
Evidence of differential mass change rates between human breast
cancer cell lines in culture
Elise A. Corbin
1,2,3
& Olaoluwa O. Adeniba
1,2
& Olivia V. Cangellaris
2,4
&
William P. King
1,2
& Rashid Bashir
2,4
#
Springer Science+Business Media New York 2017
Abstract Investigating the growth signatures of single
cells will determine how cell g rowth is regulated and cell
size is maintained. The ability to preci sely measure such
changes and alterations in cell size and cell mass could be
important fo r applications in cancer and drug screening.
Here, we measure the mass growth rate of individual be-
nign (MCF-10A), non-invasive (MCF-7), and highly-
invasive malignant (MDA-MB-231) bre ast cancer cells.
A micro-patterni ng technique was employed to allow for
the long-term growth of motile cells. Results show ma ss
growth rates at 4.8%, 1.2%, and 2.8% for MCF-10A,
MCF-7, and MDA-MB-231, demonstrating t hat normal
cells have a higher mass growth rate than cancerous cells.
All the cell lines show an increase in mass change rate
indicating that the mass accumulation rate is exponential
over a single cell cycle. The growth rates measured with
our MEMS sensor are compared with doubling times ob-
tained through conventional bulk analysis techniques, and
exhibit excellent agreement.
Keywords MEMS mass sensor
.
Breast cancer
.
Cell growth
rate
.
Cell micromechanics
.
Resonant frequency
1 Introduction
Cell growth is necessary for l ife, but the mechanism s that
control the regulation of cell mass, volume and growth rate
are still poorly understood (Lloyd 2013; Zangle and Te itell
2014). Cancer arises from a number of mutations in the
genetic makeup of a cell; through the cell cycle the mutated
genetic makeup is transferred to daughter cells and prolif-
eration causes these cells to mult iply (Lodish et al. 2003;
Alberts et al. 2002;Weinberg200 6 ).
Currently, a great deal is known about the conse-
quences of the mutations, but on a fundamental level there
is st ill much to learn. C ancerous mutations alter relevant
signaling pathways, which in-turn influence the interac-
tion and response of a cell to mechanical stimuli and
growth factors, cell fate, proliferation, transcription, mi-
gration, or differentiation. These pathways can change
how the cell cycle is regulated. In cancer, cells exhibit
unregulated growth and division as a consequence of
disrupted checkpoints within t he cell cycle, w hich is trig-
gered by the loss of proper signaling cues (Hanahan and
Weinberg 2000, 2011).
Cell growth consists of coordinated changes in both
mass and volume. A standard approach to measuring cell
growth is through accurate monitoring of cell size. A
Coulter counter is a well-established technique to measure
cell volume and has deepened our understanding as to how
cells regulate growth. However, cell mass is a more direct
and p recise indicator of ce ll size and it is more closely
indicative of biosynthetic processes in a cell. There are
many different approaches for measuring cellular growth
Electronic supplementary material The online version of this article
(doi:10.1007/s10544-017-0151-x) contains supplementary material,
which is available to authorized users.
* Rashid Bashir
rbashir@illinois.edu
1
Department of Mechanical Science and Engineering, University of
Illinois Urbana-Champaign, Urbana, IL 61801, USA
2
Micro and Nanotechnology Laboratory, University of Illinois
Urbana-Champaign, Urbana, IL 61801, USA
3
Perelman School of Medicine, University of Pennsylvania,
Philadelphia, P A 19104, USA
4
Department of Bioengineering, University of Illinois
Urbana-Champaign, Urbana, IL 61801, USA
Biomed Microdevices (2017) 19:10
DOI 10.1007/s10544-017-0151-x

and there is a debate as to whether looking at an aggregate
population is more correct and necessary (Cooper 2006;
Mitchison 2003; Mitchison 2005). When dealing with
time-dependent measurements, it is known that studying
bulk properties will often overlook single cell events and
provide misleading analysis (Di Carlo et al. 2012;DiCarlo
and Lee 200 6 ). Measuring the growth of single cells has
many challenges to overcome (Popescu et al. 2014), includ-
ing the ability to handle and manipulate individual cells in
real time. H owever, the use of microelectromechanical sys-
tems (MEMS) has provided opportunities to make signifi-
cant advances (Bryan et al. 2010; So n et al. 2012). Several
precise MEMS measurement techniques have been recently
developed t o better understand how the biophysical prop-
erties of a cell affect its cycle progression and behavior in
disease. This includes magnetic twisting cytometry (Wang
and Thampatty 2006), optomechanical measures (Park
et al. 2015), micropipette aspiration (Evans and Yeung
1989; Hochmuth 2016;Satoetal.2016), quartz crystal
microbalance (QCM) (Li et al. 2005), atomic force m icros-
copy (AFM) (Cross et al. 2007; Kuz netsova et al. 2007;
Plodinec et al. 2012), suspended microchannel resonator
(SMR) (Son et al. 2012), and quantitative phase microsco-
py (QPM) (Popescu et al. 2008). Although QPM m ethods
are widely used to study adherent cells, they can only mea-
sure the ‘dry mass’ of live cel ls; thus, they rely on a refrac-
tive index difference to measure the non-aqueous (proteins,
nucleic acids, and lipid m olecules) mass density of the cell.
