
Tom Cook, associate professor of horticulture, Oregon State University, and
Brian McDonald, turfgrass research assistant, Oregon State University.
EC 1278 • Revised January 2005
$2.50
Fertilizing, along with mowing
and irrigating, is one of the basic
cultural practices used to produce
healthy, dense, green lawns. The
goals of this publication are to
help you better understand the
following:
• Reasons for fertilizing
• Optimum application rates and
timing
• Types of fertilizer materials
• How to read a fertilizer label
• Application techniques
• How to avoid turf damage and
environmental pollution result-
ing from improper fertilization
Do I really need to
fertilize my lawn?
In terms of turf survival, the
answer probably is no, since
the majority of lawns in Oregon
receive little or no fertilizer
and are more or less functional.
However, unfertilized lawns tend
to be thin, light green or brown in
color, and have high weed popula-
tions. Perennial ryegrass, the most
widely planted grass in much of
Oregon, is especially prone to
diseases such as red thread, rust,
and brown blight when under-
fertilized. Adequately fertilized
lawns look better than underfertil-
ized lawns, compete better against
weeds, hold up better under
wear and tear, and recover more
quickly from damage.
Visual
turf
quality J F M A M J J A S O N D
High
Medium
Utility
= Planned application = Optional application
Horizontal bars indicate time for each application. Adjust timing based on your
goals and personal experience with your lawn. Each application is assumed to be
at 1 lb N per 1,000 sq ft. On hungry lawns, 1.5 to 2 lb N per 1,000 sq ft can be
used to stimulate density and color. Unless lawns are very weak, avoid early-spring
applications since grass normally grows vigorously by itself at that time.
Visual
turf
quality J F M A M J J A S O N D
High
Medium
Utility
= Planned application = Optional application
Horizontal bars indicate time for each application. Adjust timing based on your
goals and personal experience with your lawn. Each application is assumed to be
at 1 lb N per 1,000 sq ft. On hungry lawns, 1.5 to 2 lb N per 1,000 sq ft can be
used to stimulate density and color. Unless lawns are very weak, avoid early-spring
applications since grass normally grows vigorously by itself at that time.
T. Cook and B. McDonald
Figure 1. Fertilizer calendar for irrigated lawns in western Oregon.
Figure 2. Fertilizer calendar for irrigated lawns in central and eastern
Oregon.
FERTILIZING LAWNS

2
How often do I need
to fertilize my lawn?
The ultimate goal is to apply
the least amount of fertilizer
needed to produce healthy turf
and meet your personal aesthetic
standards. The proper rates,
frequency, and timing of fertil-
izer application depend on your
desired turf quality, the type of
fertilizer you use, the type of
grass in your lawn, and whether
you leave clippings on the lawn.
Fertilizer needs are lowest on
older lawns where clippings have
been regularly returned to the
surface during mowing. In this
case, bentgrass lawns growing in
clay soils often get by nicely with
a single application of nitrogen
fertilizer each year. If perennial
ryegrass or Kentucky bluegrass is
planted on imported sandy loam
soil, and clippings are removed,
multiple applications of fertilizer
may be needed on new lawns to
keep them dense and attractive.
The key is to decide the quality
of lawn you want. Figures 1 and 2
show potential fertilizer applica-
tion schedules for western Oregon
(Figure 1) and central and eastern
Oregon (Figure 2). These sched-
ules are not intended to be rigid;
rather, they are examples of logi-
cal application times for different
levels of turf quality.
If your goal is to maintain a
consistently dense, dark green
turf, keep in mind that a nitrogen
fertilizer application generally
produces a visual response for
at least 4 weeks and sometimes
as long as 8 weeks. Response is
longer when fertilizer is applied
in the cool temperatures of fall
or early spring. Applications to
weak, hungry grass generally do
not last as long as applications to
dense, healthy turf.
When soluble fertilizers
such as ammonium sulfate are
applied at normal application
rates of 1 lb nitrogen (N) per
1,000 sq ft, expect to see green-up
in as little as a week, followed
by about 4 weeks of vigorous,
dark green growth. Fertilizers
containing slow-release nitrogen
sources generally react more
slowly but last slightly longer.
The differences between soluble
and slow-release nitrogen are
discussed under “Types of nitro-
gen fertilizer,” page 3.
If you remove clippings, as
most people do, you will see
reduced color and growth after
4 weeks. Your lawn still will
be dense and healthy, but color
will be less intense. If you leave
clippings on the lawn, color and
growth enhancement may last
6 to 8 weeks. Returned clippings
extend the effect of fertilizers by
recycling nutrients during decom-
position. This is an easy way
to prolong fertilizer responses.
