
Air separation plants
History and technological progress
in the course of time
Making our world more productive


N₂
O₂
Ar
Linde based his experiment on findings
discovered by J. P. Joule and W. Thomson
(1852). They found that compressed air
expanded in a valve cooled down by approx.
0.25°C with each bar of pressure drop. This
proved that real gases do not follow the
Boyle-Mariotte principle, according to which
no temperature decrease is to be expected
from expansion. An explanation for this effect
was given by J. K. van der Waals (1873), who
discovered that the molecules in compressed
gases are no longer freely movable and
the interaction among them leads to a
temperature decrease after decompression.
Carl von Linde in 1925.
History and technological progress of air separation 03
When and how did air separation start?
Liquefaction process of air separation
Water cooler
p₂t
5
p₁t₄
p₂t₁
p₁t
3
p₂t₂
t₃
For his experiment, air was compressed
from 20 bar [p₁] [t₄] to 60 bar [p₂] [t₅] in
the compressor and cooled in the water
cooler to ambient temperature [t₁]. The pre-
cooled air was fed into the countercurrent
heat exchanger, further cooled down [t₂]
and expanded in the expansion valve
(Joule-Thomson valve) [p₁] to liquefaction
temperature [t₃]. The gaseous content of the
air was then warmed up again [t₄] in the heat
exchanger and fed into the suction side of
the compressor [p₁]. The hourly yield from
this experiment was approx. three litres of
liquid air.
In May 1895, Carl von Linde performed an experiment in his laboratory in Munich that
led to his invention of the first continuous process for the liquefaction of air based on the
Joule-Thomson refrigeration effect and the principle of countercurrent heat exchange. This
marked the breakthrough for cryogenic air separation.
Nitrogen 78.08%
Oxygen 20.95%
Argon 0.93%
Neon 0.0018%
Helium 0.0005%
Krypton 0.00011%
Xenon 0.000009%
Composition of air
Separator
Expansion valve
Compressor
Liquid air
Heat exchanger
Gaseous air

04 History and technological progress of air separation
What are the physical properties of air
required for liquefaction?
To enable air to be separated into its
constituents by means of rectification – the
actual separation process – a large part
of the air volume used must be liquefied.
A gas can only be transformed into a
liquid state at temperature and pressure
conditions below those of its critical point.
The critical point of air is T
crit
= –140.7ºC
(132.5 K) and P
crit
= 37.7 bar. In other words,
air can be liquefied only at temperatures
below –140.7ºC (132.5 K).
The vapour pressure curve illustrates the
temperatures and pressures at which a gas
condenses or a liquid evaporates.
→ Air below atmospheric pressure (1 bar)
must be chilled to –192ºC (81.5 K) before it
starts to condense
→ Air below a pressure of 6 bar must be
chilled to –172ºC (101 K) before it starts to
condense
The boiling point and condensation conditions
of gas mixtures such as air are not identical.
A condensation line and a boiling point line
delineate the boiling point range.
Vapour pressure curves of atmospheric gases
Critical point V Start of evaporation K Start of condensation
0°C Water freezes
Boiling points
–107°C Xenon
–152°C Krypton
–183°C Oxygen
–186°C Argon
–196°C Nitrogen
–246°C Neon
–273°C Absolute zero
50
10
6
1
5
0.5
60 100 14080 160
Pressure in bar
Temperature in K
120
101
81.5
V K
Air
Vapour
Liquid
N₂
O₂
Ar

