
Wayne State University Wayne State University
Wayne State University Theses
January 2022
Spatial Localization Of Markers And 3d-Cell Model For Study Of Spatial Localization Of Markers And 3d-Cell Model For Study Of
The Human Cytomegalovirus Cytoplasmic Virion Assembly The Human Cytomegalovirus Cytoplasmic Virion Assembly
Compartment Compartment
Taylor Alan Vensko
Wayne State University
Follow this and additional works at: https://digitalcommons.wayne.edu/oa_theses
Part of the Virology Commons
Recommended Citation Recommended Citation
Vensko, Taylor Alan, "Spatial Localization Of Markers And 3d-Cell Model For Study Of The Human
Cytomegalovirus Cytoplasmic Virion Assembly Compartment" (2022).
Wayne State University Theses
.
854.
https://digitalcommons.wayne.edu/oa_theses/854
This Open Access Thesis is brought to you for free and open access by DigitalCommons@WayneState. It has been
accepted for inclusion in Wayne State University Theses by an authorized administrator of
DigitalCommons@WayneState.

SPATIAL LOCALIZATION OF MARKERS AND 3D-CELL MODEL FOR STUDY OF THE
HUMAN CYTOMEGALOVIRUS CYTOPLASMIC VIRION ASSEMBLY COMPARTMENT
by
TAYLOR ALAN VENSKO
THESIS
Submitted to the Graduate School
of Wayne State University,
Detroit, Michigan
in partial fulfillment of the requirements
for the degree of
MASTER OF SCIENCE
2022
MAJOR: IMMUNOLOGY AND
MICROBIOLOGY
Approved By:
Advisor Date
ii
ACKNOWLEDGMENTS
Both graduate school and research are impossible to do alone, and I am thankful for everyone I had
and have through this part of life.
If it weren’t for my mother Roxanne, brother Billy, and sister-in-law Olya, I would probably not
be here right now. Their support has pushed me to pursue more in my life, and they always made sure I had
a roof over my head and a fridge full of food.
The Pellett Lab has been a nice place to call home with a great family for the last few years. Ian
McCusker showed me the ropes in my early days and started the weekly Saturday lunch tradition. Amina
Wofford was there to hear my crazy ideas, no matter how early in the morning, more than anyone else, and
she made a significant impact on my writing. Jillian Green brought a new side and perspective to the lab
that has benefitted us all. Former members of the lab, Christina Lim, William Close, and Ashley Anderson,
gave me a platform to expand from during my time here. Lastly, Phil Pellett has provided me with the
freedom to act and think independently, offer guidance when needed, and push me to grow. I have learned
as much about life as I have about herpesviruses from him.
My fellow graduate students at WSU SoM have made surviving graduate school a whole lot easier.
Friendships, teaching, and collaboration through the BMI student body and outside students have
considerably impacted my time here.
iii
TABLE OF CONTENTS
Acknowledgments ......................................................................................................................................... ii
List of Figures ................................................................................................................................................ v
CHAPTER 1: INTRODUCTION TO HUMAN HERPESVIRUSES ........................................................... 1
Introduction ............................................................................................................................................... 1
Overview ................................................................................................................................................... 1
Herpesviruses ............................................................................................................................................ 1
Herpesvirus taxonomy ............................................................................................................................... 2
HCMV ....................................................................................................................................................... 3
Virion structure.......................................................................................................................................... 3
Replication Cycle ...................................................................................................................................... 5
The cytoplasmic virion assembly compartment ........................................................................................ 5
Thesis......................................................................................................................................................... 7
CHAPTER 2: HCMV MODIFIES CELLULAR METABOLISM DURING ASSEMBLY AND
EGRESS. ....................................................................................................................................................... 8
Introduction ............................................................................................................................................... 8
Metabolism ................................................................................................................................................ 8
Mitochondria ............................................................................................................................................. 8
Glycolysis ................................................................................................................................................ 10
Apoptosis ................................................................................................................................................. 10
Methods ................................................................................................................................................... 11
CHAPTER 3: VALIDATION OF MARKERS FOR cVAC ANALYSIS .................................................. 12
iv
Introduction ............................................................................................................................................. 12
Materials & Methods ............................................................................................................................... 12
Results and Discussion ............................................................................................................................ 14
CHAPTER 4: DEVELOPING A 3D CELL MODEL FOR cVAC VISUALIZATION ............................. 15
Introduction and Preliminary Data .......................................................................................................... 15
Materials & Methods ............................................................................................................................... 16
Results and Discussion ............................................................................................................................ 16
CHAPTER 5: CONCLUSIONS .................................................................................................................. 18
Appendix ..................................................................................................................................................... 29
References.................................................................................................................................................... 31
ABSTRACT ................................................................................................................................................ 35
Autobiographical Statement ........................................................................................................................ 36
v
LIST OF FIGURES
Fig. 1: False Colored Electron Micrograph of HSV-1 Virion. .................................................................... 19
Fig. 2: Herpesvirus replication cycle. .......................................................................................................... 20
Fig. 3: Diagram of HCMV cVAC. .............................................................................................................. 21
Fig. 4: Colocalization of nuclear and cellular markers during HCMV infection. ....................................... 22
Fig. 5: Overlap of nuclear and Golgi-markers are an artifact of 2D microscopy. ....................................... 23
Fig. 6: cVAC in human colon tissue infected with HCMV. ........................................................................ 24
Fig. 7: Autofluorescence of HCMV-positive lung tissue control slides. ..................................................... 26
Fig. 8: Illustration of a cell spheroid. ........................................................................................................... 26
Fig. 9: GFP-tagged HCMV infection of a cell monolayer and spheroid. .................................................... 27
Fig. 10: General workflow for spheroid generation, infection, and processing. ......................................... 28
1
CHAPTER 1: INTRODUCTION TO HUMAN HERPESVIRUSES
Introduction
Much of chapters 1 and 2 is from chapter 10, Betaherpesvirus assembly and egress: Recent
advances illuminate the path, of Advances in Virus Research, Volume 108 "Virus Assembly and Exit
Pathways" [1]. My writing included: the abstract, introduction, virus-modified cellular metabolic shifts and
mitochondrial regulation, apoptosis, and virion envelope structure. The published work includes human
cytomegalovirus (HCMV), the focus of the thesis work, and the human roseoloviruses (human
herpesviruses 6A, 6B, and -7). The following text under the headings Overview, Herpesviruses,
Herpesvirus taxonomy, and Envelope are from the chapter, with some edits and updates.
Overview
The human betaherpesviruses include human cytomegalovirus (HCMV) and the human
roseoloviruses (human herpesviruses 6A, 6B, and -7; HHV-6A, HHV-6B, and HHV-7). They are important
human pathogens that cause congenital damage due to fetal infection during gestation, early life infections,
disease in immune-compromised individuals, and disease that may connect to chronic infection. These
viruses have extraordinary genetic and biologic complexity linked to their long co-evolutionary history with