Meanwhile, allowable sample sizes in QCM are based on
electrode dimensions, which limit its adoption. SMR uses a
flow-through technique that senses the ‘buoyant mass’ of a
cell – the volumetric difference between the densities of a
cell and that of the media replaced by the cell – which is
similar to the optically measured ‘dry m ass’ as described in
QPM. This limits the types of cel ls able to be studied with
SMR. As an alternative to these methods, our pedestal
resonant sensor measures the whole cell apparent mass
and thus could potentially illustrate critical elements of
cell growth.
In this paper, we use our MEMS resonant mass sensors
to compare t he long-term growth of benign epithelial cells
(MCF-10A) with cancer cells of both high (MDA-MB-
231) a nd low invasive properties (MCF-7). In order to
measure long-term growth, we confine and trap single cells
on the pedestal of the MEMS sensor and repeatedly ob-
serve its resonant frequency to explore the way that cells
accumulate mass, grow, and divide. This approach is aided
by the use of cell patterning on the suspended resonant
devices in order to maintain capture of the cells over
time (Corbin et al. 2014a). From this approach, we find
that all the cell lines examined exhibit a grow th rate that
increases with cell mass, but that each cell line exhibits its
own unique mass change rate.
2 Experimental methods
2.1 MEMS resonant pedestal sensor
The MEMS resonant mass sensor comprises four beam-
springs suspending a square platform (60 × 60 μm
2
), as has
been described previously (Corbin et al. 2014b;Milletetal.
2012;Parketal.2010). The pedestal design both allows for a
large cell capture region and also minimizes the variation of
the displacement amplitude across the vibrating platform to
provide uniform mass sensitivity. These sensors are arrayed
on a chip in a 9 × 9 format of 81 sensors to maximize through-
put and capture efficiency. It operates in a first resonance
mode for mass sensing, which is a vertical displacement vi-
bration with resonant frequencies of approximately 160 kHz in
air and 60 kHz in liquid. This sensor is driven by passing an
actuation current through the sensor in a static magnetic field
to generate a Lorentz force that forces vibration.
2.2 Micro-patterned surface functionalization
In order to improve cell capture and retention, cells are
micro-patterned on the pedestal sensors through a selec-
tive functionalization and backfill passivation technique
(Corbin et al. 2014a, b). First, a hyd rophobic layer of
hexamethyldisilizane (HMDS) was applied to the sensor
surface through vapor deposition to promote efficient de-
position of Pluronic® F127 (Dorvel et al. 2010). Due to
the delicate nature of the structure, a photoresist transfer
technique (Yeom and Shannon 2010)wasusedto
provide a uniform layer of photoresist to be patterned.
The sample was then developed and exposed to oxygen
plasma to remove HMDS from the openings in the pho-
toresist for deposition of collagen type I. After rinsing
the surface with PBS, the chip was soaked in acetone to
lift-off the photoresist, leaving collagen selectively on
the pedestals of the sensors and surrounded by HMDS
everywhere else. At this point, pluronic was deposited on
the HMDS covering the beam springs and a thin edge of
the pedestal sensor through a backfill technique. This has
the effect of selectively promoting cell adhesion on the
collagen-functionalized pedestal while blocking unwant-
ed adhesion at sites with pluronic.
2.3 Cell mass measurement
The mass measurement procedure involves comparing the
resonant frequency of a loaded sensor with its original,
unloaded state to determine the mass of the loaded object.
