
A review of flower bud initiation in blueberry
Susan McCallum
The James Hutton Institute, Invergowrie, Dundee, DD2 5DA
B
ackg
r
o
un
d
In blueberry, knowledge of what determines vegetative vs. reproductive bud development under UK
conditions is difficult to obtain. Bud determination is under significant environmental control across
seasons leading to excessive yield instability. Unpredictable profit projections have hampered further
investment in the UK. We need to understand the environmental triggers of the phenotype prior to their
effect on yield and quality being realised, allowing mitigation strategies to be determined. Unstable
yields in high yielding environments raise serious concerns about the ability of existing crop systems to
sustain and expand. Unpredicted phenotypic variation in a range of developmental traits that have a
direct impact on yield has been evident for several seasons across a range of crops. In blueberry, yield
variation between seasons can be as much as 50%, valued around £36,000 per ha per year.
Summary
of main
f
i
nd
i
ng
s
• Flower bud formation occurs under short days/ long nights only.
• High temperature or water shortage during short days reduces flower bud initiation and results
in smaller flower bud formation.
• Cold temperature at night keeps growth slow and increases flower bud initiation.
• Number of flower buds per shoot is related to the number of days good weather for flower bud
initiation (longer developmental period).
• Factors including day length, temperature and plant health are all critical for flower bud initiation.
• Careful selection of cultivars depending on expected environmental conditions (early, mid-
season or late) can optimise light levels and resultant yield.
• Genetic potential of new selections can be determined through the study of key regulatory
pathways.
• Irrigation, fertigation, and pruning are areas a grower can control to maximise flower bud
initiation and subsequent yield.
A
ppr
o
ac
h
This desk study was commissioned by AHDB to fill a knowledge gap in our understanding of factors
influencing bud initiation and yield in highbush blueberries (Vaccinium Corymbosum) and how
management regimes may influence these.
The study was undertaken by performing literature searches of Web of Science, Google Scholar and
the websites of grower organisations (e.g. AHDB), using search terms such as flower bud initiation, bud
development, photoperiod and plant signaling. Some plant characteristics found to be influenced by
light and temperature within flowering plants are listed in Table 1.
The study summarises information gathered from these literature searches into environmental and
management factors that have been found to influence (positively or negatively) the initiation of flower and
vegetative buds in blueberries. Although this study focusses primarily on highbush blueberry (Vaccinium
Corymbosum) some information related to Southern highbush (Vaccinium corymbosum hybrid with V.
darrowii) and Rabbiteye (Vaccinium ashei) has been reported if thought to be relevant to the study.
Finally, the study summarises additional crop management strategies that could be used to encourage
flower bud initiation and hence improve fruit yield.
Introduction
Throughout their life cycle, plants are subjected to many adverse environmental conditions including
low light levels and periods of drought or extreme temperatures which can dramatically affect plant
survival and limit productivity. To cope with such stresses, plants adjust metabolically and
physiologically. Unanticipated variation in crop development is already in evidence in a range of crop
cultivars resulting in yield instability with significant negative impacts on the rural economy,
environment, and wellbeing.
The lack of accessible information in this area severely limits the capacity for active crop management
to optimise yield or to breed for future environmental resilience. Factors influencing bud initiation and
hence yield, along with management practices to mitigate these could help inform growers how to
maximise yield and reduce crop losses.
Currently no methodologies exist to predict when a plant's development will lead to unwanted
phenotypes. In blueberry the environment has a significant role in yield determination and while we
have some understanding of yield traits, we have little knowledge in the UK of what determines the
development of vegetative vs. reproductive buds which has a great impact on yield. This is under
significant environmental control so varies greatly between seasons. Increasingly unpredictable
seasonal conditions have an impact on yield stability through the expression of inferior phenotypes
(erratic bud break, early fruit drop etc.). In blueberries, unpredictable profit projections have hampered
further investment in the UK.
During vegetative growth, one bud develops on the stem for each leaf produced. The bud is located at
the leaf axil, which is just above the point where the petiole meets the stem. These axillary buds first
develop vegetatively. Depending on the day length, temperature, and plant health, some of these
vegetative buds will convert to flower buds and this happens during late summer and throughout
autumn. Flower buds develop on the older current-season’s wood that grew during the spring first, and
then on the vigorous upright growth that develops following fruit harvest (Williamson et al., 2004). Day
length is measured by special plant pigments called phytochromes which are located within the leaves
and buds. As day length shortens in the autumn, vegetative buds are gradually converted to flower
buds with flower bud number being positively correlated with the length of exposure to short days (Strik,
2012). High temperatures and water deficit can both reduce flower bud initiation, even when days are
short enough to induce it. Moreover, flower buds initiated under high temperatures are smaller and do
not develop as well as those initiated under lower temperatures.