their host. Studies of these viruses are important, not only for the development of new methods of
preventing or controlling infections, but also because a deeper understanding of the host has repeatedly
emerged from studies of virus-host interactions that have evolved over millennia.
Herpesviruses
Viruses are obligate, intracellular parasites that can inhabit all forms of cellular life and replicate
in various cell types in their host. They manipulate and modulate host-cell systems to enable their successful
replication. Herpesviruses do this in the context of life-long infections in which the virus establishes a latent,
or quiescent, state during which the virus genome is maintained, but no infectious virions are produced.
Intermittently, and in response to a variety of stimuli, in a subset of latently infected cells, the virus
2
reactivates to lytic replication, during which new infectious virions are produced that can be transmitted to
other cells in that individual and can also be transmitted to another susceptible host.
Virions are complete, infectious virus particles. Herpesvirus virions have four major components,
from inside to outside: (1) a dsDNA genome that is packaged into, (2) an icosahedral nucleocapsid
surrounded by (3) a layer of proteins and viral RNAs called the tegument, all enclosed within (4) an
envelope derived from host membranes (Fig. 1). Virions play several critical roles, including protecting the
virus genome and interacting with cell surface molecules to deliver virion contents to the cell interior at the
start of the infection.
Herpesvirus taxonomy
Members of the Herpesviridae family infect a broad host range of mammals, birds, and reptiles.
More than 300 identified herpesviruses belong to the order Herpesvirales [2], which also includes viruses
of amphibians and fish in the family Alloherpesvirdae, and mollusk viruses in the family
Malacoherpesviridae. Family Herpesviridae is further divided into three subfamilies, the Alpha-, Beta- and
Gammaherpevirinae. Alphaherpesviruses include e.g., herpes simplex viruses 1 and 2 (HSV-1 and -2),
varicella-zoster (VZV), and pseudorabies virus (PRV). Gammaherpesviruses include Epstein-Barr virus
(EBV) and Kaposi sarcoma-associated herpesvirus (KSHV), which are both associated with cancers.
Betaherpesviruses include cytomegaloviruses (CMV) of humans (HCMV), mice/rats (MCMV), guinea pigs
(GPCMV), and various non-human primates (chimpanzee, rhesus, simian), as well as members of the
Roseolovorus genus, which includes human herpesviruses 6A, 6B, and 7 (HHV-6A, HHV-6B, and HHV7).
Key distinctions between the subfamilies of the Herpesviridae are the duration of their replication
cycles, cell tropisms, and genome lengths and complexity. Alphaherpesviruses have relatively short
replication cycles, less than 10 hours for HSV-1, compared to 48 hours for HCMV. They undergo lytic
replication in epithelial cells and establish latency in neurons. Among the betaherpesvirus, HCMV lytic
replication occurs in epithelial and endothelial cells, and latency is established in monocytes and T-cells;
roseoloviruses replicate in T-cells and cells of other lineages, and like HCMV, establish latency in lymphoid
cells and monocytes. An uncommon characteristic of HHV-6A and -6B is their ability to integrate into
3
human chromosomes, occasionally in germ cells, which results in the presence of the virus in every cell of
the offspring. Gammaherpesviruses are generally lymphotropic. EBV and KSHV lytic replication occur in
B cells and epithelial cells, and latency is established in B cells, although EBV latency can also occur in
other lymphocytes.
HCMV
HCMV is a ubiquitously expressed human virus, but it does not often cause disease.
Immunocompromised and immunonaive populations are most at risk for severe disease. HCMV is a leading
cause of non-genetic birth defects, child deaths, is associated with various pregnancy complications,
developmental abnormalities, and cognitive disabilities [3-5]. Children infected with HCMV during the
first trimester of pregnancy are at high risk for severe disease. Disease outcomes are less severe as
pregnancy progresses, but fetal infections also become more likely. Many of the molecular and cellular
mechanisms of congenital HCMV are not well understood. HCMV pathology is likely due to drastic
changes in the cellular microenvironment, including, but not limited to, changes in cell signaling,
differentiation capacity, immune response, and metabolism at the maternal/fetal interface [6-10].
Virion structure
As with all herpesviruses, the HCMV virion comprises a dsDNA genome, nucleocapsid,
proteinaceous tegument layer, and a lipid-envelope (Fig. 1).
Genome. Of the human betaherpesviruses, HCMV has the largest, most complex genome (236 kb),
which has over 160 long-recognized (canonical) protein-coding open reading frames that can be expressed
in a multitude of ways via variations in splicing patterns and the use of alternative transcriptional and
translational starts and stops. In addition, the virus expresses at least 23 miRNAs, plus four RNAs that have
been described as long non-coding RNAs, but can be translated to produce abundant small proteins [11].
The broad tropism, prevalence, and complex host-virus interactions of HCMV can be attributed to its
genomic capacity.
4
Capsid. The genome is protected by and packaged within an icosahedral nucleocapsid. Capsids
contain a portal protein (pUL104) that is used for delivery of the virus genome and major capsid protein
(MCP) that interacts with capsid-associated tegument complexes to provide structural support [1, 12, 13].
Tegument. There is a large region of host and viral proteins and RNAs between the capsid and
envelope; this layer is the tegument. The tegument is made up of capsid-associated tegument and tegument.
Many proteins and RNAs that assist in viral replication are found in the tegument [14], and they are released
into the infected cell after viral entry [15].
Envelope. The envelope acquired during secondary envelopment at the final stage of virion
maturation surrounds the viral nucleocapsid along with its surrounding tegument. Virion envelopes are
comprised of cholesterol containing phospholipid membranes and lipid raft-embedded viral glycoproteins
that are necessary for cell-surface adhesion and membrane fusion/virion endocytosis during virion entry at
the initiation of infection. Among the numerous glycoproteins encoded by HCMV and the roseoloviruses,
glycoproteins gB, gH, gL, gM, and gN are conserved across the herpesvirus family; all play roles in entry.
gO is conserved among betaherpesviruses, and gQ1 and gQ2 are only found in roseoloviruses [16, 17].
The complex biology of HCMV includes the ability to replicate in diverse cell types. This is enabled
in part by shifts in the balance between the complexes made by the gH/gL complex with other HCMV
glycoproteins; these shifts are regulated in complex ways by viral factors and by host cell factors [18-24]
(reviewed in [17]). gH/gL can form a trimeric complex with gO (gH/gL/gO), and a pentameric complex
with the small glycoproteins expressed from UL128, UL130, and UL131 (gH/gL/UL128-131). On
fibroblasts, cell-to-cell spread is dependent on gH/gL/gO or gH/gL/UL128-131, while gH/gL/UL128-131
is necessary for cell-to-cell spread on endothelial or epithelial cells.
Although at least eight glycoproteins are present in HHV-6A and HHV-6B envelopes, only gB,
gH/gL, gQ1, and gQ2 are required for cell-to-cell fusion [16, 25].
Phosphatidylethanolamine (PE) makes up almost half of the glycerophospholipids found in HCMV
virions, compared to ~20% in uninfected cell membranes [26]. PEs are non-bilayer lipids, which can
influence the curvature of lipid bilayers [27]; this is particularly important for the inner leaflet of herpesvirus
5
virion envelopes that faces the tegument [26]. Virion envelopes are mainly comprised of saturated and
monounsaturated fatty acids. HCMV virion glycerophospholipid head group composition is similar to that
of synaptic vesicles [28]; this is consistent with other evidence that HCMV uses synaptic vesicle-like
secretory pathways for virion egress [26, 29].
Cholesterol is a critical envelope component that promotes virion envelope fusion with the host
cell plasma membrane following virion attachment. For HCMV [30] and HHV-6A [31], depletion of
envelope cholesterol using methyl-
-cyclodextrin (M
CD) resulted in only a minor inhibition of virion
adhesion but almost entirely abolished virion entry, with almost no detectable viral IE protein production
in either case.
Replication Cycle
All herpesviruses follow the same general replication cycle with specifics for each virus (Fig. 2).
Infection is initiated by virion glycoproteins attaching to the cell surface, followed by endocytosis or
membrane/envelope fusion. The naked capsid is translocated to the nucleus, where the viral genome is
deposited. Tegument proteins act as cellular modulators and transcription factors for viral genes. Viral gene
expression is done in a kinetic cascade of immediate-early (IE), then early (E), and lastly late (L), with
proteins from each class influencing transcription of the next class of genes. Following the production of
viral structural proteins and genome replication, the capsid is assembled, and the viral genome is packaged
inside. The genome-containing capsid undergoes nuclear egress, involving a primary, or nuclear,
envelopment. After nuclear egress, virion maturation occurs with the formation of the tegument, and the
virion gains its secondary, or cytoplasmic, envelope. Following complete assembly, the virion follows
cellular outbound pathways for egress. HCMV virion maturation and envelopment occur in a specialized
viral compartment.
The cytoplasmic virion assembly compartment
Many viruses induce cellular alterations upon infection, but HCMV infected cells have near-global
reorganization of intracellular organelles and changes in organelle morphology. Part of this reorganization
leads to the formation of the juxtanuclear site of HCMV virion maturation and egress: the cytoplasmic
6
virion assembly compartment (cVAC). The cVAC was first described as an accumulation site for viral