Contrary to popular belief,
clippings do not contribute signifi -
cantly to thatch buildup.
In my own landscape, I use a
watch-and-wait approach, with
the goal of applying the least
amount of fertilizer per year to
maintain a functional turf. I also
use the “special event” approach;
I coast along with as little fer-
tilizer as I can and then make
a strategic application about
3 weeks before my in-laws visit.
The lawn looks good when they
show up, and in between visits I
donʼt have to mow so much grass!
Should I have my
soil tested before
fertilizing?
In the best of all worlds, every-
one would get a soil test before
planting a lawn and would repeat
the test each year and adjust the
fertilizer program according to the
results. This approach is common
at many golf courses and athletic
fi eld complexes with professional
turf managers.
Unfortunately, it takes some
effort for homeowners to fi nd a
testing laboratory and interpret
test results. As a result, very few
people get their soil tested regu-
larly. If you suspect you have a
problem soil, contact a local
professional analytical lab or
estimate your soil nutrient content
by using a soil test kit purchased
from a retail nursery center.
What are the
important fertilizer
elements for lawns?
Nitrogen (N)
Nitrogen is the most impor-
tant nutrient in most fertilization
programs. Applied at proper
rates, nitrogen stimulates verti-
cal growth, improves turf density,
and makes the grass darker green.
By stimulating growth, nitrogen
reduces the severity of diseases

3
such as red thread, rust, and
brown blight.
Soil tests for nitrogen are
available, but theyʼre seldom
used. Nitrogen levels in soil
fl uctuate rapidly, which makes
interpretation for fertilizer recom-
mendations diffi cult.
Annual nitrogen needs. Of
the commonly used turf grasses,
Kentucky bluegrass and perennial
ryegrass need the most nitrogen,
tall fescue is intermediate, and
the fi ne fescues and bentgrasses
persist well at low levels of nitro-
gen. All grasses turn darker green
when fertilized with nitrogen.
For top-quality ryegrass or
bluegrass lawns, you may have
to apply up to 6 lb nitrogen per
1,000 sq ft per year. Medium-
quality turf can be achieved with
3 to 4 lb nitrogen per 1,000 sq ft
per year. Functional turf can be
produced with as little as 1 to
2 lb nitrogen per 1,000 sq ft per
year. Bentgrass and fi ne fescue
lawns require about half as much
nitrogen fertilizer as ryegrass or
bluegrass for any given quality.
Nitrogen fertilizer application
rates. The quantities given in the
previous paragraph are for total
annual nitrogen. In most cases,
the total amount is split between
two or more applications (see
Figures 1 and 2).
Rates per application gener-
ally range from 0.5 to 1 lb soluble
nitrogen per 1,000 sq ft of turf.
Below 0.5 lb soluble N, initial and
residual response is poor. Above
1 lb soluble N, the chance of
foliar burn and excessive growth
increases. Most commercial
product directions are based on
applying 0.9 to 1 lb of total N per
1,000 sq ft per application. If you
follow package directions, you
generally will get good results.
Application rates for weak, thin
lawns and lawns fertilized in late
fall or early winter may be up to
2 lb total N per 1,000 sq ft.
Note that the rates discussed
here give the amount of nitrogen
to apply. Fertilizer labels indicate
the percentage of nitrogen in the
product (see “How can I deci-
pher a fertilizer label?” page 6).
Table 1 shows how to estimate
the amount of fertilizer to apply
based on the productʼs percentage
N content and your desired rate of
application.
Types of nitrogen fertil-
izer. Nitrogen fertilizers are
classifi ed by the rate of nitro-
gen release (i.e., availability to
plants). The two general types are
water-soluble nitrogen and slow-
release nitrogen.
Water-soluble nitrogen is
available immediately after being
watered in, and it rapidly turns the
lawn green and stimulates growth.
Nitrogen in soluble fertilizers
doesnʼt last long, so you need to
apply it more frequently at lower
rates to maintain a steady supply
of nitrogen to the turf. Examples
of water-soluble nitrogen fertil-
izer materials include ammonium
nitrate, urea, ammonium phos-
phate, and ammonium sulfate.
Water-soluble fertilizers have
salt-like characteristics and can
cause desiccation injury (burn-
ing) by drawing water out of
leaf tissue. This problem is most
common when people apply
ammonium sulfate or urea to
moist turf and fail to water it in
Table 1. Pounds of product per 1,000 sq ft needed to apply
1, 1.5, or 2 lb N to turf.