Rectification is synonymous with counter-
current distillation. This special distillation
separation process enables the individual
components of a mixture to be separated
with a high purity combined with a good
yield, even when their boiling points are
relatively close to each other.
As a result of the different vapour pressures
of the individual components (pN₂ > pO₂), the
composition of the vapour differs from that of
the liquid mixture.
The vapour produced from a boiling liquid
mixture of O₂/N₂ will thus have a higher N₂
concentration than the liquid mixture from
which it originates.
History and technological progress of air separation 05
What is rectification of air?
Liquid
Condensation
temperature
T
S
at P
S
= 1 bar
Boiling point
temperature
T
S
at P
S
= 1 bar
Vapour
77
0
Temperature in K
O₂ concentration in O₂/N₂ mixture % by volume
20 40 60 80 100
90
Boiling point diagram of O₂/N₂ mixtures
06
Process air
Nitrogen
with 7% O₂
Pure oxygen
Single column
Air separation by rectification in a single/double column
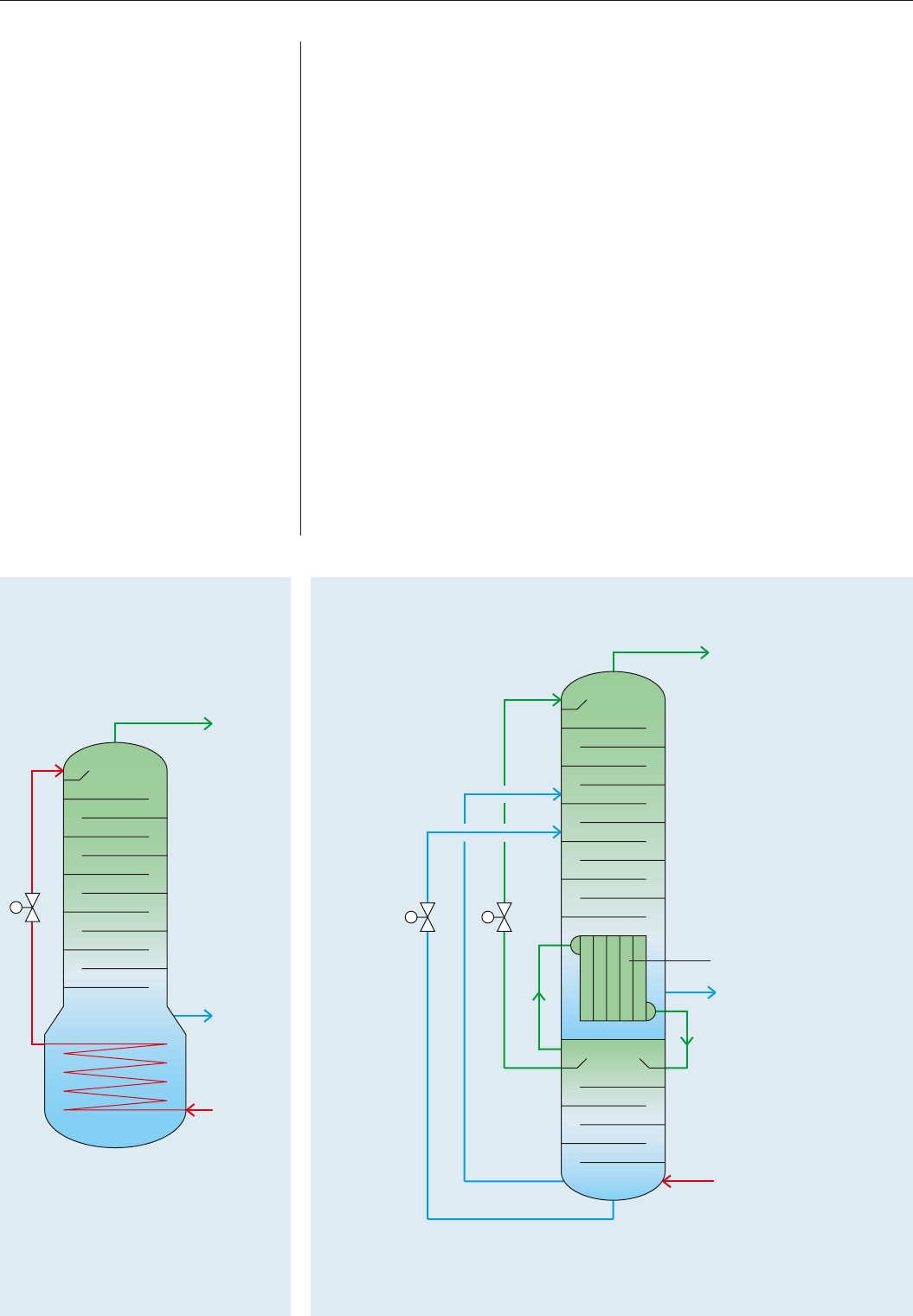
Using his air liquefaction principle as a basis, Carl von Linde constructed the first air separation
plant for oxygen production in 1902 using a single-column rectification system.
In 1910, he established the basis for cryogenic air separation with the development of a double-
column rectification system. Now it was possible to produce pure oxygen and pure nitrogen
simultaneously.
This involves installing a pressure column below the low-pressure column. At the top of this
pressure column, pure nitrogen was drawn off, liquefied in a condenser and fed to the top
low-pressure column as reflux. At the top of the low-pressure column, pure gaseous nitrogen
was withdrawn, while liquid oxygen evaporated at the bottom of this column to deliver pure
gaseous oxygen. This principle of double-column rectification combining the condenser and
evaporator to form a heat exchanger unit is still used today.
History and technological progress of air separation
Liquid N₂
Pure nitrogen
Condenser
Pure
oxygen
Process air
Liquid with 35–40% O₂
Double column
What are the principles of air separation?
1.5 bar
5.6 bar
Low-pressure column
Pressure column

