
COGNITION
SYSTEMS ENGINEERING
TEMPLATE KITS FOR
SMALL ENTERPRISES
JANUARY 21, 2016

COGNITION CORP
© 2016
Background
• Supported large enterprises who directed our work
• Provided a tool kit allowing users to create their world
• They had procedures, process, work instructions, SOPs
• Small enterprises saw little value in a world builder tool
• Startup CEO told me “Just throw me a lifeline”
• Further discussions revealed the need for a lean approach
• We needed to have a pre built compliance world for them
• We needed to guide them through the steps to compliance
How I came to my epiphany

COGNITION CORP
© 2016
Large v Small Enterprises
• All enterprises must bring their product “to compliance”
• Large enterprises have tribal knowledge
• Large enterprises have rigorous(?) processes in place
• Large enterprises have big teams, including systems engineers
• Small enterprises have minimal formal processes
• Small enterprises have limited staffs and budgets
• Small enterprises do not have “time” for early action
• Small enterprises need to move fast to survive/be purchased
Important differences in needs

COGNITION CORP
© 2016
Why Template Kits
Enabling a systems engineering approach to compliance
Small enterprises do not have the sophistication to “go it alone”
• Checklists for regulatory compliance needs
• Applicable standards
• Self guided templates for product development activities
• Auto generation of compliance outputs
• Secure SaaS: lean IT, no user setup, no user maintenance
• Validated for each enterprise, not by each enterprise
• “FDA Early” approach; can support Early Feasibility Studies
• Online regulatory advisor for product development
• No special training or ramp up period; “speed to compliance”

COGNITION CORP
© 2016
Checklists
• Series of questions about the system/device
• Answers trigger standards that apply
• Generates template kits
• Completing kits results in compliance outputs/artifacts
• Can be used standalone or with template kits
• Can be independent, not coupled with only one software tool
• Cognition is engaged in this work now
• Open to working with INCOSE or independent people
Guide users to applicable standards and deliverables

COGNITION CORP
© 2016
EXAMPLE: DESIGN CONTROLS
• Design Controls Template kits exist to allow users to easily begin
capturing requirement, verification test, and validation test traces in
accordance with the requirements of 21 CRF 820.30.
• The Template kits alone do not ensure total compliance:
• They do not include the complete procedures that detail the design and
development process(es). These are added by users.
• They do not include custom workflow to support access rights, change
management, electronic signatures or version control. This can be
added by users.
• They can be configured/modified to suit existing procedures and
processes as required by users.
• NOTE: wherever you see “by users” implies opportunity for INCOSE or
consultants to engage with small enterprises.

COGNITION CORP
© 2016
DESIGN CONTROLS TRACE MATRIX
Key deliverable is automatically generated by the template kit

COGNITION CORP
© 2016
• Out of the box, the Template kit
has the structure as shown in
the screenshot.
• Inside each directory is a
document/set of documents
which have been pre-configured
to easily facilitate the capture of
requirements and tests along
with their full traceability
according to the requirements of
21 CFR 820.30.
DESIGN CONTROLS PROJECT TEMPLATE
OUT OF THE BOX

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain plans that describe or reference the design
and development activities and define responsibility for implementation. The plans shall
identify and describe the interfaces with different groups or activities that provide, or result
in, input to the design and development process. The plans shall be reviewed, updated, and
approved as design and development evolves.
REQUIREMENTS
§ 820.30(b) Design and development planning.
• Each manufacturer shall establish and maintain plans that describe or reference the
design and development activities and define responsibility for implementation.
• The plans shall identify and describe the interfaces with different groups or activities that
provide, or result in, input to the design and development process.
• The plans shall be reviewed, updated, and approved as design and development
evolves.
PART (b) - DESIGN AND DEVELOPMENT PLANNING

COGNITION CORP
© 2016
• The Template kit includes a
document for the Design and
Development Plan (DDP). This
document is completed by users
early in the process.
• It is the responsibility of the
users to review, update, and
approve as design and
development evolves.
DESIGN AND DEVELOPMENT PLAN
PART (b) - DESIGN AND DEVELOPMENT PLANNING