Blueberry is a key crop with great potential for UK production, but current supply only meets around
15% of the UK market. Demand for blueberries is at record levels with UK fresh sales valued at £450
million in 2020 representing growth of over 11% year on year. As the cost of production for the fresh
market is high, and labour is increasingly difficult to secure, there is a significant requirement to develop
cultivars which are quick to establish and produce consistent yield and quality. The changing UK
climate presents additional challenges to plant growth, particularly for woody perennial species like
blueberries. There are already indications that the trend towards warmer winters in UK and Europe is
affecting the dormancy cycle and subsequent fruit development of some berry crop species.
Due to increasing demand, prices for fresh-market blueberries have been relatively high, ranging from
£6.00 per kg early in the season to £11.00 per kg as the season comes to an end. Average five-year-old
plants would be expected to yield between 2.5-5.0 kg per plant, but this is subject to cultivar,
environment, and management conditions. Increases of 0.5kg per mature bush at the average growing
density of 4,000 bushes per ha could therefore result in the addition of up to £16,000 per ha per year
based on a rather conservative blueberry value of £8 per kg.
During each growing season, blueberry bushes form buds for the following year’s production. In spring
and early summer, new shoots which produce leaf buds are formed. Later in the summer if both light
levels and temperature are favourable, the plant produces fruit buds at the tips of these new shoots.
Heavy fruit set this year means the bush had enough energy to produce adequate fruit buds the
previous year.
In many crops, flower bud initiation (FBI) and flower bud differentiation (FBD) are clearly affected by
photoperiod and temperature with these often having interactive effects. Manipulation of photoperiod
and temperature during the FBI period has been used to alter fruiting season. In blueberry, FBI occurs
under short day length (it is the long night that is of most value) around late summer and early autumn,
beginning at the tip of the current season’s shoot (Aalders and Hall, 1964; Banados and Strik, 2006).
FBI is found to increase after eight weeks of 8-, 10-, or 12-hour photoperiod, compared with 14- or 16-
hours (Hall et al., 1963). Flower bud number has been reported to positively correlate with the length of
exposure to short days in northern highbush blueberries (Banados and Strik, 2006). The same study
was found to show FBD was incomplete in plants exposed to only four weeks of short days compared to
eight and bloom was also delayed.
In early, mid and late season northern highbush blueberries (NHB), FBI was found to only occur under
short days (SD) at a constant temperature of 22
o
C with the number of flower buds correlated to the
length of exposure. Plants grown under SD (8 hours light and 16 hours dark) for four or more weeks
ceased growth and entered endo-dormancy, whereas those grown under long days (LD) of 16 hours
light and 8 hours dark continued to grow but did not initiate flower buds or enter dormancy. FBI proceeds
basipetally with the number of floral buds per shoot affected by cultivar, climate, and production
practices. Shoot growth often slows under short days but this may be in response to a reduction in daily
photosynthesis as opposed to a direct photoperiod response (Strik, 2012). Flower bud development will
then continue until temperatures become too cool in late autumn. An experiment conducted over nine
years following eight different cultivars (early, mid and late ripening) reported variations in duration from
crop maturity to the end of the growing period as well as length of thermal time (heat sum of temperature
above 0
o
C). Early cultivars took between 99-133 days and received 1,166-1,640
o
C, mid cultivars 95-127
days with 977-1,664
o
C and late cultivars spanned 62-102 days with only 816-1,146
o
C (Kurlovich, 2020).
Constant 6
o
C for 1,000 hours chilling has been reported as being more successful for bud break than
10
o
C or 12
o
C in NHB. Once chilling has been reached, bud break and bloom will occur after six and 26
days with little difference seen between cultivars. Insufficient chilling will result in delayed and erratic bud
break as well as a reduction in the overall number of buds that break. Flower buds tend to have a lower
chilling requirement than vegetative buds. FBI and FBD requires at least two weeks of shoot cessation
before a flower bud can be visually identified at the apex. Equal hours of chilling in cold storage are not
equal to similar hours in the field. Currently little is known about the effect of fluctuating temperatures
and how best to simulate more “natural” chilling (Strik, 2012).
Environmental factors
Plants are sessile organisms which are unable to migrate to new locations should they find their
current environmental conditions unfavorable. They are exposed to an everchanging environment
throughout any given growing season, to which they must adjust accordingly if they are to thrive. These
responses are tightly regulated by a number of complex signaling pathways and those are initiated into
action depending on the stimuli received. During its lifetime, a plant must make numerous decisions,
based on the input they receive from the environment, in order to survive. Weather events such as
extremes in temperature, water availability or sub-optimal soil pH leads to the plants suffering from
abiotic stress which can result in poor plant growth (Lamers et al., 2020).
