical care and receiving teams
5. Arrangement of ambulance for inter-hospital transfer
6. Consider staff (medical & nursing), drugs (iv calcium, salbutamol nebules, 20% dextrose
in event of hypoglycaemia) and equipment (cardiac monitor/ defibrillator, blood
glucose monitor) required for safe transfer
7. Ensure medical and nursing records are complete and are kept confidential, as
governed by the Data Protection Act 2018
8. Inform patient’s relatives of transfer
9. Provide ongoing treatment and care as necessary during transfer, including maintaining
clinical appropriate records
10. Maintaining patient dignity
11. Hand-over to receiving team
12. Return of transfer staff and equipment

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 114
References
1. Faculty of Intensive Care Medicine. Guidance On: The Transfer of the Critically Ill Adult. 2019.
https://www.ficm.ac.uk/sites/default/files/transfer_critically_ill_adult_2019.pdf
2. Bourn, S., S. Wijesingha, and G. Nordmann, Transfer of the critically ill adult patient. Bja Education,
2018. 18(3): p. 63-68.
3. Dunn, M.J., C.L. Gwinnutt, and A.J. Gray, Critical care in the emergency department: patient
transfer. Emerg Med J, 2007. 24(1): p. 40-4.
4. Whiteley, S. Guidelines for the transport of the critically ill adult (3rd Edition 2011). Intensive Care
Society 2011.
5. National Institute for Health and Care Excellence. Standardised systems of care for intra- and inter-
hospital transfers. [NG94]. July 2017. https://www.nice.org.uk/guidance/ng94
Prevention (Guidelines 21.1-21.4)
Guideline 21.1 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend that the need for prescribed medication which can cause hyperkalaemia are reviewed in the
context of the current illness and level of renal function both on and during hospital admission. (1B)
Guideline 21.2 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend a low potassium diet for hospitalised patients with moderate or severe hyperkalaemia. (1C)
Guideline 21.3 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend that community blood monitoring is arranged on discharge for all patients who have
required treatment for hyperkalaemia during hospital admission. (1B)
Guideline 21.4 – Hyperkalaemia: Prevention of hyperkalaemia in hospitalised patients
We recommend that the risk of recurrence of hyperkalaemia is considered before reinstating previous
medication that may have contributed to the episode. (1B)
Audit Measures
1. The frequency of hyperkalaemia developing beyond 24 hours of hospital admission.
2. The frequency of prescribed drugs potentially contributing to hyperkalaemia.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 115
Rationale (Guideline 21.1 – 21.4)
The NCEPOD Report (2009), ‘Adding Insult to Injury’, highlighted the risk of AKI in acute hospital admissions.
1
Acute illness (e.g. sepsis, diarrhoea and vomiting) with systemic hypotension can result in reduced renal
blood flow and ultimately AKI with hyperkalaemia. Patients may present with hyperkalaemia at the time of
hospital admission or it may develop after hospital admission in patients in whom the K+ level was normal
on arrival. Clinicians should be alert to the potential development of hyperkalaemia in the context of
intercurrent illness in patients receiving drugs known to exacerbate hyperkalaemia. Early recognition and
treatment of AKI can reduce morbidity and mortality.
Hyperkalaemia often occurs after hospital admission. A study of in-patients with hyperkalaemia showed that
33.3% of cases developed after hospital admission.
4
Most cases were mild, but 15.4% were moderate or
severe (K+ ≥ 6.0 mmol/l). AKI was present in 73% of cases with a pre-renal cause in 50% of these. The
aetiology was often multifactorial, but hyperkalaemia was more common in the elderly and patients with
diabetes and/or CKD. Prescribed medication was implicated in 76% of patients receiving potentially
hyperkalaemia-inducing drugs (e.g. RAASi) and 55% of these patients were taking two or more of such
medications.
5
Furthermore, this study demonstrated that the severity of hyperkalaemia was found to be
significantly correlated (p< 0.01) with the number of potentially hyperkalaemia-inducing drugs used
concurrently. Medications frequently implicated in hyperkalaemia are summarised in Table 25.
Hospital acquired hyperkalaemia is common in the elderly, aged 75 years or more. Robert et al investigated
the factors predisposing to the development of hyperkalaemia occurring 3 or more days after hospital
admission in an elderly cohort.
5
Hyperkalaemia developed during 4.5% of hospital admissions and 27% of
episodes were considered severe (K+ ≥ 6.0 mmol/l). AKI was present in 51% of cases and hyperkalaemia-
inducing drugs were implicated in 80.5% of cases.
5
Overall, 79.9% of hyperkalaemic events were potentially
avoidable.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 116
Table 25: Drugs commonly associate with hyperkalaemia (Adapted from [2, 3]).
Hyperkalaemia is particularly common in patients with CKD. Furuland et al reported hyperkalaemia (K+ > 5.0
mmol/l) in 48.4% of patients admitted with renal impairment with multiple episodes occurring in 28.8% of
patients with CKD Stage 3-5 .
6
The main risk factors for recurrence were decline in renal function, diabetes
and treatment with RAASi drugs. Patients with hyperkalaemia were shown to have a longer duration of
hospital stay and higher mortality risk than those without hyperkalaemia
.6
Pharmacists can also play a role in the prevention of hospital-acquired hyperkalaemia during medicines
reconciliation. Reviewing drug therapy and dosage early in the course of hospital admission, especially in
patients at risk of AKI, allows time to consider if any medications should be withheld.
7, 8
Dietary intervention is a further preventative measure in hospitalised patients with moderate or severe
hyperkalaemia. Institution of a low potassium diet may help to control hyperkalaemia and avoid recurrence.
This requires provision of a renal menu and restrictions on food items brought in by family members. A
formal assessment by a renal dietician would ensure appropriate guidance is given.
Drugs that affect aldosterone secretion
• ACE inhibitors
• Angiotensin Receptor Blockers
• Non-steroidal anti-inflammatory drugs
• Calcineurin inhibitors
• Heparins
• Antifungals (e.: ketoconazole, fluconazole and itraconazole)
Drugs that block aldosterone binding to mineralocorticoid receptor (MRA)
• Spironolactone
• Eplerenone
• Drospirenone
Drugs that inhibit activity of epithelial sodium channel
• Potassium sparing diuretics (e.g. amiloride and triamterene)
• Trimethoprim; Co-trimoxazole
• Pentamidine
Drugs that alter transmembrane potassium movement
• β-blockers
• Digoxin
• Intravenous cationic amino acids
• Hyperosmolar solutions (e.g. mannitol, glucose)
• Suxamethonium
Potassium containing agents
• Potassium supplements (e.g. Sando-K®, Kay-Cee L Liquid ®)
• Salt substitutes
• Herbal medicines (e.g. alfalfa, dandelion, horsetail, milkweed and nettle)
• Stored red blood cells

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 117
Patients may also be at risk of hyperkalaemia after hospital discharge. Amongst patients who were
normokalaemic and prescribed a RAAS inhibitor on discharge from hospital, 12.3% of patients have been
shown to develop hyperkalaemia during the early period after discharge.
9
Risk increases in the presence of
impaired renal function, use of drug combinations that can exacerbate hyperkalaemia or in patients with a
higher baseline K+ level.
9
Patient education and community monitoring should be in place before hospital
discharge.
Re-instating RAASi or other medication following an acute illness associated with hyperkalaemia is another
important consideration. This decision balances the risk-benefit ratio and original indication of the drug (e.g.
heart failure).
10
It is reasonable to consider re-introduction and re-titration of the drug, on recovery, in
patients who previously had stable renal function and K+ levels prior to the acute illness.
9
Whether
treatment is re-started in hospital or intended in the community, clear communication with primary care or
specialist clinic (e.g. Heart Failure service) is required on hospital discharge.
References
1. National Confidential Enquiry into Patient Outcome and Death. Adding Insult to Injury.2009.
www.ncepod.org.uk
2. Nyirenda, M.J., et al., Hyperkalaemia. BMJ, 2009. 339: p. b4114.
3. Ben Salem, C., et al., Drug-induced hyperkalemia. Drug Saf, 2014. 37(9): p. 677-92.
4. de Sequera, P., et al., Hyperkalaemia in hospitalised patients. How to avoid it? Nefrologia, 2014.
34(3): p. 417-9.
5. Robert, L., et al., Hospital-Acquired Hyperkalemia Events in Older Patients Are Mostly Due to
Avoidable, Multifactorial, Adverse Drug Reactions. Clin Pharmacol Ther, 2019. 105(3): p. 754-760.
6. Furuland, H., et al., Recurrent Hyperkalaemia and Association with Length-of-Stay and Mortality
Following Hospitalisation: Real-World Evidence from UK Patients with Ckd. Nephrology Dialysis
Transplantation, 2018. 33: p. 157-157.
7. Think Kidneys: Communities at risk of developing acute kidney injury. 2018.
www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/11/Nov-18-Communities-at-risk.
8. Think Kidneys: Guidelines for medicines optimisation in patients with acute kidney injury. 2016.
www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2016/03/Guidelines-for-medicines-
optimisation-in-patients-with-AKI-final.
9. Saito, Y., et al., Incidence of and risk factors for newly diagnosed hyperkalemia after hospital
discharge in non-dialysis-dependent CKD patients treated with RAS inhibitors. PLoS One, 2017. 12(9):
p. e0184402.
10. Think Kidneys: When or if to re-start ACEI, ARB, diuretics and other antihypertensive drugs after an
episode of acute kidney injury. 2018. www.thinkkidneys.nhs.uk/aki/wp-
content/uploads/sites/2/2018/11/Nov-18-restarting-diuretics.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 118
Treatment Algorithm: Hospital (Guidelines 22.1)
Guideline 21.1 – Hyperkalaemia; Algorithm in Hospital
We recommend that hyperkalaemia in hospitalised patients is managed using the treatment
algorithm which provides guidance on the medical therapies and the need for initiation of renal
replacement therapy. (1C)
Rationale (Guideline 21.1)
Treatment algorithms provide a systematic approach for managing medical emergencies and can improve
consistency in clinical practice. This strategy has been utilised in resuscitation for decades and the provision
of clear user-friendly instructions is helpful in stressful and time-critical situations. Algorithms may also be
used as an aide memoire and as a teaching tool.
The response to hyperkalaemia in hospitalised patients is guided by its severity and ECG appearances. The
algorithm provides actions to be taken at different time intervals (i.e. first 15-30 minutes, next 30-60
minutes) to avoid delay. It follows the 5-step approach in treating hyperkalaemia to ensure that each
priority is addressed as shown in Appendix 7.
The 2019 algorithm provides guidance for the new treatment recommendations:
• 10% glucose infusion following insulin-glucose for patients with a pre-treatment blood glucose <
7.0 mmol/l
• Sodium zirconium cyclosilicate for life-threatening hyperkalaemia

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 119
Section 3
Management of Hyperkalaemia
in Resuscitation