proteins [32]. It is now known that most, if not all, cellular organelles are a part of this structure, and much
work has been done characterizing its genesis, composition, and function over the last two decades [33-37].
Structurally, the cVAC consists of a central microtubule-organizing center (MTOC) with
microtubules anchoring at cellular organelles and moving them inward into a ring- or barrel-like shape.
This is phenotype includes: reniform nuclei, Golgi, trans-Golgi, endoplasmic reticulum (ER), and
mitochondria forming the ring, and various endosomes localized to the center of the ring (Fig. 3) [35, 36].
The accumulation of cellular and viral materials and cellular membranes enable tegumentation,
envelopment, and trafficking of the mature virion to designated secretory vesicles. The complete
composition and function of the cVAC are still unknown, but virion production is significantly reduced
without this structure [33]. Viral proteins are required for the biogenesis of the cVAC, likely by hijacking
cellular machinery or altering normal cellular gene expression [29, 33].
The cVAC has been visualized utilizing brightfield and fluorescent light microscopy and confocal
microscopy with computer-generated 3D reconstructions [35]. Conventional models for HCMV culture use
adherent primary fibroblasts grown in a monolayer but provide little evidence about whether the cVAC
might form in a 3D tissue matrix in vivo. HCMV infections can be examined in tissue specimens obtained
from humans with HCMV disease. However, there are many problems in visualization, including
autofluorescence from fixatives, mixed cell types, a minimal number of infected cells per section, and
cellular orientations where infected markers are hidden.
Thesis
My thesis work includes published written material on betaherpesvirus induced alterations to
cellular metabolism (Chapter 2), validation of markers for cVAC analysis (Chapter 3), and an introduction
of a model to examine the cVAC in a 3D cell-culture model (Chapter 4).
7
CHAPTER 2: HCMV MODIFIES CELLULAR METABOLISM DURING ASSEMBLY AND
EGRESS.
Introduction
The following text is adapted to exclusively HCMV from what I wrote on modified mitochondria
and the effects on metabolism and apoptosis from [1].
Metabolism
Host cell metabolism is required for the generation of materials used for virion production. Many,
if not all viruses, including herpesviruses, induce alterations to cytoplasmic and mitochondrial metabolic
systems to modulate glycolysis and the tricarboxylic acid (TCA) cycle.
Mitochondria
Producing several thousand new virions per infected cell is energetically expensive and requires
large quantities of molecular building blocks such as nucleotides and lipids. Mitochondria are involved in
several critical cellular metabolic activities, including glucose metabolism and ATP production, nucleotide
synthesis, Ca
2+
homeostasis, and fatty acid (FA) biosynthesis. Viruses often manipulate mitochondrial
morphology and function to favor their replication [38]. HCMV pUL37x1, also known as the viral
mitochondria-localized inhibitor of apoptosis, has multiple roles in mitochondria-linked aspects of HCMV
biology, including roles in managing apoptotic pathways and metabolic processes such as the FA elongation
required for the biosynthesis of the lipids that form viral envelopes, as well as regulation of Ca
2+
flux during
virion exocytosis [39].
During HCMV infection, mitochondria undergo fragmentation that links to autophagy (mitophagy)
[40], and become much smaller, more circular, and less tubular 48 to 96 hours post-infection [41]. HCMV-
infected cells show increased glycolysis and glutaminolysis [42-45] and exhibit a phenotype similar to the
Warburg effect seen in cancer cells. The accompanying aerobic glycolysis increases glutamine production
for use in the TCA cycle [46, 47]. Citrate produced from extracellular glucose is shuttled out of the TCA
cycle for FA and cholesterol synthesis.
8
Lipids
Lipids are critical components of cellular and organelle structures. Herpesviruses rely on host lipid
metabolism for FAs and phospholipids in envelope structure, lipidification of viral proteins, lipid rafts for
glycoprotein localization [48], and cholesterol, all of which are necessary during virion envelopment.
Herpesvirus virions are large and require a substantial amount of lipids throughout the maturation phase.
HCMV infection alters TCA cycle metabolic flux; enhanced glucose uptake is followed by a nearly
complete global metabolic flux increase during infection [45]. The drastic increase in citrate and malate
production favors FA synthesis [49]. FA synthesis increases twenty-fold, compared to a three-fold increase
in nucleotide biosynthesis [49]; this suggests that the demand for FA synthesis is due to the production of
HCMV envelopes, which are mostly comprised of newly-synthesized, rather than recycled, membranes
[50].
As a possible explanation for the drastic differences in FA and nucleotide demands, it has been
hypothesized that the extended replication cycle of HCMV allows viral DNA to accumulate over a longer
time [50]. It is unknown if HCMV is solely dependent on de novo synthesis of nucleotides or if there is a
connection to autophagic recycling processes. The high abundance of non-infectious enveloped particles
(NIEPs) and dense bodies, consisting of only virion envelopes and tegument proteins, may contribute to
the difference in the degree of alterations in FA and nucleotide synthesis during HCMV infection.
As suggested by the metabolic analyses, concentrations of saturated very-long-chain FAs
(VLCFAs) increase significantly during HCMV infection [51, 52], and they are further enriched in virions
[51]. The most abundant phospholipid in uninfected cell membranes is phosphatidylcholine (PC). During
HCMV infection, PC production is drastically increased by 19- to 60-fold for PC(44:1) (numbers represent
carbon chain length and the number of double bonds) and by as much as 270- 1,400-fold for PC(46:1). In
infections with a UL37x1-null virus, the changes were much less drastic, with a ~10-fold change for
PC(44:1) and only a 200-fold change in PC(46:1) [53].
Cholesterol metabolism is a critical component of HCMV infectivity. Cholesterol is accompanied
by sphingolipids and glycolipid-anchored proteins to form lipid rafts [48, 54]. HCMV infection triples
9
cellular cholesterol abundance at 24 hpi [30]. Host low-density lipoprotein related receptor 1 (LRP1) is a
plasma membrane receptor that regulates lipid metabolism and levels of intracellular cholesterol [55-59].
During the early stages of infection (24 hpi), LRP1 expression increases and is then downregulated at 72
hpi [30]. This coincides with increased virion production and the associated need for virion envelopes to
contain cholesterol-rich lipid rafts required for efficient membrane fusion during infection [48, 60].
Glycolysis
HCMV has pronounced effects on cellular glycolysis [45, 49, 61]. As stated above, although
HCMV increases cellular glucose uptake and early stages of metabolism, the focus is directed to feeding
the TCA cycle for FA synthesis. HHV-6A significantly increases glucose uptake after 48 hpi and
dramatically increases expression and membrane localization of glucose transporters GLUT1 and GLUT3
while reducing GLUT2 and GLUT4 mRNAs to nearly undetectable levels [61]. HCMV utilizes GLUT4
primarily for glucose uptake and eliminates GLUT1 expression [44]. HCMV drastically increases the
production of lactate [49, 61]), which can be used in the TCA cycle after conversion into pyruvate. The
results of Wu et al. suggest that, similar to HCMV, these glycolytic shifts precede the elevation in TCA
cycle intermediates necessary for increased synthesis of nucleotides, FAs, and other materials needed for
virion assembly. During infection, major metabolic alterations focus on the building blocks of virion
envelopes, allowing for cellular egress and complete replication.
Apoptosis
Apoptosis is a well-characterized response to virus infections. Maintenance of infected cells as
virus-producing factories is dependent on early and ongoing inhibition of apoptotic or “self-destruct”
signals by viral factors. HCMV encodes two immediate-early proteins, vICA (viral inhibitor of caspase-8-
induced apoptosis, UL36) and vMIA/pUL37x1 (viral mitochondria-localized inhibitor of apoptosis, UL37),
that are expressed throughout the infectious cycle and inhibit death-receptor-mediated apoptosis in HCMV
infected cells [62]. Virion replication and egress are dependent on the capacity of HCMV to circumvent
host cell death pathways, including apoptosis and inflammasome-induced pyroptosis [63].
No table of figures entries found.
10
Methods
Writing this chapter began with an outline made by the Pellett lab. Each lab member was assigned
a section based on their interests or experience. For each section, the goal was to find advances in the
specific subject and apply them to the volume's overall context: virus assembly and exit pathways.
Literature searches started with research done by the lab or papers that were critical to that research.
Part of the search was a continuation of work done by key references. Searches with keywords were
essential for finding new research and many other review papers that were a great source of information
and references. This process involved a significant amount of curiosity and speculation that led to many
interesting possible connections.
Editing was done continuously and was a crucial component of completing this large chapter. Most
of my contributed sections were not co-authored, so the majority of editing was done between myself and
my mentor. The process involved reading through the written material together, usually aloud, and
modifying the text to be clear, engaging, and succinct while following a logical flow.
The AI text editing app Grammarly was invaluable during the writing process. The app is
configurable based on your writing context and your preference in tone. This app acts as a helpful guide to
edit while writing and act as an extra pair of eyes on the material. Grammarly not only helps during the
writing process, but it also helped me become a better writer while unassisted.