Photoperiod
Photoperiod is an environmental factor that changes in a predictable manner year on year for a
particular combination of latitude and day. Plants have evolved to sense these changes and adapt their
physiology and metabolism to the oncoming season. Shortened photoperiods in the autumn precede
cold winters. This is especially important in deciduous woody perennial plants native to high latitudes,
which alternate their annual growth cycle between a period of active growth in the spring and summer
and one of dormancy in the autumn and winter. Highbush blueberry is native to latitudes of 40 to 45
o
N,
where the natural photoperiod ranges from 16 to 8 hours of light. The UK has a latitude between 51 to
56
o
N and also experiences between 8 to 16 hours of daily light levels. The influence of short-day
photoperiod on flower bud induction in blueberry shows that lowbush (Vaccinium angustifolium Ait.),
highbush (Vaccinium corymbosum L.) and rabbiteye (Vaccinium ashei Read) all develop flower buds
under an 8 hour photoperiod but not under 16 hours (Strik, 2015).
Vegetative growth was highest under 16-hour photoperiods and least under 8-hour. Maximum flower
bud initiation occurred under 10-hour photoperiods and this was attributed to the greater shoot growth
produced under 10-hour photoperiod which provided more shoots on which to produce flower buds
(Darnell, 1991).
Light
Without light plants cannot survive. Plants use light as a trigger for all stages of growth and development
such as seed germination, juvenility and flowering. Physiological acclimation responses to the
environment are also triggered by light and include stress tolerance, pigment and secondary metabolite
synthesis (Table 1) (Pocock, 2015). Decreasing levels of light intensity reduces FBI and flower bud
number as well as rate of fruit maturation (Aalders et al., 1969). In rabbiteye blueberries 83% of all
flower buds are formed in the top 0.6m of the canopy with relatively few buds formed in the lower
canopy where light levels tend to be less than 9 to 18% of full sun (Yanez et al., 2009). Principal
component analysis and multiple linear regression can be used to determine links between variable
temperature and luminosity (sunshine) and effect on growth and production. Development of fruit
consists of two phases: cell division and cell expansion. Both phases are related to the number of hours
the fruit was exposed to different temperature levels. Phytochrome is a light-sensitive, blue-green
pigment. It occurs in plant tissues in minute quantities - about one part in 10 million. The pigment acts as
an enzyme in other words, it activates certain processes without itself being used up. During daylight,
phytochrome is converted to an active form, and in darkness it is converted to an inactive form. If the
active form of phytochrome gets to a specific level for that plant, the enzyme can start changes in the
plant (Gettens and Klein, 1974).

Table 1. A selection of processes involved in temperature and light sensing in flowering plants.
Process
Level
Description
References
Photoreceptors
Cellular
Detect and respond to
dynamic changes of spectral
composition, light direction and
duration.
Galvao and
Fankhauser, 2015
Stomatal
opening
Biochemical
Opening and closing of
stomata for the control of gas
exchange and aid in
photosynthesis depending on
available light.
Zoulias et al.,
2018
Photoperiod
response
Molecular
Alignment of development for
favourable times of the year.
Bastow and
Dean, 2002
Hormone
signalling
Cellular
Regulation of plant growth and
development relies on energy
from light.
Liu et al., 2017
Seed
germination
Cellular and
molecular
Light activates and regulates
cellular and molecular
functions involved in cell
proliferation and growth.
Vandebrink et al.,
2014
Photosynthesis
Molecular,
biochemical
and cellular
Complex dynamic process
transforming radiant energy
into chemical bond energy
within carbohydrate molecules.
Brown and
Schwartz, 2008
Protein
Interactions
Molecular
Interactions between protein –
protein or protein – DNA can
be affected. Lower affinity
between proteins and weaker
DNA binding by transcription
factors.
Struk et al., 2019
Protein Stability
Molecular
Protein function can be
disturbed, or proteins
degraded by temperature
changes.
Vogt et al., 1997
Shade
Blueberries are an under storey crop which are found growing naturally in deciduous forests across
northern areas of the United States. The use of shade netting can be used to protect plants from some
environmental stress (temperature, radiation and relative humidity) without reducing potential yield.
Retamales et al. (2008) looked at different coloured shade netting in a blueberry plantation in Chile over
two seasons and reported an increase in yield using white, grey, or red nets (Retamales et al., 2008).
The positive effect of shading under extreme radiation is attributed to avoiding supra-optimal light levels,
plant heating and photosynthesis inhibition (Dale, 1992; Retamales et al., 2008). It was found that the
use of black netting however, reduced the level of PAR radiation by around 50% and saw decreases in
yield. It also saw an increase in shoot and leaf growth as more carbohydrates were partitioned towards
vegetative growth. Light is a major factor affecting leaf traits and photosynthesis, regulating plant growth
and survival and determining the geographical distribution of a plant. Photosynthetic photon flux density
(PPFD) is often below the saturation level of photosynthesis because of canopy shade and weed
shading, which often curtail growth and production in plants (Xu, 2019).