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain procedures to ensure that the design requirements
relating to a device are appropriate and address the intended use of the device, including the needs of
the user and patient. The procedures shall include a mechanism for addressing incomplete,
ambiguous, or conflicting requirements. The design input requirements shall be documented and shall
be reviewed and approved by a designated individual(s). The approval, including the date and
signature of the individual(s) approving the requirements, shall be documented.
REQUIREMENTS
§ 820.30(c) Design input.
• Each manufacturer shall establish and maintain procedures to ensure that the design requirements
relating to a device are appropriate and address the intended use of the device, including the needs
of the user and patient.
• The procedures shall include a mechanism for addressing incomplete, ambiguous, or conflicting
requirements.
• The design input requirements shall be documented and shall be reviewed and approved by
designated individual(s).
• The approval, including the date and signature of the individual(s) approving the requirements, shall
be documented.
PART (c) - DESIGN INPUT

COGNITION CORP
© 2016
• The Template kit includes pre-
configured documents for
capturing and linking the User
Needs and Design Inputs.
• The sections can be renamed, re-
ordered and deleted as required.
• The User Needs are validatable
requirements and the Design
Inputs are verifiable requirements.
• It is the responsibility of the user to
review, update, and approve the
User Needs and Design Inputs.
USER NEEDS AND DESIGN INPUTS DOCUMENTS
PART (c) - DESIGN INPUT

COGNITION CORP
© 2016
• The Template kit includes a pre-
configured document for capturing and
linking the lower level requirements that
drive Design Outputs.
• Engineering Requirements at any level
can link to a Design Output.
• Engineering Requirements can also link to
the Engineering Requirements at the next
level.
• Only Level 1 Engineering Requirements
can link to a Design Input.
• The Engineering Requirements can be
verifiable requirements if required.
• It is the responsibility of the user to review,
update, and approve the Engineering
Requirements.
DESIGN ACTIVITY DOCUMENT
PART (c) - DESIGN INPUT

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain procedures for defining and documenting
design output in terms that allow an adequate evaluation of conformance to design input
requirements. Design output procedures shall contain or make reference to acceptance criteria
and shall ensure that those design outputs that are essential for the proper functioning of the
device are identified. Design output shall be documented, reviewed, and approved before
release. The approval, including the date and signature of the individual(s) approving the output,
shall be documented.
REQUIREMENTS
§ 820.30(d) Design output.
• Each manufacturer shall establish and maintain procedures for defining and documenting design output
in terms that allow an adequate evaluation of conformance to design input requirements.
• Design output procedures shall contain or make reference to acceptance criteria and shall ensure that
those design outputs that are essential for the proper functioning of the device are identified.
• Design output shall be documented, reviewed, and approved before release.
• The approval, including the date and signature of the individual(s) approving the output, shall be
documented.
PART (d) – DESIGN OUTPUT

COGNITION CORP
© 2016
• The Template kit includes a pre-
configured document for capturing
and linking the Design Outputs.
• The sections can be renamed, re-
ordered and deleted as required.
• The Design Outputs are verifiable
requirements.
• It is the responsibility of the user to
review, update, and approve the
Design Outputs.
DESIGN OUTPUTS DOCUMENT
PART (d) - DESIGN OUTPUT

COGNITION CORP
© 2016
• The Template kit includes a pre-
configured document for displaying
a number of traces.
• The sections can be renamed, re-
ordered and deleted as required.
• New sections showing new tables
can be added as required.
• It is the responsibility of the user to
review, update, and approve the
Design Trace Matrix document.
REQUIREMENTS –TRACE MATRICES
USEFUL TOOLS

