
INFECTION AND IMMUNITY, Sept. 1995, p. 3388–3395 Vol. 63, No. 9
0019-9567/95/$04.0010
Copyright q 1995, American Society for Microbiology
Analysis of Human Immunoglobulin-Degrading Cysteine
Proteinases of Trichomonas vaginalis
DANIELE PROVENZANO AND J. F. ALDERETE*
Department of Microbiology, The University of Texas Health Science Center, San Antonio, Texas 78284-7758
Received 9 February 1995/Returned for modification 4 April 1995/Accepted 13 June 1995
Trichomonas vaginalis is a protozoan parasite that causes a widely distributed sexually transmitted disease
(STD). Since immunoglobulin G (IgG) antibodies to specific trichomonad immunogens are found in serum and
vaginal washes (VWs) from patients with trichomoniasis, a potential mechanism of immune evasion by this
parasite might be the ability of T. vaginalis proteinases to degrade human immunoglobulins (Igs). Incubation
of human IgG with lysates of T. vaginalis organisms resulted in time- and concentration-dependent degradation
of the heavy chain. Secretory IgA was degraded similarly. Inhibitors of cysteine proteinases, when added to
trichomonal lysates, abolished IgG and IgA degradation, while EDTA, a metalloproteinase inhibitor, did not.
Substrate-gel electrophoresis with human IgG, IgM, or IgA copolymerized with acrylamide revealed several
distinct cysteine proteinases in both lysates and culture supernatants from logarithmically growing parasites
that degraded all classes of human antibodies. Trichomonal lysates and supernatants of numerous isolates
tested all had Ig-degrading activity. Finally, proteolytic activity against IgG was detected in most (26 of 33;
78%) VWs from patients with trichomoniasis. In contrast, 18 of 28 (65%) VWs from women without tricho-
moniasis or from patients infected with other STDs had no detectable proteinases when tested in an identical
manner. The other 10 of these 28 VWs (35%) had smaller amounts of detectable Ig-degrading proteinases.
These differences in Ig-degrading proteinase activity between patients with and without trichomoniasis, re-
gardless of coinfecting STDs, were statistically significant (P 5 0.001). These results illustrate that T. vaginalis
is capable of degrading human Igs.
Trichomoniasis is an important sexually transmitted disease
(STD) of worldwide distribution caused by the protozoan
Trichomonas vaginalis. This non-self-limiting disease is associ-
ated with a broad symptomatology, ranging from a relatively
asymptomatic carrier state to severe inflammation and irrita-
tion with foul-smelling discharge (27). Adverse pregnancy out-
come (20, 37, 45) and enhanced predisposition to human im-
munodeficiency virus infection (28, 55) are recent noteworthy
findings that illustrate the importance of this STD. Although
immunoglobulin G (IgG) and IgA antibodies to immunogenic
trichomonad surface proteins and to proteinases have been
found in sera and vaginal washes (VWs) from patients with
trichomoniasis (5, 6, 7, 12, 30, 47), these antibodies appear to
have little or no protective value.
Numerous cysteine proteinases of T. vaginalis that have been
identified are possible virulence factors (17, 33, 38). Up to 23
distinct activities have been resolved by two-dimensional sub-
strate-gel electrophoresis (38). The in vivo role of these pro-
teolytic enzymes has not been elucidated. However, several or
all trichomonad cysteine proteinases are expressed in vivo (5,
6), and at least one appears necessary for trichomonal cytoad-
herence (8) and resistance to complement (29). Since nothing
has been reported regarding a role for trichomonad protein-
ases in degrading human immunoglobulins (Igs), we felt that
examining for this property would be highly relevant to the
chronic, non-self-limiting nature of this infection in the uro-
genital tract.
In this report, we document that T. vaginalis cysteine pro-
teinases degrade human IgG, IgM, and IgA. We show that all
trichomonal isolates possess numerous Ig-degrading cysteine
proteinases of various molecular weights. Isolates with and
without the double-stranded RNA (dsRNA) virus (54), which
can be distinguished by certain biological properties (3, 25, 26),
were found to have different Ig-degrading proteinase patterns.
Finally, Ig-degrading proteinases were found in VWs of pa-
tients with trichomoniasis, reinforcing the view that this prop-
erty has in vivo relevance, possibly as a mechanism of immune
evasion. The significance of these trichomonad Ig-degrading
proteinases in predisposing the vagina to other coinfecting
STD agents and their role in pathogenesis are discussed.
MATERIALS AND METHODS
Cells and supernatants. Fresh T. vaginalis isolates were obtained from the
STD-Collaborative Research Center (STD-CRC) of the Department of Micro-
biology at the University of Texas Health Science Center at San Antonio, and
some isolates have been used before (3, 7, 32, 38). Axenized parasites were
cultured in Trypticase-yeast extract-maltose (TYM) medium supplemented with
10% heat-inactivated horse serum (TYM-serum) (15) and passaged daily. One
isolate, labeled M, was obtained from the pellet of a centrifuged urine sample
from a male patient. A small aliquot (100 ml) of the resuspended urine debris was
inoculated into TYM-serum or the In-Pouch (Biomed Diagnosis, San Jose,
Calif.). Only parasites at the mid- to late-logarithmic phase of growth were
utilized. To prepare cell lysates, parasites (2 3 10
7
) were washed three times in
cold phosphate-buffered saline (PBS), pelleted at 500 3 g for 5 min, and stored
immediately at 2708C until utilized. Storage of cell lysates had no effect on
proteinase activities, as described elsewhere (33). Multiple cell lysates of
trichomonads grown under identical conditions and frozen at 2708C at different
times were tested simultaneously or on different days. The lysates gave highly
reproducible patterns of proteinase activity. Duplicate gels, as described below,
of the same lysate or supernatant always gave identical and reproducible pro-
teinase patterns.
Isolates were differentiated on the basis of criteria established recently for the
presence (type II) and absence (type I) of dsRNA virus (25, 26). Type II
trichomonads are infected with a multisegmented dsRNA virus (25) and undergo
phenotypic variation (3). After centrifugation of parasites that reached densities
of 1.5 3 10
6
/ml, a 1-ml aliquot of supernatant was filtered through 0.22-mm-
* Corresponding author. Mailing address: Department of Microbi-
ology, UTHSCSA, 7703 Floyd Curl Dr., San Antonio, TX 78284-7758.
Phone: (210) 567-3940. Fax: (210) 567-6612.
3388
pore-size filters (Gelman Sciences, Ann Arbor, Mich.). Supernatants were then
dialyzed for 30 h with three changes of double-distilled water in 3,500-molecular-
weight-cutoff membranes (Spectrum Medical Industries, Inc., Los Angeles, Ca-
lif.). Dialyzed culture supernatants were lyophilized and suspended in 200 mlof
electrophoresis dissolving buffer without b-mercaptoethanol (bME; 125 mM
Tris-HCl, 4% sodium dodecyl sulfate [SDS], 20% glycerol, 10% bromophenol
blue). Only 20 ml of supernatant was used immediately for the substrate-gel
electrophoresis described below. One-milliliter volumes of culture supernatants
to be evaluated for degradation of iodinated Igs were frozen at 2708C immedi-
ately after filtration.
Growth of T. vaginalis in the chemostat has been described (31). Isolates
T068-II (type II) and T056 (type I) were first grown in TYM-serum, pelleted, and
resuspended in a small volume for inoculation of the chemostat vessel.