When plants are exposed to low PPFD, most plants show shade avoidance syndromes (decrease or
increase of leaf blade area and elongation of the petiole). The shade avoidance syndromes are typical
examples of leaf shape plasticity. Leaf responses to shade vary widely among plant species. In general,
leaf adaption to shade during leaf development considerably differs in structure, photosynthetic pigment
content, electron carriers and photosynthetic rates as compared to sun leaves. Shapes and sizes of
leaves are important factors influencing the absorption of light energy. To absorb sufficient light energy,
leaves must be as wide as possible. At the same time, to facilitate gas exchange (CO
2
, O
2
and H
2
0),
leaves must be as flat and thin as possible. However, if leaves are too wide and too thin, they will quickly
become desiccated. Thus, leaf area and thickness are mainly restricted by the availability of water.
Shade leaves usually invest assimilates in increasing leaf size to capture the maximum amount of
sunlight available for photosynthesis in a place where light levels are low (Jin Kim et al., 2011). The use
of shade nets has been shown to alter both the quality and quantity of light which a plant receives
(Shahak et al., 2004). Plants can detect these subtle changes as well as the orientation of the light and
this helps them to optimise their growth and development in any given environment as they determine
the distribution of carbohydrates, water and nitrogen to survive (Rajapakse et al., 1999).
Day length
Research has shown that the conversion of vegetative buds to flower buds is controlled by day length
and temperature. Day length is measured by special photoreceptors found within the leaves and buds.
As days shorten during the Autumn, vegetative buds gradually convert to flower buds. Experiments have
shown that high temperatures reduce flower bud initiation, even when days are short enough to induce
them. Additionally, flower buds initiated under high temperature are smaller and do not develop as well
as those initiated under lower temperature (Williamson et al., 2004).
In Autumn, blueberry bushes become dormant and prepare for winter. The blueberry bush uses the cue
of shortened day length in the late summer to prepare for winter. The next is lower temperatures near
freezing. Freezing temperatures are the final cue, and the bushes go dormant waiting for winter.
Providing the cold weather does not arrive too abruptly the bushes have little to worry about. The
cultivars can easily handle temperatures down to 0
o
C while below freezing temperatures stimulate the
plant to acclimate and increase its cold hardiness. Significantly colder temperatures of -15
o
C and below
can result in winter injury to buds and shoots.
Temperature
Perennial woody fruit species cultivated in temperate zones synchronize their annual growth patterns
with seasonal environmental changes. During unfavourable winter conditions, temperate fruit species
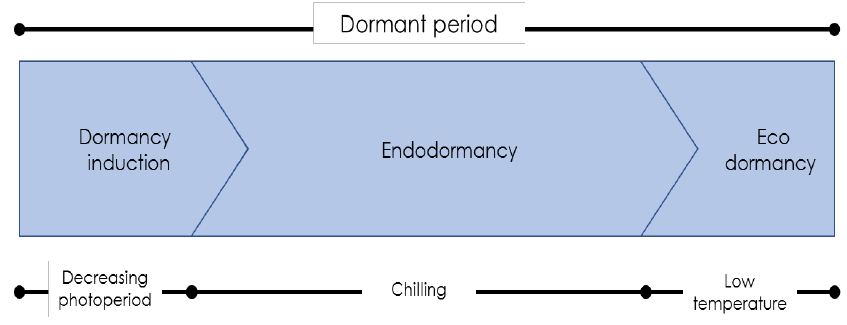
use bud dormancy as a defensive mechanism. Bud dormancy is classified in two stages (Figure 1):
Endodormancy: Buds are latent due to internal factors, and even under favourable environmental
conditions they are unable to grow. Dormant buds will not grow until certain biochemical changes occur.
Accumulation of chilling promote the changes that break dormancy of buds.
Ecodormancy: After chilling is completed the plants are dormant only because environmental factors
(cold or cool weather, day length) are preventing growth. Buds can be induced to break by exposure to a
specific ‘amount’ of heat. Evidence suggests that chilling and heat needs are not entirely fixed, but can
partially compensate for each other, but the exact nature of this compensation is poorly understood.
Figure 1. The relative contribution of the various types of dormancy during a hypothetical dormant
period (Lang et al., 1987).
Climate change is resulting in declining chilling (causing poor and erratic bud break) seen in
some environments and is predicted to continue to decline, which has resulted in winter chill
being recognised as a potential limiting factor for fruit production in deciduous woody plants.
Adequate winter chill is a pre-requisite for successful commercial production of many
temperature fruit crops (Atkinson et al., 2013). Chilling requirement (CR) generally calculated as
cumulative hours at temperatures of 7.2
o
C or below varies between 150 and 1500 hours across
blueberry species. Blueberry floral bud initiation often starts before endodormancy. Sufficient
chill accumulation during endodormancy is required to break dormancy along with a warming of
temperature. Hormone and flowering pathways are also involved in dormancy release.
Crop management strategies
An appropriate balance of vegetative and reproductive buds is required to maximise yields of high-
quality fruit. If there are too few flower buds, then the yield potential will not be maximised, too many,
relative to the vegetative buds, then the berries may exceed what the bush can support. This can result
in poor leafing, small berries, delayed harvest, poor fruit quality and plant stress or even plant death.