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 120
Hyperkalaemia in Resuscitation
Introduction
Hyperkalaemia is an uncommon, but potentially reversible cause of cardiac arrest.
1, 2
It most often
occurs in patients with pre-existing renal disease or in the context of an acute kidney injury. Patients
receiving long-term haemodialysis (HD) are most at risk of hyperkalaemia. Cardiac arrest can occur in
hospital, within an out-patient dialysis unit or out of hospital, but hyperkalaemia should be considered
in all settings in patients at risk. Patients on long-term HD are one of the highest risk groups for out-
of-hospital cardiac arrest, occurring 20 times more frequently than in the general population.
3
The reported incidence of in-hospital hyperkalaemic cardiac arrest is variable. Wallmuller et al found
hyperkalaemia as the primary aetiology in only 1% of in-hospital cardiac arrests (n=1041) although it
was the most common metabolic cause (47%).
4
In contrast, Wang et al
5
reported an incidence of 12%
(n=1114) and Saarinen et al
6
reported an incidence of 13% (n=104) in patients with PEA as the initial
rhythm following in-hospital cardiac arrest (IHCA).
Patients with all stages of CKD have a higher prevalence of cardiovascular disease, but the mortality
risk is estimated to be 57% higher in patients with eGFR < 60 ml/min per 1.73 m2 compared with the
general population without CKD.
7
Cardiovascular disease is also highly prevalent in the dialysis
population. The added insult of hyperkalaemia in patients with pre-existing heart disease may
contribute to sudden death in dialysis patients, presumably from cardiac arrest.
Pre-dialysis hyperkalaemia and hypokalaemia have both been shown to be associated with higher all-
cause mortality.
8
Pun et al demonstrated a 49% increase in risk of cardiac arrest with each 1 mmol/l
decrease in serum K+ below 5.1 mmol/l and a 38% increased risk with each 1 mmol/l increase above
5.1 mmol/l.
9
There was no advantage of using a low K+ dialysate. The intermittent nature of HD
treatment is a further consideration. Bleyer et al demonstrated that HD patients are susceptible to
SCD in the first 12 hours from start of the HD session, but the highest risk period is the last 12 hours of
the 2-day inter-dialytic interval.[10] In this study, hyperkalaemia (K+ ≥ 6.0 mmol/l) was present in
6.5% of patients with SCD.
Optimising and controlling K+ levels in dialysis patients is challenging. Kovesdy et al demonstrated
greater survival in maintenance HD patients with a pre-dialysis serum K+ of 4.6 – 5.3 mmol/l.
8
The
conventional thrice-weekly HD schedule is difficult to overcome, but evidence suggests that careful
dialysis prescription with the avoidance of low K+ dialysates and fistula access reduces the risk of
cardiac arrest. Other factors associated with a favourable outcome after cardiac arrest in dialysis
patients were the use beta-blockers, RAASi and calcium channel blockers at the time of the event.
11
This section of the guideline will cover:
1. special considerations in the resuscitation of patients receiving dialysis including aetiology,
out-patient dialysis setting, dialysis access, and defibrillation practice,
2. medical management of hyperkalaemic cardiac arrest, and
3. approach to treatment of refractory hyperkalaemic cardiac arrest including dialysis initiation
during CPR and the use of ECMO.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 121
References
1. Tirkkonen, J., et al., Aetiology of in-hospital cardiac arrest on general wards. Resuscitation,
2016. 107: p. 19-24.
2. Bergum, D., et al., Causes of in-hospital cardiac arrest - incidences and rate of recognition.
Resuscitation, 2015. 87: p. 63-8.
3. Pun, P.H., et al., Outcomes for Hemodialysis Patients Given Cardiopulmonary Resuscitation for
Cardiac Arrest at Outpatient Dialysis Clinics. J Am Soc Nephrol, 2019.
4. Wallmuller, C., et al., Causes of in-hospital cardiac arrest and influence on outcome.
Resuscitation, 2012. 83(10): p. 1206-11.
5. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia
during cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac
arrest. Resuscitation, 2016. 98: p. 105-11.
6. Saarinen, S., et al., Does appropriate treatment of the primary underlying cause of PEA during
resuscitation improve patients' survival? Resuscitation, 2012. 83(7): p. 819-22.
7. Webster, A.C., et al., Chronic Kidney Disease. The Lancet, 2017. 389(10075): p. 1238-1252.
8. Kovesdy, C.P., et al., Serum and dialysate potassium concentrations and survival in
hemodialysis patients. Clin J Am Soc Nephrol, 2007. 2(5): p. 999-1007.
9. Pun, P.H., et al., Modifiable risk factors associated with sudden cardiac arrest within
hemodialysis clinics. Kidney Int, 2011. 79(2): p. 218-27.
10. Bleyer, A.J., et al., Characteristics of sudden death in hemodialysis patients. Kidney Int, 2006.
69(12): p. 2268-73.
11. Pun, P.H., et al., Predictors of survival after cardiac arrest in outpatient hemodialysis clinics.
Clin J Am Soc Nephrol, 2007. 2(3): p. 491-500.
Hyperkalaemic cardiac arrest – Special circumstance (Guidelines 23.1-23.2)
Guideline 23.1 – Hyperkalaemia; Cardiac Arrest - special circumstance
We recommend that hyperkalaemia is considered in all patients who have a cardiac arrest, as part of
identifying and treating a reversible cause using the 4 Hs and 4 Ts approach. (1A)
Audit Measure
1. All cardiac arrests should be audited – hospital participation in the National Cardiac Arrest Audit is
encouraged as part of quality improvement and benchmarking.
Rationale (Guidelines 23.1)
Hyperkalaemia is an important and potentially reversible cause of cardiac arrest, therefore should be
considered in all patients, particularly in the presence of renal failure. Recognition of hyperkalaemia as the
aetiology cardiac arrest may be pre-arrest or during the resuscitation attempt. Early detection of

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 122
hyperkalaemia before cardiac arrest provides a window of opportunity to prevent arrhythmias or cardiac
arrest, but delays in treatment are well recognised.
The National Patient Safety Alert resource for hyperkalaemia (2018) highlighted 35 cases of cardiac arrest in
patients with hyperkalaemia which were reported due to concerns related to treatment and/ or monitoring.
1
Wang et al (2016) reported that 20% (5/25) of dialysis patients who suffered a hyperkalaemic cardiac arrest
did not receive either intravenous calcium or sodium bicarbonate.
2
Saarinen et al (2011) investigated the
impact of appropriate treatment in cases where a reversible cause of cardiac arrest was identified and found
that no patients received appropriate treatment when the aetiology was hyperkalaemia.
3
The ECG may be
helpful in assessing the risk of cardiac arrest in patients with hyperkalaemia. However, the progressive ECG
changes frequently described may not be present and the first sign of hyperkalaemia may be cardiac arrest.
An et al reported that approximately 20% of patients presented with cardiac arrest at the time of diagnosis
of hyperkalaemia.
4
Durfey et al demonstrated that arrhythmias or cardiac arrest occurred within 6 hours of
the presenting ECG in 15% of patients with serum K+ ≥ 6.5 mmol/l before IV calcium was administered and
before K+-lowering treatment was initiated in all but one patient.
5
The probability of cardiac arrest is likely to correlate with the severity of hyperkalaemia, but the threshold
for arrhythmias in hyperkalaemia appears to vary from patient to patient. For these reasons, arrhythmias
should be anticipated and cardiac monitoring is essential for all patients with severe hyperkalaemia. Prompt
treatment of hyperkalaemia can avoid arrhythmias and cardiac arrest. Avoid delays in treatment and seek
specialist help early.
References
1. NHS Improvement: Patient Safety Alert - Resources to support safe and timely management of
hyperkalaemia (high level of potassium in the blood). August 2018. Alert reference number:
NHS/PSA/RE/2018/006. http://improvement.nhs.uk/news-alert/safe-and-timely-management-of-
hyperkalaemia/
2. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia during
cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest.
Resuscitation, 2016. 98: p. 105-11.
3. Saarinen, S., et al., Does appropriate treatment of the primary underlying cause of PEA during
resuscitation improve patients' survival? Resuscitation, 2012. 83(7): p. 819-22.
4. An, J.N., et al., Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care, 2012.
16(6): p. R225.
5. Durfey, N., et al., Severe Hyperkalemia: Can the Electrocardiogram Risk Stratify for Short-term
Adverse Events? West J Emerg Med, 2017. 18(5): p. 963-971.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 123
Resuscitation strategy in dialysis patients (Guidelines 24.1-24.2)
Guideline 24.1 – Hyperkalaemia; Cardiac Arrest – Resuscitation strategy in haemodialysis patients
We recommend that standard ALS practice in cardiac arrest be applied to patients requiring dialysis. (1A)
Guideline 24.2 – Hyperkalaemia; Cardiac Arrest – Defibrillation practice in haemodialysis patients
We recommend disconnection from dialysis equipment prior to defibrillation unless the dialysis machine is
defibrillator-proof. (1C)
Rationale (Guidelines 24.1 – 24.2)
The incidence of cardiac arrest in dialysis patients is higher than in the general population, therefore
vigilance and staff training is essential. The incidence of cardiac arrest in the out-patient setting ranges from
3.4 – 7.8 / 100,000 HD sessions as shown in Table 26.
1-3
Sparse data is available on the incidence of in-
hospital cardiac arrest in patients on long-term HD. Wong et al reported a rate of 1.4 events per 1000 in-
hospital days with a survival to hospital discharge of 22%.
4
An early study of in-hospital CPR in patients with
ESRD showed a survival to hospital discharge of only 8%.
5
Study
Number of HD
sessions
Number of cardiac
arrests
Incidence of CPR
/100,000 dialysis
sessions
Survival to Hospital
Discharge
Karnik
2001
1
5, 744,708
400
7
NA
La France
2006
3
307,553
24
7.8
75%
Davis
2008
2
2, 611,119
110
3.4
24%
Table 26: Incidence and outcome of cardiac arrest in out-patient dialysis units.
NA – not available.
Within the out-patient setting, most cardiac arrests occur during the dialysis session as shown in Table 27.
Karnik et al reported that the mean time into dialysis at cardiac arrest was 123 ± 77 minutes.
1
The mean time
to cardiac arrest was shorter in patients with central venous catheters compared with arteriovenous
fistulas.
1
Electrolyte and fluid shifts may also play a role in the timing of events.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 124
Study
N=
Before HD
During HD
After HD
Karnik 2001
1
400
7%
81%
12%
Davis 2008
2
152
10%
70%
20%
La France 2006
3
38
8%
78%
14%
Table 27: Timing of cardiac arrest during dialysis in out-patient centres.
HD – haemodialysis
Shockable cardiac arrest rhythms (pulseless VT or VF), have been reported to be more common in the
dialysis population than non-shockable rhythms (PEA or asystole). Davis et al demonstrated a shockable
primary arrest rhythm in 65% of arrests.
2
Karnik et al reported the arrest rhythm in only 16% of cases but of
these, the initial rhythm was VF in 42%, VT in 20% and asystole in 15%.
1
LaFrance et al reported data on the
first cardiac arrest rhythm in only 12 patients - VF/VT (6/12 patients), PEA/ asystole (6/12 patients).
3
PEA/Asystole
VF/VT
Study
Events
%
ROSC
Achieved
(%)
Survival to
D/C (%)
Events
%
ROSC
Achieved
(%)
Survival to
D/C (%)
Davis 2008
2
HD patients
Out-pt HD unit
n= 152
35
37
11
65
51
31
La France 2006
3
HD patients
Out-pt HD unit
n= 24
*50
NA
NA
*50
NA
NA
Meaney 2010
6
US gen pop IHCA
n= 51,919
76
42
11
24
64
37
Nolan 2014
7
UK gen pop
IHCA
n= 23,554
72
26
11
17
76
49
Table 28: Outcome of cardiac arrest in patients receiving haemodialysis (HD) in an outpatient dialysis
facility versus all in-hospital cardiac arrests.
PEA – pulseless electrical activity; VF – ventricular fibrillation; VT – ventricular tachycardia;
ROSC – return of spontaneous circulation; IHCA – In hospital cardiac arrest
NA – not available; D/C – discharge; Out-pt – out-patient; gen pop – general population
* Data available for primary cardiac arrest rhythm in only 12/24 patients

