
Centers for Disease Control and Prevention
Office of Readiness and Response
2023-2024 Recommendations for Influenza
Prevention and Treatment in Children:
An Update for Pediatric Providers
Clinician Outreach and Communication Activity (COCA) Call
Thursday, August 31, 2023

Free Continuing Education
▪ Free continuing education is offered for this webinar.
▪ Instructions on how to earn continuing education will be provided at the end of
the call.

Continuing Education Disclosure
▪ In compliance with continuing education requirements, all planners and presenters must disclose
all financial relationships, in any amount, with ineligible companies over the previous 24 months
as well as any use of unlabeled product(s) or products under investigational use.
▪ CDC, our planners, and presenters wish to disclose they have no financial relationship(s) with
ineligible companies whose primary business is producing, marketing, selling, re-selling, or
distributing healthcare products used by or on patients with the exception of Dr. Kristina Bryant
who would like to disclose that she is an investigator on multicenter vaccine trials with Pfizer and
Enanta, and receives royalties from Oxford University Press. All of the relevant financial
relationships listed for this individual have been mitigated.
▪ Content will not include any discussion of the unlabeled use of a product or a product under
investigational use except Dr. Fatimah Dawood who would like to disclose that she will discuss
neuraminidase inhibitor medications (antivirals) that are FDA approved only for treating
uncomplicated influenza.
▪ CDC did not accept financial or in-kind support from ineligible companies for this continuing
education activity.

At the conclusion of today’s session, the participant will be able to accomplish the
following:
1. Highlight key recommendations in the AAP influenza policy statement, “Recommendations for
Prevention and Control of Influenza in Children, 2023–2024” and in the CDC Advisory
Committee on Immunization Practices’ document, “Prevention and Control of Seasonal
Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization
Practices—United States, 2023-2024 Influenza Season.”
2. Review strategies to increase influenza vaccination rates and highlight current health disparities
in vaccination coverage.
3. Describe considerations and best practices for coadministering influenza vaccines and other
childhood immunizations.
Objectives

To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button
– Type your question in the “Q&A” box
– Submit your question
▪ If you are a patient, please refer your question to your healthcare provider.
▪ If you are a member of the media, please direct your questions to CDC Media
Relations at 404-639-3286 or email med[email protected].

Today’s Presenters
▪ Fatimah Dawood, MD, FAAP
Pediatrician and Medical Officer
Influenza Prevention and Control Team
Influenza Division
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
▪ Kristina A. Bryant, MD, FAAP, FPIDS
Member, Committee on Infectious Diseases, American Academy of Pediatrics
Professor of Pediatrics, University of Louisville School of Medicine
Hospital Epidemiologist at Norton Children’s Hospital
Director for System Pediatric Epidemiology and Infectious Diseases, Norton Children’s
Medical Group, Louisville, KY

National Center for Immunization & Respiratory Diseases
2023-2024 Recommendations for Influenza Prevention
and Treatment in Children:
An Update for Pediatric Providers
Fatimah S. Dawood, MD, FAAP
Influenza Division, CDC
Clinician Outreach and Communication Activity (COCA) Call
August 31, 2023

Influenza (Flu) in Children
▪ Millions of children in the US get sick with seasonal flu during typical seasons
– 7,000 to 26,000 estimated flu-related hospitalizations per season in children
aged <5 years during 2010-2011 to 2019-2020
– 37 to 199 reported flu-related deaths in children per season during 2004-2005
to 2019-2020
▪ Flu vaccination is the best way to prevent flu in
children
– Studies show that getting vaccinated
reduces flu illnesses, doctor’s visits, flu-
related hospitalizations, life-threatening
flu episodes, and death*
*Key Facts About Seasonal Flu Vaccine | CDC

A Review of Last Influenza Season
▪ A/H3N2 predominant, with some A/H1N1 circulation
▪ Early influenza season
– Single epidemic wave
– Activity began to increase in early October and subsided by early January
– Reports of high pediatric hospitalization rates in some areas
▪ Influenza virus circulation coincided with other respiratory virus circulation
– CDC Health Alert Notification issued about early respiratory disease incidence
caused by multiple viruses, especially among children

US Clinical Laboratory and Public Health Laboratory Surveillance,
October 2022–August 2023
Time Period Percent Positive at Clinical Labs
Median (Range)
Peak Number of Positives at PHLs
Median (Range)
Pre-COVID: 2015-16 – 2019-20 26.3 (23.6 – 30.3) 3,482 (3,274 – 4,334)
Early COVID: 2020-21 & 2021-22 0.3 – 9.9 24 – 1,528
This Season: 2022-23 26.3 3,387