Blueberry yields (Kg per plant) are dominated by two principal factors: the number of fruits per plant and
their fresh weight. The number of fruits available can be estimated from the number of flower buds left
after pruning and by the bud density per branch (Bowen and Eaton, 1983).
The yield of blueberry plants depends on a series of both internal (genetic) and external factors (growing
practices, stimulants, climate). The flower buds in blueberries contain clusters of flowers. There are five
to 12 flowers per cluster depending on cultivar. The flowers emerge as a tightly packed cluster. As the
flowers develop, the corolla (petal tube) is visible as pink tissue at the tip of the flowers (pink bud). As
the flowers develop, the corolla expands and the pink fades to white. Bloom begins when the tip of the
corolla opens (first bloom). The flower clusters at the shoot tip bloom first, with bloom then progressing
down the shoot. The flowers at the base of each cluster open first. The first flowers pollinated have the
potential to be the largest fruit. Fruit from the later blooms are smaller. The early period of fruit growth is
very important in determining final fruit size. For several weeks, the fruit grows quickly by cell division.
Then, cell division in the fruit stops, and the fruit switches to growth by cell enlargement. Bigger fruits
have more cells, so the final size of the fruit is determined in the month immediately after bloom
(Longstroth, 2020).
As the fruit begins to grow, the fruit and shoots compete for the available resources and shoot growth
slows down. Maintaining good soil moisture during the growing season allows the roots to maintain an
adequate flow of water to the leaves, sustaining growth. By the time the fruit starts its final swell before
harvest, most of the shoot and leaf growth for the season has stopped. After harvest, the plant prepares
for next year’s growth by storing reserves, but this has been found to be limited in some
blueberry cultivars, at least in the UK (Petridis et al., 2018). In the autumn, flower buds are formed for
next year’s crop. The terminal shoot bud becomes fat and plump as it changes from a leaf bud to a
flower bud and under favourable conditions, other leaf buds below the terminal bud will also change to
fruit buds (Longstroth, 2020).
Nutrients
Nutrient management in blueberry production is crucial for successful and productive plantings. When
various chemical elements are inadequate, the general health, yield and quality of the crop may be
reduced so long as other factors are not limiting. Severe nutrient deficiency can lead to discoloured
leaves, wilted flowers, reduced yields, decreased cane initiation and growth, and eventually plant death.
Calcium and Boron have been used as supplemental fertilisers in blueberry production and have the
potential to positively influence fruit set and yield. The absorption of nutrients into the fruit tissue relies
on the passage of nutrients through the cuticle and stomata (Toselli et al., 2009). For effective uptake of
nutrients to fruit tissue with foliar applications, adequate surface contact is required. A soil application of
Nitrogen (4.5-6.5kg per Acre) may help to stimulate leaf growth. Frequent light fertilizer applications
throughout the summer are recommended to promote new growth of fruit-bearing wood and for longer
leaf retention into autumn. A warning however, that excessive late summer or autumn fertilization could
delay cessation of terminal growth and reduce or delay flower bud formation (Williamson et al., 2004).
Calcium is an essential macronutrient in blueberry production. The roles include structural (calcium
bound to pectin cell walls), defence and communication (between cells and tissues). Calcium is
particularly important for the function of calcium ion transporters and their subsequent role in
environmental and biotic responses. A major concern with commercial production of some cultivars i.e.
Draper, is abscission of unripe fruit just prior to berry colouring. Reports of crop losses due to
premature fruit drop in ‘Draper’ have been reported as high as 65%. While the main cause of premature
fruit drop remains unclear, research has suggested a calcium (Ca) deficiency in fruit is involved and that
foliar applications of Ca can reduce subsequent drop. One study showed that fruit drop was reduced
following the application of Ca while the exposure to 50% shade was found to exacerbate fruit drop
(Arrington and DeVetter, 2017). Strik and Vance (2015) previously demonstrated a predisposition to
poor Ca partitioning in ‘Draper’ and some cultivars with ‘Draper’ parentage have shown a similar pre-
disposition. Calcium is immobile in the plant and tends to be found in elevated concentrations in tissue
adjacent to the xylem (Shear and Faust, 1970). Speculations surrounding the Ca deficiencies in ‘Draper’
pointed towards the result of many competing shoot tips during fruit development. This is likely to result
in Ca movement in the xylem to transpiring leaves, thus supporting observations of increased fruit drop
in vigorous plantings with excessive growth. Seasons with reduced solar radiation and daytime air
temperature can see increased fruit drop as transpiration rates are reduced under such conditions.
Carbohydrate status
Blueberry leaves are the major organs that produce photosynthates and perceive changes in day length.