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 125
Shockable rhythms are associated with a higher incidence of return of spontaneous circulation (ROSC) and
survival to hospital discharge in the general population as well as in patients with ESRD as shown in Table 28.
Non-shockable cardiac arrest rhythms are associated with a poor outcome. Registry data in the general
population in the UK and USA demonstrate survival to hospital discharge of 11% in patients presenting with
PEA/ asystole.
6, 7
In contrast, Wang et al reported a non-shockable rhythm in 92.7% of IHCA in
hyperkalaemic patients which in part accounts for the survival to hospital discharge of only 3.7% in this
study.
8
Modifications to ALS in Renal Failure
The universal ALS algorithm applies to all patients and the initial steps of recognition of cardiac arrest,
initiating high-quality CPR with minimal interruption, and attempting defibrillation if required, are
independent of the cause of cardiac arrest.
During CPR, reversible causes should be considered and treated. If the serum potassium is ≥ 6.5 mmol/L
before or early in the resuscitation attempt, hyperkalaemia should be considered to be the potential cause
of the cardiac arrest. Hyperkalaemia occurring late in the resuscitation attempt may be the consequence of
progressive acidosis and hypoxia, and may not be the precipitant of the cardiac arrest or require specific
intervention.
Special considerations during resuscitation in dialysis patients is shown in Table 29. The cardiac arrest team
may have little knowledge of these considerations in dialysis patients, therefore expert help is essential for
optimising care and safety.
The practice of defibrillation in HD units is variable across the UK and many staff are unaware of the safety
considerations.
9
The ERC Guidelines (2015) recommends disconnection from dialysis equipment prior to
defibrillation, unless defibrillator-proof, in keeping with the International Electrotechnical Committee (IEC)
standards 60601-2-4.
10
Most haemodialysis equipment is not defibrillator-proof.
Shockable cardiac arrest rhythms are more common in
haemodialysis patients than in the general population.
Survival after cardiac arrest is better with shockable rhythms.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 126
Table 29: Special considerations during resuscitation in haemodialysis patients.
Automated external defibrillators (AED) are now widely available for non-expert use worldwide to facilitate
early defibrillation. Many dialysis centres are predominantly nurse-led. For this reason, the National Kidney
Foundation KDOQI Guidelines (2005) recommended that all dialysis facilities should have on-site capability
of defibrillation and the use of AEDs is the simplest and most cost effective device.
11
The implementation of
AEDs within dialysis facilities was mandated within one year of this guideline. Shortly thereafter, Lehrich et
al investigated the use of AEDs in dialysis centres and reported that the presence of AEDs alone did not
independently improve survival and suggested that further measures are required to affect outcome.
12
The impact of dialysis unit staff initiating resuscitation before arrival of paramedics has recently been
reported to assess outcomes of staff-led CPR and AED use. In this study of OHCA in out-patient dialysis
clinics (n=398 events), dialysis staff initiated CPR in 81% of events, but applied an AED before paramedics
arrived in only 52.3%.
13
The timing of events in relation to dialysis is not available. When dialysis staff were
the first to apply the AED, there was a greater proportion of shockable rhythms (41% vs 25%), reinforcing
Special considerations during resuscitation of haemodialysis patients
Reversible causes – 4 Hs & 4 Ts – electrolyte disorder (hyperkalaemia,
hypokalaemia, calcium disorder), pulmonary oedema
Dialysis access – arteriovenous fistulas and dialysis lines can be used in
life-threatening emergencies.
Defibrillation practice – disconnect prior to defibrillation unless dialysis
machine is ‘defibrillator proof’ (check for these symbol on machine)
IEC 60417-5841
DEFIBRILLATION-PROOF TYPE B APPLIED PART
IEC 60417-5334
DEFIBRILLATION-PROOF TYPE BF APPLIED PART
IEC 60417-5336
DEFIBRILLATION-PROOF TYPE CF APPLIED PART
Post-resus care – repeat serum K
+
, blood glucose and ECG; preserve
dialysis access; move to an area with dialysis facilities (ICU or Renal HDU);
consider timing and need for dialysis after ROSC

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 127
early application of AED. The odds of survival to hospital discharge was 3-fold higher with staff-initiated CPR,
but there was only a non-significant trend towards improved survival to discharge with staff-initiated AED.
This may be explained by the low usage of AED by nursing staff.
Within out-patient dialysis centres, cardiac arrest occurs most often during dialysis thereby are witnessed
events. Shockable rhythms are more common, therefore early defibrillation using safe practice should be
attempted. Patients with a shockable rhythm have the best chance of survival, therefore prompt and
effective action by first responders is crucial.
References
1. Karnik, J.A., et al., Cardiac arrest and sudden death in dialysis units. Kidney Int, 2001. 60(1): p. 350-7.
2. Davis, T.R., et al., Outcome of cardiac arrests attended by emergency medical services staff at
community outpatient dialysis centers. Kidney Int, 2008. 73(8): p. 933-9.
3. Lafrance, J.P., et al., Predictors and outcome of cardiopulmonary resuscitation (CPR) calls in a large
haemodialysis unit over a seven-year period. Nephrol Dial Transplant, 2006. 21(4): p. 1006-12.
4. Wong, S.P., et al., Trends in in-hospital cardiopulmonary resuscitation and survival in adults receiving
maintenance dialysis. JAMA Intern Med, 2015. 175(6): p. 1028-35.
5. Moss, A.H., J.L. Holley, and M.B. Upton, Outcomes of cardiopulmonary resuscitation in dialysis
patients. J Am Soc Nephrol, 1992. 3(6): p. 1238-43.
6. Meaney, P.A., et al., Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med, 2010.
38(1): p. 101-8.
7. Nolan, J.P., et al., Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National
Cardiac Arrest Audit. Resuscitation, 2014. 85(8): p. 987-92.
8. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia during
cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest.
Resuscitation, 2016. 98: p. 105-11.
9. Bird, S., et al., Defibrillation during renal dialysis: a survey of UK practice and procedural
recommendations. Resuscitation, 2007. 73(3): p. 347-53.
10. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
11. National Kidney Foundation: Kidney Disease Outcomes Quality Initiative. Clinical Practice Guidelines
for Cardiovascular Disease in Dialysis Patients. NKF DDOQI: Section I, Guideline 8, 2005.
Cardiac arrest in dialysis centres are witnessed events.
CPR should be initiated by nursing staff.
First responders require regular training in use of an AED.
Ensure safety: Disconnect patient from haemodialysis machine prior to
defibrillation (most machines are not ‘defib-proof’).

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 128
12. Lehrich, R.W., et al., Automated external defibrillators and survival from cardiac arrest in the
outpatient hemodialysis clinic. J Am Soc Nephrol, 2007. 18(1): p. 312-20.
13. Pun, P.H., et al., Outcomes for Hemodialysis Patients Given Cardiopulmonary Resuscitation for
Cardiac Arrest at Outpatient Dialysis Clinics. J Am Soc Nephrol, 2019.
Treatment: Calcium chloride (Guidelines 25.1)
Guideline 25.1 – Cardiac Arrest: Treatment - Intravenous calcium
We recommend that intravenous calcium chloride is administered if hyperkalaemia is known or suspected to
be the cause of cardiac arrest. (1C)
Audit measures
1. The proportion of patients treated with intravenous calcium for hyperkalaemic cardiac arrest.
Rationale (Guidelines 25.1)
This guideline extrapolates from management in the non-arrested patient, recognising the sparsity of
evidence for the use of specific medical interventions in hyperkalaemic cardiac arrest. Intravenous calcium is
widely recommended for treatment of hyperkalaemia in the context of toxic ECG changes, arrhythmias and
cardiac arrest.
1-3
However, in the absence of hyperkalaemia or other specific indication, IV calcium can have
deleterious effects in cardiac arrest with coronary vasospasm and worsening cerebral hypoxic damage.
The quality of evidence for the general use of IV calcium in cardiac arrest was reviewed using the 2010
International Liaison Committee on Resuscitation (ILCOR) evidence evaluation process.
4
Only 10 studies were
adequate for inclusion and only two studies had a blinded randomised design. The analysis was further
limited by the wide variation in sample size, reported data and outcomes. The conclusion was that there is
no evidence that IV calcium during CPR improves survival after cardiac arrest. Its role in specific settings of
hyperkalaemia, calcium channel blocker intoxication, hypocalcaemia and hypermagnesaemia remain unclear
due to limited data.
More recently, Wang et al (2016) have reported the outcome of IV calcium in hyperkalaemic IHCA.
5
In this
study, 56% of patients received IV calcium either alone (4/ 109; 4%) or more frequently in combination with
sodium bicarbonate (57/ 109; 52%). ROSC was achieved in only one patient who received IV calcium alone
(1/4; 25%), but this patient did not survive > 24 hours. In comparison, ROSC was achieved in a higher
proportion of patients who received both drugs (12/57; 21%).
Despite the limited evidence-base, IV calcium has become standard practice for preventing and treating
arrhythmias in hyperkalaemia. Its effect is evidenced by the improvement in the ECG changes in the non-
arrested patient. Its effects last only 30-60 minutes, therefore further doses may be required if
hyperkalaemia persists or during prolonged resuscitation attempts.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 129
References
1. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
2. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
3. Vanden Hoek, T.L., et al., Part 12: cardiac arrest in special situations: 2010 American Heart
Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.
Circulation, 2010. 122(18 Suppl 3): p. S829-61.
4. Kette, F., J. Ghuman, and M. Parr, Calcium administration during cardiac arrest: a systematic review.
Eur J Emerg Med, 2013. 20(2): p. 72-8.
5. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia during
cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest.
Resuscitation, 2016. 98: p. 105-11.
Treatment: Insulin-glucose (Guidelines 25.2)
Guideline 25.2.1 – Cardiac Arrest: Treatment – Insulin-glucose
We recommend that 10 units soluble insulin and 25g glucose is administered if hyperkalaemia is known or
suspected to be the cause of cardiac arrest. (1B)
Guideline 25.2.2 – Cardiac Arrest: Treatment – Insulin-glucose
We suggest 10% glucose infusion be initiated if the blood glucose is < 7.0 mmol/l at the time of cardiac
arrest. (2C)
Rationale (Guidelines 25.2.1 – Guideline 25.2.2)
Insulin and glucose is the most effective treatment for hyperkalaemia in the non-arrested patient as
discussed in Guideline 16.3. The onset of action is within 15 minutes
1, 2
with a peak reduction in serum K+
ranging from 0.65 – 1.0 mmol/l by 60 minutes.
1-5
Although several studies have shown equivalent efficacy
with standard (10 units) vs low dose (5 units) insulin, Garcia et al have found a trend towards greater efficacy
with 10 units compared with 5 units insulin in patients with a serum K > 6.0 mmol/l.
6
Moussavi et al also
demonstrated significantly greater K+-lowering with 10 units insulin compared with low-dose insulin.
7
These
observations are important in patients with life-threatening hyperkalaemia. The main adverse effect is
hypoglycaemia, therefore blood glucose monitoring is essential.
International resuscitation guidelines have historically recommended the use of insulin-glucose for
hyperkalaemic cardiac arrest based on treatment in the non-arrested patient.
8-10
The efficacy of insulin-
glucose is augmented with the use of salbutamol and novel potassium binders in the non-arrested patient.
In cardiac arrest, the use of adrenaline has an analogous effect to salbutamol and will likely enhance K+-