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews
of the design results are planned and conducted at appropriate stages of the device's design
development. The procedures shall ensure that participants at each design review include
representatives of all functions concerned with the design stage being reviewed and an individual(s)
who does not have direct responsibility for the design stage being reviewed, as well as any specialists
needed. The results of a design review, including identification of the design, the date, and the
individual(s) performing the review, shall be documented in the design history file (the DHF).
REQUIREMENTS
§ 820.30(e) Design review.
• Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of
the design results are planned and conducted at appropriate stages of the device's design development.
• The procedures shall ensure that participants at each design review include representatives of all
functions concerned with the design stage being reviewed and an individual(s) who does not have direct
responsibility for the design stage being reviewed, as well as any specialists needed.
• The results of a design review, including identification of the design, the date, and the individual(s)
performing the review, shall be documented in the design history file (the DHF).
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
• The Template kit includes a pre-
configured document template for
both formal and informal design
reviews.
• The Template also includes a pre-
configured log of all design
reviews.
• It is the responsibility of the user to
perform, review, update, and
approve any Design Reviews.
DESIGN REVIEWS
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
• The Information section allows basic information about the review to be
captured.
• Out of the box the Information section automatically records the project
name as well as giving fields for capturing the date and location of the
review, however, this table can be modified by users to show different
information as desired.
DESIGN REVIEW DOCUMENT TEMPLATE –INFORMATION
SECTION
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
• The Attendees section allows all review attendees to be captured. New
Attendees can be added using the ‘Add item here…’ prompt.
• Out of the box the Attendees section shows the name, job title and the role