Target rate (lb N per 1,000 sq ft)
1 1.5 2
% nitrogen
in fertilizer Pounds of product per 1,000 sq ft
5 20.0 30.0 40.0
8 12.5 18.8 24.0
10 10.0 15.0 20.0
15 6.8 10.2 13.6
20 5.0 7.5 10.0
25 4.0 6.0 8.0
30 3.4 5.1 6.8
35 2.9 4.3 5.7
40 2.5 3.8 5.0
45 2.2 3.3 4.4

4
immediately (Figure 3). Avoid
foliar burn by irrigating turf
thoroughly after applying any
water-soluble fertilizer. Water
long enough to wash granules off
foliage or until granules com-
pletely dissolve.
Slow-release nitrogen fertil-
izers have relatively low water
solubility and release nitrogen
slowly over a longer period of
time than soluble fertilizers.
Therefore, they may give less
initial color and growth response
but are less likely to cause fertil-
izer burn. Theyʼre signifi cantly
more expensive than soluble
nitrogen fertilizers.
Common slow-release nitro-
gen fertilizers include methylene
ureas (Nitroform and Nutralene),
sulfur-coated urea (SCU),
polymer-coated urea (PCU),
polymer-coated SCU (PCSCU),
isobutylidenediurea (IBDU), and
natural organic, protein-based
sources such as activated sewage
sludge and mixtures of feather
meal, dried poultry waste, and
dried blood.
Synthetic methylene ureas
release nitrogen when soil micro-
organisms break them down.
Since microbes are more active
in warm weather, these products
work well in the summer but
poorly in winter and early spring.
SCU nitrogen release depends
largely on water that permeates
the sulfur shell and dissolves the
urea. These products release nitro-
gen faster at higher temperatures.
Common SCU materials release
nitrogen slightly faster than
other slow-release sources. This
source appears in many common
fertilizers.
PCU and PCSCU sources
depend on water movement
through the polymer shell to
dissolve the urea, which then
diffuses into the soil, where the
nitrogen is taken up by plant
roots. Nitrogen release is faster
in warm weather and slows down
dramatically when soil tempera-
tures drop to about 40°F.
IBDU depends primarily
on water hydrolysis to release
nitrogen and is less affected by
soil temperatures than natural or
polymer-coated fertilizers. It is
effective at all times of the year,
but fi rst-time applications tend to
produce a weak response. Used
repeatedly over time, this is a
highly effective material.
Protein-based natural products
release nitrogen when soil micro-
organisms break them down.
Thus, they are highly temperature-
dependent and release nitrogen
faster during summer and fall
when soil temperatures tend to be
high. They produce effects much
like those of methylene ureas,
PCU, PCSCU, and IBDU. Natural
organic materials work well and
are widely used in Oregon.
Commercial fertilizers often
combine soluble and slow-release
nitrogen sources to give good ini-
tial and residual response, lower
burn potential, and intermediate
cost.
Phosphorus (P)
Healthy turf does not show
a growth or color response to
phosphorus. Contrary to the
popular notion expressed in many
newspapers, magazines, and gar-
den books, phosphorus does not
enhance root growth of grasses
unless it is added to defi cient turf.
Since the vast majority of lawn
soils contain adequate P, supple-
mental applications usually are
wasteful.
Figure 3. This turf was severely burned when a soluble nitrogen fertil-
izer was applied to dew-covered grass and not thoroughly watered in.

5
Phosphorus is best applied only
when soil tests indicate it is low.
In all regions of Oregon, a soil
test level above 20 ppm indicates
that P is adequate and does not
need to be applied.
If you do apply phosphorus,
use it sparingly. Turf rarely ben-
efi ts from more than 1 lb P
2
O
5
per
1,000 sq ft per year.
Most fertilizers are “complete,”
meaning they contain nitrogen,
phosphorus, and potassium. For
routine lawn applications, look for
fertilizers with little or no P. (Note
that phosphorus is listed on fertil-
izer labels as P
2
O
5
, or phosphoric
acid.) Avoid products such as
15-15-15 or similar ratios because
they contain too much P for lawn
needs and increase the potential
for P pollution of surface waters.
Be cautious when selecting
natural organic fertilizer products
and composts because they often
contain relatively high P levels.
Potassium (K)
Potassium is an important ele-
ment for healthy turf and often
is applied in relatively large
amounts. Although it causes no
color or growth response, potas-
sium seems to slightly enhance
plant hardiness to heat and cold.
It also may reduce drought-
induced wilting and increase
wear tolerance.
A general rule for soils in
Oregon is that soil potassium
levels below 250 ppm K indicate
a need for potassium fertiliza-
tion. Based on this standard,
many lawns in eastern Oregon do
not need potassium fertilizer. In
western Oregon, soil potassium
often is low, indicating potassium
should be applied regularly.