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 130
lowering, but unfortunately there are no clinical trials to confirm this. For consistency, the treatment
protocol in hyperkalaemic cardiac arrest is the same as in the non-arrested patient.
The ERC recommendation for insulin-glucose during cardiac arrest has changed over the past two decades.
The ERC Resuscitation Guidelines (2000, 2005) for managing life-threatening electrolyte abnormalities
recommended 10 units insulin with 50g glucose.
11, 12
Subsequent ERC guidelines (2010, 2015) altered the
dose of glucose to 25g based on the available evidence and the Cochrane review on the emergency
interventions for hyperkalaemia published in 2005.
10, 13, 14
Given the sparsity of evidence for medical treatments in hyperkalaemic cardiac arrest, it is interesting to
consider an analogous circumstance. Cardiac arrest is induced to facilitate cardiopulmonary bypass. The
standard technique for induction of cardiac arrest includes the delivery of a high concentration of K+ to the
myocardium.
15
Therefore, hyperkalaemia frequently occurs after cardioplegia.
15, 16
This scenario is essentially
an iatrogenic hyperkalaemic cardiac arrest. The 2019 European Guidelines on cardiopulmonary bypass in
adult cardiac surgery suggests treatment with IV calcium and insulin-glucose (dose unspecified) if the serum
K+ exceeds 6.5 – 7.0 mmol/l.
15
The optimal dose of insulin and glucose during cardioplegia is unclear. Morgan et al suggested 30-50g per 10
units of insulin.
17
Davis et al suggested that if the glucose dose is 0.5 – 2g/kg, then the appropriate ratio is 1
unit insulin to 4g glucose.
18
Kocoglu et al suggested 2g of glucose for 1 unit of insulin, but hypoglycaemia
was common and required treatment with 10% glucose.
16
This data demonstrates that 25g glucose was
insufficient to prevent hypoglycaemia when administered with 10 units insulin
10, 15, 16
and in one study 10%
glucose infusion was required.
16
The current RA Hyperkalaemia guideline (2020) recommends 10 units insulin with 25g glucose for treating
acute hyperkalaemia (Guideline 16.3.1). An infusion of 10% glucose (50ml/hr for 5 hours) is suggested if the
pre-treatment blood glucose < 7.0 mmol/l to avoid iatrogenic hypoglycaemia (Guideline 16.3.3). Although it
is important to prevent hypoglycaemia in cardiac arrest, there is some evidence that the administration of
glucose during resuscitation results in lower rates of survival and worse neurological outcome.
19
In this
observational study, it was not possible to determine the reason, timing or dosage for glucose administration
and the effect was more prominent in patients without diabetes mellitus.
Hyperkalaemic cardiac arrest usually requires prolonged resuscitation and often occurs in patients with
other risk factors for iatrogenic hypoglycaemia including renal failure. The first available blood glucose post
arrest and subsequent monitoring, should guide the need for initiation and rate of a 10% glucose infusion
during the resuscitation attempt. In practical terms, a blood glucose range of 6 – 10 mmol/l is accepted for
critically ill patients.
20
References
1. Allon, M. and C. Copkney, Albuterol and insulin for treatment of hyperkalemia in hemodialysis
patients. Kidney International, 1990. 38(5): p. 869-872.
2. Allon, M. and N. Shanklin, Effect of bicarbonate administration on plasma potassium in dialysis
patients: Interactions with insulin and albuterol. American Journal of Kidney Diseases, 1996. 28(4): p.
508-514.
3. Lens, X.M., et al., Treatment of Hyperkalemia in Renal-Failure - Salbutamol V Insulin. Nephrology
Dialysis Transplantation, 1989. 4(3): p. 228-232.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 131
4. Ljutic, D. and Z. Rumboldt, Should glucose be administered before, with, or after insulin, in the
management of hyperkalemia? Ren Fail, 1993. 15(1): p. 73-6.
5. Kim, H.J., Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia
in end-stage renal disease patients. Nephron, 1996. 72(3): p. 476-482.
6. Garcia, J., et al., Reduced Versus Conventional Dose Insulin for Hyperkalemia Treatment. J Pharm
Pract, 2018: p. 897190018799220.
7. Moussavi, K., et al., Comparison of IV Insulin Dosing Strategies for Hyperkalemia in the Emergency
Department. Crit Care Explor, 2020. 2(4): p. e0092.
8. Special resuscitation situations. An advisory statement on conditions which may require
modifications in resuscitation procedures or techniques. Prepared by members of the International
Liaison Committee on Resuscitation. Resuscitation, 1997. 34(2): p. 129-49.
9. Vanden Hoek, T.L., et al., Part 12: cardiac arrest in special situations: 2010 American Heart
Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.
Circulation, 2010. 122(18 Suppl 3): p. S829-61.
10. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
11. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 8:
advanced challenges in resuscitation: section 1: life-threatening electrolyte abnormalities. The
American Heart Association in collaboration with the International Liaison Committee on
Resuscitation. Circulation, 2000. 102(8 Suppl): p. I217-22.
12. Soar, J., et al., European Resuscitation Council Guidelines for Resuscitation 2005 - Section 7. Cardiac
arrest in special circumstances. Resuscitation, 2005. 67: p. S135-S170.
13. Soar, J., et al., European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac
arrest in special circumstances: Electrolyte abnormalities, poisoning, drowning, accidental
hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution.
Resuscitation, 2010. 81(10): p. 1400-33.
14. Mahoney, B.A., et al., Emergency interventions for hyperkalaemia - art. no. CD003235.pub2.
Cochrane Database of Systematic Reviews, 2005(2).
15. Authors/Task Force, M., et al., 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in
adult cardiac surgery. Br J Anaesth, 2019.
16. Kocoglu, H., et al., Insulin dose versus rate of potassium decrease in the treatment of hyperkalemia
with IV insulin during extracorporeal circulation: An observational study. Current Therapeutic
Research-Clinical and Experimental, 2002. 63(9): p. 549-555.
17. Morgan GE, et al. Clinical Anesthesiology, 2nd ed. Stamford, Conn: Aplpleton & Lange; 1996: 533-
536.
18. Davis, R.F. Etiology and treatment of perioperative cardiac arrhythmias. In: Kaplan JA, ed. Cardiac
Anesthesia. 4th ed. Philadelphia, Pa: WB Saunders; 1999: 177-213.
19. Peng, T.J., et al., The administration of dextrose during in-hospital cardiac arrest is associated with
increased mortality and neurologic morbidity. Crit Care, 2015. 19: p. 160.
20. Investigators, N.-S.S., et al., Intensive versus conventional glucose control in critically ill patients. N
Engl J Med, 2009. 360(13): p. 1283-97.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 132
Treatment: Sodium bicarbonate (Guidelines 25.3)
Guideline 25.3 – Hyperkalaemia; Cardiac Arrest – Sodium bicarbonate
We suggest that sodium bicarbonate is administered if hyperkalaemia is known or suspected to be the cause
of cardiac arrest. (2C)
Audit measure
1. The proportion of patients treated with sodium bicarbonate for hyperkalaemic cardiac arrest.
Rationale (Guidelines 25.3)
The use of sodium bicarbonate in cardiac arrest has evolved over the past few decades. The rationale for
using sodium bicarbonate (SB) is to counteract the worsening metabolic acidosis in cardiac arrest as a result
of hypoxia, poor perfusion and increased lactate production. The potential deleterious effects of using SB in
cardiac arrest are an increase in intracellular acidosis, reduced cardiac output and worsening tissue acidosis.
1
Sodium bicarbonate was commonly used in the early resuscitation guidelines in the 1970’s – 1980’s, but use
declined in the 1990’s in light of concerns related to potential harm. A review by Adgey et al in 1998
recommended that treatment with SB should be reserved for cardiac arrest in one of four settings: 1) severe
acidosis (pH < 7.1), 2) prolonged cardiac arrest (> 10-20 minutes), 3) hyperkalaemia and 4) overdose of
tricyclic antidepressants.
2
More recently, Weng et al (2013) showed no benefit of sodium bicarbonate
during prolonged CPR.
3
Velissaris et al (2016) conducted a comprehensive review of the literature and found
that there was little evidence to support the routine use of SB during CPR.
1
Clinical practice is guided by international resuscitation guidelines. The 2010 ACLS Guidelines for adults
published by the American Heart Association stated that ‘the routine use of sodium bicarbonate is not
recommended for patients in cardiac arrest’, but supported its use in hyperkalaemia and tricyclic overdose
with or without cardiac arrest.
4
Similarly, the European Resuscitation Council (ERC) guidelines (2015) have
also recommended the use of SB for these specific indications.
5
Although there is little evidence that sodium bicarbonate lowers serum K+, the rationale for its use in
hyperkalaemia cardiac arrest is to mitigate the effects of metabolic acidosis which exacerbates
hyperkalaemia. The largest study of hyperkalaemic cardiac arrest undertaken by Wang et al (2016)
demonstrated that approximately 82% of patients received SB either alone (32/109; 29%) or in combination
with intravenous calcium (57/ 109; 52%).
6
SB was administered early in the course of resuscitation (within 10
minutes) and ROSC was achieved in 47% of patients who received SB alone and 21% who received both
drugs.
The treatment of hyperkalaemic cardiac arrest is multi-modal and both American and European resuscitation
guidelines recommend the use of sodium bicarbonate in the setting of hyperkalaemic cardiac arrest.
References
1. Velissaris, D., et al., Use of Sodium Bicarbonate in Cardiac Arrest: Current Guidelines and Literature
Review. J Clin Med Res, 2016. 8(4): p. 277-83.
2. Adgey, A.A., Adrenaline dosage and buffers in cardiac arrest. Heart, 1998. 80(4): p. 412-4.
3. Weng, Y.M., et al., The effects of sodium bicarbonate during prolonged cardiopulmonary
resuscitation. Am J Emerg Med, 2013. 31(3): p. 562-5.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 133
4. Neumar, R.W., et al., Part 8: adult advanced cardiovascular life support: 2010 American Heart
Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.
Circulation, 2010. 122(18 Suppl 3): p. S729-67.
5. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015: Section 4.
Cardiac arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
6. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia during
cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest.
Resuscitation, 2016. 98: p. 105-11.
Treatment: Initiation of dialysis during cardiac arrest (Guidelines 25.4)
Guideline 25.4 – Hyperkalaemia; Cardiac Arrest – Initiation of dialysis during CPR
We suggest that renal replacement therapy with ongoing CPR may be considered for hyperkalaemic cardiac
arrest, if hyperkalaemia is resistant to medical therapy and appropriate staff and facilities are available. (2C)
Audit measure
1. The number and outcome of patients with refractory hyperkalaemic cardiac arrest treated with
dialysis initiation during CPR.
Rationale (Guidelines 25.4)
The outcome of hyperkalaemic cardiac arrest is poor, therefore urgent action is required to prevent cardiac
arrest. Prompt medical treatment and initiation of dialysis in patients with severe hyperkalaemia are crucial
steps in avoiding cardiac arrest. If cardiac arrest occurs, survival is dependent on urgent control of the serum
K+ level. Intravenous calcium does not alter serum K+ level and there is little evidence that sodium
bicarbonate significantly lowers serum K+. Therefore, the only drugs administered during CPR which may
lower the serum K+ are insulin-glucose and adrenaline.
In the largest study of hyperkalaemic cardiac arrest (n=109), dialysis was not instituted during CPR.
1
Patients
were analysed by the severity of hyperkalaemia - K+ 6.5 – 7.9 mmol/l (72/ 109; 66%), K+ 7.9 – 9.4 mmol/l
(30/109; 28%) and K+ > 9.4 mmol/l (7/ 109; 6%). Overall, ROSC > 20 minutes was achieved in 37% of
patients, but only 4 patients (3.7%) survived to hospital discharge. The incidence of ROSC declined with
increasing severity of hyperkalaemia and was achieved in: 32/72 (44%) patients with a serum K+ 6.5 – 7.9
mmol/l, 7/30 (23%) patients with a serum K+ 7.9 – 9.4 mmol/l and in 1/7 (14%) patients with a serum K+ >
9.4 mmol/l. No patients with a K+ > 9.4 mmol/l survived beyond 24 hours. The authors suggested that there
might be a threshold for medical therapies and beyond this level, dialysis may be an alternative option.
There have been several case reports of successful resuscitation following hyperkalaemic cardiac arrest in
adults and children as shown in Table 30.
2-14
Survival with good neurological outcome after both pulseless VT
or VF and asystole or PEA cardiac arrest has been reported. In many of these reports, patients were
refractory to defibrillation until the potassium was controlled. Resuscitation efforts were frequently
prolonged, and in recent years, extra-corporeal membrane oxygenation (ECMO) support has been used to
augment systemic perfusion.
6,11-14

