TOXICOLOGICAL PROFILE FOR
MANGANESE
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Public Health Service
Agency for Toxic Substances and Disease Registry
September 2012
ii MANGANESE
DISCLAIMER
Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic
Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human
Services.
iii MANGANESE
UPDATE STATEMENT
A Toxicological Profile for Manganese, Draft for Public Comment was released in September 2008. This
edition supersedes any previously released draft or final profile.
Toxicological profiles are revised and republished as necessary. For information regarding the update
status of previously released profiles, contact ATSDR at:
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences (proposed)
Environmental Toxicology Branch (proposed)
1600 Clifton Road NE
Mailstop F-62
Atlanta, Georgia 30333
iv MANGANESE
This page is intentionally blank.

MMAN
v MANGANESE
FOREWORD
This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic
Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The
original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised
and republished as necessary.
The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects
information for the toxic substances each profile describes. Each peer-reviewed profile identifies and
reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is
also presented but is described in less detail than the key studies. The profile is not intended to be an
exhaustive document; however, more comprehensive sources of specialty information are referenced.
The profiles focus on health and toxicologic information; therefore, each toxicological profile begins with
a public health statement that describes, in nontechnical language, a substance's relevant toxicological
properties. Following the public health statement is information concerning levels of significant human
exposure and, where known, significant health effects. A health effects summary describes the adequacy
of information to determine a substance's health effects. ATSDR identifies data needs that are significant
to protection of public health.
Each profile:
(A) Examines, summarizes, and interprets available toxicologic information and
epidemiologic evaluations on a toxic substance to ascertain the levels of significant human
exposure for the substance and the associated acute, subacute, and chronic health effects;
(B) Determines whether adequate information on the health effects of each substance is
available or being developed to determine levels of exposure that present a significant risk to
human health of acute, subacute, and chronic health effects; and
(C) Where appropriate, identifies toxicologic testing needed to identify the types or levels of
exposure that may present significant risk of adverse health effects in humans.
The principal audiences for the toxicological profiles are federal, state, and local health professionals;
interested private sector organizations and groups; and members of the public.
This profile reflects ATSDR’s assessment of all relevant toxicologic testing and information that has been
peer-reviewed. Staff of the Centers for Disease Control and Prevention and other federal scientists also
have reviewed the profile. In addition, this profile has been peer-reviewed by a nongovernmental panel
and was made available for public review. Final responsibility for the contents and views expressed in
this toxicological profile resides with ATSDR.
Christopher J. Portier, Ph.D.
Assistant Administrator
Agency for Toxic Substances and
Disease Registry

C
vi MANGANESE
*Legislative Background
The toxicological profiles are developed under the Comprehensive Environmental Response,
Compensation, and Liability Act of 1980, as amended (CERCLA or Superfund). CERCLA section
104(i)(1) directs the Administrator of ATSDR to “…effectuate and implement the health related
authorities” of the statute. This includes the preparation of toxicological profiles for hazardous
substances most commonly found at facilities on the CERCLA National Priorities List and that pose the
most significant potential threat to human health, as determined by ATSDR and the EPA. Section
104(i)(3) of CERCLA, as amended, directs the Administrator of ATSDR to prepare a toxicological profile
for each substance on the list. In addition, ATSDR has the authority to prepare toxicological profiles for
substances not found at sites on the National Priorities List, in an effort to “…establish and maintain
inventory of literature, research, and studies on the health effects of toxic substances” under CERCLA
Section 104(i)(1)(B), to respond to requests for consultation under section 104(i)(4), and as otherwise
necessary to support the site-specific response actions conducted by ATSDR.

vii MANGANESE
QUICK REFERENCE FOR HEALTH CARE PROVIDERS
Toxicological Profiles are a unique compilation of toxicological information on a given hazardous
substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation
of available toxicologic and epidemiologic information on a substance. Health care providers treating
patients potentially exposed to hazardous substances will find the following information helpful for fast
answers to often-asked questions.
Primary Chapters/Sections of Interest
Chapter 1: Public Health Statement: The Public Health Statement can be a useful tool for educating
patients about possible exposure to a hazardous substance. It explains a substance’s relevant
toxicologic properties in a nontechnical, question-and-answer format, and it includes a review of
the general health effects observed following exposure.
Chapter 2: Relevance to Public Health: The Relevance to Public Health Section evaluates, interprets,
and assesses the significance of toxicity data to human health.
Chapter 3: Health Effects: Specific health effects of a given hazardous compound are reported by type
of health effect (death, systemic, immunologic, reproductive), by route of exposure, and by length
of exposure (acute, intermediate, and chronic). In addition, both human and animal studies are
reported in this section.
NOTE: Not all health effects reported in this section are necessarily observed in the clinical
setting. Please refer to the Public Health Statement to identify general health effects observed
following exposure.
Pediatrics: Four new sections have been added to each Toxicological Profile to address child health
issues:
Section 1.6 How Can (Chemical X) Affect Children?
Section 1.7 How Can Families Reduce the Risk of Exposure to (Chemical X)?
Section 3.7 Children’s Susceptibility
Section 6.6 Exposures of Children
Other Sections of Interest:
Section 3.8 Biomarkers of Exposure and Effect
Section 3.11 Methods for Reducing Toxic Effects
ATSDR Information Center
Phone: 1-800-CDC-INFO (800-232-4636) or 1-888-232-6348 (TTY) Fax: (770) 488-4178
E-mail: [email protected]ov Internet: http://www.atsdr.cdc.gov
The following additional material can be ordered through the ATSDR Information Center:
Case Studies in Environmental Medicine: Taking an Exposure History—The importance of taking an
exposure history and how to conduct one are described, and an example of a thorough exposure
history is provided. Other case studies of interest include Reproductive and Developmental
Hazards; Skin Lesions and Environmental Exposures; Cholinesterase-Inhibiting Pesticide
Toxicity; and numerous chemical-specific case studies.

viii MANGANESE
Managing Hazardous Materials Incidents is a three-volume set of recommendations for on-scene
(prehospital) and hospital medical management of patients exposed during a hazardous materials
incident. Volumes I and II are planning guides to assist first responders and hospital emergency
department personnel in planning for incidents that involve hazardous materials. Volume III—
Medical Management Guidelines for Acute Chemical Exposures—is a guide for health care
professionals treating patients exposed to hazardous materials.
Fact Sheets (ToxFAQs) provide answers to frequently asked questions about toxic substances.
Other Agencies and Organizations
The National Center for Environmental Health (NCEH) focuses on preventing or controlling disease,
injury, and disability related to the interactions between people and their environment outside the
workplace. Contact: NCEH, Mailstop F-29, 4770 Buford Highway, NE, Atlanta,
GA 30341-3724 • Phone: 770-488-7000 • FAX: 770-488-7015.
The National Institute for Occupational Safety and Health (NIOSH) conducts research on occupational
diseases and injuries, responds to requests for assistance by investigating problems of health and
safety in the workplace, recommends standards to the Occupational Safety and Health
Administration (OSHA) and the Mine Safety and Health Administration (MSHA), and trains
professionals in occupational safety and health. Contact: NIOSH, 200 Independence Avenue,
SW, Washington, DC 20201 • Phone: 800-356-4674 or NIOSH Technical Information Branch,
Robert A. Taft Laboratory, Mailstop C-19, 4676 Columbia Parkway, Cincinnati, OH 45226-1998
• Phone: 800-35-NIOSH.
The National Institute of Environmental Health Sciences (NIEHS) is the principal federal agency for
biomedical research on the effects of chemical, physical, and biologic environmental agents on
human health and well-being. Contact: NIEHS, PO Box 12233, 104 T.W. Alexander Drive,
Research Triangle Park, NC 27709 • Phone: 919-541-3212.
Referrals
The Association of Occupational and Environmental Clinics (AOEC) has developed a network of clinics
in the United States to provide expertise in occupational and environmental issues. Contact:
AOEC, 1010 Vermont Avenue, NW, #513, Washington, DC 20005 • Phone: 202-347-4976
• FAX: 202-347-4950 • e-mail: [email protected] • Web Page: http://www.aoec.org/.
The American College of Occupational and Environmental Medicine (ACOEM) is an association of
physicians and other health care providers specializing in the field of occupational and
environmental medicine. Contact: ACOEM, 25 Northwest Point Boulevard, Suite 700, Elk
Grove Village, IL 60007-1030 • Phone: 847-818-1800 • FAX: 847-818-9266.
ix MANGANESE
CONTRIBUTORS
CHEMICAL MANAGER(S)/AUTHOR(S):
Malcolm Williams, DVM, Ph.D.
G. Daniel Todd, Ph.D.
Nickolette Roney, M.P.H.
Jewell Crawford, M.D.
Charleton Coles, Ph.D.
ATSDR, Division of Toxicology and Human Health Sciences (proposed), Atlanta, GA
Peter R. McClure, Ph.D., DABT
Joan D. Garey, Ph.D.
Kimberly Zaccaria, Ph.D.
Mario Citra, Ph.D.
SRC Inc. (formerly known as Syracuse Research Corporation), North Syracuse, NY
THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS:
1. Health Effects Review. The Health Effects Review Committee examines the health effects
chapter of each profile for consistency and accuracy in interpreting health effects and classifying
end points.
2. Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to
substance-specific Minimal Risk Levels (MRLs), reviews the health effects database of each
profile, and makes recommendations for derivation of MRLs.
3. Data Needs Review. The Environmental Toxicology Branch (proposed) reviews data needs
sections to assure consistency across profiles and adherence to instructions in the Guidance.
4. Green Border Review. Green Border review assures the consistency with ATSDR policy.
x MANGANESE
This page is intentionally blank.
xi MANGANESE
PEER REVIEW
A peer review panel was assembled for manganese. The panel consisted of the following members:
1. David Dorman, D.V.M., Ph.D., Associate Dean for Research and Graduate Studies, College of
Veterinary Medicine, Professor of Toxicology, Department of Molecular Biomedical Sciences,
North Carolina State University, Raleigh, North Carolina 27606,
2. Donald Smith, Ph.D., Professor of Environmental Toxicology, University of California, Santa
Cruz, California 95064, and
3. Wei Zheng, Ph.D., Director of Graduate Studies, School of Health Sciences, Purdue University,
West Lafayette, Indiana 47907.
These experts collectively have knowledge of manganese’s physical and chemical properties,
toxicokinetics, key health end points, mechanisms of action, human and animal exposure, and
quantification of risk to humans. All reviewers were selected in conformity with the conditions for peer
review specified in Section 104(I)(13) of the Comprehensive Environmental Response, Compensation,
and Liability Act, as amended.
Scientists from the Agency for Toxic Substances and Disease Registry (ATSDR) have reviewed the peer
reviewers' comments and determined which comments will be included in the profile. A listing of the
peer reviewers' comments not incorporated in the profile, with a brief explanation of the rationale for their
exclusion, exists as part of the administrative record for this compound.
The citation of the peer review panel should not be understood to imply its approval of the profile's final
content. The responsibility for the content of this profile lies with the ATSDR.
xii MANGANESE
This page is intentionally blank.
xiii MANGANESE
CONTENTS
DISCLAIMER .............................................................................................................................................. ii
UPDATE STATEMENT .............................................................................................................................iii
FOREWORD ................................................................................................................................................ v
QUICK REFERENCE FOR HEALTH CARE PROVIDERS....................................................................vii
CONTRIBUTORS....................................................................................................................................... ix
PEER REVIEW ........................................................................................................................................... xi
CONTENTS............................................................................................................................................... xiii
LIST OF FIGURES .................................................................................................................................. xvii
LIST OF TABLES..................................................................................................................................... xix
1. PUBLIC HEALTH STATEMENT.......................................................................................................... 1
1.1 WHAT IS MANGANESE?............................................................................................................ 2
1.2 WHAT HAPPENS TO MANGANESE WHEN IT ENTERS THE ENVIRONMENT? .............. 3
1.3 HOW MIGHT I BE EXPOSED TO MANGANESE? ................................................................... 3
1.4 HOW CAN MANGANESE ENTER AND LEAVE MY BODY? ................................................ 4
1.5 HOW CAN MANGANESE AFFECT MY HEALTH? ................................................................. 4
1.6 HOW CAN MANGANESE AFFECT CHILDREN? .................................................................... 6
1.7 HOW CAN FAMILIES REDUCE THE RISK OF EXPOSURE TO MANGANESE? ................ 7
1.8 IS THERE A MEDICAL TEST TO DETERMINE WHETHER I HAVE BEEN EXPOSED
TO MANGANESE?....................................................................................................................... 8
1.9 WHAT RECOMMENDATIONS HAS THE FEDERAL GOVERNMENT MADE TO
PROTECT HUMAN HEALTH? ................................................................................................... 8
1.10 WHERE CAN I GET MORE INFORMATION? .......................................................................... 9
2. RELEVANCE TO PUBLIC HEALTH ................................................................................................. 11
2.1 BACKGROUND AND ENVIRONMENTAL EXPOSURES TO MANGANESE IN THE
UNITED STATES ....................................................................................................................... 11
2.2 SUMMARY OF HEALTH EFFECTS......................................................................................... 12
2.3 MINIMAL RISK LEVELS (MRLs) ............................................................................................ 19
3. HEALTH EFFECTS.............................................................................................................................. 39
3.1 INTRODUCTION........................................................................................................................ 39
3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE ..................................... 41
3.2.1 Inhalation Exposure .............................................................................................................. 42
3.2.1.1 Death.............................................................................................................................. 60
3.2.1.2 Systemic Effects............................................................................................................. 61
3.2.1.3 Immunological and Lymphoreticular Effects ................................................................ 67
3.2.1.4 Neurological Effects ...................................................................................................... 67
3.2.1.5 Reproductive Effects...................................................................................................... 94
3.2.1.6 Developmental Effects................................................................................................... 97
3.2.1.7 Cancer ............................................................................................................................
98
3.2.2 Oral Exposure........................................................................................................................ 98
3.2.2.1 Death.............................................................................................................................. 98
3.2.2.2 Systemic Effects........................................................................................................... 147
3.2.2.3 Immunological and Lymphoreticular Effects .............................................................. 157
3.2.2.4 Neurological Effects .................................................................................................... 158
3.2.2.5 Reproductive Effects.................................................................................................... 192
3.2.2.6 Developmental Effects................................................................................................. 197
3.2.2.7 Cancer .......................................................................................................................... 204
xiv MANGANESE
3.2.3 Dermal Exposure................................................................................................................. 204
3.2.3.1 Death............................................................................................................................ 205
3.2.3.2 Systemic Effects........................................................................................................... 205
3.2.3.3 Immunological and Lymphoreticular Effects .............................................................. 207
3.2.3.4 Neurological Effects .................................................................................................... 207
3.2.3.5 Reproductive Effects.................................................................................................... 207
3.2.3.6 Developmental Effects................................................................................................. 207
3.2.3.7 Cancer .......................................................................................................................... 208
3.2.4 Diagnostic Uses................................................................................................................... 208
3.2.4.1 Death................................................................................................................................ 209
3.2.4.2 Systemic Effects .............................................................................................................. 210
3.2.4.3 Immunological and Lymphoreticular Effects.................................................................. 214
3.2.4.4 Neurological Effects........................................................................................................ 214
3.2.4.5 Reproductive Effects ....................................................................................................... 215
3.2.4.6 Developmental Effects .................................................................................................... 216
3.3 GENOTOXICITY ...................................................................................................................... 218
3.4 TOXICOKINETICS................................................................................................................... 224
3.4.1 Absorption........................................................................................................................... 224
3.4.1.1 Inhalation Exposure ..................................................................................................... 224
3.4.1.2 Oral Exposure .............................................................................................................. 228
3.4.1.3 Dermal Exposure ......................................................................................................... 232
3.4.2 Distribution ......................................................................................................................... 232
3.4.2.1 Inhalation Exposure .....................................................................................................
235
3.4.2.2 Oral Exposure .............................................................................................................. 247
3.4.2.3 Dermal Exposure ......................................................................................................... 250
3.4.2.4 Other Routes of Exposure............................................................................................ 250
3.4.3 Metabolism.......................................................................................................................... 255
3.4.4 Elimination and Excretion................................................................................................... 259
3.4.4.1 Inhalation Exposure ..................................................................................................... 260
3.4.4.2 Oral Exposure .............................................................................................................. 262
3.4.4.3 Dermal Exposure ......................................................................................................... 263
3.4.4.4 Other Routes of Exposure............................................................................................ 263
3.4.5 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models ...........
264
3.5 MECHANISMS OF ACTION ................................................................................................... 293
3.5.1 Pharmacokinetic Mechanisms............................................................................................. 293
3.5.2 Mechanisms of Toxicity...................................................................................................... 296
3.5.3 Animal-to-Human Extrapolations....................................................................................... 304
3.6 TOXICITIES MEDIATED THROUGH THE NEUROENDOCRINE AXIS........................... 305
3.7 CHILDREN’S SUSCEPTIBILITY............................................................................................ 308
3.8 BIOMARKERS OF EXPOSURE AND EFFECT ..................................................................... 321
3.8.1 Biomarkers Used to Identify or Quantify Exposure to Manganese .................................... 322
3.8.2 Biomarkers Used to Characterize Effects Caused by Manganese....................................... 327
3.9 INTERACTIONS WITH OTHER CHEMICALS ..................................................................... 329
3.10 POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE................................................ 331
3.11 METHODS FOR REDUCING TOXIC EFFECTS.................................................................... 336
3.11.1 Reducing Peak Absorption Following Exposure............................................................. 336
3.11.2 Reducing Body Burden ................................................................................................... 337
3.11.3 Interfering with the Mechanism of Action for Toxic Effects ..........................................
339
3.12 ADEQUACY OF THE DATABASE ........................................................................................ 340
3.12.1 Existing Information on Health Effects of Manganese ................................................... 340
3.12.2 Identification of Data Needs............................................................................................ 342
MANGANESE xv
3.12.3 Ongoing Studies .............................................................................................................. 360
4. CHEMICAL AND PHYSICAL INFORMATION.............................................................................. 365
4.1 CHEMICAL IDENTITY............................................................................................................ 365
4.2 PHYSICAL AND CHEMICAL PROPERTIES......................................................................... 365
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL .......................................................... 373
5.1 PRODUCTION .......................................................................................................................... 373
5.2 IMPORT/EXPORT .................................................................................................................... 379
5.3 USE ............................................................................................................................................ 379
5.4 DISPOSAL................................................................................................................................. 381
6. POTENTIAL FOR HUMAN EXPOSURE ......................................................................................... 383
6.1 OVERVIEW...............................................................................................................................383
RELEASES TO THE ENVIRONMENT................................................................................... 385 6.2
6.2.1 Air ....................................................................................................................................... 390
6.2.2 Water................................................................................................................................... 392
6.2.3 Soil ...................................................................................................................................... 393
6.3 ENVIRONMENTAL FATE ...................................................................................................... 394
6.3.1 Transport and Partitioning................................................................................................... 394
6.3.2 Transformation and Degradation ........................................................................................ 396
6.3.2.1 Air ................................................................................................................................ 396
6.3.2.2 Water............................................................................................................................ 397
6.3.2.3 Sediment and Soil ........................................................................................................ 397
6.4 LEVELS MONITORED OR ESTIMATED IN THE ENVIRONMENT .................................. 397
6.4.1 Air .......................................................................................................................................
398
6.4.2 Water................................................................................................................................... 400
6.4.3 Sediment and Soil ............................................................................................................... 404
6.4.4 Other Environmental Media................................................................................................ 404
6.5 GENERAL POPULATION AND OCCUPATIONAL EXPOSURE ........................................ 407
6.6 EXPOSURES OF CHILDREN.................................................................................................. 413
6.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES .............................................. 415
6.8 ADEQUACY OF THE DATABASE ........................................................................................ 419
6.8.1 Identification of Data Needs ............................................................................................... 419
6.8.2 Ongoing Studies .................................................................................................................. 423
7. ANALYTICAL METHODS................................................................................................................ 425
7.1 BIOLOGICAL MATERIALS.................................................................................................... 426
7.2 ENVIRONMENTAL SAMPLES .............................................................................................. 428
7.3 ADEQUACY OF THE DATABASE ........................................................................................ 432
7.3.1 Identification of Data Needs ............................................................................................... 433
7.3.2 Ongoing Studies .................................................................................................................. 434
8. REGULATIONS, ADVISORIES, AND GUIDELINES..................................................................... 435
9. REFERENCES .................................................................................................................................... 443
10. GLOSSARY ...................................................................................................................................... 501
xvi MANGANESE
APPENDICES
A. ATSDR MINIMAL RISK LEVELS AND WORKSHEETS .............................................................A-1
B. USER’S GUIDE.................................................................................................................................. B-1
C. ACRONYMS, ABBREVIATIONS, AND SYMBOLS...................................................................... C-1
D. INDEX ................................................................................................................................................ D-1
xvii MANGANESE
LIST OF FIGURES
3-1. Levels of Significant Exposure to Inorganic Manganese – Inhalation............................................... 57
3-2. Levels of Significant Exposure to Inorganic Manganese – Oral ...................................................... 136
3-3. Levels of Significant Exposure to Organic Manganese-MMT – Oral.............................................. 144
3-4. Metabolism of MnDPDP.................................................................................................................. 258
3-5. Conceptual Representation of a Physiologically Based Pharmacokinetic (PBPK) Model for a
Hypothetical Chemical Substance.................................................................................................... 266
3-6. Qualitative PBPK Model for Manganese ......................................................................................... 268
3-7. Schematic Structures of Nong et al. (2008) PBPK Models A and B for Manganese in CD Rats .... 270
3-8. Schematic of Models for Nasopharyngeal and Lung Deposition of Manganese and Transport to
Blood in the Nong et al. (2008) PBPK Models A and B for Manganese in CD Rats ...................... 271
3-9. Schematic of the Leavens et al. (2007) Model to Describe Olfactory and Blood Delivery of
Manganese to the Left Side of the Brain Isilateral to the Olfactory Mucosa (OM) in the Left
Nasal Cavity ..................................................................................................................................... 279
3-10. Physiologically Based Pharmacokinetic Model Structure Describing Tissue Manganese
Kinetics in Adult Rats .................................................................................................................... 284
3-11. Model Structure for Simulating Manganese Exposure During Gestation in the Rat...................... 287
3-12. Model Structure for Predicting Manganese Tissue Levels in Lactating Rat Dams and Pups ........ 288
3-13. Physiologically Based Pharmacokinetic Model Structure Describing Manganese Tissue
Kinetics in Adult Monkeys and Humans ....................................................................................... 290
3-14. Simulated End-of-Exposure Tissue Total Manganese Levels in Rat Striatum and Monkey and
Human Globus Pallidus ................................................................................................................. 292
3-15. Existing Information on Health Effects of Inorganic Manganese .................................................. 341
6-1. Frequency of NPL Sites with Manganese Contamination................................................................ 384
xix MANGANESE
LIST OF TABLES
2-1. Adequate Intake (AI) for Manganese ................................................................................................. 13
3-1. Levels of Significant Exposure to Inorganic Manganese – Inhalation............................................... 43
3-2. Levels of Significant Exposure to Inorganic Manganese – Oral ........................................................ 99
3-3. Levels of Significant Exposure to Organic Manganese-MMT – Oral.............................................. 142
3-4. Scores on Intelligence Tests ............................................................................................................. 166
3-5. Genotoxicity of Manganese In Vitro ................................................................................................ 220
3-6. Genotoxicity of Manganese In Vivo ................................................................................................. 222
3-7. Manganese Levels in Human and Animal Tissues........................................................................... 233
3-8. Manganese Levels in Human Serum/Plasma.................................................................................... 236
3-9. Terminal Mean (±Standard Error on the Mean) Tissue Manganese Concentrations (µg
Manganese/g Tissue Wet Weight) in Maternal CD Rats Exposed to Aerosols of Manganese
Sulfate 6 Hours/Day, 7 Days/Week Starting 28 Days Prior to Breeding Through Postnatal
Day 18 .............................................................................................................................................. 241
3-10. Mean (±Standard Error on the Mean) Tissue Manganese Concentrations (µg Manganese/g
Tissue Wet Weight) in Young Male Rhesus Monkeys Exposed to Aerosols of Manganese
Sulfate (1.5 mg Manganese/m
3
) 6 Hours/Day, 5 Days/Week for Up to 65 Days .......................... 242
3-11. Manganese Concentrations in Brain Tissues of Lactating CD Rats and Offspring Exposed to
Aerosols of Manganese Sulfate...................................................................................................... 245
3-12. Manganese Levels in Rat Tissue After Oral Exposure................................................................... 248
3-13. Levels of Manganese in Exposed and Non-Exposed Workers....................................................... 261
3-14. Parameter Values in the Teeguarden et al. (2007c) PBPK Model for Manganese in CD Rats
(Nong et al. 2008) Model A ........................................................................................................... 272
3-15. Refined Parameter Values in Nong et al. (2008) Model A............................................................. 276
3-16. Parameter Values in Nong et al. (2008) Model B........................................................................... 277
3-17. Parameter Values for Manganese Chloride in the Leavens et al. (2007) PBPK Model for
Olfactory Transport of Manganese in Rats ....................................................................................
280
3-18. Parameter Values for Manganese Phosphate in the Leavens et al. (2007) PBPK Model for
Olfactory Transport of Manganese in Rats .................................................................................... 281
xx MANGANESE
3-19. Parameter Values for Describing Blood Concentrations in the Leavens et al. (2007) PBPK
Model for Olfactory Transport of Manganese in Rats ................................................................... 283
3-20. Ongoing Studies on Manganese ..................................................................................................... 361
4-1. Chemical Identity of Manganese and Compounds........................................................................... 366
4-2. Physical and Chemical Properties of Manganese and Compounds.................................................. 369
5-1. Facilities that Produce, Process, or Use Manganese......................................................................... 374
5-2. Facilities that Produce, Process, or Use Manganese Compounds .................................................... 376
5-3. Manganese Import/Export Data for 2003–2007 ............................................................................... 380
6-1. Releases to the Environment from Facilities that Produce, Process, or Use Manganese ................. 386
6-2. Releases to the Environment from Facilities that Produce, Process, or Use Manganese
Compounds....................................................................................................................................... 388
6-3. Average Levels of Manganese in Ambient Air ................................................................................ 399
6-4. Levels of PM
2.5
and PM
10
in Indoor and Outdoor Air in Toronto, Canada and Indianapolis,
Indiana .............................................................................................................................................. 401
6-5. Manganese Detections and Concentrations in Surface Water and Groundwater in the United
States................................................................................................................................................. 403
6-6. Mean Concentrations of Manganese for FDA’s Total Diet Study Market Baskets 1991 through
1997 .................................................................................................................................................. 405
6-7. Summary of Typical Human Exposure to Manganese ..................................................................... 408
6-8. Estimated 3-Day PM
2.5
Manganese Exposure Distribution for a Population (n=922) in Toronto,
Canada .............................................................................................................................................. 410
7-1. Analytical Methods for Determining Manganese in Biological Materials....................................... 427
7-2. Analytical Methods for Determining Manganese in Environmental Samples ................................. 429
8-1. Regulations, Advisories, and Guidelines Applicable to Manganese ................................................ 438
1 MANGANESE
1. PUBLIC HEALTH STATEMENT
This public health statement tells you about manganese and the effects of exposure to it.
The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the
nation. These sites are then placed on the National Priorities List (NPL) and are targeted for long-term
federal clean-up activities. Manganese has been found in at least 869 of the 1,699 current or former NPL
sites. Although the total number of NPL sites evaluated for this substance is not known, the possibility
exists that the number of sites at which manganese is found may increase in the future as more sites are
evaluated. This information is important because these sites may be sources of exposure and exposure to
this substance may harm you.
When a substance is released either from a large area, such as an industrial plant, or from a container,
such as a drum or bottle, it enters the environment. Such a release does not always lead to exposure. You
can be exposed to a substance only when you come in contact with it. You may be exposed by breathing,
eating, or drinking the substance, or by skin contact.
If you are exposed to manganese, many factors will determine whether you will be harmed. These factors
include the dose (how much), the duration (how long), and how you come in contact with it. You must
also consider any other chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and
state of health.

MANGANESE 2
1. PUBLIC HEALTH STATEMENT
1.1 WHAT IS MANGANESE?
Description
Manganese is a naturally occurring substance found in many
types of rocks and soil. Pure manganese is a silver-colored
metal; however, it does not occur in the environment as a pure
metal. Rather, it occurs combined with other substances such
as oxygen, sulfur, and chlorine. Manganese is a trace element
and is necessary for good health.
Uses
• Manufacturing
• Consumer products
Manganese is used principally in steel production to improve
hardness, stiffness, and strength. It is used in carbon steel,
stainless steel, high-temperature steel, and tool steel, along
with cast iron and superalloys.
Manganese occurs naturally in most foods and may be added
to food or made available in nutritional supplements.
Manganese is also used in a wide variety of other products,
including:
• fireworks
• dry-cell batteries
• fertilizer
• paints
• a medical imaging agent
• cosmetics
It may also be used as an additive in gasoline to improve the
octane rating of the gas.
Small amounts of manganese are used in a pharmaceutical
product called mangafodipir trisodium (MnDPDP) to improve
lesion detection in magnetic resonance imaging of body organs.
Chapters 4, 5, and 6 have more information on the properties and uses of manganese and how it behaves
in the environment.
MANGANESE 3
1. PUBLIC HEALTH STATEMENT
1.2 WHAT HAPPENS TO MANGANESE WHEN IT ENTERS THE ENVIRONMENT?
Sources
Manganese is a normal constituent of air, soil, water, and food.
Additional manganese can be found in air, soil, and water after
release from the manufacture, use, and disposal of manganese-
based products.
Breakdown
As with other elements, manganese cannot break down in the
environment. It can only change its form or become attached or
separated from particles. The chemical state of manganese
and the type of soil determine how fast it moves through the soil
and how much is retained in the soil. In water, most of the
manganese tends to attach to particles in the water or settle into
the sediment.
The manganese-containing gasoline additive may degrade in
the environment quickly when exposed to sunlight, releasing
manganese.
For more information on manganese in the environment, see Chapter 6.
1.3 HOW MIGHT I BE EXPOSED TO MANGANESE?
Food – primary
source of exposure
The primary way you can be exposed to manganese is by eating food
or manganese-containing nutritional supplements. Vegetarians who
consume foods rich in manganese such as grains, beans and nuts, as
well as heavy tea drinkers, may have a higher intake of manganese
than the average person.
Workplace air
Certain occupations like welding or working in a factory where steel is
made may increase your chances of being exposed to high levels of
manganese.
Water and soil
Because manganese is a natural component of the environment, you
are always exposed to low levels of it in water, air, soil, and food.
Manganese is routinely contained in groundwater, drinking water and
soil at low levels. Drinking water containing manganese or swimming
or bathing in water containing manganese may expose you to low
levels of this chemical.

