
BTS Clinical Statement on air travel for passengers
with respiratorydisease
Robina Kate Coker,
1
Alison Armstrong,
2
Alistair Colin Church,
3
Steve Holmes,
4
Jonathan Naylor,
5
Katharine Pike,
6
Peter Saunders,
7
Kristofer John Spurling,
8
Pamela Vaughn
9
INTRODUCTION
BTS recommendations for managing passengers
with stable respiratory disease planning air travel
were published in Thorax in 2011.
1
This followed
original guidance published in 2002
2
and an online
update in 2004.
3
The 2011 recommendations
provided an expert consensus view based on litera-
ture reviews, aimed at providing practical advice for
lung specialists in secondary care. Recognising that
knowledge in this area has grown since 2011, and
that updated, pragmatic advice regarding which
respiratory patients need specialist assessment is
required, the Society has commissioned a new clin-
ical statement.
Although air travel appears generally safe for
those with respiratory disease assessed previously
by a lung specialist,
4
a decision to undertake air
travel should not be taken lightly. Diverted flights
incur significant expense and inconvenience, and a
patient whose condition deteriorates during flight
can pose huge challenges to airline crew and other
passengers. High altitude destinations may also be
problematic.
European and North American regulatory
authorities limit maximum cabin altitude to 2438 m
(8000 ft) under normal operating conditions.
5–7
The choice of 2438 m was based on the oxyhae-
moglobin dissociation curve, which shows that
up to this level arterial oxygen saturations (SaO
2
)
remain >90% in the average healthy individual.
8
Some newer commercial aircraft have a lower
normal cabin altitude, for example, the Boeing 787
Dreamliner. However, passengers booking such
flights should note that airlines may, for operational
reasons, switch at short notice to an aircraft with a
higher normal cabin altitude.
Besides the passenger’s respiratory condition and
significant comorbidities, a decision regarding suit-
ability for air travel should consider flight duration
and timings, destination (especially if at altitude
or subject to extreme weather conditions), equip-
ment and medications, and whether equipment will
operate effectively and safely at altitude.
There have been developments in three key
areas over the last decade. The first is an attempt,
with research from several groups, to define more
precisely the value and role of the hypoxic challenge
test (HCT). This has included examining the accu-
racy of other, more routinely available lung func-
tion parameters, in predicting hypoxaemia during
air travel. HCT can be expensive in terms of equip-
ment and consumables; and demands additional
staff time. A ‘negative’ HCT (where in- flight
oxygen is not considered necessary) takes around
30 min; if oxygen titration is needed it takes around
60 min. In contrast, spirometry requires 20 min, a
walk test 30 min, and ‘full’ lung function testing
45 min.
9
Results of such assessments may already be
available as part of routine clinical care.
The second development has been increasing
recognition that, although early research in this
area focused on patients with chronic obstructive
pulmonary disease (COPD), other patient groups
may respond differently to altitude- related hypox-
aemia. Although data remain limited, available
evidence no longer appears to support a ‘one size
fits all’ approach.
Finally, the equipment used to deliver oxygen
has changed significantly over the last decade,
with much greater availability of portable oxygen
concentrators (POCs). For overseas travel, patients
usually need to lease a POC privately, since UK
companies do not generally allow their equipment
to be taken out of the country. If a POC is to be used
in- flight, the equipment must be approved by the
airline before travel. There are now a wide variety
of such devices, providing varying flow rates and
modes of delivery (continuous flow vs pulse- dose),
and not all are suitable for all individual patients.
Attention has, therefore, been drawn in this State-
ment to newer data, especially those published since
the 2011 BTS recommendations.
1
Readers wanting
more detailed background information on physi-
ology and the flight environment should consult the
2002 and 2011 BTS documents.
1 2
Scope
The clinical statement provides practical advice for
healthcare professionals in primary and secondary
care managing passengers with pre- existing respi-
ratory conditions planning commercial air travel,
including those recovering from an acute event/
exacerbation. It provides information for patients
and carers; and is also intended to be helpful to
patient support groups, airlines and associated
medical services. Passengers returning home with a
new diagnosis should be reviewed in the light of the
presenting condition and individual circumstances.
The document does not cover emergency aero-
medical evacuation, or travel on non- commercial
flights. Pregnant passengers with respiratory disease
should also consult Royal College of Obstetricians
and Gynaecologists guidance (see online supple-
mental appendix 1).
BTS Clinical Statement
To cite: CokerRK,
ArmstrongA, ChurchAC,
etal. Thorax Epub ahead of
print: [please include Day
Month Year]. doi:10.1136/
thoraxjnl-2021-218110
► Additional supplemental
material is published online
only. To view, please visit the
journal online (http:// dx. doi.
org/ 10. 1136/ thoraxjnl- 2021-
218110).
1
Respiratory Medicine,
Hammersmith Hospital, Imperial
College Healthcare NHS Trust,
London, UK
2
The Newcastle upon Tyne
Hospitals NHS Foundation Trust,
Newcastle upon Tyne, UK
3
Scottish Pulmonary Vascular
Unit, Golden Jubilee Hospital,
Clydebank, UK
4
The Park Medical Practice,
Shepton Mallet, UK
5
Queen Elizabeth Hospital,
Birmingham, UK
6
Department of Paediatric
Respiratory Medicine, Bristol
Royal Hospital for Children,
Bristol, UK
7
Churchill Hospital, Oxford, UK
8
Respiratory Physiology
Department, North Middlesex
University Hospital, London, UK
9
Glasgow Royal Infirmary,
Glasgow, UK
Correspondence to
Dr Robina Kate Coker,
Respiratory Medicine,
Hammersmith Hospital, Imperial
College Healthcare NHS Trust,
London, London, UK;
robina. coker@ imperial. ac. uk
© Author(s) (or their
employer(s)) 2022. Re- use
permitted under CC BY- NC. No
commercial re- use. See rights
and permissions. Published
by BMJ.
1CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
The Statement addresses adults and children with the following
conditions or undergoing the following procedures:
► Airflow obstruction including asthma and COPD.
► Bronchopulmonary dysplasia.
► Cystic fibrosis (CF).
► Non- CF bronchiectasis.
► Restrictive respiratory disease including interstitial lung
disease (ILD), respiratory muscle and chest wall disorders.
► Thoracic surgery or other interventional procedures.
► Pleural disease including pneumothorax and pleural effusion.
► Respiratory infections.
► Obstructive sleep apnoea syndrome (OSAS) and obesity
hypoventilation syndrome (OHS).
► Venous thromboembolism (VTE).
► Pulmonary hypertension (PH).
► Lung cancer and mesothelioma.
► Hyperventilation and dysfunctional breathing (DB).
Preflight assessment is described. Appendix A provides infor-
mation on logistics for air travel with equipment (nebulisers,
oxygen and ventilators); Appendix B provides technical infor-
mation for respiratory physiologists. Sources of useful informa-
tion, Information for primary care healthcare practitioners and
for patients are provided in online supplemental appendices 1–3.
Heart disease and HIV are excluded, as are emergency repa-
triation and travel on military or other non- commercial flights
including helicopter travel. The Terrence Higgins Trust and
British Heart Foundation provide advice on travel with HIV and
heart conditions respectively (see online supplemental appendix
1).
METHODOLOGY
Dr Robina Coker chaired the clinical statement group (CSG).
Membership was drawn from respiratory medicine, paediat-
rics, nursing, respiratory physiology, physiotherapy and primary
care. The CSG identified key areas requiring Clinical Practice
Points. The group reviewed previous BTS recommendations on
this topic
1–3
and supplemented the evidence with up- to- date
literature searches. The overall content was developed to reflect
the scope approved by the BTS Standards of Care Committee
(SOCC). Following discussions of broad statement content, indi-
vidual sections were drafted by group members. A final edited
draft was reviewed by the BTS SOCC before posting for public
consultation and peer review on the BTS website in January
2020. The document was revised in the light of consultation
feedback and approved by the BTS Standards of Care Committee
in July 2021 before final publication.
Summary of clinical practice points
Preflight screening
► All patients should undergo careful initial evaluation with
history and physical examination by a clinician who is
competent. The history should include:
– Review of symptoms, baseline exercise capacity, recent
exacerbation history, treatments and previous experience
of air travel.
– Consideration of the logistics of the intended journey, to
include (if known):
– Number and duration of flights, including whether
daytime or overnight,
– Location of stop- over(s) and destination: these deter-
mine air quality, altitude and available medical facil-
ities,
– Time away from home
– Return journey.
► Further assessment by a respiratory specialist is advised for
those in whom screening raises concerns, and HCT may be
advised.
The following clinical practice points are specific to infants and
children
► For infants born at term (>37 weeks) it is prudent to delay
flying for 1 week after birth to ensure they are healthy.
► Infants born prematurely (<37 weeks) with or without a
history of respiratory disease who have not reached their
expected date of delivery at the time of flying should have
in- flight oxygen available. HCT may not be a reliable guide
of oxygen requirement in this group. If air travel is essential,
they should travel with oxygen at a tolerable low flow, recog-
nising that this may be a minimum of 1 L/min depending on
equipment.
► Infants under 1 year with a history of chronic respiratory
problems should be discussed with a respiratory paediatri-
cian and HCT considered. Those with SpO
2
<85% on HCT
should have in- flight oxygen available; paediatrician discre-
tion should be used for infants with SpO
2
85%–90% recog-
nising that sleep or respiratory infection may further reduce
saturations in this group.
► In children with chronic lung disease able to perform
spirometry whose forced expiratory volume in 1 s (FEV
1
) is
consistently <50% predicted, HCT should be considered.
This includes children with CF and primary ciliary dyski-
nesia (PCD). Children with chronic lung disease who are too
young to perform spirometry reliably should have a clinical
assessment of disease severity and their likely tolerance of
hypoxia. In children with CF the disease is rarely severe
enough to compromise lung function significantly at this
age.
► Infants and children who have required long- term oxygen
in the last 6 months should be discussed with a respiratory
paediatrician and HCT considered.
Patient selection for HCT
See figures 1 and 2.
The following patients should not require HCT
► Those with stable disease who have previously undergone
HCT (no recent hospital admissions, exacerbations, or
significant changes to treatment).
► Patients with COPD with baseline SpO2 ≥95% and either
MRC score 1–2 or desaturation to no less than 84% during
6 min walk test (6MWT) or shuttle walking test (SWT),
should be able to travel without in- flight oxygen.
► Those with previous significant intolerance to air travel,
such as mid- air emergency oxygen or diversion. These
should have in- flight oxygen available at 2 L/min provided
there is no history of hypercapnia.
► Preterm infants who have not reached their due date at the
time of travel, as testing is not a reliable guide of oxygen
requirement in these infants. These should have in-flight
oxygen available, delivered at 1–2 L/min if they develop
tachypnoea, recession, or other signs of respiratory distress.
HCT should be considered for the following patients
► Patients with COPD with resting SpO
2
≤95%, MRC score 3
or greater, or desaturation to <84% on 6MWT or SWT, and
in whom there are concerns about hypercapnia.
2 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
► Infants and children with a history of neonatal respiratory
problems, or existing severe chronic lung disease including
those with FEV1 persistently <50% predicted.
► Adults and children with severe asthma, evidenced by
persistent symptoms and/or frequent exacerbations despite
optimal treatment regardless of resting sea level SpO
2
.
► Patients with ILD in whom SpO
2
falls to <95% on exercise,
and whose resting sea level arterial oxygen tension (PaO
2
)
is ≤9.42 kPa or whose TLCO is ≤50%.
► Those with severe respiratory muscle weakness or chest wall
deformity in whom forced vital capacity (FVC) is <1 L.
► Those with existing or previous hypercapnia and those at
risk of hypercapnia, including those taking medication(s)
which can cause respiratory depression.
► Patients with a history of type 2 respiratory failure already
on LTOT at sea level. However, if there is no evidence of
hypercapnia, it seems reasonable to recommend an increase
in flow rate by 2 L/min in- flight, provided the equipment can
provide it (see Appendix A)
HCT results
► PaO
2
≥6.6 kPa (≥50 mm Hg) or SpO
2
≥85%: in- flight
oxygen not required.
► PaO
2
<6.6 kPa (<50 mm Hg) or SpO
2
<85%: in- flight
oxygen recommended.
► Where required, titrate oxygen to maintain PaO
2
≥6.6 kPa
or SpO
2
≥85% in adults, SpO
2
90% in children aged 1 year
or more.
Asthma
► The patient’s condition should be optimised before travel,
with attention paid to inhaler technique and smoking cessa-
tion referral as required.
► All medications and spacer devices should be carried in
hand luggage to mitigate the risk of lost or missing hold
baggage.
► Emergency medications, including salbutamol inhalers and
spacers, must be immediately accessible.
► Individuals prescribed epinephrine auto- injectors should
have them readily available.
► For acute exacerbations on board, the passenger’s own
bronchodilator inhaler should be given, with a spacer if
needed.
► The passenger should alert the cabin crew if symptoms do
not respond rapidly to use of the inhaler, or if they recur
after a short interval.
Figure 1 Preflight assessment of patients with chronic airflow obstruction.
3CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from
BTS Clinical Statement
► If the passenger does not have their own inhaler with them,
or if it is inaccessible, the airline may carry an inhaler in the
emergency medical kit. Spacers are not commonly available.
► Those with severe asthma should consult their respiratory
specialist beforehand and consider taking an emergency
supply of oral corticosteroid in their hand luggage in addi-
tion to their usual medication.
► Passengers with severe asthma are advised to carry copies of
their asthma management plan and/or relevant clinic letters.
Information can be held securely as scanned copies on a
mobile phone, or on a digital platform such as the National
Health Service (NHS) App.
► Food allergy affects up to 8.5% of children and adults with
asthma and asthma is a risk factor for severe or fatal anaphy-
laxis. Appropriate precautions for those affected include
wiping tray tables and hands, informing the airline before-
hand and the cabin crew of allergies, and not eating during
flights or bringing known ‘safe’ foods from home.
Chronic obstructive pulmonary disease
► The patient’s condition should be optimised before travel,
with attention paid to inhaler technique and smoking cessa-
tion referral where appropriate.
► All medications and spacer devices should be carried in hand
luggage to mitigate the risk of missing hold baggage.
► Emergency medications, including salbutamol inhalers and
spacers, must be immediately accessible.
► For acute exacerbations on board, the passenger’s own bron-
chodilator inhaler should be given, with a spacer if appropriate.
► Passengers with severe COPD are advised to carry a copy of
their COPD management plan and/or relevant clinic letters.
This information can be held securely as scanned copies on
their mobile phone A history of previous pneumothorax or
bullous lung disease necessitates assessment by a respiratory
specialist to determine the potential risk of complications
from reduced cabin pressure.