Influenza-associated hospitalization rates among children
aged 0-4 years by season, 2012-2023
Influenza-associated hospitalization rates among
children
aged 0-4 years by season, 2012-2023
Early and large peak
during last season
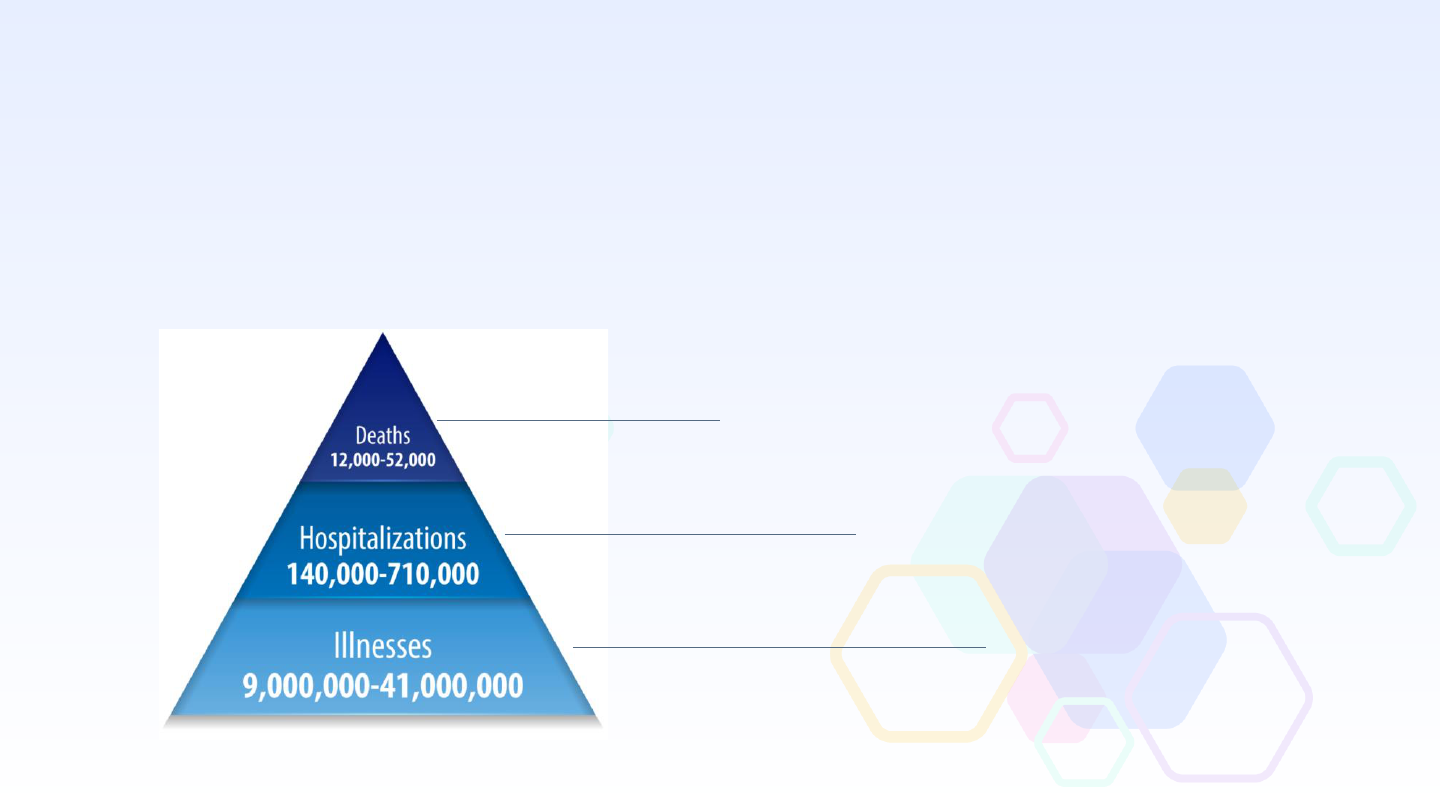
Annual U.S. Influenza Burden Estimates
Estimated Range from
2010 – 2020
Preliminary
2022 – 2023 Estimates
At least 19,000 deaths
At least 300,000 hospitalizations
At least 27 million illnesses