4 MANGANESE
1. PUBLIC HEALTH STATEMENT
Air
Air also contains low levels of manganese, and breathing air may
expose you to it. Releases of manganese into the air occur from:
• industries using or manufacturing products containing manganese,
• mining activities, and
• automobile exhaust.
Lifestyle traits may also lead to exposure to manganese. People who
smoke tobacco or inhale second-hand smoke are typically exposed to
manganese at levels higher than those not exposed to tobacco smoke.
See Chapter 6 for more information on how you might be exposed to manganese or its compounds.
1.4 HOW CAN MANGANESE ENTER AND LEAVE MY BODY?
Enter your body
• Inhalation
When you breathe air containing manganese, a small amount of the
manganese will enter your body through your lungs and the remainder
can become trapped in your lungs. Some of the manganese in your
lungs can also be trapped in mucus which you may cough up and
swallow into your stomach.
• Ingestion
Manganese in food or water may enter your body through the digestive
tract to meet your body’s needs for normal functioning.
• Dermal contact
Only very small amounts of manganese can enter your skin when you
come into contact with liquids containing manganese.
Leave your body
Once in your body, manganese-containing chemicals can break down
into other chemicals. However, manganese is an element that cannot
be broken down. Most manganese will leave your body in feces within
a few days.
For more information on how manganese enters and leaves the body, see Chapter 3.
1.5 HOW CAN MANGANESE AFFECT MY HEALTH?
This section looks at studies concerning potential health effects in human and animal studies.
General population
Manganese is an essential nutrient, and eating a small amount of it
each day is important to stay healthy.
5 MANGANESE
1. PUBLIC HEALTH STATEMENT
Workers
• Inhalation
The most common health problems in workers exposed to high levels
of manganese involve the nervous system. These health effects
include behavioral changes and other nervous system effects, which
include movements that may become slow and clumsy. This
combination of symptoms when sufficiently severe is referred to as
“manganism.” Other less severe nervous system effects such as
slowed hand movements have been observed in some workers
exposed to lower concentrations in the work place.
The inhalation of a large quantity of dust or fumes containing
manganese may cause irritation of the lungs which could lead to
pneumonia.
Loss of sex drive and sperm damage has also been observed in men
exposed to high levels of manganese in workplace air.
The manganese concentrations that cause effects such as slowed hand
movements in some workers are approximately twenty thousand times
higher than the concentrations normally found in the environment.
Manganism has been found in some workers exposed to manganese
concentrations about a million times higher than normal air
concentrations of manganese.
Laboratory animals
• Inhalation
Respiratory effects, similar to those observed in workers, have been
observed in laboratory monkeys exposed to high levels of manganese.
Laboratory animals
• Oral
Manganese has been shown to cross the blood-brain barrier and a
limited amount of manganese is also able to cross the placenta during
pregnancy, enabling it to reach a developing fetus.
Nervous system disturbances have been observed in animals after very
high oral doses of manganese, including changes in behavior.
Sperm damage and adverse changes in male reproductive
performance were observed in laboratory animals fed high levels of
manganese. Impairments in fertility were observed in female rodents
provided with oral manganese before they became pregnant.
Illnesses involving the kidneys and urinary tract have been observed in
laboratory rats fed very high levels of manganese. These illnesses
included inflammation of the kidneys and kidney stone formation.
Cancer
The EPA concluded that existing scientific information cannot
determine whether or not excess manganese can cause cancer.
Further information on the health effects of manganese in humans and animals can be found in
Chapters 2 and 3.
MANGANESE 6
1. PUBLIC HEALTH STATEMENT
1.6 HOW CAN MANGANESE AFFECT CHILDREN?
This section discusses potential health effects in humans from exposures during the period from
conception to maturity at 18 years of age.
Effects in children
Studies in children have suggested that extremely high levels of
manganese exposure may produce undesirable effects on brain
development, including changes in behavior and decreases in the
ability to learn and remember. In some cases, these same manganese
exposure levels have been suspected of causing severe symptoms of
manganism disease (including difficulty with speech and walking). We
do not know for certain that these changes were caused by manganese
alone. We do not know if these changes are temporary or permanent.
We do not know whether children are more sensitive than adults to the
effects of manganese, but there is some indication from experiments in
laboratory animals that they may be.
Birth defects
Studies of manganese workers have not found increases in birth
defects or low birth weight in their children.
No birth defects were observed in animals exposed to manganese
In one human study where people were exposed to very high levels of
manganese from drinking water, infants less than 1 year of age died at
an unusually high rate. It is not clear, however, whether these deaths
were attributable to the manganese level of the drinking water. The
manganese toxicity may have involved exposures to the infant that
occurred both before (through the mother) and after they were born.
Avoid inhalation of
manganese at work
High levels of airborne manganese are observed in certain
occupational settings such as steel factories or welding areas. You
should take precautions to prevent inhalation of manganese by
wearing an appropriate mask to limit the amount of manganese you
breathe.
Avoid wearing
manganese dust-
contaminated work
clothing in your home
or car
Workers exposed to high levels of airborne manganese in certain
occupational settings may accumulate manganese dust on their work
clothes. Manganese-contaminated work clothing should be removed
before getting into your car or entering your home to help reduce the
exposure hazard for yourself and your family.
Avoid inhalation of
If you weld objects around your home, do so in a well-ventilated area
welding fumes at
and use an appropriate mask to decrease your risk of inhaling
home
manganese-containing fumes. Children should be kept away from
welding fumes.
Diet
Children are not likely to be exposed to harmful amounts of
manganese in the diet. However, higher-than-usual amounts of
manganese may be absorbed if their diet is low in iron. It is important
to provide your child with a well-balanced diet.
Water
While tap and bottled water generally contain safe levels of
manganese, well water may sometimes be contaminated with
sufficiently high levels of manganese to create a potential health
hazard. If drinking water is obtained from a well water source, it m
be wise to have the water checked for manganese to ensure the le
is below the current guideline level established by the EPA.
ay
vel
Smoking
Manganese is a minor constituent of tobacco smoke. Avoiding
tobacco smoke may reduce your family’s exposure to manganese.
MANGANESE 7
1. PUBLIC HEALTH STATEMENT
1.7 HOW CAN FAMILIES REDUCE THE RISK OF EXPOSURE TO MANGANESE?
If your doctor finds that you have been exposed to significant amounts of manganese, ask whether your
chi
ldren might also be exposed. Your doctor might need to ask you state health department to investigate.

MANGANESE 8
1. PUBLIC HEALTH STATEMENT
1.8 IS THERE A MEDICAL TEST TO DETERMINE WHETHER I HAVE BEEN EXPOSED TO
MANGANESE?
Detecting exposure
Several tests are available to measure manganese in blood, urine,
hair, or feces. Because manganese is normally present in our body,
some is always found in tissues or fluids.
Normal ranges of manganese levels are about 4–15 μg/L in blood, 1–
8 μg/L in urine, and 0.4–0.85 μg/L in serum (the fluid portion of the
blood).
Measuring exposure
Because excess manganese is usually removed from the body within
a few days, past exposures are difficult to measure with common
laboratory tests.
A medical test known as magnetic resonance imaging, or MRI, can
detect the presence of increased amounts of manganese in the brain.
However, this type of test is qualitative, and has not been shown to
reliably reflect or predict toxicologically meaningful exposures.
Information about tests for detecting manganese in the body is given in Chapters 3 and 7.
1.9 WHAT RECOMMENDATIONS HAS THE FEDERAL GOVERNMENT MADE TO
PROTECT HUMAN HEALTH?
The federal government develops regulations and recommendations to protect public health. Regulations
can be enforced by law. The EPA, the Occupational Safety and Health Administration (OSHA), and the
Food and Drug Administration (FDA) are some federal agencies that develop regulations for toxic
substances. Recommendations provide valuable guidelines to protect public health, but cannot be
enforced by law. The Agency for Toxic Substances and Disease Registry (ATSDR) and the National
Institute for Occupational Safety and Health (NIOSH) are two federal organizations that develop
recommendations for toxic substances.
Regulations and recommendations can be expressed as “not-to-exceed” levels, that is, levels of a toxic
substance in air, water, soil, or food that do not exceed a critical value that is usually based on levels that
affect animals; they are then adjusted to levels that will help protect humans. Sometimes these not-to-
exceed levels differ among federal organizations because they used different exposure times (an 8-hour
workday or a 24-hour day), different animal studies, or other factors.

9 MANGANESE
1. PUBLIC HEALTH STATEMENT
Recommendations and regulations are also updated periodically as more information becomes available.
For the most current information, check with the federal agency or organization that provides it. Some
regulations and recommendations for manganese include the following:
Drinking water
The EPA has established that exposure to manganese in drinking water at
concentrations of 1 mg/L for 1 or 10 days is not expected to cause any
adverse effects in a child.
The EPA has established that lifetime exposure to 0.3 mg/L manganese is
not expected to cause any adverse effects.
Bottled water
The FDA has established that the manganese concentration in bottled
drinking water should not exceed 0.05 mg/L.
Workplace air
OSHA set a legal limit of 5 mg/m
3
manganese in air averaged over an
8-hour work day.
For more information on regulations and advisories, see Chapter 8.
1.10 WHERE CAN I GET MORE INFORMATION?
If you have any more questions or concerns, please contact your community or state health or
environmental quality department, or contact ATSDR at the address and phone number below.
ATSDR can also tell you the location of occupational and environmental health clinics. These clinics
specialize in recognizing, evaluating, and treating illnesses that result from exposure to hazardous
substances.
Toxicological profiles are also available on-line at www.atsdr.cdc.gov and on CD-ROM. You may
request a copy of the ATSDR ToxProfiles
TM
CD-ROM by calling the toll-free information and technical
assistance number at 1-800-CDCINFO (1-800-232-4636), by e-mail at [email protected]ov, or by writing
to:
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences (proposed)
1600 Clifton Road NE
Mailstop F-62
Atlanta, GA 30333
Fax: 1-770-488-4178
11 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
2.1 BACKGROUND AND ENVIRONMENTAL EXPOSURES TO MANGANESE IN THE
UNITED STATES
Manganese is a naturally occurring element and an essential nutrient. Comprising approximately 0.1% of
the earth’s crust, it is the twelfth most abundant element and the fifth most abundant metal. Manganese
does not exist in nature as an elemental form, but is found mainly as oxides, carbonates, and silicates in
over 100 minerals with pyrolusite (manganese dioxide) as the most common naturally-occurring form.
As an essential nutrient, several enzyme systems have been reported to interact with or depend on
manganese for their catalytic or regulatory function. As such, manganese is required for the formation of
healthy cartilage and bone and the urea cycle; it aids in the maintenance of mitochondria and the
production of glucose. It also plays a key role in wound-healing.
Manganese exists in both inorganic and organic forms. An essential ingredient in steel, inorganic
manganese is also used in the production of dry-cell batteries, glass and fireworks, in chemical
manufacturing, in the leather and textile industries and as a fertilizer. The inorganic pigment known as
manganese violet (manganese ammonium pyrophosphate complex) has nearly ubiquitous use in
cosmetics and is also found in certain paints. Organic forms of manganese are used as fungicides, fuel-oil
additives, smoke inhibitors, an anti-knock additive in gasoline, and a medical imaging agent.
The average manganese soil concentrations in the United States is 40–900 mg/kg; the primary natural
source of the manganese is the erosion of crustal rock. Its presence in soil results in vegetable and animal
foods reliably containing varying amounts of the mineral. As an essential nutrient, manganese is added to
certain foods and nutritional supplements. Vegetarians often have diets richer in manganese than those
who select omnivorous diets.
The most important source of manganese in the atmosphere results from the air erosion of dusts or soils.
The mean concentration of manganese in ambient air in the United States is 0.02 μg/m
3
; however,
ambient levels near industrial sources can range from 0.22 to 0.3 µg/m
3
. Manganese is released into
waterways mainly through the erosion of rocks and soils, mining activities, and industrial waste, or by the
leaching of manganese from anthropogenic materials discarded in landfills or soil, such as dry-cell
batteries. Surface waters in the United States contain a median manganese level of 16 μg/L, with
99
th
percentile concentrations of 400–800 μg/L. Groundwater in the United States contains median
12 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
manganese levels of 5 to 150 μg/L, with the 99
th
percentile at 2,900 or 5,600 μg/L in rural or urban areas,
respectively.
The general population is exposed to manganese through consumption of food and water, inhalation of
air, and dermal contact with air, water, soil, and consumer products that contain manganese. The primary
source of manganese intake is through diet. The Food and Nutrition Board (FNB) of the Institute of
Medicine (IOM) has set adequate intake (AI) levels for manganese for humans. These levels are
presented in Table 2-1.
The inhalation of air contaminated with particulate matter containing manganese is the primary source of
excess manganese exposure for the general population in the United States. Populations living in close
proximity to mining activities and industries using manganese may be exposed by inhalation to high
levels of manganese in dust. Workers in these industries are especially vulnerable to exposure to
manganese dust. Manganese concentrations in soil may be elevated when the soil is in close proximity to
a mining source or industry using manganese and may therefore pose a risk of excess exposure to children
who ingest contaminated soil. Manganese is ubiquitous in drinking water in the United States. Although
certain water sources in the United States are contaminated with excess manganese, there is little risk of
excessive exposure to manganese through ingestion of fish or shellfish emanating from contaminated
waters, unless the manganese levels in the fish are extremely high and/or the fish are eaten as subsistence.
Although many forms of manganese are water-soluble, there is little evidence that dermal contact with
manganese results in significant absorption through the skin. Thus, dermal contact with manganese is not
generally viewed as an important source of exposure to the population at large.
Excess exposure to manganese may be revealed by tests to detect heightened levels in body fluids as well
as in hair samples. Normal ranges of manganese levels in body fluids are 4–15 μg/L in blood, 1–8 μg/L
in urine, and 0.4–0.85 μg/L in serum. Excess manganese in the body characteristically accumulates in the
brain region known as the basal ganglia. This accumulation can be revealed by magnetic resonance
imaging (MRI) as a distinctive symmetrical high-signal lesion in the globus pallidus region of the basal
ganglia on T1- but not T2-weighted MRI.
2.2 SUMMARY OF HEALTH EFFECTS
Although low levels of manganese intake are necessary for human health, exposures to high manganese
levels are toxic. Reports of adverse effects resulting from manganese exposure in humans are associated