07
Condenser
1.5 bar
5.6 bar
History and technological progress of air separation
Condenser/reboiler
The principle of double-column rectification is characterised by the combination of condenser
and evaporator to form a common heat exchanger unit. This divides the rectification into two
separate areas with different pressures.
Condenser
N₂
1.0
70
Pressure in bar
Temperature in K
75 80 85 95 120
9.0
Vapour pressure of N₂ and O₂
0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
11511010510090
O₂
dT=1.4K
95.5
5.6
94.1
1.5

08
What happens inside a column?
Any tray of the rectification column follows
the same principle:
The O₂ concentration of the boiling O₂/N₂
liquid mixture F is greater than the O₂
concentration of the vapour D. A certain
volume of liquid corresponding to the same
volume of reflux constantly flows from the
tray above into the liquid mixture below with
an equivalent volume flowing down over a
weir onto the tray below.
The vapour D
u
coming from the bottom tray
penetrates the liquid mixture F and has a
higher O₂ content than the vapour mixture D.
The O₂ concentration of the vapour D
o
rising
from the upper tray is in turn less than that
of the vapour D. Thus a gas rich in nitrogen
is obtained in the head of the column and a
liquid rich in oxygen is obtained in the sump
of the column.
History and technological progress of air separation
Vapour
Liquid
D
O
F
O
D
F
D
U
F
U
Fabrication of sieve tray column.
Principle of sieve trays

09History and technological progress of air separation
Structured packings
1991
World’s largest
air separation plant with
packed columns
Packed column.
Significant progress in air separation
technology was made in the mid-1980s. For
the first time, structured packings were used
in cryogenic rectification. Packed columns
work in a similar way to sieve trays. The
intensive contact between liquid and vapour
required for the rectification takes place on
the huge surface area of the packing material.
Liquid flowing down becomes increasingly
richer in oxygen, whereby the ascending
vapour is enriched with nitrogen. The main
benefits of packed columns compared with
sieve trays are a lower pressure drop and
consequently a lower power consumption for
the air separation process. Another important
advantage of packed columns is the possible
loading range including a very high turn down
to nearly 30%. This also forms the basis for a
new process for argon separation.
Principle of structured packings
Downflow of liquid O₂
Rising N
2
gas