11
CHAPTER 3: VALIDATION OF MARKERS FOR cVAC ANALYSIS
Introduction
The ring-like rearrangement of the Golgi-apparatus is a defining characteristic of the HCMV
cVAC. Much is still unknown about the composition, regulation, and function of the cVAC. When studying
host-virus interactions, a viral marker should be included in the analysis of virus-induced changes, proving
that the cell is unquestionably infected. Multiplexing antibodies for use in fluorescent microscopy can be
limited when observing multiple cellular targets while also having an infection marker.
During Dr. Ashley Anderson’s dissertation work in the Pellett lab, she was trying to develop a
robust method for quantifying HCMV infected cells with cVACs and establish a temporal window for
biogenesis. This process included validation of the simultaneous use of antibodies against the juxtanuclear
Golgi marker GM130 and the viral nuclear marker IE2 (Immediate-Early Protein 2) so another marker
could be used against her protein of interest HCMV pUL103. The proximity of these markers posed an
issue of fluorescent signal bleed from cytoplasmic GM130 or nuclear IE2 that may interfere with qualitative
and quantitative analysis of the Golgi in infected cells.
To address potential colocalization of our markers, I performed immunofluorescent microscopy
with either IE2 or GM130 in HCMV-infected and mock-infected cells.
Materials & Methods
Cell lines. Human foreskin fibroblasts (HFFs) passage < 15 were used for experiments. HFFs were
used for growing virus stocks, plaque assays, and immunofluorescence assays. HFFs were grown in
Dulbecco's modified Eagle medium (DMEM) containing high glucose, L-glutamine, and sodium pyruvate
(HyClone, SH30243.FS, Pittsburgh, PA) supplemented with 10% fetal bovine serum (FBS, S11150, Atlanta
Biologicals, Flowery Branch, GA), 1x GlutaMAX (Gibco, 35050061, Waltham, MA), and 1x nonessential
amino acids (NEAA, HyClone, SH3023801, Pittsburgh, PA).
Viruses. Virus stocks were obtained by infecting 100% confluent HFFs at MOI of 0.001. When
100% cytopathic effect (CPE) was reached, cells and supernatant were removed from flasks and centrifuged
at 1280xg for 15 minutes at 4°C. A milliliter of 10% 3x autoclaved milk was used to resuspend the pellet.
12
The cell suspension was sonicated in a Branson analog 450 sonifier cup horn filled with ice water at an
output control of 10 and 30% duty cycle for 10 seconds, followed by 10 seconds of rest, three times total.
The sonicated samples were centrifuged at 1280xg for 10 minutes at 4°C, and the supernatant was removed
and stored at -80°C. Virus titers were determined by performing plaque assays using confluent layers of
HFFs.
Immunofluorescence microscopy. 8-well glass chamber slides (Nunc LabTek II-154534, Thermo
Fisher Scientific, Waltham, MA) were incubated with 300 µl of 0.2% gelatin in 1x PBS for 1 hour at room
temperature before seeding human foreskin fibroblasts (HFFs) to 80% confluence. The following day, cells
were infected with UL103-V5-His HCMV at multiplicity of infection (MOI) of 0.1 for 120 hpi.
At the end of the infections, the cells were fixed with 300 µl of 4% paraformaldehyde in 1x PBS
for 15 min., autofluorescence quenched with 300 µl of 50 mM ammonium chloride for 15 min.,
permeabilized with 300 µl of blocking buffer (5%, glycine, 10% normal goat serum, 0.1% sodium azide in
1x PBS) containing 0.2% Triton X for 15 min. Samples were blocked with blocking buffer for 1 hour before
addition of 75 µl of primary antibodies (GM130, BD Biosciences, 610823; IE2, Millipore, MAB810)
diluted in blocking buffer for 1 hour. Wells were washed twice for 7 min. in 1x PBS and then 75 µl of
fluorescence-tagged secondary antibody Alexa Fluor 568-conjugated goat anti-mouse IgG,( A-11031,
Invitrogen, Waltham, MA) diluted in blocking buffer for 1 hour was added and then washed twice for 7
min. in 1x PBS. The chamber was removed from the slide, was mounted with VectaShield mounting
medium with DAPI (Vector Laboratories, H1200, Burlingame, CA), and the coverslip (Fisherbrand, 12-
548-5E, L-50 x W-22 mm, thickness 0.13 to 0.17mm, Waltham, MA) was sealed using clear topcoat nail
polish (wet n wild, Los Angeles, CA)(Loreal, Paris, France).
Imaging was done on a Nikon E800 and microscope and CoolSNAP EZ grayscale-camera using
MetaMorph (Molecular Devices, San Jose, CA) imaging software. Colorations came from ImageJ Lookup
Tables, and a linear contrast adjustment was made using a consistent macro for all experimental conditions
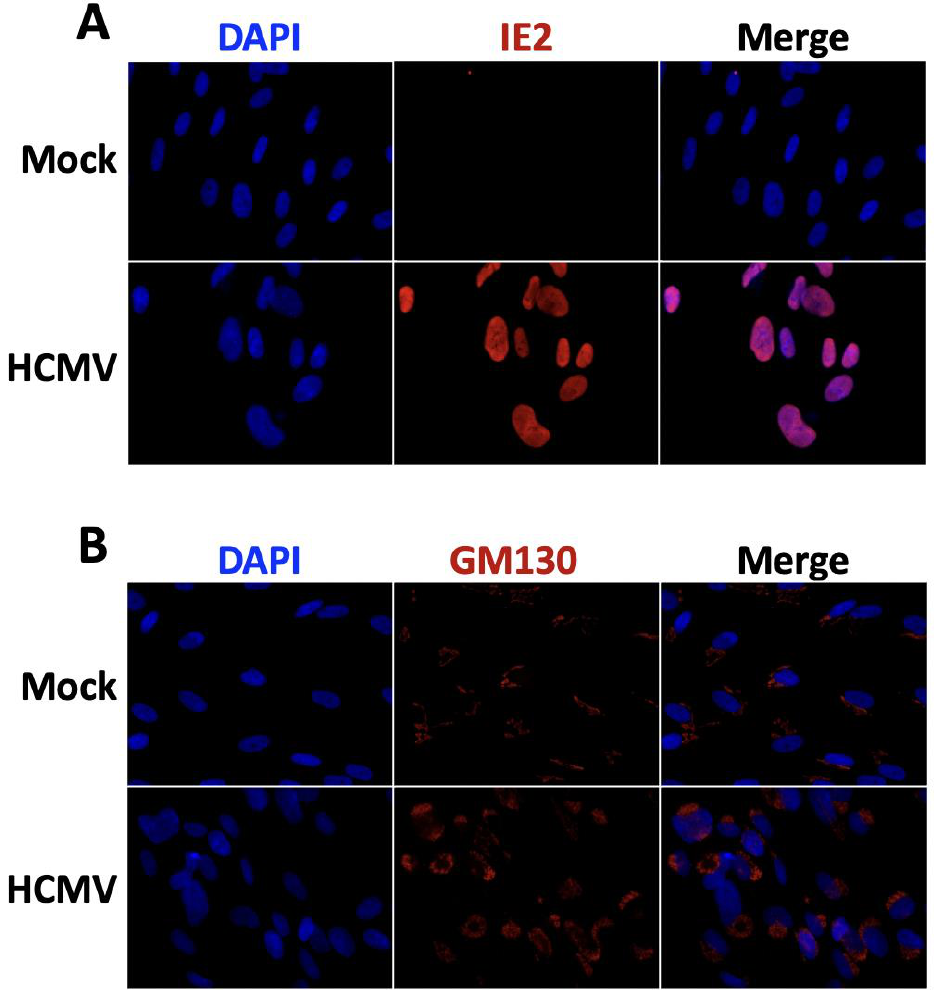
in Fig. 4.
13
Results and Discussion
The purpose of this work was to assess localization of common cellular and viral markers for
concurrent use. In chamber slides, separate wells of mock-infected and infected cells were incubated with
individual primary antibodies while using the same fluorescent secondary antibody. Colocalization was
done on either marker compared to DAPI, nuclear DNA stain, allowing for observation of signal spill-over
from IE2 out of the nucleus and GM130 into the nucleus.
IE2 staining overlapped and colocalized with nuclear DAPI staining, and GM130 staining is only
present in the cytoplasm and does not colocalize with nuclear DAPI (Fig. 4). These data indicate that these
nuclear and perinuclear markers are unrelated in localization and can be detected using the same secondary
fluorophore without interfering with the analysis of one or the other.
Any Golgi signal in the nuclear area is likely due to overlap in X and Y planes, but not in the same
Z plane. 3D-microscopy shows this overlap is due to the Golgi-apparatus wrapping above or below the
nucleus (Fig. 5) and not the invasion of immunofluorescent markers. Human interpretation of these images
may seem obvious, but the unbiased computation of these data has added layers of complexity.
Discrete localization of these markers allows the same secondary antibody to detect two separate
signals, allowing for a third marker using a different secondary antibody. Although not shown, it is possible
to segment the IE2-positive nuclei from the IE2 and GM130 positive channel using ImageJ, allowing for
separate coloring of IE2 and GM130 without affecting the image metadata, i.e., a three-color image from
only two detectable fluorophores.
Future Directions. An expansion of this work would include the potential label-free identification
of HCMV infected cells based on nuclear morphology. Automated recognition of a Golgi-ring may be
possible using neural networks and computer vision to classify cells as infected with a viral nuclear signal
and having a cVAC by morphologic features outside the nucleus.
14
CHAPTER 4: DEVELOPING A 3D CELL MODEL FOR cVAC VISUALIZATION
Introduction and Preliminary Data
At this point, visualizations of ERC reorganization and cVAC biogenesis have only been in
traditional two-dimensional monolayers of adherent fibroblasts, which are not representative of living
tissues. It is still unknown if the cVAC exists or has a pathologic role in tissues. After staining nearly 100
human tissue slides collectively between myself and Christina Lim, there was only one instance of a cVAC
in human tissue as part of Christina’s M.S. thesis work [64] (Fig. 6).
During this process, tissue staining protocols were optimized using commercially acquired HCMV-
positive lung tissue slides (Azer Scientific). Accurate intracellular imaging of these slides proved to be
impossible due to the background fluorescence found in unstained and stained samples (Fig. 7). The
hospital-acquired tissues from other organs of HCMV-positive children are available in small amounts and
are not appropriate for troubleshooting or large-scale replication. Specimen availability quantity and
technical limitations are a major roadblock to this work.
To overcome the issues above, we evaluated use of a three-dimensional cell-based model in place
of sectioned tissue samples using cell spheroids.
Cell spheroids are self-assembling aggregates of cells formed by promoting cell-to-cell interactions
in three dimensions rather than cell-surface interactions in a culture vessel [65] (Fig. 8). When the cells
cluster together, they form a 3D sphere structure. Spheroids are typically homogenous, although co-cultures
can be used. The simplest models consist of a single cell type growing in three dimensions. Spheroids have
been used as in vivo models in drug delivery [66], and in modeling diseases like Alzheimer’s [67] and
Parkinson Disease [68].
We developed a three-dimensional cell-spheroid model based on a liquid overlay spheroid cell
culture technique that uses cells grown in a non-adherent vessel. I added molten agarose into multi-well
plates and placed on a rotating shaker to form a concave surface as the agarose cools. Once the agarose is
solidified, cells are be seeded into the wells where they attach to each other rather than the coating on the