Trichomonads were grown aerobically at 378C at dilution rates of 0.02 and 0.007
h
21
, corresponding to doubling times (t
d
s) of 24 and 100 h, respectively. t
d
s were
based on flow rates of TYM medium–5% heat-inactivated horse serum added to
the growth vessel (50). After inoculation, the cells were allowed to acidify the
chemostat medium to pH 6.0, after which the pH was maintained with sterile 1.25
N NaOH. High- and low-iron media (30, 32) were each prepared by the addition
of 100 mM ferric chloride or 50 mM 2,2-dipyridal to the medium, respectively.
Fresh growth medium was maintained in reservoirs at 48C throughout the study.
VWs. VWs acquired through the STD-CRC were from patients with tricho-
moniasis, patients with other STD pathogens as indicated below, or control
uninfected individuals, obtained during gynecologic examinations as described
before (6). VWs stored at 2708C for processing were thawed to room temper-
ature and centrifuged at 500 3 g for 10 min. Ten milliliters of each VW was then
passed through a 0.22-mm-pore-size filter before being dialyzed for 30 h with
three changes of double-distilled water as described above. Finally, VWs were
lyophilized, resuspended in 200 ml of electrophoresis dissolving buffer, and eval-
uated by substrate SDS-polyacrylamide gel electrophoresis (SDS-PAGE), as
described below. Although different VWs have distinct proteinase patterns, they
nonetheless had highly reproducible patterns when run simultaneously in dupli-
cate or when the samples were evaluated on different days. Controls were
generated by boiling an aliquot of each VW sample prior to electrophoresis or by
omitting activation of the electrophoresed gels. Boiled and nonactivated samples
never displayed any type of degradation of the IgG substrate.
To obtain a more accurate reflection of the extent of IgG-degrading protein-
ases among different patient VWs, undiluted samples were examined as well as
samples diluted 1:2, 1:5, and 1:10 (vol/vol) for analysis. Each VW was classified
as 41 (or 1111), 31,or21if proteinases were detected up to dilutions of 1:10
(vol/vol), 1:5, and 1:2, respectively. Proteinases detected only in undiluted VW
were classified as 11 reactions. Samples without detectable proteinases were
classified as negative. Only 2 of 61 VW samples analyzed were without T.
vaginalis and had no other STDs; both of these were negative for Ig degradation
under the different conditions evaluated in this report. Thirty-three VWs were
from patients with trichomoniasis. Twenty-eight VWs were from patients without
trichomoniasis but who had, as did some of the VWs from patients with tricho-
moniasis, any of the following coinfecting organisms, as shown previously (6):
Ureaplasma urealyticum, Candida albicans, Streptococcus agalactiae, Neisseria
gonorrhoeae, herpes simplex virus type I, Mycoplasma hominis, Gardnerella vagi-
nalis, Chlamydia trachomatis, Escherichia coli, Serratia odorifera, Citrobacter
freundii, and Klebsiella pneumoniae.
Iodination of human IgG antibodies. Purified human IgG (Sigma Chemical
Co., St. Louis, Mo.) was iodinated with the chloramine T-
125
I labeling kit (ICN,
Costa Mesa, Calif.), as recommended by the manufacturer. Briefly, 100 mlofa
2.0-mg/ml concentration of chloramine T was added to a Sephadex G-25 column
(1 by 10 cm; ISOLAB Inc., Akron, Ohio), followed by a solution containing 10
ml (1 mCi) Na
125
I (ICN). After 2 min, the reaction was stopped by the addition
of 100 ml of a 4.0-mg/ml solution of sodium metabisulfide. After 1 min, 1 ml of
IgG (1 mg/ml) was added to the column, and fractions of 0.5 ml each were
collected. The specific activity of iodinated IgG fractions ranged from 10
5
to 10
6
cpm/mg of protein. The fractions were pooled and frozen at 2208C until utilized.
The iodination of both the heavy chain (HC) and the light chain (LC) of IgG was
always monitored by SDS-PAGE autoradiography.
Trichomonal degradation of IgG and secretory IgA. IgG or secretory IgA was
added to 100 ml of lysate of T. vaginalis organisms, either with or without 1 mM
dithiothreitol (DTT; United States Biochemicals, Cleveland, Ohio). DTT is
needed to activate the trichomonad cysteine proteinases (14, 38). The mixture
was incubated at 378C for different times. The presence of DTT did not alter the
reactivity of antibody, as determined by indirect immunofluorescence, on live
organisms (2–7). To further show that the concentrations of DTT employed in
the assays did not enhance the susceptibility of IgG to degradation, IgG as
substrate in agar diffusion plates was first treated with 1 mM DTT before the
addition of a 1-mg/ml solution of trypsin to a well. The same extent of IgG
degradation was evident in the presence or absence of DTT.
Controls were generated by boiling lysates for 3 min prior to incubation with
IgG or IgA. An additional control included iodinated Igs in PBS with 1 mM DTT
but without lysate. For time- and concentration-dependent degradation experi-
ments, lysates (100 ml) equivalent to 5 3 10
6
,1310
7
, and 2 3 10
7
organisms
were utilized in the same final volume for reaction mixtures incubated at differ-
ent times. Inhibitor studies were performed identically to the degradation ex-
periments, except for the presence of 0.1 mM cystatin, 1 mM leupeptin, 1 mM
TLCK (N-a-p-tosyl-
L-lysine chloromethyl ketone), 1 mM TPCK (L-1-tosylamide-
2-phenylethyl chloromethyl ketone), and 5 mM EDTA (all from Sigma). The
extent of IgG degradation was monitored for up to 24 h to ensure complete
inhibition of the proteinases. To determine the lytic activity of the trichomonal
culture supernatant equivalent in cell number to lysates, frozen aliquots were
thawed and incubated with 5 mgof
125
I-IgG in the presence or absence of 1 mM
DTT. The highly reproducible nature of these experiments was confirmed, and
each assay and sample of each condition were done at least in duplicate and
repeated no less than three separate times.
SDS-PAGE autoradiography and immunoblotting. Samples for SDS-PAGE
were prepared every 2 or 4 hours during incubation with iodinated IgG or
secretory IgA by resuspending aliquots of the incubated samples in dissolving
buffer (1:1 [vol:vol]). Resuspended samples were then boiled for 3 min to prevent
further degradation by proteinases and stored at 2208C until electrophoresed.
Stacking gels of 4% acrylamide and separating gels of either 7.5 or 10% acryl-
amide were prepared. Samples up to 10 ml were added to gels and electropho-
resed at 100 V, as described before (38), in mini-Protean II units (Bio-Rad
Laboratories, Richmond, Calif.) containing 25 mM Tris-HCl, 192 mM glycine,
and 10% SDS (pH 8.7). For samples incubated with iodinated IgG, gels stained
in Coomassie brilliant blue for 2 h prior to destaining were dried and exposed to
Kodak XRP-40 X-ray film (Kodak, Rochester, N.Y.). Molecular weight markers
(Bio-Rad) were always included. For quantitative determination of IgG diges-
tion, radioactive gels were exposed to a phosphorimager screen (Molecular
Dynamics, Sunnyvale, Calif.) before analysis. Percent degradation was calculated
as follows: [(volume of IgG HC in control sample 2 volume of IgG HC in
experimental sample)/(volume of IgG HC in control sample)] 3 100.