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 134
Success has been reported using all modes of RRT: haemodialysis (HD), haemofiltration (CVVH),
haemodiafiltration (HDF), as well as peritoneal dialysis (PD). Dialysis has also been used successfully for re-
warming in accidental hypothermia without cardiac arrest
15-17
and in cardiac arrest.
18, 19
. In one of these
cases, manual CPR was performed for 5.5 hours and CVVH was achieved with no technical difficulties for
over 3 hours.
18
This patient made a full neurological recovery, returned to work within 6 weeks and has
become a parent.
It is important to acknowledge that this evidence is limited, but large scale studies to demonstrate efficacy of
dialysis during CPR is not feasible. Despite advances in resuscitation practice in recent years, ROSC remains
unlikely if hyperkalaemia is not controlled. Although these reports likely reflect publication bias illustrating
good outcomes, they do show that dialysis with and without ECMO is technically feasible in cardiac arrest.
These reports also illustrate the evolution of the use of dialysis during CPR with ECMO providing a method to
enhance resuscitation alongside conventional dialysis in recent years.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 135
Table 30: Outcome of hyperkalaemic cardiac arrest with RRT during CPR.
(ns = not specified)
The severity of hyperkalaemia is a good indicator of the likelihood of achieving and sustaining ROSC.
Analysis of the case reports shown above in Table 30 reveals that the mean serum K+ at the time of cardiac
arrest was 9.2 mmol/l (range 8.3-10.2 mmol/l). The mean serum K+ at ROSC was 6.1 mmol/l (range 4.2-7.6
mmol/l) in patients who received a haemodialysis modality. Therefore, the mean reduction in K+ required to
achieve ROSC was 3.01 mmol/l (range 1.3-5.0 mmol/l) and this would be difficult to achieve with drugs
alone.
The term ‘extreme hyperkalaemia’ has been used in the literature.[20-22] It has been defined as a serum K+
> 9.0 mmol/l.
23
Wang et al reported no survivors in patients with a serum K+ > 9.4 mmol/l treated without
dialysis during CPR.
1
In contrast, in the series of patients treated with dialysis during CPR (Table 30), 9/15
Study
Age
(yrs)
Arrest
Rhythm
[K] at
arrest
(mmol/L)
CPR
pre-
RRT
(min)
Dialysis
modality
Dialysis
duration
(min)
[K] at
ROSC
(mmol/L)
Outcome
Gomez-
Arnau
1981
2
36
Asystole
9.7
70
HD
75
6.6
Full recovery
Torrecilla
1989
3
53
Asystole
10.2
15
HD
90
6.5
Full recovery
Lin
1994
4
27
VT
9.6
55
HD
25
7.6
Full recovery
58
VF
8.5
35
HD
30
7.2
Full recovery
77
VT
8.5
155
HD
25
5.2
Died
Costa
1994
5
57
Asystole
9.6
15
HD
95
7.2
Survived
(3 days)
Lee
1994
6
11
Asystole
10.2
140
HF on CPB
ns
ns
Full recovery
Jackson
1996
7
16
Asystole
9.8
165
PD
60
4.3
Full recovery
Kao
2000
8
68
VT
8.3
150
HD
40
5.1
Full recovery
Schummer
2000
9
68
ns
9.0
ns
HDF
15
ns
Full recovery
Iwanczuk
2008
10
53
ns
8.5
ns
HD
40
5.4
Full recovery
Chiu
2014
11
66
VF
8.6
ns
CVVH on
VA-ECMO
ns
ns
Full recovery
Tijssen
2017
12
17
Asystole
8.3
ns
CRRT on
ECMO
ns
ns
Full recovery
Kim
2019
13
13
Sine wave
9.6
90
HF on VA-
ECMO
ns
ns
Full recovery
Klingkowski
2019
14
5
VF
9.2
ns
CVVH and
ECMO
25
(ECMO
prolonged)
4.2
Full recovery

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 136
(60%) had a serum K+ > 9.0 mmol/l and 7/9 (78%) survived with full neurological recovery. Although this
evidence is limited and subject to publication bias, it would suggest that dialysis during CPR can potentially
improve the outcome for patients with extreme hyperkalaemia.
The ERC Guidelines (2015) suggest considering dialysis initiation for hyperkalaemic cardiac arrest resistant to
medical therapy.
24
This recommendation was based on several considerations:
• Firstly, the reports of successful outcomes of hyperkalaemic cardiac arrest have demonstrated that it
is technically feasible to dialyse during CPR. With the aid of the blood pump, a blood flow rate of up
to 200 ml/min can be achieved with a chest compression rate of 100/min.
• Secondly, it seems logical to consider the most effective intervention for the most serious
complication of hyperkalaemia, particularly when unresponsive to medical therapies.
• Thirdly, other invasive procedures are recommended for other special circumstances of cardiac
arrest - cardiopulmonary bypass for hypothermia, chest drain insertion for tension pneumothorax
and pericardiocentesis for cardiac tamponade. ECMO has also become increasingly utilised in
resuscitation, including in hyperkalaemic cardiac arrest. Therefore, there is a clear rationale to
considering dialysis in refractory hyperkalaemia.
• Fourthly, survival in patients with extreme hyperkalaemia is very low without the initiation of dialysis
during CPR.
• Lastly, the evidence base for other interventions for hyperkalaemia, particularly calcium salts, is also
limited, but has become standard medical practice. Large scale studies are unlikely to be feasible to
demonstrate the efficacy of dialysis during CPR.
The practical approach to resuscitation for refractory hyperkalaemic cardiac arrest is not included in renal
specialist training programs. There may also be a reluctance to consider dialysis during CPR with the
anticipation of technique failure. The resuscitation team will rely on the renal team for guidance. Given the
sparsity of information available, a review of the modifications in advanced life support in dialysis patients
was previously reported.
25
An update and summary of the procedure is outlined in Table 31.
Once CPR is underway, initiate medical treatment for hyperkalaemia and seek expert help early. If
hyperkalaemia is suspected, treat even before the serum K+ is known. Monitor serum K+ (using blood gas
analyser) every 15 minutes to assess response to treatment. Monitor blood glucose to assess for
hypoglycaemia.
Next, consider if medical treatment alone is likely to be effective. Ultimately, the severity of hyperkalaemia,
the initial response to medical therapy, the suitability of the patient and the availability of dialysis facilities
provide the best guide for considering dialysis in cardiac arrest. This intervention is unlikely to be available
outwith a Renal Unit or Critical Care area.
Next, plan ahead and consider the timing for initiation of dialysis. Analysis of the case reports suggest that
the mean duration of CPR before initiation of dialysis was 89 minutes (range 15-165 minutes). The mean
duration of dialysis to achieve ROSC was 50 minutes (range 15-95 minutes). There appeared to be an inverse
relationship between duration of CPR and duration of dialysis required to achieve ROSC. Given that dialysis
initiation will require some planning, it is reasonable to start preparations early and to consider initiation if
ROSC is not achieved within 15 minutes.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 137
Use existing dialysis access (i.e. fistula or tunnel dialysis catheter) to initiate dialysis if available. If dialysis
access is not available, the most practical approach during cardiac arrest is the insertion of a femoral line
using ultrasound guidance.
Anticipate that the resuscitation attempt will be prolonged. Consider the use of mechanical devices to
perform chest compressions (e.g. LUCAS2, Autopulse). Where available, use ECMO to optimise perfusion
during prolonged cardiac arrest management.
6, 11-14
Given that defibrillation is frequently unsuccessful until the serum K+ is controlled, analogous to rewarming
for hypothermic cardiac arrest, RRT should be considered for refractory hyperkalaemic cardiac arrest if
deemed clinically appropriate and suitable facilities and staff are available.
“Like most things in life, you may not always succeed, but failure is usually guaranteed if you do not try.”
26