They are therefore vital for flower bud initiation and development. In Rabbiteye plants, net CO
2
assimilation was found to increase dramatically in leaves of ‘Beckyblue’ and ‘Climax’ developed under
short days. This increase in net CO
2
was also accompanied by an increase in stomatal conductance
(Darnell, 1991). For woody, perennial crops such as blueberry, naturally shortened photoperiods
precede the onset of dormancy, and carbohydrate reserves required for dormancy and subsequent
spring bud break begin to increase. Therefore the increase in CO
2
assimilation found in blueberry under
short days may be in response to the onset of dormancy and the initiation of carbohydrate reserve
accumulation. It has been reported however, that blueberries grown in the UK tend to have limited
carbohydrate reserves and instead work on a “current account” of newly assimilated carbon (Petridis et
al., 2018).
Irrigation
Water stress can occur quickly in blueberries and takes only a few days without irrigation to result in
reduced growth and fruit production due to photosynthetic limitation. Over watering is equally damaging
in blueberries and can result in root function impairment, and an increase in soil erosion and
nutrient leaching (Bryla, 2011). During periods of warm weather, the net photosynthesis in blueberries
declines significantly and the higher leaf temperatures results in an increase in water use. If these high
temperatures coincide with fruiting, as they often do in the UK, this can result in both water and
carbohydrates being diverted away from the fruit to the leaves (Bryla, 2011). Plant water deficit during
FBI period can also reduce flower bud and fruit number for the following season (Perrier et al., 2000). It
is important to maintain good soil moisture in August and September. While the demand for water
declines with cooler and shorter days, there is still a significant demand for water. Daily water losses can
often exceed 0.2 inches per day during the hot summer days in a well irrigated field. This can fall to less
than 0.1 inch per day during the cooler days. This means the bushes still need approximately half-inch
of water or more every week. Continuing irrigation throughout Autumn is a good way to guarantee you
have a good number of flowers for the following spring’s bloom. Autumn is also when the plant develops
the cold hardiness necessary to withstand winter cold. Plants that are suffering from drought-stress have
less reserves and cannot withstand as much cold as healthier plants. Having good soil moisture leading
into winter also ensures the plant will be better able to withstand winter cold by moderating soil
temperatures (Bryla, 2011).
Pruning
One of the goals of winter pruning is to bring fruit and leaf buds into balance on the bush. Heavier
pruning reduces fruit but increases leaf canopy for the following year. A bush without leaves cannot
produce the energy it needs to ripen the berries. So even though there may be many berries, they will
likely be undersized and under sweet if they ripen at all. Further, the bush will not have sufficient energy
to put into a) next year’s buds or b) root system which could lead to plant stress (Figure 2).

Fruit removal could also be carried out manually to reduce fruit load in overburdened bushes. This
should be done by early July especially in bushes less than three years old or the excess stress could
limit future plant growth. Fruit removal will also increase the quality of remaining fruit as well as reducing
stress and increase potential yields for the following year.
Figure 2. Dormant blueberry pruning of “Bluecrop” bushes, before and after (Pavlis, 2005).
Plant growth regulators
Plant flowering and size are affected by a range of phytohormones such as abscisic acid (ABA),
auxin, cytokinin, ethylene, brassinosteroid, jasmonic acid, nitric oxide, gibberellic acid (GA),
peptide hormone and salicylic acid. Pathway genes for the major phytohormones have been
associated with the regulation of transcript levels (upregulated or downregulated) in plants found
to have altered plant growth (dwarfing habit) and delayed flowering (Gao et al., 2016).
Stimulants are often used across the USA to encourage vegetative budding and accelerate the
development of flower buds, an example of which is hydrogen cyanamide
sprays (H
2
CN
2
). The concentration required and its application rate and timing, would depend on
the amount of the cold weather to which the plant was exposed to during the winter (Williamson
et al., 2001). Hydrogen cyanamide however, is not approved for use in the UK. Gibberellic acid
can also be used to control the number of flower buds in early-fruiting cultivars, improving the
yield in subsequent harvests which currently has UK approval (Black and Ehlenfeldt, 2007).
Plant size is determined by genetic potential as well as environmental (biotic or abiotic)
conditions. The development of new genomic and biotechnological tools will facilitate the
knowledge within blueberry genetics and genomics and this will lead to an increase in breeding
efficiency. Traditional breeding is both time consuming and expensive but as genetic resources
develop it may be possible to select for genes within flowering pathways that can influence the
regime of blueberry flowering and increase fruit production (Walworth et al., 2016). Targets
could include flowering pathway genes like flowering locus C (FLC) and MADS affecting
flowering-2 like (MAF2), phytohormone related genes such as ABA related 2 (ABF2) and
ethylene responsive and brassinosteroid related (RAV1) or photoperiod pathways like constans-
like 5 (col5).
C
o
nc
l
us
i
o
ns
The floral transition is an essential process in the life cycle of flowering plants because their
reproductive success depends on it. Flowering plants must therefore respond to many environmental
signals and stimuli to adapt and cope effectively. While growers are limited by their own environment, in
terms of light and temperature, there are certainly measures that can be put in place to optimise bud
initiation and fruit yield in blueberries. Maximising the light levels by using reflected mulches or
appropriate tunnel polythene alongside selecting cultivars proven to be adaptive to local climate is a
good place to start but further work is required to confirm how well they would work under UK
conditions.