Gels of samples incubated with secretory IgA (Sigma) were immunoblotted as
described previously (1, 7, 51). Electrophoretic transfer of proteins from acryl-
amide to nitrocellulose was carried out in blotting buffer (20 mM Tris-HCl, 150
mM glycine, 20% methanol [pH 8.3]) at 170 mA for 2 h. Transferred gels were
stained to ensure complete blotting of all proteins, while the membranes were
incubated overnight in Tris-buffered saline (TBS; 20 mM Tris-HCl [pH 7.4], 500
mM NaCl) containing 5% nonfat dry milk at 48C and washed three times in TBS.
Membranes were incubated for2hinTBS–5% milk containing a 1/1,000 dilution
of anti-human secretory IgA HC polyvalent antibody (Sigma) and then washed
three times in TBS before development.
Substrate-gel electrophoresis. Analysis of the Ig-degrading proteinases of T.
vaginalis was performed by substrate-SDS-PAGE (14, 22, 33). Separating gels of
7% acrylamide were copolymerized with 300 mg each of human IgG, IgM, or
secretory IgA per ml (each from Sigma), and these substrates were compared
with the activity of cysteine proteinases on 1.8 mg of gelatin (Bio-Rad) per ml, as
described before (38). For Ig substrates, it was necessary to prepare samples by
resuspending 2 3 10
7
trichomonads in dissolving buffer without bME. Cell
equivalents of 3 3 10
5
of each sample were loaded per lane and electrophoresed
as described above. Control samples washed with 1 mM TLCK in PBS prior to
storage never degraded gelatin or Ig substrate. It is noteworthy that, for sub-
strate-SDS-PAGE, identical results were obtained for experiments performed
identically on the same lysate but at different times and/or as multiple samples
run simultaneously. All experiments were repeated no less than three separate
times. Furthermore, similar results were obtained in separate experiments using
previously stored trichomonal lysates as well as with new lysates of parasites
grown under the same conditions. This shows the highly reproducible nature of
the proteinase patterns generated from in vitro-grown trichomonads, as has been
shown before (38).
The absence of bME from dissolving buffer was necessary to prevent reduction
of the IgG substrate in the separating gel, avoiding migration of the copolymer-
ized IgG during electrophoresis. In addition, this modification allowed for im-
proved resolution of the proteinase activities and the detection of proteinases
previously indistinguishable on both gelatin and Ig substrates. After electro-
phoresis, gels were incubated at 378Cfor2hinreducing buffer (2.5% Triton
X-100, 100 mM Na acetate, 1 mM DTT [pH 6.0]). VWs electrophoresed similarly
in acrylamide copolymerized with IgG were incubated at 378C in reducing buffer
for 5 h. Gels were then stained for2hinCoomassie brilliant blue prepared in
40% methanol and 10% acetic acid before destaining.
RESULTS
T. vaginalis lysates degrade human IgG. We first wanted to
determine whether trichomonal lysates, known to contain nu-
merous proteinases (14, 38), had the ability to degrade human
IgG. As shown in a representative experiment (Fig. 1A),
trichomonal lysates incubated in the presence of DTT, which
activates cysteine proteinases (14, 33), resulted in the complete
disappearance of the IgG HC (Fig. 1A, lane 3). No similar
degradation was ever detected in controls without lysate (Fig.
1A, lane 5) or with heat-inactivated cell lysates (see Fig. 2, lane
5), which gave autoradiogram patterns identical to the one
seen in Fig. 1A, lane 5. Supernatant also degraded the HC of
VOL. 63, 1995 TRICHOMONIASIS AND HUMAN Ig DEGRADATION 3389
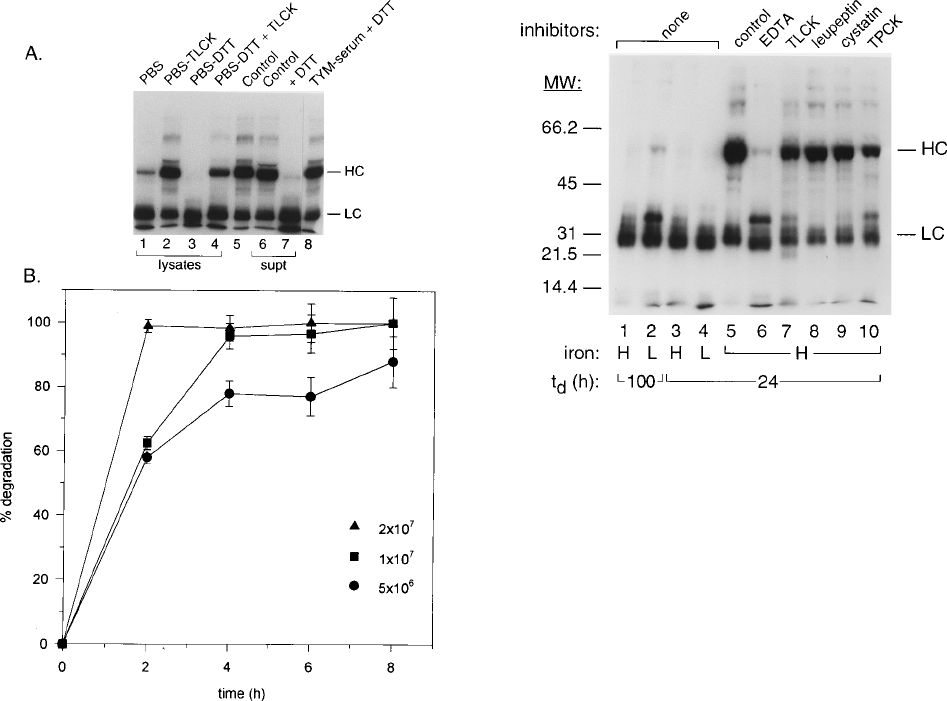
IgG in the presence of DTT (Fig. 1A, lane 7). As expected,
degradation of labeled IgG was not detected in complex me-
dium alone, whether without or with DTT (Fig. 1A, lane 8).
Furthermore, the absence of DTT from lysates added to iodi-
nated IgG did not result in significant HC degradation (Fig.
1A, lanes 1 and 6), indicating a requirement of reducing agent
for optimal activation of proteinases. Finally, TLCK was found
to significantly reduce the degradation of the IgG HC (Fig. 1A,
lane 4), suggesting that trichomonad cysteine proteinases de-
grade human IgG. Importantly, identical results for these and
other experiments were obtained with lysates and supernatants
of another fresh clinical isolate, T. vaginalis T056.
A representative experiment with standard errors derived
from analysis of triplicate samples shows the time- and con-
centration-dependent degradation of the IgG HC (Fig. 1B).
Cell lysate concentrations of 2 3 10
7
trichomonads digested
the HC of a 50-mg/ml concentration of IgG by 2 h, as compared
with longer incubation times required for decreased amounts
of lysate. For example, lysates of 5 3 10
6
organisms degraded
.80% of the IgG HC by 8 h. Lysates containing 2 3 10
6
trichomonads required 16 h to achieve similar levels of IgG
degradation, as shown in Fig. 1A. As expected, degradation of
IgG was never detected in identical experiments using heat-
inactivated lysates and lysates pretreated with TLCK.