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 138
Initial Approach
• Follow ALS Algorithm
• Give medical treatment for hyperkalaemia during CPR as per Hyperkalaemic Cardiac Arrest Algorithm
• Refer for Expert Help
• Consider mechanical chest compression device
Preparation for Dialysis Initiation
• If ROSC not achieved within 15 minutes consider initiating dialysis if clinically appropriate.
• Choose RRT modality depending on local availability
• Consider ECMO if available
• Use renal trained nurse (preferably two) to deliver dialysis treatment
• Prepare dialysis machine with a low K
+
dialysate
• Use existing dialysis access (i.e fistula or line) if available or alternatively insert dialysis line whilst
machine is being prepared - use femoral vein with ultrasound guidance; easier site during CPR
Initiation of Dialysis during CPR
• Give fluid bolus (250ml) once connected to dialysis machine and record starting time
• Start with pump speed of 100ml/min and gradually increase aiming for 200ml/min
• Give anticoagulation unless contraindicated (e.g. history of trauma)
• Give further IV Calcium Chloride if resuscitation is prolonged
• Check K
+
level at least every 15 min using arterial blood gas analyser and monitor blood glucose
• Allow time for K
+
-lowering on dialysis before attempting further defibrillation
Defibrillation
• Do not perform defibrillation during dialysis unless machine is defibrillation-proof
• Disconnect patient from dialysis machine just before defibrillation, then immediately reconnect
• If ROSC achieved, resume dialysis until serum K
+
< 6.5 mmol/L to maintain ROSC
• If ROSC not achieved, resume dialysis until serum K
+
< 6.5 mmol/L and attempt defibrillation again if
shockable rhythm
Post-resuscitation care
• Re-assess serum K
+
, blood glucose and ECG when ROSC achieved
• Terminate dialysis when serum K
+
controlled (K
+
< 6.5 mmol/L) and cardiac rhythm stable
• Record time of termination of dialysis and serum K
+
at ROSC
• Transfer to ICU
Table 31: Summary of procedure for initiation of dialysis during CPR.
References
1. Wang, C.H., et al., The effects of calcium and sodium bicarbonate on severe hyperkalaemia during
cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest.
Resuscitation, 2016. 98: p. 105-11.
2. Gomez-Arnau, J., et al., Hyperkalemic cardiac arrest: prolonged heart massage and simultaneous
hemodialysis. Crit Care Med, 1981. 9(7): p. 556-7.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 139
3. Torrecilla, C. and J.L. de la Serna, Hyperkalemic cardiac arrest, prolonged heart massage and
simultaneous hemodialysis. Intensive Care Med, 1989. 15(5): p. 325-6.
4. Lin, J.L., et al., Outcomes of Severe Hyperkalemia in Cardiopulmonary-Resuscitation with
Concomitant Hemodialysis. Intensive Care Medicine, 1994. 20(4): p. 287-290.
5. Costa, P., E. Visetti, and C. Canavese, Double simultaneous hemodialysis during prolonged cardio-
pulmonary resuscitation for hyperkalemic cardiac arrest. Case report. Minerva Anestesiol, 1994.
60(3): p. 143-4.
6. Lee, G., J.F. Antognini, and G.A. Gronert, Complete Recovery after Prolonged Resuscitation and
Cardiopulmonary Bypass for Hyperkalemic Cardiac-Arrest. Anesthesia and Analgesia, 1994. 79(1): p.
172-174.
7. Jackson, M.A., R. Lodwick, and S.G. Hutchinson, Hyperkalaemic cardiac arrest successfully treated
with peritoneal dialysis. BMJ, 1996. 312(7041): p. 1289-90.
8. Kao, K.C., et al., Hyperkalemic cardiac arrest successfully reversed by hemodialysis during
cardiopulmonary resuscitation: case report. Chang Gung Med J, 2000. 23(9): p. 555-9.
9. Schummer, W.J. and C. Schummer, Cardiac arrest in multiple visceral organ transplantation:
successful treatment with continuous venovenous hemodiafiltration. Anesthesiology, 2000. 93(2): p.
589.
10. Iwanczuk, W., [Haemodialysis during resuscitation from hyperkalemic cardiac arrest]. Anestezjol
Intens Ter, 2008. 40(3): p. 169-72.
11. Chiu, C.C., et al., Severe hyperkalemia with refractory ventricular fibrillation: successful resuscitation
using extracorporeal membrane oxygenation. Am J Emerg Med, 2014. 32(8): p. 943 e5-6.
12. Tijssen, J.A. and G. Filler, When CRRT on ECMO Is Not Enough for Potassium Clearance: A Case
Report. Can J Kidney Health Dis, 2017. 4: p. 2054358117722559.
13. Kim, S.H., J.H. Song, and K.T. Jung, Combination of extracorporeal membrane oxygenation and inline
hemofiltration for the acute hyperkalemic cardiac arrest in a patient with Duchenne muscular
dystrophy following orthopedic surgery -a case report. Korean Journal of Anesthesiology, 2019.
72(2): p. 178-183.
14. Klingkowski, U., et al., Refractory hyperkalaemic cardiac arrest - What to do first: Treat the reversible
cause or initiate E-CPR? Resuscitation, 2019. 142: p. 81.
15. Murakami, T., et al., Accidental Hypothermia Treated by Hemodialysis in the Acute Phase: Three
Case Reports and a Review of the Literature. Intern Med, 2019.
16. Rahman, S., et al., Early use of hemodialysis for active rewarming in severe hypothermia: a case
report and review of literature. Ren Fail, 2012. 34(6): p. 784-8.
17. Singh, T. and K.R. Hallows, Hemodialysis for the treatment of severe accidental hypothermia. Semin
Dial, 2014. 27(3): p. 295-7.
18. Alfonzo, A., et al., Survival after 5-h resuscitation attempt for hypothermic cardiac arrest using CVVH
for extracorporeal rewarming. Nephrology Dialysis Transplantation, 2009. 24(3): p. 1054-1056.
19. Hughes, A., P. Riou, and C. Day, Full neurological recovery from profound (18.0 degrees C) acute
accidental hypothermia: successful resuscitation using active invasive rewarming techniques. Emerg
Med J, 2007. 24(7): p. 511-2.
20. Kes, P., D. Orlic-Cunovic, and N. Trubelja, A life-threatening complication of extreme hyperkalemia in
a patient on maintenance hemodialysis. Acta Med Croatica, 1995. 49(3): p. 147-50.
21. Tran, H.A., Extreme hyperkalemia. South Med J, 2005. 98(7): p. 729-32.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 140
22. Fennelly, J.J., H. Smyth, and F.P. Muldowney, Letter: Extreme hyperkalaemia due to rapid lysis of
leukaemic cells. Lancet, 1974. 1(7845): p. 27.
23. Ahmed, J. and L.S. Weisberg, Hyperkalemia in dialysis patients. Seminars in Dialysis, 2001. 14(5): p.
348-356.
24. Truhlar, A., et al., European Resuscitation Council Guidelines for Resuscitation 2015 Section 4. Cardiac
arrest in special circumstances. Resuscitation, 2015. 95: p. 148-201.
25. Alfonzo, A.V.M., et al., Modifications to advanced life support in renal failure. Resuscitation, 2007.
73(1): p. 12-28.
26. Alfonzo, A., Survival after in-hospital hyperkalaemic cardiac arrest-Does intravenous calcium or
sodium bicarbonate influence outcome? Resuscitation, 2016. 98: p. A1-A2.
Prevention (Guidelines 26.1-26.2)
Guideline 26.1 – Hyperkalaemia; Prevention of Cardiac Arrest in Hyperkalaemia
We recommend that hyperkalaemia is treated urgently in patients with severe hyperkalaemia (K+ ≥ 6.5
mmol/l) and in those with ECG changes suggestive of severe hyperkalaemia. (1C)
Guideline 26.2 – Hyperkalaemia; Prevention of Cardiac Arrest in Hyperkalaemia
We recommend continuous cardiac monitoring for patients with severe hyperkalaemia (K+ ≥ 6.5 mmol/l) in a
setting appropriate for the level of care required. (1C)
Rationale (Guidelines 26.1 – 26.2)
The outcome of hyperkalaemic cardiac arrest is generally poor, therefore efforts to avoid its occurrence is
the best approach. Primary measures to prevent the development of hyperkalaemia in patients at risk is a
key step. Patients with renal failure, heart failure and/ or diabetes have a high risk, particularly when
treated with RAASi drugs. Cautious prescribing and blood monitoring is essential in these patients.
Preventing cardiac arrest in patients who have become hyperkalaemic is dependent on prompt recognition
and treatment. The initial clinical presentation may be overshadowed by the acute illness, but severe
hyperkalaemia is likely to be more immediately life-threatening. Limb weakness is an ominous sign. Look
for toxic ECG changes which may precede cardiac arrest - wide QRS complex, bradycardia or sine wave
(Guidelines 14.1-14.2; Figure 3), but some patients may have a normal ECG despite severe hyperkalaemia.
The rationale for cardiac monitoring is to detect arrhythmias before cardiac arrest ensues, therefore a higher
level of care is required.
Delays in treatment are well recognised and has resulted in patient harm.
1,2
The potential for clinical
deterioration may not be appreciated by medical or nursing staff prior to cardiac arrest. Time is frequently
lost whilst awaiting repeat bloods to confirm hyperkalaemia even in the presence of renal failure and ECG
changes. Refer for specialist advice early in patients with severe hyperkalaemia with ECG changes, end-stage
renal disease, oliguric AKI, and in patients who do not respond to medical treatment.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 141
IV calcium is a crucial step in the prevention of arrhythmias and cardiac arrest in hyperkalaemia.
3
It is
important to re-assess the ECG after administration of IV calcium as a further dose may be necessary if toxic
changes persist. Vigilance is also required as toxic ECG changes may recur when the effects of the drug have
worn off after approximately 30-60 minutes. IV calcium may buy time, but does not lower the serum K+.
Therefore, other therapeutic measures should not be delayed.
Adverse events related to severe hyperkalaemia has been evaluated to determine if the ECG is helpful in risk
stratification. Durfey et al reported the frequency of adverse events within 6 hours of the presenting ECG.
4
The study included 188 patients with a serum K+ ≥ 6.5 mmol/l (mean K+ 7.1 mmol/l). Adverse events
occurred in 15% of patients including symptomatic bradycardia (n=22), VT (n=2), CPR (n=2) and death (n=4).
All of these events occurred prior to administration of IV calcium and all but one occurred before
administration of K+-lowering medication. This highlights the importance of timely treatment to prevent
arrhythmias and cardiac arrest.
The lack of blood monitoring after initiating medical treatment is a common pitfall in the management of
hyperkalaemia. If the serum K+ is not repeated at approximately one hour after treatment when the drugs
have taken its maximum effect, then the efficacy of treatment cannot be assessed. Patients who are
refractory to medical treatment are potentially at risk of cardiac arrest. Furthermore, there is a tendency for
rebound hyperkalaemia once the effects of insulin-glucose and salbutamol have worn off. Failure to repeat
the serum K+ at 4-6 hours will miss this rebound and could result in arrhythmias or cardiac arrest. Rebound
may also occur after dialysis and may be exaggerated if temporising drugs have been used.
5
There are a few fallacies related to hyperkalaemia that require clarification:
• Patients with pacemakers are not protected from hyperkalaemic cardiac arrest. Indeed, pacemaker
failure has been well documented in this circumstance.[6, 7]
• The presence of a normal ECG in the context of severe hyperkalaemia is not protective against
arrhythmias.
• Severe hyperkalaemia can occur in the presence of near normal renal function, but may be assumed
to be spurious. An urgent ECG and repeat blood sample using a blood gas analyser should confirm
the presence of hyperkalaemia.
• Patients receiving longterm haemodialysis do not have a ‘tolerance’ to severe hyperkalaemia and are
also at risk of cardiac arrest. Medical treatment will only temporarily lower the serum K+, therefore
urgent dialysis is indicated.
References
1. NHS Improvement. Patient Safety Alert. Resources to support safe and timely management of
hyperkalaemia (high level of potassium in the blood). August 2018. Alert reference number:
NHS/PSA/RE/2018/006
2. Freeman, K., et al., Effects of presentation and electrocardiogram on time to treatment of
hyperkalemia. Acad Emerg Med, 2008. 15(3): p. 239-49.
Treat severe hyperkalaemia as a medical emergency.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 142
3. Batterink, J., T.A. Cessford, and R.A.I. Taylor, Pharmacological interventions for the acute
management of hyperkalaemia in adults. Cochrane Database of Systematic Reviews, 2015.
4. Durfey, N., et al., Severe Hyperkalemia: Can the Electrocardiogram Risk Stratify for Short-term
Adverse Events? West J Emerg Med, 2017. 18(5): p. 963-971.
5. Ahmed, J. and L.S. Weisberg, Hyperkalemia in dialysis patients. Seminars in Dialysis, 2001. 14(5): p.
348-356.
6. Barold, S.S. and B. Herweg, The effect of hyperkalaemia on cardiac rhythm devices. Europace, 2014.
16(4): p. 467-76.
7. Schiraldi, F., G. Guiotto, and F. Paladino, Hyperkalemia induced failure of pacemaker capture and
sensing. Resuscitation, 2008. 79(1): p. 161-4.
Treatment Algorithm: Resuscitation (Guideline 27.1)
Guideline 27.1 – Hyperkalaemia; Algorithm in Cardiac Arrest
We recommend that cardiac arrest attributable to hyperkalaemia is managed using the treatment algorithm
which provides guidance on the medical therapies and the need for initiation of renal replacement therapy
during CPR. (1C)
Rationale (Guideline 27.1)
Hyperkalaemia is a potentially reversible cause of cardiac arrest, but achieving and sustaining ROSC is
dependent on controlling the serum K+ level. In this way, this special circumstance is analogous to
hypothermic cardiac arrest. There are fewer drug therapy options for controlling hyperkalaemia during
cardiac arrest (Guidelines 25.1 - 25.3) and the degree of K+-lowering required to achieve ROSC may not be
achievable with drugs alone (Guideline 25.4). The hyperkalaemic cardiac arrest algorithm outlines the
modifications to ALS and the specific interventions to address hyperkalaemia as illustrated in Appendix 7.
.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 143
Lay summary
Hyperkalaemia is a medical disorder in which the potassium (K+) level in the blood is raised. The higher the
level, the greater the risk of life-threatening consequences. Hyperkalaemia may be mild (K+ 5.5 – 5.9
mmol/l), moderate (K+ 6.0 – 6.4 mmol/l) or severe (K+ ≥ 6.5 mmol/l). Severe hyperkalaemia can affect
muscle function in the limbs causing weakness, or in the heart causing potentially fatal heart rhythms.
Therefore early recognition and treatment of hyperkalaemia can save lives.
The kidneys are largely responsible for removing potassium from the body, therefore the most common
cause of hyperkalaemia is impaired kidney function. Some commonly prescribed drugs used to treat heart
failure, kidney disease and diabetes mellitus can also contribute to hyperkalaemia, therefore regular blood
monitoring is required.
Hyperkalaemia can occur in the community or in the hospital. Most cases of mild or moderate
hyperkalaemia can be managed without the need for hospital admission unless it occurs in the setting of an
acute illness. Hospital assessment is required for severe hyperkalaemia as prompt treatment may prevent
adverse events. Hyperkalaemia detected in hospital may be present at the time of hospital admission or
occur during the course of admission, therefore vigilance is required during acute illness.
The treatment for hyperkalaemia is evolving. Two new oral potassium lowering drugs (sodium zirconium
cyclosilicate and patiromer) have recently been approved for specific indications by the National Institute for
Health and Care Excellence (NICE). Insulin and glucose infusion remains the most effective emergency
treatment, but hypoglycaemia (low blood glucose) is a common adverse event. A continuous infusion of
glucose in patients with a low pre-treatment blood glucose level (< 7 mmol/l) is now recommended.
Treatment algorithms are useful in many medical emergencies and have been applied to the treatment of
hyperkalaemia in the community, in hospital and in resuscitation. Prevention of hyperkalaemia is the best
approach. This requires careful drug prescribing, blood monitoring, a low potassium diet when indicated and
the provision of patient information and education. Standardised protocols will provide a more consistent
approach to treating hyperkalaemia and improve patient safety.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 144
Appendices
1. Oral potassium lowering drugs
2. Summary of clinical trials of oral potassium lowering drugs
3. Drug administration and safety
A. Intravenous Calcium – Chloride and Gluconate solutions
B. Insulin-glucose infusion
C. Salbutamol
D. Patiromer
E. Sodium zirconium cyclosilicate
F. Calcium resonium
4. ECG in Hyperkalaemia – sine wave.
5. Algorithm – Management of Hyperkalaemia in the Community.
6. Algorithm – Management of Hyperkalaemia in Hospital.
7. Algorithm – Management of Hyperkalaemia in Resuscitation.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 145
Appendix 1: Oral potassium lowering drugs.
Characteristic
Calcium resonium
Patiromer
SZC
Mechanism of
action
Entraps K
+
in exchange
for Ca
2+
Non-specific binding of K
+
in exchange for Ca
2+
Selective K
+
binding in
exchange for Na
+
Site of action
Distal Colon
Distal colon
Entire intestinal tract
Administration
Oral or rectal
Oral
Oral
Dosing
15-60g/ day
8.4-25.2 g/day
2.5-30 g/day
Onset of effect
>4 hours
4-7 hours
1 hour
Efficacy
Unpredictable and
variable
−1.01 mmol/l in 4 weeks
[OPAL-HK]
- 1.1 mmol/l in 48 hours
[ZS-003, ZS-004]
Median time to
normalisation of serum K
+
is 2.2 hours [ZS-004]
Common adverse
effects
Gastrointestinal disorders
Hypokalaemia
Gastrointestinal
disorders
Hypokalaemia
Hypomagnesaemia
Gastrointestinal
disorders
Hypokalaemia
Oedema
Serious adverse
effects
Colonic necrosis
No episodes of colonic
perforation or necrosis
reported
No episodes of colonic
perforation or necrosis
reported
FDA Approval
1958
2015
2018
NICE Appraisal
status
N/A
Pending
Approved
Comparison between potassium-binding agents for treatment of hyperkalaemia.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 146
Appendix 2: Summary of clinical trials of oral potassium lowering drugs
Trials of oral potassium lowering drugs, representative comorbidities and use of RAASi drugs.
NA – not available
STUDY
N=
INTERVENTION
CKD
(eGFR
<60)
DIABETES
HEART
FAILURE
RAASi
Lepage 2015
RCT
33
SPS
100%
72%
9%
76%
Nasir 2014
RCT
97
CPS
SPS
100%
65%
NA
0%
(excluded)
Gruy-Kapral 1998
RCT
6
SPS
HD
NA
NA
NA
Ash 2015
Phase II RCT
ZS-002
90
SZC
100%
56%
NA
62%
Packman 2015
Phase III RCT
ZS-003
753
SZC
75%
60%
40%
67%
Kosiborod 2014
Phase III RCT
HARMONIZE
ZS-004
258
SZC
66%
66%
36%
70%
Fishbane 2017
ZS-005
751
SZC
73%
62%
38%
64%
Pitt 2011
PEARL-HF (RCT)
104
Patiromer
27%
32%
100%
NA
Bakris 2015
AMETHYST-DN
(RCT)
222
Patiromer
87%
100%
35%
71%
Bushinsky 2015
Phase I Trial
25
Patiromer
100%
60%
28%
100%
Weir 2015
OPAL-HK (RCT)
243
Patiromer
100%
57%
42%
100%
Pergola 2017
TOURMALINE
(RCT)
112
Patiromer
76%
82%
9%
59%
Pitt 2018
Open-label
63
Patiromer
100%
43%
100%
98%