This is a well-characterized method described fully elsewhere
(Corbin et al. 2013;Milletetal.2012), therefore it will only be
briefly introduced here. The measurement system combines
electromagnetic actuation, a lock-in amplifier, and a laser
10 Page 2 of 7 Biomed Microdevices (2017) 19:10
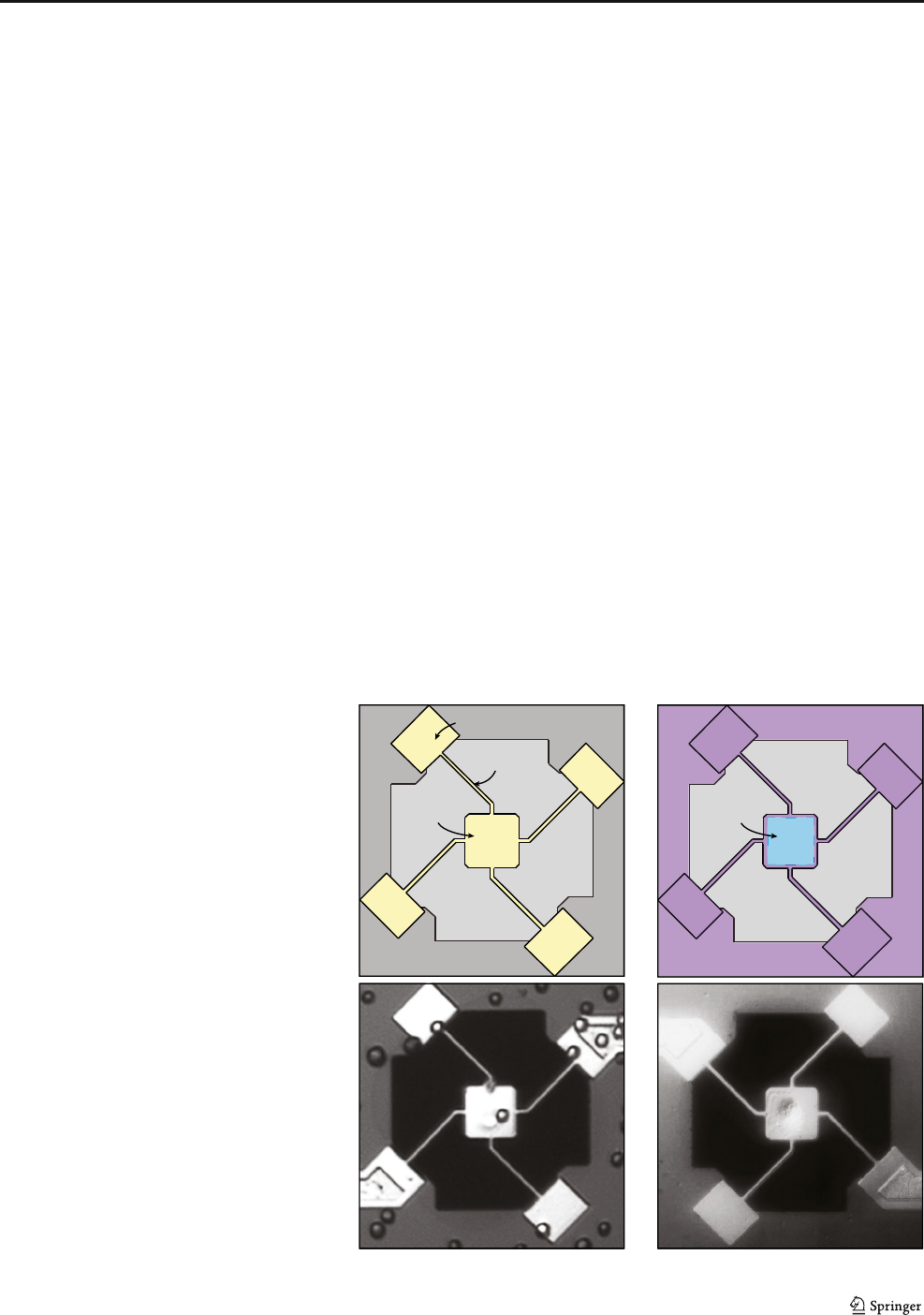
Doppler vibrometer (LDV) to measure the velocity of the
vibrating MEMS sensor platform to ultimately determine the
resonant frequency of the device. This is achieved by moni-
toring the difference in phase between applied actuation cur-
rent and sensor vibration. The excitation frequency is updated
based on this phase until converging upon the resonant fre-
quency. This procedure is used to estimate the resonant fre-
quency of the devices in a series of different states to extract
the mass of the adhered cell.
Calculating object mass requires three separate resonant
frequency measurements. First, the empty sensor resonant fre-
quency was measured in air to obtain the spring constant.
Next, the micro-pa tterning procedure was completed by
backfilling with pluronic to passivate the remaining chip sur-
faces. We then placed a PDMS chamber over the sensor area,
added fresh warm complete cell culture media, and sealed the
chamber with a sterile glass coverslip. The sensor was then
loaded into the incubation chamber of our system and allowed
to stabilize to 37 °C prior to the measurement. The second
empty sensor frequency was then measured in liquid to deter-
mine the reference frequency, which is reduced from the in-air
frequency due to hydrodynamic loading. At this point cells
were randomly seeded on the sensor area and allowed to at-
tach for 1 h before being gently rinsed to remove non-adhered
cells. The sensor is then restabilized at 37 °C in the incubation
chamber with the necessary conditions for each cell type (i.e.
humidity and CO
2
level). Finally, the in-liquid resonant fre-
quency of the mass loaded sensors were measured for com-
parison with the empty reference sensor frequency to calculate
the attached mass. This measurement of the loaded sensor
frequency was repeated over time to produce a growth profile.