Cysteine proteinases degrade IgG. We wanted to determine
whether degradation of the IgG HC was influenced by growth
parameters and nutrients, such as iron, which has recently been
shown to regulate numerous trichomonal properties (29–32),
including expression of a proteinase(s) involved in T. vaginalis
resistance to complement (29). For these experiments, cell
lysates of parasites from chemostat cultures were tested (Fig. 2,
lanes 1 to 4). Lysates of trichomonads grown in either high-
iron (lanes H) or low-iron (lanes L) medium and at two dif-
ferent t
d
s resulted in degradation of the HC. However, lysates
of parasites grown in low-iron medium at a t
d
of 100 h had a
slightly reduced degradative ability (Fig. 2, lane 2). These re-
sults suggested that expression of the IgG-degrading proteases
was not regulated by t
d
and the iron level.
In addition, complete inhibition of IgG HC degradation was
achieved in the presence of trichomonad cysteine proteinase
FIG. 1. Representative results showing degradation of human IgG by cell
lysates of T. vaginalis isolate T068-II. (A) Autoradiograms after SDS-PAGE in
7.5% acrylamide gels of samples prepared from incubations for 16 h of
125
I-
labeled human IgG with trichomonal lysates (lanes 1 through 4) and 24 h for
culture supernatant (lanes 6 and 7) as described in Materials and Methods. The
HC and LC are indicated. The lysate sample was equal to 2 3 10
6
parasites (in
100 ml) incubated with 5 mgof
125
I-IgG in 100 ml of PBS at 378C. Cysteine
proteinase inhibitor (TLCK) and DTT were each ata1mMconcentration.
Lanes 6 and 7 were loaded with a sample prepared by incubation of 100 mlof
filter-sterilized supernatant with the same volume of
125
I-IgG in the absence
(lane 6) and presence (lane 7) of 1 mM DTT. Lane 5 shows a control consisting
of iodinated IgG in PBS and 1 mM DTT but without trichomonal lysate incu-
bated in parallel with the other samples. Lane 8 is the pattern of iodinated IgG
incubated with medium alone plus 1 mM DTT. (B) Concentration- and time-
dependent degradation of
125
I-labeled IgG as determined by densitometric scan-
ning by phosphorimager (see Materials and Methods) of the HC band from
autoradiograms, such as that seen in panel A (lane 3). Cell lysates equivalent to
2 3 10
7
,1310
7
, and 5 3 10
6
organisms are represented. Results were from
autoradiograms of triplicate samples run simultaneously. Identical results were
obtained from lysates derived from another trichomonal isolate. For the data
presented here, degradation experiments involving iodinated IgG and IgA were
highly reproducible on duplicate samples run simultaneously and on at least
three separate occasions.
FIG. 2. Inhibitor studies and evaluation of chemostat growth conditions for
degradation of IgG. Autoradiograms of samples after SDS-PAGE in 10% acryl-
amide gels prepared from a 24-h incubation of lysates equivalent to 2 3 10
6
trichomonads of T. vaginalis isolate T068-II grown in a chemostat under high-
iron (H) versus low-iron (L) conditions at t
d
s of 100 and 24 h are shown. Lanes
1 to 4 consist of cell lysates of parasites from chemostat cultures with no added
inhibitors. Lanes 5 through 10 consist of parasites grown in the chemostat under
high-iron conditions at a t
d
of 24 h. A control consisting of cells boiled for 3 min
prior to incubation with iodinated IgG is shown in lane 5. Inhibitors were added
to lysates (lanes 6 to 10) as detailed in Methods and Materials. The HC and LC
are indicated on the right. The numbers on the left indicate the mobilities of
molecular weight (MW) standards (Bio-Rad) in thousands. For the data pre-
sented here, degradation experiments involving iodinated IgG and IgA were
highly reproducible on duplicate samples run simultaneously and on at least
three separate occasions.
3390 PROVENZANO AND ALDERETE INFECT.IMMUN.

inhibitors (Fig. 2, lanes 8 and 9) (14, 39). The control (lane 3)
exhibited extensive degradation of IgG HC, as described be-
fore (Fig. 1). Partial inhibition was noted for the inhibitors
TLCK (Fig. 2, lane 7) and TPCK (Fig. 2, lane 10). Inclusion of
EDTA (lane 6) to neutralize T. vaginalis metalloproteinases
that have also been described (11) did not result in any inhi-
bition.
The appearance of an iodinated protein band with a relative
molecular mass of ;37,000 Da upon HC degradation in the
presence of TLCK (Fig. 2, lane 7), TPCK (lane 10), and EDTA
(lane 6) was of interest. This band was always seen in assays
performed on different days with duplicate samples or newly
derived lysates and was never detected in samples with other
inhibitors (lanes 8 and 9) or in positive controls (lane 3).
Identical results were obtained with lysates from parasites
grown in low-iron medium with extended generation times
(lane 2). These results suggest that numerous proteinases,
some with distinct degrading activities, were likely involved in
the degradation of the HC. This notion is reinforced by the
known differential inhibition of the trichomonad cysteine pro-
teinases (14, 39).
Trichomonad cysteine proteinases degrade secretory IgA.
Because antitrichomonal antibody has also been found to be
IgA (47), we tested whether proteinases in lysates degraded
this Ig. Figure 3 shows that trichomonal lysates degraded the
HC of IgA under experimental conditions similar to those for
IgG HC degradation (lane 5). As controls, boiled lysate (lane
4), IgA alone (lane 2), and IgA plus DTT (lane 3) showed no
evidence of any degradation, as compared with the tricho-
monal lysates (lane 5). As before, cysteine proteinases were
involved, as evidenced by the inhibition of IgA digestion by
pretreatment of lysates with 1 mM leupeptin (lane 6) or 1 mM
TLCK (lane 7).
Analysis of Ig-degrading activities. Substrate-gel electro-
phoresis was performed to evaluate the trichomonal IgG deg-
radation activities. Optimization for proteinase detection on
IgG substrate necessitated that trichomonal lysates be pre-
pared in the absence of bME prior to electrophoresis. Reduc-
tion of substrate resulted in clearing of the top half of the gel
during electrophoresis, which prevented visualization of medi-
um- to high-M
r
proteinase activities (Fig. 4). A lack of resolu-
tion was seen when comparing IgG- and gelatin-degrading
proteinases in samples with bME (Fig. 4A2 and B2) and with-
out bME (Fig. 4A1 and B1). Samples without bME possessed
numerous proteinase bands regardless of substrate. No quali-
tative differences were observed in clearing of the substrates
when gelatin was compared with IgG degradation (Fig. 4A1
and B1). Quantitatively more clearing was evident with gelatin
FIG. 3. Degradation of human secretory IgA by cell lysates of T. vaginalis
isolate T068-II. An immunoblot done after SDS-PAGE with 10% separating
acrylamide gels of proteins prepared by incubating secretory IgA with
trichomonal lysates (lanes 5 through 7) is shown. The purified IgA after SDS-
PAGE is marked HC and LC in the Coomassie-brilliant-blue-stained gel (lane
1). Immunoblots were probed with specific anti-IgA HC antiserum. Lysates for
each reaction were prepared from 2 3 10
7
parasites per 100-ml sample added to
100 mg of IgA dissolved in 100 ml of PBS. This amount of IgA and the number
of organisms in the lysate were different from that used for the IgG assays (Fig.
1 and 2) and were required for visualization of IgA degradation by immunoblot.
Reactions were performed at 378C for 16 h. Boiled lysate incubated with IgA is
shown in lane 4. Inhibitors of trichomonad cysteine proteinases added to lysates
included leupeptin (lane 6) and TLCK (lane 7). IgA alone incubated in a manner
identical to other samples with 1 mM DTT (lane 3) is compared with IgA without
DTT (lane 2). For the data presented here, degradation experiments involving
iodinated IgG and IgA were highly reproducible on duplicate samples run si-
multaneously and on at least three separate occasions.