10
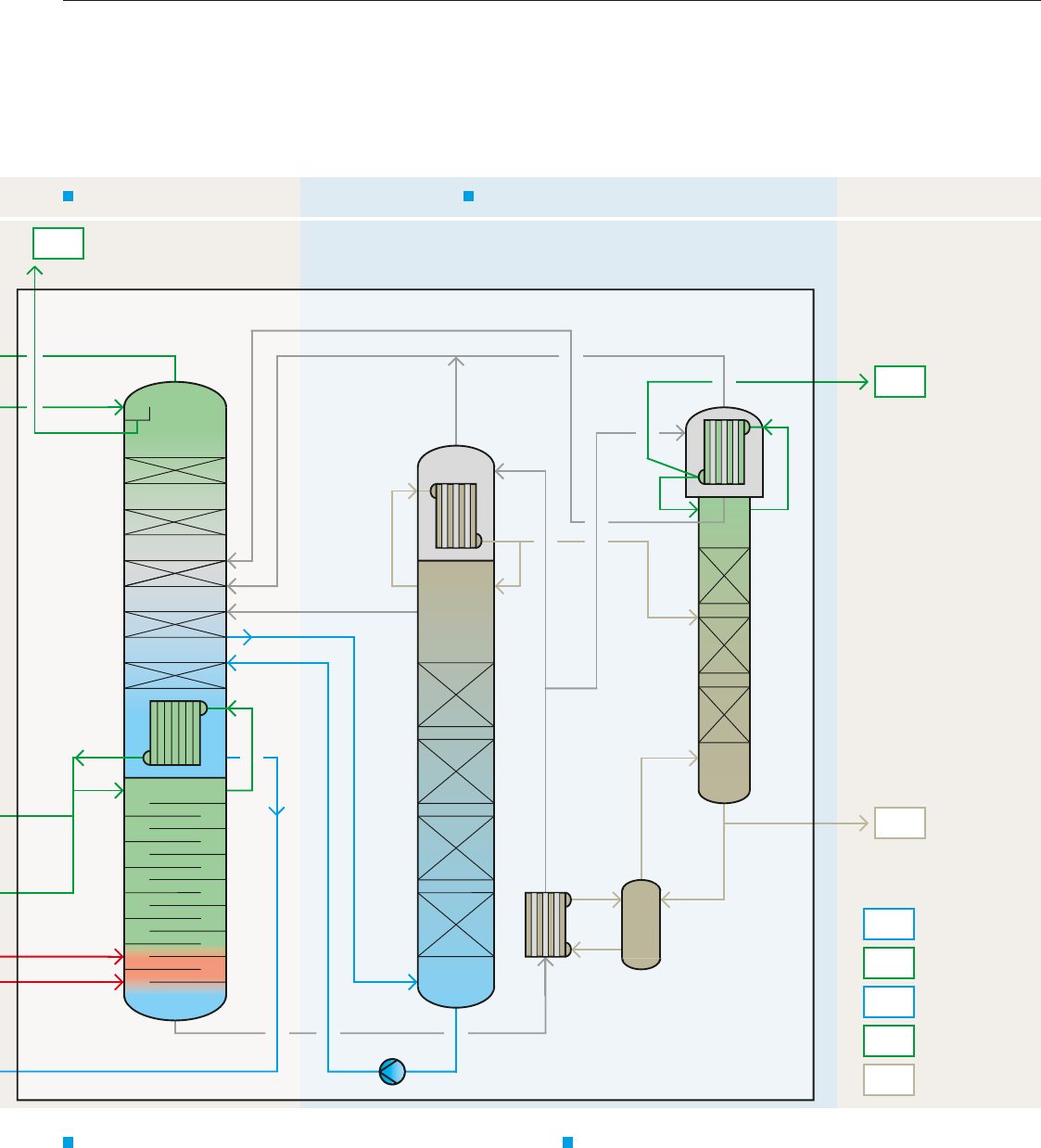
What does a typical cryogenic air separation process look like?
GOX GAN LOX
AIR
Air compression
Air compressor
Molecular sieve
unit
Direct
contact
cooler
Evaporation cooler
Water pump
Air booster
compressor
Coldbox
Expansion
turbine
Heat
exchanger
Sub-
cooler
Cryo pump for
int. compression
Cryo pump for
int. compression
1
Air cooling and purification
2
Cold production and internal product compression
3
1
Air compression
→ Compression of ambient air by a multi-stage turbo compressor with
intercoolers at a supply pressure of approx. 6 bar.
→ Removal of dust particles by a mechanical air filter at the inlet of
the compressor.
2
Air cooling and purification
→ Cooling of process air with water in a direct contact cooler and
removal of water soluble air impurities.
→ Chilling of cooling water in an evaporation cooler against dry
nitrogen waste gas from the rectification process.
→ Removal of CO₂, water and hydrocarbons from the process air in
periodically loaded/regenerated molecular sieve adsorbers.
3
Cold production and internal product compression
→ Cooling of process air in heat exchangers down to nearly
liquefaction temperature by means of countercurrent with gas
streams from the rectification process.
→ Further compression of a sidestream of process air by an air booster
compressor. Expansion and cold production of the boosted air
stream in an expansion turbine.
→ Expansion and liquefaction of a sidestream of the boosted air in a
liquid separator.
→ Evaporation and warming to ambient temperature of the pumped
oxygen and nitrogen product in high-pressure heat exchangers.
History and technological progress of air separation
11
LIN
ATM
LAR
Cryogenic rectification of air Cryogenic rectification of argon
GOX
GAN
LOX
LIN
LAR
gaseous oxygen
gaseous nitrogen
liquid oxygen
liquid nitrogen
liquid argon
Pressure
column
Condenser/
reboiler
Low-pressure
column
Crude argon
column
Pure argon
column
4 5
4
Cryogenic rectification of air
→ Pre-separation of the cooled and liquefied air within the pressure
column into oxygen-enriched liquid in the column sump and pure
nitrogen gas at the column top.
→ Liquefaction of the pure nitrogen gas in the condenser/reboiler
against boiling oxygen in the sump of the low-pressure column.
Liquefied nitrogen provides the reflux for the pressure column and
(after sub-cooling) for the low-pressure column.
→ Different types of condenser are described in detail on page 16.
→ Further separation of the oxygen-enriched liquid within the low-
pressure column into pure oxygen in the sump and nitrogen waste
gas at the top.
5
Cryogenic rectification of argon
→ Argon-enriched gas from the low-pressure column is transformed
into oxygen-free crude argon by means of separation within the
crude argon column.
→ Pumping back liquid oxygen from the crude argon column sump into
the low-pressure column. Removal of the remaining nitrogen in the
pure argon column.
History and technological progress of air separation
Coldbox