Previous studies (Sato et al. 2016)showthatthrough250in-
media measurements, the sensor resonant frequency can be
determined within ±0.94 Hz (95% Confidence Internal (CI)),
yielding a mass resolution of 8.5 pg within a confidence in-
terval of 95% in liquid. With this sensor mass resolution per-
centage of ~1% of a typical mammalian cell mass (~1 ng), we
can guarantee that within a CI = 95%, an exponential and
linear growth model can be differentiated by our resonant
mass sensor (See Supplemental Information).
2.4 Breast epithelial cell culture
Normal human breast epithelial cells (MCF-10A) were cul-
tured in Dulbecco’s M odified Eagle Medium/Ham’sF-12
(Gibco) with 5% horse serum, 20 ng/mL EGF, 0.5 mg/mL
hydrocortisone, 100 ng/mL cholera toxin, 10 μg/mL insulin,
and 1% penicillin streptomycin. Human breast adenocarcino-
ma cells (MCF-7) were cultured in Dubecco’s Modified Eagle
Medium (Gibco) with 10% fetal bovine serum and 1% peni-
cillin streptomycin. Highly metastatic human breast adenocar-
cinoma cells (MDA-MB-231) were cultured in Leibovitz’s
Pluronic® F127
collagen
Standard Sensor
Micro-patterned Sensor
pedestal
spring
reference pad
a
c
b
d
Fig. 1 Overview of sensor
design with select
functionalization and passivation:
a Cartoon of the sensor layout;
and b cartoon of the desired
patterning with collagen
selectively patterned in the center
of the pedestal and with pluronic
backfilled everywhere else c
Bright field image of a single
released non-patterned pedestal
sensor that has been seeded with
human colon cancer cells (HT29).
It is shown that cells are able to
attach to the springs and
anywhere else that could
ultimately compromise the cell
measurement. d Bright Field
image of a single released
patterned pedestal sensor seeded
with human breast cells (MCF-
10A) and then rinsed to remove
non-attached cells, leaving only
the cell captured on the platform
Biomed Microdevices (2017) 19:10 Page 3 of 7 10

L-15 Medium (Sigma-Aldrich) with 10% fetal bovine serum
and 1% penicillin streptomycin. MCF-7 and MCF-10A were
cultured at 5% CO
2
, 100% humidity, while MDA-MB-231
were cultured at 100% humidity and no CO
2
.
Cells were introduced onto the sensors at a total of 9000
cells p er chip and allowe d t o a dhere. The sensors were
rinsed with fresh growth media and the culture chamber
was sealed with a sterilized glass cover slip for the
measurement. Mass measurements were taken approxi-
matelyevery20minforupto24h.
2.5 Bulk doubling time measurements
Since cell-collagen interaction can strongly change cellular
behavior, we performed doubling time experiments on colla-
gen coated plastic dishes and non-collagen coated dishes for
reference. We plated the all the cell lines at the same starting
density of 318 cells/mm
2
and culture conditions as with the
single cell analysis experiments and imaged them for 24 h.
Using ImageJ we determined the cell count and found that
MCF-7, MCF-10A, and MDA-MB-231 had doubling times
of 43.7 ± 7.4, 20.1 ± 1.9, and 26.7 ± 8.8, respectively.
3 Results and discussion
Direct, long-term growth profiles of breast epithelial cell lines
are measured using MEMS resonant pedestal sensors with
micro-patterned surfaces for selective functionalization and
passivation (Fig. 1). The cell lines st udied include MDA-
MB-231 and MCF-7, which are cancerous with high and
low invasive potential, respectively, and a benign cell line,
MCF-10A, for comparison. Repeated mass measurements of
single cells captured on the mass sensor reveal the increase of
cellular mass due to growth over the cell cycle. Figure 2 shows
examples of individual cell growth curves that continue until
the cell division where, interestingly, a temporary sharp de-
crease in apparent mass is detected (Movie S1-S3; Online
Resource 1–3). These sharp decreases in apparent mass can
be seen in the selected growth profiles at approximately 10,
13, and 9 h for MDA-MB-231, MCF-7, and MCF-10A, re-
spectively; however, each investigated cell divided at a differ-
ent time as our populations were not synchronized. During
mitosis the dividing cell will partially detach from the plat-
form, thus decreasing the contact area and altering the shape
of the cell (Park et al. 2010). This geometry change can lead to
a reduction of the inertial loading of the cell decreasing the
apparent mass as described by a 2-DOF model (Corbin et al.