FIG. 4. Evaluation of proteinase activities by substrate-SDS-PAGE with gel-
atin (A1 and A2) and IgG (B1 and B2). Cell lysates of chemostat-grown T.
vaginalis isolate T068-II were electrophoresed on gelatin and copolymerized with
7.5% acrylamide in the absence (A1 and B1) and presence (A2 and B2) of bME.
Each gel shows four lanes of T. vaginalis isolate T068-II grown under high-iron
(H) (lanes 1 and 2) and low-iron (L) (lanes 3 and 4) conditions at two t
d
s, and
samples were as described in the legend to Fig. 2. Lane 1 for each gel represents
the same cell equivalent of a sample washed with 1 mM TLCK in PBS and stored
at 2708C prior to electrophoresis. Samples of lane 1 not treated with TLCK
resulted in degradation patterns identical to those seen for the lysates from a t
d
of 24 h (lanes 2). An asterisk indicates one low-M
r
proteinase activity that was
consistently affected by the absence of iron. An arrow indicates a second pro-
teinase that was expressed when the t
d
played a role in low-iron-grown parasites.
Finally, for the data presented here and in Fig. 5 and 6, substrate-gel degradation
experiments were highly reproducible regardless of substrate, and all experi-
ments were done on duplicate samples run simultaneously and on at least three
separate occasions.
VOL. 63, 1995 TRICHOMONIASIS AND HUMAN Ig DEGRADATION 3391

than with IgG. This may be consistent with the fact that only
the HC of IgG is degraded (Fig. 1).
Minor qualitative and quantitative variations in clearing ar-
eas were evident between trichomonads grown under high-iron
(Fig. 4, lanes 2) and low-iron (lanes 3 and 4) conditions. One
low-M
r
proteinase activity was consistently affected by the ab-
sence of iron (Fig. 4, asterisk). Although not shown, high-iron-
grown organisms produced identical degradation patterns, re-
gardless of t
d
. However, the t
d
played a role for low-iron-grown
parasites in the expression of a second proteinase (Fig. 4,
arrow). As expected, pretreatment of samples with TLCK neu-
tralized the cysteine proteinase activity (lanes 1). Finally, it is
noteworthy that stained gels of total proteins of T. vaginalis for
each condition gave almost identical banding patterns, showing
that equivalent numbers of cells were used in the lysates. These
controls illustrated that iron had some effect in overall Ig-
degrading proteinase activity.
Heterogeneity of Ig-degrading proteinase patterns among
isolates. In view of the known heterogeneity in expression of
cysteine proteinases by T. vaginalis isolates (5, 6, 14, 38), we
evaluated 20 isolates for Ig degradation patterns. Isolates with
(type I) and without (type II) a dsRNA virus were compared
(25, 54).
Figure 5 shows the complex proteinase patterns on each Ig
substrate. All proteinase bands were absent upon pretreatment
of lysates with TLCK or by heat inactivation, as described
before (Fig. 2). For each Ig substrate, five degradation patterns
representative of several virus-harboring (Fig. 5, lanes 6 to 10)
and virus-negative (Fig. 5, lanes 1 to 5) isolates are shown. One
half of the isolates displayed more heterogeneity in proteinase
patterns than the others and, interestingly, were those with the
dsRNA virus (Fig. 5, lanes 6 to 10, labeled type II). All patterns
were reproducible by use of the same lysate in duplicate gels,
with lysates from the same frozen parasites examined at dif-
ferent days, and with different lysates of the same isolate ob-
tained from different cultures. Thus, the variations in patterns
were unique to the isolates.
Two proteinases with M
r
s of 65,000 and 69,000 (Fig. 5, in-
dicated by brackets on the right) were detected consistently in
all isolates and degraded all four substrates. The 56- and 60-
kDa proteinases (Fig. 5, indicated by .) were specific for IgA
substrate (Fig. 5B) and were found among all trichomonal
isolates. A low-M
r
proteinase was seen only on the IgM sub-
strate (Fig. 5C, arrow). Finally, three high-M
r
proteinase ac-
tivities (lanes 8, 9, and 10) were detected on IgG, secretory
IgA, and gelatin, but not IgM, substrates (Fig. 5, asterisks).
That the patterns were reproducible for duplicate lysates run
simultaneously or at different times strongly suggests that het-
erogeneity, especially among the lower-M
r
proteinase activi-
ties, was not due to autodegradation of higher-M
r
proteinases.
It was important to determine whether all agar clones (3) of
a representative isolate expressed the full repertoire of Ig-
degrading activities. In data not shown, all clones had patterns
identical to those of the parental isolates for gelatin and for the
Igs. This suggests that individual trichomonads of an isolate are
all capable of expressing the full complement of the proteinase
genes (38).
Of relevance was that supernatants were also tested. In data
not shown, supernatants with cell numbers equivalent to those
used in lysates (1.5 3 10
5
) gave proteinase patterns identical to
those seen in Fig. 5. No lysis of trichomonads was evident
during growth and multiplication of mid- to late-logarithmical-
ly-growing parasites, as was shown previously (1), or during
centrifugation procedures as evidenced by cell enumeration.
Furthermore, placement of highly motile trichomonads in
PBS-maltose for 30 to 60 min, conditions that do not result in
lysis or death of trichomonads, as determined by enumeration
and trypan-blue exclusion, yields large amounts of released
proteinases. These data suggest the possibility that Ig-degrad-
ing proteinases are actively released or secreted by live
trichomonads, consistent with earlier work (1, 14, 17, 33, 39).
FIG. 5. Analysis of T. vaginalis isolates for proteinases degrading different Ig
substrates (A, B, and C) compared with those degrading gelatin (D). Isolates
were defined on the basis of the absence (type I, lanes 1 to 5) or presence (type
II, lanes 6 to 10), respectively, of a dsRNA virus (25, 26, 54). All are fresh
isolates, except for IR78 and NYH286, which have been grown in vitro for
extended periods. All samples were harvested from cultures at the mid- to
late-logarithmic phase of growth and processed identically. The brackets indicate
proteinase activities present in all isolates, which reacted with all substrates. The
proteinase unique to IgM is indicated by an arrow on the right. The symbol .
indicates the appearance of proteinases with specificity toward IgA. Proteinases
denoted by an asterisk degrade all substrates except IgM. The numbers on the
left are molecular weight (MW) standards as described in the legend to Fig. 2. (k
5 1,000).
3392 PROVENZANO AND ALDERETE INFECT.IMMUN.

Patient VWs also contain proteinases that degrade human
IgG. Finally, to explore a potential in vivo role for these en-
zymes, we tested for the detection of Ig-degrading proteinases
in VWs from patients with trichomoniasis and compared them
with VWs from control, uninfected women or those with other
STDs. As shown in Fig. 6B1 and B2 and Table 1, degradation
of IgG was observed in 26 of 33 (78%) VWs from patients with
trichomoniasis. All of the positive reactions for trichomoniasis
patient VWs were highly reactive and classified as 41 to 11
(see Materials and Methods), as shown for representative sam-
ples in Fig. 6B1, lanes 5 through 8. The presence of proteinases
upon 1:10 dilution (for 41 VW), 1:5 dilution (for 31), and 1:2
dilution (for 21) of VWs is also presented for the same rep-
resentative samples illustrated in Fig. 6B1 (lanes 9 through 11).