15
bottom of the plate. Cells maintain their typical morphology, and cell-to-cell interactions have an added z-
dimension, similar to the multiple layers of cells in tissues.
Spheroid cultures can provide a more accurate representation of living tissue, removing fixed
positions of traditional monolayers, and allowing cells to interact and orient more naturally. In this work, I
developed a system that allows control of cell type, density, number of infected cells, infection time points,
and fixatives used during processing.
Materials & Methods
Spheroid formation. Spheroids were generated using a liquid overlay method based on a protocol
from Ibidi Biosciences (https://ibidi.com/img/cms/support/AN/AN32_Generation_of_spheroids.pdf). 1%
Melted agarose in PBS was added to a pre-heated (110F for one hour) multi-well culture plate (Corning
Costar 96-Well, Cell Culture-Treated, Flat-Bottom Microplate; 09-761-145, Corning, NY). After adding
agarose, the multi-well plate was placed on a rotating shaker to form a concave surface as the agarose
cooled. After complete solidification of the agarose, 15 minutes, cells can be seeded into wells. Cells are
incubated at 37C for at least 24 hours for stabilization of spheroids before handling.
Spheroid infection. HFFs <15 passages were seeded in a 24-well culture plate (Corning Costar
24-Well, Cell Culture-Treated, Flat-Bottom Microplate; 09-761-146, Corning, NY). The seeded cell
density is equal between 3D and 2D cell cultures at 70% confluency, about 70,000 cells/well. 24 hours
after seeding, viral infections at MOI of 0.1 were done as described in Chapter 3. GFP-HCMV positive
cells were observed and compared over a five-day infection using an inverted microscope with a GFP-
epifluorescent filter. Images were obtained through the eyepiece of the microscope with an iPhone X
camera.
Results and Discussion
To overcome the previous issues with human tissue samples, we evaluated use of a three-
dimensional cell-based model.
To verify that this model can support viral infection comparable to normal cell culture, I seeded an
equal number of cells into uncoated or agarose coated wells in a 24-well plate. After 24 hours, the cells
16
were infected with GFP-tagged HCMV and were imaged over a five-day time course (Fig. 9). GFP-tagged
HCMV infected cells similarly, whether they were in a spheroid or a monolayer when observed under low-
magnification light microscopy. GFP expression is seen first in cells on the exterior of the spheroid, but
throughout infection cells deep within also become infected. The virus is able to infect the cells and progress
through layers similar to in a tissue. HCMV infection does not cause dissociation of spheroids even in
infections over 20 days (images not available).
After establishing HCMV infection in spheroids, I optimized culturing, infected, and processing to
develop the current best workflow and protocols (Fig. 10 and Appendix). The spheroid model is inexpensive
and reproducible. The model has potential to be handled and processed like a tissue specimen or it may also
be imaged using 3D-confocal microscopy to address cVAC biogenesis in a tissue-like structure.
In my current workflow, the spheroids are processed like human tissue, but using 3D-confocal
microscopy, it is also possible to optically section a sample and image the entirety of a spheroid. Confocal
microscopes can image through an entire sample up to 300 microns thick and the spheroids I generate are
typically 100-150 microns in diameter. This method could eliminate the complications of histological
processing and staining, although this method should be technically possible, I haven’t done it, nor have I
seen anyone else do it. If I were to do this process again, I would pursue optical sectioning with microscopy
rather than physical sectioning.
Future directions. A potential follow-up to this work is to use human trophoblasts spheroids as an
in vivo representation of a blastocyst during the early stages of pregnancy [10, 69, 70]. These methods of
culturing and infecting spheroids combined with established in vitro reproductive models could be an
effective model to study congenital HCMV infection during the early stages of pregnancy.