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 147
Appendix 3A: Drug administration and safety - IV calcium preparations
Calcium Chloride
Available as
• Calcium chloride 10% pre-filled syringe 10mL (contains 6.8mmol of calcium in
10mL)
Preparation
• Can be used undiluted
Flush solutions
• Flush well with sodium chloride 0.9% to reduce vein irritation.
• Incompatible with many solutions (including sodium bicarbonate and
phosphate).
Administration
• Give by intravenous injection over 3-5 minutes.
• Give as a bolus injection during resuscitation.
• Preferably administer via a central venous device (if already in-situ).
• For peripheral administration, choose a large vein and monitor closely for
phlebitis.
• Ensure patient is supine and closely observed during injection.
• Monitor ECG and blood pressure.
Specialist
technical
information
• Extravasation can cause tissue damage because of the high osmolarity.
Cautions and
side effects
• Cautions: - Hypercalcaemia. Digoxin.
• Side Effects: - Too rapid administration may lead to symptoms of
hypercalcaemia and may cause cardiac arrhythmias or arrest, hypotension and
vasomotor collapse, sweating, hot flushes, nausea and vomiting.
Calcium Gluconate
Available as
• Calcium gluconate 10% ampoules (contains 2.2mmoL of calcium in 10mL)
Preparation
• Can be used undiluted.
Flush solutions
• Flush well with sodium chloride 0.9% or glucose 5% to avoid vein irritation.
• Incompatible with many solutions (including sodium bicarbonate and
phosphate).
Administration
• The rate of administration should not exceed 2mL per minute (equivalent to
10mL of undiluted injection over 5 minutes).
• For peripheral administration, choose a large vein and monitor closely for
phlebitis.
• Ensure patient is supine and closely observed during injection.
• Monitoring ECG and blood pressure.
Specialist
technical
information
• Extravasation can cause tissue damage because of the high osmolarity.
Cautions and side
effects
• Cautions: - Hypercalcaemia. Digoxin.
• Side-Effects: - Administer slowly to minimise peripheral vasodilation, cardiac
depression and circulatory collapse

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 148
References
1. Electronic Medicines Compendium (2018) ‘Summary of Product Characteristics – Calcium
Gluconate Injection BP – Hameln Pharmaceuticals Ltd’. Available at
https://www.medicines.org.uk/emc/product/6264/smpc. Accessed on 06.05.19.
2. Injectable Medicines Guide. Monograph for Calcium gluconate. Version 6 05.02.2019. Available at
http://www.injguide.nhs.uk/IVGuidePrint.asp?Drugno=3225&format=3. Accessed 06.05.2019
3. Guidelines and Audit Implementation Network (2014) Guidelines for the treatment of
hyperkalaemia in adults. Belfast, GAIN.
4. Injectable Medicines Guide. Monograph for Calcium chloride. Version 5 22.06.2016. Available at.
http://www.injguide.nhs.uk/IVGuidePrint.asp?Drugno=2562&format=3 Accessed 06.05.2019
5. Electronic Medicines Compendium (2018) ‘Summary of Product Characteristics – Calcium Chloride
Intravenous Infusion 10% w/v – Martindale Pharma’. Available at
https://www.medicines.org.uk/emc/product/4126/smpc Accessed 06.05.2019
6. University College London Hospitals NHS Foundation Trust. Injectable Medicines Administration
Guide. 3
rd
edition. 2010. Wiley-Blackwell. Chichester.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 149
Appendix 3B: Drug administration and safety –Insulin-glucose infusion
10 units of Soluble Insulin in 50mL Glucose 50% (25g)
Available as
Vials containing human soluble insulin 100 units per mL (Actrapid®)
Vials containing 50mL glucose 50% (25g)
Preparation
• Withdraw 10 units of Actrapid® insulin. This should be done only
using an insulin syringe which is graduated in units. Due to the
potential for dosing errors, it is recommended that this is
independently checked by another healthcare professional.
• Inject the insulin into a 50mL glucose 50% vial and mix well.
• Withdraw contents of vial into 50mL intravenous syringe.
Concentration of final
solution
10 units soluble insulin in 50mL
Dilution/flush solutions
Sodium chloride 0.9% - flush well to reduce vein irritation
Administration
IV Injection: Administered over 5-15 minutes intravenously into a large
vein
Monitor for phlebitis if 50% glucose is given peripherally.
Storage and handling
Do not use unless solution is clear and without visible particles.
Specialist technical
information
Glucose 50% has a high osmolarity and administration into a peripheral
vein may result in vein irritation, vein damage and thrombosis.
Cautions and side effects
• Hypoglycaemia – follow monitoring recommendations in guideline
and treat according to local guidelines.
• Infusion site reactions including phlebitis, erythema and
thrombophlebitis.
• Hypersensitivity/ anaphylactic reactions have been reported
thought to be due to corn allergy. Should be used with caution, if at
all in patients with a known allergy to corn products.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 150
Alternative Glucose preparations
20% Glucose
Available as
100 ml bottle
Volume required for 25g glucose
125 ml (two bottles required)
10% Glucose
Available as
500 ml bag
Volume required for 25g glucose
250 ml
References
1. Electronic Medicines Compendium (2017) ‘Summary of Product Characteristics – Glucose 50% w/v
Concentrate for solution for infusion – Baxter Healthcare Ltd’ Available at
https://www.medicines.org.uk/emc/product/1826/smpc. Accessed 29.04.2019
2. Electronic Medicines Compendium (2018) ‘Summary of Product Characteristics – Glucose
intravenous infusion BP 50% w/v – Hameln Pharmaceutical Ltd’. Available at
https://www.medicines.org.uk/emc/product/6266/smpc. Accessed 29.04.2019
3. Guidelines and Audit Implementation Network (2014) Guidelines for the treatment of
hyperkalaemia in adults. Belfast, GAIN.
4. Injectable Medicines Guide. Monograph for Insulin (soluble) Human. Version 5 22.01.2019.
Available at http://www.injguide.nhs.uk/IVGuidePrint.asp?Drugno=2612&format=3. Accessed
29.04.2019