Influenza-Associated Pediatric Deaths* Reported to CDC
No. of
Pediatric
Deaths*
2004
-
05
47
2005
-
06
46
2006
-
07
77
2007
-
08
88
2008
-
09
137
2009
-
10
288
2010
-
11
124
2011
-
12
37
2012
-
13
171
2013
-
14
111
2014
-
15
148
2015
-
16
95
2016
-
17
110
2017
-
18
188
2018
-
19
144
2019
-
20
199
2020
-
21
1
2021
-
22
49
2022
-
23
168

Global Influenza Surveillance in Southern Hemisphere
Locations in 2023
South Africa
Australia
Chile
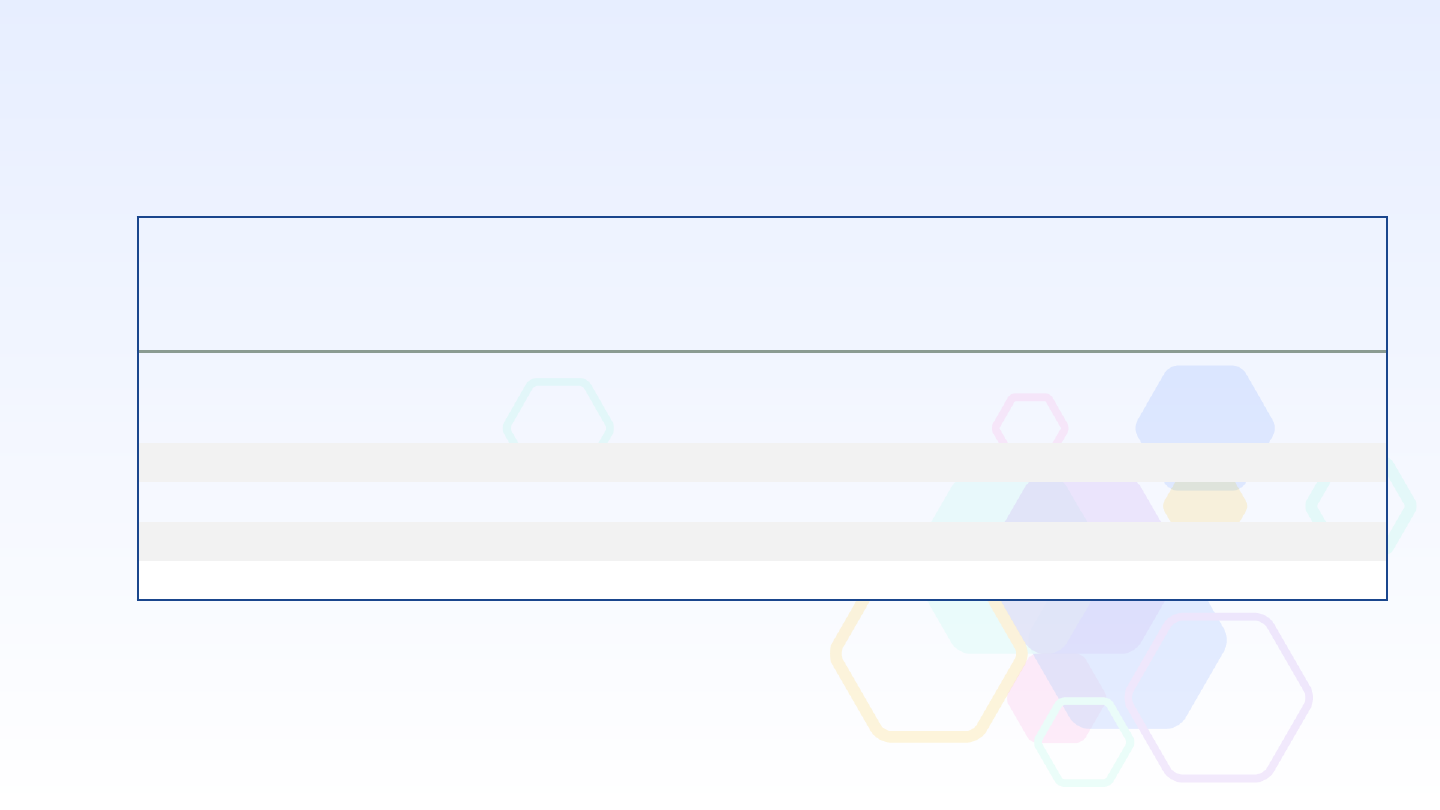
2022-2023 Influenza Vaccine Effectiveness Against Influenza-Associated
Emergency Department Visits and Hospitalizations in Children Aged 6 mos–17
years, New Vaccine Surveillance Network (NVSN)
Preliminary Data
Outcomes
Vaccinated/
Total (%)
Influenza
positive
Vaccinated/
Total (%)
Influenza
negative
Effectiveness against laboratory confirmed
Influenza A* in hospital and ED settings,
VE % (95% CI)**
Influenza A
All 6 mos
–
17 years
123/640 (19) 750/2256 (33)
49 (36 to 60)
Inpatient
19/131 (15) 288/913 (32)
68 (46 to 81)
ED
104/507 (21) 461/1330 (35)
42 (25 to 56)
A/H3N2
98/478 (21) 750/2256 (33)
45 (29 to 58)
A/H1N1
23/139 (17) 750/2256 (33)
56 (28 to 72)
* Of 335 influenza-positive specimens sequenced, 250 were A(H3N2) clade 3C.2a1b.2a.2b and 32 were clade 3C.2a1b.2a.2a.1 and 38 were
A(H1N1) clade 6B.1A.5a.2a.1. There were 16 coinfections with Influenza and SARS-CoV-2 that were excluded from the VE estimate.
**
Multivariable logistic regression models adjusted for site, age, and calendar time.