17
CHAPTER 5: CONCLUSIONS
HCMV is a highly prevalent pathogen that causes severe disease in immunocompromised and
immune naive populations. The latency capacity of all herpesviruses allows them to persist in the host for
the totality of its life with phases of reactivation and lytic replication. HCMV has many complex
interactions with its host cell and is a master of manipulating multiple cellular systems. One of these
includes the reorientation of cellular secretory machinery to form the cVAC. Much work has been done in
the Pellett lab to characterize the origin, identity, and function of the cVAC during virion assembly and
egress. This work validated the concurrent use of the same secondary antibody to detect the nuclear viral
marker IE2 with the cellular Golgi-marker GM130 in fluorescence microscopy. The cVAC has not been
well established in human tissues, so we have developed a 3D cell culture model to study HCMV infection
and cVAC biogenesis in a tissue-like environment. I showed that HCMV infection progresses similarly in
cells in a monolayer or spheroid. I have also established methods to generate, infect, and process these
spheroids for immunofluorescent microscopy of cVAC markers.

18
Fig. 1: False Colored Electron Micrograph of HSV-1 Virion.
A false-colored electron micrograph of a herpesvirus virion. Virion components include the genome
(orange), capsid (blue), tegument (teal), envelope (yellow), and glycoproteins (green). Imaged adapted from
https://www.sciencemagazinedigital.org/sciencemagazine/06_april_2018/MobilePagedArticle.action?arti
cleId=1371646#articleId1371646.

19
Fig. 2: Herpesvirus replication cycle.
The general life cycle of herpesviruses and some host cellular systems altered during infection [71].

20
Fig. 3: Diagram of HCMV cVAC.
The cVAC is bounded by a ring of Golgi (green) and trans-Golgi (red) membranes that surround a collection
of early endosomes (blue). Microtubules (brown) radiate from the microtubule organizing center (MTOC)
(green rectangles) to anchor points at the cell periphery and nuclear envelope. The cross-sectional
representation depicts the direction of virion maturation from nuclear egress, tegumentation, envelopment,
and transport out of the cVAC. Captions and diagram adapted from [35].
21
Fig. 4: Colocalization of nuclear and cellular markers during HCMV infection.
Infected or mock-infected HFFs were fixed and stained at 120 hpi with UL103-V5-His HCMV at 0.1 MOI.
All wells were stained with DAPI, and individual wells were either stained for (A) IE2 or (B) GM130.

22
Fig. 5: Overlap of nuclear and Golgi-markers are an artifact of 2D microscopy.
Human lung fibroblasts were infected with HCMV and stained at 120 hpi. 3D reconstructions of single-
plane confocal microscopy images visualize an entire cell nucleus (blue), Golgi-apparatus (red), and EEA1-
positive endosomes (green). (A) and (B) are rotations of the same image, showing that the Golgi-apparatus
does wrap around the nucleus in some cases, contributing to nuclear and Golgi markers occupying the same
space in X and Y dimensions but different spaces in the Z dimension. Images taken from [34].

23
Fig. 6: cVAC in human colon tissue infected with HCMV.
Human colon tissue infected with HCMV. Orientation of the tissue section showed that characteristic
reniform nucleus and Golgi and EEA1-positive endosome localization show the formation of a cVAC.
Images and caption adapted from [64].

24
Fig. 7: Autofluorescence of HCMV-positive lung tissue control slides.
(A) The merge of blue, green, and red channels in unstained HCMV-positive lung tissue at 40x. (B) HCMV-
positive lung tissue was stained with DAPI (blue), IE2 (red), GM130 (red), and EEA1 (green). Eighty
images per channel at 10x were stitched together to show the entire tissue sample.

25
Fig. 8: Illustration of a cell spheroid.
Spheroids are three-dimensional cell cultures with a filled interior. Image created in BioRender.

26
Fig. 9: GFP-tagged HCMV infection of a cell monolayer and spheroid.
HFFs were seeded to equal density in either a monolayer or a spheroid. After 24 hours, HFFs were infected
with GFP-tagged HCMV at MOI of 0.1 over five days. Days post-infection (DPI).

27
Fig. 10: General workflow for spheroid generation, infection, and processing.
Diagram accompanying the protocol in Appendix. Image generated in BioRender.

28
APPENDIX
Spheroid Cell Culture Protocol
(12/2/19 TV)
Cell Culture Preparation
1. Choose appropriate culture vessel, place vessel and pipette tips needed in oven at 150
0
F for 20-60
minutes.
2. Add molten agarose to wells of the culture vessel
a. Use ¼- ½ of the minimum liquid needed. See “Cell Culture Important Numbers”
3. Place culture vessel on circular shaker in the main lab, set speed so liquid gently coats the walls of
the vessel to form a concave surface
4. Allow agarose to solidify before adding cells
Cell Culture
1. Calculate the desired number of cells per spheroid.
a. Spheroids can be composed of more than 5x the number of cells in a normal monolayer.
Add enough so you can see the spheroid with the naked eye. (50,000 cells/spheroid is a
good minimum).
2. Follow the Cell Passage: Adherent Cell Lines protocol
3. Seed cells into agarose-coated vessel.
4. Place culture in the incubator undisturbed for at least 24 hours to allow spheroids to form.
Virus Infections
1. Follow “Infecting Fibroblasts” protocol.
a. Take care when doing liquid transfers to not disturb the spheroid.
b. HAND ASPIRATE ALL LIQUIDS. Take care to not aspirate the spheroid.
Processing and Embedding
1. Fix spheroid culture in 4% paraformaldehyde for 24-48 hours.
2. Incubate spheroids in increasingly hydrophobic reagents to dehydrate and allow for paraffin
penetration. HAND ASPIRATE ALL LIQUIDS.
a. 70% Ethanol for 30 minutes
b. 80% Ethanol for 30 minutes
c. 90% Ethanol for 30 minutes
d. 95% Ethanol for 30 minutes
e. 100% Ethanol for 20 minutes, twice
f. Transfer spheroids to a new container without agarose
i. Xylene will dissolve the agarose.
g. Xylene for 20 minutes, twice.
h. Add molten paraffin to spheroids in a mold, incubate overnight.
i. Section paraffin block to desired thickness.
Deparaffinization
1. Wash Slides for 3 minutes each in:
1. 100% Xylene 3 times
2. 100% Alcohol twice
3. 95% Alcohol
4. 70% Alcohol
5. ddH20 twice
In a short coplin jar, needed volume is about 35mL per wash:
95% alcohol = 33.25mL alcohol +1.75mL ddH20
70% alcohol = 24.5mL alcohol + 10.5mL ddH20
Antigen Retrieval
1. Add 350uL of 100x Citrate buffer to 34.650mL of ddH20 in a tall coplin jar.
2. Place slides in jar, then put in the microwave at 20% power for 5 minutes.