2015; Corbin et al. 2013;Parketal.2010; Corbin et al. 2016).
The result of the 2-DOF model revealed that geometry and
viscoelasticity of the target, or cell, influences the mass mea-
surement. Cells are known to be soft and cancer cells are
known to be even softer ( Cross et al. 2007;Gucketal.
2016; Wirtz et al. 2011). The viscoelastic properties of cells
can lead to the cell operating out of phase with our sensor
causing a geometry change of the cell. However, it is impor-
tant to note that the increase in apparent mass during the cell
growth represents a true increase in cell mass and is not a
geometry or contact area artifact (Park et al. 2010).
To better investigate the growth dynamics of the benign
and cancerous epithelial cell populations, we analyzed the
MDA-MB-231
a
MCF-7
b
Cell Mass (ng)
MCF-10A
c
Time (hr)
Cell Mass (ng) Cell Mass (ng)
Time (hr)
Time (hr)
Fig. 2 Mass measurement of adherent cells versus time for each cell line:
a MDA-MB-231; b MCF-7; and c MCF-10A. Each growth profile shows
an increase of a single adherent cell, then will go through a cell division
that is marked by a sudden decrease in cell mass, once division has
completed, the growth profile continues
10 Page 4 of 7 Biomed Microdevices (2017) 19:10
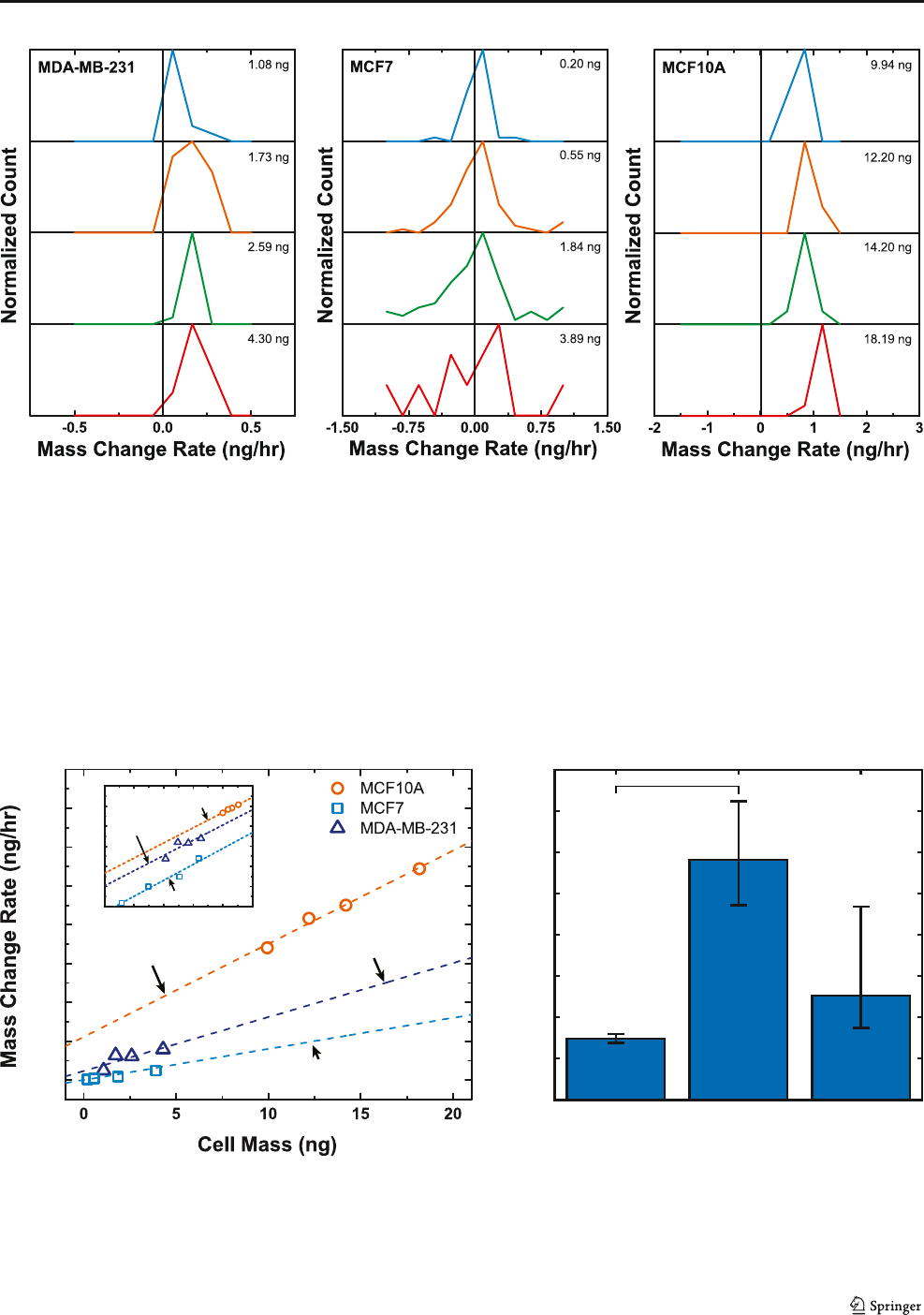
individual temporal mass profiles of all the cell lines. The
derivative of the cell mass profiles provides the instantaneous
mass change rate. For a given cell line, the data from each
individual measured cell is pooled together, and Fig. 3 (a -
c) show histograms of the instantaneous mass change rate data
divided into four groups based on their instantaneous mass. It
is clearly evident from these histograms that mass change rate
does depend on the instantaneous cell mass. The small oscil-
lations in mass data observed in Fig. 2 are expected and can
lead to negative mass change rates. These oscillations can
arise from a combination of short-term variations in cell vis-
coelasticity, density, or adhesion of the cell over the cell cycle.