In contrast, IgG degradation was detected in only 10 of 29
(35.5%) patients without evidence for trichomoniasis, regard-
less of the presence of other STDs (see Materials and Meth-
ods) (6). As further shown in Table 1, no VWs from patients
negative for T. vaginalis were 41, and only 1 of 28 (3%) was
31. Furthermore, it is noteworthy that 18 of 28 (64%) were
totally unreactive by the assays used in this study. Finally,
statistical analysis revealed highly significant (P 5 0.001) dif-
ferences in the extent of reactivity between the samples from
patients with trichomoniasis and those without T. vaginalis.Itis
noteworthy that the reactivities for all of the undiluted as well
as the diluted VWs were highly reproducible, as evidenced by
identical gel patterns obtained from duplicate samples handled
similarly and on different occasions. Not surprisingly, VWs
with proteolytic activity on IgG also readily degraded gelatin
(data not shown), consistent with previous findings (6). That
the Ig-degrading activity was due to proteinases was verified by
the use of inhibitors and lack of degradation observed with
boiled and nonactivated samples, which gave reactions like
those shown for boiled trichomonal lysate (Fig. 6A, lane 2).
FIG. 6. VWs of patients demonstrating detectable IgG-degrading activity by substrate-SDS-PAGE. VWs of patients with trichomoniasis (B1) and VWs of women
without a history of T. vaginalis infection or with other STDs (C) were processed as described in Materials and Methods before testing for the presence of proteinases
against IgG. Representative samples of VW reactivities classified from 41 to 11 are given in panel B1 (lanes 3 through 8) to show the extent of proteinase degradation
of IgG. Furthermore, the presence of IgG degradation of samples in panel B1 upon 1:10 dilution (for 41 reactivity), 1:5 dilution (for 31), and 1:2 dilution (for 21)
of VW is shown in panel B2 (lanes 9 through 11). Electrophoresis was performed as described before (Fig. 3 legend), except for incubation in reducing buffer for 5
h prior to staining. For comparison, panel A shows a control total lysate (lane 1) and boiled lysate (lane 2) of T. vaginalis prepared as described in Materials and Methods
and processed in parallel with the VWs. The numbers on the left are molecular weight (MW) standards as described in the legend to Fig. 2 (k 5 1,000).
TABLE 1. Protease activities against IgG in VWs of patients
infected with T. vaginalis
Level of IgG
degradation
a
VWs
b
No. infected (%) No. uninfected (%)
41 5 (15.2) 0 (0)
31 4 (12.1) 1 (3.6)
21 6 (18.2) 5 (17.8)
11 11 (33.3) 4 (14.3)
0 7 (21.2) 18 (64.3)
Total 33 (100) 28 (100)
a
Degradation based on the detection by substrate-gel electrophoresis of di-
luted samples with IgG as substrate is as described in Materials and Methods.
b
VWs were from patients with trichomoniasis (infected) or without trichomo-
niasis (uninfected). Twenty-six of the 28 uninfected individuals without T. vagi-
nalis had other coinfecting STDs, as indicated in Materials and Methods and as
has been described previously by us (6). Mean levels of degradation (6 standard
error) of the infected and uninfected VWs were 11.7 6 0.2 and 10.6 6 0.2,
respectively (P 5 0.001, two-tailed t test of difference in means).
VOL. 63, 1995 TRICHOMONIASIS AND HUMAN Ig DEGRADATION 3393
DISCUSSION
Mucosal pathogens have been reported to synthesize pro-
teinases that cleave host antibodies as a mechanism contribut-
ing to virulence (13, 19, 24, 34, 42, 44). Proteinases degrading
antibodies have also been found in protozoan parasites (23,
36). A recent report describes human IgA HC degradation by
Entamoeba histolytica, a mucosal protozoan parasite (23).
Tritrichomonas foetus, the mucosal trichomonad that causes
fetal wastage in cattle (48), is known to secrete cysteine pro-
teinases that digest the HC of host IgG (49). Although the
exact function for many of the trichomonad cysteine protein-
ases remains unknown, the existence of up to 23 cysteine pro-
teinases (38) makes it plausible that specific functions may
exist for subsets of these molecules. We now show the ability of
cysteine proteinases synthesized by T. vaginalis to degrade hu-
man Igs. Lysates of trichomonads degraded IgG under all
experimental conditions tested here. Furthermore, the detec-
tion in all isolates of distinct proteinases that degrade either
only IgA (Fig. 5B) or only IgM (Fig. 5C) shows some specificity
in expression of proteinase(s) activity toward Igs.
It may be noteworthy that some qualitative differences in
proteinase degradation patterns of IgG were detected for
trichomonads with and without the dsRNA virus (Fig. 5) (25,
26). Unlike dsRNA viruses of other protozoa (54), the T.
vaginalis dsRNA virus is multisegmented (25), illustrating an
increased coding capacity of the viral genome. Since the virus
has been shown to influence expression of a trichomonad gene
(26), it is conceivable that the virus may affect overall Ig-
degrading activity through expression of virally encoded prod-
ucts, some of which may be proteinases, or in up-regulation of
trichomonad proteinases.
Iron has been found to be an important factor in the regu-
lation of many properties of T. vaginalis (29, 30, 32). Only a
slight reduction in IgG degradation was detected under iron-
limiting conditions at a low t
d
(Fig. 2 and 4). Thus, iron is not
an on-off signal, as for other properties described before (29–
31). That IgG degradation is independent of certain growth
conditions may be important for trichomonads since the pres-
ence of mucosal antibody is independent of the iron status of
the vagina during the menstrual cycle (46, 52). Constitutive
expression of Ig-degrading proteinases may be advantageous
and result in parasite resistance to antibody regardless of the
immune status of the host at the time of infection.
The finding that VWs possessed IgG-degrading activity (Fig.
6) strengthens the notion that these observations are relevant
in vivo, and these results may be noteworthy in light of the
non-self-limiting nature of infection by this parasite. Earlier
work has shown that soluble cysteine proteinases can be de-
tected in patient VWs (6), and antibodies to proteinases and
immunogens have been demonstrated in both sera and VWs
from patients with trichomoniasis (5–7, 12, 47). Because anti-
body to immunogens has been shown, in vitro, to be tricho-
monicidal (2), any ability to degrade Igs would promote the
survival of the parasite. It was notable that patients without
trichomoniasis, as controls, and most VWs from patients with
other STDs (Table 1) did not possess any activity detectable
under identical experimental conditions (6). Of those VWs
from patients with other STDs that had detectable proteinases,
most were poorly reactive in comparison with the trichomoni-
asis patient VWs. It is noteworthy that the differences in the
levels of proteinases present in T. vaginalis-infected women
and those with other STDs were highly significant (P 5 0.001)
(Table 1), further strengthening the view that this class of
molecules deserves continued attention as possible virulence
factors.
Interestingly, most, if not all, serum and vaginal antibody to
T. vaginalis proteinases and immunogens detected by immu-
noprecipitation experiments is predominantly IgG (5–7, 12).
The absence of vaginal antibody in some women with tricho-
moniasis (4, 6) may be a direct or indirect result of tricho-
monad Ig degradation activity reported here. In this scenario,
conclusions reached by investigators about the absence of vag-
inal antibody to T. vaginalis or other coinfecting STDs may be
incorrect. These observations possibly necessitate a reevalua-
tion of antibody responses in trichomoniasis patients to get a
more accurate assessment of host antibody responses.