Further work
1) Demonstrate that good management practices such as nutrition, pruning and cultivar
selection can influence bud initiation in blueberries and look at optimising light levels
in tunnels using reflective mulches.
2) Assess the effect of different polythene on light levels and temperatures within
tunnels and the impact on bud initiation, yield stability and fruit quality.
3) Compare the performance of a wide range of commercial cultivars and how they can
be grown for optimum yield under UK conditions.
4) Study the genetic potential for flowering and yield by identifying key genes across the
regulatory pathways and comparing their expression between cultivars and under
differing environmental conditions.
R
eferen
c
es
Aalders, L.E. and Hall, I.V. (1964). A comparison of flower bud development in the lowbush
blueberry V. angustifolium Ait. Under greenhouse and field conditions. Proc. Amer. Soc. Hort.
Sci. 85: 281-284
Aalders, L.E., Hall, I.V. and Forsyth, F.R. (1969). Effects of partial defoliation and light intensity
on fruit set and berry development in the lowbush blueberry. Hort, Res. 9: 124-129
Arrington, M., & DeVetter, L. (2017). Foliar Applications of Calcium and Boron Do Not Increase
Fruit Set or Yield in Northern Highbush Blueberry (Vaccinium corymbosum), HortScience horts,
52(9), 1259-1264.
Atkinson, C.J., Brennan, R.M. and Jones, H.G. (2013). Declining chilling and its impact on
temperate perennial crops. Environ. and Exp. Bot. 91: 48-62,
Banados, M.P. and Strik, B. (2006). Manipulation of the annual growth cycle of blueberry using
photoperiod. Acta. Hort.715: 65-71
Bastow,R. and Dean, C (2002).The Molecular Basis of Photoperiodism, Dev. Cell,3 (4): 461-462
Black, B.L. and Ehlenfeldt, M.K. (2007). Foliar applications of GA (4+7) reduce flowering in
highbush blueberry. Hortscience 42 (3), 555-558
Bowen, P.A. and Eaton, G.W. (1983). Yield component analysis of winter damage and flower
buds in highbush blueberry. Sci. Hortic. 19 (3-4), 279-286
Brown, M.H. and Schwartz, R.S. (2008). Connecting photosynthesis and cellular
respiration:Preservice teachers perception. J. Rs Sci. Teach. 46 (7): 791-812
Bryla, D. (2011). Crop Evapotranspiration and Irrigation Scheduling in Blueberry. 10.5772/18311.
Dale, J.E. (1992). How do leaves grow: advances in cell and molecular biology are unraveling
some of the mysteries of leaf development. BioScience. 42: 423-432
Darnell, R.L. (1991). Photoperiod, carbon partitioning, and reproductive development in
rabbiteye blueberry. J. Amer. Soc. Hort. Sci. 116: 856-860
Galvão, V.C. and Fankhauser, C. (2015). Sensing the light environment in plants:
photoreceptors and early signaling steps, Curt Op in Neuro. 34:46-53
Gao, X., Walworth, A.E., Mackie, C. and Song, G. (2016). Overexpression of blueberry
FLOWERING LOCUS T is associated with changes in the expression of phytohormone-related
genes in blueberry plants. Hortres. 3,
Gettens Hayes, R. and Klein, W.K.(1974) Spectral quality influence of light during development of
Arabidopsis thaliana plants in regulating seed germination, Plant and Cell Phys. 15 (4) : 643–653
Hall, I.V., Craig, D.L. and Aalders, L.E. (1963). The effect of photoperiod on the growth and
flowering of the highbush blueberry (Vaccinium corymbosum L.) Proc. Amer. Soc. Hort. Sci. 82:
260-263
Jin Kim, S., Jun Yu, D., Kim, T.C. and Jae Lee, H. (2011). Growth and photosynthetic
characteristics of blueberry (Vaccinium corymbosum cv. Bluecrop) under various shade levels.
Sci. Horti 129, 486-492
Kurlovich, T. (2020). Effect of length and thermal time on the growing season on blueberry
production. BIO Web of Conferences 24, 1-5
Lamers, J., van der Meer, T. and Testerink, C. (2020). How plants sense and respond to
stressful environments. Plant Phys. 182: 1624-1635
Lang, G.A., Early, J.D.,Martin, G.C. and Darnell, R.L. (1987). Endo, para and ecodormancy:
physiological terminology and classification for dormancy research. Hort Sci. 22, 371-377
Liu, X., Li, Y. and Zhong, S. (2017). Interplay between Light and Plant Hormones in the Control
of Arabidopsis Seedling Chlorophyll Biosynthesis. Front. in Plant Sci. 8:1433
Longstroth, M. (2020). Annual growth cycle of northern highbush blueberry. MSU Extension.