Flu Vaccines Protect Graphic

Sources of 2022‐2023 Influenza Season Data
▪ Updated surveillance information is available each Friday
– FluView, static report: https://www.cdc.gov/flu/weekly/
– FluView Interactive, online application:
https://www.cdc.gov/flu/weekly/fluviewinteractive.htm
▪ Vaccine effectiveness estimates
– Morbidity and Mortality Week Report (MMWR) updates:
https://www.cdc.gov/mmwr/index.html
– Advisory Committee on Immunization Practices (ACIP) meetings:
https://www.cdc.gov/vaccines/acip/meetings/index.html

2023-2024 CDC Antiviral Treatment
Recommendations

CDC Antiviral Treatment Recommendations
▪ Antiviral treatment is recommended as early as possible for any
patient with confirmed or suspected influenza who is:
– Hospitalized
– Has severe, complicated, or progressive illness
– Is at high risk for influenza complications
▪ Antiviral treatment can be considered for previously healthy,
symptomatic outpatient not at high risk with confirmed or suspected
influenza, if treatment can be initiated within 48 hours of illness onset
▪ Clinical benefit is greatest when antiviral treatment is given early

People at High Risk for Influenza Complications for
whom Antiviral Treatment is Recommended
▪ Children aged <2 years old (although all children aged <5 years are
considered at high risk for complications, highest risk is for children aged <2
years)
▪ Adults aged 65 years and over
▪ Pregnant/postpartum persons
▪ Children aged <18 years receiving long-term aspirin therapy
▪ American Indians/Alaska Natives
▪ People with underlying medical conditions (e.g., pulmonary, cardiac,
immunosuppression, neurologic and neurodevelopment conditions)
▪ Residents of nursing homes/chronic care facilities

Influenza Antiviral Medication Treatment,
Route and Age Indications
* Oral oseltamivir phosphate is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of
illness onset in people 14 days and older. Although not part of the FDA-approved indications, use of oral oseltamivir for
treatment of influenza in infants less than 14 days old is recommended by the CDC and the American Academy of
Pediatrics.
** Intravenous peramivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness
onset in people 6 months and older.
*** Oral baloxavir marboxil is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness
onset in people aged ≥5 years who are otherwise healthy, or in people aged ≥12 years who are high risk of developing
influenza-related complications.
Drug
Route
Age Indication for Treatment
Oseltamivir*
Oral
Any age
Zanamivir
Inhaled
>
7 years
Peramivir**
Intravenous
>
6 months
Baloxavir***
Oral
>
5 years

2023-2024 ACIP Influenza Vaccination
Recommendations Update

Groups Recommended for Vaccination
▪ Routine annual influenza vaccination is recommended for all persons ≥6
months of age who do not have contraindications
▪ Vaccination is recommended for all— if supply is limited, vaccinate those
at highest risk for influenza complications
– People aged ≥6 months who are at increased risk of influenza
complications and severe illness
– Contacts and caregivers of persons
• <5 years of age
• ≥50 years of age
• with medical conditions that put them at higher risk for severe
complications from influenza