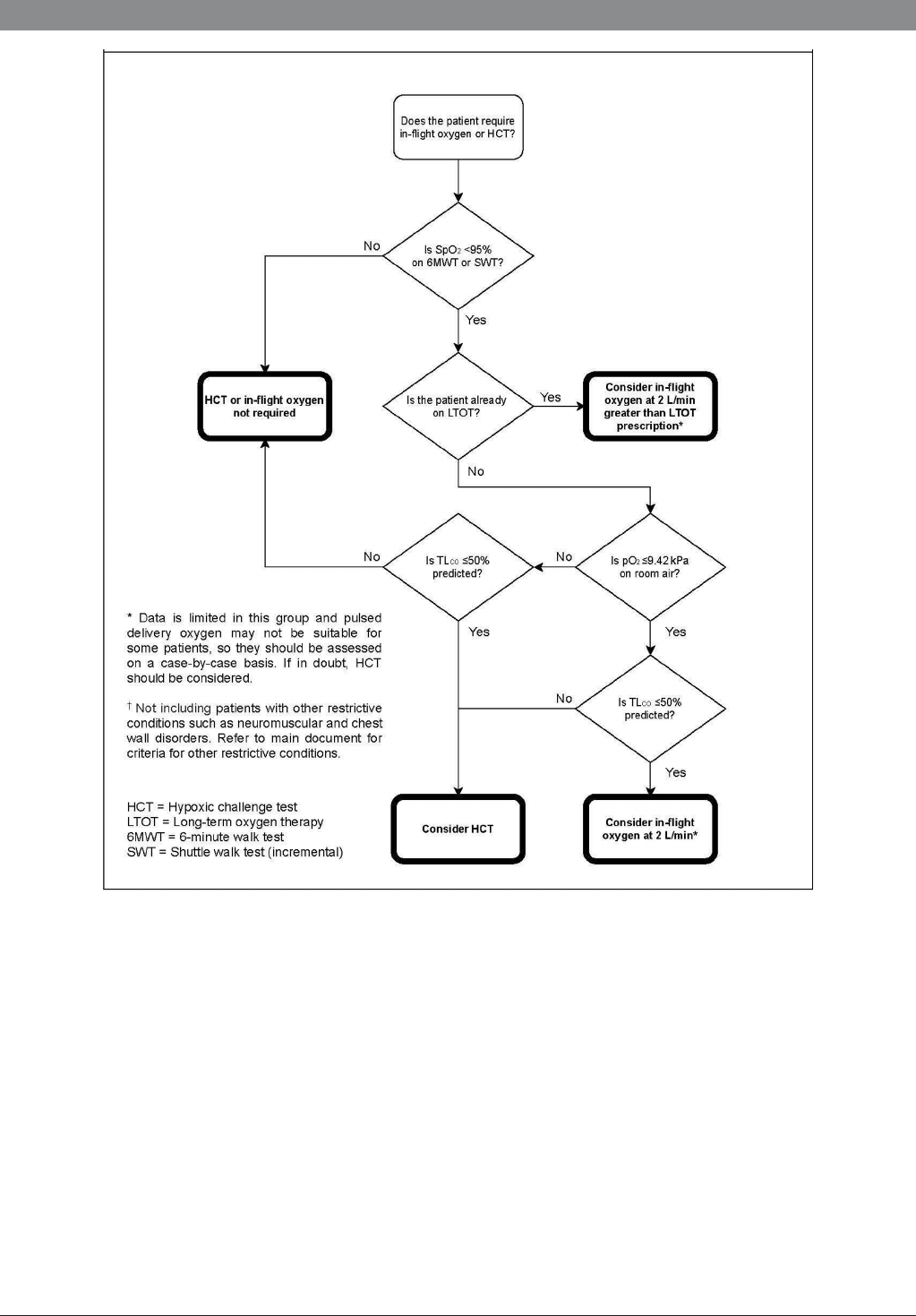
Figure 2 Preflight assessment of patients with restrictive respiratory disease.
4 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
► Patients with COPD are at greater risk of VTE as a direct
consequence of the underlying condition, as well as after an
exacerbation. They should be advised accordingly, especially
if planning longer flights when the risk is further enhanced.
► Patients requiring long- term oxygen therapy should also
plan for oxygen supplementation at their destination (see
online supplemental appendix 1).
► Wherever possible, those who have had a recent exacerba-
tion of their condition should not fly until their condition
is stable and use of reliever therapy has returned to their
usual baseline. If their condition deteriorates while overseas,
medical advice should be sought before undertaking the
return flight.
Cystic fibrosis
► All medications and spacer devices should be carried in hand
luggage to mitigate the risk of missing hold baggage.
► Patients with CF under the age of 6 are likely to be well
enough to fly at the paediatrician’s discretion.
► In those with CF who are old enough for spirometry and
whose FEV1 is <50% predicted, HCT is recommended.
If SpO2 falls below the 90% cut- off, as outlined above,
in- flight oxygen is advised.
► In children with chronic lung disease able to perform
spirometry whose FEV
1
is consistently <50% predicted,
HCT should be considered. This includes children with
CF and non- CF bronchiectasis. Children with chronic lung
disease who are too young to reliably perform spirometry
should have a clinical assessment of assess disease severity
and their likely tolerance of hypoxia. For children with CF
disease is rarely severe enough to severely compromise lung
function at this age.
Non-CF bronchiectasis
► Regular airway clearance is essential for those dealing with
overproduction of mucus.
► Advice from a respiratory physiotherapist on adapting
airway clearance techniques should be sought for long- haul
flights.
► Portable nebulisers and positive expiratory pressure (PEP)
devices may be considered, but use of these devices in- flight
must be approved by the airline before travel.
Interstitial lung disease
► In patients with comorbidity, including PH and/ or cardio-
vascular disease, attention should also be paid to the impact
of air travel on these conditions.
► Physicians may wish to consider HCT in those whom
SpO2 falls to <95% on exercise, and/or in those in whom
either Transfer Factor Carbon Monoxide (TLCO) ≤50% or
PaO
2
≤9.42 kPa (if available).
► Patients with TLCO <50% of predicted or PaO
2
≤9.42 kPa
are likely to need in- flight oxygen. If there are no concerns
about hypercapnia it may be reasonable to recommend 2 L/
min without recourse to HCT. In those in whom there are
concerns about CO2 retention, titration HCT is advised to
determine the oxygen flow rate.
Thoracic surgery
► The opinion of the surgeon or interventionalist should be
obtained before the patient travels by air. Patients, profes-
sionals and their carers should be aware that this may result
in a delay of 4 weeks for non- essential air travel and 2 weeks
for essential air travel.
► Careful clinical assessment of the patient is required. This
should include consideration of their baseline status including
comorbidities, SpO2, postprocedure complications such as
infection and/or pain, flight duration and destination.
Other interventional procedures
► The opinion of the interventionalist should be obtained
before the patient travels by air.
► Careful clinical assessment of the patient is required. This
should include consideration of baseline status including
co- morbidities, SpO2, postprocedure complications such as
infection or pain, flight duration and destination.
► Patients with no pneumothorax seen on the postprocedure
chest X- ray should wait for 1 week before air travel.
► Patients with a pneumothorax seen on the post- procedure
chest X- ray should wait for one1 week after resolution on
chest X- ray before air travel.
Trapped lung
► The opinion of the interventionalist should be obtained
before the patient travels by air.
► Patients should be assessed carefully and advised on a case-
by- case basis.
► Patients should be clinically stable before air travel.
Bronchoscopic procedures
► The opinion of the interventionalist should be obtained
before the patient travels by air.
► Patients should be clinically stable before they travel.
► After interventional bronchoscopy including Transbronchial
Needle Aspiration (TBNA), Transbronchial Lung Biopsy
(TBB), Endobronchial Ultrasound Bronchoscopy (EBUS)
and endobronchial valve insertion, those with no pneumo-
thorax seen on the postprocedure chest X- ray should wait
for 1 week before air travel.
► After interventional bronchoscopy including TBNA, TBB
and EBUS, those with a pneumothorax seen on the post-
procedure chest X- ray should wait for 1 week after resolu-
tion on chest X- ray before air travel.
Pneumothorax
► Passengers should not travel by air until 7 days after full reso-
lution on chest X- ray.
► Those at higher risk of recurrent pneumothorax should be
advised accordingly.
► Higher- risk groups, including those with cystic lung disease
such as lymphangioleiomyomatosis (LAM) and Birt- Hogg-
Dubé (BHD) syndrome, should be advised accordingly.
► Patients with trapped lung and a chronic air space thought
to present a low risk should be evaluated in secondary care
before travel.
Upper respiratory infection including otitis media and sinusitis
► In passengers who develop sinus barotrauma after flying, it
may be helpful to consider topical and oral decongestants as
well as appropriate analgesia. Prolonged use of decongest-
ants is not advised owing to the risk of rebound congestion
on withdrawal.
► If there is an allergic component, intranasal steroids used
for a week prior to travel, and/or oral corticosteroids may
be considered.
► Symptoms and signs of barotrauma should have resolved
before flying again. This usually takes between 1 and 6 weeks.
5CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
► After an episode of acute otitis media, patients are usually
advised not to fly for 2 weeks.
Viral infections
► Patients with highly contagious infections including measles,
chickenpox, mumps, SARS, Middle East respiratory
syndrome (MERS) or COVID- 19 should not be allowed to
travel until they are considered non- infectious.
► Passengers should familiarise themselves with current
national and international regulations regarding air travel,
which should always be observed.
Tuberculosis
► Smear positive patients must not fly until they have provided
two smear negative samples on treatment.
► Those starting treatment for pulmonary tuberculosis (TB),
where not all the information is yet available, should not
travel by air for the first 2 weeks.
► For those who are smear negative and have a fully sensitive
organism, treatment would be expected to render them non-
infectious after 2 weeks.
► For patients with multidrug resistant/extensive drug resistant
(MDR/XDR) TB, travel is prohibited until two negative
culture samples have been produced and there is clinical
evidence of improvement on treatment.
► Extrapulmonary TB does not usually warrant additional
precautions before air travel.
Pneumonia
► All but essential travel should be postponed for 7 days in
those who have reduced baseline sea level SpO2 (<94%).
Obstructive Sleep Apnoea (OSAS) and Obesity Hypoventilation
Syndrome (OHS)
► Daytime flights are advised wherever possible.
► The patient should be advised to carry their continuous posi-
tive airway pressure (CPAP) device as hand luggage, and a
hospital letter to advise that the patient uses CPAP.
► Careful planning and preparation are required, and use of
the patient’s own CPAP device is advised.
► Alcohol and sedatives should be avoided in the 12 hours
before, and during, airline travel.
► Patients should use their CPAP device on board if they are
travelling overnight, and avoid sleeping during daytime
flights.
► Consideration should be given to device settings and whether
adjustment is required for operation at altitude.
► Airline approval for carriage and use of device, including
battery specification, must be gained before travel.
► Consideration should be given to the whole journey. If
driving is required the following day, an overnight stay at
destination may be advisable. Patients are advised to refrain
from driving if tired and sleepy.
Respiratory muscle and chest wall disorders
► HCT is recommended for all adult patients with FVC <1 L,
pending further data, and may be considered in others
thought to be at particular risk, including children with
reduced FVC due to respiratory muscle or chest wall
disorders.
► If patients are unable to perform spirometry reliably, a walk
test may be considered as an alternative.
► Patients should be advised to take daytime flights where
possible.
► Further planning and support are required for those estab-
lished on non- invasive ventilation (NIV) (see Appendix A).
(online supplemental appendix 2
Prevention of VTE during air travel
See table 1.
► Limit the risk of dehydration with adequate fluid intake.
► Avoid alcohol.
► Keep mobile, if possible, by walking around or doing seat-
based exercises once an hour.
► Consider graduated compression stockings (class 1 with
15–30 mm Hg).
► Low molecular weight heparin (LMWH) or a Direct Acting
Oral Anticoagulant (DOAC) are advised for both outward
and return long haul flights (long haul defined as flights
of 6–12 hours) in high- risk patients including those with a
history of VTE; local policy should be followed regarding
liaison with primary care and/or haematology services to
teach the patient how to administer the injection and dispose
safely of the equipment. There is no formally recommended
dose, but enoxaparin at a dose of 40 mg or weight based
1 mg/kg injected once 4–5 hours before the flight has been
suggested.
► The prophylactic doses of the DOAC may also be used.
► All patients with a recent (<6 weeks) history of VTE, espe-
cially any who presented with significant right ventricular
strain and decompensation should be reassessed before air
travel.
Air travel after VTE
► Air travel should be delayed for 2 weeks after a diagnosis of
DVT or pulmonary embolism (PE).
Pulmonary hypertension
► Those in New York Heart Association (NYHA) WHO
functional class 3 or 4 are usually advised to have in- flight
oxygen. If there is no evidence of hypercapnia it seems
reasonable to suggest 2 L/min by nasal cannulae. If there are
concerns about hypercapnia, HCT should be considered if
available.
► Those eligible for LTOT (sea level PaO
2
<8 kPa at rest on
air) should have in flight oxygen at double the flow rate
recommended at sea level, provided there is no evidence of
hypercapnia.
Table 1 Summary of risk factors for VTE during air travel
Risk status Risk factors Advice
All passengers Low Avoid excess alcohol and
caffeine- containing drinks
Remain mobile/exercise legs
Moderate risk Examples include:
Aged over 60
Extensive varicose veins
Recent minor surgery
Pregnancy
Above plus consider:
Take short periods of sleep
Support hosiery/graduated
compression stockings
High risk Examples include:
Previous VTE (and not on current
anticoagulation)
Thrombophilia
Within 6 weeks of major surgery
Current malignancy
If travel is essential, consider:
low- molecular- weight heparin
or formal anticoagulation
(including return journey)
This requires careful clinical
assessment
VTE, venous thromboembolism.
6 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
Lung cancer and mesothelioma
► Patients undergoing chemotherapy should not travel while
they are at increased risk of infection or suffering from
significant side effects, such as vomiting.
Hyperventilation and DB
► Patients with DB, inducible laryngeal obstruction (ILO) and/
or vocal cord dysfunction (VCD) should be referred to a
respiratory physiotherapy specialist for advice on symptom
management before travel.
► Those with anxiety disorders should be reviewed before
travel; compliance with medication assessed; and use of
short acting anxiolytics encouraged.
► Other life- threatening conditions presenting with dyspnoea
should be excluded on board as far as possible.
► Supplemental oxygen should be given on board if the cause
of breathlessness is unclear
► Rebreathing via a paper bag is not recommended.
HCT outcomes
Preflight respiratory screening
Why?
Medical incidents have been reported in around 1 in 600 flights,
10
or 1 in 30 000 passengers.
11 12
Estimates vary and reliable data
are difficult to obtain, but respiratory events account for around
12% of in- flight incidents. In a recent study of 1260 healthy
volunteers, no significant changes occurred in pulse oximetry
(SpO
2
) during a simulated 8- hour flight at cabin altitudes up to
2438 m (8000 ft).
13
However, if cabin altitude exceeds 3048 m
(10 000 ft), hypoxaemia becomes more prominent and SaO
2
falls to∼89% in healthy individuals.
14
Other potential hazards
for passengers with respiratory conditions include low rela-
tive humidity, and altitude- related expansion of gases within
enclosed pulmonary parenchymal spaces. It follows from Boyle’s
Law that a cabin altitude of 2438 m (8000 ft) will result in a 38%
expansion of humidified gas.
Who?
There is no good- quality evidence to determine who should have
a formal respiratory review before air travel. Experts generally
advise preassessment or screening for the following adults, chil-
dren and infants:
► Those with a respiratory condition with the potential to
deteriorate acutely resulting in incapacitation and/or the
need for medical intervention. This includes (but is not
exclusive to):
– Severe (FEV1 <50% predicted
15
or poorly controlled
obstructive airway disease (evidenced by symptoms, oxy-
gen requirements, severe and/or frequent exacerbations).
– Symptomatic restrictive lung or chest wall conditions, or
known respiratory muscle weakness causing breathless-
ness and exercise limitation.
– PH.
– Comorbid conditions which may be worsened by hypox-
aemia (cerebrovascular or cardiac disease).
– Recent (<6 weeks) hospital treatment for a respiratory
condition.
– Requirement for CPAP or ventilator support such as NIV.
– Active cancer with lung involvement.
– Patients requiring domiciliary oxygen.
► Recent (<6 weeks) pneumothorax and those at higher risk
of pneumothorax (cystic lung disease or recurrent pneumo-
thorax), and patients with trapped lung and a chronic air
space.
► Recent (<6 weeks) pulmonary embolus or deep venous
thrombosis, or increased risk of VTE.
► Anyone who has experienced significant symptoms during
previous air travel, or whose condition is of concern to their
physician.
The following are generally considered contraindications to
air travel:
► Untreated respiratory failure.
► Untreated pneumothorax.
► Active infection representing a risk to others for example,
TB, SARS, MERS, COVID- 19.
► Bronchogenic cysts. Cerebral air embolism, in some cases
fatal, has been reported in aircraft passengers after rupture
of a bronchogenic cyst.
16
► Patients with severe hypoxaemia requiring >4 L/min
in- flight oxygen were previously advised against air travel,
because 4 L/min was the maximum fixed flow rate routinely
available on commercial aircraft. With the availability of
flight approved POCs delivering a range of continuous
and intermittent flow rates, this cut- off no longer applies.
