
Stephanie Chisolm:
Early stage tumors in non-muscle invasive bladder cancer, or NMIBC, are generally confined to the lining
of the bladder, and they may be papillary and look a little bit like tiny finger-like clusters or flat, velvety
patches known as carcinoma in situ or CIS. Tumors that are CIS have a very high rate of recurrence and
possible disease progression, so it's really helpful to understand your diagnosis. Tonight, BCAN is
welcoming urologist Peter Black from the Vancouver General Hospital in Canada and Pathologist Dr.
Hikmat Al-Ahmadie from Memorial Sloan Kettering Cancer Center in New York City. We're delighted to
have you here to help us talk about CIS and what patients need to know. So welcome to both of you. I'm
going to turn my video off, and Dr. Black, if you want to take it away.
Dr. Peter Black:
Terrific. Thank you, Stephanie, very much
for the invitation and the opportunity. I
think I can speak for Hikmat as well, that
we're both passionate about bladder
cancer and so we're always very happy to
explain what we know and educate, so
hopefully the attendees tonight will find
this interesting. Hikmat and I are going to
go back and forth a little bit. I'll start and
then we'll switch to Hikmat and we'll carry
on like that. What is carcinoma in situ?
Stephanie alluded to it a little bit at the
beginning. We have this broad spectrum of
disease stages for bladder cancer from the
very superficial on the left and carcinoma
in situ is also called TIS. It's just right on the
surface of the bladder, to tumors that

actually extend into the lumen of the bladder but still sit on the surface. That's the Ta just next to that.
Then, the T1 tumors that are already invading the first layer of the bladder wall. Those three together
are all non-muscle invasive, but there's a difference between actually invasive T1 and the other two that
are really non-invasive. And then on the right, we have the muscle-invasive, which we're not going to
talk about this evening.
Dr. Peter Black:
There's special things about carcinoma
in situ that make it really worth having
a dedicated session just on this. So it's
a flat tumor, there's no tumor
extending into the lumen of the
bladder. It's often red and velvety, but
sometimes it's invisible, so we struggle
to see it in a lot of patients. Although
it's an early stage of bladder cancer, it
is high grade. By definition, it's always
high grade and therefore, it has a
significant malignant potential. It has
the potential to develop into muscle-
invasive bladder cancer, so even
though it's early stage, we take it very
seriously and we treat it quite
aggressively because we need to
eradicate it. One of the problems that
we also struggle with is that this
transition from a non-invasive,
superficial carcinoma in situ into a
potentially invasive muscle-invasive
tumor is very unpredictable.
Some other key features is that it's
often... if it's found together with the
other non-muscle invasive bladder
cancer stages, so Ta and T1, it indicates
a higher risk of recurrence and
progression. The fact that the cancer
could come back or it can turn into
something more invasive if there's
carcinoma in situ there. It's an
additional risk factor in patients who
have other disease stages.
Carcinoma in situ we assume is
multifocal, so it's at different locations
in the bladder, or even diffuse,
meaning anywhere you would sample
the bladder wall, you would find
carcinoma in situ. That's a very