29
3. Cook at 30% power until the solution boils (only a few seconds).
4. Cook at 20% power for 5 minutes.
Immunofluorescence
*Unless an incubation is done overnight, all incubations are done covered and at room temperature*
1. Dilute 30% H202 to 3% in PBS.
2. Circle tissues with hydrophobic barrier pen.
3. Add 100-300uL of H202 to each tissue and incubate.
4. Drain off H202, wash in 100-300uL of PBS for 5 minutes.
5. Drain off PBS, add 300uL of Blocking Buffer with 6% Triton X to slides and incubate for 1 hour.
6. Drain off Blocking Buffer with Triton-X, add 300 uL of Blocking buffer and incubate for 1 hour
7. Drain off Blocking Buffer, add Primary Antibody and incubate for 1-24 hours.
8. Drain off Primary Antibody, wash 3 times in PBS for 5 minutes each.
9. Incubate Secondary Antibody for 1 hour.
10. Wash 3 times in PBS for 5 minutes each.
11. Prepare Vector Labs TrueVIEW Autofluorescence Quenching Kit.
Add 1:1 dilution of Reagent A to Reagent B then mix for 10 seconds, and the same
amount of Reagent C and mix for 10 seconds. (About 150uL total per tissue is needed,
should be 1:1:1)
12. Add 150uL of TrueVIEW to each slide, incubate for 5 minutes.
13. Drain off TrueVIEW, wash in PBS for 5 minutes.
14. Add mounting medium with DAPI to coverslip, lay slides on their coverslips and allow the medium to
spread.
15. Seal the coverslip with clear nail-polish, allow to dry before imaging.
30
REFERENCES
1. Wofford, A.S., et al., Betaherpesvirus assembly and egress: Recent advances illuminate the path.
Adv Virus Res, 2020. 108: p. 337-392.
2. Davison, A.J., Evolution of sexually transmitted and sexually transmissible human herpesviruses.
Ann N Y Acad Sci, 2011. 1230: p. E37-49.
3. Walsh, H., et al., Congenital Cytomegalovirus and Human Immunodeficiency Virus: Effects on
Hearing, Speech and Language Development, and Clinical Outcomes in Children. Front Pediatr,
2021. 9: p. 771192.
4. Pereira, L., Have we overlooked congenital cytomegalovirus infection as a cause of stillbirth? J
Infect Dis, 2011. 203(11): p. 1510-2.
5. Pereira, L., Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu Rev
Virol, 2018. 5(1): p. 273-299.
6. Berkebile, Z.W., et al., The Placental Response to Guinea Pig Cytomegalovirus Depends Upon
the Timing of Maternal Infection. Front Immunol, 2021. 12: p. 686415.
7. Silasi, M., et al., Viral infections during pregnancy. Am J Reprod Immunol, 2015. 73(3): p. 199-
213.
8. Pereira, L., et al., Congenital cytomegalovirus infection undermines early development and
functions of the human placenta. Placenta, 2017. 59 Suppl 1: p. S8-S16.
9. Pereira, L., et al., Intrauterine growth restriction caused by underlying congenital
cytomegalovirus infection. J Infect Dis, 2014. 209(10): p. 1573-84.
10. Tabata, T., et al., Human cytomegalovirus infection interferes with the maintenance and
differentiation of trophoblast progenitor cells of the human placenta. J Virol, 2015. 89(9): p.
5134-47.
11. Ingolia, N.T., et al., Ribosome profiling reveals pervasive translation outside of annotated
protein-coding genes. Cell Rep, 2014. 8(5): p. 1365-79.
31
12. Liu, W., et al., Atomic structures and deletion mutant reveal different capsid-binding patterns and
functional significance of tegument protein pp150 in murine and human cytomegaloviruses with
implications for therapeutic development. PLoS Pathog, 2019. 15(2): p. e1007615.
13. Zhang, Y., et al., Atomic structure of the human herpesvirus 6B capsid and capsid-associated
tegument complexes. Nat Commun, 2019. 10(1): p. 5346.
14. Varnum, S.M., et al., Identification of proteins in human cytomegalovirus (HCMV) particles: the
HCMV proteome. J Virol, 2004. 78(20): p. 10960-6.
15. Kalejta, R.F., Tegument proteins of human cytomegalovirus. Microbiol Mol Biol Rev, 2008.
72(2): p. 249-65, table of contents.
16. Tang, H. and Y. Mori, Glycoproteins of HHV-6A and HHV-6B. Adv Exp Med Biol, 2018. 1045:
p. 145-165.
17. Nguyen, C.C. and J.P. Kamil, Pathogen at the Gates: Human Cytomegalovirus Entry and Cell
Tropism. Viruses, 2018. 10(12).
18. Wille, P.T., et al., A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the
virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J Virol,
2010. 84(5): p. 2585-96.
19. Li, G., et al., A viral regulator of glycoprotein complexes contributes to human cytomegalovirus
cell tropism. Proc Natl Acad Sci U S A, 2015. 112(14): p. 4471-6.
20. Laib Sampaio, K., et al., The contribution of pUL74 to growth of human cytomegalovirus is
masked in the presence of RL13 and UL128 expression. J Gen Virol, 2016. 97(8): p. 1917-1927.
21. Chidiac, C. and E. Braun, [Atherosclerosis, multiple sclerosis, and Alzheimer's disease: what role
for Herpesviridae?]. Pathol Biol (Paris), 2002. 50(7): p. 463-8.
22. Zhang, L., et al., Expression Levels of Glycoprotein O (gO) Vary between Strains of Human
Cytomegalovirus, Influencing the Assembly of gH/gL Complexes and Virion Infectivity. J Virol,
2018. 92(15).
32
23. Day, L.Z., et al., Polymorphisms in Human Cytomegalovirus Glycoprotein O (gO) Exert Epistatic
Influences on Cell-Free and Cell-to-Cell Spread and Antibody Neutralization on gH Epitopes. J
Virol, 2020. 94(8).
24. Schultz, E.P., et al., Specialization for Cell-Free or Cell-to-Cell Spread of BAC-Cloned Human
Cytomegalovirus Strains Is Determined by Factors beyond the UL128-131 and RL13 Loci. J
Virol, 2020. 94(13).
25. Tanaka, Y., et al., Herpesvirus 6 glycoproteins B (gB), gH, gL, and gQ are necessary and
sufficient for cell-to-cell fusion. J Virol, 2013. 87(19): p. 10900-3.
26. Liu, S.T., et al., Synaptic vesicle-like lipidome of human cytomegalovirus virions reveals a role
for SNARE machinery in virion egress. Proc Natl Acad Sci U S A, 2011. 108(31): p. 12869-74.
27. Yang, Q., et al., Effects of lipid headgroup and packing stress on poly(ethylene glycol)-induced
phospholipid vesicle aggregation and fusion. Biophys J, 1997. 73(1): p. 277-82.
28. Takamori, S., et al., Molecular anatomy of a trafficking organelle. Cell, 2006. 127(4): p. 831-46.
29. Close, W.L., et al., Infection-Induced Changes Within the Endocytic Recycling Compartment
Suggest a Roadmap of Human Cytomegalovirus Egress. Front Microbiol, 2018. 9: p. 1888.
30. Gudleski-O'Regan, N., et al., Increased expression of LDL receptor-related protein 1 during
human cytomegalovirus infection reduces virion cholesterol and infectivity. Cell Host Microbe,
2012. 12(1): p. 86-96.
31. Huang, H., et al., Human herpesvirus 6 envelope cholesterol is required for virus entry. J Gen
Virol, 2006. 87(Pt 2): p. 277-285.
32. Sanchez, V., et al., Accumulation of virion tegument and envelope proteins in a stable
cytoplasmic compartment during human cytomegalovirus replication: characterization of a
potential site of virus assembly. J Virol, 2000. 74(2): p. 975-86.
33. Das, S., et al., Identification of human cytomegalovirus genes important for biogenesis of the
cytoplasmic virion assembly complex. J Virol, 2014. 88(16): p. 9086-99.
33
34. Das, S. and P.E. Pellett, Spatial relationships between markers for secretory and endosomal
machinery in human cytomegalovirus-infected cells versus those in uninfected cells. J Virol,
2011. 85(12): p. 5864-79.
35. Das, S., A. Vasanji, and P.E. Pellett, Three-dimensional structure of the human cytomegalovirus
cytoplasmic virion assembly complex includes a reoriented secretory apparatus. J Virol, 2007.
81(21): p. 11861-9.
36. Jean Beltran, P.M., R.A. Mathias, and I.M. Cristea, A Portrait of the Human Organelle Proteome
In Space and Time during Cytomegalovirus Infection. Cell Syst, 2016. 3(4): p. 361-373 e6.
37. Lucin, P., et al., Cytomegalovirus Generates Assembly Compartment in the Early Phase of
Infection by Perturbation of Host-Cell Factors Recruitment at the Early Endosome/Endosomal
Recycling Compartment/Trans-Golgi Interface. Front Cell Dev Biol, 2020. 8: p. 563607.
38. Glingston, R.S., et al., Organelle dynamics and viral infections: at cross roads. Microbes Infect,
2019. 21(1): p. 20-32.
39. Sharon-Friling, R., et al., Human cytomegalovirus pUL37x1 induces the release of endoplasmic
reticulum calcium stores. Proc Natl Acad Sci U S A, 2006. 103(50): p. 19117-22.
40. Zorov, D.B., et al., Lessons from the Discovery of Mitochondrial Fragmentation (Fission): A
Review and Update. Cells, 2019. 8(2).
41. Combs, J.A., et al., Human Cytomegalovirus Alters Host Cell Mitochondrial Function during
Acute Infection. J Virol, 2020. 94(2).
42. Chambers, J.W., T.G. Maguire, and J.C. Alwine, Glutamine metabolism is essential for human
cytomegalovirus infection. J Virol, 2010. 84(4): p. 1867-73.
43. Munger, J., et al., Dynamics of the cellular metabolome during human cytomegalovirus infection.
PLoS Pathog, 2006. 2(12): p. e132.
44. Yu, Y., T.G. Maguire, and J.C. Alwine, Human cytomegalovirus activates glucose transporter 4
expression to increase glucose uptake during infection. J Virol, 2011. 85(4): p. 1573-80.
34
45. Rodriguez-Sanchez, I. and J. Munger, Meal for Two: Human Cytomegalovirus-Induced
Activation of Cellular Metabolism. Viruses, 2019. 11(3).
46. Young, C.D. and S.M. Anderson, Sugar and fat - that's where it's at: metabolic changes in
tumors. Breast Cancer Res, 2008. 10(1): p. 202.
47. Warburg, O., On the origin of cancer cells. Science, 1956. 123(3191): p. 309-14.
48. Kawabata, A., et al., y Human herpesvirus 6 envelope components enriched in lipid rafts:
evidence for virion-associated lipid rafts. Virol J, 2009. 6: p. 127.
49. Munger, J., et al., Systems-level metabolic flux profiling identifies fatty acid synthesis as a target
for antiviral therapy. Nat Biotechnol, 2008. 26(10): p. 1179-86.
50. Vastag, L., et al., Divergent effects of human cytomegalovirus and herpes simplex virus-1 on
cellular metabolism. PLoS Pathog, 2011. 7(7): p. e1002124.
51. Koyuncu, E., et al., Saturated very long chain fatty acids are required for the production of
infectious human cytomegalovirus progeny. PLoS Pathog, 2013. 9(5): p. e1003333.
52. Purdy, J.G., T. Shenk, and J.D. Rabinowitz, Fatty acid elongase 7 catalyzes lipidome remodeling
essential for human cytomegalovirus replication. Cell Rep, 2015. 10(8): p. 1375-85.
53. Xi, Y., et al., Human Cytomegalovirus pUL37x1 Is Important for Remodeling of Host Lipid
Metabolism. J Virol, 2019. 93(21).
54. Simons, K. and E. Ikonen, Functional rafts in cell membranes. Nature, 1997. 387(6633): p. 569-
72.
55. Herz, J., et al., Surface location and high affinity for calcium of a 500-kd liver membrane protein
closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO
J, 1988. 7(13): p. 4119-27.
56. Franchini, M. and M. Montagnana, Low-density lipoprotein receptor-related protein 1: new
functions for an old molecule. Clin Chem Lab Med, 2011. 49(6): p. 967-70.
57. Lillis, A.P., et al., LDL receptor-related protein 1: unique tissue-specific functions revealed by
selective gene knockout studies. Physiol Rev, 2008. 88(3): p. 887-918.
35
58. Terrand, J., et al., LRP1 controls intracellular cholesterol storage and fatty acid synthesis
through modulation of Wnt signaling. J Biol Chem, 2009. 284(1): p. 381-388.
59. Zhou, L., et al., LRP1 controls cPLA2 phosphorylation, ABCA1 expression and cellular
cholesterol export. PLoS One, 2009. 4(8): p. e6853.
60. Tang, H., et al., Human herpesvirus-6 infection induces the reorganization of membrane
microdomains in target cells, which are required for virus entry. Virology, 2008. 378(2): p. 265-
71.
61. Wu, Z., et al., Human herpesvirus 6A promotes glycolysis in infected T cells by activation of
mTOR signaling. PLoS Pathog, 2020. 16(6): p. e1008568.
62. Goldmacher, V.S., vMIA, a viral inhibitor of apoptosis targeting mitochondria. Biochimie, 2002.
84(2-3): p. 177-85.
63. Brune, W. and C.E. Andoniou, Die Another Day: Inhibition of Cell Death Pathways by
Cytomegalovirus. Viruses, 2017. 9(9).
64. Lim, M.C., Human Cytomegalovirus Cytoplasmic Virion Assmebly Complex: Structure in vivo
and Role of pUL103 in its Biogenesis. 2017.
65. Bialkowska, K., et al., Spheroids as a Type of Three-Dimensional Cell Cultures-Examples of
Methods of Preparation and the Most Important Application. Int J Mol Sci, 2020. 21(17).
66. Fang, Y. and R.M. Eglen, Three-Dimensional Cell Cultures in Drug Discovery and Development.
SLAS Discov, 2017. 22(5): p. 456-472.
67. Choi, S.H., et al., 3D culture models of Alzheimer's disease: a road map to a "cure-in-a-dish".
Mol Neurodegener, 2016. 11(1): p. 75.
68. Bolognin, S., et al., 3D Cultures of Parkinson's Disease-Specific Dopaminergic Neurons for High
Content Phenotyping and Drug Testing. Adv Sci (Weinh), 2019. 6(1): p. 1800927.
69. Liu, H., et al., Establishment and characterization of a new human first trimester Trophoblast cell
line, AL07. Placenta, 2020. 100: p. 122-132.
36
70. You, Y., et al., Novel 3D in vitro models to evaluate trophoblast migration and invasion. Am J
Reprod Immunol, 2019. 81(3): p. e13076.
71. Krug, L.T.P., P. E., Fields Virology. 2021, Wolters Kluwer. p. 212-234.