In- flight oxygen delivery is more varied, and maximum flow
rate is determined by the equipment available. Pulse- dose
delivery systems can however complicate determination of
the flow delivered and may not be well tolerated. The effects
of mouth- breathing, speech, snoring and/or sleeping should
be considered. High- flow nasal oxygen (HFNO) cannot be
delivered on board commercial aircraft.
In- flight oxygen may be contraindicated in adults and chil-
dren with a history of type 2 respiratory failure.
17 18
Hypoxic
challenge with arterial carbon dioxide tension (PaCO
2
) measure-
ment was advised for this group in 1996
17
but there has been
little research since. This document, therefore, follows the 2015
BTS Guideline for Home Oxygen Use in Adults
19
when making
recommendations for managing patients with previously docu-
mented hypercapnia.
Clinical practice points
► All patients should undergo careful initial evaluation with
history and physical examination by a clinician who is
competent. The history should include:
– Review of symptoms, baseline exercise capacity, recent
exacerbation history, treatments and previous experience
of air travel.
– Consideration of the logistics of the intended journey, to
include (if known):
– Number and duration of flights, including whether
daytime or overnight.
– Location of stop- over(s) and destination: these deter-
mine air quality, altitude and available medical facil-
ities.
– Time away from home.
– Return journey.
► Further assessment by a Respiratory Specialist is advised for
those in whom screening raises concerns, and hypoxic chal-
lenge testing may be advised.
Infants and children
In general, similar considerations apply to both adults and
children if they have severe chronic airway disease, or require
chronic supplementary oxygen, or non- invasive or tracheos-
tomy ventilation. Both children and adults with these condi-
tions require a preflight assessment. Similarly, unless otherwise
stated, recommendations for individuals with previous thoracic
surgery, pneumothorax or empyema apply to both adults and
7CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
children. There are, however, some specific considerations for
infants and younger children since several factors place infants
at greater risk of developing hypoxia. These factors include left
shift of the oxygen dissociation curve (due to the presence of
foetal haemoglobin), smaller airway diameter, relatively fewer
alveoli, compliant rib cage and increased tendency to pulmo-
nary vasoconstriction and bronchoconstriction and thus ventila-
tion–perfusion mismatch under hypoxic conditions. Moreover,
preterm infants and infants under 2 months of age may develop
apnoea/hypoventilation in response to hypoxia or infection.
20 21
Beyond 3 months of age there is no evidence that ex- preterm
infants, without bronchopulmonary dysplasia, are at significantly
greater risk of desaturation during a HCT than term infants.
22
In addition to very young and ex- preterm infants, the chil-
dren most at risk of hypoxia are those with anaemia, congenital
heart disease with an actual or potential right to left shunt,
23
neuromuscular disorders or chronic or acute lung disease. Low
humidity during air travel can also present a problem for chil-
dren with respiratory conditions such as CF. Those most at risk
of complications associated with reduced air pressure are chil-
dren with upper respiratory tract infections, or trapped intra-
thoracic air, including those with recent pneumothorax or cystic
lung disease.
24
For infants born at term (>37 weeks) it is prudent to delay
flying for 1 week after birth to ensure they are healthy.
25
In view
of their greater risk of apnoea and hypoxia, infants born prema-
turely (<37 weeks) with or without a history of respiratory
disease who have not reached their expected date of delivery
at the time of flying should have in- flight oxygen available.
HCT may not be a reliable guide of oxygen requirement in this
group.
26
If air travel is essential, they should travel with oxygen
at a tolerable low flow, recognising that this may be a minimum
of 1 L/min depending on equipment.
The following Clinical Practice Points are specific to infants
and children.
Clinical practice points
► For infants born at term (>37 weeks) it is prudent to delay
flying for 1 week after birth to ensure they are healthy.
► Infants born prematurely (<37 weeks) with or without a
history of respiratory disease who have not reached their
expected date of delivery at the time of flying should have
in- flight oxygen available. HCT may not be a reliable guide
of oxygen requirement in this group. If air travel is essen-
tial, they should travel with oxygen at a tolerable low flow
rate, recognising that this may be a minimum of 1 L/min
depending on equipment.
► Infants under 1 year with a history of chronic respiratory
problems should be discussed with a respiratory paediatri-
cian and HCT considered. Those with SpO
2
<85% on HCT
should have in- flight oxygen available; paediatrician discre-
tion should be used for infants with SpO
2
85%–90% recog-
nising that sleep or respiratory infection may further reduce
saturations in this group.
► In children with chronic lung disease able to perform
spirometry whose FEV
1
is consistently <50% predicted,
HCT should be considered. This includes children with CF
and PCD. Children with chronic lung disease who are too
young to perform spirometry reliably should have a clinical
assessment of disease severity and their likely tolerance of
hypoxia. In children with CF the disease is rarely severe
enough to compromise lung function significantly at this
age.
► Infants and children who have required long- term oxygen
in the last 6 months should be discussed with a respiratory
paediatrician and HCT considered.
How?
Pulse oximetry is the easiest and usually the first screening
test.
4
It has generally been accepted in the past that those with
resting SpO
2
>95% at sea level should not require in- flight
oxygen.
2 25 27–30
Spirometry results may already be available
in patients with known acute or chronic lung disease, or with
symptoms suggesting lung disease.
31 32
However, lung function
parameters are in many cases poor at predicting hypoxaemia or
complications.
28 33–35
Many airlines have historically considered that those able
to walk 50 m or climb up 10–12 steps without distress have
sufficient cardiopulmonary reserve to fly.
2 36
The role of the
6MWT in preflight evaluation, widely used to assess functional
capacity and exercise- induced hypoxaemia in COPD
37–40
and
ILD including IPF,
41–43
has also been examined. Current data
suggest that the 50 m walk test is an insensitive assessment of
‘fitness to fly’
38 44 45
although still sometimes referenced.
36 46 47
Several studies show no correlation between walking distance
and HCT outcome in patients with COPD, ILD or extrapul-
monary restriction.
38 44 45 48
One study showed no correla-
tion between exertional dyspnoea and HCT outcome.
38
The
50 m walk test alone thus appears unsuitable for preflight
assessment.
The 6MWT and externally paced incremental SWT may be
of value. Baseline values do not reliably predict in- flight hypox-
aemia in a number of respiratory conditions
1 4 33 34 44 49–51
but
changes in SpO
2
during 6MWT and SWT may correlate with
HCT outcome in COPD, ILD and chest wall deformity.
30 38 44 45
Walk tests cannot predict the in- flight oxygen flow rate required,
but they may help inform the decision as to who needs further
assessment.
A walk test is not always practical. Data from one small study in
COPD suggest that MRC scores may help predict the likelihood
of exercise desaturation.
52
From this it appears that patients with
COPD, MRC score 1 or 2 and resting oxygen saturations >95%
do not usually need further testing before air travel. If there
are still concerns, a walk test may help decide whether HCT is
required. In those with COPD who do undergo 6MWT or SWT
and do not desaturate below 84%, in flight oxygen should not be
required and they should not need HCT.
If resting oxygen saturations are SpO2 92%–95% and they
desaturate <84% but have no evidence of CO
2
retention, data
from Edvardsen et al
30
suggest it is reasonable to recommend
in- flight oxygen at 2 L/min without proceeding to HCT. Patients
in whom there are concerns about hypercapnia should proceed
to HCT.
Data are much more limited in restrictive disease, including
ILD, and baseline SpO2 does not appear to predict outcome.
In general, it seems reasonable to suggest that if baseline satu-
rations are >95% at rest and there is no desaturation below
95% on 6MWT or SWT, HCT should not be required. Those
with ILD and TLco ≤50% of predicted and PaO
2
≤9.42 kPa are
likely to need in- flight oxygen or HCT. If there are no concerns
about hypercapnia it may be reasonable to recommend 2 L/min
without recourse to HCT. If there are concerns about CO
2
reten-
tion, titration HCT will be required to determine the oxygen
flow rate.
8 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
Hypoxic challenge testing
HCT is performed using an inspired gas mixture containing
15% oxygen, which gives an approximate similar inspired
oxygen tension (PO
2
) to breathing air at the maximum allowable
cabin pressure altitude (2438 m or 8000 ft).
53 54
HCT is usually
performed in a specialist respiratory physiology unit. The provi-
sion of a 15% oxygen gas mixture can be achieved using one of
the methods described in online supplemental appendix B.
The closest approximation to aircraft cabin conditions entails
exposure to simulated altitude in a hypobaric chamber, but such
chambers are not available for clinical assessment. A reasonable
substitute is the normobaric HCT, described by Gong et al
55
in patients with chronic airflow obstruction. This assesses the
response to hypoxaemia achieved by breathing a hypoxic gas
mixture at sea level. Various methods of hypoxic gas delivery
produce equivalent results to tests in a hypobaric chamber or
during real flights in adults with COPD.
1 39 56–58
Data are limited
in other conditions as well as for children and neonates.
The HCT is used to help decide whether passengers with
respiratory disease need in- flight oxygen and at what flow rate.
It does not assess fitness for air travel, despite its reputation as
a ‘fitness to fly’ test. If healthcare providers give this impres-
sion in patient information, they must manage patient and carer
expectations accordingly. A ‘preflight oxygen test’ is a more
accurate description. Most patients do not require HCT as part
of preflight medical assessment, and there should not be pressure
on physicians to arrange, or healthcare professionals to perform,
unnecessary HCT.
The physiological response to hypobaric hypoxia
(PaO
2
<8 kPa) is increased ventilation.
59
Alterations in respi-
ratory pattern may adversely impact on lung mechanics,
60
which may be further impaired by gas expansion, reducing vital
capacity and increasing residual volume.
61
The increase in venti-
latory drive is likely to be limited on commercial flights,
62
but a
modest increase in ventilation can exhaust an already reduced
ventilatory reserve.
16 60 63
The usual consensus is to recommend in- flight oxygen if
PaO
2
is predicted to fall below 6.6 kPa (50 mm Hg) or SpO2
below 85% in flight. There is little high- quality evidence
supporting these cut- off values, but this PaO
2
value ensures
that SpO2 remains above the steep portion of the oxyhaemo-
globin dissociation curve.
64
Some authors consider 6.6 kPa
to represent the lower safe limit for hypoxaemia,
65 66
as PVR
increases sharply in response to arterial pO2 below this level,
67
with the potential for an acute increase in right ventricle after-
load and right ventricular dysfunction.
16 29
As many patients
with COPD have cardiac comorbidity,
68
hypoxaemia in these
patients could precipitate cardiac ischaemia; this is unlikely in
those with stable disease in NYHA functional class I or II (no or
mild limitation of physical activity).
69
70
In the absence of new
evidence to the contrary, the cut- off PaO
2
of 6.6 kPa during
HCT appears reasonable.
HCT outcomes do not predict respiratory symptoms during
air travel.
71
72
73
Such symptoms do not appear to result directly
from hypoxaemia,
62
but from a combination of poor respira-
tory mechanics and reduced respiratory reserve impairing the
response to hypoxaemia. Symptoms are more likely to occur in
those with more severe breathlessness at sea level.
4 72
Limited
evidence suggests that those who desaturate during HCT and
have previously experienced respiratory symptoms during air
travel can avoid these by using in- flight oxygen.
29 71
Symptoms
may also result from anxiety regarding air travel (see section on
hyperventilation and DB).
Patient selection for HCT
Those with stable respiratory disease without history of air travel
intolerance, normal resting and exercise SpO2 at sea level and
no significant cardiac comorbidity, are unlikely to need in- flight
oxygen and should not require HCT. Those who have had HCT
in the past should not need it repeated unless their clinical
condition has changed. The patient’s plans should, however, be
discussed with the patient’s respiratory physician, paediatrician
or specialist nurse.
Those already using LTOT will need in- flight oxygen. Ideally,
the flow rate required at cruising altitude should be determined
using HCT. If HCT is not readily available and there are no
concerns about hypercapnia, passengers already on LTOT should
be advised that they will need a flow rate 2 L/min greater than
their baseline flow rate. This should be sufficient to compen-
sate for the relative hypoxia at normal cabin altitude. However,
current POCs do not routinely offer continuous flow rates above
3 L/min, and a pulse- dose delivery mode at higher levels may not
always be suitable.
74
As noted above, it is not practical for all patients with COPD
who want to fly to undergo 6MWT. Respiratory physicians may
however wish to consider 6MWT if there has been a significant
change in the patient’s condition since the last assessment, or
in new patients previously unknown to the service. Those who
desaturate below 84% may then be referred for HCT at the
discretion of the respiratory physician.
Some data are available in smaller numbers of patients with
restrictive lung disease, but there is currently no consensus
regarding the best walk test or cut- off values. In a study of 14
patients with primary thoracic scoliosis, Bandyopadhyay et
al found that resting SpO2 >95% did not accurately identify
those who do not desaturate during HCT, and recommend a
low threshold for performing HCT on patients with thoracic
scoliosis.
44
Likewise, in a study of 13 patients with OHS, base-
line SpO2 did not predict HCT outcome.
49
In a study including
42 patients with ILD and 20 with extra- pulmonary restric-
tion
35
before and after ‘2 min of moderate exercise’, Ling et al
proposed that a postexercise SpO2 of no less than 95% could be
used to exclude the need for HCT. Further research is required
to determine the most appropriate assessments for patients with
a variety of restrictive lung diseases, including which (if any) can
reliably eliminate the need for HCT.
Clinical practice points: patient selection for HCT
See figures 1 and 2
The following patients should not require HCT
► Those with stable disease who have previously undergone
HCT (no recent hospital admissions, exacerbations, or
significant changes to treatment).
► Patients with COPD with baseline SpO2 ≥95% and either
MRC score 1–2 or desaturation to no less than 84% during
6MWT or SWT, should be able to travel without in- flight
oxygen.
► Those with previous significant intolerance to air travel,
such as mid- air emergency oxygen or diversion. These
should have in- flight oxygen available at 2 L/min provided
there is no history of hypercapnia.
► Preterm infants who have not reached their due date at the
time of travel, as testing is not a reliable guide of oxygen
requirement in these infants. These should have in-flight
oxygen available, delivered at 1–2 L/min if they develop
tachypnoea, recession, or other signs of respiratory distress.
9CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
HCT should be considered for the following
► Patients with COPD with resting SpO
2
≤95%, MRC score 3
or greater, or desaturation to <84% on 6MWT or SWT, and
in whom there are concerns about hypercapnia
► Infants and children with a history of neonatal respiratory
problems, or existing severe chronic lung disease including
those with FEV1 persistently <50% predicted (see page 7).
► Adults and children with severe asthma, evidenced by
persistent symptoms and/or frequent exacerbations despite
optimal treatment (see BTS/SIGN Asthma Guideline
75
)
regardless of resting sea level SpO
2
.
► Patients with ILD in whom SpO
2
falls to <95% on exer-
cise, and whose resting sea level PaO
2
is ≤9.42 kPa or whose
TLCO is ≤50%.
► Those with severe respiratory muscle weakness or chest wall
deformity in whom FVC is <1 L.
► Those with existing or previous hypercapnia and those at
risk of hypercapnia, including those taking medication(s)
which can cause respiratory depression.
► Patients with a history of type 2 respiratory failure already
on LTOT at sea level. However, if there is no evidence of
hypercapnia, it seems reasonable to recommend an increase
in flow rate by 2 L/min in- flight, provided the equipment can
provide it (see Appendix A).
Clinical practice points: HCT results
► PaO
2
≥6.6 kPa (≥50 mm Hg) or SpO
2
≥85%: in- flight
oxygen not required.
► PaO
2
<6.6 kPa (<50 mm Hg) or SpO
2
<85%: in- flight
oxygen recommended.
► Where required, titrate oxygen to maintain PaO
2
≥6.6 kPa
or SpO
2
≥85% in adults, SpO
2
90% in children aged 1 year
or more.
Air travel may be contraindicated in infrequent cases when
supplementary oxygen, at the flow rate needed to maintain
PaO
2
≥6.6 kPa or SpO
2
≥85%, causes significant changes to pH
and pCO
2
. The 2015 BTS Guidelines for Home Oxygen Use in
Adults
19
consider that a pH <7.35, and a PaCO
2
increase >1 kPa
from baseline (within 20 min) is significant. Specialist respiratory
physicians should use their discretion to determine the risk in
individual cases and advise accordingly. Where hyperventilation
is suspected, especially in response to anxiety rather than hypox-
aemia, results should be interpreted with caution as there is a
risk of false negative results.