Life stage
Age
Males (mg/day)
Females (mg/day)
Infants
0–6 Months
0.003
0.003
Infants
7–12 Months
0.6
0.6
Children
1–3 Years
1.2
1.2
Children
4–8 Years
1.5
1.5
Children
9–13 Years
1.9
1.6
Adolescents
14–18 Years
2.2
1.6
Adults
19 Years and older
2.3
1.8
Pregnancy
All ages
—
2.0
Lactation
All ages
—
2.6
13 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Table 2-1. Adequate Intake (AI) for Manganese
Source: FNB/IOM 2001
14 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
primarily with inhalation in occupational settings. Inhaled manganese is often transported directly to the
brain before it is metabolized by the liver. The symptoms of manganese toxicity may appear slowly over
months and years. Manganese toxicity can result in a permanent neurological disorder known as
manganism with symptoms that include tremors, difficulty walking, and facial muscle spasms. These
symptoms are often preceded by other lesser symptoms, including irritability, aggressiveness, and
hallucinations. Some studies suggest that manganese inhalation can also result in adverse cognitive
effects, including difficulty with concentration and memory problems. Although the workplace is the
most common source of excess inhalation of manganese, frequent inhalation of fumes from welding
activities in the home can produce a risk of excess manganese exposure leading to neurological
symptoms. Environmental exposures to airborne manganese have been associated with similar preclinical
neurological effects and mood effects as are seen in occupational studies. Acute or intermediate exposure
to excess manganese also affects the respiratory system. Inhalation exposure to high concentrations of
manganese dusts (specifically manganese dioxide [MnO
2
] and manganese tetroxide [Mn
3
O
4
]) can cause
an inflammatory response in the lung, which, over time, can result in impaired lung function. Lung
toxicity is manifested as an increased susceptibility to infections such as bronchitis and can result in
manganic pneumonia. Pneumonia has also been observed following acute inhalation exposures to
particulates containing other metals. Thus, this effect might be characteristic of inhalable particulate
matter and might not depend solely on the manganese content of the particle.
A number of reports indicate that oral exposure to manganese, especially from contaminated water
sources, can produce significant health effects. These effects have been most prominently observed in
children and are similar to those observed from inhalation exposure. An actual threshold level at which
manganese exposure produces neurological effects in humans has not been established. However,
children consuming the same concentration of manganese in water as adults are ultimately exposed to a
higher mg/kg-body weight ratio of manganese than adults (as a consequence of the lower body weight of
children as well as their higher daily consumption volume and greater retention of manganese). Children
are also potentially more sensitive to manganese toxicity than adults. A study conducted in infant
monkeys suggests that soy-based infant formula, which contains a naturally higher concentration of
manganese than human or cow’s milk, may produce mild effects on neurological development, although
such effects have not been documented in humans. While many of the studies reporting oral effects of
excess manganese have limitations that preclude firm conclusions about the potential for adverse effects,
these studies collectively suggest that ingestion of water and/or foodstuffs containing increased
concentrations of manganese may result in adverse neurological effects.
15 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
There is indirect evidence that reproductive outcomes might be affected (decreased libido, impotence, and
sexual dysfunction have been observed in manganese-exposed men). The available studies on the effect
that manganese has on fertility (as measured by birthrate) is inconclusive. Two studies in men
occupationally exposed to manganese show adverse effects on reproductive parameters: one found
increased sexual dysfunction and the other found reduced sperm quality, but neither measured birthrate in
wives of affected workers. Impaired sexual function in men may be one of the earliest clinical
manifestations of manganese toxicity, but no dose-response information is currently available, so it is not
possible to define a threshold for this effect. There is a lack of information regarding effects in women
since most data are derived from studies of male workers. Developmental data in humans exposed to
manganese by inhalation are limited and consist mostly of reports of adverse pulmonary effects from
inhaling airborne manganese dust and adverse neurological effects in offspring following ingestion
exposure. Animal studies indicate that manganese is a developmental toxin when administered orally and
intravenously, but inhalation data concerning these effects are scarce and not definitive. Some studies in
children suggest that routine exposures to high levels of manganese from contaminated drinking water
may ultimately impair intellectual performance and behavior.
The few available inhalation and oral studies in humans and animals indicate that inorganic manganese
exposure does not cause significant injury to the heart, stomach, blood, muscle, bone, liver, kidney, skin,
or eyes. However, if manganese is in the (VII) oxidation state (as in potassium permanganate), then
ingestion may lead to severe corrosion at the point of contact. Studies in pigs have revealed a potential
for adverse coronary effects from excess manganese exposure.
There is no evidence that manganese causes cancer in humans. Although no firm conclusions can be
drawn from the mixed results in animal studies, there are little data to suggest that inorganic manganese is
carcinogenic. The IRIS has provided manganese with a weight-of-evidence classification D—not
classifiable as to human carcinogenicity.
It should be noted that individuals with cirrhosis of the liver, as well as children with a congenital venous
anomaly known as a portosystemic shunt, may be at heightened risk of health deficits from exposure to
dietary and environmental sources of manganese. Manganese is ordinarily eliminated from the body
through bile, but cirrhosis and portosystemic shunts impair the normal functioning of the liver and thus
limit the ability of the body to excrete manganese, which then can accumulate in the blood and,
eventually, the brain.
16 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
A more detailed discussion of the critical targets of manganese toxicity (i.e., the nervous system,
respiratory system, reproductive system, and development) follows.
Neurological Effects. There is clear evidence from studies of humans exposed to manganese dusts in
mines and factories that inhalation of high levels of manganese can lead to a series of serious and
ultimately disabling neurological effects in humans. This disease, termed manganism, typically begins
with feelings of weakness and lethargy. As the disease progresses, a number of other neurological signs
may become manifest. Although not all individuals develop identical signs, the most common are a slow
and clumsy gait, speech disturbances, a masklike face, and tremors. The neurological symptoms may
improve when exposure ceases; however, in most cases, the symptoms are found to persist for many years
post-exposure. In addition, a syndrome of psychological disturbances (hallucination, psychosis)
frequently emerges, although such symptoms are sometimes absent. As the disease progresses, patients
develop severe muscle tension and rigidity and may be completely and permanently disabled. Workplace
inhalation exposure levels producing overt symptoms of manganism have been on the order of 2–22 mg
manganese/m
3
. While manganese neurotoxicity has clinical similarities to Parkinson’s disease, it can be
clinically distinguished from Parkinson’s. Manganism patients present a hypokinesia and tremor that is
different from Parkinson’s patients. In addition, manganism patients sometimes have psychiatric
disturbances early in the disease, a propensity to fall backward when pushed, less frequent resting tremor,
more frequent dystonia, a “cock-walk”, and a failure to respond to dopaminomimetics.
Subclinical neurological effects have been observed in numerous studies of workers exposed to
manganese dusts at lower exposure levels than those associated with symptoms of overt manganism.
These effects include decreased performance on neurobehavioral tests; significantly poorer eye-hand
coordination, hand steadiness, and reaction time; poorer postural stability; and lower levels of cognitive
flexibility. Manganese air concentrations producing these effects in chronically exposed workers range
from about 0.07 to 0.97 mg manganese/m
3
.
Studies in communities surrounding manganese industries have also reported associations between
manganese exposure and subclinical neurological effects in adults and children. In a study of men and
women living close to a manganese alloy production plant, a blood manganese level-age interaction was
observed, with the poorest performance on neurological tests occurring among those >50 years old who
had the highest blood manganese levels. Additional studies of environmentally exposed adults reported
attention impairments, poorer postural stability, and subclinical motor impairments at environmental air
exposures >0.1 μg manganese/m
3
; however, other potential sources of environmental exposure were not
17 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
accounted for. In several studies of children, associations have been reported between manganese
concentrations in blood or hair and motor impairment and deficits in neurodevelopment and intellectual
functions.
There is also an accumulating body of evidence suggesting that exposure to excess levels of manganese in
drinking water (≥0.2 mg/L) may lead to neurological deficits in children, including poor school
performance, impaired cognitive function, abnormal performance on neurobehavioral tests, and increased
oppositional behavior and hyperactivity. Several cases of apparent manganism in both children and
adults have been reported where exposures to high levels of manganese in drinking water were implicated
as the probable cause. The symptoms in these case reports are similar to those in individuals with high
levels of exposure in manganese mining operations. Taken together, these studies provide added weight
to the evidence for the neurotoxic potential of excessive manganese in children, but one or more of the
following uncertainties preclude the characterization of causal and dose-response relationships between
the observed effects and manganese exposure: (1) whether or not the observed effects were solely due to
excess manganese alone or could have been influenced by other drinking water or dietary components;
(2) the lack of quantitative information about manganese levels from different environmental sources
(food, water, and air); and (3) the small sample sizes.
Respiratory Effects. Inhalation exposure to manganese dusts often leads to an inflammatory
response in the lungs of both humans and animals. This generally leads to an increased incidence of
cough and bronchitis and can lead to mild-to-moderate injury of lung tissue along with minor decreases in
lung function. In addition, susceptibility to infectious lung disease may be increased, leading to increased
pneumonitis and pneumonia in some manganese-exposed worker populations. These effects have been
reported primarily in workers exposed to fairly high concentrations of manganese dusts in the workplace,
although there are some data that indicate that, in populations living and attending school near
ferromanganese factories, there was an increased prevalence of respiratory effects. The risk of lung
injury in people exposed to the levels of manganese typically found in the general environment is
expected to be quite low. However, exposure to manganese-containing dusts from factories, mining
operations, automobile exhaust, or other sources may be of concern. It should be noted that these effects
on the lung are not unique to manganese-containing dusts but are produced by a variety of inhalable
particulate matter. On this basis, it seems most appropriate to evaluate the risk of inflammatory effects on
the lung in terms of total suspended particulate matter (TSP) or particulate matter <10 μm in diameter
(PM
10
), as well as the concentration of manganese in the air. Studies involving controlled inhalation
exposures in humans or animals to methylcyclopentadienyl manganese tricarbonyl (MMT), a gasoline
18 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
additive that improves combustion efficiency, are not available because the compound breaks down
readily in light to form inorganic manganese compounds. Rats exposed to high concentrations of car
exhaust containing oxidation products from MMT-containing fuel exhibited labored breathing.
Reproductive Effects. Impotence and loss of libido are common symptoms in male workers
afflicted with clinically identifiable signs of manganism. These symptoms could lead to reduced
reproductive success in men. Impaired fertility (measured as a decreased number of children/married
couple) has been observed in male workers exposed for 1–19 years to manganese dust (0.97 mg/m
3
) at
levels that did not produce frank manganism. This suggests that impaired sexual function in men may be
one of the earliest clinical manifestations of manganese toxicity, but no dose-response information is
available; therefore, it is not possible to define a threshold for this effect. Evidence obtained in laboratory
mammals indicates that exposure to high levels of manganese may adversely effect sperm quality,
produce decreased testicular weights, and impair development of the male reproductive tract.
No direct effect of manganese toxicity has been observed on fertility in women. Although many studies
in laboratory mammals have attempted to detect effects of manganese on female fertility, only one study
demonstrated the possibility that excess manganese exposure outside of pregnancy may impair future
fertility (decreased number of offspring).
Developmental Effects. There is evidence to suggest that children exposed to high levels of
manganese from environmental sources (airborne, drinking water, dietary) may develop a variety of
adverse developmental effects, particularly neurological effects (as discussed above). Many studies
suggest that children exposed to particularly high levels of manganese over a long period of time (months
or years) will eventually develop one or more symptoms, including general cognitive impairment,
diminished memory, attention deficit, motor impairments, aggressiveness, and/or hyperactivity.
However, it is not clear from any of these studies whether other factors, perhaps environmental or genetic,
are responsible for these changes in the presence of manganese, or whether manganese alone can produce
these effects.
A potentially serious developmental effect of manganese was suggested by the results of a study where
high infant mortality in a Bangladesh community was reported in conjunction with the presence of a local
drinking water supply containing high levels of manganese (concentration up to 8.31 mg/L). Infants
exposed to levels of manganese equal to or greater than those recommended by the World Health
Organization (WHO) were at the highest risk of mortality prior to 1 year of age. The nature of this
19 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
epidemiological study, with nutritional deficits in the population anticipated but not documented, prevents
a determination that manganese alone was responsible for the high rate of infant mortality.
Developmental studies involving the use of laboratory animals have detected subtle changes in growth;
(e.g., diminished body weight, in animals provided with relatively high doses of manganese). These
changes have been observed both when the animals were exposed while in utero or postpartum when the
animals have already been born.
2.3 MINIMAL RISK LEVELS (MRLs)
Estimates of exposure levels posing minimal risk to humans (MRLs) have been made for manganese. An
MRL is defined as an estimate of daily human exposure to a substance that is likely to be without an
appreciable risk of adverse effects (noncarcinogenic) over a specified duration of exposure. MRLs are
derived when reliable and sufficient data exist to identify the target organ(s) of effect or the most sensitive
health effect(s) for a specific duration within a given route of exposure. MRLs are based on
noncancerous health effects only and do not consider carcinogenic effects. MRLs can be derived for
acute, intermediate, and chronic duration exposures for inhalation and oral routes. Appropriate
methodology does not exist to develop MRLs for dermal exposure.
Although methods have been established to derive these levels (Barnes and Dourson 1988; EPA 1990),
uncertainties are associated with these techniques. Furthermore, ATSDR acknowledges additional
uncertainties inherent in the application of the procedures to derive less than lifetime MRLs. As an
example, acute inhalation MRLs may not be protective for health effects that are delayed in development
or are acquired following repeated acute insults, such as hypersensitivity reactions, asthma, or chronic
bronchitis. As these kinds of health effects data become available and methods to assess levels of
significant human exposure improve, these MRLs will be revised.
A User’s Guide has been provided at the end of this profile (see Appendix B). This guide should aid in
the interpretation of the tables and figures for Levels of Significant Exposure and the MRLs.
Inhalation MRLs for Inorganic Manganese
Acute and Intermediate Inhalation Exposure. MRL values were not derived for acute- or intermediate-
duration inhalation exposures to manganese. The available data on the toxicity of inhaled manganese
20 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
were considered inadequate for derivation of acute- or intermediate-duration inhalation MRLs. Data are
lacking on whether exposure to inhaled manganese across these durations has any significant adverse
effects on numerous end points including reports on developmental and reproductive effects.
Reports of human exposure at acute and intermediate durations (i.e., 15–364 days) indicate adverse
respiratory and neurological effects, but these reports consist of anecdotal case studies and lack
quantitative exposure values.
A few animal studies for these durations also evaluated respiratory effects in rodents and monkeys and
reported no-observed-adverse-effect levels (NOAELs). Inhalation of particulate manganese compounds
such as manganese dioxide or manganese tetroxide leads to an inflammatory response in the lungs of
animals, although inhalation of MnCl
2
did not cause lung inflammation in rabbits (Camner et al. 1985).
Several acute- and intermediate-duration studies in animals report various signs of lung inflammation
following periods ranging from 1 day to 10 months at manganese concentrations ranging from 0.7 to
69 mg/m
3
(Bergstrom 1977; Camner et al. 1985; Shiotsuka 1984; Suzuki et al. 1978; Ulrich et al. 1979a,
1979b). Bergstrom (1977) and Ulrich et al. (1979a, 1979b) determined NOAELs, which are reported in
the levels of significant exposure (LSE) table and figure. Increased susceptibility to lung infection by
bacterial pathogens following inhalation of manganese dusts has been noted in acute animal studies
(Maigetter et al. 1976). Conversely, Lloyd Davies (1946) reported no increase in the susceptibility of
manganese-treated mice to pneumococci or streptococci.
More recently, reversible inflammation (pleocellular inflammatory infiltrates and fibrinonecrotic debris)
in the nasal respiratory epithelium (but not the olfactory epithelium) was observed in young adult male
Crl:CD(SD)BR rats following 13 weeks of inhalation exposure to 0.5 mg manganese/m
3
as manganese
sulfate, but not in rats exposed to 0.1 mg manganese/m
3
as manganese sulfate or manganese phosphate
(hureaulite) (Dorman et al. 2004b). The lesions were not apparent in groups of rats assessed 45 days after
the end of exposure, indicating their transient nature. In studies with young male rhesus monkeys
exposed to 0, 0.06, 0.3, or 1.5 mg manganese/m
3
as manganese sulfate 6 hours/day, 5 days/week for
65 days, no nasal histological effects were found in exposed monkeys, but the high exposure level
induced lesions in the lower respiratory tract (mild subacute bronchiolitus, alveolar duct inflammation,
and proliferation of bronchus-associated lymphoid tissue) (Dorman et al. 2005b). The lower airway
lesions from intermediate-duration exposure appear to have been transient, because they were not found
in monkeys assessed 45 days after the end of exposure (Dorman et al. 2005b). These findings in rats and
21 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
monkeys are consistent with the understanding that inflammation of respiratory tissues from high-level
exposure to inhaled manganese particulates is likely a consequence of the inhaled particulate matter.
Bredow et al. (2007) reported that nose-only inhalation exposure to 2 mg manganese/m
3
as manganese
chloride aerosols 6 hours/day for 5 consecutive days did not cause lung lesions in female GVB/N mice,
but induced a 2-fold increase in pulmonary levels of mRNA for vascular endothelial growth factor
(VGEF), a regulator of proliferation, migration, and formation of new capillaries. Elevated levels of
VGEF have been associated with respiratory diseases, but current understanding is inadequate to
understand if this pulmonary gene expression response to manganese is adverse or benign.
There are limited evaluations of neurological end points in animals following intermediate-duration
inhalation exposure to manganese. Neurological effects comparable to those observed in humans have
been reported in monkeys exposed to manganese by parenteral routes (intravenous) for intermediate
duration (Newland and Weiss 1992), but no reports of the application of sensitive neurobehavioral test
batteries to animals following acute or intermediate-duration inhalation exposure to inorganic manganese
were located.
In monkeys exposed to manganese oxide aerosol concentrations as high as 1.1 mg manganese/m
3
24 hours/day for 9 months, no exposure-related effects on limb tremor or electromyograms were
observed, even though blood manganese levels were 5-fold higher in exposed compared with control
monkeys (Ulrich et al. 1979a, 1979b, 1979c). No gross signs of neurological impairment were observed
in rats exposed by the same protocol to manganese oxide aerosol concentrations as high as 1.1 mg
manganese/m
3
(Ulrich et al. 1979a, 1979b, 1979c).
More recent studies of monkeys exposed to concentrations up to 0, 0.06, 0.3, or 1.5 mg manganese/m
3
as
manganese sulfate 6 hours/day for 65 days reported: (1) no obvious signs of gross toxicity in the exposed
monkeys; (2) about 2-fold higher manganese concentrations in most brain regions at 1.5 mg
manganese/m
3
, except for the globus pallidus which showed manganese concentrations 6-fold greater
than control concentrations; and (3) a spectrum of exposure-related changes in biochemical markers of
neurotoxicity in various regions of the exposed monkeys, compared with control monkeys (Dorman et al.
2006a, 2006b; Erikson et al. 2007, 2008). No published accounts of the application of sensitive
neurobehavioral test batteries to these animals are available and there are no studies in monkeys reporting
NOAELs and lowest-observed-adverse-effect level (LOAELs) for neurological effects following chronic-
duration exposure.
22 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Increased locomotor activity has been observed in Sprague-Dawley rats exposed for 90 days (6 hours/day,
5 days/week) to a manganese phosphate/manganese sulfate mixture at concentrations ≥0.03 mg
manganese/m
3
(Salehi et al. 2003) and to manganese sulfate at concentrations ≥0.009 mg manganese/m
3
(Tapin et al. 2006), but this effect was not observed with exposure to hureaulite (manganese phosphate) at
aerosol concentrations as high as 1 mg manganese/m
3
(Normandin et al. 2002). Significant neuronal cell
loss in the globus pallidus and caudate putamen was also observed in Sprague-Dawley rats exposed for
90 day (6 hours/day, 5 days/week) to the manganese phosphate/manganese sulfate mixture at an aerosol
concentration of 3 mg manganese/m
3
; these changes, however, were not accompanied with signs of
tremor as assessed with electromyographic techniques (Salehi et al. 2006).
MRL values for acute or intermediate durations based on animal studies were not derived, because an
MRL based on animal data would be lower than the proposed chronic-duration inhalation MRL that is
based on effects observed in humans. It is uncertain if this is due to species differences in susceptibility
to the neurotoxic properties of inhaled manganese or to the testing of humans with sensitive
neurobehavioral tests that have not been applied to animals following inhalation exposures to manganese.
It is expected that the chronic MRL for inhaled inorganic manganese would provide protection for
intermediate-duration exposure scenarios. The MRL is based on an analysis of dose-response data for
subtle neurological deficits in occupationally exposed workers with durations of employment from about
5 to 24 years (see Appendix A); the average duration of employment in workers in the principal study was
5.3 years.
• An MRL of 0.0003 mg manganese/m
3
(manganese in respirable dust; 0.3 μg manganese/m
3
) has
been derived for chronic inhalation exposure (365 days or more) to manganese.
The study chosen to derive the MRL is from an investigation of an occupational cohort involving 92 male
workers in a dry alkaline battery plant (Roels et al. 1992). They and the 101 age- and area-matched
controls (with no industrial exposure to manganese) were observed for performance on a battery of
neurobehavioral tests. Manganese workers were exposed for an average (geometric mean) of 5.3 years
(range: 0.2–17.7 years) to a respirable dust concentration of 215 μg manganese/m
3
and a total dust
concentration of 948 μg manganese/m
3
. Manganese concentrations were measured with personal
samplers, with respirable dust being <5 microns in diameter. The authors noted that plant exposure
conditions had not changed considerably in the last 15 years, suggesting that past exposures were
consistent with those measured at the time of the study. Performance in measured neurobehavioral tests,
23 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
especially on measures of simple reaction time, eye-hand coordination, and hand steadiness, was
significantly worse in manganese-exposed workers than in the comparison group.
Manganese-exposed workers performed significantly worse than the controls on the neurobehavioral
tests, with particular differences in simple reaction time, eye-hand coordination, and hand steadiness.
Dr. Harry Roels provided the data on the manganese-exposed group evaluated in this study. These data
included individual exposure levels and whether the individual had an abnormal performance in the
neurobehavioral tests (scores below the 5
th
percentile score of the control group). Percent precision score
in the eye-hand coordination test was the most sensitive end point among the end points showing
statistically significantly elevated incidences of abnormal scores and was selected as the basis of the
MRL. Average exposure concentration for each worker was calculated by dividing the individual lifetime
integrated respirable concentration (LIRD; calculated by Dr. Roels from occupational histories and
measurements of workplace air manganese concentrations) by the individual’s total number of years
working in the factory. Individuals were grouped into six exposed groups and the control group, and the
average of the range in each group was used in benchmark dose (BMD) modeling of the incidence data
for number of workers with abnormal percent precision eye-hand coordination scores (see Table A-1 in
Appendix A).
Available dichotomous models in the EPA Benchmark Dose Software (BMDS version 1.4.1c) were fit to
the incidence data for abnormal eye-hand coordination scores in workers exposed to respirable
manganese (Roels et al. 1992, Table A-1). Results from the modeling using a benchmark dose response
of 10% are shown in Table A-2 in Appendix A. Based on the chi-square and Akaike’s Information
Criterion (AIC) measures of fit, all of the models provided adequate and comparable fits to the data (the
quantal linear and Weibull models had the same parameter values). BMCL
10
estimates from the different
models showed an approximate 2-fold range from 73 µg/m
3
from a one-stage multistage model to 142
µg/m
3
from the logistic model. The logistic model was indicated as the best fitting model by the AIC
measure (Table A-2) and was used to provide the point of departure (POD) for the MRL. Previous BMD
analyses of exposure data and incidence data for abnormal eye-hand coordination test scores from the
Roels et al. (1992) study used a quantal linear model to arrive at a BMCL
10
value of about 74 µg
respirable manganese/m
3
(Agency for Toxic Substances and Disease Registry 2000; EPA 1994c; WHO
2001). This value is virtually the same as the BMCL
10
of 73.2 µg manganese/m
3
obtained from the
equivalent multistage model in the current analysis (Table A-2).
24 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
The MRL of 0.3 µg manganese/m
3
was derived by adjusting the POD to a continuous exposure basis
(142 µg manganese/m
3
x 5/7 x 8/24) and dividing by an uncertainty factor of 100.
An uncertainty factor of 10 was used for uncertainty about human variability including possibly enhanced
susceptibility of the elderly, infants, and children; individuals with chronic liver disease or diminished
hepatobiliary function; and females and individuals with iron deficiency. The current assessment does
not use an additional modifying factor of 5 for potentially increased susceptibility in children based on
differential kinetics in the young (which was used in the Agency for Toxic Substances and Disease
Registry [2000] assessment), because recent toxicokinetic studies in lactating rats and their offspring
exposed to manganese by the oral or inhalation routes suggest that the human variability factor of 10
provides sufficient protection for differential kinetics in children and adults. For example, in neonatal rats
orally exposed to 25 or 50 mg manganese/kg/day (as manganese chloride) from postnatal day (PND) 1
through 21, manganese concentrations in various brain regions were about 2-fold higher than brain
manganese concentrations in adult rats exposed to the same oral dose levels for 21 days (Dorman et al.
2000). Similarly, 18-day-old neonatal rats exposed from birth to aerosols of manganese sulfate at 1 mg
manganese/m
3
, 6 hours/day showed a 2.6-fold increase in striatum manganese concentrations, compared
with controls, while lactating adults exposed to the same aerosol concentration showed a 1.7-fold increase
compared with controls (Dorman et al. 2005a). Likewise, simulations with physiologically based
pharmacokinetic (PBPK) models for inhaled manganese in lactating rat dams and offspring indicate that
manganese concentrations in the striatum and olfactory bulb of the brains of PND 19 offspring begin to
increase when air concentrations exceed 50–100 µg manganese/m
3
, whereas maternal concentrations
begin to increase at air concentrations between 100 and 300 µg manganese/m
3
(Yoon et al. 2009b). These
results indicate that at air concentrations above about 0.05–0.1 mg/m
3
, brain concentrations in neonates
may be elevated, compared with controls, to a greater degree than in lactating dams, but the age-specific
difference in the tested air concentration range does not appear to be large. Simulations from a human
PBPK model for inhaled manganese in lactating mothers and their offspring indicate that average daily
areas under the curve (AUCs) for manganese concentrations in the globus pallidus of the fetus, suckling
neonate, and 3-year-old child from manganese air concentrations increased beyond 10% of background
concentrations in fetuses and 3-year-old children when air concentrations exceeded 0.01 mg/m
3
(10 µg/m
3
) and in suckling neonates when air concentrations exceeded 0.001 mg/m
3
(1 µg/m
3
) (Yoon et
al. 2011). Thus, the inhalation MRL derived herein, 0.3 µg/m
3
, is below the air concentrations at which
brain concentrations in human fetuses (10 µg/m
3
) and nursing infants (1 µg/m
3
) are predicted to begin to
rise under normal dietary manganese exposure conditions.
25 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
An uncertainty factor of 10 was applied for limitations/uncertainties in the database including the lack of
epidemiological data for humans chronically exposed to soluble forms of manganese and the concern that
the general population may be exposed to more soluble forms of manganese than most of the manganese-
exposed workers in the principal and supporting studies. Evidence from several rat studies indicate that
inhalation of more soluble forms of manganese (e.g., manganese sulfate and manganese chloride) results
in higher brain manganese concentrations in brains than inhalation of less soluble forms, such as
manganese phosphate, manganese tetroxide or manganese dioxide (Dorman et al. 2004a, 2001a; Roels et
al. 1997). In addition, data on developmental toxicity for this route and duration of exposure are lacking.
There is limited information on reproductive effects in females (one study in rat dams) and reported
effects on male reproductive organs have not been clearly associated with decreased reproductive
function. Though it is clear that the neurological system is the most sensitive identified target organ for
effects from sub-chronic to chronic-duration inhalation exposure to manganese, data are lacking to fully
characterize the potential risk for all organ systems from chronic inhalation exposure.
Several BMD analyses of results from other epidemiological studies of neurobehavioral end points in
manganese-exposed workers provide support for the MRL (Clewell and Crump 1999; Clewell et al. 2003;
Health Canada 2010). Estimated BMCL
10
values in these analyses were within an approximate 2–4-fold
range of the POD (142 µg manganese/m
3
) selected for the chronic inhalation MRL herein.
Dr. Anders Iregren provided ATSDR with individual worker data on total dust manganese exposure and
performance on neurobehavioral tests for the occupational cohort that participated in his study (Iregren
1990; Wennberg et al. 1991). A BMD analysis was performed with these data under contract with
ATSDR (Clewell and Crump 1999) and the lowest BMCL
10
value among the end points analyzed was
0.07 mg respirable manganese/m
3
for a 10% change in simple reaction time. The BMD analysis applied
K-power and Weibull models to continuous variable data (from 11 different test scores collected by Dr.
Iregren) using current respirable manganese exposure estimates, age, and vocabulary test results as
explanatory variables, an assumption that 5% of unexposed subjects had adverse responses, and a
benchmark response of 10% change from unexposed mean scores. For each dataset, BMCL
10
values from
the Weibull model were lower (by 2–3-fold at the most) than BMCL
10
values from the K-Power model.
Weibull BMCL
10
values for the different test score datasets ranged from 0.07 to 0.67 mg respirable
manganese/m
3
. Thus, the lowest BMCL
10
value from this analysis of test score data from manganese-
exposed workers collected by Iregren (1990; Wennberg et al. 1991) is within a 2-fold range of the
selected POD of 142 µg manganese/m
3
for the MRL.
26 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Clewell et al. (2003) conducted BMD analyses on data from three neuromotor tests in the Roels et al.
(1992) study (visual reaction time, eye-hand coordination, and hand steadiness) and from five neuromotor
tests in the Gibbs et al. (1999) study (hole 6 of the hand steadiness test, percent precision of the eye-hand
coordination test, reaction time in the complex reaction test, root mean square amplitude in the steady
test, and tap time). Exposure measures in these analyses were recent measures of manganese
concentrations in respirable dust. BMCL
10
values were 0.257, 0.099, and 0.202 mg manganese/m
3
for the
visual reaction time, eye-hand coordination, and hand steadiness data from the Roels et al. (1992) study;
these results were obtained after fitting incidence data for abnormal scores in these tests to a Weibull
model for dichotomous data. The reported BMCL
10
value of 0.099 mg manganese/m
3
for the eye-hand
coordination test is similar to the BMCL
10
value of 0.091 mg manganese/m
3
obtained with the Weibull
model in the current ATSDR analysis (Table A-2). BMCL
10
values from the analyses of outcomes from
the Gibbs et al. (1999) study ranged from 0.09 to 0.27 mg manganese/m
3
(averaging the BMCLs within
end points across different BMD models applied to the data). Clewell et al. (2003) did not have
individual worker data from the Iregren (1990) or Mergler et al. (1994) studies, but based on some
assumptions about exposures (e.g., all workers were exposed to average concentrations for the facilities
and respirable manganese concentrations were calculated for the workers in the Iregren [1990] study
based on an assumption that 50% of total dust manganese was respirable), they calculated BMCL
10
values
for six end points from the Mergler et al. (1994) study and the simple reaction time end point in the
Iregren (1990) study. BMCL
10
values ranged from about 0.1 to 0.3 mg manganese/m
3
from the Mergler et
al. (1994) study end points to 0.1 mg manganese/m
3
for the reaction time end point in the Iregren (1990)
study.
Health Canada (2010) published a human health risk assessment for inhaled manganese in which BMD
analyses were conducted on data for neurobehavioral end points from the study of manganese alloy
workers by Lucchini et al. (1999). Dose-response data for six tests of fine motor control, two aspects of
memory tests, one test of mental arithmetic, and measured serum prolactin levels were fit to linear models
using exposure metrics based on an average overall occupational history (ARE) or an average over the
latest 5 years of occupation (ARE5). Using a linear model, BMCL
10
values for the various end points
were 32–59 and 85–98 µg manganese/m
3
for the ARE5 and ARE exposure metrics, respectively.
Regardless of exposure metric, the values are within an approximate 2–4-fold range of the selected POD
of 142 µg manganese/m
3
, based on eye-hand coordination test scores in workers in the Roels et al. (1992)
study.
27 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Neurological effects from repeated inhalation exposure to manganese are well recognized as effects of
high concern based on case reports and epidemiological studies of groups of occupationally exposed
workers. A number of epidemiological studies have used batteries of neurobehavioral tests of
neuromotor, cognition, and mood states to study the psychological or neurological effects of exposure to
low levels of manganese in the workplace (Bast-Pettersen et al. 2004; Beuter et al. 1999; Blond and
Netterstrom 2007; Blond et al. 2007; Bouchard et al. 2003, 2005, 2007a, 2007b; Chia et al. 1993a, 1995;
Crump and Rousseau 1999; Deschamps et al. 2001; Gibbs et al. 1999, Iregren 1990; Lucchini et al. 1995,
1999; Mergler et al. 1994; Myers et al. 2003a, 2003b; Roels et al. 1987a, 1992, 1999; Summers et al.
2011; Wennberg et al. 1991). Some of these studies found statistically significant differences between
exposed and non-exposed groups or significant associations between exposure indices and neurological
effects (Bast-Pettersen et al. 2004; Chia et al. 1993a; Iregren 1990; Lucchini et al. 1995, 1999; Mergler et
al. 1994; Roels et al. 1987a, 1992; Wennberg et al. 1991), whereas others have not found significant
associations (Deschamps et al. 2001; Gibbs et al. 1999; Myers et al. 2003a, 2003b; Summers et al. 2011;
Young et al. 2005). Table A-3 in Appendix A summarizes results from these studies. The neurological
effects associated with prolonged low-level manganese exposure generally have been subtle changes
including deficits in tests of neuromotor or cognitive functions and altered mood states; they have been
referred to by various authors as preclinical or subclinical neurological effects. Manganese air
concentrations associated with these effects in chronically exposed workers range from about 0.07 to
1.59 mg manganese/m
3
(manganese in total or inhalable dust measurements; values for manganese in
respirable dust are noted in parentheses in Table A-3). Comparison of the effect levels in these studies
provides support for selection of the Roels et al. (1992) as the basis of the MRL. The advantage of the
Roels et al. (1992) study is that individual worker data were available to support a BMD analysis, but
BMD analyses of other epidemiological data for performance on tests of neurobehavior provided
potential PODs within about 2–4-fold of the POD selected as the basis of the MRL.
Studies in communities surrounding manganese industries have also reported evidence for associations
between deficits in neurological end points (such as attention impairments, postural stability, and motor
impairments) and increasing biomarkers of manganese exposure in adults and children, but all potential
sources of exposure (e.g., air, diet, drinking water) could not be accounted for in these studies and they do
not provide useful dose-response data for deriving an MRL for inhaled manganese (Baldwin et al. 1999;
Beuter et al. 1999; Bowler et al. 1999; Hernández-Bonilla et al. 2011; Kim et al. 2011; Menezes-Filho et
al. 2011; Mergler et al. 1999; Solís-Vivano et al. 2009; Standridge et al. 2008; Riojas-Rodríguez et al.
2010; Rodríguez-Agudelo et al. 2006).
28 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Oral MRLs for Inorganic Manganese
Overview. No oral MRLs were derived for acute-, intermediate-, or chronic-duration oral exposure to
manganese, even though the limited human data and extensive animal data clearly identify
neurobehavioral changes as the most sensitive effect from intermediate- and chronic-duration oral
exposure to excess inorganic manganese. However, inconsistencies in the dose-response relationship
information across studies evaluating different neurological end points under different experimental
conditions in different species, as well as a lack of information concerning all intakes of manganese
(e.g., dietary intakes plus administered doses), make it difficult to derive intermediate- or chronic-