The present report now calls attention to the plausible re-
lationship between trichomoniasis and Ig-degrading ability
with the neutralization of protective host antibody responses to
coinfecting STD pathogens (9, 16, 20, 21, 35, 40, 41). Future
vaccination for antibody production in the vaginal mucosa (10)
must consider the possibility presented here, i.e., that infection
by T. vaginalis may subvert the protective antibody response(s)
of humans to other pathogens. Therefore, Ig-degrading activity
might lead to enhancement of infection and pathogenesis by
coinfecting STD pathogens (43, 53), like C. trachomatis (20, 21,
35), human immunodeficiency virus (28, 55), human papilloma
virus (16), herpes simplex virus (9, 41), and others (21). Anti-
bodies (18, 52) to these STD pathogens may be found in the
vaginas of patients (40). Reports already describe multiple
STDs coinfecting at-risk individuals (43, 52, 55). The existence
of numerous proteinases possibly released or secreted by T.
vaginalis during vaginal infections (1) may also have other
consequences, such as contributing significantly to the reported
adverse pregnancy outcome (37, 45) and enhanced predispo-
sition to human immunodeficiency virus infection (28, 55).
This report provides additional support for the need for con-
tinued research on the cysteine proteinases of this STD pro-
tozoan.
ACKNOWLEDGMENTS
We gratefully acknowledge Ed Newton and Jeanna Piper of the
STD-CRC at our institution for the contribution of fresh clinical iso-
lates and vaginal washes. We thank Suzanne Dakin for her secretarial
assistance. We want especially to acknowledge Barbara Elizondo for
her expertise in the statistical analysis. We are also thankful to the
STD-CRC clinical staff for collecting clinical specimens from patients,
William Peairs for providing detailed data on the patient materials,
and Mike Lehker for helpful suggestions and spirited discussions on
the role of proteinases in the biology of the T. vaginalis-host interre-
lationship.
This study was supported by Public Health Service grants AI 31498
and AI 18768 from the National Institute of Allergy and Infectious
Diseases.
REFERENCES
1. Alderete, J. F., and G. Garza. 1984. Soluble Trichomonas vaginalis antigens
in cell-free culture supernatant. Mol. Biochem. Parasitol. 13:147–158.
2. Alderete, J. F., and L. Kasmala. 1986. Monoclonal antibody to a major
glycoprotein immunogen mediates differential complement-independent ly-
sis of Trichomonas vaginalis. Infect. Immun. 53:697–699.
3. Alderete, J. F., L. Kasmala, E. Metcalfe, and G. Garza. 1986. Phenotypic
variation and diversity among Trichomonas vaginalis isolates and correlation
of phenotype with trichomonal virulence determinants. Infect. Immun. 53:
285–293.
4. Alderete, J. F., E. Newton, C. Dennis, J. Engbring, and K. A. Neale. 1991.
Vaginal antibody of patients with trichomoniasis is to a prominent surface
immunogen of Trichomonas vaginalis. Genitourin. Med. 67:220–225.
5. Alderete, J. F., E. Newton, C. Dennis, and K. A. Neale. 1991. Antibody in sera
of patients infected with Trichomonas vaginalis is to trichomonad protein-
ases. Genitourin. Med. 67:331–334.
6. Alderete, J. F., E. Newton, C. Dennis, and K. A. Neale. 1991. The vagina of
women infected with Trichomonas vaginalis has numerous proteinases and
antibody to trichomonad proteinases. Genitourin. Med. 67:469–474.
7. Alderete, J. F., L. Suprun-Brown, L. Kasmala, J. Smith, and M. Spence.
3394 PROVENZANO AND ALDERETE INFECT.IMMUN.
1985. Heterogeneity of Trichomonas vaginalis isolates and subpopulations
with sera of patients and experimentally infected mice. Infect. Immun. 49:
463–468.
8. Arroyo, R., and J. F. Alderete. 1989. Trichomonas vaginalis proteinase activity
is necessary for parasite adherence to epithelial cells. Infect. Immun. 57:
2991–2997.
9. Ashley, R. L., L. Corey, J. Dalessio, P. Wilson, M. Remington, G. Barnum,
and P. Tretheway. 1994. Protein-specific cervical antibody responses to pri-
mary genital herpes simplex virus type 2 infections. J. Infect. Dis. 170:20–26.
10. Bouvet, J.-P., L. Belec, R. Pires, and J. Pillot. 1994. Immunoglobulin G
antibodies in human vaginal secretions after parenteral vaccination. Infect.
Immun. 62:3957–3961.
11. Bozner, P., and P. Demes. 1991. Proteinases in Trichomonas vaginalis and
Tritrichomonas mobilensis are not exclusively of cysteine type. Parasitology
102:113–115.
12. Bozner, P., A. Gambosova, M. Valent, P. Demes, and J. F. Alderete. 1992.
Proteinases of Trichomonas vaginalis: antibody response in patients with
urogenital trichomoniasis. Parasitology 105:387–391.
13. Cole, M. F., M. Evans, S. Fitzsimmons, J. Johnson, C. Pearce, M. J. Sheri-
dan, R. Wientzen, and G. Bowden. 1994. Pioneer oral streptococci produce
immunoglobulin A1 protease. Infect. Immun. 62:2165–2168.
14. Coombs, G. H., and M. J. North. 1983. An analysis of the proteinases of
Trichomonas vaginalis by polyacrylamide gel electrophoresis. Parasitology
86:1–6.
15. Diamond, L. S. 1957. The establishment of various trichomonads of animal
and man in axenic cultures. J. Parasitol. 43:488–490.
16. Dillner, L., A. Fredriksson, E. Persson, O. Forslund, B. G. Hansson, and J.
Dillner. 1993. Antibodies against papillomavirus antigens in cervical secre-
tions from condyloma patients. J. Clin. Microbiol. 31:192–197.
17. Garber, G. E., and L. T. Lemchuck-Favel. 1989. Characterization of extra-
cellular protease of Trichomonas vaginalis. Can. J. Microbiol. 35:903–909.
18. Govers, J., and J. P. Girard. 1972. Some immunological properties of human
cervical and vaginal secretions. Gynecol. Invest. 3:184–194.
19. Grenier, D. 1992. Inactivation of human serum bactericidal activity by a
trypsinlike protease isolated from Porphyromonas gingivalis. Infect. Immun.
60:1854–1857.
20. Hardy, P. H., J. B. Hardy, E. E. Nell, D. A. Graham, M. R. Spence, and R. C.
Rosenbaum. 1984. Prevalence of six sexually transmitted disease agents
among pregnant inner-city adolescents and pregnancy outcome. Lancet ii:
333–337.
21. Heisterberg, L., P. E. Branebjerg, A. Bremmelgaard, J. Scheibel, and L. Hoy.
1987. The role of vaginal secretory immunoglobulin A, Gardnerella vaginalis,
anaerobes, and Chlamydia trachomatis in postabortal pelvic inflammatory
disease. Acta Obstet. Gynaecol. Scand. 66:99–102.
22. Heussen, C., and E. B. Dowdle. 1980. Electrophoretic analysis of plasmino-
gen activators in polyacrylamide gels containing sodium dodecyl sulfate and
copolymerized substrates. Anal. Biochem. 102:196–202.
23. Kelsall, B. L., and J. I. Ravdin. 1993. Degradation of human IgA by Enta-
moeba histolytica. J. Infect. Dis. 168:1319–1322.