Application rates should be
about two-thirds the rate of
nitrogen (i.e., 2 lb K
2
0 for every
3 lb N). For example, a fertilizer
labeled 12-3-8 or 24-3-16 would
give the proper ratio of N to K
2
0.
(Note that fertilizer labels list
the potassium content as K
2
0, or
soluble potash.)
Sulfur (S)
Sulfur is an important plant
nutrient, particularly for bent-
grass, which is very common
in most old lawns west of the
Cascades. Long-term research
at Washington State University
shows that bentgrass turf receiv-
ing 2.5 to 3.5 lb sulfur per
1,000 sq ft per year has less
Take-all and Fusarium patch dis-
eases and less encroachment by
annual bluegrass than turf receiv-
ing less sulfur. Many commonly
used turf fertilizers contain sulfur,
so you often can meet the need
for S as part of a regular fertilizer
program.
Excessive use of sulfur-
containing fertilizers such as
ammonium sulfate will decrease
the pH of the soil and thatch,
resulting in excessively acidic
soil. As a rule, apply no more than
3.5 lb S per 1,000 sq ft per year.
pH, calcium (Ca), and
magnesium (Mg)
Calcium and magnesium rarely
cause a growth or color response
in grass. Their main impact is
their effect on soil pH. When
added as limestone, they help
raise the pH of acid soils and indi-
rectly improve turf fertility.
Turfgrasses will grow in a
wide range of soil pH, but most
have an optimum range, as noted
in Table 2. Soil pH in the opti-
mum range is important because
it increases availability and soil
retention of many of the nutrients
needed for turfgrass growth.
A low soil pH indicates acidic
soil. Acidic soils are common in
western and coastal Oregon, but
are rare in central and eastern
Oregon.
If your soil test pH value falls
below the optimum range for
the grasses in your lawn, a lime
application will help raise the pH.
It often takes 2 or more years to
signifi cantly change turfgrass soil
pH with lime, because lime is not
very soluble and canʼt be mixed
into the soil in lawns.
There are two common types of
lime—agricultural limestone and
dolomitic limestone. Agricultural
lime contains calcium, while
dolomitic lime contains both
calcium and magnesium.
Soil test values for calcium
and magnesium can help you
determine which type to apply.
If magnesium levels are above
1.5 meq/100 grams of soil, apply
Table 2. Optimum soil pH ranges
for commonly grown turfgrasses.
Optimum
Grass pH range
Bentgrasses 5.0–6.5
Perennial ryegrass 5.5–6.5
Fine fescues 5.5–6.5
Tall fescue 5.5–6.5
Annual bluegrass 6.0–7.0
Kentucky bluegrass 6.0–7.0

6
agricultural limestone. If Mg is
below 1.5 meq/100 grams of soil,
apply dolomite. Coastal coun-
ties usually are the only areas
in Oregon where defi ciencies of
magnesium are found; conse-
quently, they are most likely to
benefi t from dolomitic limestone.
As a general rule, donʼt exceed
50 lb lime per 1,000 sq ft per
application or a total of 100 lb
lime per 1,000 sq ft per year on
established turf. During a single
year, split applications between
spring and fall to avoid lime accu-
mulation at the soil surface.
Donʼt apply nitrogen fertilizers
that contain ammonium sulfate
or urea with or immediately after
liming. The presence of lime may
cause ammonium to turn to vapor
(volatilize) and be lost to the
atmosphere.
Iron (Fe)
Iron is known as a micronutri-
ent because it is required in very
small amounts. Iron defi ciency in
turfgrasses is not widespread, but
it may occur in eastern Oregon,
where soil pH can be above 7.0.
(High soil pH limits the availabil-
ity of iron to plants.) Most lawns
do not need supplemental iron to
produce healthy turf.
Nonetheless, iron is a common
supplement in commercial fertil-
izers because it provides a rapid,
short-lived, greening response.
Many fertilizers contain iron in
soluble form. If you get these
materials on concrete sidewalks
or driveways and then apply
water, you can count on having
brown stains (Figure 4). Like tat-
toos, iron stains are diffi cult and
expensive to remove. To prevent
stains, buy fertilizers that do not
contain iron or use a blower to
blow the fertilizer back onto the
lawn before you irrigate.
How can I decipher a
fertilizer label?
State law requires that certain
information be printed on the
fertilizer bag or label. This is your
guarantee that you will get what
you pay for. Fertilizer companies
are fi ned by the state if the analy-
sis on the bag does not match the
contents. The generic label in
Figure 5 explains key information
contained on all fertilizer bags.
Next time you look at fertilizers,
study several labels to see how
products vary.