37
ABSTRACT
SPATIAL LOCALIZATION OF MARKERS AND 3D-CELL MODEL FOR STUDY OF THE
HUMAN CYTOMEGALOVIRUS CYTOPLASMIC VIRION ASSEMBLY COMPARTMENT
by
TAYLOR ALAN VENSKO
May 2022
Advisor: Dr. Philip E. Pellett
Major: Immunology and Microbiology
Degree: Master of Science
Human cytomegalovirus (HCMV) causes severe disease in immunocompromised individuals and
is a leading cause of congenital disease. Efficient assembly of virions (viral particles) is an intricate process
that requires modulation and modification of host systems. HCMV induces an extensive rearrangement of
the cellular endocytic recycling compartment into the site of virion maturation and egress: the cytoplasmic
virion assembly compartment (cVAC). The HCMV cVAC is a distinguishing characteristic of infection,
although studies have only been performed in traditional cell culture with limited observations in infected
human tissues. To study the cVAC, viral and cellular markers that are discrete to their respective
compartments are required. This work uses immunofluorescent microscopy to determine colocalization of
two cellular and viral markers. We also propose a method using cell spheroids as a tissue-like 3D model to
examine the cVAC. This work validates the concurrent use of viral IE2, a nuclear viral marker, along with
the cellular Golgi-marker GM130 using the same secondary antibody in fluorescence microscopy and
establishes a workflow for culture and processing of HCMV-infected spheroids.
38
AUTOBIOGRAPHICAL STATEMENT
I wonder if my classmate remembers calling me “science boy” in the third grade; I never thought
much of it back then. Science and math were always there at a high level in my life, but my enthusiasm
wasn’t apparent to me. In high school, my Trigonometry teacher changed my perspective on math, saying,
“Math isn’t just a bunch of numbers with some letters mixed in. It’s about applying logic to solve the
problem in front of you; the subject and values are arbitrary.” All of my years of puzzle-solving video
games started to make sense; I enjoy the challenge that comes with learning how systems work and
watching pieces fit together to solve a problem.
I have lived a unique life that pointed seemingly every direction away from the lab, while at the
same time, each step has provided me with the tools needed where I am now. My mother taught me how to
think and act for myself, leave things better than I found them, and make what I want of my life.
Weightlifting taught me discipline and how to apply biochemistry and kinesiology in real-world
physiology. Video games and fantasy taught me to be creative, imaginative, and visionary. “Your world is
a culmination of everything you’ve been exposed to.”
My first exposure to viruses was the horrific “T-Virus” from the Resident Evil series as a child;
now I get to study how they work. I stumbled into microbiology when I didn’t care about much in my life,
and it sounded challenging. Spoiler alert: it was. Understanding these bacteria, viruses, and parasites was
excitingly arduous and gave me something to be enthusiastic about. Since then, learning the new pieces of
the puzzle that is biology and how they fit together pushes me to keep going. The unknown is exciting to
me, as long as sharks aren’t involved, and exploring human and microbial systems and their infinitely
complex interplay can keep me stimulated for years to come.