Groups at Increased Risk for Influenza Complications
and Severe Illness
▪ Children aged 6 through 59 months and adults aged ≥65 years
– children <6 months of age are also at high risk but cannot be vaccinated.
– adults ≥50 years are a priority group if supply is limited
▪ Persons with chronic medical conditions, including pulmonary (including asthma) or
cardiovascular (excluding isolated hypertension), renal, hepatic, neurologic, hematologic, or
metabolic disorders (including diabetes mellitus)
▪ Persons who are immunocompromised
▪ Persons who are or will be pregnant during the influenza season
▪ Children and adolescents (aged 6 months–18 years) who are receiving aspirin- or
salicylate-containing medications (who might be at risk for Reye syndrome after influenza
virus infection)
▪ Residents of nursing homes and other long-term care facilities
▪ American Indians/Alaska Natives
▪ Persons with extreme obesity (BMI ≥40)

2023‒2024 ACIP Influenza Statement –
Updates to Pediatric Vaccination
▪ Influenza vaccine composition for 2023-2024
▪ Administration of influenza vaccines to people with egg allergy

2023-2024 Influenza Vaccine Composition
▪ Egg-based IIVs and LAIV4:
– An A/Victoria/4897/2022 (H1N1)pdm09-like (updated)
– An A/Darwin/9/2021 (H3N2)-like virus
– A B/Austria/1359417/2021 (Victoria lineage)-like virus
– A B/Phuket/3073/2013 (Yamagata lineage)-like virus.
▪ Cell-culture-based IIV4 and RIV4:
– An A/Wisconsin/67/2022 (H1N1)pdm09-like virus (updated)
– An A/Darwin/6/2021 (H3N2)-like virus
– A B/Austria/1359417/2021 (Victoria lineage)-like virus
– A B/Phuket/3073/2013 (Yamagata lineage)-like virus.

Updates to Guidelines for Influenza Vaccine
Administration to People with Egg Allergy
▪ Most influenza vaccines are produced with an egg-based manufacturing
process and contain a small amount of egg proteins
– Additional safety measures were previously recommended for
administration of egg-based influenza vaccine to people who had a history
of severe allergic reactions to egg
▪ UPDATE People with egg-allergy may receive any influenza vaccine (egg-
based or non-egg based) that is otherwise appropriate for their age and
health status; additional safety measures are no longer recommended
▪ All vaccines should be given in settings where allergic reactions can be
recognized and treated quickly.

Influenza Vaccines for Children 6 through 35 months
▪ Five IIVs licensed for this age group
▪ Licensed dose volumes for this age group differ
– FluLaval Quadrivalent (IIV4, GSK) 0.5 mL
– Fluarix Quadrivalent (IIV4, GSK) 0.5 mL
– Flucelvax Quadrivalent (ccIIV4, Seqirus) 0.5 mL
– Afluria Quadrivalent (IIV4, Seqirus) 0.25 mL
– Fluzone Quadrivalent (IIV4, Sanofi Pasteur) 0.25 mL or 0.5 mL
▪ Afluria Quadrivalent and Fluzone Quadrivalent 0.25 mL prefilled syringes are no
longer available
• Afluria Quadrivalent: a 0.25 mL dose must be obtained from a multidose vial.
• Fluzone Quadrivalent: can give 0.5 mL; or 0.25 mL can be taken from multidose
or single dose vial.

Coadministration of Influenza and Recommended
Vaccines in Children
▪ Routine administration of all age-appropriate doses of vaccines
simultaneously is recommended for persons for whom no specific
contraindications exist
▪ COVID-19 vaccines and nirsevimab may be administered regardless of
timing of influenza vaccines, including simultaneous administration of
COVID-19 vaccine, nirsevimab and influenza vaccines on the same day
▪ Administer each vaccine in a different injection site (at least 1 inch apart)

Self-knowledge Check #1
A child with a history of severe allergic reaction to eggs should not receive an
annual influenza vaccine according to ACIP guidelines:
A. True
B. False

Self-knowledge Check #1 (Answer)
The correct answer is B.
The Advisory Committee on Immunization Practices (ACIP) recommends that all
people 6 months and older with egg allergy receive an annual influenza vaccine
in the absence of contraindications to vaccination.
In addition, the 2023-2024 ACIP Influenza Vaccination Recommendations have
been updated to state that people with egg-allergy may receive any influenza
vaccine (egg-based or non-egg based) that is otherwise appropriate for their age
and health status without the need for additional safety measures beyond those
recommended for receipt of any vaccine.