Milestones in air separation.
12
1902
1904
1910
1930
1950
World’s first air separation
unit (ASU) for oxygen production
World’s first air separation plant for
the recovery of nitrogen
World’s first air separation plant
using the double-column rectification
process
Development of the Linde-Fränkl
process for air separation
First Linde-Fränkl oxygen plant
without pressure recycling and stone-
filled reactors
1954
1968
1981
1984
World’s first air separation plant with
air purification by means of adsorbers
Introduction of the molecular sieve
technology for pre-purification of air
Introduction of the elevated pressure
process
World’s largest VAROX air separation
plant with variable oxygen flow
adjustment
History and technological progress of air separation
1978
Internal compression of oxygen applied
to tonnage air separation plants, p. 14
1988
First columns with structured packings

13
1997
Largest air separation plant built for
N₂ with capacity of 5 x 10,000 tpd,
fifth train added in 2004, p. 18–19
2012
2015
2016
2017
Flexible high air pressure process,
p. 14
Simple filling of dual-bed radial
adsorber
Optimised fins for high-pressure
PFHEs in ASUs
Trouble-free start-up of largest ASU
complex in the world
6 x 3,600 tpd of oxygen , p. 22–23
Start-up of world’s largest
air separation plant
5 x 5,250 tpd of oxygen, p. 24–25
History and technological progress of air separation
1990
1991
1992
1993
Linde introduced argon
production by rectification, p. 15
World’s first remotely controlled air
separation plant with unmanned operation
World’s largest air separation plant
with packed columns
Ultra-pure gases production in
air separation plants
First world-scale radial adsorbers in
large air separation plants
2006
2008
2010
2011
Largest EPC contract in history
of air separation with
8 x 3,800 tpd O₂, p. 20–21
Reflux condenser in crude argon
column, p. 16
Advanced cryogenic process,
efficiency optimised for
CCS application (oxyfuel, IGCC)
Argon production without
pure argon system, p. 15
2000
Development of the advanced
multi-stage bath-type condenser, p. 16