important and special feature. The other tumors generally we consider to be confined to what we see
and what we resect. Because it's multifocal and diffuse, we general believe that we cannot resect it
completely with a transurethral resection, which again, is different from the other bladder tumors that
typically we can resect at least all visible disease.
Dr. Peter Black:
Since we can't resect it, we either need to treat it with intravesical therapy such as BCG, or we need to
remove the bladder if we really want to get rid of it all. That's why it's really tricky and a bit different
than the other bladder tumors.
I'm going to present a patient profile,
and then Hikmat and I will go back
and forth a little bit about some of the
issues related to this patient's bladder
cancer. So this patient is an 87-year-
old gentleman, retired chartered
accountant, who has been a longtime
smoker, more than 50 pack/year
history of smoking. He was found just
on a regular check with his primary
care provider to have red blood cells
in his urine under microscopy. He's
never seen blood himself, so there's
no gross hematuria, just microscopic
hematuria. He has symptoms that are
common for his age. Nothing
particularly severe or noteworthy,
extreme. He gets up a couple times at
night. There's some urgency to void.
He has very significant other health
issues. He has heart disease, diabetes,
high blood pressure, and he is even on home oxygen because he has emphysema also related to his
smoking history. One thing that is relatively clear when we look at this gentleman is that he's probably
not going to be fit from a general health point of view to undergo radical cystectomy to remove the
bladder. We're not necessarily at that point yet, but I just wanted to highlight that in his medical history.
His family doctor, based on the microhematuria and the risk factors, sent his urine for urine cytology.
Can you go back for a second? The diagnosis of the urine cytology is high grade urothelial carcinoma.
Hikmat's going to run us through a little bit what that means.
Dr. Hikmat Al-Ahmadie:
All right. Thank you, Peter for the lead-in and hello everyone. Thanks for tuning in and spending the
evening with us. It's my pleasure to share this hour with you and talk to you about urothelial carcinoma
in situ and different aspects of the disease, and hopefully you'll find it helpful. As the story started, a
quick test is urine sample. It's a very easy type of specimen to get. It just requires the urine sample that
you may get in the clinic or the office. It is sent to the pathology department. It's spun down, just to
concentrate the cells in that container, and then it will give us a better opportunity to detect any
atypical cells in there, and when slides are prepared, the tissue is stained, then we'll be able to have the

ability to visualize cells that can tell us what they constitute, they're benign or reactive or abnormal cells,
and we follow a system. There is a system.
These type of specimens have been
studied for a long time and there
are criteria that we apply every time
we analyze these type of samples.
And then we follow the most recent
system is the Paris System, and it
has different categories and every
case, every urine sample that goes
to the lab, the result come back
with one of these categories. Of
course the red flag would be, or
when we highly suspect a urothelial
carcinoma is in these two categories
that are in bold now, the number
four and five. Either we see some
atypical cells that are atypical
enough to suspect urothelial
carcinoma but there are not as
many of them as you would want.
The cutoff has been made as 10
cells. If you see more than 10 cells
that are very atypical, and I'm going to show in some of these features what we mean by atypical in the
next slide, these are what you say suspicious. When you see these atypical cells in a quantity that is high
enough, and as I said, this is typically more than 10 cells, then you can render the diagnosis of high grade
urothelial carcinoma.
Dr. Hikmat Al-Ahmadie:
During cytology, you will be not able to say in situ or papillary because most of the times, that
designation requires more architectural assessment rather than individual cells, so I'm going to show
you example how the urothelial carcinoma cells in the urine may look like.
Yes, so this is a high magnification image from a urine cytology specimen taken from under the
microscope. These are the individuals, as these dark purple or blue structures in the middle of the
picture. These are the nucleus, the nuclei of tumor cells. You can see how they're different in size and
shape, different in the darkness quality just of the chromatin quality. They have indentations,
projections. These are all atypical features that do not resemble normal cells that we are very familiar in
how they look like, and with these type of features altogether, with the abundance of these cells in the
urine sample, we can comfortably say that this is cyg-diagnostic of a high grade urothelial carcinoma.
Dr. Hikmat Al-Ahmadie:
Urine cytology, as I said, it's a very simple process. It's a simple test. Doesn't require much. It's not
invasive. You don't stick a needle or anything, just a urine sample in the office. It's very sensitive for high
grade urothelial carcinoma. It's less sensitive for lower grade lesions, because again, you need to see
atypical features that are obvious here. In low grade lesions, you don't see the same features. And it's
also specific, which means if you don't see these atypical cells, you have a high confidence that you're