Upcoming 2023-2024 U.S. Influenza Season
• Influenza remains unpredictable
• Last season reminded us that influenza viruses can
• circulate early
• result in high rates of medically attended illnesses in children
• co-circulate with other respiratory viruses such as SARS-CoV-2 and
respiratory syncytial virus, placing increased strain on healthcare systems
• Annual influenza vaccination is the most
effective way to prevent influenza

Additional CDC Resources
▪ CDC Influenza homepage: https://www.cdc.gov/flu/
▪ Influenza surveillance: https://www.cdc.gov/flu/weekly/fluactivitysurv.htm
▪ Influenza vaccination coverage: https://www.cdc.gov/flu/fluvaxview/index.htm
▪ For Professionals: https://www.cdc.gov/flu/professionals/index.htm
– Vaccination homepage:
https://www.cdc.gov/flu/professionals/vaccination/index.htm
– 2023-24 ACIP Influenza Recommendations:
https://www.cdc.gov/mmwr/volumes/72/rr/rr7202a1.htm?s_cid=rr7202a1_w
– Antiviral homepage:
https://www.cdc.gov/flu/professionals/antivirals/index.htm
▪ For Children (created by CDC and endorsed by the AAP): activity book
– https://www.cdc.gov/phpr/readywrigley/documents/ready_wrigley_flu.pdf

2023-2024 Recommendations for
Influenza Prevention and Treatment in
Children:
An Update for Pediatric Providers
Kristina Bryant, MD, FAAP, FPIDS
Committee on Infectious Diseases
American Academy of Pediatrics
August 31, 2023

LEARNING OBJECTIVES
In this presentation, participants will:
• Understand AAP recommendations for influenza
immunization and treatment during the 2023-2024 season
• Learn strategies to increase immunization rates
• Recognize ongoing health disparities with vaccination

AAP RECOMMENDATIONS FOR INFLUENZA SEASON 2023-2024
Policy Statement and Technical Report:
• Early release online, August 29
th
:
https://publications.aap.org/pediatrics/online-
first
• October issue of Pediatrics
American Academy of Pediatrics. Recommendations for Prevention and Control of Influenza in Children, 2023-2024. American Academy of
Pediatrics, 2023

WHAT’S NEW FOR 2023-2024?
• Vaccine composition updated
• Recommendations for immunocompromised hosts clarified
• Strategies for improving access to vaccine emphasized
• Indications for influenza testing highlighted, including at-home
testing

WHAT’S THE SAME FOR 2023-2024?
• Influenza continues to cause morbidity and mortality in children
• Annual influenza vaccination is recommended for all persons 6
months and older
• Any vaccine appropriate for age and health status can be used
• Influenza vaccine can be administered at the same time as
other vaccines, including the COVID-19 vaccine
• Antiviral treatment is recommended for certain children with
influenza

IMPACT OF INFLUENZA ON CHILDREN
• ~9% develop symptomatic infection
annually
• Significant morbidity in hospitalized
children
▪ 20% require ICU care
▪ 17% with pneumonia
▪ 5% require mechanical ventilation
▪ 8-10% experience neurologic complication
▪ 0.5% die
• Post-discharge sequelae (critical influenza)
Photo Credit: Red Book Online
Influenza pneumonia in a 12-year-old with respiratory
failure. Courtesy of Benjamin Estrada, MD
Tokars JI, et al. Clin Infect Dis. 2018;66(10):1511-1518. Kamidani S, et al. Clin Infect Dis. 2022 ; Maddux AB et al. J Allergy Clin Immunol Pract.
2023;11(3):836-843.e3

HEALTH DISPARITIES AND INFLUENZA
Hospitalization rates higher
in Black, Hispanic, American
Indian/Alaska Native and
Asian/Pacific Islander
children
Rate of in-hospital death is 3- to
4-fold higher in Black, Hispanic,
and Asian/Pacific Islander
children compared with white
children.
This Photo by Unknown Author is licensed under CC BY-NC
O'Halloran AC et al. JAMA Netw Open. 2021;4(8):e2121880

INFLUENZA IMMUNIZATION RATES FALLING 1/2
Source: Centers for Disease Control and Prevention, NIS-Flu
Influenza vaccination coverage in children 6 months to 17 years of age in the United States, 2020–2021 to
2022 -2023

INFLUENZA IMMUNIZATION RATES FALLING 2/2
Influenza vaccination coverage in children 6 months to 17 years of age in the United States, 2019-2023
Source: Centers for Disease Control and Prevention, NIS-Flu

INFLUENZA VACCINE ADMINISTRATION PEARLS
• When two doses are required in a season, use of the same
brand or type is not required.
• The maximum number of doses drawn from a multidose vial is
specified in the package insert and should not be exceeded.
• Residual product must be discarded regardless of the
remaining volume in the vial.
• A 0.5-mL unit dose of any IIV should not be split into 2
separate 0.25-mL doses.