Complete fertilizers contain-
ing N, P
2
0
5
, and K
2
0 in ratios of
about 6-1-4 are good for balanced
turf fertility, although many of the
top brands have lower K
2
0. You
can feel confi dent using complete
fertilizers with ratios ranging from
24-4-16 (6-1-4) to 24-3-6 (8-1-2)
or anything close to that range.
Fertilizers often are marketed
as spring, summer, or fall and
winter fertilizers. Usually, sum-
mer fertilizers contain a higher
proportion of slow-release nitro-
gen, and winter fertilizers contain
more potassium. These adjust-
ments are essentially meaningless
and are simply used to get cus-
tomers to buy different products
for each season. If you stick with
a good basic ratio, you can use
the same product year-round and
produce beautiful turf.
What is the best way
to apply fertilizer?
For small lawns, the most
accurate way to apply fertilizer is
with a drop-type spreader. Drop
spreaders are highly effi cient and
dramatically reduce the possibility
of misapplying fertilizer to drive-
ways, sidewalks, or streets.
Because fertilizer goes right
where you put it and does not
spread laterally once it hits the
ground, you need to overlap
slightly with each consecutive
Figure 4. Brown rust stains where iron contacted concrete and then was
watered in.
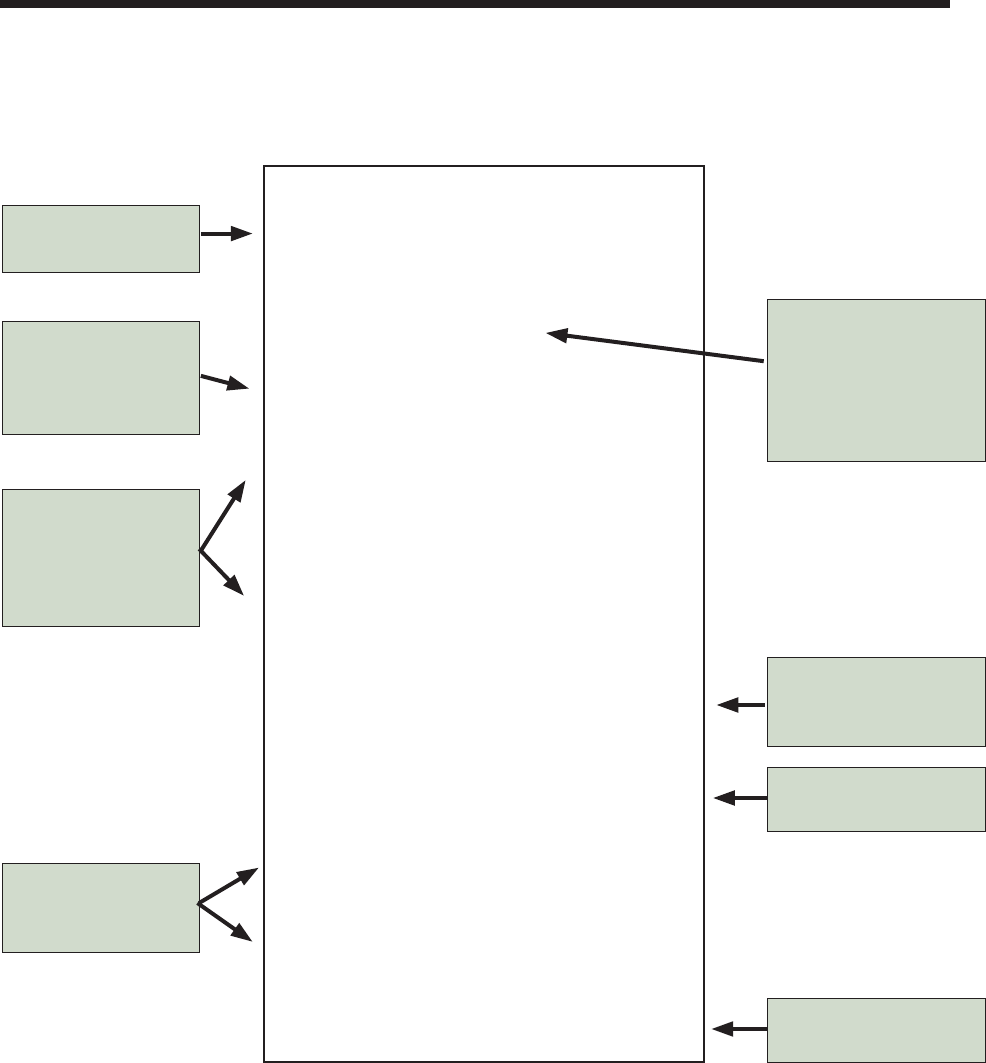
7
Brand X
Fertilizer
24-3-15
Guaranteed analysis:
Total Nitrogen (N) .............................24.0 %
4% Nitrate nitrogen
8% Ammoniacal nitrogen derived from
ammonium phosphate and ammonium
sulfate
12% Coated slow-release nitrogen derived
from polymer-coated sulfur-coated
urea
Available Phosphoric Acid (P
2
O
5
) .......3.0 %
derived from ammonium phosphate
Soluble Potash (K
2
O) ........................15.0 %
derived from potassium chloride
Sulfur (S) ..........................................14.0 %
derived from sulfates and sulfi des
Iron (Fe) .............................................2.0 %
derived from ferrous sulfate
Net weight 20 lbs.