not seeing a high grade urothelial
carcinoma, and that's why you can say
it's very specific. If we go to the next
slide, just to show you where these
cells might come, this is a tissue
section. This is a biopsy. There's also
the biopsy processed in a similar way,
a little bit different because you have
to prepare, you have to fix the tissue
and cut the sections and make them
into a slide. As you can see at the top,
in both these images, all these dark
cells that have different sizes and
shapes, these are all malignant cells.
With this tissue section, I can easily
call it urothelial carcinoma in situ
because I have architecture. But as
you can see, the individual cells are
coming off of the main tissue
fragment and they'll be sloughed off
or shed into urine, and that's what will
make up the positive urine cytology.
I'll turn it back to you, Peter, I think,
for the next set of slides.
Dr. Peter Black:
Great. Yeah, I think from the
urologist's perspective, the urine
cytology is so important because we
often don't see carcinoma in situ.
That's something we'll come back to
while we're talking. This gentleman
had a CT scan. He had blood in his
urine, so that's part of the usual
workup, and everything was normal
with the kidneys and ureters.
Remember that carcinoma in situ and
indeed any cancer of the bladder, you
can get very similar lesions in the renal
pelvis and the ureters, and then upper
tracts. On cystoscopy, however, there
was a red patch on the back of the
bladder. It was a little bit raised. It
wasn't the typical sort of cauliflower
tumor that we often see, but it was
certainly suspicious, especially given