INFLUENZA VACCINE ADMINISTRATION PEARLS CONT.
• IIV may be administered simultaneously with or at any time
before or after other inactivated or live vaccines (including
COVID-19 vaccines and RSV monoclonal antibody).
• LAIV may be administered simultaneously with other live or
inactivated vaccines.
– If not administered simultaneously, 4 weeks should pass between the
administration of LAIV and other non-oral live vaccines.

INFLUENZA VACCINE: IMMUNOCOMPROMISED HOSTS
• Malignant neoplasms: In general, administer influenza
vaccine ≥ 2 weeks before cytotoxic chemotherapy
• Anti-B cell therapies in previous 6 months: defer IIV until B cell
recovery
• Hematopoietic stem cell: IIV beginning 4 to 6 months after
transplantation
• Solid organ transplant (SOT): IIV beginning 3 months after
transplant
– considered ≥1 month after transplant during the influenza season
American Academy of Pediatrics. Recommendations for Prevention and Control of Influenza in Children, 2023-2024. American Academy of
Pediatrics, 2023

2023–2024 SEASONAL INFLUENZA VACCINE FOR CHILDREN:
NUMBER OF DOSES
• Must be at least 6 months
of age to be eligible for
influenza vaccine
• Second dose still required
for children who turn 9
between first and second
dose
American Academy of Pediatrics. Recommendations for Prevention and Control of Influenza in Children, 2023-2024. American Academy of
Pediatrics, 2023

2023–2024 SEASONAL INFLUENZA VACCINE FOR CHILDREN:
TIMING
• Offer influenza vaccine as soon as it becomes available,
especially children who require 2 doses.
• Administer recommended dose(s) ideally by the end of
October.
• Continue offering vaccine to unvaccinated children and
families throughout the season.

CHILDREN AT HIGH RISK FOR INFLUENZA COMPLICATIONS
▪
Any child < 5 years
▪
Especially < 2 years
▪
Residents of chronic care
facilities or nursing homes
▪
Children born early-term or
late-preterm
Photo credit: AAP
Underlying Condition or
Treatment
Chronic pulmonary disease
Metabolic disorders
Cardiovascular disease
Neurologic and
neurodevelopmental
conditions
Kidney disease
Extreme obesity
2
Hepatic disease
Immunosuppression
Hematologic disease
Pregnancy and post
-partum
Receipt of aspirin or
salicylate
-containing
therapies
1
1
<19 years who may be at increased
risk of Reye syndrome
2
Could consider BMI ≥99% for age
American Academy of Pediatrics. Recommendations for Prevention and Control of Influenza in Children, 2023-2024. American Academy of
Pediatrics, 2023

STRATEGIES FOR INCREASING INFLUENZA IMMUNIZATION:
PROVIDER / CARE TEAM
Photo Credit: Heather Hazzan, SELF Magazine
• Offer a strong, presumptive recommendation
• Bundle recommendation for influenza vaccine with
recommendations for other needed vaccines
• Use consistent messaging across care team members
• Identify influenza champions

STRATEGIES FOR INCREASING INFLUENZA IMMUNIZATION:
PRACTICE/HEALTH SYSTEM
• Review influenza vaccination status at all visits
• Bundle influenza vaccine with other needed vaccines
• Vaccinate at all visit types
• Vaccinate in all healthcare settings
• Increase access to influenza vaccine (eg, expanded hours,
vaccine-only clinic)
• Provide evidence-based information to patients and families
(eg, office-based educational handout)
Photo Credit: Heather Hazzan, SELF Magazine

STRATEGIES FOR INCREASING INFLUENZA IMMUNIZATION:
PRACTICE/HEALTH SYSTEM CONT.
This Photo by Unknown Author is
licensed under CC BY-NC-ND
• Send influenza vaccine reminder/recall messages
• Use electronic health records (EHR)-based tools to identify and
classify high-risk patients for targeted outreach
• Utilize standing orders
• Implement influenza vaccine provider prompts/clinical decision
support
• Perform audits or share feedback reports
• Integrate EHR with regional or state immunization systems

STRATEGIES FOR INCREASING INFLUENZA IMMUNIZATION:
COMMUNITY/ PUBLIC HEALTH
Photo Credit: CDC
• Partner with stakeholders to support vaccine
initiatives within the community, including school-
based programs and pharmacies.
• Engage with communities affected by health
disparities to develop tailored strategies that
promote trust, encourage dialogue, and increase
access to preventative services.