76
HCT methods
These are described in Appendix B.
Exertion on board
Studies in adults with COPD
33 77 78
or CF
79
have shown that
patients can develop profound hypoxaemia when exercising
under hypoxic conditions, whether on board a commer-
cial aircraft at cruising altitude or during HCT. This has been
reported in patients whose HCT results would otherwise not
warrant oxygen. In some cases, PaO
2
values as low as 3.9 kPa
have been recorded.
33 78
The combination of further hypoxaemia and increased venti-
latory demand from exertion while flying may challenge those
already approaching the limits of their respiratory reserve. It
therefore seems prudent to recommend that passengers with
significant respiratory limitation, regardless of whether they
travel with in- flight oxygen, should request an aisle seat near a
toilet to avoid long periods of walking.
1 29 80
Passengers should
keep active by undertaking seat- based exercises and/or standing
at intervals if flight conditions permit.
Patients who cannot tolerate withdrawal of supplemental
oxygen for even a short period of time should not travel by air,
as there will be periods of time when oxygen cannot be supplied.
POC use below 10 000 ft may in some circumstances be prohib-
ited by cabin crew. The reduction in cabin pressure between an
aircraft taking off and reaching 10 000 ft is small (10%) and
unlikely to have any clinical impact on those who do not usually
require oxygen at rest at sea level. Aircraft descent may however
take longer than ascent, and the time to landing may be less
predictable.
Disease/condition-specific advice
Chronic airflow obstruction including asthma and COPD
Most adults and children with well- controlled mild or moderate
airflow obstruction and no other co- morbidities should have no
problem with commercial air travel, but they should be prepared
for the possibility of an exacerbation of their condition. Air
travel presents a theoretical risk of bronchospasm because of
mucosal water loss due to low cabin humidity.
Cigarette smokers are at a physiological disadvantage during
exposure to altitude.
81
Every opportunity should be taken, when
reviewing travel plans, to take a smoking history and offer brief
intervention and smoking cessation referral as appropriate.
Asthma
While asthma is prevalent and has the potential to be life-
threatening, most episodes are not.
82 83
Most passengers with asthma will have relatively mild disease
and do not require HCT. HCT should however be considered
for those with severe asthma, regardless of baseline sea level
oxygen saturation. In a retrospective study of 37 adults with
severe asthma (as defined in the BTS/SIGN Asthma guide-
line
75
) undergoing HCT, two- thirds who fulfilled the criteria for
in- flight oxygen on HCT had baseline sea level oxygen satura-
tions of >95%.
84
The role of HCT has not been studied in children with severe
stable asthma. A study in 51 children aged 2–12 years requiring
transient oxygen therapy during an acute asthma attack
(SpO2 <92%) showed that although 5% failed HCT within 24
hours of discontinuing oxygen therapy, all passed the HCT when
retested at 48 hours.
85
Food allergy affects up to 8.5% of children and adults with
asthma,
86
and asthma is a risk factor for severe or fatal anaphy-
laxis.
87
Appropriate precautions for those affected include
wiping tray tables and hands, informing the airline beforehand
and the cabin crew of allergies, and not eating during flights or
bringing known ‘safe’ foods from home
88
Clinical practice points
► The patient’s condition should be optimised before travel,
with attention paid to inhaler technique and smoking cessa-
tion referral as required.
► All medications and spacer devices should be carried in hand
luggage to mitigate the risk of lost or missing hold baggage.
► Emergency medications, including salbutamol inhalers and
spacers, must be immediately accessible.
► Individuals prescribed epinephrine auto- injectors should
have them readily available.
► For acute exacerbations on board, the passenger’s own bron-
chodilator inhaler should be given, with a spacer if needed.
10 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
► The passenger should alert the cabin crew if symptoms do
not respond rapidly to use of the inhaler, or if they recur
after a short interval.
► If the passenger does not have their own inhaler with them,
or if it is inaccessible, the airline may carry an inhaler in the
emergency medical kit. Spacers are not commonly available.
► Those with severe asthma should consult their respiratory
specialist beforehand and consider taking an emergency
supply of oral corticosteroid in their hand luggage in addi-
tion to their usual medication.
► Passengers with severe asthma are advised to carry copies of
their asthma management plan and/or relevant clinic letters.
Information can be held securely as scanned copies on a
mobile phone, or on a digital platform such as the NHS App.
► Food allergy affects up to 8.5% of children and adults with
asthma and asthma is a risk factor for severe or fatal anaphy-
laxis. Appropriate precautions for those affected include
wiping tray tables and hands, informing the airline before-
hand and the cabin crew of allergies, and not eating during
flights or bringing known ‘safe’ foods from home.
Chronic obstructive pulmonary disease
Patients with COPD planning air travel need careful evaluation,
not only because of their respiratory disease, but also because of
their high levels of comorbidity.
71 89
Respiratory symptoms in those with COPD are common
during air travel, but Edvardsen et al have shown that HCT does
not predict respiratory symptoms during air travel in patients
with moderate to very severe COPD.
71
They suggest that exac-
erbation of comorbidities such as cardiovascular disease (the
most common cause of death in COPD) is the most threatening
consequence of severe hypoxaemia. This is consistent with data
showing a risk of cardiac arrhythmias and ischaemic chest pain
in patients with COPD unable to respond to the physiological
stressors of air travel.
55 70
Work by Robson et al shows that resting
sea level saturations alone do not predict HCT outcome.
28
Spirometry does not reliably predict hypoxaemia or compli-
cations in COPD.
29
It seems prudent to avoid air travel within 6
weeks of an exacerbation although there are few data to support
this recommendation. Patients with COPD are at greater risk
of VTE as a direct consequence of the underlying condition, as
well as after an exacerbation. They should be advised accord-
ingly, especially if planning longer flights when the risk is further
enhanced (see section on VTE).
90–92
See Figure 1.
Clinical practice points
► The patient’s condition should be optimised before travel,
with attention paid to inhaler technique and smoking cessa-
tion referral where appropriate.
► All medications and spacer devices should be carried in hand
luggage to mitigate the risk of missing hold baggage.
► Emergency medications, including salbutamol inhalers and
spacers, must be immediately accessible.
► For acute exacerbations on board, the passenger’s own
bronchodilator inhaler should be given, with a spacer if
appropriate.
► Passengers with severe COPD are advised to carry a copy of
their COPD management plan and/or relevant clinic letters.
This information can be held securely as scanned copies on
their mobile phone A history of previous pneumothorax or
bullous lung disease necessitates assessment by a respiratory
specialist to determine the potential risk of complications
from reduced cabin pressure.
► Patients with COPD are at greater risk of VTE as a direct
consequence of the underlying condition, as well as after an
exacerbation. They should be advised accordingly, especially
if planning longer flights when the risk is further enhanced.
► Patients requiring long- term oxygen therapy should also
plan for oxygen supplementation at their destination (see
online supplemental appendix 1).
► Wherever possible, those who have had a recent exacerba-
tion of their condition should not fly until their condition
is stable and use of reliever therapy has returned to their
usual baseline. If their condition deteriorates while overseas,
medical advice should be sought before undertaking the
return flight.
CF (adults and children)
The risks associated with air travel are greater for those with
CF than for healthy individuals.
93
This is despite the fact that
people with CF have been shown to tolerate PaO
2
values below
6.6 kPa (50 mm Hg) for several hours without cardiac decom-
pensation or cerebral symptoms
94
; do not usually have cardio-
vascular comorbidities; and are generally younger than patients
with other respiratory conditions. Hypoxaemia results mainly
from ventilation/perfusion mismatch attributable to chronic
inflammation and mucus plugging. It is not clear which physio-
logical values measured at sea level best predict hypoxaemia or
complications during flight.
In 1 study of 30 adults with CF undergoing HCT, four fulfilled
the study’s criteria for supplemental oxygen (PaO
2
<6.6 kPa) at rest
and a further 11 dropped below this threshold while walking slowly.
Variables obtained during CPET (including SpO
2
and PaO
2
) showed
a stronger correlation with arterial oxygen tension (Pao
2
) during
HCT than baseline SpO
2
or spirometry.
79
However, in children
with CF the sensitivity and specificity of preflight HCT have been
reported as 20% and 99% (using a cut- off of SpO
2
<90% during
HCT with FiO2 0.15), compared with 70% and 96% for spirom-
etry (cut- off FEV1 <50% predicted).
95
Combining spirometry
and HCT increased sensitivity to 80%. Spirometry may, therefore,
usefully predict who may desaturate during flight, and a cut- off of
FEV1 50% has been used to recommend HCT.
Passengers with CF should practise good hand hygiene using
soap and water or an alcohol- based hand gel, and avoid touching
their face, particularly after touching arm rests, food trays or
toilet doors to minimise risk of infection. These measures are
included within recommendations from the European Centres
of Reference Network for Cystic Fibrosis project, endorsed
by the European Cystic Fibrosis Society.
93
These also advise
checking the relevant airline policy and levels of CF healthcare
provision at the proposed destination before travel (see online
supplemental appendix 1).
Clinical practice points
► All medications and spacer devices should be carried in hand
luggage to mitigate the risk of missing hold baggage.
► Patients with CF under the age of 6 are likely to be well
enough to fly at the paediatrician’s discretion.
► In those with CF who are old enough for spirometry and
whose FEV1 is <50% predicted, HCT is recommended.
If SpO2 falls below the 90% cut- off, as outlined above,
in- flight oxygen is advised.
► In children with chronic lung disease able to perform
spirometry whose FEV
1
is consistently <50% predicted,
HCT should be considered. This includes children with
CF and non- CF bronchiectasis. Children with chronic lung
disease who are too young to reliably perform spirometry
11CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
should have a clinical assessment of assess disease severity
and their likely tolerance of hypoxia. For children with CF
disease is rarely severe enough to severely compromise lung
function at this age.
Non-CF bronchiectasis
Passengers with bronchiectasis should not necessarily be discour-
aged from flying, but air travel can pose challenges.
Clinical practice points
► Regular airway clearance is essential for those dealing with
overproduction of mucus.
► Advice from a respiratory physiotherapist on adapting
airway clearance techniques should be sought for long- haul
flights.
► Portable nebulisers and PEP devices may be considered, but
use of these devices in- flight must be approved by the airline
before travel.
Interstitial lung disease
Like individuals with airflow limitation, patients with ILD,
including pulmonary fibrosis, respond to hypoxaemia at
altitude with increased heart rate and minute ventilation.
In severe disease the ability to increase minute ventilation
is limited and the resulting hypoxaemia may be marked.
However, unlike COPD, where many patients appear to be
able to tolerate marked hypoxia,
65
patients with ILD may
have acute or subacute disease and be less able to withstand
marked hypoxia. There are fewer relevant studies available
in ILD, and patient numbers are smaller than in COPD
studies.
Two studies in patients with ILD (n=15 and 10, respectively)
have shown that sea level oxygen saturations do not reliably
predict HCT outcome, and that oxygen saturations fall signifi-
cantly after light exercise performed under conditions of normo-
baric hypoxia.
73 96
These findings are consistent with those from
the UK Flight Outcomes Study,
4
a prospective observational
study of 431 patients including 186 with ILD. This showed that
neither FEV1 nor sea level SpO
2
reliably predict desaturation at
altitude, and that patients with ILD were more likely than others
to require unscheduled healthcare for respiratory events within
4 weeks of air travel.
In a study including 15 patients with ILD, Martin et al found
that predictive equations overestimated the need for in- flight
oxygen in patients with ILD, as they did for those with COPD
and CF.
97
More recently, Barratt et al examined the predictive
value of various parameters for HCT outcome in 106 ILD
patients (69 with IPF).
98
Only the combined parameters
of TLCO >50% predicted and sea level PaO
2
>9.42 kPa
independently predicted a successful HCT outcome. From
analysis of a subset of 88 patients with a complete dataset
available the authors propose a new prelight algorithm for
patients with ILD with a sensitivity of 86% and specificity
of 84%. In patients with both sea level PaO
2
≤9.42 kPa and
TLCO ≤50% predicted, in- flight oxygen is recommended
without recourse to an initial diagnostic HCT. HCT for titra-
tion of the oxygen flow rate required on board is still advised.
For patients in whom either TLCO ≤50% or PaO
2
≤9.42 kPa,
diagnostic HCT is advised. This promising approach requires
further validation in a larger, prospective cohort of patients
with ILD, preferably supported by patient reported outcomes
from actual flight(s).
Clinical practice points
► In patients with comorbidity, including PH and/or cardiovas-
cular disease, attention should also be paid to the impact of
air travel on these conditions.
► Physicians may wish to consider HCT in those whom SpO2
falls to <95% on exercise, and/or in those in whom either
TLCO ≤50% or PaO
2
≤9.42 kPa (if available).
► Patients with TLco <50% of predicted or PaO
2
≤9.42 kPa
are likely to need in- flight oxygen. If there are no concerns
about hypercapnia it may be reasonable to recommend 2 L/
min without recourse to HCT. In those in whom there are
concerns about CO2 retention, titration HCT is advised to
determine the oxygen flow rate.
Thoracic surgery and other interventional procedures
There is no high- quality evidence in this area and further
research and/or data collection are needed. The following are
suggested time periods before which a medically unaccompanied
commercial flight can safely be undertaken after the specific
thoracic interventions described below. The advice is conserva-
tive. Shorter recovery periods may be appropriate in individual
cases, but only if approved by the doctor/surgeon carrying out
the procedure. It is also important to note that the potential
risks of travel are not just those associated with a postprocedure
pneumothorax, but include wound infection and pain, which
could require medical attention at destination and would need
approval by the travel insurer.
Thoracic surgery, including VATS procedures
In the absence of published evidence, we advocate a conserva-
tive and safe minimum time interval, with the caveat that flying
sooner after such procedures may be possible and/or desirable,
but that this should be agreed with the surgeon and discussed
with the airline. At least two UK centres independently advise
against non- essential air travel for 4 weeks after removal of
drains (Jon Naylor, personal communication). If air travel is
essential, a minimum delay of 2 weeks is advised, depending on
the type of surgery and the surgeon’s advice.
Clinical practice points
► The opinion of the surgeon or interventionalist should be
obtained before the patient travels by air. Patients, profes-
sionals, and their carers should be aware that this may result
in a delay of 4 weeks for non- essential air travel and 2 weeks
for essential air travel.
► Careful clinical assessment of the patient is required. This
should include consideration of their baseline status including
comorbidities, SpO2, postprocedure complications such as
infection and/or pain, flight duration and destination.
Percutaneous lung biopsy, pleural procedures (including
thoracocentesis, medical thoracoscopy and insertion of
indwelling pleural catheter)
A North American study of 179 patients, who between them
underwent 183 percutaneous transthoracic needle biopsies,
suggested that air travel was safe within 24 hours of procedure,
even in the 65 patients (35%) who developed a small, stable
postbiopsy pneumothorax.
99
Fifty (77%) of them flew within
4 days of the final postbiopsy chest radiograph. During a brief
telephone survey after the flight, 14 (8%) reported worsening of
pre- existing respiratory symptoms or new respiratory symptoms.
There were no reported events requiring in- flight medical atten-
tion or flight diversion. These were, however short, internal
North American flights over land, where diversion is relatively
12 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
straightforward if required. The situation for a UK- based patient
travelling on a long- haul flight to the Middle East, USA, Far East
or Australasia is quite different.
Clinical practice points
► The opinion of the interventionalist should be obtained
before the patient travels by air.
► Careful clinical assessment of the patient is required. This
should include consideration of baseline status including
comorbidities, SpO2, postprocedure complications such as
infection or pain, flight duration and destination.
► Patients with no pneumothorax seen on the postprocedure
chest X- ray should wait for 1 week before air travel.
► Patients with a pneumothorax seen on the postprocedure
chest X- ray should wait for 1 week after resolution on chest
X- ray before air travel.
‘Trapped lung’ after drainage of pleural space
Only very limited data are available, from a report of two patients
with a small chronic pneumothorax.