Small fluctuations of these properties occur naturally and have
a small impact on the apparent mass detected by our sensors.
y = 0.028x + 0.047
y = 0.012x + 0.0008
y = 0.048x + 0.22
0.0
0.2
0.4
0.6
0.8
1.0
1.4
1.6
1.2
0.50.0-0.5
1.0
0.5
-0.5
-1.5
-2.5
y = 0.76x - 1.227
y = 0.757x - 0.9137
y = 0.793x - 1.846
0
10
20
30
40
50
60
70
80
MCF7MCF10A MDA-MB-231
*
Doubling Time (hr)
ba
Fig. 4 Analysis of mass change rate per unit mass of individual cells. A
five point moving average of changes in mass from all culture data points
of individual breast cells. a Apparent values: Average cells acquire 1.2%,
2.8%, 4.8% additional mass every hour for MCF-7, MDA-MB- 231, and
MCF-10A, respectively. (Inset) Log-log plot shows a power law of the
different cell lines where the slopes are less than unity verifying
consistency with scaling rules of energy consumption versus size of an
organism. b Average doubling times found through mass measurement
for MCF-7, MDA-MB- 231, and MCF- 10Awith error bars representing
standard error
Apparent
abc
Fig. 3 Analysis of cell growth rate and mass change rate versus mass. Four histograms accounting for the mass accumulation at specific mass values: a
MDA-MB-231; b MCF-7; and c MCF10-A
Biomed Microdevices (2017) 19:10 Page 5 of 7 10

Despite these mass fluctuations, there is a clearly identifiable
increase in cell mass over long periods of time, as confirmed
by simultaneous optical imaging.
Fig. 4a translates the distributions found in Fig. 3 to find the
cell mass growth rate indicated by the linear trends of the bin
max peaks for both the apparent and corrected masses. These
trends suggest that cells with greater mass also have an in-
creased rate of mass accumulation, regardless of cell line.
However, the cell lines do have different exponential growth
rates, which is the slope of the mass change rate against mass.
Figure 4a shows that MCF-10A, MCF-7, and MDA-MB-231
on average accumulate 4.8%, 1.2%, and 2.8% of their mass
every hour for the apparent mass values. Figure 4bpresents
how the growth rates translate to mass doubling times of 14.8,
58.2, and 25.1 h (= log (2)/ log (1 + rate)). To determine if
doubling times measured with the sensor differed between cell
lines, we used analysis of covariance (ANCOVA) to compare
the fitted mass change rates. Through this analysis we found
that the mass change rate, and thus the doubling time, signif-
icantly depended on cell line (p = 0.012). Post hoc tests be-
tween each cell line, with Bonferroni correction for multiple
comparisons, found that MCF-7 and MCF-10A differed sig-
nificantly (p = 0.013), but neither differed from MDA-MB-
231 significantly (p = 0.434 for MCF-7 and p = 0.182 for
MCF-10A). The inset of Fig. 4a presents log-log plots com-
paring mass change rate with cell mass. The growth follows
the rules of scaling energy consumption by having a slope less
than unity(Hou et al. 2008).