duration MRLs using standard MRL derivation methodology from the human or animal studies. New
reports of neurobehavioral effects in children associated with elevated concentrations of manganese in
drinking water were evaluated as the possible basis of an oral MRL for intermediate and/or chronic
durations of exposure. However, the data were assessed to be unsuitable for MRL derivation due to
uncertainties about other possible confounding exposures to neurotoxic agents in the drinking water or via
food and/or the lack of information about dietary intakes of manganese by the children. An interim
guidance value of 0.16 mg manganese/kg/day, based on the Tolerable Upper Intake Level for 70 kg adults
of 11 mg manganese/day (established by the U.S. FNB/IOM [2001]) is recommended to be used for
ATSDR public health assessments of oral exposure to inorganic forms of manganese.
Acute Oral Exposure. Although neurological effects are expected to be the most sensitive end points
based on epidemiological studies in humans (see Section 3.1), only two acute studies reported
neurological end points in rodents. Moreno et al. (2009) administered 0, 4.4, or 13.1 mg manganese/
kg/day (as manganese chloride) via gavage for 2 weeks to juvenile C57Bl/6 mice. Increased novelty
seeking behavior in an open arena was reported in males exposed to 4.4 or 13.1 mg/kg/day (time in center
increased 10 and 8%, respectively; 8–10 animals/group). These data identify a free-standing LOAEL of
4.4 mg/kg/day for behavioral alterations; however, the response did not increase with increasing dose.
Additionally, mice receiving 13.1 mg/kg/day had significantly increased concentrations of dopamine,
decreased concentration of its metabolite dihydroxyphenylacetic acid (DOPAC), and increased
concentration of the serotonin metabolite 5-hydroxyindolacetic acid (5-HIAA) in the stratum compared
with control mice (altered 60, 20, and 68%, respectively; 3–4 mice/group). Additionally, Shukakidze et
al. (2003) reported that a single dose of 50 mg manganese chloride/kg (13.9 mg manganese/kg) to a group
of 10 white rats caused worsened acquisition of an avoidance reaction in response to unconditioned and
condition stimuli, increased latent period of a conditioned reflex activity, and increased numbers of errors
29 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
and time taken to navigate a maze (compared with controls), beginning on day 5 after dose administration
and lasting until days 10–15.
Other acute-duration oral studies found only decreased liver and body weight and decreased leukocyte
and neutrophil counts in rats at dietary doses of 1,300 mg manganese/kg/day and no effects in mice at
dietary doses up to 2,600 (males) or 3,900 (females) mg manganese/kg/day after 14 days of exposure to
manganese sulfate in the diet (NTP 1993). No signs of developmental or maternal toxicity were observed
in a standard developmental toxicity study of pregnant rats given daily gavage doses of 2,200 mg
manganese/kg/day as manganese chloride on gestation days (GDs) 6–17 (Grant et al. 1997a). With
intermediate-duration, no exposure-related effects on fetal body weight or skeletal development or
anomalies were found in pregnant rabbits exposed to 33 mg manganese/kg/day on GDs 6–20, but some
evidence for delayed fetal skeletal development was found in pregnant Sprague-Dawley rats exposed to
the same dose of manganese chloride on GDs 0–21 (Szakmáry et al. 1995).
Of the acute studies, the lowest LOAEL identified was 4.4 mg manganese/kg/day for decreased increased
novelty-seeking behavior in an open field in male juvenile C57Bl/6 mice exposed for 2 weeks by gavage
(Moreno et al. 2009). If this was used as the POD for the intermediate-duration oral MRL, a value of
0.004 mg manganese/kg/day would be derived if an uncertainty factor of 1,000 was used (10 for use of a
LOAEL, 10 for extrapolating across species, and 10 for human variability). However, this rodent-based
value of 0.004 mg manganese/kg/day would be 7.5-fold below the FNB/IOM (2001) recommended AI of
1.8 and 2.3 mg manganese/day for women and men, respectively (approximately 0.03 mg
manganese/kg/day) and 40-fold below the FNB/IOM (2001) recommended Tolerable Upper Intake Level
(UL) of 11 mg/day for adults ≥19 years of age (approximately 0.16 mg manganese/kg/day). Part of the
apparent discrepancy between this prospective MRL and the recommended dietary intakes is that the
MRL is based only on manganese intakes above the normal dietary intakes. Unfortunately, the dietary
intakes of manganese by the rats in the Moreno et al. study (2009) cannot be estimated from the
information provided in the published report.
Intermediate Oral Exposure. With intermediate-duration oral exposure, effects on neurobehavior are
expected to be the most sensitive effects from excessive manganese, particularly during early
developmental periods, based on findings for subtle neurobehavioral effects in epidemiological studies on
manganese-exposed workers (see Section 3.1), higher brain manganese levels and altered brain dopamine
levels in neonatal rats, compared with adult rats, due to immaturity of the blood-brain barrier and the lack
of biliary excretion in preweanling rats (Aschner et al. 2005; Dorman et al. 2000, 2005a; Kontur and
30 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Fechter 1985, 1988), and results from studies of the effects of intermediate-duration oral exposure on
systemic toxicity end points and neurobehavioral, neurochemical, and neurodevelopmental end points in
adult and young laboratory animals (Anderson et al. 2007a, 2009; Avila et al. 2008; Calibresi et al. 2001;
Kern and Smith 2011; Kern et al. 2010; Moreno et al. 2009; Reichel et al. 2006; Tran et al. 2002a,
2002b).
The discussion that follows provides evidence that, while systemic effects of manganese are not typically
the most sensitive end point of action, some evidence exists to support adverse cardiovascular effects of
manganese at relatively low dose levels, followed by a review of the large number of studies that most
consistently support neurobehavior effects as the most sensitive effects from excessive oral manganese
exposure.
In standard toxicity studies of intermediate-duration oral exposure to inorganic manganese, marginal
evidence for systemic toxicity was found in rats at doses ≥33 mg manganese/kg/day (increased neutrophil
count and decreased liver weight in males; decreased body weights at higher doses) and in mice at the
highest administered dose of 1,950 mg manganese/kg/day (decreased hemoglobin, mild hyperplasia of
forestomach, decreased liver and body weight) (NTP 1993). Corroborative evidence comes from reports
of decreased red blood cell counts and body weight in mice following 100 days of dietary exposure to one
of several forms of inorganic manganese (manganese acetate, carbonate, oxide, or chloride) at a dose
level of 284 mg manganese/kg/day (Komura and Sakamoto 1991).
However, other animal studies indicate that excessive oral intake of manganese may present a
cardiovascular hazard. Under magnesium deficiency conditions (4.1 mmol Mg/kg diet), swine fed
moderately elevated levels of manganese (about 500 mg manganese/kg diet) died suddenly within
5 weeks and showed necrosis and mineralization of the heart (Miller et al. 2000). This finding was
supported with subsequent findings of myocardial necrosis and mitochondrial swelling in magnesium-
deficient pigs fed a diet high in manganese (500 mg manganese/kg diet) for 8 weeks (Miller et al. 2004)
and of depressed heart muscle mitochondrial O
2
consumption and decreased red blood cells in rats
consuming a high manganese diet (250 mg manganese/kg diet) under marginal magnesium dietary
conditions; the manganese-induced effects on hematological end points in rats were absent when adequate
dietary magnesium was provided (Miller et al. 2006). In another study involving rats supplied with
adequate and excessive Mn in the diet (10–15 and 45–50 mg manganese/kg diet), aortas from rats with
excessive dietary manganese showed less expression and sulfation of heparin sulfate glycosaminoglycans,
compared with the adequate condition (Kalea et al. 2006). The results from these studies suggest that
31 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
excessive intermediate-duration oral intake of manganese may present a cardiovascular hazard, especially
under magnesium-deficient dietary conditions, but their use as the basis of an intermediate-duration oral
MRL for inorganic manganese is limited due to the lack of reported information to accurately calculate
daily intakes. Myocardial lesions were not found in rats or mice provided manganese sulfate in the diet
for 2 years at dose levels up to 232 or 731 mg manganese/kg/day, respectively (NTP 1993).
Numerous studies support the sensitivity of neurobehavioral end points to intermediate-duration oral
doses of manganese. In humans and nonhuman primates exposed orally for intermediate durations,
neurobehavioral end points have been examined in healthy adult female subjects given low (0.01 mg
manganese/kg/day) or high (0.3 mg manganese/kg/day) manganese diets for 8 weeks (Finley et al. 2003)
and in infant monkeys fed either a commercial cow’s milk formula (17.5 mg manganese/kg/day), a
commercial soy formula (107.5 mg manganese/kg/day), or a soy formula with added magnesium chloride
(328 mg manganese/kg/day) for 4 months with monkeys tested through 18 months of age (Golub et al.
2005). No differences between the low and high dietary-intake states were found in the adult females on
scores for hand-steadiness and self-reported traits such as assertiveness and anger (Finley et al. 2003).
Monkeys provided the highest manganese dose level showed no marked differences from the cow’s milk
controls in gross motor maturation, growth, cerebrospinal fluid levels of dopamine or serotonin
metabolites, or performance on tests of cognitive end points, but showed decreased activity during sleep
at 4 months and decreased play activity between 1 and 1.5 months. These results suggest that daily
intakes of 328 mg manganese/kg/day (but not 107.5 mg manganese/kg/day) during neonatal periods may
cause subtle neurobehavioral changes in primates.
In neurobehavioral assessments of rodents orally exposed to inorganic manganese for intermediate
durations during neonatal periods, subtle neurobehavioral effects have been observed at supplemental
dose levels as low as about 10–20 mg manganese/kg/day (Brenneman et al. 1999; Dorman et al. 2000;
Kern et al. 2010; Kristensson et al. 1986; Moreno et al. 2009; Pappas et al. 1997; Reichel et al. 2006; Tran
et al. 2002a, 2002b). Although there are some inconsistencies in the results obtained in these studies
(e.g., Brenneman et al. [1999] found increased motor activity with exposure to 22 mg manganese/kg/day
after exposure on PNDs 1–49, but Dorman et al. [2000] found no effects of the same dose level on motor
activity after exposure on PNDs 1–21), the weight of evidence suggests that subtle neurobehavioral
effects can occur in rats with intermediate-duration neonatal exposures at doses ≥10–20 mg
manganese/kg/day.
32 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
Findings for histopathological changes in the rat brain following intermediate-duration oral exposure to
inorganic manganese during neonatal periods are less consistent than the findings for subtle
neurobehavioral effects. Chandra and Shukla (1978) reported neuronal degeneration in cortical and
cerebellar sections from the brains of young rats orally exposed to 0.3 mg manganese/kg/day as
manganese chloride between PND 21 and 51. In contrast, Kristensson et al. (1986) reported no adverse
histological changes in cerebellum or hippocampus in rats exposed to a much higher dose level of
manganese chloride (150 mg manganese/kg/day) between PND 3 and 44. Pappas et al. (1997) reported a
decreased cortical thickness in the offspring of rat dams exposed to 120 or 650 mg manganese/kg/day
from GD 1 through PND 30, but found no immunohistological evidence for increased glial fibrillary
acidic protein in the cortex, caudate, or hippocampus. Dorman et al. (2000) reported that no adverse
histological changes were found in sections of the following brain regions in Sprague-Dawley rats
exposed to 11 or 22 mg manganese/kg/day on PNDs 1–21: olfactory bulbs, cerebral cortex,
hippocampus, basal ganglia, thalamus, hypothalamus, midbrain, and cerebellum. However, Lazrishvilli et
al. (2009) reported neuronal damage in small proportion of cells (7–10%) and marked gliosis throughout
the brain in the offspring of rat dams exposed to 10 mg manganese/kg/day in feed for 15–20 days before
pregnancy, during pregnancy, and for 1 month after parturition. The weight of evidence from these
studies indicates that subtle neurobehavioral effects in neonatally exposed rats are not consistently
associated with histological changes in the brain.
Neurobehavioral effects have also been observed in adult rats orally exposed to inorganic manganese for
intermediate durations. In several studies, doses inducing these effects were higher than those inducing
subtle neurobehavioral effects after neonatal exposure (Calabresi et al. 2001; Centonze et al. 2001;
Torrente et al. 2005), but in two other studies, neurobehavioral effects were observed at doses as low as
5.6 mg manganese/kg/day (Shukakidze et al. 2003) and 6.5 mg manganese/kg/day (Vezér et al. 2005,
2007). Increased open field activity, increased interest in a novel object, and increased signs of fear were
observed in adult male Wistar rats exposed to drinking water containing 20 mg manganese chloride/L for
10 weeks (estimated doses of 1,310 mg manganese/kg/day), but no effects on radial maze performance,
numbers of neuronal cells or levels of glial fibrillary acidic protein in striatum, or intrinsic
electrophysiological membrane properties of striatal neurons with the exception of a manganese-induced
increase in the frequency and amplitude of spontaneous excitatory postsynaptic potentials (Calabresi et al.
2001; Centonze et al. 2001). In an earlier study of adult male Wistar rats exposed to 20 mg manganese
chloride/L for 13 weeks, no neuronal loss or gliosis was evident in the globus pallidus by either
histological or immunohistochemical examination (Spadoni et al. 2000). Decreased open field activity
and impaired spatial learning were observed in restraint stressed adult male Sprague-Dawley rats exposed
33 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
to 153 mg manganese/kg/day (but not 76 mg manganese/kg/day) as manganese chloride in drinking water
for 19 weeks (Torrente et al. 2005). Similarly, decreased locomotor activity, as well as decreased tongue
protrusion frequency (orofacial dyskinesia measure), were reported in adult Wister rats exposed to
1,280 mg manganese/kg/day (as manganese chloride in drinking water) for 30 days (Avila et al. 2008).
No changes in motor activity or performance in a passive avoidance test were observed in adult male
Sprague-Dawley rats exposed to 11 or 22 mg manganese/kg/day for 21 days; these doses induced
increased pulse-elicited acoustic startle response with neonatal exposure, but exposure during adulthood
did not (Dorman et al. 2000). The lowest intermediate-duration daily dose associated with
neurobehavioral effects in adult rats is 5.6 mg manganese/kg/day for severely impaired cognitive
performance in a maze test following a 30-day exposure of white rats to manganese chloride in the diet
(strain not otherwise indicated) (Shukakidze et al. 2003). In another study, decreased open-field
locomotor activity and acoustic startle response and impaired performance in maze learning (a test of
spatial memory) were observed in male adult Wistar rats exposed to gavage doses of 6.5 or 25.9 mg
manganese/kg/day for 10 weeks, compared with controls (Vezér et al. 2005, 2007). Decreased acoustic
startle response and impaired spatial memory were still evident in exposed rats, compared with controls,
after 5–7 weeks without exposure (Vezér et al. 2005, 2007). The only intermediate-duration study in
mice reported no changes in open field activity following adult exposure up to 13.1 mg/kg/day (as
manganese chloride) via gavage for 8 weeks (Moreno et al. 2009). However, if adults were previously
exposed as juveniles (PNDs 20–34), subsequent exposure in males (but not females) at 4.4 mg/kg/day for
8 weeks resulted in decreased novelty seeking behavior in the open field. Additionally, at 13.1 mg/kg/
day, total overall movement in the open field was decreased in males.
Several types of reproductive effects have been reported for manganese. A study by Hafeman et al.
(2007) reported a high mortality rate among infants <1 year of age in a Bangladesh community where
manganese levels in drinking water were high, but the actual association between the manganese levels in
drinking water and infant mortality is difficult to make with certainty. The average level of manganese
intake was calculated to be 0.26 mg manganese/kg/day. Similarly, Spangler and Spangler (2009) reported
that with every log increase in groundwater manganese concentration in North Carolina counties, there
was a 2.074 increase in county level infant deaths per 1,000 live births. Other reproductive effects
reported for manganese in intermediate-duration animal studies include 25% decreased pregnancy rate in
Long-Evans rats (males and females) exposed to manganese oxide in the diet at 180 mg manganese/
kg/day (but not 55 mg manganese/kg/day) for 100–224 days (Laskey et al. 1982), increased incidence of
testicular degeneration in male Sprague-Dawley rats exposed to manganese acetate at gavage doses of
137 (but not 69) mg manganese/kg/day for 63 days (Ponnapakkam et al. 2003c), and delayed growth of
34 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
testes and sex accessory glands in CD-1 mice exposed to manganese oxide in the diet at 205 mg
manganese/kg/day (Gray and Laskey 1980). In Swiss mice exposed for 12 weeks to manganese chloride
in drinking water, impaired fertility was observed in males at 309 mg manganese/kg/day (but not a
154 mg manganese/kg/day) and in females at 277 mg manganese/kg/day (Elbetieha et al. 2001).
Decreased sperm motility and sperm counts were observed in CD-1 mice exposed to 4.8 or 9.6 mg
manganese/kg/day as manganese acetate, but no effects on the ability of exposed males to impregnate
unexposed female mice were found at these doses (Ponnapakkam et al. 2003a). The results from the
intermediate-duration animal studies suggest that oral exposure to manganese may produce adverse
effects on reproduction, but at much higher doses than those inducing subtle neurobehavioral effects in
adult or neonatal rats.
In summary, results from animal studies identify subtle neurobehavioral effects as the critical effect in
rodents from intermediate-duration oral exposure to inorganic manganese. Potential points of departure
for an intermediate-duration oral MRL include LOAEL values of 5.6 mg manganese/kg/day for severely
impaired cognitive performance in a maze test following 30-day dietary exposure of adult white rats
(Shukakidze et al. 2003); 6.5 mg manganese/kg/day for decreased open-field locomotor activity and
acoustic startle response and impaired performance in maze learning (a test of spatial memory) in male
adult Wistar rats exposed for 10 weeks by gavage (Vezér et al. 2005, 2007); and 11 mg manganese/
kg/day for increased pulse-initiated acoustic startle response in Sprague-Dawley rats exposed (orally by
pipette) on PNDs 1–21 (Dorman et al. 2000). In contrast, hand steadiness or self-reported scales for
assertiveness or anger were not different in adult female subjects following 8 weeks of exposure to dietary
doses of 0.01 or 0.3 mg manganese/kg/day (Finley et al. 2003). In young monkeys, decreased activity
during sleep at 4 months and decreased play activity between 1 and 1.5 months were observed following
daily intakes of 328 mg manganese/kg/day (but not 107.5 mg manganese/kg/day), but no effects on gross
motor maturation or performance in cognitive tests were observed at either dose level compared with
controls (Golub et al. 2005).
The effects noted in the rat study by Shukakidze et al. (2003) are much more severe than effects noted in
adult rats at reportedly higher dose levels of 1,310 mg manganese/kg/day (Calabresi et al. 2001; Centonze
et al. 2001) or 153 mg manganese/kg/day (Torrente et al. 2005) or in adult rats at comparable reported
doses of 6.5 mg manganese/kg/day (Vezér et al. 2005, 2007). Shukakidze et al. (2003) reported that the
exposed rats “showed increased aggresivity, frequently fell from the platform in the maze, and were
unable to perform the maze test.” Because the reporting of the experimental conditions in the Shukakidze
et al. (2003) study is sparse and the severity of effects is so unusual, the results are considered to be
35 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
outlying results that are not consistent with the rest of the database and not appropriate as the basis of an
MRL.
If the LOAEL of 6.5 mg manganese/kg/day for decreased open-field locomotor activity and acoustic
startle response and impaired performance in maze learning in male adult Wistar rats exposed for
10 weeks by gavage (Vezér et al. 2005, 2007) was used as the POD for the intermediate-duration oral
MRL, a value of 0.007 mg manganese/kg/day would be derived if an uncertainty factor of 1,000 were
used (10 for use of a LOAEL, 10 for extrapolating across species, and 10 for human variability).
However, this rodent-based value of 0.007 mg manganese/kg/day would be about 4-fold below the
FNB/IOM (2001) recommended AI of 1.8 and 2.3 mg manganese/day for women and men, respectively
(approximately 0.03 mg manganese/kg/day) and about 23-fold below the FNB/IOM (2001) recommended
UL of 11 mg/day for adults ≥19 years of age (approximately 0.16 mg manganese/kg/day). Part of the
apparent discrepancy between this prospective MRL and the recommended dietary intakes is that the
MRL is based only on manganese intakes above the normal dietary intakes. Unfortunately, the dietary
intakes of manganese by the rats in the Vezér et al. study (2005, 2007) cannot be estimated from the
information provided in the published report.
Alternatively, using the monkey NOAEL of 107 mg manganese/kg/day for decreased activity during
sleep at 4 months and decreased play activity between 1 and 1.5 months in formula-fed infant monkeys
provided soy-based formula from birth to 4 months of age (Golub et al. 2005), a value of 1 mg
manganese/kg/day would be derived if an uncertainty factor of 100 were used (10 for extrapolating across
species and 10 for human variability). The monkey-based value would be about 6-fold higher than the
FNB/IOM (2001) UL of 11 mg manganese/day for adults (0.16 mg manganese/kg/day assuming a 70-kg
body weight). The formulas fed to the infant monkeys in this study are expected to have been the
principal source of manganese.
For children and adolescents, FNB/IOM (2001) scaled the adult UL values according to reference body
weights for children and adolescents, noting that there were no reports of manganese toxicity in children
and adolescents and that it was not possible to establish UL values for infants (0–12 months).
Based on several surveys, FNB/IOM (2001) reported that average intakes of adults with typical “Western-
type” and vegetarian diets ranged from 0.7 to 10.9 mg/day (0.01–0.156 mg manganese/kg/day, assuming
a 70-kg body weight). WHO (2004b) recently calculated an estimated daily intake of about 0.0003 mg
36 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
manganese/kg/day for 70-kg subjects drinking 2 L of water per day at a concentration of 0.010 mg
manganese/L, the median of a survey of manganese in drinking water.
Chronic Oral Exposure. Data on the effects of manganese following chronic oral exposure are less
extensive than intermediate-duration data, but these reports do suggest that neurological effects similar to
those seen after intermediate-duration exposure may be anticipated following chronic oral exposure to
excess manganese. In the reports of neurological effects in humans following chronic oral exposure,
there is either uncertainty regarding the exposure level (He et al. 1994; Zhang et al. 1995) or uncertainty
that the effects observed were solely attributable to manganese (Bouchard et al. 2007c, 2011; Holzgraefe
et al. 1986; Kawamura et al. 1941; Kilburn 1987; Kondakis et al. 1989; Wasserman et al. 2006, 2011;
Wright et al. 2006). There is also no clear understanding of the threshold for manganese
deficiency/sufficiency or toxicity. Males consuming 0.35 and 0.11 mg manganese/day exhibited
symptoms of manganese deficiency (Doisy 1973; Friedman et al. 1987, respectively). But Davis and
Greger (1992) did not report any deficiency symptoms among female subjects, 20% of whom consumed
<1 mg manganese/day, and Finley et al. (2003) did not observe signs of manganese deficiency or toxicity
in adult females with dietary intakes of 0.8 or 20 mg manganese/day for 8 weeks. Authors of a case study
suspected abuse of vitamin and mineral preparations to be the source for excess manganese and
neurological symptoms observed in their patient (Banta and Markesbery 1977).
Four epidemiological reports of manganese neurotoxicity in children resulting from manganese exposure
in drinking water have been recently published. In two separate cross-sectional studies, Wasserman et al.
(2006, 2011) reported statistically significant relationships for decreasing intelligence scores with
increasing manganese levels in drinking water in 142–151 children (ages 8–11 years) in Bangladesh.
Similarly, in a cross-sectional study conducted by Bouchard et al. (2011), a significant negative
association was found between manganese levels in the home tap water and intelligence scores in
362 children from Quebec, Canada. In previous study by Bouchard et al. (2007c), a statistically
significant relationship between increased levels of oppositional behaviors and hyperactivity and
increased levels of manganese in drinking water in an epidemiological study of 46 children (ages 6–
15 years), also from Quebec, Canada.
Additionally, three recent case studies suggest that certain children are particularly susceptible to
manganese neurotoxicity from high levels in drinking water, including: (1) severe neurotoxic symptoms
(inability to walk independently, tendency to fall backward, and development of a “cock-like” walk) and
MRI scan findings consistent with a diagnosis of hypermanganism in a previously healthy 5-year-old
37 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
female that were associated with elevated drinking water concentrations of manganese (1.7–2.4 mg
manganese/L), pica, emotional lability, polycythemia, iron deficiency, and elevated levels of plasma
manganese (Brna et al. 2011); (2) a similar case of severe manganism-like neurotoxic symptoms in a
previously healthy 6-year-old female that were associated with elevated drinking water concentrations of
manganese (1.7–2.4 mg manganese/L), pica, a diet high in manganese-rich foods, and elevated levels of
plasma manganese (Sahni et al. 2007); and (3) inattentiveness and lack of focus in the classroom and low-
percentile performance in tests of memory in a 10-year-old male with no history of learning problems
associated with elevated manganese in drinking water (1.21 mg manganese/L) (Woolf et al. 2002).
Although these recent reports cannot causally link the observed neurotoxic effects to excessive
manganese intakes, they provide added weight to the evidence for the neurotoxic potential of excessive
manganese in children.
As shown in the chronic exposure section of the oral LSE table and figure in Chapter 3, estimated daily
intakes from drinking water were calculated as 0.05 mg manganese/kg/day based on the mean manganese
drinking water concentration for high exposure group of Bangladesh children ages 8–11 (1.111 mg
manganese/L), reference daily water intakes (1.3 L/day), and reference body weights (31.19 kg); 0.07 mg
manganese/kg/day based on the mean manganese drinking water concentration for the fourth quartile
group of Bangladesh 10-year-old children (1.923 mg manganese/L), reference daily water intakes
(1.3 L/day), and average body weights (22.4 kg) (Wasserman et al. 2006); 0.0003 mg manganese/kg/day
based on the reported 50th percentile monthly exposure value (8.0 μg/kg/month), assuming 30 days in a
month (Bouchard et al. 2011); 0.02 mg manganese/kg/day for the high-manganese intake children in
Quebec (0.5 mg manganese/L), reference daily water intakes (1.3 L/day) and reference body weights
(37.2 kg) (Bouchard et al. 2007c); 0.104 mg/ manganese/kg/day for the 5-year-old female (Brna et al.
2011); 0.103 mg manganese/kg/day for the 6-year-old female (Sahni et al. 2007), and 0.06 mg
manganese/kg/day for the 10-year-old male (Woolf et al. 2002).
To derive an oral MRL for intermediate and chronic durations, an average of the drinking water LOAELs
for neurobehavioral effects in the three case reports (Brna et al. 2011; Sahni et al. 2007; Woolf et al.
2002), the cross-sectional studies of children in Bangladesh (Wasserman et al. 2006, 2011), and the
studies of children in Quebec (Bouchard et al. 2007c, 2011) could potentially serve as a POD for the
MRL. However, one or more of the following uncertainties associated with these studies of children
preclude their use as the basis for an intermediate- or chronic-duration MRL: (1) whether or not the
observed effects were solely due to excess manganese alone or could have been influenced by other
38 MANGANESE
2. RELEVANCE TO PUBLIC HEALTH
drinking water or dietary components; (2) the lack of information about manganese levels in food and air;
and (3) the small sample sizes.
Interim Guidance Value for Oral Exposure to Inorganic Manganese. As discussed in the preceding
sections, no oral MRLs were derived for acute-, intermediate-, or chronic-duration exposure to inorganic
manganese, but it is recommended that an interim guidance value of 0.16 mg manganese/kg/day be used
for ATSDR public health assessments. The interim guidance value is based on the Tolerable Upper
Intake Level for adults of 11 mg manganese/day established by the U.S. Food and Nutrition Board/
Institute of Medicine (FNB/IOM 2001) based on a NOAEL for Western diets (0.16 mg manganese/kg/day
assuming an adult body weight of 70 kg). The interim guidance value is well above the FNB/IOM AI
value for manganese for men and women of 2.3 and 1.8 mg manganese/day, respectively (for 70-kg
individuals, this would result in exposures of 0.033 and 0.026 mg manganese/kg/day, respectively). The
interim guidance value is necessary because of the prevalence of manganese at hazardous waste sites and
the fact that manganese is an essential nutrient. It is recommended that this value be used until more
information on actual intake levels across environmental media can be obtained.
MRLs for MMT
Inhalation and oral MRL values for acute, intermediate, or chronic exposures to MMT have not been
derived. There are currently insufficient data regarding the systemic toxicity and carcinogenicity of this
compound via inhalation or oral exposures and no reliable data concerning current environmental or
occupational exposures with appropriate dose-response information.
MRLs for Mangafodipir
MRL values for mangafodipir are not believed to be warranted. This compound is used in a clinical
environment, is administered intravenously only, and is restricted to a very limited population. Thus, it is
believed unlikely that this compound would be found at hazardous waste sites or other environmental
settings.
39 MANGANESE
3. HEALTH EFFECTS
3.1 INTRODUCTION
The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and
other interested individuals and groups with an overall perspective on the toxicology of manganese. It
contains descriptions and evaluations of toxicological studies and epidemiological investigations and
provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health.
A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile.
Manganese is a naturally occurring element found in rock, soil, water, and food. In humans and animals,
manganese is an essential nutrient that plays a role in bone mineralization, protein and energy
metabolism, metabolic regulation, cellular protection from damaging free radical species, and formation
of glycosaminoglycans (Wedler 1994). Manganese acts as both a constituent of metalloenzymes and an
enzyme activator. Enzymes that contain manganese include arginase, pyruvate carboxylase, and
manganese-superoxide dismutase (MnSOD) (Keen and Zidenberg-Cher 1990; NRC 1989; Wedler 1994).
Manganese, in its activating capacity, can bind either to a substrate (such as adenosine triphosphate,
ATP), or to a protein directly, thereby causing conformational changes (Keen and Zidenberg-Cher 1990).
Manganese has been shown to activate numerous enzymes involved with either a catalytic or regulatory
function (e.g., transferases, decarboxylases, hydrolases) (Wedler 1994). The nutritional role of
manganese is discussed in Section 3.4. Although manganese is an essential nutrient, exposure to high
levels via inhalation or ingestion may cause some adverse health effects.
It has been suggested that these adverse health effects, especially neurologic effects, are occurring on a
“continuum of ...dysfunction” that is dose-related (Mergler et al. 1999). In other words, mild or unnotice-
able effects may be caused by low, but physiologically excessive, amounts of manganese, and these
effects appear to increase in severity as the exposure level or duration of exposure increases. Case reports
and occupational studies address this continuum of nervous system dysfunction and help to characterize
the apparent dose-response relationship. It is clear that chronic exposure to manganese at very high levels
results in permanent neurological damage, as is seen in former manganese miners and smelters. Chronic
exposure to much lower levels of manganese (as with occupational exposures) has been linked to deficits
in the ability to perform rapid hand movements and some loss of coordination and balance, along with an
increase in reporting mild symptoms such as forgetfulness, anxiety, or insomnia.
40
MANGANESE
3. HEALTH EFFECTS
Chemical Forms of Concern. Manganese can exist in both inorganic and organic forms. This
profile will discuss key manganese compounds in both forms, with inorganic compounds discussed first.
The inorganic forms include manganese chloride (MnCl
2
), manganese sulfate (MnSO
4
), manganese
acetate (MnOAc), manganese phosphate (MnPO
4
), manganese dioxide (MnO
2
), manganese tetroxide
(Mn
3
O
4
), and manganese carbonate (MnCO
3
). Emphasis has been placed on the health effects of
compounds containing inorganic manganese in the Mn(II), Mn(III), or Mn(IV) oxidation states, since
these are the forms most often encountered in the environment and the workplace. There is evidence in
animals and humans that adverse neurological effects can result from exposure to different manganese
compounds; much of this information on toxicity differences between species of manganese is from
reports and experiments of acute exposures to very high doses. Results from animal studies indicate that
the solubility of inorganic manganese compounds can influence the bioavailability of manganese and
subsequent delivery of manganese to critical toxicity targets such as the brain; however, the influence of
manganese oxidation state on manganese toxicity is not currently well understood. Manganese in the
form of permanganate produces toxic effects primarily through its oxidizing capacity. However, because
of its tendency to oxidize organic material, the permanganate ion is not stable in the environment; thus,
the probability of exposure to this species around waste sites is considered very low. For this reason, data
on exposures to permanganate are only briefly discussed.
The organic compounds that will be discussed are methylcyclopentadienyl manganese tricarbonyl (MMT)
and mangafodipir. The latter is a chelate of Mn(II) and an organic ligand, dipyridoxyl diphosphate
(MnDPDP; Mn(II) N,N’-dipyridoxylethylenediamine-N,N’-diacetate 5,5'bis(phosphate)). These
compounds were chosen for this profile because their toxicity is expected to be mediated by excess
exposure to elemental manganese. Organic fungicides containing manganese, such as maneb, were not
chosen for discussion in this profile, because their critical toxic effects are expected to be mediated by the
organic moities of their chemical structure, not by excessive elemental manganese.
MMT is a fuel additive developed in the 1950s to increase the octane level of gasoline and thus improve
the antiknock properties of the fuel (Davis 1998; Lynam et al. 1999). Additional information on the
chemical, physical, and environmental properties of MMT is included in Chapter 4. Exposure to MMT is
expected to be primarily through inhalation or oral pathways, although occupational exposure for gasoline
attendants or mechanics may be more significant via dermal absorption. Engines using MMT-containing
gasoline and equipped with catalytic converters primarily emit manganese in inorganic phosphate and
sulfate forms and smaller amounts of manganese dioxides can be detected (Mölders et al. 2001; Ressler et
41
MANGANESE
3. HEALTH EFFECTS
al. 2000; Zayed et al. 1999a, 1999b). These findings and observations that MMT is very unstable in light
and degrades quickly in air (Garrison et al. 1995) suggest that human exposure to manganese from the use
of MMT in gasoline is most likely to occur in inorganic forms as a result of the combustion of MMT,
with the exception of people occupationally exposed to uncombusted gasoline containing MMT.
However, despite this evidence, there are some reports that MMT levels in the environment increase with
traffic density (Garrison et al. 1995; Zayed et al. 1999a, 1999b); therefore, inhalation and/or ingestion
exposures to the parent compound are possible. Exposure and resultant toxicity from MMT’s inorganic
combustion products are covered under the inorganic subsections, while toxicity attributable to MMT is
covered under the organic subsections.
Mangafodipir is a contrast agent for magnetic resonance imaging (MRI) used primarily (after intravenous
administration) to detect and characterize neoplastic liver lesions; it has also been found to aid in the
identification of kidney and pancreatic tumors (Federle et al. 2000; Grant et al. 1997a, 1997b; Ni et al.
1997). The compound is only used in the diagnosis of organ-specific cancers and is found exclusively in
a clinical setting. Mangafodipir is injected intravenously; therefore, inhalation, oral, and dermal pathways
of exposure are not a concern. Because exposure to this compound is pathway-specific and the exposure
population is inherently limited, toxicity arising from exposure to mangafodipir will be discussed in a
separate subsection to Section 3.2.4, Diagnostic Uses.
3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE
To help public health professionals and others address the needs of persons living or working near
hazardous waste sites, the information in this section is organized first by route of exposure (inhalation,
oral, and dermal) and then by health effect (death, systemic, immunological, neurological, reproductive,
developmental, genotoxic, and carcinogenic effects). These data are discussed in terms of three exposure
periods: acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more).
Levels of significant exposure for each route and duration are presented in tables and illustrated in
figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-
observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies.
LOAELs have been classified into "less serious" or "serious" effects. "Serious" effects are those that
evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress
or death). "Less serious" effects are those that are not expected to cause significant dysfunction or death,
or those whose significance to the organism is not entirely clear. ATSDR acknowledges that a
42
MANGANESE
3. HEALTH EFFECTS
considerable amount of judgment may be required in establishing whether an end point should be
classified as a NOAEL, "less serious" LOAEL, or "serious" LOAEL and that, in some cases, there will be
insufficient data to decide whether the effect is indicative of significant dysfunction. However, the
Agency has established guidelines and policies that are used to classify these end points. ATSDR
believes that there is sufficient merit in this approach to warrant an attempt at distinguishing between
"less serious" and "serious" effects. The distinction between "less serious" effects and "serious" effects is
considered to be important because it helps the users of the profiles to identify levels of exposure at which
major health effects start to appear. LOAELs or NOAELs should also help in determining whether or not
the effects vary with dose and/or duration and place into perspective the possible significance of these
effects to human health.
The significance of the exposure levels shown in the Levels of Significant Exposure (LSE) tables and
figures may differ depending on the user's perspective. Public health officials and others concerned with
appropriate actions to take at hazardous waste sites may want information on levels of exposure
associated with more subtle effects in humans or animals (LOAELs) or exposure levels below which no
adverse effects (NOAELs) have been observed. Estimates of levels posing minimal risk to humans
(Minimal Risk Levels or MRLs) may be of interest to health professionals and citizens alike.
A User's Guide has been provided at the end of this profile (see Appendix B). This guide should aid in
the interpretation of the tables and figures for Levels of Significant Exposure and the MRLs.
3.2.1 Inhalation Exposure
Inorganic manganese compounds are not volatile, but they can exist in the air as aerosols or suspended
particulate matter. Table 3-1 and Figure 3-1 summarize the available quantitative information on the
health effects that have been observed in humans and animals following inhalation exposure to various
inorganic manganese compounds. All exposure levels are expressed as milligrams of manganese per
cubic meter (mg manganese/m
3
).
Many of the studies, especially those dealing with occupational exposures, make the distinction between
respirable and total manganese dust. Respirable dust is usually defined by a particular dust particle size
that varies from study to study. It is typically defined as those particles ≤5 microns; these smaller dust
particles can enter the lower areas of the lungs, including the bronchioles and the alveoli. These particles
can be absorbed by the lung and will enter the bloodstream immediately, thus avoiding clearance by the