100
This suggests that such
patients may be able to travel safely by air, but require thor-
ough clinical assessment, CT imaging and HCT as a minimum
beforehand.
Clinical practice points
► The opinion of the interventionalist should be obtained
before the patient travels by air.
► Patients should be assessed carefully and advised on a case-
by- case basis.
► Patients should be clinically stable before air travel.
Bronchoscopic procedures
The risks associated with air travel are not only those of a
possible pneumothorax, but also the effects of sedation, exacer-
bation of pre- existing or new symptoms such as cough, hoarse
voice haemoptysis and dyspnoea, respiratory infection and the
consequences of arrhythmias observed during the procedure.
Clinical practice points
► The opinion of the interventionalist should be obtained
before the patient travels by air.
► Patients should be clinically stable before they travel.
► After interventional bronchoscopy including TBNA, TBB,
EBUS and endobronchial valve insertion, those with no
pneumothorax seen on the postprocedure chest X- ray
should wait for 1 week before air travel.
► After interventional bronchoscopy including TBNA, TBB
and EBUS, those with a pneumothorax seen on the post-
procedure chest X- ray should wait for 1 week after resolu-
tion on chest X- ray before air travel.
Pleural disease
Pleural effusion
Patients with stable pleural disease and normal resting oxygen
saturations should be able to fly without further precautions.
Those who have indwelling long- term drainage catheters should
be reminded that the manufacturers do not advise air travel.
Those who choose to travel should be encouraged to take a
supply of drainage bottles for their time away.
In those with a recent onset pleural effusion, investigation
should be delayed if air travel is planned within 2 weeks, since
intervention may increase the risk of pneumothorax. The risk of
delaying investigation should be discussed with the individual to
determine whether travel plans can be modified.
Pneumothorax
The prevalence of in- flight pneumothorax in passengers with
existing lung disease appears low overall, being zero in the
UK Flight Outcomes Study.
4
It increases, however, in those at
increased risk: 3.6% in a study of 276 patients with LAM
101
; and
9% within 1 month of air travel in a retrospective survey of 145
patients with BHD syndrome.
102
Most individuals with an untreated, closed pneumothorax
should not travel by air. In exceptional cases where the pneu-
mothorax is long- standing and thought to present a low risk,
secondary care evaluation is strongly advised before travel.
In individuals with a treated pneumothorax, exposure to alti-
tude poses a risk of recurrence. The 2010 BTS Pleural Disease
guidelines state that patients ‘…should be cautioned against
commercial flights … until full resolution of the pneumothorax
has been confirmed by a chest X- ray”.
103
These guidelines state
that patients should wait a week after pneumothorax resolution
before flying. There is limited, more recent evidence to suggest
that in the case of traumatic pneumothorax, air travel as early
as 72 hours after chest drain removal with full lung inflation
may be safe.
104
A prospective observational study of 20 patients
with a small residual traumatic pneumothorax, exposed to hypo-
baric hypoxia for 2 hours suggested no significant clinical effects
despite expansion of up to 171%.
105
The data should, however,
be interpreted with caution given the small numbers involved,
the small size of the pneumothorax in each case, and the limited
duration of hypobaric exposure. They are not sufficiently robust
to justify overriding current BTS guidance.
In those who have undergone thoracotomy and surgical
pleurodesis, the recurrence rate is so low that no subsequent
restriction on travel is necessary.
1
The recurrence rate has been
reported to be four times greater after video- assisted thoracos-
copy,
106
suggesting that this procedure may not be as definitive.
The risk of recurrence is greatest in those with pre- existing lung
disease, cigarette smokers and taller men.
1
Clinical practice points
► Passengers should not travel by air until 7 days after full reso-
lution on chest X- ray.
► Those at higher risk of recurrent pneumothorax should be
advised accordingly.
► Higher- risk groups, including those with cystic lung disease
such as LAM and BHD syndrome, should be advised
accordingly.
► Patients with trapped lung and a chronic air space thought
to present a low risk should be evaluated in secondary care
before travel.
Respiratory tract infections
Upper respiratory infection including otitis media and sinusitis
During air travel with acute infection of the upper airway, the
main risks are unpredictable, but may reflect previous experi-
ence. They are of pain and potential rupture of the tympanic
membrane. Those with significant symptomatic viral upper
respiratory tract infection may wish to delay travel because of
the risk of pain and disseminating infection to others.
Barotrauma, characterised by otalgia, is a consequence of
inability to equilibrate the pressure differential between the
external and middle ear. This is usually more severe during
landing than take- off. Most passengers, including older chil-
dren, can equilibrate the pressure through yawning, swallowing,
chewing or a Valsalva manoeuvre (eg, pinching the nose and
blowing). Infants and young children may be unable to perform
these manoeuvres, but swallowing may be encouraged by
13CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
drinking. It is also more frequent when the child is awake and/
or crying.
It has been estimated that 10% of adults and 22% of children
may have changes to the ear drum after a flight, although perfo-
ration is rare and symptoms usually resolve spontaneously.
107 108
Historically, oral (and to a lesser extent topical) decongestants
have been recommended for adults with risk factors for sinus or
middle ear barotrauma.
1
The evidence is very weak, and there
is no evidence in children. Treatment with intranasal steroids
(commenced at least a week before the flight) can however
improve symptoms, as for inflammatory rhinosinusitis. After an
episode of acute otitis media, patients are usually advised not to
fly for 2 weeks
107
Clinical practice points
► In passengers who develop sinus barotrauma after flying, it
may be helpful to consider topical and oral decongestants as
well as appropriate analgesia. Prolonged use of decongest-
ants is not advised owing to the risk of rebound congestion
on withdrawal.
► If there is an allergic component, intranasal steroids used
for a week prior to travel, and/or oral corticosteroids may
be considered.
► Symptoms and signs of barotrauma should have resolved
before flying again. This usually takes between 1 and 6 weeks.
► After an episode of acute otitis media, patients are usually
advised not to fly for 2 weeks.
Viral infections
Although viral infections may be transmitted on board, as in
any environment where people are in proximity for prolonged
periods, available data suggest this is not common on modern
commercial aircraft. This may reflect lack of face- to- face
contact, the barriers afforded by seat backs, and the characteris-
tics of cabin airflow on board, which is not front to back. Viruses
are within the particle size range captured by HEPA filters on
modern commercial aircraft, which are like those used in hospi-
tals. Transmission by droplet spread, including via fomites, is
applicable to all environments.
100 101
This may be reduced by
passengers wearing masks, frequent use of hand sanitiser and
disinfectant wipes for hard surfaces, and by regular deep cleaning
of the aircraft cabin. The risk of infection in airport facilities on
departure, during stopovers, and on arrival should also be consid-
ered. More general hygiene practices, such as handwashing and
covering the mouth and nose when coughing or sneezing, have
also been shown to reduce spread of viral infections.
109 110
Some respiratory viral infections may be more infectious than
others. A review of passengers on a flight carrying a confirmed
case of SARS in 2003 reported 16 cases of SARS developing in
fellow passengers,
111
but it seems likely that affected individuals
were in close proximity in the airport lounge, so transmission
may have occurred before boarding. To date there is just one
reported case of possible aircraft transmission of COVID- 19,
112
but the literature is clearly evolving.
The principal public health concern around air travel is the
role it plays in carrying infected persons (who may be asymp-
tomatic and are not always contagious) long distances within a
short space of time, with the associated risk of disseminating
novel contagious disease to new locations. This has been espe-
cially evident during the COVID- 19 pandemic. Special attention
should therefore be paid to the clearance of people wishing to fly
who have respiratory tract symptoms during outbreaks of such
infections. At any time, and not just during outbreaks of serious
infectious respiratory disease, airport screening measures may be
implemented and travellers with a fever can be refused boarding
by the airline. In cases of serious epidemics and/or pandemics
such as MERS and COVID- 19, even urgent travel may be
prohibited. The Centers for Disease Control and Prevention
website has regular updates on air travel (www.cdc.gov). The
2020 BTS COVID- 19 Statement on Air Travel contains prac-
tical advice for potential passengers with lung disease during the
COVID- 19 pandemic.
113
Clinical practice points
► Patients with highly contagious infections including measles,
chickenpox, mumps, SARS, MERS or COVID- 19 should not
be allowed to travel until they are considered non- infectious.
► Passengers should familiarise themselves with current
national and international regulations regarding air travel,
which should always be observed.
Tuberculosis
WHO provides comprehensive information about the risk of air
travel with TB.
114
Risk is determined by two factors: whether
acid fast bacilli are present on smears of respiratory samples, or
a sputum smear is culture positive; and whether drug resistance
is present. Patients with sputum smear or culture positivity are
considered potentially infectious.
Clinical practice points
► Smear positive patients must not fly until they have provided
two smear negative samples on treatment.
► Those starting treatment for pulmonary TB, where not all
the information is yet available, should not travel by air for
the first 2 weeks.
► For those who are smear negative and have a fully sensitive
organism, treatment would be expected to render them non-
infectious after 2 weeks.
► For patients with MDR/XDR TB, travel is prohibited until
two negative culture samples have been produced and there
is clinical evidence of improvement on treatment.
► Extrapulmonary TB does not usually warrant additional
precautions before air travel.
Pneumonia
Patients suffering from acute lobar bacterial pneumonia present
a low risk to other passengers. They are, however, more likely to
experience in- flight desaturation during flight.
Clinical practice points
► All but essential travel should be postponed for 7 days in
those who have reduced baseline sea level SpO2 (<94%).
OSAS and OHS
The most recent available guidance states that for patients with
OSAS, the potential risks during commercial airline travel are
worsening hypoxaemia when asleep, and exacerbation of jet lag
with potential adverse effects on driving.
1
Data are sparse regarding risks for passengers with OSAS
during air travel. Some data suggest there is a risk of cardiovas-
cular and other adverse events in this group when staying at high
altitude destinations. Hypobaric hypoxia can promote central
apnoeas in addition to obstructive events, which may cause
tachycardia, cardiac arrhythmia and systemic hypertension.
115
It
is not, however, clear how quickly this response develops, and
therefore whether the findings are relevant to air travel.
Using hypobaric chamber simulation testing, studies have
shown that there is an association between hypoxaemia,
decreased sleep time and an increased frequency of hypopneas
14 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
for patients with OSAS who are acutely exposed to high alti-
tude.
116
There are further adverse effects on sleep and OSAS if
alcohol
117
or sedatives
118
are taken.
Many patients with OSAS are already established on CPAP.
Some studies have shown that patients with OSAS have lower
oxygen saturations at baseline and at cabin altitude simulation
than normal subjects.
119
The changes are more marked in those
with severe OSAS. Use of CPAP at altitude is associated with
decreased central sleep apnoea and increased sleep efficiency.
116
Consideration must be given to the whole journey including
the return flight. This is especially important if the flight involves
an overnight element and patients expect to drive the next day.
Studies have identified that not using CPAP for one night during
the flight increases the risk of drowsiness at destination the
following day.
120
Careful planning is required. A retrospective survey of 394
patients who undertook air travel with CPAP reported that over
a third encountered problems with their equipment, power cord,
adapter or transport of the CPAP machine.
121
These findings
highlight the need for clinical teams to understand the logistics
so that they can support safe patient travel (see Appendix A).
In summary, the potential physiological risks for this group
include cardiac stress; increased frequency of hypopnoeas;
possible central apnoeas; hypoxaemia and exacerbation of jet
lag.
There are no data relating specifically to air travel in OHS,
which is considered a restrictive disorder. For these patients,
physicians should refer to guidance around the use of NIV in
those with respiratory muscle and chest wall disorders.
Clinical practice points
► Daytime flights are advised wherever possible.
► The patient should be advised to carry their CPAP device as
hand luggage, and a hospital letter to advise that the patient
uses CPAP.
► Careful planning and preparation are required, and use of
the patient’s own CPAP device is advised.
► Alcohol and sedatives should be avoided in the 12 hours
before, and during, airline travel.
► Patients should use their CPAP device on board if they are
travelling overnight, and avoid sleeping during daytime
flights.
► Consideration should be given to device settings and whether
adjustment is required for operation at altitude.
► Airline approval for carriage and use of device, including
battery specification, must be gained before travel.
► Consideration should be given to the whole journey. If
driving is required the following day, an overnight stay at
destination may be advisable. Patients are advised to refrain
from driving if tired and sleepy.
Respiratory muscle and chest wall disorders
There is little good evidence to guide decision- making around the
need for oxygen or NIV during air travel for patients with severe
extrapulmonary restriction resulting from chest wall disorder
or respiratory muscle weakness. One case report suggests that a
long- haul flight may have precipitated a first episode of PH and
right heart failure requiring intubation and ventilator support in
a man aged 59 with congenital kyphoscoliosis and apparently
stable cardiorespiratory function before travel. FVC was docu-
mented as 0.98 L on recovery.
122
Some work has been conducted to understand which patients
require HCT. The authors of a study of 21 adults with idiopathic
kyphoscoliosis or neuromuscular disease
123
concluded that
those with FVC <1 L, even with resting SpO2 >95%, are likely
to desaturate significantly at cabin altitude. In some restric-
tive conditions, for example, bulbar MND, FVC is difficult to
reproduce. Walk tests may aid decision- making in patients with
scoliosis,
35
but may also be inaccessible to those with MND and
similar conditions where spirometry is a challenge.
Since the 2011 BTS recommendations,
1
several studies have
tried to identify factors that may predict the need for in- flight
oxygen for patients with neuromuscular disease. One study
aimed to identify parameters that predict HCT outcome in 40
patients with MND. Baseline PaCO2 was the only independent
predictor of hypoxaemia during HCT.
124
This appears to be
supported by a more recent study examining baseline PaCO2
as a predictor of HCT outcome.
125
Patients likely to fail HCT
have a higher baseline PaC02, but the authors were unable to
determine an absolute threshold PaC02 value that could identify
patients needing in- flight supplementary oxygen.
Another study in 36 patients with MND examined baseline
lung function as a predictor of hypoxaemia in response to alti-
tude simulation.
126
The authors concluded that maximumin-
spiratory pressure (MIP) and sea level SpO2 may help identify
MND patients who will develop hypoxaemia at altitude. Despite
the small numbers, none of the patients with an MIP >30 mm
Hg or with sea level SpO2 >96% desaturated below 85% during
HCT. Preliminary data from a smaller study of 12 patients with
MND suggested that sniff nasal inspiratory pressure (SNIP) may
more accurately predict the risk of hypoxia during air travel
in those with neuromuscular disease and respiratory muscle
weakness.
127
While evidence to date addresses specific patient groups, the
principles may be applied to any individual with a restrictive
disorder resulting from respiratory muscle weakness or chest
wall deformity. Further research on the value of FVC, PaCO2,
MIP and/or SNIP in predicting HCT outcome in this group is
desirable. In the meantime, it seems reasonable to recommend
that individuals with severe respiratory muscle weakness or
chest wall deformity (FVC <1 L) should undergo HCT before
air travel. Physicians should use their discretion for considering
HCT if there are additional reasons for concern, such as a history
of previous travel intolerance, hypoxaemia or hypercapnia.
In those with respiratory muscle weakness, the possibility
of respiratory failure should also be considered. For patients
established on NIV, further planning and advice are required to
support the use of NIV during flight. If continuous flow oxygen
cannot be provided by the airline or by POC, oxygen and NIV
cannot be used simultaneously. A decision around whether NIV
or supplementary oxygen is of greatest physiological impor-
tance to the patient is then required on an individual basis. The
previous travel history, current clinical condition and the pres-
ence or absence of overnight travel should also be considered.
(see Appendix B, table 2).
In summary, the potential physiological risk for patients with
restrictive respiratory disease is respiratory failure resulting
from inadequate ventilation. It is therefore essential to assess
ventilatory requirements before deciding whether supplemen-
tary oxygen is required. The risk of respiratory failure must be
understood and assessed before travel, and there are currently no
absolute predictors to guide which patients are likely to require
supplementary oxygen.
Clinical practice points
► HCT is recommended for all adult patients with FVC <1 L,
pending further data, and may be considered in others
15CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
thought to be at particular risk, including children with
reduced FVC due to respiratory muscle or chest wall
disorders.
► If patients are unable to perform spirometry reliably, a walk
test may be considered as an alternative.