Brand name of the
fertilizer
Law requires that the
guaranteed analysis
be equal to or greater
than the stated values
Nitrogen sources are
listed starting with
soluble sources and
moving to slow-
release sources
Other fertilizer
elements and
micronutrients
Fertilizer analysis
24% N (Nitrogen)
3% P
2
O
5
(Phosphorus)
15% K
2
O (Potassium)
Ratio = 8-1-5
Phosphorus is listed
as phosphoric acid
equivalent (P
2
O
5
)
Potassium is listed as
soluble potash (K
2
O)
Total weight of fertilizer
in the bag
Notice that half of the total nitrogen in this example is from soluble sources (nitrate and ammonium), and half is from
slow-release sources (polymer-coated sulfur-coated urea). If you apply 1 lb of total nitrogen from this product, you will apply
0.5 lb of soluble nitrogen.
To fi nd out how much of any given nutrient you are applying, multiply your fertilizer application rate times the percentage
of that nutrient in the fertilizer. For example, if you apply 6 lb of the fertilizer represented by the sample label, you would be
applying 0.84 lb of sulfur (6 lb x 0.14 = 0.84).
Figure 5. Generic fertilizer label with explanation of terms.

8
pass to avoid skips (Figure 6).
A good method is to apply the
fertilizer at half the application
rate and go over the area twice at
different angles to minimize the
possibility of skips (Figure 7).
Make sure you are moving before
opening the spreader to avoid
dropping a large quantity of fertil-
izer in one spot.
When buying a spreader, pur-
chase only top-of-the-line name
brands and avoid discount spe-
cials. In extensive spreader testing
at OSU, we have found that many
cheap spreaders are so poorly
constructed that they cannot be
calibrated to apply fertilizer accu-
rately. The top brands generally
are fairly accurate. Most commer-
cial fertilizers have instructions
for settings on common spreaders.
For larger areas, it is eas-
ier to apply fertilizer with a
rotary spinner-type spreader
(Figure 8). These spreaders are
not very accurate and tend to
throw fertilizer past the lawn into
fl ower and shrub beds, or on side-
walks and streets. They are best
used only where lawns are sur-
rounded by other plant materials
that can trap and use the fertilizer.
Because consumer models of
rotary spreaders are so inaccu-
rate, it is hard to give advice on
how to use them. Trial and error
may be the only way to learn
with any given brand. Try using
the half-rate setting and making
consecutive passes at the spacing
recommended on the fertilizer
bag. Spacing can range from 5 to
15 feet, depending on the machine
and fertilizer product. After cover-
ing the entire area at the half rate,
go over it again at a different
angle.
What should I know
before I fertilize?
Start by reading the pack-
age directions so you know how
much area the fertilizer will cover.
Adjust your spreader based on the
bag directions. Fill the spreader
on a level surface away from the
lawn. Make sure the spreader is in
the closed position so you do not
spill fertilizer at the loading site.
Figure 6. Drop spreaders are very accurate but must be operated properly. The lawn on the right has stripes
because the operator failed to overlap each pass. You could say it got a half-rate application!
Figure 7. Typical application
pattern for a drop spreader. By
overlapping each pass slightly
and covering the area twice in
different directions, you can
achieve thorough coverage
without skipped areas.

9
When using soluble fertilizers,
avoid burning grass by apply-
ing the fertilizer when the lawn
is dry and temperatures are cool.
Evening is better than morning,
when there often is dew on the
grass.
Avoid fertilizing during very
hot summer weather. Grass nor-
mally doesnʼt need fertilizer in
the heat of summer, and growth
at that time tends to favor shoots
over roots. If you do fertilize in
midsummer, use lower rates.
After you apply the fertilizer,
use a blower or broom to remove
fertilizer from sidewalks or other
hard surfaces, where it might
stain concrete or get washed into
storm sewers. After cleaning hard
surfaces, water the lawn to reduce
the chance of foliar burn and to
wash the fertilizer into the root
zone, where the grass roots can
use it. If the area is dry, irrigate
Figure 8. Rotary spinner spreaders are great for large open areas (left) but are not suited for confi ned areas
next to roads and sidewalks (right). Near these areas use a drop spreader (Figure 6) to make sure the fertilizer
goes on the lawn and not in the street, where it can get into storm sewers and pollute rivers.
thoroughly just as you would
when watering your lawn. If the
area is moist, water just enough to
wash the fertilizer off the foliage
and into the root zone.