32
43
138
20
2.8
1100
2
16
14
25
69
1116
0.71
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation
Exposure/
Duration/
a
Key to
Figure
Species
(Strain)
Frequency
(Route)
ACUTE EXPOSURE
Systemic
1
Rat
(Sprague-
Dawley)
10 d
6 hr/d
System
Resp
NOAEL
(mg/m³)
Less Serious
(mg/m³)
LOAEL
Serious
(mg/m³)
43 (pneumonitis and
increased lung weight)
Reference
Chemical Form
Shiotsuka 1984
MnO2
Comments
Hemato 138
2
Mouse
(CD-1)
2 hr
Resp 2.8 F
A
dkins et al. 1980b
Mn3O4
3
Mouse
(FVB/N)
5 d
6 h/d
Resp 2 F
Bredow et al. 2007
(MnCl2)
No significant
treatment-related
histopathic lesions in
lungs.
4
Gn Pig
(NS)
Immuno/ Lymphoret
5
Mouse
(CD-1)
1 hr
24 hr/d
1-4 d
3 hr/d
Resp 14
69
M (increased susceptibility
to pneumonia)
Bergstrom 1977
MnO2
Maigetter et al. 1976
MnO2
Neurological
6
Rat
(Sprague-
Dawley)
Gd 9-10 or pnd
37-47 or Gd
9-10 and pnd
37-47
0.71 (decreased APP, COX-2,
nNOS, GFAP, TGF-beta
mRNA in the brain)
HaMai et al. 2006
(MnSO4)
Increased transcription
of genes related to
oxidative stressor
inflammation in brain of
rats exposed during
gestation or early
adulthood.
3. HEALTH EFFECTS
MANGANESE
43