► Patients should be advised to take daytime flights where
possible.
► Further planning and support are required for those estab-
lished on NIV (see Appendix A).
VTE (DVT and PE)
Prevention of VTE during air travel
The risk of VTE during air travel appears low overall, and
prophylaxis is unnecessary for most travellers. Prolonged travel
(exceeding 6 hours) and/or the coexistence of another risk factor
for VTE increase the risk. The incidence of symptomatic VTE
has been estimated at 0.5% on flights over 12 hours, but asymp-
tomatic rates may be higher.
128–130
The reasons for the increased
risk are not entirely clear. Potential contributory factors include
prolonged immobility and dehydration, but these are not conclu-
sively proven. Other risk factors for VTE such as obesity, recent
surgery, pregnancy, malignancy and previous VTE all increase
the overall risk for travel- related VTE and may necessitate addi-
tional prophylaxis.
General measures, including getting up and walking around
where possible every 2–3 hours; ankle and calf exercises and
avoidance of alcohol or sedating drugs; are advisable for most
travellers. Although there is no conclusive evidence that flying
causes dehydration, the fall in cabin humidity along with alcohol
consumption and reduced fluid intake, may increase the risk on
long haul flights. Remaining well hydrated is, therefore, advis-
able. Wearing graduated compression stockings during travel
may reduce the incidence of deep venous thrombosis.
131
Data
are sparse regarding the method or duration of pharmacolog-
ical prophylaxis, and recommendations rely on consensus expert
opinion. Physicians may wish to recommend pharmacological
prophylaxis for those at higher risk of VTE, for example an
obese patient planning a flight exceeding 6 hours with a history
of recent surgery. The risks of prophylaxis are thought to be low.
There is limited evidence for LMWH as prophylaxis.
132
There is
no formally recommended dose, but enoxaparin 40 mg at a dose
of 40 mg or weight based 1 mg/kg injected once 4–5 hours before
the flight has been suggested.
132
The use of factor Xa inhibitors
is off- licence in this situation and currently has no evidence base.
Clinical practice points
► Limit the risk of dehydration with adequate fluid intake.
► Avoid alcohol.
► Keep mobile, if possible, by walking around or doing seat-
based exercises once an hour.
► Consider graduated compression stockings (class 1 with
15–30 mm Hg).
► LMWH or a DOAC are advised for both outward and return
long haul flights (long haul defined as flights of 6–12 hours)
in high- risk patients including those with a history of VTE;
local policy should be followed regarding liaison with
primary care and/or haematology services to teach the
patient how to administer the injection and dispose safely
of the equipment. There is no formally recommended dose,
but enoxaparin at a dose of 40 mg or weight based 1 mg/kg
injected once 4–5 hours before the flight has been suggested.
► The prophylactic doses of the DOAC may also be used.
► All patients with a recent (<6 weeks) history of VTE, espe-
cially any who presented with significant right ventricular
strain and decompensation should be reassessed before air
travel.
Air travel after VTE
Although the risks of prolonged air travel and development of
VTE are well known, there are fewer data on the risks associated
with flying after a diagnosis of VTE.
A patient with a confirmed diagnosis of PE is highly likely to
start anticoagulation, with the aim of preventing the formation
of new deep venous thrombi and further PEs. There is a general
acceptance that flying immediately after a diagnosis of PE/DVT
should be avoided. It appears reasonable to assume that the
sooner air travel occurs after a PE the greater the likelihood that
hypoxic pulmonary vasoconstriction will exacerbate ventilation-
perfusion mismatch and raise pulmonary pressures, affecting
cardiac output.
Some authors, but not all, suggest that most clots are resolved
after 14–21 days.
133
Consensus opinion is to delay air travel,
if possible, usually for at least 2 weeks, although there are no
concrete data to support a safe time interval. Clearly the risk-
benefit ratio needs to be assessed if more urgent air travel is
needed. Clot resolution depends principally on in vivo fibrino-
lysis. Consideration should be given to the severity of the initial
presentation. It is good practice, before any proposed air travel,
to reassess clinically a patient who has presented with significant
right ventricular strain and decompensation.
The probability of recurrent VTE while anticoagulated is
extremely low. Recovered, stable patients who remain on antico-
agulation should be reassured accordingly and advised to follow
the above general measures.
Clinical practice point
► Air travel should be delayed for 2 weeks after a diagnosis of
DVT or PE.
Pulmonary hypertension
Data are sparse, and recommendations are largely based on
expert consensus opinion. The concern in PH is the risk of
hypoxia causing increased pulmonary arterial pressure and right
ventricular strain. Most studies have employed HCT. They have
shown that most patients with PH can tolerate this degree of
hypoxia with minor increases in dyspnoea.
134
Furthermore, the
effect on the right ventricle in one study has been shown to be
minimal.
134
However, most of these studies only covered a short
Table 2 Clinical practice points: Hypoxic challenge test (HCT)
outcomes
PaO
2
≥ 6.6 kPa (≥ 50 mm Hg);
SpO
2
≥ 85%
In- flight oxygen not required
PaO
2
< 6.6 kPa (<50 mm Hg);
SpO
2
<85%
Repeat HCT with oxygen (flow rate and modality
ideally the same as that to be given on board
aircraft):
► Titrate oxygen to maintain PaO
2
≥ 6.6 kPa or
SpO
2
≥ 85%
► Monitor pH and pCO
2
if there is a history of
hypercapnia
Consider advising against air travel if pH falls to
<7.35 and PCO
2
increases by > 1 kPa (7.5 mm Hg)
from baseline when PaO
2
is maintained at ≥ 6.6
kPa or SpO
2
≥85%
The use of arterial or capillary blood gas sampling is preferred if available. Blood
gases are not routinely measured in children.
Recommendations on pCO
2
and pH changes are based on the 2015 BTS guideline
for home oxygen use in adults.
19
16 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
time period.
134
Longer exposure to hypoxia on long haul flights
may have more significant effects.
One study has monitored patients during commercial
flights.
135
This showed that up to a quarter of patients with
PH desaturate during short haul flights, with higher altitude,
ambulation and longer flights correlating with desaturations.
Most tolerated this well, with fewer than 40% of participants
reporting symptoms. A larger questionnaire based retrospective
study has also confirmed that in most patients with stable PAH,
flight is well tolerated with minimal clinical effects.
136
Around
half those surveyed travelled with supplementary oxygen.
Hypoxia reduces exercise capability; breathing oxygen-
enriched air improves exercise capacity.
134
It therefore appears
logical to give patients with impaired functional capacity supple-
mental oxygen on board the aircraft. Whether patients should
have oxygen while walking around as well as when sitting is
unknown; ambulatory oxygen on board presents obvious logis-
tical challenges.
The 2011 BTS Recommendations advised that patients in
NYHA WHO functional class 3 or 4 should have supplemental
oxygen during air travel.
1
This recommendation is pragmatic
rather than evidence based; and may result in over- prescribing
of in- flight oxygen. One study suggests that more than double
the number of patients would be recommended in- flight oxygen
based on functional class rather than HCT outcome.
134
If HCT is not readily available and there are no concerns about
hypercapnia, passengers already on LTOT should be advised that
they will need a flow rate 2 L/min greater than their baseline
flow rate. This should be sufficient to compensate for the rela-
tive hypoxia at normal cabin altitude.
Clinical practice points
► Those in NYHA WHO functional class 3 or 4 are usually
advised to have in- flight oxygen. If there is no evidence of
hypercapnia it seems reasonable to suggest 2 L/min by nasal
cannulae. If there are concerns about hypercapnia, HCT
should be considered if available.
► Those eligible for LTOT (sea level PaO
2
<8 kPa at rest on
air) should have in flight oxygen at double the flow rate
recommended at sea level, provided there is no evidence of
hypercapnia.
Lung cancer and mesothelioma
When evaluating those with lung cancer or mesothelioma it is
important to consider the nature and extent of their condition as
well as their treatment. A pragmatic approach is to evaluate their
risk of haemorrhage, pneumothorax, pleural effusion, VTE and
any recent surgical and/or bronchoscopic interventions.
If taking medication, and particularly controlled drugs,
patients must be aware of the appropriate documentation
required for the countries they are visiting. Advance- planning
is essential.
Clinical practice point
► Patients undergoing chemotherapy should not travel while
they are at increased risk of infection or suffering from
significant side effects, such as vomiting.
Hyperventilation and DB
There are few data on the implications of functional breathing
disorders for air travel, whether DB, VCD or ILO. Asthma should
not be overlooked as a possible association in those with DB.
137
Acute shortness of breath is one of several symptoms for which
flight diversion is advised.
138
Diversions are costly, typically
ranging from £10 000–£80 000 depending on aircraft size and
diversion destination.
139
Dyspnoea caused by DB or hyperven-
tilation is unlikely to have serious clinical consequences; but
it must be distinguished from dyspnoea attributable to life-
threatening acute medical conditions such as acute coronary
syndrome or PE.
1 140
Data from the last two decades suggest that 65% of in- flight
medical emergencies were due to exacerbations of pre- existing
conditions and that respiratory problems were most common;
half were due to asthma or ‘asthma- like’ presentations.
141–143
Air travel can be stressful. Physiological or psychological stress
may precipitate acute breathlessness in patients with respiratory
disease.
144
Acute hyperventilation can be a response to stress
independent of lung pathology, usually in those with known
panic and anxiety disorders.
145
Hyperventilation can cause bronchoconstriction resulting in
‘asthma- like’ symptoms
146
which are unresponsive to standard
asthma medication.
147 148
A perception that the usual ‘rescue’
medication is ‘not working’ may worsen an individual’s breathing
pattern, causing concern to them, other passengers and air crew.
Similar situations can arise with ILO or VCD, and onset of symp-
toms is often sudden.
149
Where DB is linked to respiratory conditions, particularly
asthma, national and international guidelines endorse breathing
exercise programmes provided by a specialist respiratory physio-
therapist as an adjuvant to pharmacological treatment. Patients
should be advised to use breathing techniques in situations where
breathlessness may become problematic.
75 150
ILO and VCD,
which can present with acute respiratory distress and stridor,
may be treated with breathing exercises taught by a respiratory
physiotherapist or a speech therapist with specialist expertise in
paradoxical vocal cord movement.
151
Paper bag rebreathing is no longer recommended, because
inspired oxygen concentration decreases sufficiently to endanger
hypoxic patients. There are data reinforcing that significant
harm to patients can result from acute myocardial infarction,
pneumothorax and PE being misdiagnosed as hyperventilation.
152
153
Clinical practice points
► Patients with DB, ILO and/or VCD should be referred to a
Respiratory Physiotherapy Specialist for advice on symptom
management before travel.
► Those with anxiety disorders should be reviewed before
travel; compliance with medication assessed; and use of
short acting anxiolytics encouraged.
► Other life- threatening conditions presenting with dyspnoea
should be excluded on board as far as possible.
► Supplemental oxygen should be given on board if the cause
of breathlessness is unclear.
► Rebreathing via a paper bag is not recommended.
Appendix A Logistics of air travel with equipment
Oxygen
Passengers requiring oxygen and travelling overseas will usually
need to lease a POC privately, since UK companies do not gener-
ally allow equipment provided through the NHS to be taken
out of the country. Furthermore, most airlines have moved away
from supplying routine medical oxygen.
Where battery powered mechanical devices are required
in- flight, including a POC, sufficient batteries should be carried
for 1.5 times the anticipated duration of the journey to cope
with delays or diversions. For example, a patient using a POC
on a 4- hour flight should have 6 hours of battery life. Any spare
17CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
batteries must be correctly packaged and should be carried in
cabin baggage. The airline must be notified in advance of these
plans, or airline staff can refuse to allow the equipment to be
taken on board.
Pulse- dose oxygen may not be suitable for patients with a fast
and shallow respiratory pattern, or during sleep.
154 155
Pulse-
dose settings do not equate to the equivalent continuous flow
rates,
74
and not every POC functions well at altitude.
156
In
contrast, pulse- dose oxygen functions reliably when provided
by a cylinder and conserving device.
156
One author found
significantly lower PaO
2
values when using a POC, compared
with compressed oxygen with a conserving device. Acceptable
in- flight values are achievable with POCs, but the dose may need
to be increased.
56
Pulse- dose delivery systems can complicate determination of
the flow delivered; and may not be well tolerated. The effects
of mouth- breathing, speech, snoring and/or sleeping should be
considered. HFNO cannot be delivered on board commercial
aircraft.
Pulse- dose oxygen has not been studied in infants and chil-
dren; and should not be used unless they have been shown to
trigger the device’s inspiratory flow.
Currently available POCs that do supply continuous flow
oxygen cannot provide flow rates above 3 L/min. Passengers
must refer to POC documentation to check that the equipment
meets their requirements before they lease it for air travel.
For all these reasons, assessments would ideally take place
using the same equipment as that which will be used on board
the aircraft. This is only likely to be possible when the patient
has leased or purchased a POC for their own long term, private
use.
Continuous positive airway pressure
Few airlines, if any, allow any medical device to be powered via
the aircraft power supply. An appropriate battery must, there-
fore, be used. The device and battery specifications must be
approved for use by the airline before travel. Battery perfor-
mance should be checked by the user beforehand, so there is an
understanding of operating times on their usual settings.
Further consideration needs to be given to CPAP use during
flight and at high altitude destinations, as it requires a machine
that will perform adequately at low ambient pressure. The 2011
BTS guidance
1
reported that a fixed- pressure CPAP machine
without pressure compensation, set to deliver a pressure of
12 cm H
2
O at sea level, may deliver only 9 cm H
2
O at 8000
ft. The machine may therefore require adjustment to ensure a
safe level of treatment throughout the flight. If continuous flow
oxygen cannot be provided by the airline or by POC, oxygen and
CPAP cannot be used simultaneously. The availability of distilled
water for humidifiers may be restricted.
Non-invasive ventilation
For patients established on NIV, further planning and advice
are required to support the use of NIV during flight. If contin-
uous flow oxygen cannot be provided by the airline or by POC,
oxygen and NIV cannot be used simultaneously. A decision
around whether NIV or supplementary oxygen is of greatest
physiological importance to the patient is then required on an
individual basis. Previous travel history, current clinical condi-
tion and the presence or absence of overnight travel should also
be considered. The level of clinical and personal dependency
must be considered in the context of requirements for trained
supervision and assistance by the caregiver.
Appendix B Quick reference guide for respiratory
physiologists
Most patients with respiratory conditions are able to fly safely
without any additional support. The following guide provides
specific information for respiratory physiologists regarding
patients who do need further investigation before embarking on
air travel. See figure 3.
HCT methods
The HCT uses an inspired gas mixture containing 15% oxygen,
which gives an approximate similar PO
2
to breathing air at
the maximum allowable cabin pressure altitude (2438 m or
8000ft).
53 54
HCT is usually performed in a specialist respiratory
physiology unit. The provision of a 15% oxygen gas mixture can
be achieved as follows:
► A premixed cylinder containing a 15% oxygen gas mixture
can be obtained from medical gas providers, or Douglas bags
can be mixed with air and nitrogen to reduce the percentage
of inspired oxygen to 15%, both to supply a tight- fitting face
mask in a closed circuit.
1
► Pure nitrogen can be introduced into a sealed chamber such
as a body plethysmograph for paediatric or mask- intolerant
patients, removing the need for a face mask.
17
Paediatric
patients can be sat in a body plethysmograph on an adult’s
lap throughout;
1
the adult should also undergo SpO
2
moni-
toring to avoid excessive hypoxaemia. A body box is gener-
ally used for children, although some paediatric laboratories
use masks
► A 40% Venturi oxygen mask can be used with pure nitrogen
as the driving gas, giving a resultant gas mixture containing
approximately 15% oxygen.
11 58
► A hypoxic gas generator, like an oxygen concentrator, can
be used to provide a continuous supply of variable hypoxic
gas mixtures to supply a mask or closed chamber. This can
be the most cost- effective method for centres with a high
demand for HCT.
12
The patient usually breathes the hypoxic gas mixture for
20 min, or until SpO
2
reaches 85%. Although this is shorter
than the briefest commercial flight, oxygenation equilibrium is
usually reached within this time.