of the attendee, however, this table can be altered by users to show
different information as required.
DESIGN REVIEW DOCUMENT TEMPLATE –ATTENDEES
SECTION
PART (e) - DESIGN REVIEW
COGNITION CORP
© 2016
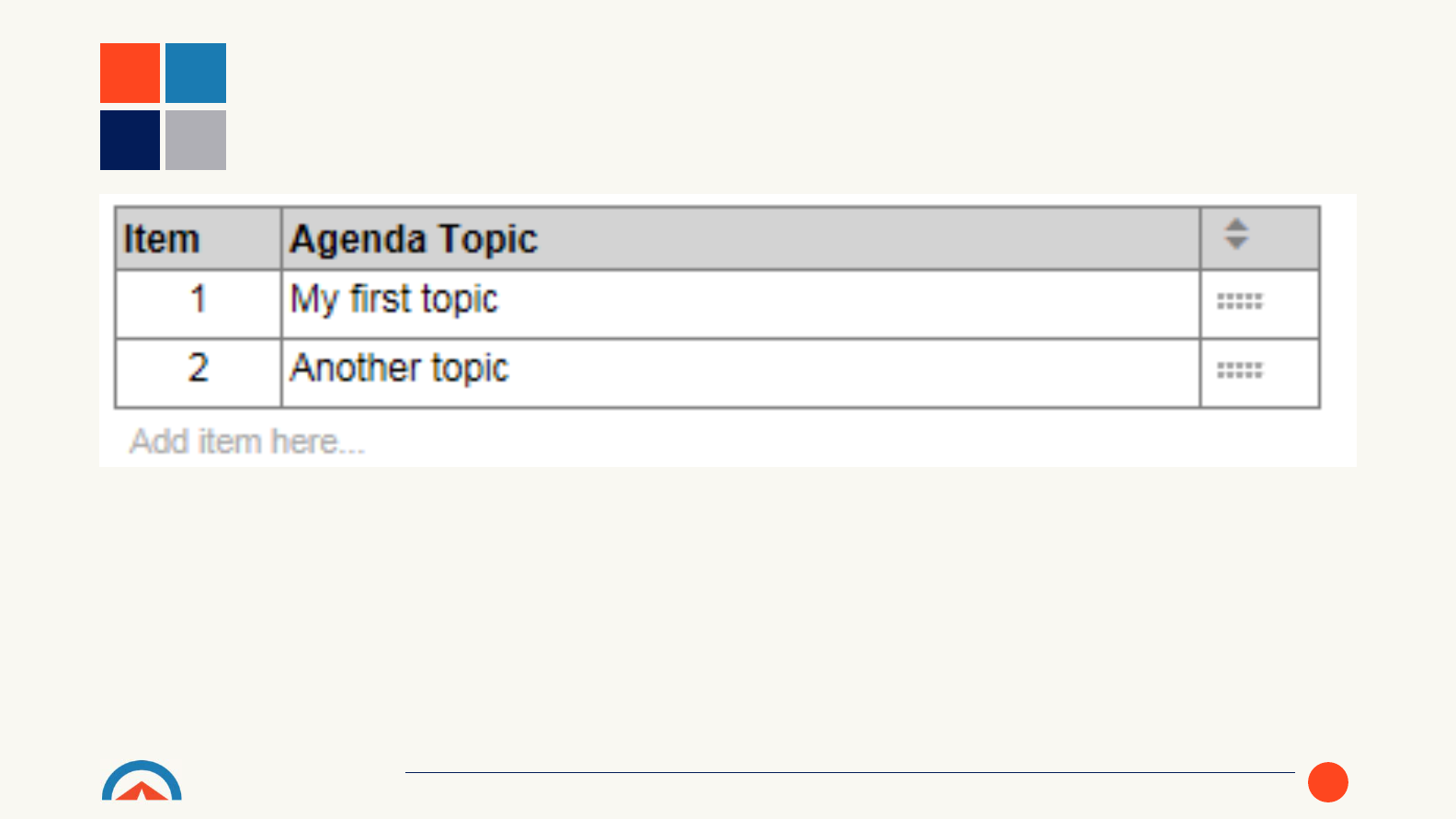
• The Agenda section allows all the topics to be discussed during the review
to be captured. New Agenda Topics can be added using the ‘Add item
here…’ prompt.
• Out of the box the Agenda section shows the item number and agenda
topic, however, this table can be altered by users to show different
information as required.
DESIGN REVIEW DOCUMENT TEMPLATE –AGENDA SECTION
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
• The Item(s) Under Review section shows both internal template kit
documents and external attachments which are part of the review.
• Internal template kit documents can be added using the ‘Add Internal kit
Document(s) to Review’ view (available on the Item(s) Under Review
section) using the picker.
• External attachments can be added using the ‘Add External Attachment(s)
to Review’ view (available on the Item(s) Under Review section) using the
link.
• Out of the box the Item(s) Under Review section shows the name of the
item, however, this table can be altered by users to show different
information as required.
DESIGN REVIEW DOCUMENT TEMPLATE –ITEM(S) UNDER
REVIEW SECTION
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
• The Minutes section allows the minutes for all the agenda topics to be
captured and Action Items to be created.
• Out of the box the Minutes section shows the name of the agenda item(s),
the minutes for each agenda item and any associated action items,
however, this table can be altered by users to show different information as
required.
DESIGN REVIEW DOCUMENT TEMPLATE –MINUTES SECTION
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
• The Design Review Log is a record of all the formal and informal
reviews created.
• Out of the box the table shows the review document name, the
agenda items (as identified in the review document), the last
modified date of the review document and all items that were
reviewed (as identified in the review document), however, this table
can be altered by users to show different information as required.
DESIGN REVIEW LOG
PART (e) - DESIGN REVIEW