the positive cytology. So this gentleman we took to the operating room for a resection of this area that
you see in the micrograph here. Next slide please.
An important consideration, again,
this is particularly with carcinoma in
situ, is the use of enhanced
cystoscopy. There are two different
methods with which we can
enhance a cystoscopy. On the left-
hand side, you see the cysview,
which is the trade name. It's also
called blue light cystoscopy or
fluorescent cystoscopy. PDD is often
used in Europe as a terminology. It's
photo-dynamic diagnosis. But here,
you put a substance into the
bladder prior to the surgery, prior to
the TRBT, and it's taken up and
metabolized by cancer cells so that
these cells then fluoresce. When
you shine a blue light on it, a
fluorescent light, or, well, a blue
light, the cells will fluoresce and
they appear a bright pink. They
really stand out. We know that we can detect more tumors this way, and especially more carcinoma in
situ. Up to 40% more carcinoma in situ. It's particularly valuable, again, because we often overlook them
on regular white light cystoscopy.
Dr. Peter Black:
On the right-hand side, we have narrow band imaging, which is a little bit different. It uses filters to pull
out the blue and the green wavelengths that really accentuates blood vessels and changes in blood
vessel patterns. Vascularity will often accentuate tumors so that we can see them better. It's a little bit
easier to use because it's just a flip of a switch on the device and it doesn't require putting anything into
the bladder, but the evidence for its use is not as widespread. Next slide.
I just want to highlight some of the
differences in fluorescent cystoscopy.
And this is I think particular to the US
market actually, because it's not
necessarily available everywhere, but you
can actually do fluorescent cystoscopy at
the time of a surveillance cystoscopy in
the office. If a patient has had a prior
bladder cancer and they're undergoing
their routine cystoscopy, you can do
fluorescent cystoscopy that, for example,
is not available for us in Canada. Or, you
can do it, which is more common, more
universal, you do it at the time of a
broader tumor resection or biopsy, so if a patient has an identified tumor, you go to the operating room,
you give them the reagent beforehand and then you use it at the time of resection. So there are two
different uses, and I think as patients and caregivers, you need to be clear on the differences.
On the left-hand side, if you're talking about what we do at the time of surveillance cystoscopy, so it's
used to detect a recurrence. It's primarily patients with intermediate and high risk disease. So they
they've had tumors before. Based on the prior tumor characteristics, we know that they're high risk for
recurrence and we especially do it early on in their disease course. So if they had a tumor three months
ago and it's the first look, then that might be a time when we do it. We always have to consider the cost
and the treatment burden of all these tests. We can also do it, for example, if we see something on
white light that we're not, and white light's that the usual cystoscopy, if we're not quite sure what it is,
this might help us decide, yes, we need to biopsy that or no, we don't need to biopsy it. And overall,
there's especially one very good American trial that shows that it enhances the detection of high-grade
recurrence.
Dr. Peter Black:
On the right-hand side now, when we're using this at the time of resection, there's very good data from
multiple trials, North American and European trials that tell us that we'll detect more tumors, we'll
resect more thoroughly, patients will have a lower risk of recurrence, and that's a very important
endpoint. In particular for what we're talking about, we'll also find more carcinoma in situ. Next slide.
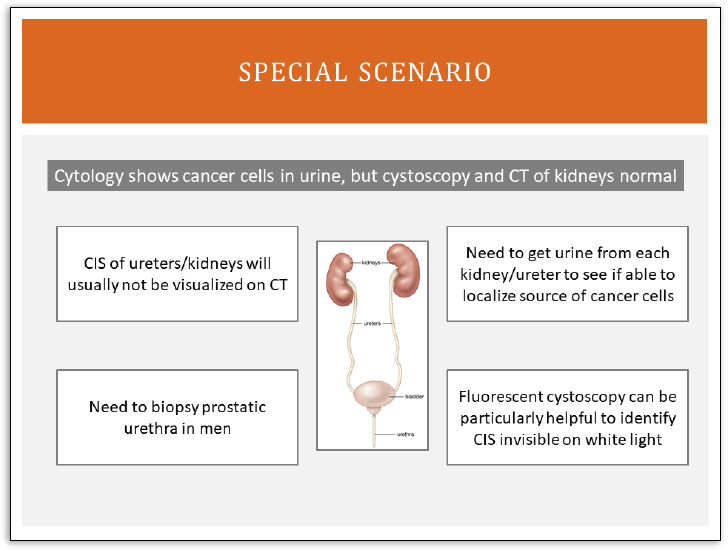
I wanted to highlight one
specific scenario that comes up
routinely and is particularly
relevant for carcinoma in situ. I
know there was already a
question in the chat or the Q&A
about carcinoma in situ of the
upper tracts. Upper tracts are
the ureters and the renal pelvis.
One scenario that we see
sometimes are patients who
come in to the office with a
urine cytology that clearly
shows abnormal cells, so it says
high-grade urothelial
carcinoma, so there are
malignant cells there, but we
don't see anything on
cystoscopy and we don't see
anything on a CT scan.
There, we have a specific
algorithm that we run through and we know, for example, that carcinoma in situ is something flat in the
ureter or renal pelvis is not going to be visualized on CT scan. We might not even see it, actually, when
we look at it with a camera. We can't do the fluorescent cystoscopy in the upper tract, so it can be
particularly tricky.
So what we do is we go to the operating room, we get urine from the right ureter and separately from
the left ureter so that if it is coming from one side or the other, we can determine that definitively. If we

find urine, let's say from the right renal pelvis, then the cytology is positive, but we don't see anything
with the camera and we don't see anything on CT scan, then we call that carcinoma in situ. We assume
there's carcinoma in situ there even though we don't see it.
Dr. Peter Black:
The other thing we do, then, so, if in a patient like this it's still more likely that it's actually from the
bladder and we're just not seeing it. So in the bladder, we will do the fluorescent cystoscopy and we'll
biopsy whatever we see there. And then in men, we always have to consider that it could be coming
from the prostatic urethra, where you can also get carcinoma in situ. It can be quite tricky. Upper tracts,
bladder, and prostatic urethra. Next slide.
So our patient, you'll recall he
had the positive cytology and
the red patch from the posterior
bladder wall. We took him to
the operating room for a
resection and we used the
fluorescent cystoscopy. It lit up
the patch that we saw, but it
also lit up a second patch bright
pink. And you can get an idea
from these pictures here, which
of course are not actually from
this patient. I stole these, but on
the left-hand side, the regular
white light, you don't really
appreciate much with even an
experienced eye, yet on the
right-hand side, it's really night
and day. It's very clear that
there's something there. So this
lesion was resected in addition
to the other one that we saw,
and the patient did well with the surgery, no complications. Next slide.
Dr. Peter Black:
So we sent the sample off to Dr. Al-Ahmadie, who's going to go through the pathology for us.