134 157
The patient is advised to
have in- flight oxygen if PaO
2
falls below 6.6 kPa (<50 mm Hg)
or SpO
2
remains <85%
1 17
(see page 11).
HCT with oxygen
If PaO
2
or SpO
2
values meet the criteria, in- flight oxygen is
recommended.
1
The flow rate required can be assessed as part
of the HCT.
17
Previous BTS recommendations advised in- flight
oxygen to be supplied at two or 4 L/min via nasal cannulae,
which were for many years the only fixed flow rates routinely
available on commercial aircraft. As airline- supplied in- flight
oxygen becomes less common and greater numbers of patients
travel with flight- approved POCs delivering a wide range of
continuous and intermittent flow rates, these figures are less crit-
ical. The HCT should ideally be performed with the modality
that is intended for use in- flight.
Most airlines have moved away from supplying routine medical
oxygen. In- flight oxygen is thus now likely to be supplied by
an FAA approved POC, leased by the patient. These are mostly
pulse- dose delivery. It is, therefore, advisable to conduct a titrated
HCT with pulsed dose oxygen to maintain PaO
2
at ≥6.6 kPa or
SpO2 ≥85%, using setting 2 as the starting point. This approx-
imates to the 2 L/min originally stated. If pulse- dose oxygen at
higher settings is insufficient to maintain PaO
2
at ≥6.6 kPa or
18 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
SpO2 ≥85%, then continuous oxygen should be considered. It
should be noted that POC models supplying continuous flow are
limited, and they do not currently supply >3 L/min.
Pulse- dose delivery is not suitable for young children, for use
during sleep
154 155
or for certain adults. Not all POCs function
as expected under conditions of simulated altitude
156
and pulse-
dose settings may not equate to equivalent continuous flow rates
74
(see Appendix A).
Patients with hypercapnia
Previous BTS advice was to err on the side of recommending
oxygen if in doubt,
1
and other authors have recommended
doubling oxygen flow rates for patients with a pre- existing
oxygen requirement.
1 29
However, there is a potential risk of
developing hypercapnia and respiratory acidosis from oxygen
during HCT in patients with type 2 respiratory failure.
18
Patients
with a history of hypercapnia should ideally undergo HCT with
blood gas sampling. In these cases, the minimum amount of
oxygen should be delivered to maintain PaO
2
≥6.6 kPa while
monitoring PaCO
2
and pH. If blood gas sampling is not available
then care should be taken not to raise SpO
2
above the resting
level with supplementary oxygen in this group of patients .
17
In
some cases it may be unsafe to undertake air travel even if good
oxygenation can be achieved, if adverse PCO
2
and pH changes
are evident.
18
Figure 3 HCT for physiologists.
19CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
An incidental finding of an elevated COHb during HCT
represents an important opportunity to take a smoking history
and offer smoking cessation referral as appropriate.
Twitter Robina Kate Coker @RobinaCoker1
Acknowledgements The assistance of the British Thoracic Society Standards of
Care Committee is gratefully acknowledged. The Society and the Air Travel Clinical
Statement Group is also grateful to the organisations that provided feedback as part
of the consultation process.
Contributors RKC was the lead author responsible for the final document. All
authors agreed the outline and content of the document and authored sections of
the document.
Funding The authors have not declared a specific grant for this research from any
funding agency in the public, commercial or not- for- profit sectors.
Disclaimer A Clinical Statement reflects the expert views of a group of
specialists who are well versed in the topic concerned, and who carefully
examine the available evidence in relation to their own clinical practice. A Clinical
Statement does not involve a formal evidence review and is not developed in
accordance with clinical practice guideline methodology. Clinical Statements
are not intended as legal documents or a primary source of detailed technical
information. Readers are encouraged to consider the information presented and
reach their own conclusions.
Competing interests AA declared funding from Fisher Paykel and Breas. CC
declared funding from Pfizer, GSK, Janssen, MSD. SH declared funding from Astra
Zeneca, GSK, Roche, Chiesi, Trudell, Boehringer Ingelheim, Mylan, Teva.
Patient consent for publication Not applicable.
Provenance and peer review Not commissioned; externally peer reviewed.
Open access This is an open access article distributed in accordance with the
Creative Commons Attribution Non Commercial (CC BY- NC 4.0) license, which
permits others to distribute, remix, adapt, build upon this work non- commercially,
and license their derivative works on different terms, provided the original work is
properly cited, appropriate credit is given, any changes made indicated, and the use
is non- commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
REFERENCES
1 Ahmedzai S, Balfour- Lynn IM, Bewick T, etal. Managing passengers with stable
respiratory disease planning air travel: British thoracic Society recommendations.
Thorax 2011;66 Suppl 1:i1–30.
2 British Thoracic Society Standards of Care Committee. Managing passengers with
respiratory disease planning air travel: British thoracic Society recommendations.
Thorax 2002;57:289–304.
3 Coker RK. Managing passengers with respiratory disease planning air travel: British
thoracic Society recommendations: British thoracic Society, 2004. Available: www.
brit-thoracic.org.uk
4 Coker RK, Shiner RJ, Partridge MR. Is air travel safe for those with lung disease? Eur
Respir J 2007;30:1057–63.
5 Federal Aviation Administration. Federal Aviation Administration. pressurized cabins
section 25.841. Washington DC: FAA, 2006. www.ecfr.gov
6 CAA. Guidance for health professionals: physiology of flight: civil aviation authority,
2020. Available: https://www.caa.co.uk/Passengers/Before-you-fly/Am-I-fit-to-fly/
Guidance-for-health-professionals/Physiology-of-flight/
7 EASA. Easy access rules for large aeroplanes (CS- 25) (Amendment 21), 2018.
Available: https://www.easa.europa.eu/document-library/easy-access-rules/easy-
access-rules-large-aeroplanes-cs-25
8 Aerospace Medical Association. Cabin cruising altitudes for regular transport aircraft.
Aviat Space Environ Med 2008;79:433–9.
9 ARTP. ARTP workforce and staffing survey and toolkit 2018, 2018. Available: https://
www.artp.org.uk/Reports/a5056393-cb7b-401a-bd4a-c114b383fc55
10 Peterson DC, Martin- Gill C, Guyette FX, etal. Outcomes of medical emergencies on
commercial airline flights. N Engl J Med 2013;368:2075–83.
11 Vohra KP, Klocke RA. Detection and correction of hypoxemia associated with air
travel. Am Rev Respir Dis 1993;148:1215–9.
12 Spurling KJ, Zammit C, Lozewicz S. Mains- powered hypoxic gas generation: a cost-
effective and safe method to evaluate patients at risk from hypoxia during air travel.
Thorax 2011;66:731–2.
13 Health effects of airline cabin environments in simulated 8- Hour flights. Aerosp Med
Hum Perform 2017;88:651–6.
14 ICAO. Manual of Civil Aviation Medicine. International Civil Aviation Organization
2012;2012.
15 NICE. Chronic obstructive pulmonary disease in over 16S: diagnosis and
management. London: NICE, 2018Contract No.: NG115.
16 Tzani P, Pisi G, Aiello M, etal. Flying with respiratory disease. Respiration
2010;80:161–70.
17 Cramer D, Ward S, Geddes D. Assessment of oxygen supplementation during air
travel. Thorax 1996;51:202–3.
18 Spurling KJ, Moonsie IK, Perks JL. Hypercapnic respiratory acidosis during an In- Flight
oxygen assessment. Aerosp Med Hum Perform 2016;87:144–7.
19 Hardinge M, Annandale J, Bourne S, etal. British thoracic Society guidelines for
home oxygen use in adults. Thorax 2015;70 Suppl 1:i1–43.
20 Zhao J, Gonzalez F, Mu D. Apnea of prematurity: from cause to treatment. Eur J
Pediatr 2011;170:1097–105.
21 Samuels MP. The effects of flight and altitude. Arch Dis Child 2004;89:448–55.
22 Bossley CJ, Cramer D, Mason B, etal. Fitness to fly testing in term and ex- preterm
babies without bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed
2012;97:F199–203.
23 Naqvi N, Doughty VL, Starling L, etal. Hypoxic challenge testing (fitness to fly) in
children with complex congenital heart disease. Heart 2018;104:1333–8.
24 Loo S, Campbell A, Vyas J, etal. Case report of a hypobaric chamber fitness to fly
test in a child with severe cystic lung disease. Pediatrics 2017;140. doi:10.1542/
peds.2016-2376. [Epub ahead of print: 08 06 2017].
25 Aerospace Medical Assocation. Medical Guidelines for Air Travel. In: Aviation, Space
and Environmental Medicine. . 2nd Edition, 2003: 74. 1–19.
26 Resnick SM, Hall GL, Simmer KN, etal. The hypoxia challenge test does not
accurately predict hypoxia in flight in ex- preterm neonates. Chest 2008;133:1161–6.
27 Josephs LK, Coker RK, Thomas M, etal. Managing patients with stable respiratory
disease planning air travel: a primary care summary of the British thoracic Society
recommendations. Prim Care Respir J 2013;22:234–8.
28 Robson AG, Lenney J, Innes JA. Using laboratory measurements to predict in- flight
desaturation in respiratory patients: are current guidelines appropriate? Respir Med
2008;102:1592–7.
29 Ergan B, Akgun M, Pacilli AMG, etal. Should I stay or should I go? COPD and air
travel. Eur Respir Rev 2018;27:180030.
30 Edvardsen A, Akerø A, Christensen CC, etal. Air travel and chronic obstructive
pulmonary disease: a new algorithm for pre- flight evaluation. Thorax
2012;67:964–9.
31 Mohr LC. Hypoxia during air travel in adults with pulmonary disease. Am J Med Sci
2008;335:71–9.
32 Nicholson TT, Sznajder JI. Fitness to fly in patients with lung disease. Ann Am Thorac
Soc 2014;11:1614–22.
33 Christensen CC, Ryg M, Refvem OK, etal. Development of severe hypoxaemia in
chronic obstructive pulmonary disease patients at 2,438 M (8,000 ft) altitude. Eur
Respir J 2000;15:635–9.
34 Robson AG, Hartung TK, Innes JA. Laboratory assessment of fitness to fly in patients
with lung disease: a practical approach. Eur Respir J 2000;16:214–9.
35 Ling IT, Singh B, James AL, etal. Vital capacity and oxygen saturation at rest and
after exercise predict hypoxaemia during hypoxic inhalation test in patients with
respiratory disease. Respirology 2013;18:507–13.
36 IATA. IATA Medical Manual2018.
37 Ergan B, Nava S. Long- Term oxygen therapy in COPD patients who do not meet the
actual recommendations. COPD 2017;14:351–66.
38 Chetta A, Castagnetti C, Aiello M, etal. Walking capacity and fitness to fly in
patients with chronic respiratory disease. Aviat Space Environ Med 2007;78:789–92.
39 Kelly PT, Swanney MP, Seccombe LM, etal. Air travel hypoxemia vs. the hypoxia
inhalation test in passengers with COPD. Chest 2008;133:920–6.
40 Brown CD, Wise RA. Field tests of exercise in COPD: the six- minute walk test and the
shuttle walk test. COPD 2007;4:217–23.
41 du Bois RM, Weycker D, Albera C, etal. Six- minute- walk test in idiopathic pulmonary
fibrosis: test validation and minimal clinically important difference. Am J Respir Crit
Care Med 2011;183:1231–7.
42 Holland AE, Dowman L, Fiore J, etal. Cardiorespiratory responses to 6- minute walk
test in interstitial lung disease: not always a submaximal test. BMC Pulm Med
2014;14:136.
43 Lancaster LH. Utility of the six- minute walk test in patients with idiopathic
pulmonary fibrosis. Multidiscip Respir Med 2018;13:45.
44 Bandyopadhyay D, Oscroft NS, Shneerson JM, etal. Is there an alternative
to pre- flight hypoxic challenge testing in scoliotic patients? Respir Med
2010;104:1566–70.
45 Edvardsen A. Air travel and COPD: exercise SPO2 and walking distance as predictors
for in- flight desaturation. European Respiratory Journal 2011;38 https://erj.
ersjournals.com/content/38/Suppl_55/p3477.article-info
46 British Airways. Information sheet for passengers requiring medical clearance
(MEDIF), 2012. Available: http://www.britishairways.com/cms/global/pdfs/health/
BA_Medif_Oct2012.pdf
47 Civil Aviation Authority. Information for health care professionals on assessing
fitness to fly, 2019. Available: https://www.caa.co.uk/Passengers/Before-you-fly/Am-
I-fit-to-fly/Guidance-for-health-professionals/Assessing-fitness-to-fly/
48 Bradi AC, Faughnan ME, Stanbrook MB, etal. Predicting the need for supplemental
oxygen during airline flight in patients with chronic pulmonary disease: a comparison
of predictive equations and altitude simulation. Can Respir J 2009;16:119–24.
49 Ali M, Smith IE, Gulati A, etal. Pre- flight assessment in patients with obesity
hypoventilation syndrome. Respirology 2014;19:1229–32.
20 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
50 Schwartz JS, Bencowitz HZ, Moser KM. Air travel hypoxemia with chronic obstructive
pulmonary disease. Ann Intern Med 1984;100:473–7.
51 Akerø A, Christensen CC, Edvardsen A, etal. Pulse oximetry in the preflight
evaluation of patients with chronic obstructive pulmonary disease. Aviat Space
Environ Med 2008;79:518–24.
52 Hussein K, Farouk Alkarn A, El- Sokkary R, etal. Factors predicting exercise- induced
oxygen desaturation in stable COPD. Egypt J Chest Dis Tuberc 2013;62:59–63.
53 Civil Aviation Authority. Cap 393 the air navigation order 2016 and regulations,
2016. Available: http://publicapps.caa.co.uk/modalapplication.aspx?catid=1&appid=
11&mode=detail&id=7523
54 Far code of US federal regulations Washington DC: Federal Aviation Administration,
2019. Available: https://www.ecfr.gov/cgi-bin/text-idx?SID=82d757ba11a578e0
4151050a42c48741&mc=true&tpl=/ecfrbrowse/Title14/14cfrv1_02.tpl#0
55 Gong H, Tashkin DP, Lee EY, etal. Hypoxia- altitude simulation test. evaluation of
patients with chronic airway obstruction. Am Rev Respir Dis 1984;130:980–6.
56 Akerø A, Edvardsen A, Christensen CC, etal. Copd and air travel: oxygen equipment
and preflight titration of supplemental oxygen. Chest 2011;140:84–90.
57 Dillard TA, Moores LK, Bilello KL, etal. The preflight evaluation. A comparison of the
hypoxia inhalation test with hypobaric exposure. Chest 1995;107:352–7.
58 Dine CJ, Kreider ME. Hypoxia altitude simulation test. Chest 2008;133:1002–5.
59 Gradwell DP. Hypoxia and hyperventilation. In: Ernsting’s Aviation Medicine. 4th ed,
2006.: 41–56.
60 Agusti A, Soriano JB. Dynamic hyperinflation and pulmonary inflammation: a
potentially relevant relationship? European Respiratory Review 2006;15:68–71.
61 Dillard TA, Rajagopal KR, Slivka WA, etal. Lung function during moderate hypobaric
hypoxia in normal subjects and patients with chronic obstructive pulmonary disease.
Aviat Space Environ Med 1998;69:979–85.
62 Howard LS. Last call for the flight simulation test? Eur Respir J 2013;42:1175–7.
63 Soffler MI, Hayes MM, Schwartzstein RM. Respiratory sensations in dynamic
hyperinflation: physiological and clinical applications. Respir Care 2017;62:1212–23.
64 Luks AM, Swenson ER. Travel to high altitude with pre- existing lung disease. Eur
Respir J 2007;29:770–92.
65 Murphy R, Driscoll P, O’Driscoll R. Emergency oxygen therapy for the COPD patient.
Emerg Med J 2001;18:333–9.
66 Beasley R, Chien J, Douglas J, etal. Thoracic Society of Australia and New Zealand
oxygen guidelines for acute oxygen use in adults: ’Swimming between the flags’.
Respirology 2015;20:1182–91.