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain procedures for verifying the device design.
Design verification shall confirm that the design output meets the design input requirements.
The results of the design verification, including identification of the design, method(s), the
date, and the individual(s) performing the verification, shall be documented in the DHF.
REQUIREMENTS
§ 820.30(f) Design verification.
• Each manufacturer shall establish and maintain procedures for verifying the device
design.
• Design verification shall confirm that the design output meets the design input
requirements.
• The results of the design verification, including identification of the design, method(s), the
date, and the individual(s) performing the verification, shall be documented in the Design
History File.
PART (f) - DESIGN VERIFICATION

COGNITION CORP
© 2016
• Verification Tests can be created/linked using the ‘Add item here…’ prompt
or picker on the ‘Create/Link Verification Tests’ view (in either the Design
Inputs, Design Activity or Design Outputs documents) and are
automatically displayed in a table.
• Out of the box the table shows the requirement ID, requirement name,
acceptance criteria, attachments and the verification test ID and name,
however, this table can be altered by users to show different information as
required.
VERIFICATION TEST CREATION
PART (f) - DESIGN VERIFICATION

COGNITION CORP
© 2016
• The Template kit includes pre-
configured documents to record
the verification test results for
Design Inputs, Design Activities
and Design Outputs.
• It is the responsibility of the user to
perform, review, update, and
approve all verification test results.
VERIFICATION TEST SUMMARY DOCUMENTS
PART (f) - DESIGN VERIFICATION

COGNITION CORP
© 2016
• The results of Verification Tests can be recorded using the
Verification Summary documents.
• Out of the box the table shows the test ID, test name, requirement
ID, requirement name and results, however, this table can be altered
by users to show different information as required.
VERIFICATION TEST SUMMARY
PART (f) - DESIGN VERIFICATION

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain procedures for validating the device design. Design validation shall
be performed under defined operating conditions on initial production units, lots, or batches, or their equivalents.
Design validation shall ensure that devices conform to defined user needs and intended uses and shall include
testing of production units under actual or simulated use conditions. Design validation shall include software
validation and risk analysis, where appropriate. The results of the design validation, including identification of the
design, method(s), the date, and the individual(s) performing the validation, shall be documented in the DHF.
REQUIREMENTS
§ 820.30(g) Design validation.
• Each manufacturer shall establish and maintain procedures for validating the device design.
• Design validation shall be performed under defined operating conditions on initial production units, lots, or batches, or their
equivalents.
• Design validation shall ensure that devices conform to defined user needs and intended uses and shall include testing of
production units under actual or simulated use conditions.
• Design validation shall include software validation and risk analysis, where appropriate.
• The results of the design validation, including identification of the design, method(s), the date, and the individual(s) performing
the validation, shall be documented in the Design History File.
PART (g) - DESIGN VALIDATION

COGNITION CORP
© 2016
• Validation Tests can be created/linked using the ‘Add item here…’ prompt
or picker on the ‘Create/Link Validation Tests’ view (in the User Needs
document) and are automatically displayed in a table.
• Out of the box the table shows the requirement ID, requirement name,
acceptance criteria, attachments and the validation test ID and name,
however, this table can be altered by users to show different information as
required.
VALIDATION TEST CREATION
PART (f) - DESIGN VALIDATION

COGNITION CORP
© 2016
• The Template includes a pre-
configured document to record the
validation test results for User
Needs.
• It is the responsibility of the user to
perform, review, update, and
approve all validation test results.
VALIDATION TEST SUMMARY DOCUMENTS
PART (f) - DESIGN VALIDATION

COGNITION CORP
© 2016
• The results of Validation Tests can be recorded using the Validation
Summary document.
• Out of the box the table shows the test ID, test name, requirement ID,
requirement name and results, however, this table can be altered by users
to show different information as required.
VALIDATION TEST SUMMARY
PART (f) - DESIGN VALIDATION