Dr. Hikmat Al-Ahmadie:
All right, so this is kind of a step-
wise process. Every sample that
is removed in the clinic or the
office goes to our pathology
department, and in the next few
slides, I'll just walk you through
some of the process and some of
the classification or how we
make the diagnosis, how we
look, how we evaluate these
specimens. And then at the end,
I'm going to show you a slide
that would represent that actual
biopsy from this gentleman.
Once we start the evaluation of
any bladder sample, in our mind
is when we find a malignant
process, where can we ask the
question, where can we fit it
from the current specification?
And as Dr. Black alluded to in the
beginning, you have non-invasive spectrum of tumors that are in the non-invasive category, and those
can be the carcinoma in situ, which is the subject of tonight's discussion, and then you can have papillary
lesions that can be low-grade and high-grade as opposed to the in situ, which is always a high-grade.
There are some rare benign tumors that we call them papillomas. They have distinct morphologic
features, and then the tumor starts invading. And then the tumor can invade into the lamina propria,
which is the most superficial layer of the bladder wall, or the one underneath the surface lining. And
then the deep invasive disease. This is our main challenges. Where can we fit this lesion that we identify
on these histologic sections in any of these categories? So if we go next slide.
Dr. Hikmat Al-Ahmadie:
This is one we come into. We identified a
malignant process. It's a urothelial
carcinoma now. Can we place it into in
situ flat disease or papillary? This is what
it is. You look at the image on the left,
this is the definition of urothelial
carcinoma in situ. We've identified these
cells that are very atypical. Again, the
features that we use are the variation in
the size and shape, the variation in the
coloring of these tumor cells, how they're

spaced. Are they overcrowded? Are they not respecting each other's borders?
Then, there are some other features that I may point to and I apologize if these may sound too
technical, but I'm happy to kind of discuss or answer any questions if you think and if it might need more
explanation. When the cells divide, they form a structure called mitosis, which is a sign of dividing in
cells and a growing tumor. You can see a lot of mitosis in these tumors as compared to the picture on
the right, which is the papillary tumors, which are basically a finger-like projections, and this is how the
tumor grows into the lumen of the bladder. It's simple recognition of the pattern that can tell you it's a
flat versus papillary disease, and these are all things that are [inaudible 00:23:04]. You can see it's be
definition high-grade disease, and it's a tumor that's just growing as if on the flat, the surface of the
bladder. Next slide.
Dr. Hikmat Al-Ahmadie:
Fortunately and unfortunately,
just to make things difficult and
challenging, urothelial carcinoma
in situ can come in different forms
and shapes. These are just
different examples. I just wanted
to show you, just to kind of put an
image to the name, so to speak.
We always rely on reference to the
normal urothelium, which is the
image on the top left. Here you
can see it doesn't take much, you
can see, comparing this image to
all the other five images. You
could see how we use the
reference normal urothelium to
help us identify abnormal lesions.
Normal urothelium is very orderly
structure, multiple layers of
different types of cells start from
the base all the way to the top. All
of these cells are important. They
have normal functions in the bladder. For example, this middle layer can be four, five cells thick, but it
can become two cells thick if the bladder is distended and becomes flattened.
All the other images on the right and the bottom, these are different shapes of urothelial carcinoma in
situ. Again, highlighting the features that we rely on and we use: the variation in the size and shape, the
discoloration, the growth pattern. Some of these patterns can be deceptive, like the one on the top left.
These large cells are individual tumor cells just growing along the normal urothelium. We use the term
spread, but this could be challenging and this could be one of these examples where not many tumor
cells go into the urine, for example, because these cells are just growing under normal urothelium,
compared to the picture in the middle and the bottom. This is when you have a lot of dis-cohesive,
disjointed tumor cells that can easily shed into the urine.
Dr. Hikmat Al-Ahmadie:

The last image on the bottom right, this is a tumor, carcinoma in situ, that is involving some normal
invaginations in the bladder. Sometimes all the surface urothelium is normal. You don't see any
malignant cells, but you could see these tumor cells inside this invagination. Sometimes that can make it
more difficult to treat. If we go next slide.
As much as we want to believe or
we want to think of urothelial
carcinoma in situ as distinct from
papillary tumors, a lot of times
they coexist or they overlap,
coexist or they develop after one
another. These are some terms
that we use, primary urothelial
carcinoma in situ or primary CIS is
one. On the first presentation, it is
urothelial carcinoma in situ with
no associated papillary tumor, or
you can have secondary CIS,
where there is urothelial
carcinoma in situ developing
concomitantly with or after a
prior diagnosis of a papillary
urothelial carcinoma. Of course
there's always attempt to try to
link this to differences in outcome
or responses to types of therapy
even though it's not necessarily conclusive yet, but it seems that some people believe that primarily
urothelial carcinoma in situ may be associated with a worse response to rate to these conservative
treatments. Next slide.
This is one example I wanted to show
you, is as I said, as much as we like to
keep them separate and unique,
sometimes they coexist. This is one
example here. This is the same TUR
specimen from the same individual.
You can see them grouped together,
coming together within the same
container from the same patient. If
you look at the higher magnification
picture on the right top, this is a
papillary tumor. You can see these
finger-like projections sticking into
the bladder wall, out the bladder
lumen, versus the picture on the
bottom, the growth is very flat, just
along the surface urothelium. That's
the distinct growth pattern for
urothelial carcinoma in situ. So a

papillary tumor and a flat disease can coexist within the same specimen. Next.
Dr. Hikmat Al-Ahmadie:
And of course another important
thing, when we assess, when we
evaluate the urothelium here,
and every time we see urothelial
carcinoma in situ, the next most
important thing is to determine
whether there is any amount of
invasive disease or whether this
tumor is purely non-invasive
urothelial carcinoma in situ. We
look carefully at the base of the
surface urothelium and we are
carefully evaluating the stroma
underneath it, these layer that
are loose underneath it. When
we start seeing these individual
cells that are highlighted by these
green arrows or arrow hats,
that's when we start calling this
invasive disease. Depending on
the level of invasion and the
amount of the invasive disease,
we can call it focal superficial, and if it goes deeper than the first layer... if it remains in the first layer,
you call it laminal invasion or T1 disease, as you might hear about it. And if it goes further deep into the
bladder wall, it may invade the muscular layer, where you call it muscle-invasive bladder cancer. These
features are not depicted here on the slide, though. Okay, next. I think we went back, so... Yes, next
slide.
Yeah, and so this is another example
that Dr. Black alluded to that could
present challenges in evaluating
urothelial carcinoma in situ. This is a
tumor that is involving... here, what we
see in the picture is involvement of
prostatic ducts, which are immediately
underneath the urethra. This is a tumor
that kept kind of crawling along the
surface urothelium, involving the
urethra and went all the way into the
prostatic ducts. It's not invasive, it just
keeps colonizing all these ducts and
makes it difficult to detect sometimes,
difficult to remove and difficult to treat
overall. Next.

This is going back now to the actual case
here in this presentation. This is the biopsy
from the gentleman, and as you can see,
after I showed you all the slides, now
everyone should be able to recognize that
yes, these cells are very atypical. They're
different sizes and shapes, different colors.
They don't respect each other's borders.
There are a lot of variations amongst them.
This is diagnostic of urothelial carcinoma in
situ. Next. Yeah, that's yours. Back to you.