67 Beachey W. Pulmonary Blood Flow. In: Respiratory care anatomy and physiology:
foundations for clinical practice. Elsevier, 2017: 115.
68 Chen W, Thomas J, Sadatsafavi M, etal. Risk of cardiovascular comorbidity in
patients with chronic obstructive pulmonary disease: a systematic review and meta-
analysis. Lancet Respir Med 2015;3:631–9.
69 Hammadah M, Kindya BR, Allard- Ratick MP, etal. Navigating air travel and
cardiovascular concerns: is the sky the limit? Clin Cardiol 2017;40:660–6.
70 Berg BW, Dillard TA, Derderian SS, etal. Hemodynamic effects of altitude exposure
and oxygen administration in chronic obstructive pulmonary disease. Am J Med
1993;94:407–12.
71 Edvardsen A, Ryg M, Akerø A, etal. Copd and air travel: does hypoxia-
altitude simulation testing predict in- flight respiratory symptoms? Eur Respir J
2013;42:1216–23.
72 Edvardsen A, Akerø A, Hardie JA, etal. High prevalence of respiratory symptoms
during air travel in patients with COPD. Respir Med 2011;105:50–6.
73 Seccombe LM, Kelly PT, Wong CK, etal. Effect of simulated commercial flight
on oxygenation in patients with interstitial lung disease and chronic obstructive
pulmonary disease. Thorax 2004;59:966–70.
74 Chatburn RL, Williams TJ. Performance comparison of 4 portable oxygen
concentrators. Respir Care 2010;55:433–42.
75 BTS/SIGN. British guideline on the management of asthma. SIGN 2019. July 2019.
76 Spurling KJ, McGoldrick VPBlood- Injection- Injury (B- I- I) specific phobia affects the
outcome of hypoxic challenge testing. Aerosp Med Hum Perform 2017;88:503–6.
77 Akerø A, Christensen CC, Edvardsen A, etal. Hypoxaemia in chronic
obstructive pulmonary disease patients during a commercial flight. Eur Respir J
2005;25:725–30.
78 Naughton MT, Rochford PD, Pretto JJ, etal. Is normobaric simulation of hypobaric
hypoxia accurate in chronic airflow limitation? Am J Respir Crit Care Med
1995;152:1956–60.
79 Edvardsen E, Akerø A, Skjønsberg OH, etal. Pre- flight evaluation of adult patients
with cystic fibrosis: a cross- sectional study. BMC Res Notes 2017;10:84.
80 Johnson AOC. Chronic obstructive pulmonary disease * 11: fitness to fly with COPD.
Thorax 2003;58:729–32.
81 Nesthus T, Garner R SM. Effects of simulated General aviation altitude hypoxia on
smokers and nonsmokers. Washington DC: Office of Aviation Medicine, 1997.
82 Cummins RO, Schubach JA. Frequency and types of medical emergencies among
commercial air travelers. JAMA 1989;261:1295–9.
83 Dowdall N. "Is there a doctor on the aircraft?" Top 10 in- flight medical emergencies.
BMJ 2000;321:1336–7.
84 George PM, Orton C, Ward S, etal. Hypoxic challenge testing for fitness to fly with
severe asthma. Aerosp Med Hum Perform 2016;87:571–4.
85 Peña- Zarza JA, Osona B, Sailer S, etal. Assessing hypoxia risk during air travel
after a severe asthma exacerbation in children. Ann Allergy Asthma Immunol
2017;119:389–90.
86 Liu AH, Jaramillo R, Sicherer SH, etal. National prevalence and risk factors for food
allergy and relationship to asthma: results from the National health and nutrition
examination survey 2005- 2006. J Allergy Clin Immunol 2010;126:e13:798–806.
87 Bock SA, Muñoz- Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to
foods. J Allergy Clin Immunol 2001;107:191–3.
88 Greenhawt M, MacGillivray F, Batty G, etal. International study of risk- mitigating
factors and in- flight allergic reactions to peanut and tree nut. J Allergy Clin Immunol
Pract 2013;1:186–94.
89 Vanfleteren LEGW, Spruit MA, Groenen M, etal. Clusters of comorbidities
based on validated objective measurements and systemic inflammation in
patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med
2013;187:728–35.
90 Sabit R, Thomas P, Shale DJ, etal. The effects of hypoxia on markers of coagulation
and systemic inflammation in patients with COPD. Chest 2010;138:47–51.
91 Bertoletti L, Quenet S, Mismetti P, etal. Clinical presentation and outcome of venous
thromboembolism in COPD. Eur Respir J 2012;39:862–8.
92 Børvik T, Brækkan SK, Enga K, etal. Copd and risk of venous thromboembolism and
mortality in a general population. Eur Respir J 2016;47:473–81.
93 Hirche TO, Bradley J, d’Alquen D, etal. Travelling with cystic fibrosis:
recommendations for patients and care team members. J Cyst Fibros
2010;9:385–99.
94 Cogo A, Fischer R, Schoene R. Respiratory diseases and high altitude. High Alt Med
Biol 2004;5:435–44.
95 Buchdahl RM, Babiker A, Bush A, etal. Predicting hypoxaemia during flights in
children with cystic fibrosis. Thorax 2001;56:877–9.
96 Christensen CC, Ryg MS, Refvem OK, etal. Effect of hypobaric hypoxia on blood
gases in patients with restrictive lung disease. Eur Respir J 2002;20:300–5.
97 Martin SE, Bradley JM, Buick JB, etal. Flight assessment in patients with
respiratory disease: hypoxic challenge testing vs. predictive equations. QJM
2007;100:361–7.
98 Barratt SL, Shaw J, Jones R, etal. Physiological predictors of hypoxic challenge
testing (hCT) outcomes in interstitial lung disease (ILD). Respir Med 2018;135:51–6.
99 Tam A, Singh P, Ensor JE, etal. Air travel after biopsy- related pneumothorax: is it safe
to fly? J Vasc Interv Radiol 2011;22:595–602.
100 Currie GP, Kennedy A- M, Paterson E, etal. A chronic pneumothorax and fitness to fly.
Thorax 2007;62:187–9.
101 Pollock- BarZiv S, Cohen MM, Downey GP, etal. Air travel in women with
lymphangioleiomyomatosis. Thorax 2007;62:176–80.
102 Johannesma PC, van de Beek I, van der Wel JWT, etal. Risk of spontaneous
pneumothorax due to air travel and diving in patients with Birt- Hogg- Dubé
syndrome. Springerplus 2016;5:1506.
103 MacDuff A, Arnold A JH. Bts pleural disease guideline 2010: management of
spontaneous pneumothorax. Thorax 2010;65.
104 Zonies D, Elterman J, Burns C, etal. Trauma patients are safe to fly 72 hours after
tube thoracostomy removal. J Trauma Acute Care Surg 2018;85:491–4.
105 Majercik S, White TW, Van Boerum DH, etal. Cleared for takeoff: the effects of
hypobaric conditions on traumatic pneumothoraces. J Trauma Acute Care Surg
2014;77:729–33.
106 Barker A, Maratos EC, Edmonds L, etal. Recurrence rates of video- assisted
thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces:
a systematic review of randomised and non- randomised trials. Lancet
2007;370:329–35.
107 Wright T. Middle- Ear pain and trauma during air travel. BMJ Clin Evid 2015;2015.
[Epub ahead of print: 19 Jan 2015].
108 Stangerup SE, Tjernström O, Klokker M, etal. Point prevalence of barotitis in
children and adults after flight, and effect of autoinflation. Aviat Space Environ Med
1998;69:45–9.
109 Biswas D, Ahmed M, Roguski K, etal. Effectiveness of a behavior change
intervention with hand sanitizer use and respiratory hygiene in reducing Laboratory-
Confirmed influenza among schoolchildren in Bangladesh: a cluster randomized
controlled trial. Am J Trop Med Hyg 2019;101:1446–55.
110 Bin Abdulrahman AK, Bin Abdulrahman KA, Almadi MK, etal. Do various personal
hygiene habits protect us against influenza- like illness? BMC Public Health
2019;19:1324.
111 Olsen SJ, Chang H- L, Cheung TY- Y, etal. Transmission of the severe acute respiratory
syndrome on aircraft. N Engl J Med 2003;349:2416–22.
112 Eldin C, Lagier J- C, Mailhe M, etal. Probable aircraft transmission of Covid- 19
in- flight from the central African Republic to France. Travel Med Infect Dis
2020;35:101643.
113 British thoracic Society: points to consider when planning air travel by passengers
with respiratory disease during the endemic phase of COVID- 19 London: BTS, 2020.
Available: https://www.brit-thoracic.org.uk/about-us/covid-19-resumption-and-
continuation-of-respiratory-services/#planning-air-travel/
114 WHO. Tuberculosis and air travel: guidelines for prevention and control. 3rd edition.
Geneva: WHO, 2013.
21CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from

BTS Clinical Statement
115 Bloch KE, Latshang TD, Ulrich S. Patients with obstructive sleep apnea at altitude.
High Alt Med Biol 2015;16:110–6.
116 Nishida K, Lanspa MJ, Cloward TV, etal. Effects of positive airway pressure on
patients with obstructive sleep apnea during acute ascent to altitude. Ann Am
Thorac Soc 2015;12:1072–8.
117 Issa FG, Sullivan CE, Alcohol SCE. Alcohol, snoring and sleep apnea. J Neurol
Neurosurg Psychiatry 1982;45:353–9.
118 Liistro G, Aubert G, Rodenstein DO. Management of sleep apnoea syndrome. Eur
Respir J 1995;8:1751–5.
119 Bazurto Zapata MA, Dueñas Meza E, Jaramillo C, etal. Sleep apnea and oxygen
saturation in adults at 2640 m above sea level. Sleep Sci 2014;7:103–6.
120 Kribbs NB, Pack AI, Kline LR, etal. Effects of one night without nasal CPAP treatment
on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis
1993;147:1162–8.
121 Bodington R, Johnson O, Carveth- Johnson P, etal. Travel with CPAP machines: how
frequent and what are the problems? J Travel Med 2018;25.
122 Noble JS, Davidson JA. Cor pulmonale presenting in a patient with congenital
kyphoscoliosis following intercontinental air travel. Anaesthesia 1999;54:361–3.
123 Mestry N, Thirumaran M, Tuggey JM, etal. Hypoxic challenge flight assessments
in patients with severe chest wall deformity or neuromuscular disease at risk for
nocturnal hypoventilation. Thorax 2009;64:532–4.
124 Crawford E, Stone H, Cliff I. Hypoxic challenge testing in motor neurone disease.
European Respiratory Journal 2015;46 https://scholar.google.co.uk/citations?
view_op=view_citation&hl=en&user=TOYV2rAAAAAJ&citation_for_view=
TOYV2rAAAAAJ:WF5omc3nYNoC
125 Cliff I, Mustfa N, Stone H. Hypoxic challenge testing in motor neurone disease.
Thorax 2017;72.
126 Brazzale D, Ruehland W, Howard M. Resting lung function may predict the response
to altitude simulation in motor neuron disease. Respirology 2017;22.
127 Humphreys J, Agarwal S, Cliff I, etal. P131 fitness to fly assessment in patients with
neuromuscular disease. Thorax 2010;65:A133–4.
128 Pérez- Rodríguez E, Jiménez D, Díaz G, etal. Incidence of air travel- related
pulmonary embolism at the Madrid- Barajas Airport. Arch Intern Med
2003;163:2766–70.
129 Scurr JH, Machin SJ, Bailey- King S, etal. Frequency and prevention of
symptomless deep- vein thrombosis in long- haul flights: a randomised trial. Lancet
2001;357:1485–9.
130 WHO. Who reseaerch into global hazards of travel (Wright) project, 2007. Available:
https://www.who.int/cardiovascular_diseases/wright_project/phase1_report/
WRIGHT%20REPORT.pdf
131 Clarke MJ, Broderick C, Hopewell S, etal. Compression stockings for preventing
deep vein thrombosis in airline passengers. Cochrane Database Syst Rev
2016;9:CD004002.
132 Cesarone MR, Belcaro G, Nicolaides AN, etal. Venous thrombosis from air travel: the
LONFLIT3 study--prevention with aspirin vs low- molecular- weight heparin (LMWH)
in high- risk subjects: a randomized trial. Angiology 2002;53:1–6.
133 Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis
1975;17:259–70.
134 Burns RM, Peacock AJ, Johnson MK, etal. Hypoxaemia in patients with pulmonary
arterial hypertension during simulated air travel. Respir Med 2013;107:298–304.
135 Roubinian N, Elliott CG, Barnett CF, etal. Effects of commercial air travel on
patients with pulmonary hypertension air travel and pulmonary hypertension. Chest
2012;142:885–92.
136 Thamm M, Voswinckel R, Tiede H, etal. Air travel can be safe and well tolerated in
patients with clinically stable pulmonary hypertension. Pulm Circ 2011;1:239–43.
137 Heaney LG, Robinson DS. Severe asthma treatment: need for characterising patients.
Lancet 2005;365:974–6.
138 Ruskin KJ, Hernandez KA, Barash PG. Management of in- flight medical emergencies.
Anesthesiology 2008;108:749–55.
139 Civil Aviation Authority. Disruptive passengers, 2019. Available: https://www.caa.co.
uk/Passengers/On-board/Disruptive-passengers/
140 Goodwin T. In- flight medical emergencies: an overview. BMJ 2000;321:1338–41.
141 Shesser R. Medical aspects of commercial air travel. Am J Emerg Med
1989;7:216–26.
142 Gong H, Mark JA, Cowan MN. Preflight medical screenings of patients. Analysis of
health and flight characteristics. Chest 1993;104:788–94.
143 Kay RS. Safe air travel. preventing in- flight medical problems. Nurse Pract 1994;19
144 Balkissoon R, Kenn K. Asthma: vocal cord dysfunction (VCD) and other dysfunctional
breathing disorders. Semin Respir Crit Care Med 2012;33:595–605.
145 Matsumoto K, Goebert D. In- flight psychiatric emergencies. Aviat Space Environ Med
2001;72:919–23.
146 Bruton A, Holgate ST. Hypocapnia and asthma: a mechanism for breathing
retraining? Chest 2005;127:1808–11.
147 Barker N, Everard ML. Getting to grips with ’dysfunctional breathing’. Paediatr Respir
Rev 2015;16:53–61.
148 Porsbjerg C, Menzies- Gow A. Co- Morbidities in severe asthma: clinical impact and
management. Respirology 2017;22:651–61.
149 Bahrainwala AH, Simon MR. Wheezing and vocal cord dysfunction mimicking
asthma. Curr Opin Pulm Med 2001;7:8–13.
150 GINA. Global initiative for asthma, 2019. Available: https://ginasthma.org/
151 Parsons JP, Benninger C, Hawley MP, etal. Vocal cord dysfunction: beyond severe
asthma. Respir Med 2010;104:504–9.
152 Callaham M. Hypoxic hazards of traditional paper bag rebreathing in
hyperventilating patients. Ann Emerg Med 1989;18:622–8.
153 Kern B. Hyperventilation syndrome, 2016. Available: https://emedicine.medscape.
com/article/807277-print
154 Chen JZ, Katz IM, Pichelin M, etal. Comparison of pulsed versus continuous oxygen
delivery using realistic adult nasal airway replicas. Int J Chron Obstruct Pulmon Dis
2017;12:2559–71.
155 Khor YH, McDonald CF, Hazard A, etal. Portable oxygen concentrators versus oxygen
cylinder during walking in interstitial lung disease: a randomized crossover trial.
Respirology 2017;22:1598–603.
156 Fischer R, Wanka ER, Einhaeupl F, etal. Comparison of portable oxygen
concentrators in a simulated airplane environment. Respir Med 2013;107:147–9.
157 Sei K, Fujita M, Okawa S, etal. Appropriate timing of blood sampling for blood gas
analysis in the ventilated rabbit. J Surg Res 2016;206:325–36.
22 CokerRK, etal. Thorax 2022;0:1–22. doi:10.1136/thoraxjnl-2021-218110
on February 28, 2022 by guest. Protected by copyright.http://thorax.bmj.com/Thorax: first published as 10.1136/thoraxjnl-2021-218110 on 28 February 2022. Downloaded from