1117
0.71
1105
0.3
1.5
1106
1.5
0.3
1.5
0.3
1.5
1.5
1.5
1.5
1.5
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
LOAEL
a
Key to Species
Figure (Strain)
Developmental
7
Rat
(Sprague-
Dawley)
Exposure/
Duration/
Frequency
(Route)
Gd 9-10 or pnd
37-47 or Gd
9-10 and pnd
37-47
INTERMEDIATE EXPOSURE
Systemic
8
Monkey
(Rhesus)
9
Monkey
90 d
6 h/d
5 d/wk
90 d
6 h/d
5 d/wk
System
Resp 0.3 M
Resp 1.5 M
Cardio 0.3 M
Hemato 0.3 M
Hepatic 1.5 M
Renal 1.5 M
Endocr 1.5 M
Bd Wt 1.5 M
NOAEL
(mg/m³)
Less Serious Serious
(mg/m³) (mg/m³)
0.71 (decreased APP, COX-2,
nNOS, and GFAP
mRNA)
1.5 M (increased incidence of
subacute
bronchiolitis/alveolar duct
inflammation)
1.5 M (17% decrease in relative
heart weight 90 days
post-exposure)
1.5 M (decreased total bilirubin
concentrations)
Reference
Chemical Form
HaMai et al. 2006
(MnSO4)
Dorman et al. 2005c
(MnSO4)
Dorman et al. 2006a
(MnSO4)
Comments
Increased transcription
of genes related to
oxidative stressor
inflammation in brain of
rats exposed during
gestation or early
adulthood.
Only absolute and
relative organ weights
were examined for the
pituitary, liver, lung,
kidney, heart,
pancreas, hemotocrit.
MANGANESE
3. HEALTH EFFECTS
44

33
0.7
35
1.1
1102
0.1
0.5
1104
0.1
1109
0.11
1119
0.03
0.3
1134
0.9
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/m³)
Less Serious
(mg/m³)
LOAEL
Serious
(mg/m³)
Reference
Chemical Form
Comments
10
Monkey
(Rhesus)
10 mo
22 hr/d
Resp
0.7 F (mild inflammation)
Suzuki et al. 1978
MnO2
11
Monkey
(NS)
9 mo
(continuous)
Resp
1.1
Ulrich et al. 1979a
Mn3O4
No histopathological
changes in lung or
brain and no pulmonary
function changes.
12
Rat
(CD)
13 wk
6 h/d
5 d/wk
Resp
0.1 M
0.5 M (transient inflammatory
changes in the nasal
respiratory epithelium)
Dorman et al. 2004b
(MnSO4)
Inflammatory changes
were no longer present
45 days after exposure
period was over.
13
Rat
(CD)
13 wk
6 h/d
5 d/wk
Resp
0.1 M
Dorman et al. 2004b
MnPO4
There were no lesions
or inflammation
observed in the nasal
respiratory epithelium
of rats.
14
Rat
(Sprague-
Dawley)
12 wk
6 h/d
5 d/wk
Bd Wt
0.11 M (12% decreased body
weight)
El-Rahman 2004
hureaulite
15
Rat
(Sprague-
Dawley)
90 d
5 d/wk
6 hr/d
Bd Wt
0.03 M
0.3 M (10% decreased body
weight)
Salehi et al. 2003
manganese phosphate/sulfate
mixture
16
Rat
(Sprague-
Dawley)
90 d
5 d/wk
6 h/d
Bd Wt
0.9 M
Tapin et al. 2006
manganese sulfate dihydrate
3. HEALTH EFFECTS
MANGANESE
45

37
1.1
1.1
1.1
22
3.9
605
0.167
1107
1.5
1165
1.5
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to Species
Figure (Strain)
17
Rat
(NS)
18
Rabbit
(NS)
19
Pigeon
Neurological
20
Monkey
21
Monkey
(Rhesus)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
9 mo
(continuous)
Resp 1.1
Ulrich et al. 1979b
Mn3O4
Hemato 1.1
Hepatic 1.1
4 wk
5 d/wk
6 hr/d
Resp 3.9 M
Camner et al. 1985
MnCl2
5 d/wk
5, 9, or 13 wk
(IC)
Hemato 0.167 (decrease in total blood
proteins (p<= 0.05) at 13
weeks of exposure that
persisted 2 weeks after
exposure ended)
Sierra et al. 1998
Mn3O4
90 d
6 h/d
5 d/wk
1.5 M
Dorman et al. 2006a
(MnSO4)
Only absolute and
relative brain weight
were examined.
15, 33, or 65 d
1.5 (decreased brain GS,
Erikson et al 2008
with
GLT-1, and GLAST
(MnSO4)
45 or 90 d
protein and mRNA and
recovery
decreased MT mRNA;
6 hr/d
increased and decreased
5 d/wk
brain GSH; increased
inhalation
brain TH protein but
chamber
decreased mRNA)
3. HEALTH EFFECTS
MANGANESE
46

1111
0.06
34
1.1
1101
0.5
1103
0.1
1110
0.11
1.1
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to Species
Figure (Strain)
22
Monkey
(Rhesus)
23
Monkey
(NS)
24
Rat
(CD)
25
Rat
(CD)
26
Rat
(Sprague-
Dawley)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
90 d
6 h/d
5 d/wk
0.06 M (altered levels of GS,
GLT-1 mRNA, GLAST,
TH mRNA, GLT-1
mRNA, GLAST mRNA,
and TH mRNA in the
brain)
Erikson et al. 2007
(MnSO4)
9 mo
(continuous)
1.1
Ulrich et al. 1979a
Mn3O4
13 wk
6 h/d
5 d/wk
0.5 M
Dorman et al. 2004b
(MnSO4)
No changes in GFAP
levels in the olfactory
bulb, cerebellum, and
striatum.
13 wk
6 h/d
5 d/wk
0.1 M
Dorman et al. 2004b
MnPO4
No changes in GFAP
levels in the olfactory
bulb, cerebellum, and
striatum.
12 wk
6 h/d
5 d/wk
0.11 M (increased free amino
acid contents; focal glial
cell proliferation;
astrocytic nodules)
1.1 M (neuronal degeneration)
El-Rahman 2004
MnPO4
3. HEALTH EFFECTS
MANGANESE
47

1112
0.05
1115
0.05
1120
1
1118
0.03
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to Species
Figure (Strain)
27
Rat
(CD)
28
Rat
(CD)
29
Rat
(Sprague-
Dawley)
30
Rat
(Sprague-
Dawley)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Gd 0-19, pnd
1-18
6 h/d
7 d/wk
0.05 (decreased brain GS
mRNA, MT mRNA and
GHS levels in F1 females
and decreased brain MT
mRNA and GSH levels
F1 males)
Erikson et al. 2005
(MnSO4)
Gd 0-19, pnd
1-18
6 h/d
5 d/wk
0.05 (decreased brain GS and
TH protein and mRNA,
MT, and GSH and
increased GSSG levels
in F1 rats)
Erikson et al. 2006
(MnSO4)
90 d
5 d/wk
6 h/d
1 M
Normandin et al. 2002
hureaulite
90 d
0.03 M (increased locomotor
Salehi et al. 2003
5 d/wk
activity)
6 hr/d
manganese phosphate/sulfate
mixture
Comments
No differences in
neuronal cell counts
compared to controls,
and no changes in
locomotor and tremor
assessments.
There was a significant
increase in distance
traveled, but not in rest
time; increased
exposure did not result
in increased response.
3. HEALTH EFFECTS
MANGANESE
48

1121
3
1122
0.009
36
1.1
26
61
29
72
1108
1.5
27
61
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to Species
Figure (Strain)
31
Rat
(Sprague-
Dawley)
32
Rat
(Sprague-
Dawley)
33
Rat
(NS)
34
Mouse
(Swiss ICR)
35
Mouse
(Swiss ICR)
Reproductive
36
Monkey
(Rhesus)
37
Mouse
(Swiss ICR)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
90 d
5 d/wk
6 h/d
3 M (significant neuronal cell
loss in the globus
pallidus and caudate
putamen)
Salehi et al. 2006
manganese phosphate/sulfate
mixture
90 d
5 d/wk
6 h/d
0.009 M (increased locomotor
activity)
Tapin et al. 2006
manganese sulfate dihydrate
9 mo
1.1
Ulrich et al. 1979b
(continuous)
Mn3O4
18 wk
61 F (decreased maternal pup
Lown et al. 1984
5 d/wk
retrieval latency)
7 hr/d
MnO2
16-32 wk
72 M (increased open-field
Morganti et al. 1985
5 d/wk
behavior)
7 hr/d
MnO2
90 d
Dorman et al. 2006a
Only testes weight was
1.5 M
6 h/d
examined.
5 d/wk
(MnSO4)
18 wk
Lown et al. 1984
No effect on number of
61 F
5 d/wk
pups born.
7 hr/d
MnO2
3. HEALTH EFFECTS
MANGANESE
49

1113
0.05
1114
0.05
1125
90
2
3.6
7
0.97
0.97
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to
Figure
Species
(Strain)
System
Exposure/
Duration/
Frequency
(Route)
NOAEL
(mg/m³)
LOAEL
Less Serious
(mg/m³)
Serious
(mg/m³)
Reference
Chemical Form
Comments
Developmental
38
Rat
(CD)
Gd 0-19, pnd
1-18
6 h/d
7 d/wk
Erikson et al. 2005
(MnSO4)
0.05 (decreased brain GS
mRNA, MT mRNA and
GHS levels in F1 females
and decreased brain MT
mRNA and GSH levels
F1 males)
39
Rat
(CD)
Gd 0-19, pnd
1-18
6 h/d
5 d/wk
Erikson et al. 2006
(MnSO4)
0.05 (decreased brain GS and
TH protein and mRNA,
MT, and GHS and
increased GSSG levels
in F1 rats)
CHRONIC EXPOSURE
Systemic
40
Resp
Human 7.5 yr (average
duration in Mn
mine)
(occup)
Boojar and Goodarzi 2002
90 M (increased respiratory
symptoms and
prevalence of subjects
with impaired pulmonary
function)
41
Resp
Human NS
(occup)
Lloyd Davies 1946
MnO2
3.6 M (pneumonia)
42
Resp
Human 1-19 yr
(occup)
Roels et al. 1987a
Mn salts and oxides
0.97 M (cough, decreased lung
function)
Hemato 0.97 M
3. HEALTH EFFECTS
MANGANESE
50

1038
0.18
0.18
167
0.1
1124
0.07
1123
0.07
1127
0.23
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
a
Key to Species
Figure (Strain)
43
Human
44
Monkey
(Rhesus)
Neurological
45
Human
46
Human
47
Human
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
5.3 yr
(occup)
Resp 0.18
Roels et al. 1992
MnO2
Endocr 0.18
66 wk
Hemato 0.1
EPA 1977
Mn3O4
24 yr (median
employment in
steel plant)
(occup)
0.07 M (longitudinal analysis
showed impaired ability
to perform fast
pronation/supination of
the hands and fast finger
tapping compared with
controls)
Blond and Netterstrom 2007
No impairments of slow
hand and finger
movements or
increased tremor
intensity were observed
compared with
controls.
24 yr (median
employment in
steel plant)
0.07 M
Blond et al. 2007
Cognitive function
could not be
distinguished between
Mn-exposed steel
workers and controls.
19.3 yr
(average
employment in
Mn alloy plant)
(occup)
0.23 M (increased Mn
impairment with age in
1/9 neuromotor tests,
3/12 cognitive tests, and
1 or 4 sensory tests)
Bouchard et al. 2005
3. HEALTH EFFECTS
MANGANESE
51

1128
0.23
1129
0.23
355
1.59
138
22
1131
2.05
700
0.051
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
48
Human 15.7 yr
(average
employment)
(occup)
0.23 M (significantly higher
scores for 2 [depression,
anxiety] of 9
neuropsychiatric
symptoms)
Bouchard et al. 2007a
49
Human 15.3 yr
(average
employment)
(Occup)
0.23 M (impaired performance
on 1/5 neuromotor tests
and enhanced score for 1
[confusion-bewilderment]
of 6 mood states)
Bouchard et al. 2007b
50
Human 1.1-15.7 yr
(occup)
1.59 M (postural sway with eyes
closed)
Chia et al. 1995
MnO2
51
Human NS
(occup)
22 M (bradykinesia, mask-like
face)
Cook et al. 1974
NS
52
Human 19.87 yr; mean
(SD±9)
employment in
enamels
production
(occup)
2.05
Deschamps et al. 2001
53
Human 12.7 yr
(mean)
(occup)
0.051
Gibbs et al. 1999
NS
Comments
Follow-up to Mergler et
al. 1994; no significant
(p<0.05) differences
between exposed and
controls in 9 cognitive
tests.
No significant effects
on blood levels of Mn
or tests of cognition.
Tests of neuromotor
functions were not
conducted.
3. HEALTH EFFECTS
MANGANESE
52

178
0.14
403
0.149
701
0.0967
304
0.032
1132
0.21
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
(Route)
Figure (Strain)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
54
Human 1-35 yr
(2.6 median)
(occup)
0.14 M (decreased reaction time,
finger tapping)
Iregren 1990
MnO2
55
Human 1-28 yr
0.149 M (decreased
neurobehavioral
performance finger
tapping, symbol digit,
digit span, additions)
Lucchini et al. 1995
(primarily MnO2) (MnOx - Mn
oxides)
56
Human 11.5 yr
(mean)
(occup)
0.0967 M (decreased performance
on neurobehavioral
exams)
Lucchini et al. 1999
MnO2, Mn3O4
57
Human 16.7 yr (mean)
(occup)
0.032 M (decreased motor
function)
Mergler et al. 1994
NS
58
Human 10.8 yr (mean
employment in
Mn mines)
(occup)
0.21
Myers et al. 2003a
No associations
between measures of
exposure and
neurobehavioral
endpoints were found:
3 motor function and 3
cognitive tests.
MANGANESE
3. HEALTH EFFECTS
53

1133
0.85
6
0.97
266
0.179
9
2.6
10
6
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
(Route)
Figure (Strain)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
59
Human 18.2 yr; mean
(SD 7.6)
employment in
a Mn smelter
(occup)
0.85
Myers et al. 2003b
Neurobehavioral test
batteries showed
significant effects, only
in a few endpoints and
little evidence of
positive
exposure-response
relationships.
60
Human 1-19 yr
(occup)
0.97 M (altered reaction time,
short-term memory,
decreased hand
steadiness)
Roels et al. 1987a
Mn salts and oxides
61
Human 5.3 yr
(occup)
b
0.179 (impaired visual time,
eye-hand coordination,
and hand steadiness)
Roels et al. 1992
MnO2
62
Human NS
(occup)
2.6 M (tremor, decreased
reflexes)
Saric et al. 1977
NS
63
Human 1-9 yr
(occup)
6 M (psychomotor
disturbances, weakness,
pain)
Schuler et al. 1957
MnO2
MANGANESE
3. HEALTH EFFECTS
54

1170
0.123
12
5
13
3.5
21
30
166
0.1
4
0.97
428
2.82
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
Exposure/
LOAEL
Duration/
Frequency
(Route)
System
NOAEL
(mg/m³)
Less Serious
(mg/m³)
Serious
(mg/m³)
average 127.13
mo
8 hr/d
(Occup)
0.123
NS
(occup)
5 M (weakness, ataxia, pain)
1 yr
(occup)
3.5 M (weakness, anorexia,
ataxia)
2 yr
5 d/wk
6 hr/d
30 F (altered DOPA levels)
66 wk
0.1
1-19 yr
(occup)
0.97 M (decreased fertility in
males as assessed by
number of observed vs
expected children)
at least 1 yr
(occup)
2.82 M (abnormal sperm)
Reference
Chemical Form
Comments
Summers et al. 2011
No clinically relevant
alterations in
Mn dust
performance on a
battery of
neuropsychological
tests.
Tanaka and Lieben 1969
NS
Whitlock et al. 1966
NS
Bird et al. 1984
MnO2
EPA 1977
Mn3O4
Lauwerys et al. 1985
Mn salts and oxides
Wu et al. 1996
(MnO2)
a
Key to
Figure
64
65
66
67
68
Species
(Strain)
Human
Human
Human
Monkey
(Rhesus)
Monkey
(Rhesus)
Reproductive
69
Human
70
Human
MANGANESE
3. HEALTH EFFECTS
55

429
44.4
Table 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
(Route)
Figure (Strain)
System
(mg/m³) (mg/m³) (mg/m³)
Chemical Form
Comments
71
Human at least 1 yr
44.4 M (abnormal sperm)
Wu et al. 1996
(occup)
(Mn fumes)
MANGANESE
a The number corresponds to entries in Figure 3-1.
b The chronic-duration inhalation minimal risk level (MRL) of 0.0003 mg manganese/m3 was derived by using a benchmark dose analysis BMCL10 of 0.142 mg manganese/m3 for
performance deficits in an eye-hand coordination test. This value was adjusted using the following uncertainty and modifying factors: 10 for human variability, 5/7 for intermittent
exposure (5 days/week), 8/24 for intermittent exposure (8 hours/day), and 10 for potential differences in toxicity due to the different forms of manganese and other limitations in the
database.
A
PP = amyloid precursor protein; Bd Wt = body weight; Cardio = cardiovascular; COX = cyclooxygenase; d = day(s); DOPA = dihydroxyphenylalanine; Endocr = endocrine; F =
Female; Gd = gestational day; GFAP = glial fibrillary acidic protein; GLAST = glutamate/aspartate transporter; GLT-1= glutamate transporter-1; Gn pig = guinea pig; GS = glutamine
synthetase; GSH = reduced glutathione; GSSG = oxidized glutathione; Hemato = hematological; hr = hour(s); Immuno/Lymphoret = immunological/lymphoreticular; LOAEL =
lowest-observed-adverse-effect level; M = male; mo = month(s); mRNA = messenger ribonucleic acid; MT = metallothionein; nNOS = neuronal nitric oxide synthase; NOAEL =
no-observed-adverse-effect level; NS = not specified; occup = occupational; pnd = post-natal day; Resp = respiratory; TGF-beta = transforming growth factor beta; TH = tyrosine
hydroxylase; wk = week(s); yr = year(s)
3. HEALTH EFFECTS
56

Respiratory
Hematological
Immuno/Lymphor
Neurological
Developmental
1000
Figure 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation
Acute (≤14 days)
Systemic
mg/m
3
1r
100
5m
1r
4g
10
2m
3m
1
6r 7r
0.1
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
-Humans
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
Minimal Risk Level
for effects
other than
Cancer
LD50/LC50
MANGANESE
57
3. HEALTH EFFECTS