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 151
Appendix 3C: Drug administration and safety - Salbutamol
Salbutamol Nebulised Solution
Available as
• 2.5mg/2.5mL nebuliser solution
• 5mg/2.5mL nebuliser solution
Administration
• 10mg DOSE
= 10ml of 2.5mg/2.5mL nebuliser solution.
= 5ml of 5mg/2.5mL nebuliser solution.
• 20mg DOSE
= 10ml of 5mg/2.5mL nebuliser solution.
• Use a face mask or T-piece.
Cautions and side effects
• Cautions:
- Consider only giving 10mg in patients with ischaemic heart
disease.
- Tachyarrhythmia
- Open angle glaucoma
• Side-Effects:
- Tremor
- Tachycardia
- Headache
References
1. Guidelines and Audit Implementation Network (2014) Guidelines for the treatment of hyperkalaemia in
adults. Belfast, GAIN.
2. Electronic Medicines Compendium (2016) ‘Summary of Product Characteristics – Ventolin Nebules –
GlaxoSmithKline UK. Available at https://www.medicines.org.uk/emc/product/8256/smpc. Accessed
06.05.2019

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 152
Appendix 3D: Drug administration and safety – Patiromer
Patiromer
Available as
8.4g, 16.8g and 25.2g sachets
Preparation
• The dose should be poured into a glass containing approximately
40mL of water and then stirred.
• Another approximately 40mL of water should be added, and the
suspension stirred again thoroughly. The powder will not dissolve.
• More water may be added to the mixture as needed.
Administration
• Apple juice or cranberry juice can be used instead of water to prepare
the mixture (be aware of potential interactions with cranberry juice).
Other liquids should be avoided due to potential potassium content.
• Should be taken with food.
• Administration should be separated by 3 hours from other
medicines.
Storage and handling
• The reconstituted mixture should be taken within 1 hour of initial
suspension.
• Unopened storage and transportation should be refrigerated (2
o
C-
8
o
C). Patients may store below 25
o
C for up to 6 months.
Cautions and side
effects
• Cautions – Hypercalcaemia, hypomagnesaemia, GI disorders, contains
sorbitol.
• Side-effects –Hypomagnesaemia, constipation, diarrhoea, abdominal
pain and flatulence
Black label medicine subject to additional monitoring to allow quick identification of new safety
information. Report all suspected adverse reactions.
References
1. Electronic Medicines Compendium (2017) ‘Summary of Product Characteristics – Veltassa
(Patiromer) – Vifor Fresenius Medical Care Renal Pharma UK Ltd’. Available at
https://www.medicines.org.uk/emc/product/779 accessed 03.05.2019.
2. Joint Formulary Committee. British National Formulary (online) London: BMJ Group and
Pharmaceutical Press. Available at http://www.medicinescomplete.com accessed 03.05.2019

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 153
Appendix 3E: Drug administration and safety – Sodium zirconium cyclosilicate
Sodium Zirconium Cyclosilicate
Available as
5g, 10g sachets (powder oral suspension)
Preparation
• The contents of the sachet should be emptied into a glass containing
approximately 45mL of water and stirred well. The powder will not
dissolve.
• Advise patient to drink the tasteless liquid while still cloudy.
• If the suspension settles - it should be stirred again.
Administration
• The suspension can be taken with or without food.
• Administer at least 2 hours before or 2 hours after oral medications
with clinically meaningful gastric pH dependent bioavailability.
Treatment: Correction
Phase
• SZC 10g three times daily until normokalaemia (serum K
+
4.0 – 5.0
mmol/l) achieved.
• Usually duration is 24 – 48 hours, maximum duration 72 hours.
• Discontinue after 72 hours if normokalaemia not achieved.
Treatment: Maintenance
Phase
• SZC 5g daily starting dose (after normokalaemia achieved)
• Titrate up to 10g once daily or down to 5g alternate days guided by
serum K
+
levels.
• Monitor serum K level regularly.
• Discontinue of hypokalaemia develops (serum K
+
< 4.0 mmol/l)
Cautions and side effects
• Cautions – can cause QT interval lengthening as a result of a reduction
in serum potassium. May be opaque to X-rays – consider if having
abdominal X-rays.
• Side effects – Hypokalaemia, oedema, gastrointestinal disorders.
Black label medicine subject to additional monitoring to allow quick identification of new safety
information. Report all suspected adverse reactions.
References
1. Electronic Medicines Compendium (2019) ‘Summary of Product Characteristics – Lokelma 10g
powder for oral suspension – AstraZeneca UK Limited’. Available at
https://www.medicines.org.uk/emc/product/10074/smpc. Accessed 03.05.2019.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 154
Appendix 3F: Drug administration and safety – Calcium resonium
Calcium Resonium
Available as
Calcium Resonium Powder (99.934%)
Preparation
• Oral administration:-
o Each 1g of resin should be mixed with 3 to 4mL of water
or syrup (not fruit juices). This corresponds to 45 to 60mL
of liquid for a 15g dose.
• Rectal administration
o 30g of resin should be mixed with 150mL of water or
glucose 10% as a daily retention enema.
Administration
• For oral administration, administer at least 3 hours before, or 3
hours after other medication. In patients with gastroparesis
consider a 6-hour separation.
• For rectal administration, the enema should be retained for at
least 9 hours then the colon should be irrigated to remove the
resin.
Cautions and side effects
• Contra-indicated in hypercalcaemia or in obstructive bowel
disease.
• Concomitant use with sorbitol is not recommended due to gastro-
intestinal stenosis and intestinal ischaemia.
References
1. Electronic Medicines Compendium (2018) ‘Summary of Product Characteristics – Calcium Resonium
99.934% w/w powder for oral/rectal suspension – Sanofi’. Available at
https://www.medicines.org.uk/emc/product/1439. Accessed 06.05.2019
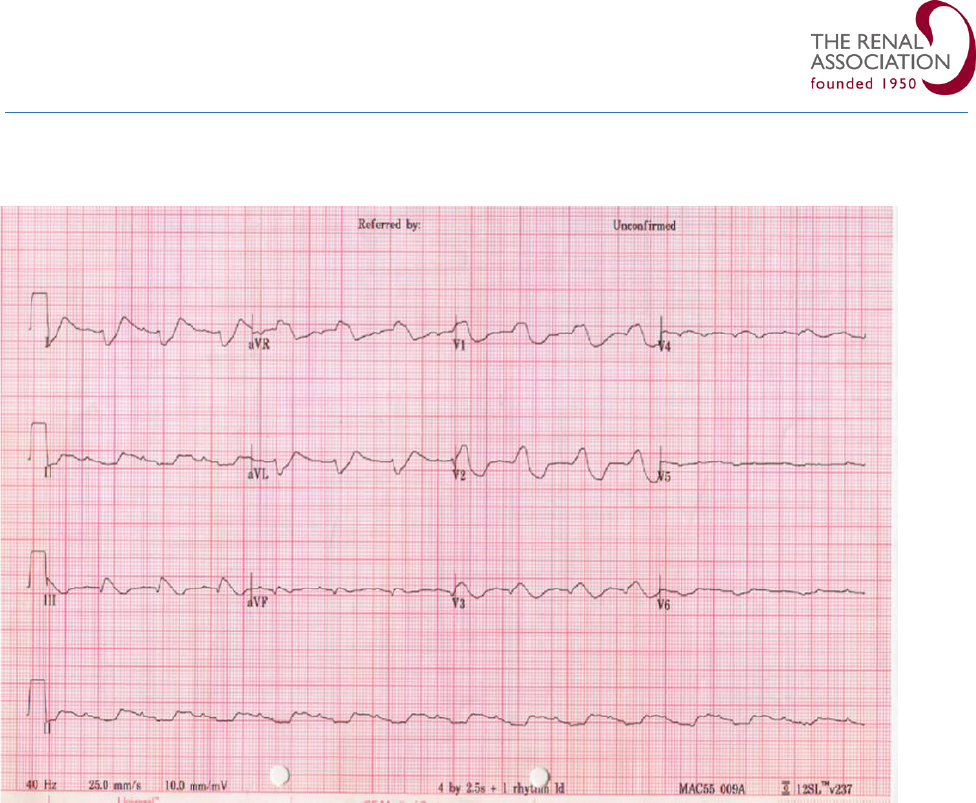
Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 155
Appendix 4 – Sine wave ECG

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 156
Appendix 5 – Hyperkalaemia Algorithm - Community

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 157
Appendix 6 – Hyperkalaemia Algorithm – Hospital

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 158
Appendix 7 – Hyperkalaemia Algorithm – Resuscitation

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 159
Abbreviations
AAGBIG Association of Anaesthetists of Great Britain and Ireland Guideline
ABCDE Airway – Breathing – Circulation – Disability – Exposure
ACC American College of Cardiology
ACE-i Angiotensin converting enzyme inhibitor
AED Automated External Defibrillator
AHA American Heart Association
AKI Acute Kidney Injury
ALS Advanced Life Support
ARB Angiotensin II receptor blocker
ARDS Adult respiratory distress syndrome
AUC Area under the curve
AV Artero-venous
AVPU Alert – Verbal – Pain - Unresponsive
BGA Blood gas analyser
BM Blood glucose
BP Blood pressure
Ca
2+
Calcium ion
CKD Chronic kidney disease
CPR Cardiopulmonary resuscitation
CPS Calcium polystyrene sulphonate
CV Cardiovascular
CVVH Continuous veno-venous haemofiltration
CVVHDF Continuous veno-venous haemodiafiltration
DM Diabetes Mellitus
DNACPR Do Not Attempt Cardiopulmonary Resuscitation
DOPPS Dialysis Outcomes and Practice Patterns Study
ECG Electrocardiogram
ECMO Extra-corporeal membrane oxygenation
eGFR Estimated glomerular filtration rate
EMA European Medicines Agency

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 160
ERC European Resuscitation Council
ESC European Society of Cardiology
ESRD End-stage renal disease
FDA Food and Drug Administration
FICM Faculty of Intensive Care Medicine
GCS Glasgow coma scale
GFR Glomerular filtration rate
HBP Hypertension
HD Haemodialysis
HDF Haemodiafiltration
HDU High dependency unit
HF Haemofiltration
HFrEF Heart failure with reduced ejection fraction
HK Hyperkalaemia
HR Hazard ratio
Hypo Hypoglycaemia
ICS Intensive Care Society
ICU Intensive Care Unit
IEC International Electrotechnical Committee
IHCA In-hospital cardiac arrest
IHD Intermittent haemodialysis
ILCOR International Liaison Committee on Resuscitation
IV Intravenous
K
+
Potassium ion
KDOQI Kidney Disease Outcomes Quality Initiative
MET Medical emergency team
Mg
+
Magnesium ion
MHRA Medicines and Healthcare products Regulatory Agency
MRA Mineralocorticoid receptor antagonist
Na
+
Sodium ion
NA Not available
NCEPOD National Confidential Enquiry into Patient Outcome and Death.

Renal Association Clinical Practice Guidelines – Treatment of Acute Hyperkalaemia in Adults – July 2020 161
NEWS National Early Warning Score
NHS National Health Service
NI Not included
NICE National Institute for Health and Care Excellence
NSAIDS Non-steroidal anti-inflammatory drugs
OHCA Out-of-hospital cardiac arrest
OR Odds ratio
PEA Pulseless electrical activity
POCT Point of care testing
RAASi Renin-Angiotensin-Aldosterone-System inhibitor
RCT Randomised controlled trial
ROSC Return of spontaneous circulation
RRT Renal replacement therapy
SB Sodium bicarbonate
SBAR Situation – Background – Assessment – Recommendation
SCD Sudden cardiac death
SMC Scottish Medicines Consortium
SPS Sodium polystyrene sulphonate
SZC Sodium Zirconium Cyclosilicate
UK United Kingdom
USA United States of America
USRDS United States Renal Data System
VF Ventricular fibrillation
VT Ventricular tachycardia