COGNITION CORP
© 2016
21 CFR 820.30
Each manufacturer shall establish and maintain a DHF for each type of device. The
DHF shall contain or reference the records necessary to demonstrate that the
design was developed in accordance with the approved design plan and the
requirements of this part.
REQUIREMENTS
§ 820.30(j) Design history file.
• Each manufacturer shall establish and maintain a DHF for each type of device.
• The DHF shall contain or reference the records necessary to demonstrate that the design
was developed in accordance with the approved design plan and the requirements of this
part.
PART (j) - DESIGN HISTORY FILE
COGNITION CORP
© 2016
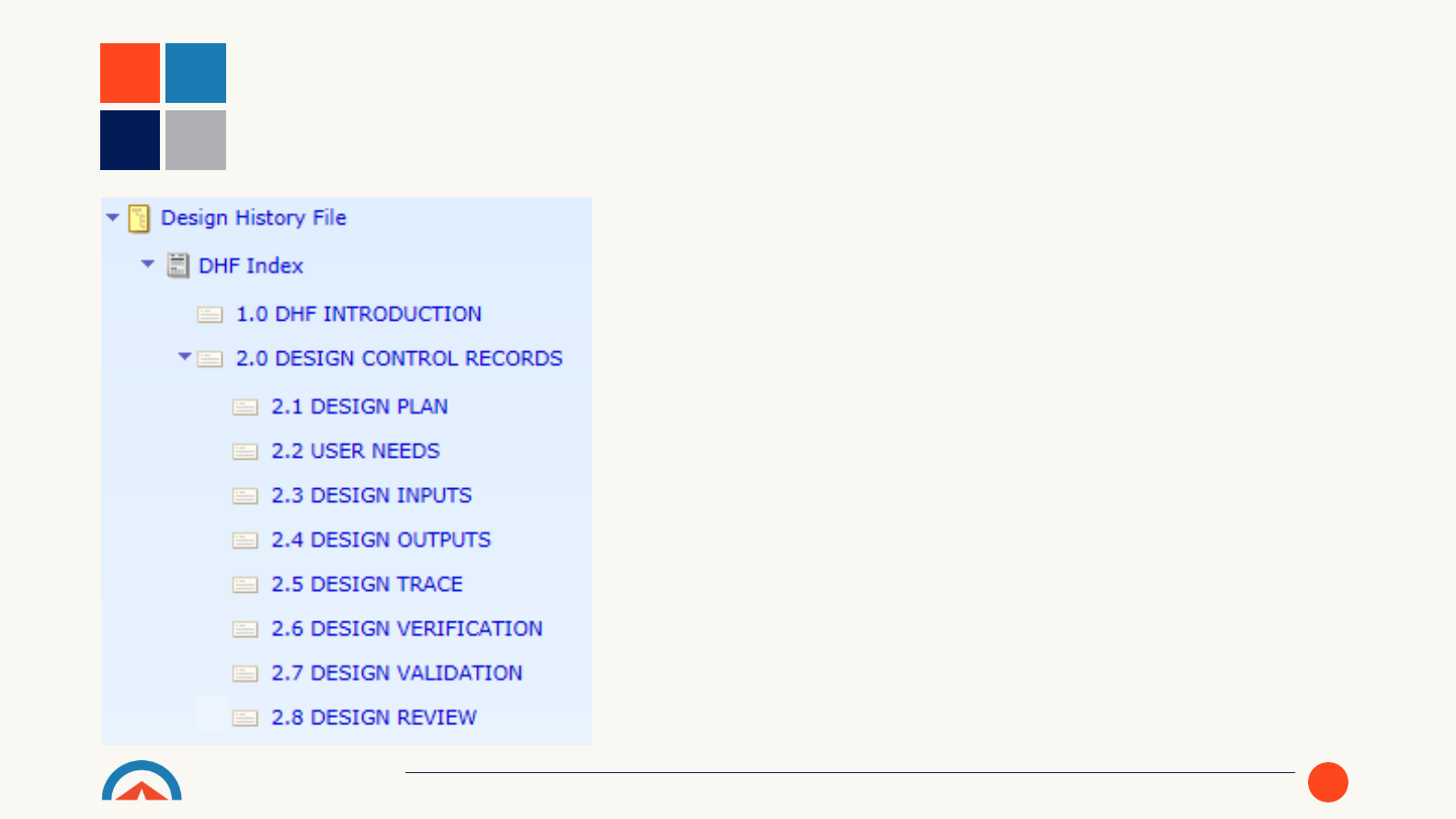
• The Template includes a pre-
configured document to index the
included content for the Design
History File.
• It is the responsibility of the user to
perform, review, update, and
approve all content of the Design
History File.
DESIGN HISTORY FILE CREATION
PART (j) - DESIGN HISTORY FILE

COGNITION CORP
© 2016
DESIGN CONTROLS TRACE MATRIX
Automatically generated by the template kit; non editable

COGNITION CORP
© 2016
Partnership Potential
• Cognition is pursuing this approach right now
• A better approach is a combined effort with INCOSE and others
• Cognition provides the SaaS platform but not all of the expertise
• Checklists will identify key compliance deliverables
• Templates will ensure automatic, accurate, rapid deliverables
• Which systems engineering tools should be included?
• MBSE? How appropriate for small enterprises?
• Device, drug, combination, software, hardware, etc.
• US v EU, FDA v Notified Bodies
• Goal: FDA accepts automated deliverables through online submissions
INCOSE, Industry, and Consultants/SMEs