24. Kharazmi, A. 1991. Mechanisms involved in the evasion of the host defense
by Pseudomonas aeruginosa. Immunol. Lett. 30:201–206.
25. Khoshnan, A., and J. F. Alderete. 1994. Multiple double-stranded RNA
segments are associated with virus particles infecting Trichomonas vaginalis.
J. Virol. 67:6950–6955.
26. Khoshnan, A., and J. F. Alderete. 1994. Trichomonas vaginalis with a double-
stranded RNA virus has upregulated levels of phenotypically variable im-
munogen mRNA. J. Virol. 68:4035–4038.
27. Krieger, J. N., P. Wolner-Hanssen, C. Stevens, and K. K. Holmes. 1990.
Characteristics of Trichomonas vaginalis isolates from women with and with-
out colpitis macularis. J. Infect. Dis. 161:307–311.
28. Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman,
F. Behets, V. Batter, M. Alary, W. L. Heyward, R. W. Ryder, and P. Piot.
1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1
transmission in women: results from a cohort study. AIDS 7:95–102.
29. Lehker, M. W., and J. F. Alderete. 1992. Iron regulates resistance to com-
plement in Trichomonas vaginalis, abstr. B-198, p. 59. In Abstracts of the
92nd General Meeting of the American Society for Microbiology. American
Society for Microbiology, Washington, D.C.
30. Lehker, M. W., and J. F. Alderete. 1992. Iron regulates growth of Trichomo-
nas vaginalis and the expression of immunogenic trichomonad proteins. Mol.
Microbiol. 6:123–132.
31. Lehker, M. W., and J. F. Alderete. 1990. Properties of Trichomonas vaginalis
grown under controlled chemostat conditions. Genitourin. Med. 66:193–199.
32. Lehker, M. W., R. Arroyo, and J. F. Alderete. 1992. The regulation by iron of
the synthesis of adhesins and cytoadherence levels in the protozoan
Trichomonas vaginalis. J. Exp. Med. 174:311–318.
33. Lockwood, B. C., M. J. North, K. I. Scott, A. F. Bremmer, and G. H. Coombs.
1987. The use of a highly sensitive electrophoretic method to compare the
proteinases of trichomonads. Mol. Biochem. Parasitol. 24:89–95.
34. Loomes, L. M., B. W. Senior, and M. A. Kerr. 1990. A proteolytic enzyme
secreted by Proteus mirabilis degrades immunoglobulins of the immunoglob-
ulin A1 (IgA1), IgA2, and IgG isotypes. Infect. Immun. 58:1979–1985.
35. McCormack, W. M., B. Rosner, D. E. McComb, J. R. Evrard, and S. H.
Zinner. 1985. Infection with Chlamydia trachomatis in female college stu-
dents. Am. J. Epidemiol. 121:107–115.
36. McKerrow, J. H., E. Sun, P. J. Rosenthal, and J. Bouvier. 1993. The pro-
teinases and pathogenicity of parasitic protozoa. Annu. Rev. Microbiol.
47:821–853.
37. Minkoff, H., A. N. Gruenebaum, R. H. Schwartz, J. Feldman, M. Cummings,
W. Cromleholme, L. Clark, G. Pringle, and M. McCormick. 1984. Risk
factors for prematurity and premature rupture of membranes: a prospective
study of the vaginal flora in pregnancy. Am. J. Gynecol. 150:965–972.
38. Neale, K. A., and J. F. Alderete. 1990. Analysis of the proteinases of repre-
sentative Trichomonas vaginalis isolates. Infect. Immun. 58:157–162.
39. North, M. J., C. D. Robertson, and G. H. Coombs. 1990. The specificity of
trichomonad cysteine proteinases analysed using fluorogenic substrates and
specific inhibitors. Mol. Biochem. Parasitol. 39:183–194.
40. O’Shea, S., M. Cordery, W. Y. Barrett, D. D. Richman, C. Bradbeer, and
J. E. Banatvala. 1990. HIV excretion patterns and specific antibody re-
sponses in body fluids. J. Med. Virol. 31:291–296.
41. Persson, E., P. Eneroth, and S. Jeansson. 1988. Secretory IgA against herpes
simplex virus in cervical secretions. Genitourin. Med. 64:373–377.
42. Pouedras, P., P. M. Andre, P. Y. Donnio, and J. L. Avril. 1992. Cleavage of
immunoglobulin A1, A2 and G by proteases from clinical isolates of Pasteu-
rella multocida. J. Med. Microbiol. 37:128–132.
43. Prins, M., C. Hooykaas, R. A. Coutinho, G. van Doornum, and A. van den
Hoek. 1994. Incidence and risk factors for acquisition of sexually transmitted
diseases in heterosexuals with multiple partners. Sex. Transm. Dis. 21:258–
267.
44. Prokesova, L., B. Potuznikova, J. Potempa, J. Zikan, J. Radl, L. Hachova, K.
Baran, Z. Porwit-Boor, and C. John. 1992. Cleavage of human immuno-
globulins by serine proteinase from Staphylococcus aureus. Immunol. Lett.
31:259–266.
45. Read, J. S., and M. A. Klebanoff. 1993. Sexual intercourse during pregnancy
and preterm delivery: effects of vaginal microorganisms. Am. J. Obstet.
Gynecol. 168:514–519.
46. Schumacher, G. F., M. H. Kim, A. H. Hosseinian, and C. Dupon. 1977.
Immunoglobulins, proteinase inhibitors, albumin, and lysozyme — methods
and results. Am J. Obstet. Gynecol. 129:629–636.
47. Sharma, P., N. Malla, I. Gupta, N. K. Ganguly, and R. C. Mahajan. 1991.
Anti-trichomonad IgA antibodies in trichomoniasis before and after treat-
ment. Folia Microbiol. 36:302–304.
48. Skirrow, S. Z., and R. H. BonDurant. 1988. Bovine trichomoniasis. Vet. Bull.
58:591–603.
49. Talbot, J. A., K. Nielsen, and L. B. Corbeil. 1991. Cleavage of proteins of
reproductive secretions by extracellular proteinases of Tritrichomonas foetus.
Can. J. Microbiol. 37:384–390.
50. Tempest, D. W. 1970. The continuous cultivation of microorganisms. 1.
Theory of the chemostat. Methods Microbiol. 2:259–276.
51. Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of
proteins from polyacrylamide gels to nitrocellulose sheets: procedure and
some applications. Proc. Natl. Acad. Sci. USA 76:4350–4354.
52. Usala, S. J., F. O. Usala, R. Haciski, J. A. Holt, and G. F. Schumacher. 1989.
IgG and IgA content of vaginal fluid during the menstrual cycle. J. Reprod.
Med. 34:292–294.
53. Vuylsteke, B., R. Bastos, J. Barreto, T. Crucitti, E. Folgosa, J. Mondlane, T.
Dusauchoit, P. Piot, and M. Laga. 1993. High prevalence of sexually trans-
mitted diseases in a rural area in Mozambique. Genitourin. Med. 69:427–
430.
54. Wang, A. L., and C. C. Wang. 1991. Viruses of the protozoa. Annu. Rev.
Microbiol. 45:251–263.
55. Wasserheit, J. N. 1992. Interrelationship between human immunodeficiency
virus infection and other sexually transmitted diseases. Sex. Transm. Dis.
19:61–77.
VOL. 63, 1995 TRICHOMONIASIS AND HUMAN Ig DEGRADATION 3395