What about
environmental
pollution?
The two nutrients of greatest
concern are nitrogen and phos-
phorus. The primary concerns are
leaching into groundwater (nitro-
gen) and runoff that contaminates
surface waters (phosphorus).
High levels of nitrogen in
groundwater can lead to high
levels of nitrogen in wells.
Numerous health disorders are
associated with elevated nitrogen
in drinking water.
Phosphorus pollution of sur-
face waters leads to plant growth
in streams, ponds, and lakes.
Algal blooms resulting from high
phosphorus levels can reduce
oxygen levels in waterways to the
point of killing fi sh. Phosphorus-
stimulated growth of aquatic
plants also can result in fouled
waterways, rendering them unus-
able for recreation purposes.
There are many sources of
nitrogen and phosphorus pollu-
tion. Research at universities has
consistently demonstrated that
synthetic fertilizers applied to
lawns are not a major source of
surface or groundwater pollution.
Grass is a very effi cient absorber
of nitrogen, and leaching and run-
off are rare when applicators use
common sense. When fertilizer is
applied properly to lawns at low
rates, leaching is unlikely to occur
and runoff is no more likely than
it is from nonfertilized lawns.
A recent report in Penn State
Agriculture (summer 1999 at

10
http://www.aginfo.psu.edu/PSA/
s99/contents.html) indicates that
99.7 percent of nitrogen pollution
comes from sources other than
golf courses and lawns.
Atmospheric deposition
and agriculture accounted for
87.9 percent of all nitrogen pol-
lution, urban stormwater run-
off accounted for 8.4 percent,
septic systems 3.4 percent, and
golf courses and lawns only
0.3 percent.
The same study found that
phosphorus pollution sources
included 68.8 percent from agri-
culture, 13.1 percent from septic
systems, 10.8 percent from urban
stormwater runoff, 7.1 percent
from atmospheric deposition, and
only 0.2 percent from golf courses
and lawns. A total of 99.8 percent
of phosphorus pollution came
from sources other than golf
courses and lawns.
The Penn State University
study is similar to many oth-
ers reported in recent years.
When measurements are made
on specifi c lawn sites, very little
nitrogen or phosphorus shows
up as either leachate or runoff.
Additional studies are listed in
“For more information,” page 11.
Does this mean that lawn
fertilizers donʼt pose a potential
pollution risk? Actually, there are
many potential problems with
fertilizer, but, if you use common
sense, you can be confi dent you
are not contributing to pollution.
See the box on this page for some
tips to help you avoid pollution.
Tips for ensuring that lawn fertilizer
doesn’t contribute to pollution
◆ Make sure the fertilizer goes on the lawn and not on sidewalks
or in the street. The single greatest source of pollution from
lawn fertilizers is the fertilizer that ends up in the street.
◆ Remove any fertilizer from hard surfaces before irrigating to
avoid fl ushing fertilizer into storm sewers.
◆ Apply fertilizer at times when grass is growing and actively
absorbing nutrients (i.e., spring through fall).
◆ Fertilize more often at lower rates rather than less often at
higher rates.
◆ Observe your lawn over time to determine the least amount of
fertilizer needed annually to provide the quality of grass you
desire.
◆ Use fertilizers with low or no phosphorus (the middle number
in the analysis). Avoid applying more than 1 lb P
2
O
5
per
1,000 sq ft per year.
◆ On sandy soils, use slow-release fertilizers to avoid an over-
load of nitrogen immediately after application. Both synthetic
slow-release and natural organic fertilizers are good choices in
this situation.
◆ Leave unfertilized buffer zones near lakes or streams. The best
solution is to eliminate mowed turf next to waterways in favor
of unmowed natural grass stands or dense native or native-like
vegetation that requires no fertilizer. Mowed grass all the way
to the water surface is an accident waiting to happen.
◆ Be cautious when selecting composts and natural organic
fertilizers, since they generally contain relatively high levels
of phosphorus. For example, 0.25 inch of compost containing
0.75 percent N and 0.75 percent P
2
O
5
translates to 2 lb of N
and 2 lb of P
2
O
5
per 1,000 sq ft per application.

11
Are synthetic
fertilizers bad
for the soil?
The question of synthetic
versus organic fertilizers is not a
matter of good versus bad. You
can achieve the same results with
either natural or synthetic sources.