14
Internal compression
The internal compression (or liquid pumping)
process allows for oxygen, nitrogen as well as
argon to be compressed within the coldbox by
means of liquid pumps, to be evaporated and
warmed up in heat exchangers, and finally to
be supplied to the end user at the required
pressure.
In order to evaporate and warm up the
compressed liquid, a countercurrent stream
of air with a higher pressure than the liquid is
required for thermodynamic reasons.
For plants that produce pressurised nitrogen,
the booster and/or recycle nitrogen
compressor also provide the countercurrent
stream for evaporation. With this method,
complex external oxygen compression is no
longer required, thus plant operation and
maintenance have become considerably
easier and more reliable. Furthermore, the risk
of dangerous hydrocarbon enrichment in the
condenser is avoided because liquid oxygen is
continuously withdrawn from the condenser
and pumped into the heat exchanger, where
it evaporates. Compared with the external
compression system, a considerably higher
level of safety has been achieved.
1978
Internal compression
of oxygen
History and technological progress of air separation
Booster compressor
75 bar
Heat exchanger
HPGAN 100 bar
HPGOX 100 bar
Rectification column
Oxygen pump
Nitrogen
pump
The ambient air is compressed by a state-of-
the-art multi-stage turbo compressor with
intercoolers at a supply pressure of approx.
20 bar. A booster air compressor is no longer
required with this process design, leading
to a reduction of investment cost. A further
advantage is the improved energy efficiency
of the main air compressor for small plants.
High air pressure process
2012
Flexible high air pressure
process used in ASUs

15History and technological progress of air separation
Conventional process
The area in the low-pressure column where
the argon concentration is at a maximum
(approx. 10%) is known as the argon belly.
From there, the gas stream is fed into the
raw argon column for further rectification.
The remaining oxygen in this gas stream
is completely removed in the packed raw
argon column. Due to the very low pressure
drop in the packings, it is possible to install
a sufficient number of “theoretical trays”
required for the rectification. In the adjoining
pure argon column, the remaining nitrogen is
removed by rectification and the pure argon is
liquefied.
Pure liquid
argon (LAR)
1ppm nitrogen
1ppm oxygen
Pure argon production by rectification
1990
Pure argon production
by rectification
Cost-optimised process for small- and
medium-sized air separation plants
As in the conventional process, a gas stream
from the low-pressure column is fed into
the raw argon system. Due to optimised
packing types, the gas stream is already free
of nitrogen. Therefore, only the remaining
oxygen needs to be removed in the argon
system.
The argon purity and recovery can be kept at
the same level as in the conventional process.
The additional pure argon column is no longer
required.
Pure liquid
argon (LAR)
1ppm nitrogen
1ppm oxygen
2011
Pure argon production
by rectification
without pure argon system

16
2000
Development of
cascade condenser
2008
Reflux condenser
for argon rectification
History and technological progress of air separation
2006
Forced flow condenser
Condenser
Forced flow condenser
→ No condenser vessel required
→ Less space necessary
→ Specially designed for total evaporation
→ Energy-saving solution
Cascade condenser
→ Multi-stage bath-type condenser
→ Suitable for medium-sized and large ASUs
→ Suitable for ASUs with internal oxygen
compression
→ Integration of large heat transfer area
into low-pressure column compared to
conventional bath-type condenser
→ No oxygen pipework
→ Energy-saving solution
→ Safe operation
Reflux condenser
→ Used instead of conventional bath-type
condenser
→ No oxygen and no nitrogen pipework
necessary
→ Space-saving design compared to
bath-type condenser
→ Very simple and stable mode of operation
→ Cost-efficient design
Low-pressure column
Liquid oxygen
Gaseous nitrogen
Pressure column
Gaseous
nitrogen
Liquid oxygen
Low-pressure column
Liquid oxygen
Gaseous nitrogen
Pressure column
1
st
stage
2
nd
stage

17History and technological progress of air separation
Condenser fabrication.

18 History and technological progress of air separation
Air separation units in Cantarell, Mexico.
5 ⨯ 10,000 t
N
2
per day for
Cantarell, Mexico

19History and technological progress of air separation
1997
Largest ASU
for nitrogen
production

20
2006
Largest EPC
contract in the
history of
air separation
History and technological progress of air separation

21
Air separation units at the Pearl GTL complex in Ras Laffan, Qatar.
History and technological progress of air separation
8 ⨯ 3,800 t
O
2
per day in
Ras Laffan, Qatar

22
Air separation units near Yinchuan, China.
History and technological progress of air separation
6 ⨯ 3,600 t
O
2
per day for a plant
near Yinchuan City, China

23History and technological progress of air separation
2016
Engineering
masterpiece
in China

24
2017
Start-up of
largest ASU
in the world
History and technological progress of air separation

25
Delivery of world’s largest coldbox weighing 800 tonnes for Jamnagar, India.
History and technological progress of air separation
5 ⨯ 5,250 t
O
2
per day for
Jamnagar, India