Accessed online: https://www.canr.msu.edu/news/annual-growth-cycle-of-northern-highbush-
blueberry
Pavlis, G. (2005). Establishing blueberries in the home garden. Factsheet from the State
University of New Jesey: https://njaes.rutgers.edu/FS750/
Perrier, C., Mingeau, M. and Ameglio, T. (2000). Effects of water stress on transpiration, radial
growth and yield in highbush blueberry. Acta Hort. 537: 923-928
Petridis, A., van der Kaay, J., Chrysanthou, E., McCallum, S., Graham, J., & Hancock, R. D.
(2018). Photosynthetic limitation as a factor influencing yield in highbush blueberries (Vaccinium
corymbosum) grown in a northern European environment. J of exp. Bot. 69 (12): 3069–3080.
Pocock, T. (2015). Light-emitting diodes and the modulation of specialty crops: Light sensing
and signalling networks in plants. Hort Sci. 50 (9) 1281-1284
Rajapaske, N.C., Young, R.E., MacMahon, M.J. and Oi, R. (1999). Plant height control by
photoselective filters: current status and future prospects. Hort Tech. 9: 618-624
Retamales, J., Montecinos, J., Lobos, G., and Rojas, L.A. (2008). Colored shading nets increase yields
and profitability of highbush blueberries. Acta horticulturae. 770. 193-197.
Shahak, Y., Gussakovsky, E.E., Cohen, Y., Laurie, S., Stern, R., Kfir, S., Naor, A., Atzmon, I., Doron, I.
and Greenblat-Avron, Y. (2004). ColorNets: a new approach for light manipulation in fruit trees. Acta
Hort. 634: 609-616
Shear, C.B. and Faust, M. (1970). Calcium Transport in Apple Trees. Plant. Phys. 45 (6) 670-674
Strik, B.C. (2012). Flowering and fruiting on command in berry crops. Acta. Hort. 926: 197-214
Strik, B.C. (2015). Manipulation of the annual growth cycle of blueberry using photoperiod. Acta.
Hort. 715: 65-71
Strik, B. C., & Vance, A. J. (2015). Seasonal Variation in Leaf Nutrient Concentration of
Northern Highbush Blueberry Cultivars Grown in Conventional and Organic Production
Systems, HortScience horts, 50(10), 1453-1466.
Struk, S., Jacobs, A., Sanchez Martín‐Fontecha, E., Gevaert, K., Cubas, P., Goormachtig, S.
(2019). Exploring the protein–protein interaction landscape in plants. Plant Cell Environ. 42:
387– 409.
Toselli, M., Scudellari, D., Fernandez, V. and Abadia, J. (2009). Foliar nutrition of fruit trees.
Italus Hortus. 16 (1): 45-54
Yanez, P., Retamales, J.B., Lobos, G.A. and del Pozo, A. (2009). Light environment within
mature rabbiteye blueberry canopies influences flower bud formation. Acta. Hort. 801: 471-473
Vandenbrink, J.P., Kiss, J.Z., Herranz, R. and Medina, F.J. (2014). Light and gravity signals
synergise in modulating plant development. Frontiers in Sci (5) 563
Vogt, G., Woell, S. and Argos, P. (1997). Protein thermal stability, hydrogen bonds, and ion
pairs. J. of Mol. Bio. 269: 631-643
Walworth, A.E., Chai, B. and Song, G. (2016). Transcript profile of flowering regulatory genes in
VcFT-Overexpressing blueberry plants. Plos One.11(6)

Williamson, J.G., Olmstead, J.W. and Lyrene, P.M. (2004). Reproductive growth and
development of blueberry. HS976. UF/IFAS Extension. Online http://edis.ifas.ufl.edu.
Williamson, J.G., Maust, B.E. and NeSmith, D.S. (2001). Timing and concentration of hydrogen
cyanamide affect blueberry bud development and flower mortality. HortScience, 36(5), 922-924
Xu,Y. (2019).Chapter 2.1 - Nature and Source of Light for Plant Factory, Editor(s): Masakazu Anpo,
Hirokazu Fukuda, Teruo Wada, Plant Factory Using Artificial Light,Elsevier,Pages 47-69,
Zoulias, N., Harrison, E. L., Casson, S. A., & Gray, J. E. (2018). Molecular control of stomatal
development. The Biochemical journal, 475(2), 441–454. https://doi.org/10.1042/BCJ20170413
While the Agriculture and Horticulture Development Board seeks to ensure that the information contained
within this document is accurate at the time of printing, no warranty is given in respect thereof and, to the
maximum extent permitted by law, the Agriculture and Horticulture Development Board accepts no liability fo
loss, damage or injury howsoever caused (including that caused by negligence) or suffered directly or
indirectly in relation to information and opinions contained in or omitted from this document.
Reference herein to trade names and proprietary products without stating that they are protected does not
imply that they may be regarded as unprotected and thus free for general use. No endorsement of named
products is intended, nor is any criticism implied of other alternative but unnamed products.
© Agriculture and Horticulture
Development Board 2020