While it is possible to create prob-
lems with any fertilizer, it isnʼt
easy to do so.
Fifty years ago, all of the avail-
able fertilizers contained soluble
nitrogen. Research found that
prolonged use of these products
could acidify the soil enough to
change the soil microbial bal-
ance. For example, we conducted
experiments using only nitrogen
from ammonium sulfate on bent-
grass turf. Over a 10-year period,
soil pH decreased from 5.4 to 3.8,
which is incredibly acidic. That
acidity level caused a shift from a
bacteria-dominated environment
to a fungus-dominated environ-
ment. How did the grass perform?
It grew just fi ne. It was dense,
dark green, and pure. It also had
very deep thatch. The thatch
developed because the environ-
ment in the thatch and soil layer
was not conducive to microbes
that decompose thatch. Was it a
dead soil? No, but it was defi -
nitely out of balance.
Experience has shown that by
using a variety of nitrogen sources
and applying lime as needed to
raise soil pH to 6.0 to 7.0, we can
produce excellent turf indefi nitely
and maintain healthy soil microbe
populations. Turf response and
soil health are the same from
organic slow-release and syn-
thetic slow-release fertilizers.
If you avoid using a steady diet
of ammonium sulfate for many
years, and you lime as needed,
soil microbiology will be similar
to that in soils treated with slow-
release organic fertilizers.
Should I add
microorganism
supplements
to my lawn?
Research does not support
the need for supplemental appli-
cations of microorganisms. A
recent review of work from fi ve
Midwestern states concluded the
following.
• Supplemental applications
of microorganisms had no
long-lasting effects on soil
microbiology.
• Pesticide applications did
not adversely affect soil
microbiology.
• Even sand-based root zones
rapidly achieve microbial ‚º‚
equal to native soils soon after
planting, even without supple-
ments (Gaussoin, 2004).
While it doesnʼt hurt to apply
products supplemented with
microorganisms, remember that it
is the fertilizer in the product, not
the microorganisms, that causes
the lawn to respond.
References
Dionis, K. 1999. Where does nutri-
ent pollution come from? Penn
State Agriculture (Summer 1999).
http://www.aginfo.psu.edu/PSA/
s99/contents.html
Gaussoin, R. 2004. Can you impact
your soil microbiology? Sportsturf
(Summer 2004), p. 12. Adams
Business Media, Chicago, IL.
For more information
Allen, A.L., F.J. Stevenson, and
L.T. Kurtz. 1973. Chemical
distribution of residual fertil-
izer nitrogen in soil as revealed
by Nitrogen-15 studies.
J. of Environmental Quality
2(1):120–124.
Cook, T. and A. VanDerZanden.
2002. Practical Lawn
Establishment and Renovation,
EC 1550. Oregon State University
Extension Service, Corvallis.
http://eesc.oregonstate.edu/
agcomwebfi le/edmat/ec1550.pdf
Gold, A.J. and P.M. Groffman. 1993.
Leaching of agrichemicals from
suburban areas. Pesticides in
Urban Environments, pp. 182–190.
American Chemical Society.
Gold, A.J., W.R. DeRagon,
W.M. Sullivan, and J.L.
Lemunyon. 1990. Nitrate nitro-
gen losses to groundwater from
rural and suburban land uses.
J. of Soil and Water Conservation
45(2):305–310.
Mancino, C.F. and J. Troll. 1990.
Nitrate and ammonium leaching
losses from N fertilizers applied
to ʻPenncrossʼ creeping bentgrass.
HortScience 25(2):194–196.
Morton, T.G., A.J. Gold, and
W.M. Sullivan. 1988. Infl uence
of overwatering and fertilization
on nitrogen losses from home
lawns. J. of Environmental Quality
19:131–135.
Starr, J.L. and H.C. DeRoo. 1981.
The fate of nitrogen fertilizer
applied to turfgrass. Crop Science
21:531–536.
Walker, W.J and B. Branham. 1992.
Environmental impacts of turf-
grass fertilization. In Balogh,
J.C. and W.J. Walker (eds.).
Golf Course Management and
Construction: Environmental
Issues, pp. 105–219. Lewis
Publishers, Chelsea, MI.

© 2005 Oregon State University.
This publication was produced and distributed in furtherance of the Acts of Congress of May 8 and June 30, 1914. Extension work is a cooperative
program of Oregon State University, the U.S. Department of Agriculture, and Oregon counties. Oregon State University Extension Service offers edu-
cational programs, activities, and materials—without discrimination based on race, color, religion, sex, sexual orientation, national origin, age, marital
status, disability, or disabled veteran or Vietnam-era veteran status. Oregon State University Extension Service is an Equal Opportunity Employer.
Revised January 2005.