0.001
0.01
0.1
1
10
100
Respiratory
8k
8k 9k
10k
11k
12r
12r
13r
17r
18h
Cardiovascular
9k
9k
Hematological
9k
9k
17r
19j
Hepatic
9k
17r
Renal
9k
Endocrine
9k
Body Weight
9k
14r
15r
15r
16r
Neurological
20k 21k
22k
23k
34m
35m
24r
25r
26r
26r
27r 28r
29r
30r
31r
32r
33r
Reproductive
36k
37m
Developmental
38r 39r
mg/m
3
Figure 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (Continued)
Intermediate (15-364 days)
Systemic
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
-Humans
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
LD50/LC50
Minimal Risk Level
for effects
other than
Cancer
3. HEALTH EFFECTS
MANGANESE
58
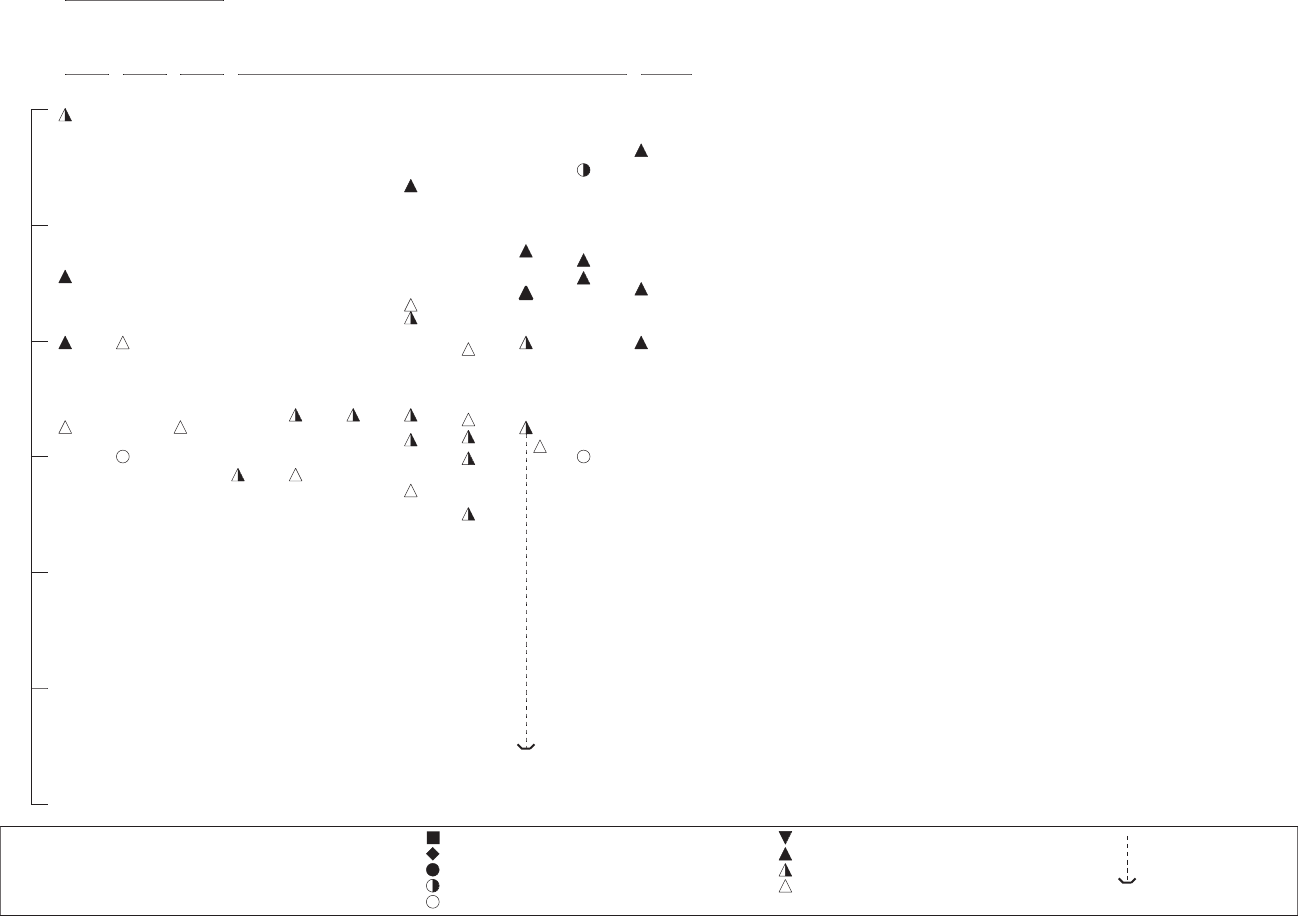
Respiratory
Hematological
Endocrine
Neurological
Reproductive
Figure 3-1 Levels of Significant Exposure to Inorganic Manganese - Inhalation (Continued)
Chronic (≥365 days)
Systemic
mg/m
3
100
40
71
67k
51
10
63
65
41
66
70
62
52
50
1
42 42 60 69
59
47 48 49
58
43 43
61
55
54
64
0.1
44k
68k
56
45 46
53
57
0.01
0.001
0.0001
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
-Humans
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
LD50/LC50 Minimal Risk Level
for effects
other than
Cancer
MANGANESE
3. HEALTH EFFECTS
59
60
MANGANESE
3. HEALTH EFFECTS
liver. Total dust represents larger particles that cannot travel as deeply into the lungs as respirable dust,
and will largely be coughed up and swallowed. Although many of the recent occupational studies have
provided information on the size of the respirable particles that are associated with the exposure levels
documented, some of the occupational studies and historical studies in miners only measure total dust.
The profile provides, where possible, the different exposure levels in terms of respirable and total dust,
but does not make a further distinction between particle sizes of the respirable dust.
3.2.1.1 Death
No conclusive studies have been located that show inhalation exposure of humans to manganese resulting
in death. Hobbesland et al. (1997a) investigated nonmalignant respiratory diseases as a cause of death in
male ferromanganese and silicomanganese workers. The authors found a slight excess in the numbers of
deaths caused by pneumonia for manganese furnace workers, but could not discount other work-related
exposures as potential causes of the pneumonia.
In analyses performed several years ago, MMT in gasoline was found to combust primarily to manganese
tetroxide, but in the low levels currently used in gasolines, it is primarily combusted to manganese
phosphate and manganese sulfate (Lynam et al. 1999). Therefore, inhalation exposures to exhaust from
gasoline containing MMT will be discussed with inorganic manganese exposures. No deaths were
observed in male outbred albino rats and male golden hamsters exposed to the exhaust (either irradiated
or non-irradiated) from automobiles that were fueled with MMT-containing gasoline (Moore et al. 1975).
No other studies were located regarding death in humans or animals after inhalation exposure to inorganic
manganese.
MMT has been used in very few inhalation studies due to the photolability of the compound; its short
half-life in air makes it a very difficult compound to administer to laboratory animals in exposure
chambers or nose-cones. Hinderer (1979) evaluated the toxicity of various unspecified MMT
concentrations administered to 10 male Sprague-Dawley rats per exposure group during 1- and 4-hour
exposure periods. The inhalation LD
50
was determined to be 62 mg manganese/m
3
(247 mg
MMT/m
3
*55 mg manganese/218.1 mg MMT=62 mg manganese/m
3
) for a 1-hour exposure and 19 mg
manganese/m
3
for a 4-hour exposure. No mention was made in the report of steps taken to prevent MMT
photodegradation during the experiment.
61
MANGANESE
3. HEALTH EFFECTS
3.2.1.2 Systemic Effects
The highest NOAEL values and all LOAEL values from each reliable study for systemic effects in each
species and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
Respiratory Effects. In humans, inhalation of particulate manganese compounds such as manganese
dioxide or manganese tetroxide can lead to an inflammatory response in the lung. This is characterized
by an infiltration of macrophages and leukocytes, which phagocytize the deposited manganese particles
(Lloyd Davies 1946). Damage to lung tissue is usually not extensive, but may include local areas of
edema (Lloyd Davies 1946). Symptoms and signs of lung irritation and injury may include cough,
bronchitis, pneumonitis, and minor reductions in lung function (Abdel-Hamid et al. 1990; Akbar-
Khanzadeh 1993; Boojar and Goodarzi 2002; Lloyd Davies 1946; Roels et al. 1987a); occasionally,
pneumonia may result (Lloyd Davies 1946). These effects have been noted mainly in people exposed to
manganese dust under occupational conditions, although there is some evidence that respiratory effects
may also occur in residential populations near ferromanganese factories (Kagamimori et al. 1973;
Nogawa et al. 1973; WHO 1987). The frequency of effects has been shown to decrease in at least one
population when concentrations of total manganese in falling dust declined (Kagamimori et al. 1973). It
is likely that the inflammatory response begins shortly after exposure and continues for the duration of the
exposure.
It is important to note that an inflammatory response of this type is not unique to manganese-containing
particles, but is characteristic of nearly all inhalable particulate matter (EPA 1985d). This suggests that it
is not the manganese per se that causes the response, but more likely the particulate matter itself.
An increased prevalence of pneumonia has also been noted in some studies of workers with chronic
occupational exposure to manganese dust (Lloyd Davies 1946) and in residents near a ferromanganese
factory (WHO 1987). It seems likely that this increased susceptibility to pneumonia is mainly secondary
to the lung irritation and inflammation caused by inhaled particulate matter, as discussed above.
Inhalation of particulate manganese compounds such as manganese dioxide or manganese tetroxide also
leads to an inflammatory response in the lungs of animals, although inhalation of manganese chloride did
not cause lung inflammation in rabbits (Camner et al. 1985). Several acute- and intermediate-duration
studies in animals report various signs of lung inflammation following periods ranging from 1 day to
10 months at manganese concentrations ranging from 0.7 to 69 mg/m
3
(Bergstrom 1977; Camner et al.
62
MANGANESE
3. HEALTH EFFECTS
1985; Shiotsuka 1984; Suzuki et al. 1978; Ulrich et al. 1979a, 1979b). Bergstrom (1977) and Ulrich et al.
(1979a, 1979b) determined NOAELs, which are reported in Table 3-1. Increased susceptibility to lung
infection by bacterial pathogens following inhalation of manganese dusts has been noted in acute animal
studies (Maigetter et al. 1976). Conversely, Lloyd Davies (1946) reported no increase in the
susceptibility of manganese-treated mice to pneumococci or streptococci. Bredow et al. (2007) reported
that nose-only inhalation exposure to 2 mg manganese/m
3
as manganese chloride aerosols 6 hours/day for
5 consecutive days did not cause lung lesions in female GVB/N mice, but induced a 2-fold increase in
pulmonary levels of mRNA for vascular endothelial growth factor (VGEF), a regulator of proliferation,
migration, and formation of new capillaries. Elevated levels of VGEF have been associated with
respiratory diseases, but current understanding is inadequate to know if this pulmonary gene expression
response to manganese is adverse or benign.
Moore et al. (1975) exposed male golden hamsters and outbred albino rats to automobile exhaust from a
car that burned MMT-containing fuel. The animals were exposed to non-irradiated exhaust or irradiated
exhaust; the irradiation served to convert hydrocarbon gases and vapors to particulate form. Controls for
each species were exposed to clean air. The animals were exposed for 8 hours/day for 56 consecutive
days. While the hamsters were fed a diet containing an adequate amount of manganese for normal
development, the rats were divided into two groups: one group was fed a manganese-sufficient diet
(42.2 μg manganese/g diet) and the other group was fed a manganese-deficient diet (5 μg manganese/g
diet). After the exposure, the authors observed a thickening of the cuboidal epithelium at the level of the
terminal bronchiole in the golden hamsters. The lesion was not classified as severe and only affected one
to two sites per lung section. Further, the lesions did not increase with length of exposure to the exhaust
products (from 1 to 9 weeks). The incidence of lesions in the lung was 21% after exposure to irradiated
exhaust, 14% after exposure to non-irradiated exhaust, and 6% after exposure to clean air.
More recently, reversible inflammation (pleocellular inflammatory infiltrates and fibrinonecrotic debris)
in the nasal respiratory epithelium (but not the olfactory epithelium) was observed in young adult male
Crl:CD(SD)BR rats following 13 weeks of inhalation exposure to 0.5 mg manganese/m
3
as manganese
sulfate, but not in rats exposed to 0.1 mg manganese/m
3
as manganese sulfate or manganese phosphate
(hureaulite) (Dorman et al. 2004b). The lesions were not apparent in groups of rats assessed 45 days after
the end of exposure, indicating their transient nature. In studies with young male Rhesus monkeys
exposed to 0, 0.06, 0.3, or 1.5 mg manganese/m
3
as manganese sulfate 6 hours/day, 5 days/week for
65 days, no nasal histological effects were found in exposed monkeys, but the high exposure level
induced lesions in the lower respiratory tract (mild subacute bronchiolitus, alveolar duct inflammation,
63
MANGANESE
3. HEALTH EFFECTS
and proliferation of bronchus-associated lymphoid tissue) (Dorman et al. 2005b). The lower airway
lesions from intermediate-duration exposure appear to have been transient, because they were not found
in monkeys assessed 45 days after the end of exposure (Dorman et al. 2005b). These findings in rats and
monkeys are consistent with the understanding that inflammation of respiratory tissues from high-level
exposure to inhaled manganese particulates is likely a consequence of the inhaled particulate matter.
No studies were located concerning respiratory effects in humans following inhalation exposure to MMT.
Male rats exposed to high concentrations of MMT (exposure doses not reported) via inhalation exhibited
labored breathing during and after 1- and 4-hour exposures (Hinderer 1979). Gross necropsy or
histopathological analyses on these animals were not performed.
Cardiovascular Effects. Three studies reported adverse cardiovascular effects after occupational
exposure to manganese. Saric and Hrustic (1975) observed a lower mean systolic blood pressure in male
workers at a ferromanganese plant. Manganese concentrations in the plant ranged from 0.4 to 20 mg/m
3
,
but specific data on exposure levels were lacking. More recently, Jiang et al. (1996a) studied the potential
cardiotoxicity of manganese dioxide exposure in 656 workers (547 males, 109 females) involved in
manganese milling, smeltering, and sintering. The authors took 181 samples of airborne manganese (not
specified if respirable or total dust), with a geometric mean of 0.13 mg/m
3
. The workers, whose work
tenure ranged from 0 to 35 years, had a greater incidence of low diastolic blood pressure. The incidence
of this effect was highest in young workers with the lowest tenure in the plant. There was no increase of
abnormal electrocardiograms between workers and their matched controls. The authors surmised that
manganese’s ability to lower the diastolic blood pressure weakens with age as the elasticity of the blood
vessels deteriorates.
Hobbesland et al. (1997b) reported a significantly increased incidence in sudden death mortality for
workers in ferromanganese/silicomanganese plants during their employment period (standardized
mortality ratio [SMR]=2.47). The sudden deaths included cardiac deaths and other natural causes. More
specifically, among furnace workers, who are more likely to be exposed to manganese fumes and dusts
than non-furnace workers, the mortality during active-person time was statistically significantly elevated
(38.7%) compared to non-furnace workers (23.3%; p<0.001). However, the authors caution that the
association of increased death and manganese exposure is speculative and the increase in sudden death
could also be caused by common furnace work conditions (heat, stress, noise, carbon monoxide, etc.).
64
MANGANESE
3. HEALTH EFFECTS
No studies on cardiovascular effects from inhalation exposure to MMT in humans or animals were
located.
Gastrointestinal Effects. There are no reports of gastrointestinal effects following inhalation
exposure to inorganic manganese in humans or animals.
There are no reports concerning the gastrointestinal effects following inhalation exposure to MMT in
humans or animals.
Hematological Effects. Examination of blood from persons chronically exposed to high levels of
manganese in the workplace has typically not revealed any significant hematological effects (Mena et al.
1967; Roels et al. 1987a; Smyth et al. 1973; Whitlock et al. 1966). The effect of manganese exposure on
erythrocyte superoxide dismutase activity remains inconsistent; some investigators observed increased
activity among male manganese smelters (Yiin et al. 1996), while others reported decreased activity in
male welders (Li et al. 2004). However, an increased plasma malondialdehyde level is consistent
between manganese-exposed smelters (Yiin et al. 1996) and welders (Li et al. 2004). Malondialdehyde is
a product of lipid peroxidation; lipid peroxidation is believed to be a mechanism for cell toxicity. The
authors observed that plasma malondialdehyde and manganese levels were strongly correlated in exposed
workers and interpreted this response to be an indicator of manganese toxicity via lipid peroxidation.
No studies on hematological effects from inhalation exposure to MMT in humans or animals were
located.
Hepatic Effects. Even though the liver actively transports manganese from blood to bile (see
Section 3.4.4), there is no information to indicate that the liver is adversely affected by manganese;
however, there are few specific studies on this subject. In a study by Mena et al. (1967), workers
chronically exposed to manganese dust in the workplace exhibited no abnormalities in serum levels of
alkaline phosphatase. Of 13 patients who were hospitalized with chronic manganese poisoning, 1 had a
20% sulfobromophthalein (SBP) retention and 1 had a 12% SBP retention, although histological
examination of a liver biopsy from the latter revealed no abnormal tissue (Mena et al. 1967). No
significance was ascribed to the elevated SBP retention.
65
MANGANESE
3. HEALTH EFFECTS
Rats exposed to manganese tetroxide dusts for 9 months exhibited no adverse effects or histopathological
lesions; however, slight increases in liver weights were noted (Ulrich et al. 1979b). These data, although
limited, indicate that the liver is not significantly injured by manganese.
No studies on hepatic effects from inhalation exposure to MMT in humans or animals were located.
Musculoskeletal Effects. No studies were located concerning musculoskeletal effects from
inhalation exposure to inorganic manganese.
No studies were located concerning musculoskeletal effects from inhalation exposure to MMT in humans
or animals.
Renal Effects. The kidney is not generally considered to be a target for manganese, but specific
studies are rare. No abnormalities in urine chemistry were detected in workers chronically exposed to
manganese dusts in the workplace (Mena et al. 1967), but other more sensitive tests of renal function
were not performed.
No studies were located regarding renal effects in animals after inhalation exposure to inorganic
manganese.
No studies on renal effects from inhalation exposure to MMT in humans or animals were located.
Endocrine Effects. Few studies have measured endocrine effects in humans exposed to inorganic
manganese. Two studies measured hormonal levels after exposure to manganese. The first study
(Alessio et al. 1989) involved chronic exposure of foundry workers to manganese for approximately
10 years. The exposure levels were reported as 0.04–1.1 mg manganese/m
3
(particulate matter) and 0.05–
0.9 mg/m
3
as fumes. These levels overlap the current American Congress of Governmental Industrial
Hygiene (ACGIH) threshold limit value-time weighted average (TLV-TWA) of 0.2 mg/m
3
for particulate,
but are less than the limit of 1 mg/m
3
for manganese fumes. The study reported both elevated prolactin
levels and elevated cortisol levels; however, no changes in the levels of follicle stimulating hormone
(FSH) and luteinizing hormone (LH) were noted.
Smargiassi and Mutti (1999) reported effects in a group of workers from a ferroalloy plant who were
exposed occupationally to elevated levels of airborne manganese. Serum prolactin levels in these workers
66
MANGANESE
3. HEALTH EFFECTS
were evaluated in a 1992 study and again in a 1997 study. Serum prolactin levels, which were
significantly elevated in the earlier analysis, had also increased significantly over the earlier measurement
(p<0.001). This difference was especially noticeable in those with abnormally high prolactin levels.
During the five year period between studies, exposure levels were consistent and were not reduced;
therefore, it is unclear whether prolactin levels reflect current or cumulative exposure.
Other elements of endocrine function (reproductive function, etc.) are discussed elsewhere in the text.
No studies were located regarding endocrine effects in animals after inhalation exposure to inorganic
manganese.
No studies on endocrine effects from inhalation exposure to MMT in humans or animals were located.
Dermal Effects. No studies have been located concerning dermal effects in humans or animals
following inhalation exposure to inorganic or organic manganese.
Ocular Effects. No studies have been located concerning ocular effects in humans or animals
following inhalation exposure to inorganic manganese.
There are no studies reporting ocular effects following inhalation exposure of humans to MMT. One- and
4-hour exposures to doses of MMT used in lethality studies resulted in conjunctivitis in rats (Hinderer
1979).
Body Weight Effects. No studies were located regarding body weight effects in humans following
exposures to inorganic manganese.
No studies were located regarding body weight effects in humans following inhalation exposure to MMT.
Hinderer (1979) observed a decrease in weight gain in Sprague-Dawley rats during the first 7 days
following a 1- or 4-hour exposure to unspecified MMT concentrations in an acute toxicity test. The rats
resumed their normal weight gain by 14 days post-exposure.
Metabolic Effects. No studies were located concerning metabolic effects from inhalation of inorganic
manganese in humans or animals.
67
MANGANESE
3. HEALTH EFFECTS
No studies were located concerning metabolic effects following inhalation exposure to MMT in humans
or animals.
3.2.1.3 Immunological and Lymphoreticular Effects
One study on immunological effects in humans following inhalation to inorganic manganese was located.
Male welders exposed to manganese (0.29–0.64 mg/m
3
for an unspecified duration), vibration, and noise
exhibited suppression of the T and B lymphocytes characterized by reductions in serum immunoglobin G
(IgG) and total E-rosette-forming cells (Boshnakova et al. 1989). However, the welders in this study
were exposed to numerous other compounds, including cobalt, carbon dioxide, and nitric oxide.
Therefore, it is impossible to determine whether exposure to manganese caused the effects. It is not
known whether any of these changes are associated with significant impairment of immune system
function. No studies were located on lymphoreticular effects in humans exposed to manganese by the
inhalation route.
No studies were located on immunological or lymphoreticular effects in animals exposed to inorganic
manganese by the inhalation route.
As noted above, inhalation exposure to particulate manganese compounds can lead to an inflammatory
response in the lung (i.e., pneumonitis), and this is accompanied by increased numbers of macrophages
and leukocytes in the lung (Bergstrom 1977; Lloyd Davies 1946; Shiotsuka 1984; Suzuki et al. 1978).
However, this is an expected adaptive response of the immune system to inhaled particulates, and these
data do not indicate that the immune system is injured. Conflicting data are reported concerning
increased susceptibility to bacterial infection after exposure to airborne manganese. Lloyd Davies (1946)
indicated that manganese exposure did not increase the susceptibility of mice to bacterial infection,
whereas Maigetter et al. (1976) reported that exposure to aerosolized manganese dioxide altered the
resistance of mice to bacterial and viral pneumonias.
No studies on immunological or lymphoreticular effects from inhalation exposure to MMT in humans or
animals were located.
3.2.1.4 Neurological Effects
Overview. Neurological effects from repeated inhalation exposure to manganese are well recognized as
effects of high concern based on case reports and epidemiological studies of groups of occupationally
68
MANGANESE
3. HEALTH EFFECTS
exposed and environmentally exposed people and results from animal inhalation studies. The highest
NOAEL values and all LOAEL values from each reliable study for neurological effects in each species
and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
There is conclusive evidence from studies in humans that inhalation exposure to high levels of manganese
compounds (usually manganese dioxide, but also compounds with Mn(II) and Mn(III)) can lead to a
disabling syndrome of neurological effects referred to as ‘manganism’.
Studies estimating the impact of low-level exposure to manganese on neurological health have employed
a number of sensitive tests designed to detect signs of neuropsychological and neuromotor deficits in the
absence of overt symptoms (Iregren 1990, 1994, 1999). These analyses allow the comparison of discrete
performance values that are associated with either biological levels of manganese or approximations of
exposure levels. Thus, they allow for the comparison of one exposure group to another without the
subjective description of neurological symptoms that were prevalent in the studies with miners and others
with frank manganism. A number of epidemiological studies have used these techniques to study the
psychological or neurological effects of exposure to low levels of manganese in the workplace (Bast-
Pettersen et al. 2004; Beuter et al. 1999; Blond and Netterstrom 2007; Blond et al. 2007; Bouchard et al.
2003, 2005, 2007a, 2007b; Chia et al. 1993a, 1995; Crump and Rousseau 1999; Deschamps et al. 2001;
Gibbs et al. 1999, Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Myers et al. 2003a,
2003b; Roels et al. 1987a, 1992, 1999; Summers et al. 2011; Wennberg et al. 1991) or in environmental
media close to manganese-emitting industries (Hernández-Bonilla et al. 2011; Lucchini et al. 2007; Kim
et al. 2011; Menezes-Filho et al. 2011; Mergler et al. 1999; Riojas-Rodríguez et al. 2010; Rodríguez-
Agudelo et al. 2006; Solís-Vivanco et al. 2009; Standridge et al. 2008). Some of these studies have found
statistically significant differences between exposed and non-exposed groups or significant associations
between exposure indices and neurological effects (Bast-Pettersen et al. 2004; Chia et al. 1993a; Iregren
1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Roels et al. 1987a, 1992; Wennberg et al. 1991),
whereas others have not found significant associations (Deschamps et al. 2001; Gibbs et al. 1999, Myers
et al. 2003a, 2003b; Summers et al. 2011; Young et al. 2005). The neurological effects associated with
prolonged low-level manganese exposure generally have been subtle changes, including deficits in tests
of neuromotor or cognitive functions and altered mood states; they have been referred to by various
authors as preclinical or subclinical neurological effects. As shown in Table 3-1 and Figure 3-1,
manganese air concentrations associated with these effects in chronically exposed workers range from
about 0.07 to 0.97 mg manganese/m
3
(manganese in total or inhalable dust measurements). For several of
69
MANGANESE
3. HEALTH EFFECTS
these work environments, values of concentrations of manganese in respirable dust (generally particulate
diameters <10 µm) represented <20–80% of the total dust values.
Manganism from High-Level Occupational Exposure to Inorganic Manganese. There is conclusive
evidence from studies in humans that inhalation exposure to high levels of manganese compounds
(usually manganese dioxide, but also compounds with Mn(II) and Mn(III)) can lead to a disabling
syndrome of neurological effects referred to as ‘manganism’. Manganism is a progressive condition that
usually begins with relatively mild symptoms, but evolves to include dull affect, altered gait, fine tremor,
and sometimes psychiatric disturbances. Some of these symptoms also occur with Parkinson’s disease,
which has resulted in the use of terms such as “Parkinsonism-like disease” and “manganese-induced
Parkinsonism” to describe those symptoms observed with manganese poisoning. Despite the similarities,
significant differences between Parkinsonism and manganism do exist (Barbeau 1984; Calne et al. 1994;
Chu et al. 1995). Barbeau (1984) reported that the hypokinesia and tremor present in patients suffering
from manganism differed from those seen in Parkinson’s disease. Calne et al. (1994) noted other
characteristics that distinguish manganism from Parkinson’s disease: psychiatric disturbances early in the
disease (in some cases), a “cock-walk,” a propensity to fall backward when displaced, less frequent
resting tremor, more frequent dystonia, and failure to respond to dopaminomimetics (at least in the late
stages of the disease).
Manganism and Parkinson’s disease also differ pathologically. In humans and animals with chronic
manganese poisoning, lesions are more diffuse, found mainly in the pallidum, caudate nucleus, the
putamen, and even the cortex with no effects on the substantia nigra and no Lewy bodies (Pal et al. 1999;
Perl and Olanow 2007). In people with Parkinson’s disease, lesions are found in the substantia nigra and
other pigmented areas of the brain (Barbeau 1984). Moreover, Lewy bodies are usually not found in
substantia nigra in manganism cases, but are almost always found in cases of Parkinson’s disease (Calne
et al. 1994; Perl and Olanow 2007). Manganese appears to affect pathways that are post-synaptic to the
nigrostriatal system, most likely the globus pallidus (Chu et al. 1995). MRI of the brain reveals
accumulation of manganese in cases of manganism, but few or no changes in people with Parkinson’s
disease; fluorodopa positron emission tomography (PET) scans are normal in cases of manganism, but
abnormal in people with Parkinson’s disease (Calne et al. 1994). Other studies suggest that manganese
produces a syndrome described as parkinsonism, distinct from Parkinson’s Disease or manganism
(Lucchini et al. 2007, Racette et al. 2005). It is likely that the terms Parkinson-like-disease and
manganese-induced-Parkinsonism will continue to be used by those less knowledgeable about the
significant differences between the two. Nonetheless, comparison with Parkinson’s disease and the use of
70
MANGANESE
3. HEALTH EFFECTS
these terms may help health providers and health surveillance workers recognize the effects of manganese
poisoning when encountering it for the first time.
Typically, the clinical effects of high-level inhalation exposure to manganese do not become apparent
until exposure has occurred for several years, but some individuals may begin to show signs after as few
as 1–3 months of exposure (Rodier 1955). The first signs of the disorder are usually subjective, often
involving generalized feelings of weakness, heaviness or stiffness of the legs, anorexia, muscle pain,
nervousness, irritability, and headache (Mena et al. 1967; Nelson et al. 1993; Rodier 1955; Tanaka and
Lieben 1969; Whitlock et al. 1966). These signs are frequently accompanied by apathy and dullness
along with impotence and loss of libido (Abdel-Hamid et al. 1990; Emara et al. 1971; Mena et al. 1967;
Nelson et al. 1993; Rodier 1955; Schuler et al. 1957). Early clinical symptoms of the disease include a
slow or halting speech without tone or inflection, a dull and emotionless facial expression, and slow and
clumsy movement of the limbs (Mena et al. 1967; Nelson et al. 1993; Rodier 1955; Schuler et al. 1957;
Shuqin et al. 1992; Smyth et al. 1973; Tanaka and Lieben 1969). In a study by Wolters et al. (1989),
6-fluorodopa (6-FD) and
18
F-2-fluoro-2-deoxyglucose (FDG) PET were used to investigate the
neurochemistry of four patients with "early manganism." FDG PET demonstrated decreased cortical
glucose metabolism. No anomalies were noted in the 6-FD scans. This led the authors to suggest that, in
early manganism, damage may occur in pathways that are postsynaptic to the nigrostriatal system, and
most likely involve striatal or pallidal neurons.
As the disease progresses, walking becomes difficult and a characteristic staggering gait develops.
Muscles become hypertonic, and voluntary movements are accompanied by tremor (Mena et al. 1967;
Rodier 1955; Saric et al. 1977a; Schuler et al. 1957; Smyth et al. 1973). Few data are available regarding
the reversibility of these effects. They are thought to be largely irreversible, but some evidence indicates
that recovery may occur when exposure ceases (Smyth et al. 1973). Manganism has been documented in
welders and in workers exposed to high levels of manganese dust or fumes in mines or foundries.
Extreme examples of psychomotor excitement have been observed in manganese miners and, to a lesser
extent, in industrial workers (Chu et al. 1995; Mena et al. 1967; Nelson et al. 1993). The behavior,
known as “manganese madness” (Mena 1979) includes nervousness, irritability, aggression, and
destructiveness, with bizarre compulsive acts such as uncontrollable spasmodic laughter or crying,
impulses to sing or dance, or aimless running (Emara et al. 1971; Mena et al. 1967; Rodier 1955; Schuler
et al. 1957). Patients are aware of their irregular actions, but appear incapable of controlling the behavior.
71
MANGANESE
3. HEALTH EFFECTS
The reports of frank manganism (Rodier 1955; Schuler et al. 1957; Smyth et al. 1973) observed in
manganese miners clearly indicate that the onset of manganism results from chronic exposure to high
concentrations of the metal. Documented cases indicate that the most important route of exposure is
inhalation of manganese dusts or fumes, while other pathways such as ingestion of the metal from
mucociliary transport of larger particles and hand-to-mouth activity, may contribute a smaller amount.
Based on the data provided by Rodier (1955) and Schuler et al. (1957), it appears that the frequency of
manganism cases increased with prolonged exposure, suggesting that the seriousness of the symptoms
presented increases with cumulative exposure. For example, Rodier (1955) reports that the highest
percentage of manganism cases (28, or 24.4%) occurred in miners with 1–2 years experience. Only six
cases of manganism (5.2%) were reported in males with 1–3 months exposure, and 68% of the cases
reported occurred after exposures >1–2 years in length. Rodier did not present statistics on the number of
men in the mine who were employed for comparable durations who did not suffer from manganism.
Schuler et al. (1957) studied fewer manganism cases, but showed that the number of men with
manganism increased with time spent mining, with the average time delay before onset of the disease
being 8 years, 2 months. In fact, the minimum duration of exposure to the metal was 9 months before
signs of manganism became recognizable, and the maximum exposure was 16 years. However, Schuler
et al. (1957) point out that their study was not intended to “suggest incidence rates” and of the 83 miners
selected for examination of potential manganism, only 9 were chosen as actually suffering from
manganese poisoning. As with the Rodier (1955) study, the Schuler et al. (1957) study did not discuss the
exposure duration or symptomatology of those men not displaying “frank manganism;” therefore, these
collective data, although suggestive of a cumulative effect of manganism neurotoxicity, must be
interpreted with caution.
Huang et al. (1998) documented the progression of clinical symptoms of manganism in five surviving
workers (from an original six) chronically exposed to manganese in a ferroalloy plant. These men were
exposed from 3 to 13 years and were examined 9–10 years after manganese exposure had ceased.
Neurologic examination revealed a continuing deterioration of health exhibited in gait disturbance, speed
of foot tapping, rigidity, and writing. The men had high concentrations of manganese in blood, urine,
scalp, and pubic hair at the time of the original neurologic evaluation. Follow-up analyses revealed a
drastic drop in manganese concentrations in these fluids and tissues (e.g., 101.9 μg/g manganese in blood
from patient 1 in 1987; 8.6 μg/g manganese in blood in 1995). Further, T1-weighted MRI analysis did
not reveal any high-signal intensity areas. These data support the progression and permanence of clinical
effects from excess manganese exposure, even when tissue levels of the metal had returned to normal.
Further, it shows that this neurotoxicity can continue in the absence of continuing manganese exposure
72
MANGANESE
3. HEALTH EFFECTS
and that a spectrum of responses to excess manganese exposure can be seen depending upon dose,
duration of exposure, and timing of the observation. While some subclinical manifestations of manganese
neurotoxicity will resolve, once neuropathology has occurred (in the form of loss of dopaminergic
neurons), then reversal becomes more limited and is restricted to functional compensation.
As shown in Table 3-1 and Figure 3-1, cases of frank manganism have been associated with workplace
exposure levels ranging from about 2 to 22 mg manganese/m
3
(Cook et al. 1974; Rodier 1955; Saric et al.
1977; Schuler et al. 1957; Tanaka and Lieben 1969; Whitlock et al. 1966). For example, Tanaka and
Lieben (1969) reported that no cases of frank manganism were diagnosed in 38 workers from
Pennsylvania industrial plants in which estimated air concentrations were below 5 mg manganese/m
3
,
whereas 7 cases were diagnosed in 117 workers from plants with air concentrations exceeding 5 mg/m
3
.
Whitlock et al. (1966) reported on two cases of frank manganism in workers who were exposed to
estimated air concentrations ranging from 2.3 to 4.7 mg manganese/m
3
.
Neurological Assessments of Workers Exposed to Low Levels of Inorganic Manganese. In a cross-
sectional epidemiological study of 141 male workers in a manganese dioxide and salt producing plant,
Roels et al. (1987a) detected preclinical neurological effects (alterations in simple reaction time,
audioverbal short-term memory capacity, and hand tremor) in workers exposed to 0.97 mg manganese
(median concentration in total dust)/m
3
as manganese dioxide, manganese tetroxide, manganese sulfate,
manganese carbonate, and manganese nitrite for a group average of 7.1 years. End points in exposed
workers were compared with end points in a matched control group of 104 non-exposed male workers
from a nearby chemical plant. The prevalences of subjective symptoms were similar in exposed and
control workers, except for the elevation of nonspecific symptoms (such as fatigue, tinnitus, trembling of
fingers, and increased irritability) in the exposed workers. Statistically significant mean deficits were
found in exposed workers (compared with controls) in tests of simple reaction time (visual), audioverbal
short-term memory capacity, eye-hand coordination, and hand steadiness. The prevalence of abnormal
values in the neurological tests were not statistically significantly correlated with manganese levels in
blood or urine or duration of employment, with the exception that blood levels of manganese were
correlated with prevalence of abnormal responses in tests of eye-hand coordination and hand steadiness.
Iregren (1990) used neurobehavioral tests (simple reaction time, digit span, finger tapping, verbal ability,
hand dexterity, and finger dexterity tests from the Swedish Performance Evaluation System, SPES) to
study adverse effects in 30 male workers from two different manganese foundries exposed to an estimated
median concentration of 0.14 mg manganese (in total dust)/m
3
as manganese dioxide for 1–35 years
73
MANGANESE
3. HEALTH EFFECTS
(mean, 9.9 years). The exposed workers had below-average scores on a number of the tests, such as
reaction time and finger tapping, when compared to matched controls with no occupational manganese
exposure.
Roels et al. (1992) provided similar results to these earlier reports. Workers in a dry alkaline battery
factory exhibited impaired visual reaction time, hand-eye coordination, and hand steadiness when
exposed to concentrations of manganese dioxide in total dust ranging from 0.046 to 10.840 mg
manganese/m
3
and in respirable dust from 0.021 to 1.32 mg manganese/m
3
(exposure ranged from 0.2 to
17.7 years). A lifetime integrated exposure (LIE) for both total manganese dust and respirable manganese
was estimated for each of the exposed workers (LIE=∑((C
job 1
x T
1
) + (C
job 2
x T
2
) + ... (C
job n
x T
n
)),
where C is concentration, T is years of exposure, and LIE is expressed as mg manganese/m
3
times year).
Based on the analysis of data by a logistic regression model, it was suggested that there was an increased
risk (odds ratio [OR]=6.43, 95% confidence interval [CI]=0.97–42.7) of decreased hand steadiness at a
lifetime integrated exposure level of 3.575 mg/m
3
*year for total dust or 0.730 mg/m
3
*year for respirable
dust. It should be noted that the LIE at which an increased risk of abnormal neurological function occurs
is based on exposures in an occupational setting. Therefore, periods of exposures would be followed by
periods that would be relatively free of manganese inhalation. Presumably, during these “rest” periods
the homeostatic mechanism would excrete excess manganese to maintain the manganese concentration
within physiologic limits. Further, the LIE for deleterious neural effects may be biased in favor of a
higher concentration due to the “healthy worker effect” (i.e., the most susceptible individuals are not
incorporated into the study).
Crump and Rousseau (1999) performed a follow-up study of 213 men occupationally exposed to
manganese, 114 of whom were subjects in the Roels et al. (1987a, 1987b) studies. Exposure data were
unavailable during the 11 years of study (1985–1996) during which blood and urine samples were taken
and neurological tests (short-term memory, eye-hand coordination, and hand steadiness) were
administered as in the Roels studies. Yearly blood and urine manganese levels remained fairly consistent
throughout the study period, and were comparable to the levels reported in the previous studies. The
authors suggest that the consistency of these data on manganese levels indicates that the airborne
manganese concentrations to which the subjects were exposed during the study period were likely
comparable to those at the time of the Roels studies. The average age and exposure duration of the
subjects increased from 36 and 7 years, respectively, in 1985, to 41 and 14 years, respectively, in 1996.
Variations in year-to-year test results were observed that were not attributable to age of the subject or
exposure to manganese. The authors observed decreases in errors in the short-term memory test (number
74
MANGANESE
3. HEALTH EFFECTS
of repeated words and number of errors). During 1987, 1988, and 1989, the average number of words
remembered on the memory test was lower than in any other year. However, there was a progressive
improvement in percent precision and percent imprecision on the eye-hand coordination test during 1985–
1988 (after 1991, the design of the test was modified and percent imprecision was lower in that year and
all subsequent testing years). The authors suggest several reasons for the inter-year variability in test
results (Crump and Rousseau 1999), including variations in test conditions, different groups of workers
being tested in different years, the mood of the workers following a plant restructuring, and increased
caution on the part of the subjects when answering test questions. When data analysis was controlled for
year of testing, older workers performed significantly worse than younger workers on total words recalled
in the memory test, and on percent precision and percent sureness in the eye-hand coordination test.
Further, blood and urine manganese levels were not significantly associated with performance on memory
or eye-hand coordination tests, but blood manganese was negatively associated with performance on the
hand steadiness test (p<0.05). Age was not a factor in hand steadiness when the year of test was
controlled for in the analysis. Crump and Rousseau (1999) investigated whether individual test scores
worsened with time by studying the group of 114 men from the original Roels et al. (1987a, 1987b)
studies and a subset of 44 long-term employees who had been given both memory and hand steadiness
tests on two occasions, 8 years apart. These analyses revealed decreases in performance over time for a
particular hole in the hand steadiness test and improvements in repetitions and errors on the memory test,
both of which were statistically significant. The authors suggest that the improvements in the memory
test were likely the result of increased caution on the part of the subject. The changes in performance
over time could not be attributed solely to manganese exposure because it was impossible to control for
age and year of testing in all of the analyses. The authors noted the lack of an age-matched control group
with which to compare test results and the absence of data caused by workers ending their terms of
employment. Some have questioned whether inter-year variability in test results, potentially caused by
different test administrators over time, would affect interpretation of the findings. While this may
contribute to the changes in performance over time seen in the Crump and Rousseau (1999) study, this
factor will potentially impact any study of this type. The lack of a control group precludes the
determination of a reliable NOAEL or LOAEL based on the results of this study.
A study by Mergler et al. (1994) also supports the work of Iregren and Roels. This epidemiologic study
included 115 (95% of the total) male workers from a ferromanganese and silicomanganese alloy factory
who were matched to other workers from the region with no history of exposure. The groups were
matched on the following variables: age, sex, educational level, smoking, and number of children. These
workers were exposed to both manganese dioxide dusts and manganese fumes. Environmental levels of
75
MANGANESE
3. HEALTH EFFECTS
manganese in total dust were measured at 0.014–11.48 mg/m
3
(median, 0.151 mg/m
3
; arithmetic mean,
1.186 mg/m
3
, geometric mean, 0.225 mg/m
3
), while manganese levels in respirable dust were 0.001–
1.273 mg/m
3
(median, 0.032 mg/m
3
; arithmetic mean, 0.122 mg/m
3
; geometric mean, 0.035 mg/m
3
), and
mean duration of exposure was 16.7 years. The exposed workers had significantly greater blood
manganese levels, but urinary manganese did not differ between groups. Manganese workers showed
decreased performance on tests of motor function (including those from the SPES) as compared to
matched control workers with no manganese exposure. Using test results obtained from performance of
the groups on the Luria-Nebraska Neuropsychological Battery and other tests, the authors reported that
manganese-exposed workers performed more poorly than controls on tests of motor function, particularly
on tests that required alternating and/or rapid hand movements and hand steadiness. The exposed workers
also differed significantly from the controls in cognitive flexibility and emotional state. They also
exhibited significantly greater levels of tension, anger, fatigue, and confusion. Further, these workers had
a significantly lower olfactory threshold than controls; this is the first study to report this effect following
inhalation exposure to manganese. Several follow-up studies of the workers from this manganese alloy
plant are described later in this section (Bouchard et al. 2005, 2007a, 2007b).
Similar effects to those observed in the Mergler et al. (1994) study were observed by Chia et al. (1993a).
Workers in a manganese ore milling plant exposed to 1.59 mg manganese (mean concentration in total
dust)/m
3
exhibited decreased scores in several neurobehavioral function tests including finger tapping,
digit symbol, and pursuit aiming. Further, the workers exhibited an increased tendency for postural sway
when walking with their eyes closed (Chia et al. 1995).
An epidemiologic study (Lucchini et al. 1995) also supports findings of these studies concerning the
preclinical neurological effects of manganese exposure. This study, which evaluated performance on
neuromotor tests (seven tests from the SPES, including simple reaction time, finger tapping, digit span,
additions, symbol digit, shapes comparison, and vocabulary) involved 58 male workers from a ferroalloy
plant. The workers had been exposed for 1–28 years (mean, 13; standard deviation [SD], 7) to geometric
mean airborne concentrations of manganese, as manganese dioxide, in total dust as high as 0.070–
1.59 mg/m
3
(geometric means in different areas). These concentrations had decreased in the last 10 years
to a range of 0.027–0.270 mg manganese (in total dust)/m
3
. At the time of the study, the exposed workers
were undergoing a forced cessation from work of 1–48 days. Blood and urine manganese levels were
analyzed. A cumulative exposure index (CEI) was calculated for each subject by multiplying the average
annual airborne manganese concentration in respirable dust characteristic of each job by the number of
years for which this activity was performed. Significant correlations were found between the log value of
76
MANGANESE
3. HEALTH EFFECTS
blood manganese concentrations in exposed workers and the tests of additions, digit span, finger tapping,
and symbol digit (log values for the last two tests); between the log value of urinary manganese levels and
the performance on the additions test; and between the log value of the CEI and the log value of the
symbol digit score. Further, a significant correlation on an individual basis was found between external
exposure, represented by CEI, and blood and urine manganese levels. These results are unique in that
they are the first to suggest that blood and urine manganese concentrations are indicative of exposure on
an individual basis. As suggested by Lucchini et al. (1995), the correlations may be observable in this
study, when they have not existed in past studies (Roels et al. 1987a, 1992), because the workers were
assessed at a time when they were not currently being exposed to manganese. In support of this
possibility, the correlation coefficients between the urine and manganese levels and the CEI increased
with time elapsed since the last exposure to airborne manganese (Lucchini et al. 1995).
Roels et al. (1999) performed an 8-year prospective study with 92 subjects exposed to manganese dioxide
at a dry-alkaline battery plant (Roels et al. 1992) to determine if poor performance on tests measuring
visual reaction time, eye-hand coordination, and hand steadiness could be improved if occupational
manganese exposure were decreased. The workers were divided into “low” (n=23), “medium” (n=55),
and “high” (n=14) exposure groups depending on location within the plant and job responsibility. At the
end of the 1987 study, technical and hygienic improvements had been implemented within the plant to
decrease atmospheric manganese concentrations. Yearly geometric mean values for airborne total
manganese dust (MnT) in the “low,” “medium,” and “high” exposure areas decreased in the following
manner, respectively: ~0.310–~0.160; ~0.900–~0.250; and ~3–~1.2 mg/m
3
. The cohort decreased from
92 subjects in 1987 to 34 subjects in 1995 due to turnover, retirement, or dismissal, but no worker left due
to neurological signs or symptoms. A separate group of workers was selected who had prior manganese
exposure (ranging from 1.3 to 15.2 years). These subjects had left the manganese processing area of the
plant prior to the end of 1992, and therefore, their exposure to manganese had ceased at that time; these
workers were still employed in other areas of the plant. The control group consisted of 37 workers
employed at the same polymer factory that had provided the control population in the previous study
(Roels et al. 1992). This group, with an average age of 38.5 (range, 32–51 years) allowed for the analysis
of age as a confounder. Exposure data (respirable manganese and total manganese dust, MnT) were taken
with personal air samplers. Time-trend analysis of air sampler data revealed a significant decrease in total
manganese from 1987 to 1995, with a more pronounced decline from 1992 forward. From 1987 to 1990,
the authors observed that the precision of the hand-forearm movement (PN1) in the eye-hand coordination
test for the whole cohort worsened, but then got progressively better. Hand steadiness and visual simple
reaction time variables were inconsistent over time, and time-trends were not observed. When the cohort
77
MANGANESE
3. HEALTH EFFECTS
was divided into exposure groups, and analyzed for performance on the eye-hand coordination test, it was
revealed that in general, the performance on the PN1 aspect of the test improved from 1987 to 1995,
especially after 1991. The performance of the “low-dose” group was comparable to that of the control
group in 1987 (Roels et al. 1992) and to that of the control group in 1997. The performance of the
“medium-dose” group was intermediate between the “low-dose” and “high-dose” group. The only
significant differences in performance were in the “high-dose” group as compared to the “low-dose”
group during the years 1988–1990 (test scores of 49–51 for the high-dose group and 63–65 for the “low-
dose” group). However, it was noted that performance on the eye-hand coordination test for the
“medium” and “high-dose” groups was considerably poorer than the controls.
Significant differences were noted in variables in the hand steadiness test between the exposure groups
during 1987–1992 (data not reported), when manganese concentrations were at their highest. However,
no readily identified temporal changes in performance among the groups on this test was found, nor with
the visual reaction time test. When the authors performed separate time-trend analysis on MnT levels and
PN1 (eye-hand coordination test) values, a significant time effect was present for each variable. An
analysis of covariance was performed for each exposure group (low, medium, and high) in which log
MnT was considered as covariate in order to adjust for estimation of PN1 variations as log MnT changed
over time. The resultant data suggested that a reduction in log MnT was associated with an improvement
in PN1 for each group. The authors also found that when time was also considered with log MnT as an
interaction term, it did not influence PN1 variations over the years and the effect of time on PN1 values
disappeared when log MnT was maintained as an ordinary covariate. The authors interpreted this to mean
that performance on the eye-hand coordination tests were only related, and inversely so, to the exposure
to manganese. In other words, when manganese exposure was increased, test performance decreased and
vice versa (Roels et al. 1999). However, in the high-exposure group, the performance increased from
71 to 83% of that of the control group, and leveled off at this point, despite decreased manganese
exposure occurring from 1991/1992 with most dramatic improvements occurring in 1994. The authors
suggest that this leveling off of performance by the high-exposure group may be indicative of a
permanent effect of manganese on eye-hand coordination. The authors tested PN1 values in exposed
subjects 3 years following a cessation of exposure. They found that in 20/24, the PN1 values were below
the mean PN1 values of the control group, but 16 of these individuals showed an improvement in 1996
(percent improvement unspecified). The remaining four subjects (three “low-exposure” and one
“medium-exposure” subjects) had PN1 values that exceeded the mean value of the control group.
However, these data indicate that although there was improvement in performance on the coordination
test, the vast majority of the exposed group still could not perform to the level of an unexposed worker
78
MANGANESE
3. HEALTH EFFECTS
3 years after manganese exposure ceased. In addition, the exposed workers who did perform as well or
better than the control subjects were among the least exposed workers while at the plant. As discussed
previously, performance of the “low-exposure” group on eye-hand coordination tests during 1992–1995
was comparable to that of the control groups from 1987 and 1997, indicating that manganese exposure of
these individuals during that time did not severely impact their ability to perform this neurobehavioral
test. Comparable performance on the tests by the same control group in 1987 and 10 years later, in 1997,
indicates that age was not a confounder in this study. None of the variables except visual reaction time
was significantly correlated with age, and the existing correlation in the visual reaction time test only
represented a 3% difference (Roels et al. 1999).
Lucchini et al. (1999) also investigated differences in neurobehavioral test performance over time as
exposure to manganese (manganese dioxide and manganese tetroxide) decreased. The study group
consisted of 61 men who worked in different areas of a ferroalloy plant. The plant was divided into three
exposure areas with total manganese dust (geometric mean) values decreasing from 1981 to 1995: “high-
exposure” values decreased from 1.6 to 0.165 mg/m
3
; “medium-exposure” values decreased from 0.151 to
0.067 mg/m
3
; and “low-exposure” values decreased from 0.57 to 0.012 mg/m
3
. The authors estimated
that the annual average manganese concentration in the “medium-exposure” group was 0.0967 mg
manganese in total dust/m
3
. Respirable dust constituted 40–60% of the total dust value. Control subjects
consisted of 87 maintenance and auxiliary workers from a nearby hospital who had not been exposed to
neurotoxins. The study and control groups were well matched except for years of education and the
percentage of subjects working night shifts. The study groups answered a questionnaire concerning
neuropsychological and Parkinsonian symptoms and underwent testing to determine the effect of
manganese on neuromotor performance. Four tests were from the SPES (addition, digit span, finger
tapping, symbol digit) and five timed tasks were from the Luria Nebraska Neuropsychological Battery
(open-closed dominant hand--Luria 1, open-closed non-dominant hand—Luria 2, alternative open-closed
hands—Luria 3, thumb-fingers touch dominant hand—Luria 4, and thumb-fingers touch non-dominant
hand—Luria 5). Individual scores were taken from these subtests, and the sum of the Luria tests was
taken (Luria sum). Postural tremor was also measured, as was visual reaction time and coordination
ability via the hand pronation/supination test. Manganese levels in blood and urine, as well as blood lead
levels were analyzed prior to each neurobehavioral test. Manganese levels in both blood and urine were
significantly elevated in exposed workers compared to controls (p<0.0001). Blood lead levels were also
significantly higher in the ferroalloy workers (p=0.0002). The authors noted that the study groups did not
report as many complaints as those reported in the Mergler et al. (1994) study.
79
MANGANESE
3. HEALTH EFFECTS