26 History and technological progress of air separation
Linde Engineering.
Facts and figures.
… World’s first air separation
unit for oxygen production
… Linde introduced argon
production by rectification.
1990
Our air separation business.
1902
Number of patents
150
new air
separation
patents in last
5 years
5,250 tpd
oxygen
World‘s largest single train
air separation unit built by
400
air separation
units owned and
operated by The
Linde Group
3,000+
air separation
plants have
been built
by Linde
Composition of air
O₂ 20.95 −183.0 °C
N₂ 78.08 −195.8 °C
Ar 0.93 −185.9 °C
Ne 0.0018 −246.1 °C
He 0.0005 −268.9 °C
Kr 0.00011 −153.2 °C
Xe 0.000009 −108.0 °C
Vol % Boiling point
N₂
O₂

27History and technological progress of air separation
Biggest
prefabricated coldbox:
Height 70 m
Weight 800 t
Number of patents
1,700 m ²/m³
Heat exchanger
max. surface
−15%
average power consumption
of our ASUs over the last
10 years
Linde air separation units
built in more than
90
countries
Read more:
linde-engineering.com/air_separation_plants
19%
TCO
(Total Cost of
Ownership)
savings in past
10
YEARS
Published by:
Linde Aktiengesellschaft
Engineering Division, Dr.-Carl-von-Linde-Strasse 6–14
82049 Pullach, Germany
Phone +49 89 7445-0, Fax +49 89 7445-4908
info@linde-le.com, www.linde-engineering.com

Plant engineering
→ Air separation plants
→ LNG and natural gas processing plants
→ Petrochemical plants
→ Hydrogen and synthesis gas plants
→ Adsorption plants
→ Cryogenic plants
→ Carbon capture and utilization plants
→ Furnaces, fired heaters, incinerators
Component manufacturing
→ Coldboxes and modules
→ Coil-wound heat exchangers
→ Plate-fin heat exchangers
→ Cryogenic columns
→ Cryogenic storage tanks
→ Liquefied helium tanks and containers
→ Air-heated vaporizers
→ Water bath vaporizers
→ Spiral-welded aluminum pipes
Your partner for the production and
processing of gases
Delivering reliable process plants for maximum capital efficiency
Linde has been optimizing gas processing technologies for 140 years, successfully delivering more than 4,000 plant
engineering projects around the globe. Favoring trusted, lasting business relationships, the company collaborates closely
with customers to enhance plant lifecycle productivity and innovate process flows. The company’s proven gas processing
expertise plays an indispensable role in the success of customers across multiple industries – from natural gas and oil
refining through petrochemicals and fertilizers to electronics and metal processing.
Operational excellence along the entire plant lifecycle
We work closely with our customers to gain an in-depth understanding of individual needs. Building on the unique
synergies of Linde as an integrated plant operator and engineering company, Linde offers innovative process technologies
and services to exceed our customers’ reliability and profitability expectations. This commitment to innovation extends
along the entire plant lifecycle. The LINDE PLANTSERV® service team supports customers every step of the way – from
maintenance and repairs to full revamps. Leveraging the latest digital technologies to offer on-site and remote operational
and support services, we consistently take asset performance to the next level.
Making the impossible possible
From the desert to the Arctic, from small- to world-scale, from standardized to customized designs, Linde’s engineering
specialists develop solutions that operate under all conditions. The company covers every step in the design, project
management and construction of gas processing plants and components. Customers can always rely on Linde to deliver the
plants, components and services that fit their needs best – anywhere in the world.
Discover how we can contribute to your success at www.linde-engineering.com
Get in touch with our air separation plant team:
Phone +49 89 7445-3526, inquiry: www.linde-engineering.com/contact
Core competencies at a glance
Services
→ Revamps and plant modifications
→ Plant relocations
→ Spare parts
→ Operational support, troubleshooting
and immediate repairs
→ Long-term service contracts
→ Expert reviews for plants, operations
and spare part inventory
→ Operator training
41273_LCS_0719
Linde is a company name used by Linde plc and its affiliates. The Linde logo, the Linde word and LINDE PLANTSERV are trademarks or registered trademarks of Linde plc or its affiliates. Copyright © 2019. Linde plc.