No permita que la gripe lo detenga

No permita que la gripe lo detenga cont.

ELIMINATING STRUCTURAL BARRIERS TO INFLUENZA VACCINE
Getty Image: Permission provided to AAP
• Eliminate disparities in influenza
vaccine supply between privately
insured patients and those eligible
for vaccination through the
Vaccines for Children (VFC)
program.

ELIMINATING STRUCTURAL BARRIERS TO INFLUENZA VACCINE CONT.
This Photo by Unknown Author is licensed under CC
BY
Public and private payers should:
• Offer adequate payment for influenza vaccine
supply and administration to pediatric
populations
• Update payment systems for influenza vaccine so
that providers are paid for administering doses in
July and August
• Eliminate remaining “patient responsibility” cost
barriers to influenza vaccination where they still
exist.

TESTING FOR INFLUENZA
• Test children with signs and symptoms of influenza
when test results are anticipated to impact clinical
management.
• When influenza is circulating, test hospitalized patients
with signs and symptoms of influenza with a molecular
assay with high sensitivity and specificity (eg, RT-PCR).
• Use of at-home test results to inform treatment
decisions should be informed by the sensitivity and
specificity of the test, the prevalence of influenza in the
community, the presence and duration of compatible
signs and symptoms, and individual risk factors and
comorbidities.
Photo Credit: Healthychildren.org

ANTIVIRAL UNDERUTILIZED
Oseltamivir use in hospitalized children, 2002-2020
Walsh PS et al. JAMA Netw Open. 2022;5(9):e2233027
Patients with certain
high-risk conditions and
patients requiring ICU
care or invasive
procedures early in their
hospitalization were
more likely to receive
oseltamivir.

AAP RESOURCES
• Influenza Resources: www.aap.org/influenza
• Red Book Online: https://publications.aap.org/redbook
• Professional Tools & Resources: www.aap.org/immunization
• Interactive Vaccination Map: www.aap.org/immunizationmap
• Promoting Vaccine Confidence: www.aap.org/vaccinecommunication
• Parenting Website: www.healthychildren.org/flu
• Infection Prevention and Control Resources:
www.aap.org/projectfirstline

SELF KNOWLEDGE CHECK #2
Influenza vaccination should only occur in the medical home.
A. True
B. False

SELF KNOWLEDGE CHECK #2 (ANSWER)
Influenza vaccination should only occur in the medical home.
A. True
B. False
Rationale: Although vaccination in the medical home is optimal, administering
influenza vaccine in diverse locations, such as subspecialty practices, urgent care
clinics, emergency departments, schools, and pharmacies, may increase uptake
among patients who do not have or cannot readily access their medical home and
those at high risk for influenza-related complications. When influenza vaccination
takes place in a nontraditional setting, appropriate documentation should be
provided to patients and to the medical home. Settings that offer influenza
vaccination should submit details about the vaccination to the appropriate IISs,
including all content needed to support communication of this information to the
patient’s medical home.

To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button
– Type your question in the “Q&A” box
– Submit your question
▪ If you are a patient, please refer your question to your healthcare provider.
▪ If you are a member of the media, please direct your questions to CDC Media
Relations at 404-639-3286 or email med[email protected].

Continuing Education
▪ All continuing education for COCA Calls is issued online through the CDC Training &
Continuing Education Online system at https://tceols.cdc.gov/.
▪ Those who participate in today’s COCA Call and wish to receive continuing education please
complete the online evaluation by October 2, 2023, with the course code WC4520-083123.
The access code is COCA083123.
▪ Those who will participate in the on-demand activity and wish to receive continuing
education should complete the online evaluation between October 3, 2023, and October 3,
2025, and use course code WD4520-083123. The access code is COCA083123.
▪ Continuing education certificates can be printed immediately upon completion of your
online evaluation. A cumulative transcript of all CDC/ATSDR CEs obtained through the CDC
Training & Continuing Education Online System will be maintained for each user.

Upcoming COCA Calls & Additional Resources
▪ Join us for our next COCA Call, Tuesday, September 19 at 2:00 P.M. ET.
▪ Topic: Preparing for the Upcoming Respiratory Disease Season: Recommendations for
Influenza, COVID-19, and RSV Vaccines for Older Adults
▪ Continue to visit https://emergency.cdc.gov/coca/ to get more details about
upcoming COCA Calls.
▪ Subscribe to receive notifications about upcoming COCA calls and other COCA
products and services at emergency.cdc.gov/coca/subscribe.asp.