The American Type Culture Collection biological resource
center (ATCC) provides doubling times, or the times for cul-
ture to double in size, for each cell line. For comparison, a
population doubling time was considered for each cell line. In
these bulk population experiments we recreated the microen-
vironment of our single cell experiments by coating collagen
on plastic dishes. Table 1 collects the doubling time data of
each cell line determined by ATCC, our population experi-
ment, and our mass measurements. The MCF-10A cells ap-
pear to agree well with the bulk doubling time from ATCC,
while they tend to grow slightly faster compared to our bulk
doubling time measurements. The low invasive MCF-7 cells
grow more slowly than the ATCC doubling times and our bulk
doubling times. MDA-MB-231 (high invasive) cancer cell
line agrees well between our measured and our bulk doubling
times, however, it exhibits fairly substantial differences be-
tween measured and ATCC values. The differences in growth
values are based on the direct measurement of mass of indi-
vidual cells while both population and ATCC values are based
on bulk cell counting. Although ATCC is a world reference for
cell lines, the growing rate for each cell line varies with culture
media and methodology. Heterogeneity exists within a homo-
geneous population implying that cells are not created equal,
and yielding differences between cells such as size or mass.
Expression of subtle signature differences in growth among
individual cells can help explain why one cell may grow dif-
ferently or lead to metastasis. Our measurements show good
agreement with our bulk measures; however, a more in depth
statistical analysis is necessary to verify differences between
cell lines.
Recently, a deeper understandin g of cell growth dy-
namics has revealed how cells grow individually and as
a population over time (Park et al. 20 1 0 ). This work has
expanded upon that initial finding and validates it through
the study of multiple cell lines. Here, we showed that the
average growth rates of the investigated adherent cell
lines increase with cell mass. It has been hypothesized
that size homeostasis can b e maintained in one of two
ways: through linear growth without regulation (Conlon
and R aff 2003), or ex ponential growth that requires
check-point regulations (Godin et al. 201 0;Tzuretal.
2009). While the specific regulatory factors defining
growth are still intangible, it is li kely that size is main-
tained by some signaling, possibly from environmental
cues or genetics; however, we observ e common trends
between different cell lines that are consistent with previ-
ous findings (Park et al. 2010).
4 Conclusion
This paper presents the use of MEMS resonant sensors to
investigate the differences in growth between benign and ma-
lignant adherent cancer cells through long-term mass mea-
surements. Cells from each investigated cell line show a n
increase in mass change rate with respect to mass; therefore,
the heavier cells accumulate more mass more quickly.
Through the measurements with the MEMS sensor, we were
able to determine that doubling time significantly depends on
cell type, suggestive of inherent differences in cell growth
depending on cancer phenotype. These doubling times also
agreed well with bulk measurements and standard reference
values from ATCC. The agreement in doubling time measures
supports the accuracy of mass measurements, and future stud-
ies can take advantage of these devices to explore instanta-
neous growth of individual cells.
Table 1 A comparison of the doubling times of the values obtained
from the MEMS resonant sensor growth measurements and the American
Type Culture Collection (ATCC) database values
Cell line Doubling time
Measured (h) Counted (h) ATCC (h)
MCF-10A 14.8 20.1 16
MCF-7 58.2 43.7 38
MDA-MB-231 25.1 26.7 38
10 Page 6 of 7 Biomed Microdevices (2017) 19:10

These measures have the ability to expand our understand-
ing of adherent cell growth using a non-destructive technique
capable of long-term observations. There are many highly
regulated processes during cell growth including the replica-
tion of intracellular material that lead to mass accumulation,
an increase in the physical size of the cell, and progression
through the cell cycle. Through direct measurement of indi-
vidual cell mass, we can better understand the mechanisms
that form the basis of uncontrolled proliferation in cancer.
The potential to expand the current system to include the
monitoring of cell cycle status through optical verification
with fluorescence promises a next generation of mass mea-
surement for identification of growth rates durin g specific
cycle phases.
References
B. Alberts, A. John son, J. Lewis, M. R aff, K. Roberts, P. Walter,
Molecular Biology of the Cell, 4th edn. (Garland Science, New
York, 2 002)
A.K. Bryan, A. Goranov, A. Amon, S.R. Manalis, Proc. Natl. Acad. Sci.
107, 999 (2010)
I. Conlon, M. Raff, J. Biol. 2 (2003)
S. Cooper, Theor. Biol. Med. Model. 3, 10 (2006)
E.A. Corbin, L.J. Millet, J.H. Pikul, C.L. Johnson, J.G. Georgiadis, W.P.
King, R. Bashir, Biomed. Microdevices. 15, 311 (2013)
E.A. Corbin, B.R. Dorvel, L.J. Millet, W.P. King, R. Bashir, Lab Chip 14,
1401 (2014a)
E.A. Corbin, L.J. Millet, K.R. Keller, W.P. King, R. Bashir, Anal. Chem.
86, 4864 (2014b)
E.A. Corbin, F. Kong, C.T. Lim, W.P. King, R. Bashir, Lab Chip 15,839
(2015)
E.A. Corbin, O.O. Adeniba, R.H. Ewoldt, R. Bashir, Appl. Phys. Lett.
108, 93701 (2016)
S.E. Cross, Y.-S. Jin, J. Rao, J.K. Gimzewski, Nat Nano 2, 780 (2007)
D. Di Carlo, L.P. Lee, Anal. Chem. 78, 7918 (2006)
D. Di Carlo, H. T. K. Tse, and D. R. Gossett, in Single-cell anal. Methods
Protoc., ed. by S. Lindström and H. Andersson-Svahn (Humana
Press, T otowa, 2012), pp. 1–10
B.Dorvel,B.Reddy,I.Block,P.Mathias,S.E.Clare,B.Cunningham,
D.E. Bergstrom, R. Bashir, Adv. Funct. Mater. 20,87(2010)
E. Evans, A. Yeung, Biophys. J. 56, 151 (1989)
M. Godin, F.F. Delgado, S. Son, W.H. Grover, A.K. Bryan, A. Tzur, P.
Jorgensen, K. Payer, A.D. Grossman, M.W. Kirschner, S.R.
Manalis, Nat Meth 7, 387 (2010)
J. Guck, S. Schinkinger, B. Lincoln, F. Wottawah, S. Ebert, M.
Romeyke, D. Len z, H.M. Erickson , R. Anan thakrishn an, D.
Mitchell, J. Käs, S. Ulvick, C. Bilby, Biophys. J. 88,3689
(2016)
D. Hanahan, R.A. Weinberg, Cell 100, 57 (2000)
D. Hanahan, R.A. Weinberg, Cell 144, 646 (2011)
R.M. Hochmuth, J. Biomech. 33, 15 (2016)
C. Hou, W. Zuo, M.E. Moses, W.H. Woodruff, J.H. Brown, G.B. West,
Science 322, 736 (2008)
T.G. Kuznetsova, M.N. Starodubtseva, N.I. Yegorenkov, S.A. Chizhik,
Micron 38, 824 (2007)
J. Li, C. Thielemann, U. Reuning, D. Johannsmann, Biosens.
Bioelectron. 20, 1333 (2005)
A.C. Lloyd, Cell 154, 1194 (2013)
H. Lodish, A. Berk, P. Matsudaira, C.A. Kaiser, M. Krieger, M.P. Scott,
S.L. Zipursky, J. Darnell, Molecular Cell Biology, 5th edn. (WH
Freeman, New York, 2003)
L.J. Millet, E.A. Corbin, R. Free, K. Park, H. Kong, W.P. King, R. Bashir,
Small 8, 2555 (2012)
J. Mitchison, Int. Rev. Cytol. 165 (2003)
J.M. Mitchison, Theor. Biol. Med. Model. 2, 4 (2005)
K.Park,L.J.Millet,N.Kim,H.Li,X.Jin,G.Popescu,N.R.Aluru,
K.J. Hsia, R. Bashir, Proc. Natl. Acad. Sci.
107, 20691 (2010)
K. Park, A. Mehrnezhad, E.A. Corbin, R. Bashir, Lab Chip 15,3460
(2015)
M. Plodinec, M. Loparic, C.A. Monnier, E.C. Obermann, R. Zanetti-
Dallenbach, P. Oertle, J.T. Hyotyla, U. Aebi, M. Bentires-Alj,
L.Y.H., C.-A. Schoenenberger, Nat Nano 7, 757 (2012)
G. Popescu, Y. Park, N. Lue, C. Best-Popescu, L. Deflores, R.R. Dasari,
M.S. Feld, K. Badizadegan, Am. J. Physiol.-Cell Physiol 295, C538
(2008)
G. Popescu, K. Park, M. Mir, R. Bashir, Lab Chip 14, 646 (2014)
M. Sato, N. Ohshima, R.M. Nerem, J. Biomech. 29, 461 (2016)
S. Son, A. Tzur, Y. Weng, P. Jorgensen, J. Kim, M.W. Kirschner, S.R.
Manalis, Nat. Methods 9, 910 (2012)
A. Tzur, R. Kafri, V.S. LeBleu, G. Lahav, M.W. Kirschner, Science 325,
167 LP (2009)
J.H.C. Wang, B.P. Thampatty, An Introductory Review of Cell
Mechanobiology, Biomechanics and Modeling in Mechanobiology
(2006), pp. 1–16
R.A. Weinberg, The biology of cancer, 1st edn. (Garland Science, New
York, 2 006)
D. Wirtz, K. Konstantopoulos, P.C. Searson, Nat. Rev. Cancer 11,512
(2011)
J. Yeom, M.A. Shannon, Adv. Funct. Mater. 20, 289 (2010)
T.A. Zangle, M.A. Teitell, Nat Meth 11, 1221 (2014)
Biomed Microdevices (2017) 19:10 Page 7 of 7 10