After correcting for age, education, alcohol, smoke, coffee, shift work, and blood lead levels, an analysis
of test results indicated that performance of the exposed workers was significantly different than that of
controls on all tests except for Luria 5 and Luria sum (Lucchini et al. 1999). A comparison of SPES test
results from workers tested in 1990 or 1991 and those from this study did not indicate any difference in
paired t-test values; this indicates that performance did not improve over time or with decreasing
exposure to manganese. CEI values were calculated (in the same manner as in Lucchini et al. [1995]) for
each exposure group and performance on the neurobehavioral tests was analyzed for correlation to these
values and to manganese levels in body fluids. Significant differences were found between those with
low CEI values of <0.5 mg/m
3
*years, mid CEI values of 0.5–1.8 mg/m
3
*years, and high CEI values of
>1.8 mg/m
3
*years and performance on the following tests: symbol digit, finger tapping, dominant and
non-dominant hand, and digit span. A positive correlation was observed between the log CEI value and
these tests, indicating that performance decreased as exposure increased. No correlations were found
between CEI values and manganese levels in blood and urine; these results differ from the correlation
between CEI and manganese levels in fluids from the previous study (Lucchini et al. 1995). Lucchini et
al. (1995) estimated a manganese dose (total dust) that would represent the annual airborne manganese
concentration indicative of neurobehavioral deficit in this study by dividing the geometric mean CEI of
the mid-exposure subgroup, 1.1 mg/m
3
*years, by the geometric mean value of years of exposure for this
same subgroup, 11.51, yielding a value of 0.096 mg/m
3
. A comparable respirable dust value would be
0.038 mg/m
3
(0.096*0.40).
Gibbs et al. (1999) studied a population of workers in a U.S. plant that produces electrolytic manganese
metal. These 75 workers and a well-matched group of control workers with no manganese exposure were
administered a computerized questionnaire concerning neurological health issues (including mood,
memory, fatigue, and other issues) and were analyzed for performance on several neurobehavioral tests
including hand steadiness (Movemap steady, Movemap square, and tremor meter), eye-hand coordination
(orthokinisimeter), and rapidity of motion (four-choice reaction time and finger tapping). The Movemap
test is a relatively recent test that has not undergone widespread use, and it has not been validated by other
researchers. Further, although technically sophisticated, the test has not been observed to discriminate
between exposure groups any better than simpler current methods (Iregren 1999). Airborne levels of total
and respirable manganese were obtained using personal samplers and were not available for years prior to
1997. Using the arithmetic mean of samples collected in 12 different job categories, exposure was
estimated for the years prior to 1997. Cumulative exposure values for each worker were estimated for the
30-day and 12-month exposure periods just prior to neurobehavioral testing. Multiple regressions of the
test scores were performed using age and each of the following manganese exposure variables
80
MANGANESE
3. HEALTH EFFECTS
individually as explanatory variables: duration of exposure; 30-day cumulative exposure; 1-year
cumulative exposure; and cumulative occupational exposure to either respirable or total manganese. Shift
work was also used as a variable in conjunction with age and cumulative 30-day exposure to respirable or
total manganese. The authors threw out outlying data points if they were >3 times the SD of the residual
after a model fit. Exposures to respirable and total dust were highly correlated (r
2
, 0.62–0.75), as were
cumulative exposures over the previous 30 days and the previous year (r
2
, 0.72–0.82); however, lifetime
integrated exposure was not correlated with either 30-day or 12-month exposure values. The average
exposure value for manganese-exposed workers was estimated at 0.066±0.059 mg/m
3
(median,
0.051 mg/m
3
) for respirable dust, and 0.18±0.21 mg/m
3
for total dust.
Responses to the questionnaire and performance on the neurobehavioral tests did not differ significantly
between exposed and control groups (Gibbs et al. 1999). Cumulative years of exposure had an effect on
tapping speed—speed increased with increased exposure, but only when outliers were included in the
analysis. The authors also reported an inverse correlation between age and performance on tests
measuring eye-hand coordination but positively correlated between age and complex reaction time. The
study by Gibbs et al. (1999) is the first to report a lack of poorer performance on neurobehavioral tests by
workers chronically exposed to manganese. Interestingly, the median exposure estimates for respirable
dust in this population (0.051 mg/m
3
) is slightly higher than the lowest level of respirable dust at which
preclinical neurological effects have been seen (0.032 mg/m
3
) as reported by Mergler et al. (1994).
Gorell et al. (1999) noted a high OR of 10.51 for the development of Parkinson’s disease in individuals
>50 years old who were occupationally exposed to manganese for >20 years, but not for those exposed
for <20 years. However, the numbers of individuals with a >20-year exposure was rather small (n=4),
and occupational exposures to other metals (copper, and lead-iron, lead-copper, and iron-copper
combinations) for >20 years were also associated with increased risk for the disease.
In a cross-sectional study of 138 (114 male and 34 female) enamels-production workers, Deschamps et al.
(2001) administered a questionnaire about neurological symptoms; evaluated performance on
psychological tests of similarity recognition, vocabulary (oral word association), geometrical figure
recognition (visual gestalts), and short-term memory (digit span); and measured levels of manganese in
blood samples. Results were compared with a control group of 137 nonexposed workers matched for age,
educational level, and ethnic group. Exposed workers were employed for a mean duration of 19.87 years
(SD±9) in enamels production. Mean manganese levels in 15 personal air samples and 15 stationary air
samples collected at the plant during the year preceding the tests were 2.05 mg manganese/m
3
(SD 2.52;
81
MANGANESE
3. HEALTH EFFECTS
range 0.5–10.2) for total dust and 0.035 mg manganese/m
3
for respirable manganese (SD 0.063; range
0.01–0.293). Symptoms of asthenia, sleep disturbance, and headache were significantly elevated in
exposed workers, compared with controls, but no significant differences in blood levels of manganese or
performance on the administered tests were found between the exposed and control groups of workers.
Clinical examination of the exposed subjects revealed no cases of obvious neurological impairment, but
sensitive psychomotor tests of simple reaction times and motor functions were not administered in this
study.
In a cross-sectional study, Myers et al. (2003a) evaluated results from a health questionnaire and a battery
of neurobehavioral tests administered to 489 workers employed as office workers, miners, surface
processors, engineers, and other service workers from two South African manganese mines. Cumulative
exposure indices for each subject were calculated based on total dust measurements and job history.
Workers were employed in the mines for a mean of 10.8 years (SD=5.5 years; range 1–41 years), had an
average cumulative exposure index of 2.2 (mg manganese/m
3
per year, SD=2.2; range=0–20.8), an
average exposure intensity of 0.21 mg manganese/m
3
(SD=0.14; range, 0–0.99), and an average blood
manganese concentration of 8.5 µg/L (SD=2.8; range, 2.2–24.1). Neurobehavioral end points included
three tests of motor function in the Luria-Nebraska battery (tests 1, 2, and 23), mean reaction time in the
SPES, and three cognitive tests (forward and backward digit span and digit-symbol score). Multiple
linear regression analysis revealed no significant (p<0.05) associations between any measure of exposure
and questionnaire or test battery outcomes.
In another cross-sectional study, Myers et al. (2003b) evaluated neurobehavioral end points in a group of
509 workers at a South African manganese smelter, compared with a group of unexposed workers from
an electrical fittings assembly plant (remote from the manganese smelter). Workers were employed for a
mean of 18.2 years (SD 7.6), compared with 9.4 years (SD 7.0) in the control group. Exposure was
assessed from manganese determinations in dust from personal air samples, blood samples, and urine
samples. Cumulative exposure indices were calculated for each exposed worker based on manganese
concentrations in “inhalable” dust from personal air samples and job histories. Mean values for exposed
workers were 16.0 mg manganese/m
3
per year (SD 22.4) for cumulative exposure index, 0.82 mg
manganese/m
3
(SD 1.04) for average intensity of exposure, 12.5 µg manganese/L (SD 5.6) for blood
manganese, and 10.5 µg manganese/L (SD 20.3) for urine manganese. Control workers had mean values
of 6.4 µg manganese/L (SD 1.7) for blood manganese and 0.96 µg manganese/L (SD 0.81) for urine
manganese. Neurobehavioral end points included the Swedish nervous system questionnaire and the
following neurobehavioral test batteries: World Health Organization (WHO) neurobehavioral core test
82
MANGANESE
3. HEALTH EFFECTS
battery, SPES, Luria-Nebraska tests, and Danish product development tests (tests of hand steadiness,
tremor, and body sway). Information collected for potential confounders included age, educational level,
alcohol and tobacco consumption, neurotoxic exposures in previous work, past medical history, and
previous head injury. Multiple linear and logistic regression analyses were conducted to examine
possible exposure-response relationships. Several tests showed significant (p<0.05) differences between
exposed and control workers, but no evidence of exposure-response relationships including the following:
the Santa Ana, Benton and digit span WHO tests; hand tapping and endurance tapping SPES tests; one
Luria-Nebraska test (item 2L); several self-reported symptoms (e.g., tiredness, depressed, irritated); and
increased sway under two conditions (eyes open with or without foot insulation). Results from two other
tests (WHO digit-symbol test and Luria-Nebraska item 1R) showed differences between exposed and
control groups and some evidence for increased deficits with increasing exposure, but the change with
increasing exposure was greater at lower exposure levels than at higher exposure levels. Results from all
of the remaining tests showed no significant adverse differences between the exposed and control groups.
The authors concluded that “the most likely explanation for few, weak and inconsistent findings with
implausible or counterintuitive exposure-response relationships is chance, and it is concluded that this is
essentially a negative study.”
Young et al. (2005) reanalyzed the data collected by Myers et al. (2003b) on the basis of estimated
exposures to manganese in “respirable” dust. Exposure estimates for each worker (cumulative exposure
indices in mg manganese/m
3
per year and average intensity of exposure in mg manganese/m
3
) were
recalculated based on manganese determinations in personal air samplings of respirable dust (collected on
37 mm, 5 µm MCEP membrane filters, as opposed to inhalable dusts of larger particle sizes used to
estimate exposure in the earlier analyses by Myers et al. [2003b]). Results from comparisons of mean
performances of exposed and control groups in the neurobehavioral tests and regression analyses to assess
exposure-response relationships were similar to results from the earlier analyses by Myers et al. (2003b)
based on manganese determinations in inhalable dust. The authors concluded that the results did not
provide evidence that exposure estimates based on respirable dust provide a more sensitive method to
detect manganese neurobehavioral effects.
A cross-sectional study by Summers et al. (2011) supports the work of Myers et al. (2003a, 2003b).
Neuropsychological tests of attention, short-term memory span, information-processing speed, and
executive functioning (Digit Symbol Coding, Controlled Oral Word Association Test, Trail Making Test,
Matrix Reasoning, and the Stroop Neuropsychological Screening Test) were used to study adverse effects
in 143 employees in a smelting plant exposed to estimated mean concentrations of 0.384 mg/m
3
inhalable
83
MANGANESE
3. HEALTH EFFECTS
manganese dust or 0.123 mg/m
3
respirable manganese dust (as per Australian standards) for 1–29 years
(mean, 10.6 years). Cumulative exposure indices for both inhalable and respirable manganese dust were
calculated for each subject based on yearly air-sampling data for each occupational position and job
history. Correlational and hierarchical linear regression analysis was conducted to assess associations of
these exposure metrics, along with age, education, and intellectual ability (estimated IQ), with
performance on the neuropsychological tests. In hierarchical analysis of performance and respirable
manganese cumulative exposure (including age, estimated IQ, and education as explanatory variables),
statistically significant relationships were found for decreasing performance with increasing exposure on
the Trail Making (Part A), Matrix Reasoning, and Stroop color-word tests (measures of attention and
executive function), and for increasing performance with increasing exposure on the Digit Symbol
Coding test. The magnitude of the effects on performance was small, as reflected by the percentages of
the variance in test scores explained by respirable cumulative manganese exposure (ranging from 0.5 to
3.7, depending on the test). In contrast, estimated IQ and education explained 3.2–24.5% of the variance.
Summers et al. (2011) concluded that the decrements in performance associated with cumulative
respirable manganese exposure were small and “not of clinical significance”, because the magnitudes of
these effects were smaller than the standard error of measurement in the tests.
Bast-Pettersen et al. (2004) cross-sectionally examined neurobehavioral end points in a group of 100 male
workers in manganese alloy plants and a group of 100 control workers (paired matched for age) from two
plants, one producing silicon metal and microsillica and another titanium oxide slag and pig iron.
Manganese alloy workers were employed for a mean of 20.2 years (SD 8.6; range 2.1– 41.0 years);
comparable statistics were not reported for the control workers. Exposure was assessed from manganese
determinations in dust from personal air samples (collected on 3 days for each subject closely before the
neurobehavioral assessment), blood samples, and urine samples. Arithmetic means for manganese
workers were 0.753 mg manganese/m
3
inhalable dust for work room air (geometric mean 0.301; range
0.009–11.5 mg manganese/m
3
), 189 nmol manganese/L in blood (range 84–426 nmol/L), and 3.9 nmol
manganese/mmol urine creatinine (range 0.1–126.3). The Institute of Occupational Medicine (IOM)
personal samplers used in this study are expected to provide estimates that are approximately 2-fold
higher than estimates using 25- or 37-mm plastic Millipore personal air samplers used in many earlier
studies to measure “total dust”. Mean levels of manganese in blood (166 nmol manganese/L) and urine
(0.9 nmol manganese/mmol creatinine) of control workers were significantly lower than levels in exposed
workers. Neurobehavioral end points included: two self-administered neuropsychiatric questionnaires;
six tests of cognitive functions (Weschlers adult intelligence scale, digit symbol, trail making test, Stroop
color-word recognition, digit span, and Benton visual retention); and eight tests of motor functions (static
84
MANGANESE
3. HEALTH EFFECTS
hand steadiness, “TREMOR” test, finger tapping, foot tapping, supination/pronation of hand, Luria-
Nebraska thumb/finger sequential touch, simple reaction time, and hand-eye coordination). Information
collected for potential confounders included age, years of education, alcohol and tobacco consumption,
and prevalence of previous brain concussions. Multiple linear regression analyses were conducted to
examine the influence of potential confounders and exposure-response relationships for test results. No
significant (p<0.05) effect of exposure was found in tests for cognitive functions, reaction time, or
symptom reporting. No statistically significant (p<0.05) differences were found in tests of motor speed,
grip strength, or reaction time. Postural tremor as measured in the hand steadiness test was significantly
(p<0.05) increased in the exposed group compared with the controls and showed an exposure-response
relationship when the exposed group was regrouped into three groups of increasing duration of
employment. Results from an alternative test of tremor (“TREMOR”) did not distinguish between the
manganese alloy group and the control group. The results indicate that the manganese-exposed group of
workers had increased hand tremor compared with the control group, but were indistinguishable from the
control group in other tests of motor function, cognitive function, or symptom reporting.
Bouchard et al. (2005) reanalyzed results from neurobehavioral tests administered by Mergler et al.
(1994) to 74 male workers in a manganese alloy plant to examine the influence of age on the tests. At the
time of testing, workers had been employed an average of 19.3 years (range 1–27 years) and 71 of the
workers were employed for >10 years. Based on personal air and stationary air samples 8-hour time-
weighted average manganese concentrations ranged from 0.014 to 11.48 mg manganese/m
3
total dust
(geometric mean=0.225 mg manganese/m
3
) and from 0.001 to 1.273 mg manganese/m
3
respirable dust
(geometric mean=0.035 mg manganese/m
3
). The referent group contained 144 workers with no history of
occupational exposure to neurotoxicants who were matched for age, educational level, smoking status,
and number of children. Mean blood manganese levels were 11.9±5.3 µg/L (range 4.4–25.9 µg/L) in
exposed workers and 7.2±0.3 µg/L (range 2.8–15.4 µg/L) in controls. Paired differences between
exposed and control workers increased significantly (p<0.05) with age for one of nine tests of neuromotor
domain (nine-hole hand steadiness test); 3 of 12 tests of cognitive domain (trail making B [test of visual
conception and visuomotor tracking], delayed word recall [test of learning, recall and attention], and
cancellation H [test of visuomotor tracking and concentration]); and 1 of 4 sensory domain tests
(vibratometer–vibrotactile perception of the index and toe). The results suggest that older workers may
be more slightly more susceptible to the neurological effects of low-level manganese exposure than
younger workers.
85
MANGANESE
3. HEALTH EFFECTS
Bouchard et al. (2007a) examined neuropsychiatric symptoms in a group of 71 male workers in a
manganese alloy plant, 14 years after cessation of exposure, and in a group of 71 unexposed referents of
similar age and education levels from the same geographical region. Based on personal air and stationary
air samples during the operation of the plant, 8-hour time-weighted average manganese concentrations
were 0.014–11.48 mg manganese/m
3
total dust (geometric mean=0.225 mg manganese/m
3
) and 0.001–
1.273 mg manganese/m
3
respirable dust (geometric mean=0.035 mg manganese/m
3
). The mean number
of years of occupational exposure to manganese was 15.7 (range, 7.4–17.3 years). The exposed workers
were participants in the earlier study by Mergler et al. (1994). Neuropsychiatric symptoms were assessed
by a self-administered questionnaire, the Brief Symptom Inventory, from which scores were determined
for somatization (psychological distress from perception of bodily dysfunction), obsessive-compulsive
behavior, interpersonal sensitivity (feeling of personal inadequacy), depression, anxiety, hostility, phobic
anxiety, paranoid ideation, and psychoticism. Former, manganese workers showed significantly (p<0.05)
higher scores (after adjustment for age, education, and alcohol consumption) for two of the nine
neuropsychiatric symptoms (depression, anxiety), compared with controls.
In a follow-up to the Mergler et al. (1994) study, Bouchard et al. (2007b) evaluated neurobehavioral end
points in a group of 77 male former workers in a manganese alloy plant, 14 years after cessation of
employment, and in a group of 81 nonexposed referents group-matched for age, education and alcohol
consumption. The groups were initially assessed in 1990 and, for the present study in 2004, in five
neuromotor tests, nine cognitive tests, and six mood state tests. Based on personal air and stationary air
samples during the operation of the plant, 8-hour time-weighted average manganese concentrations were
0.014–1.48 mg manganese/m
3
total dust (geometric mean=0.225 mg manganese/m
3
) and 0.001–1.273 mg
manganese/m
3
respirable dust (geometric mean=0.035 mg manganese/m
3
). Mean years of occupational
exposure to manganese was reported as 15.3 years (maximum=17.3 years). In the 1994 assessment,
significant (p<0.05) differences between exposed and control workers were found in scores for one of
five neuromotor tests (Luria Motor Scale), three of nine cognitive tests (cancellation H, digit span, color-
word test), and one (tension-anxiety) of six mood state tests. In 2004, significant (p<0.05) differences
between the exposed and control workers persisted for one (Luria Motor Scale) of five neuromotor tests,
none of the nine cognitive tests, and one (confusion-bewilderment) of the six mood states. These results
indicate that exposure-related effects observed initially in the manganese alloy workers did not progress
in a 14-year period following cessation of employment.
Neurological Assessments of Environmentally Exposed Populations Exposed to Inorganic Manganese.
Mergler et al. (1999) studied environmental exposure to manganese and its possible effect on mood
86
MANGANESE
3. HEALTH EFFECTS
(Bowler et al. 1999), neuromotor function (Beuter et al. 1999), and levels of the metal in biological fluids
(Baldwin et al. 1999). The study group was a community in southwest Quebec, Canada, near which a
former manganese alloy production plant served as a point source for environmental manganese pollution.
Due to the presence of MMT in gasoline in Canada, inhaled manganese from car exhaust is a potential
contributor to manganese exposures experienced in the population studied. A total of 273 persons
comprised the test population. These individuals were selected using a stratified random sampling
strategy from the Quebec Health Plan Register, which includes all residents. This strategy helped to
ensure that no selection bias was introduced. These individuals were administered a test battery including
a computerized neuromotor test, blood sampling, visual function tests from the Neurobehavioral
Evaluation System-2, an extensive neuropsychological test battery, and diverse tests covering such areas
as olfactory threshold, finger tapping, digit span, and postural sway. Blood sampling data for the study
subjects (Baldwin et al. 1999) indicated that manganese levels in women (geometric mean=7.5 μg/L)
were significantly higher than in men (6.75 μg/L). No relationship was found between the overall level of
manganese in blood and those of lead or iron in serum. However, blood manganese levels were
negatively correlated with serum iron in women and had a tendency to decrease with increasing age.
Serum iron levels in men were higher than in women. The authors analyzed manganese in drinking water
from the study subjects’ residences and analyzed air samples from four different locations for total
manganese particulates and PM
10
values. The geometric mean value for manganese in drinking water
was 4.11 μg/L; there was no correlation between individual values in drinking water manganese and
manganese blood levels. Intersite differences in manganese values in total particulate were not observed
in the air samples, but intersite differences did exist for manganese in PM
10
values. Two geographical
areas were identified where manganese in air contributed to blood manganese levels; serum iron was
negatively related to blood manganese levels in this analysis (Baldwin et al. 1999).
The Profile of Moods State and Brief Symptom Inventory self-report scales were used to assess condition
of mood in the study population (Bowler et al. 1999). The results from these analyses indicated that men
who are older (>50 years) and have higher blood manganese levels (≥7.5 μg/L) showed significant
disturbances in several mood symptoms with significantly increased values for anxiety, nervousness, and
irritability; emotional disturbance; and aggression and hostility when compared to those with lower levels
of blood manganese. Neuromotor, neurological, and neurobehavioral analyses revealed that subjects with
higher blood manganese levels (≥7.5 μg/L) performed significantly worse on a test for coordinated upper
limb movements, with poorest performance in older men (Mergler et al. 1999). Also in men, proximal
events on the qualified neurological examination, involving arm movements were significantly slower for
those with higher blood manganese, and hand movements (distal events) tended to be in the same
87
MANGANESE
3. HEALTH EFFECTS
direction. No correlation was observed in women. Other measures of motor performance (e.g., hand-arm
tremor and tapping movements) were not related to blood manganese levels, although a significant
decrease in tremor frequency dispersion was observed with log MnB (manganese blood level). For both
men and women, performance on the learning and memory tests was inversely correlated with manganese
blood level values, although performance on individual portions of the overall test varied significantly
with gender. For men, higher levels of manganese in blood were associated with poorer performance on
list acquisition, delayed auditory recall, and visual recognition following a distracter. Females, in
contrast, tended to recall fewer geometric shapes, made more errors on the visual reproduction test, but
remembered more numbers on the digit span forward test. This study is unique in that it is the first to
study both males and females in an exposed population, and it shows an association between elevated
manganese blood levels linked to elevated environmental manganese and poor performance on
neurobehavioral and neuropsychiatric tests. This study also reported that neurological effects associated
with higher levels of blood manganese were more likely to be observed in persons >50 years of age. In
contrast, Roels et al. (1999) reported that age was a significant factor only in performance of the visual
reaction time test, but not for the eye-hand coordination test or the measure of hand steadiness used in
their longitudinal studies. However, Crump and Rousseau (1999) reported that older age was a
significant factor in poor performance in tests of short-term memory and eye-hand coordination.
Although there were no statistically significant neurological effects associated with manganese exposure
among workers of a metal-producing plant evaluated by Gibbs et al. (1999), these investigators also noted
that test performance in eye-hand coordination and reaction time decreased with increasing age.
Rodríguez-Agudelo et al. (2006) examined neurobehavioral end points in 168 women and 120 men from
eight communities at various distances from manganese extraction or processing plants in the district of
Molango, Mexico. Manganese levels in PM
10
dust in air samples collected from 28 houses were
determined, and the values obtained from the closest monitor were assigned to each of the
288 participants (values ranged from 0 to 5.86 µg manganese/m
3
). Concentrations of manganese in
samples of drinking water and maize grain were mostly below detection limits, whereas soil
concentrations ranged from about 6 to 280 mg manganese/kg, with the largest concentrations noted in
samples collected close to the manganese industrial sites. Blood samples were collected from each
participant and used for manganese and lead determinations. Neuromotor tests (which were a Spanish
adaptation of Luria diagnostic procedures) were administered, and odds ratios (ORs) were calculated for
24 different end points involving hand motor functions using dichotomous assessments of performance
(e.g., normal and poor) after grouping the participants based on associated manganese concentrations in
air or blood manganese levels. No associations were found between neuromotor performance and blood
88
MANGANESE
3. HEALTH EFFECTS
levels of manganese or lead. After grouping the participants into those associated with air concentrations
between 0 and 0.1 µg manganese/m
3
and those with concentrations between 0.1 and 5.86 µg
manganese/m
3
(approximate midpoint=3 µg manganese/m
3
), significantly (p<0.05) elevated ORs for poor
performance were calculated for only 3 of the 24 neuromotor end points (two movement coordination, left
hand performance [OR=1.99, 95% CI 1.15–3.43]; change of hand position, left hand performance
[OR=1.98, 95% CI 0.99–3.95], and conflictive reaction, a test of verbal regulation of movement
[OR=2.08. 95% CI 1.17–3.71]). Although the authors concluded that the results indicate that “there is an
incipient motor deficit in the population environmentally exposed to large manganese levels,” a more
likely explanation for the few and inconsistent findings is chance. This explanation is supported by the
finding that no statistically significant associations were found between any neuromotor function end
points and blood manganese levels. In addition, the lack of air monitoring data for individual participants
in the study precludes assigning the “high” air concentration exposure level as a reliable LOAEL or
NOAEL.
Solís-Vivanco et al. (2009) evaluated the same group of subjects with a battery of neuropsychological
tests for cognitive function (general cognitive state, attention, semantic and phonological fluency,
construction, verbal memory, visual memory coding and recall, and depression). Using logistic
regression analysis with air manganese concentration as an exposure variable, no risk of poor
performance was found with a 0.05 μg/m
3
cut-off point. When using a 0.1 μg/m
3
cut-off point, only 1 of
10 cognitive measures had a significantly increased risk of poor performance (attention as measured by
the digit span test, OR = 1.75, CI 1.01–3.06). Solís-Vivanco et al. (2009) concluded that the attention
impairments associated with high levels of air manganese exposure are evidence of cognitive impairment
in the exposed population. However, similar to the study by Rodríguez-Agudelo et al. (2006), the finding
on this one measure could be due to chance, as there was no association between blood manganese levels
and cognitive performance.
In the same Molango mining district in central Mexico, a cross-sectional study assessed intellectual
function in 79 children (ages 7–11 years) exposed to an average manganese air concentration of
0.13 μg/m
3
for at least 5 years (Riojas-Rodríguez et al. 2010). The children received a medical exam, and
their height and weight were measured. Intellectual function was assessed with the revised Wechsler
Intelligence Scale for Children. Maternal intelligence was assessed with the Progressive Matrices of the
Raven test. Blood and hair samples were collected from the children to measure manganese
concentrations, along with blood concentrations of lead and hemoglobin. A control group was comprised
of 93 unexposed children (ages 7–11 years) from socioeconomically-matched communities from the
89
MANGANESE
3. HEALTH EFFECTS
Aqua Blanca district 80 km southeast from the manganese source. Children in the exposed communities
had significantly elevated mean blood (9.71 μg/L) and hair (12.13 μg/g) manganese concentrations
compared with controls (8.22 μg/L and 0.57 μg/g, respectively). Statistically significant (p<0.05)
negative associations were found between hair manganese concentrations and verbal and full scale scores.
Blood manganese concentration was inversely, but nonsignificantly, associated with verbal and full scale
scores. After adjusting for age and sex, the strongest inverse association between hair concentration and
intellectual function was in young girls, with little evidence of associations in boys at any age.
Associations with blood concentration were not modified by sex, but age adjustment suggested that the
inverse relationship was limited to younger participants. Riojas-Rodríguez et al. (2010) concluded that
findings suggest that air-borne manganese exposure is inversely associated with intellectual function in
young school-age children. However, manganese exposure from other sources (groundwater, dietary)
was not considered, and association between air concentration and test results were not explored.
Hernádez-Bonilla et al. (2011) evaluated the same groups of children for motor impairments. Parameters
assessed were manual dexterity, (fine) motor coordination, and motor speed (using the grooved pegboard,
finger tapping, and Santa Ana tests). There was a significant inverse relationship between execution of
the finger tapping test with blood, but not hair, manganese concentration. Additionally, exposed children
made significantly more errors in the grooved pegboard test than controls, but this effect was not
associated with blood or hair manganese levels. There was no correlation between manganese
concentration in blood or hair in any of the other motor function tests. Hernádez-Bonilla et al. (2011)
concluded that there was only subtle evidence of adverse effects on motor speed and coordination.
Similar cognitive findings were reported in a cross-sectional study by Menezes-Filho et al. (2011), in
which intellectual function was assessed in 83 children from 55 families (ages 6–12 years) and their
primary caregivers from the village of Cotegipe in Brazil, which is within a 2-km radius from a ferro-
manganese alloy plant that has been emitting high levels of manganese into the air for 4 decades. The
height and weight of each child was recorded, and blood and hair samples were collected to measure
manganese levels. Blood levels of lead and iron were also measured. Intellectual function was assessed
in children using the Wechsler Intelligence Scale for Children, version III. To assess intellectual function
in primary caregivers (94% mothers), the Raven Progressive Matrix was administered. Caregivers also
provided hair samples for manganese level testing and responded to a questionnaire on sociodemo-
graphics and birth history. The mean blood and hair manganese concentrations in children were 8.2 and
5.83 μg/L, respectively. The mean hair manganese concentration in caregivers was 3.5 μg/L, and levels
correlated with their children's hair manganese concentration. After adjusting for maternal education and
90
MANGANESE
3. HEALTH EFFECTS
nutritional status, there was a significant (p<0.05) negative association between hair manganese levels in
children and their verbal and full scale scores. In addition, after adjusting for education years, family
income, and age, there was a significant (p<0.05) negative association between caregiver’s hair
manganese levels and performance on the Raven Progressive Matrix. Menezes-Filho et al. (2011)
concluded that high manganese exposure, likely via air emissions from the plant, had detrimental effects
on cognition in both adults and children, especially in the verbal domain. However, they state that poor
cognitive development in children may also be due in part to lower caregiver IQs. Additionally, this
study bears the limitations of a cross-sectional design, and causal inferences cannot be made on the
relationship of manganese exposure and cognitive defects.
In a community-based study, Lucchini et al. (2007) examined possible associations between prevalence of
Parkinsonian disorders and levels of manganese in settled dust collected from communities in the vicinities
of manganese ferroalloy industrial plants in the province of Brescia, Italy. Parkinsonian patients were
identified from clinical registers from local hospitals, area neurologists, and records of exemption from
prescription payments, as well as from records of L-Dopa prescriptions; a total of 2,677 Parkinsonian cases
were identified among 903,997 residents. SMRs for each of 206 municipalities were calculated based on
national rates standardized for age and gender. Municipalities with the highest SMRs were located within
20 km and/or downwind of three manganese alloy industrial plants in the Valcamonica region of Brescia.
An average standardized prevalence of 492 cases/100,000 residents was observed in the 37 municipalities
of the Valcamonica region. Crude and standardized prevalence rates for the Valcamonica municipalities
were significantly (p<0.05) higher than rates for the other 169 municipalities of Brescia. Municipality-
based SMRs for Parkinsonian disorders were significantly (p<0.05) associated with manganese levels in
settled dust, and manganese levels in settled dust samples from the 37 municipalities in Valcamonica were
significantly (p<0.05) higher than levels in samples for the other 169 municipalities. The results suggest
that prolonged environmental exposure to excessive manganese in the Valcamonica region of Brescia may
increase the risk for Parkinsonian disorders, but the results do not identify a reliable NOAEL or LOAEL
that can be expressed in units of manganese air concentrations. The authors speculated that, even though
manganese-induced and Parkinsonian neurological disorders are expected to have two distinct target areas
in the brain (the globus pallidus and the substantia nigra, respectively), structural and chemical
interconnections between the brain areas may interact to cause increased risk for Parkinsonian disorders as
suggested by Weiss (2006).
In a preliminary cross-sectional study, Standridge et al. (2008) evaluated postural balance in 22 residents
(13 females and 9 males; ages 20–59 years old) from a manganese-exposed Ohio community where a
91
MANGANESE
3. HEALTH EFFECTS
large ferro- and silico-manganese smelter has been active for >50 years. Subjects had been living within
10 miles of the refinery for at least 3 years, were known not to have a history of manganese occupational
exposure, and had been exposed to estimated mean daily ambient manganese concentrations between
0.1 and 2.0 μg/m
3
. The control group was comprised of 22 military subjects (10 females and 12 males;
ages 24–57 years old) who were considered to be unexposed to occupational and environmental
neurotoxicants. Results from a postural sway analysis, along with blood and hair manganese levels, were
compared with unexposed controls. Several covariates (age, gender, height, weight, alcohol intake,
tobacco usage, and blood lead levels) were also recorded. Postural analysis measures of manganese-
exposed residents were significantly larger than controls in five out of eight postural balance outcomes
(sway area for eyes open on the platform, sway area for eyes open or closed on foam, sway length for
eyes open or closed on the foam). After adjustment for covariables, a significant positive association was
found between hair manganese levels and sway area and length (eyes open or closed on the platform).
Standridge et al. (2008) concluded that these preliminary findings suggest subclinical impairment in
postural balance in manganese-exposed residents.
Kim et al. (2011) conducted a cross-sectional study evaluating motor function in 100 residents from the
same manganese-exposed Ohio community. Subjects had been living in the community for 10–65 years
and had been exposed to 0.04–0.96 μg/m
3
of respirable manganese particulate (mean, 0.18 μg/m
3
; based
on U.S. EPA dispersion modeling). Results from the Unified Parkinson's Disease Rating Scale, a postural
sway test, and a comprehensive questionnaire exploring demographics and general health were compared
to 90 unexposed residents from a demographically similar comparison town in Ohio. Blood samples
were collected from all subjects for ferritin, alanine transpeptidase, gamma-glutamyl transferase,
manganese, mercury, lead, and cadmium levels. There were no significant differences between the
exposed and comparison groups in regards to manganese blood levels, demographics, or major health
outcomes. However, when adjusted for covariates (presence of other neurotoxic metals, factors
aggravating susceptibility to manganese or motor performance, demographics), the manganese-exposed
residents had a significantly increased risk of abnormal performance on the Unified Parkinson's Disease
Rating Scale and showed significantly higher postural sway scores. Kim et al. (2011) concluded that
these subclinical findings may possibly reflect early subtle effects of chronic, low-level manganese
exposure, but alternatively might be due to chance due to the cross-sectional study design, the small to
medium effect size, and the lack of association between air or blood manganese levels and motor function
performance.
92
MANGANESE
3. HEALTH EFFECTS
Neurological Studies of Animals Exposed by Inhalation to Inorganic Manganese. In several early
animal studies, intermediate or chronic inhalation exposure of monkeys and rats to manganese dusts has
not produced neurological signs similar to those seen in humans (Bird et al. 1984; EPA 1983c; Ulrich
et al. 1979a, 1979b). For example, Ulrich et al. (1979a) reported that monkeys continually exposed for
9 months to aerosols of manganese dioxide at concentrations as high as 1.1 mg manganese/m
3
showed no
obvious clinical signs of neurotoxicity, no histopathological changes in brain tissues, and no evidence for
limb (leg) tremor or electromyographic effects on flexor and extensor muscles in the arm. However, in a
chronic study with Rhesus monkeys, decreased levels of dopamine were found in several regions of the
brain (caudate and globus pallidus) (Bird et al. 1984). Behavioral tests detected signs of neurological
effects in mice (increased open-field activity and decreased maternal pup retrieval latency), although
these are only seen at relatively high exposure levels (60–70 mg manganese/m
3
) (Lown et al. 1984;
Morganti et al. 1985).
Several studies provide evidence for associations between decreased neuronal cell counts in the globus
pallidus and neurobehavioral changes (increased locomotor activity) in rats exposed by inhalation for
13 weeks to a mixture of manganese phosphate/sulfate (at 1.05 mg manganese/m
3
) or manganese sulfate
alone (at concentration between 0.009 and 0.9 mg manganese/m
3
), but not to manganese phosphate alone
at concentrations up to 1.1 mg manganese/m
3
(Normandin et al. 2002; Salehi et al. 2003, 2006; Tapin et
al. 2006). Other 13-week rat inhalation exposure studies reported increased brain manganese
concentrations and increased locomotor activity after exposure to 3.75 mg manganese/m
3
as metallic
manganese (St-Pierre et al. 2001) and increased brain manganese concentrations with no increases in
olfactory bulb, cerebellar, or striatal concentrations of glial fibrillary acidic protein (GFAP) after exposure
to 0.5 mg manganese/m
3
as manganese sulfate or 0.1 mg manganese/m
3
as manganese phosphate
(Dorman et al. 2004b). GFAP is a widely acknowledged marker of damage to astrocytes.
In male Sprague-Dawley rats, increased locomotor activity (increased distance traveled, but no change in
resting time) was observed after up to 13 weeks of exposure to 0.03 or 3 mg of a manganese
phosphate/sulfate mixture/m
3
(6 hours/day, 5 days/week), but not at 0.3 mg/m
3
(Salehi et al. 2003). These
exposure concentrations correspond to 0.01, 0.11, and 1.05 mg manganese/m
3
. Assessment of brain
manganese levels, hind limb tremor, and neuropathology of the brain (counts of neuronal cells) found no
evidence for tremor at any exposure level, but rats at the highest exposure level showed significantly
(p<0.05) increased concentrations of manganese in the frontal cortex, globus pallidus, and caudate
putamen, as well as significantly (p<0.05) decreased neuronal cell counts in the globus pallidus and
93
MANGANESE
3. HEALTH EFFECTS
caudate putamen, compared with control values or to values for rats in the lower exposure groups (Salehi
et al. 2006).
In similar experiments with male Sprague-Dawley rats exposed to 0, 0.03, 0.3, or 3 mg manganese
sulfate/m
3
(Tapin et al. 2006) or 0, 0.03, 0.3, or 3 mg manganese phosphate/m
3
(Normandin et al. 2002)
for 13 weeks by the same exposure protocol, some differences in results were obtained. These exposure
levels correspond to 0.009, 0.09, or 0.9 mg manganese/m
3
for manganese sulfate and 0.01, 0.11, or
1.1 mg manganese/m
3
for manganese phosphate. With exposure to manganese phosphate, manganese
levels were significantly (p<0.05) elevated (at 3 mg/m
3
) in the olfactory bulb, frontal cortex, globus
pallidus, caudate putamen, and cerebellum regions of the brain, but no exposure-related effects were
found on neuronal cell counts or locomotor activity (Normandin et al. 2002). In contrast, manganese
sulfate exposure significantly (p<0.05) increased manganese levels in all regions of the brain, and
decreased neuronal counts in the globus pallidus at 0.3 and 3 mg manganese sulfate/m
3
, compared with
controls (Tapin et al. 2006). In addition, the two highest exposure levels of manganese sulfate were
associated with significantly (p<0.05) increased locomotor activity (distance traveled), increased resting
time, and decreased total ambulatory counts; the lowest exposure level, 0.03 mg manganese sulfate/m
3
also increased the distance traveled end point of locomotor activity (Tapin et al. 2006). As with the
manganese phosphate/sulfate mixture, neither manganese phosphate nor manganese sulfate exposure was
associated with hind limb tremors in the rats. Earlier studies by the same research group, found that
Sprague-Dawley rats exposed to 3.75 mg aerosols of metallic manganese/m
3
(6 hours/day, 5 days/week
for 13 weeks) showed significantly (p<0.05) higher manganese concentrations in various regions of the
brain, and higher distance traveled and lower resting time in locomotor tests, compared with controls;
neuronal counts were not assessed in this earlier study (St-Pierre et al. 2001).
Several studies have examined the influence of inhalation exposure to manganese sulfate on biochemical
end points associated with oxidative stress or inflammation in the brain of rats (Erikson et al. 2005, 2006;
HaMai et al. 2006; Taylor et al. 2006) and monkeys (Erikson et al. 2007, 2008). Erikson et al. (2005,
2006) exposed neonatal rats to manganese sulfate (0, 0.05, or 1 mg manganese/m
3
) during gestation and
postnatal days (PNDs) 1–18 and examined five brain regions for several biochemical end points
associated with oxidative stress either on PND 19 (Erikson et al. 2006) or after 3 weeks without exposure
(Erikson et al. 2005). End points included levels of glutamine synthase (GS) protein and mRNA,
metallothionein (MT) mRNA, tyrosine hydroxylase (TH) protein and mRNA, and total reduced
glutathione. At PND 9, increased manganese concentrations in the striatum (the most consistently
affected region) were associated with decreases in GS, MT, and TH mRNA, and significantly decreased
94
MANGANESE
3. HEALTH EFFECTS
levels of glutathione (Erikson et al. 2006), but these were not apparent 3 weeks after cessation of
exposure (Erikson et al. 2005). However, other end points (such as decreased GS protein) were changed,
compared with control values, 3 weeks after cessation of exposure (Erikson et al. 2005). Similar end
points, as well as levels of mRNA and protein for glutamate transporters, were examined in six brain
regions of young male Rhesus monkeys exposed to 0, 0.06, 0.3, or 1.5 mg manganese/m
3
as manganese
sulfate for 65 days (Erikson et al. 2007). Exposure-related changes included decreased MT mRNA in
most regions, decreased TH protein levels in the caudate and globus pallidus, increased GSH in the
frontal cortex, and decreased GSH in the caudate. In a follow-up study, Erikson et al. (2008) examined
similar end points in groups of four Rhesus monkeys exposed to 1.5 mg manganese/m
3
for 15 or 33 days
or 65 days with 45 or 90 days of recovery. The previously reported alterations (MT mRNA, TH protein,
GSH) were confirmed after 33 days of exposure, and all but the increased GSH levels in the frontal cortex
persisted at least 90 days after treatment cessation. Additional persistent effects include decreased GS
protein and glutamate transporter (GLT-1) mRNA and protein in various brain regions and decreased
glutamate transporter GLAST protein in globus pallidus. In another study, HaMai et al. (2006) exposed
three groups of rats to 0 or 0.71 ng manganese/m
3
(2 hours/day) as manganese sulfate on gestation days
(GDs) 9 and 10, on PNDs 37–47, or on GDs 9 and 10 plus PNDs 37–47 and measured brain levels of
mRNA for gene products related to oxidative stress or inflammation. Gestational exposure was
associated with decreased mRNA for amylid precurson (APP), cyclooxygenase-2 (COX-2), neuronal
nitric oxide synthetase (nNOS), and GFAP, whereas adult exposure was associated with greater
transcriptional decreases for the same gene products as well as transcriptional growth factor beta (HaMai
et al. 2006). The results from these studies indicate that acute- or intermediate-duration inhalation
exposure to manganese sulfate concentrations ranging from about 0.1 to 1 mg manganese/m
3
can
differentially affect brain biochemical markers of neurotoxicity, but understanding of the neurotoxic
mechanism of manganese is inadequate to confidently define any one of the observed changes as
biologically adverse.
No studies on neurological effects from inhalation exposure to MMT in humans or animals were located.
3.2.1.5 Reproductive Effects
As discussed earlier (see Section 3.2.1.4), impotence and loss of libido are common symptoms in male
workers afflicted with clinically identifiable signs of manganism attributed to occupational exposure to
manganese for 1–21 years (Emara et al. 1971; Mena et al. 1967; Rodier 1955; Schuler et al. 1957). These
symptoms could lead to reduced reproductive success in men. Impaired fertility (measured as a decreased
95
MANGANESE
3. HEALTH EFFECTS
number of children/married couple) has been observed in male workers exposed for 1–19 years to
manganese dust (0.97 mg/m
3
) at levels that did not produce frank manganism (Lauwerys et al. 1985).
This suggests that impaired sexual function in men may be one of the earliest clinical manifestations of
manganism, but no dose-response information was presented so it is not possible to define a threshold for
this effect. Jiang et al. (1996b) performed a reproductive epidemiological study on 314 men in a
manganese plant. The men, from six different factories, performed milling, smeltering, and sintering
duties for up to 35 years. The geometric mean airborne manganese concentration (assumed to be total
dust) was 0.145 mg/m
3
as manganese dioxide. The researchers found no significant differences in
reproductive outcomes between exposed and control workers (controls were matched for several factors,
including age, smoking, personal hygiene, living habits, and cultural background). The incidences of
sexual dysfunction were evaluated through researchers’ questions and judged by the occurrence of two
positive responses to three potential conditions: impotence, abnormal ejaculation (early ejaculation or
nonejaculation), and lack of sexual desire. Impotence and lack of sexual desire were higher in the
exposed group than in the controls (Jiang et al. 1996b). Wu et al. (1996) reported increased semen
liquefaction time and decreased sperm count and viability in three groups of men occupationally exposed
to manganese: 63 miners or ore processors, 38 electric welders in mechanical fields, and 110 electric
welders in shipbuilding. Matched controls consisted of 99 men who were employed in the same
occupation and from the same area, but were not exposed to manganese or other reproductive toxins. The
men had been exposed to manganese for ≥1 year. Geometric means of total manganese dust (as
manganese dioxide) ranged from 0.14 mg/m
3
for mining operations to 5.5 mg/m
3
for manganese powder
processing. Manganese fume concentrations varied; the mechanical welders were exposed to a
concentration of 0.25 mg/m
3
(geometric mean), while the shipbuilding area concentrations ranged from
geometric means of 6.5–82.3 mg/m
3
, depending on the location within the ship. The miners had a
significant percentage (14.3%; p<0.01) of samples with increased liquefaction time, decreased sperm
count (34.9%; p<0.01), and decreased percentage of total viable sperm (33.3% had abnormal counts;
p<0.01) compared to controls. Welders in shipbuilding had decreased sperm viability levels that were
significantly different from controls (p<0.01). Manganese concentrations in semen were significantly
increased compared to controls in the mechanical welders; copper, nickel, chromium, and iron
concentrations were also elevated in semen in welders in both mechanical and shipbuilding careers.
Further, stepwise regression analysis of the impact of these other metals on the measured reproductive
parameters indicated that the higher the nickel concentration, the lesser the semen volume and the greater
the number of deformed sperm. Copper in the seminal fluid was also positively linked with the viable
sperm percentage, sperm viability and number of sperm. Although this study indicates that manganese
exposure can cause sperm toxicity, the presence of other metals prevents any conclusive statements
96
MANGANESE
3. HEALTH EFFECTS
concerning its importance. Gennart et al. (1992) performed a reproductive study on 70 male workers
exposed to manganese dioxide at a median concentration of 0.71 mg manganese/m
3
in total dust for an
average of 6.2 years in a dry alkaline battery plant. Results from a questionnaire answered by the workers
and controls in the study and from analysis of birthrates of exposed and control workers revealed no
difference in birthrates between the groups.
These results in human studies reveal conflicting evidence for whether occupational exposure to
manganese causes adverse reproductive effects. Effects reported may occur as a secondary result of
neurotoxicity but do not provide information on any direct effect manganese may have on the
reproductive organs. No information was found regarding reproductive effects in women.
Intratracheal instillation studies in rabbits indicate that single high doses of manganese (158 mg/kg, as
manganese dioxide) can cause severe degenerative changes in the seminiferous tubules and lead to
sterility (Chandra et al. 1973; Seth et al. 1973). This effect did not occur immediately, but developed
slowly over the course of 4–8 months following the exposure. Direct damage to the testes has not been
reported in humans occupationally exposed for longer periods, suggesting that this effect may not be of
concern under these exposure circumstances. However, it is unclear if specific studies to investigate
possible testicular damage have been performed.
None of the studies located reported adverse effects in female animals following inhalation exposure to
manganese. In a study with female mice (Lown et al. 1984), the average number of pups born to exposed
females was increased when dams were exposed to manganese dioxide before conception through
gestation. In a report of a study of tissue manganese concentrations in lactating rats and their offspring
following exposure to manganese sulfate aerosols at 0, 0.05, 0.5, or 1 mg manganese/m
3
starting 28 days
prior to breeding through PND 18, no mention was made of reproductive performance variables such as
the percentage of dams that delivered or the number of pups per litter (Dorman et al. 2005a).
The highest NOAEL values and all LOAEL values from each reliable study for reproductive effects in
each species and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
No studies were located concerning reproductive effects following inhalation exposure to organic
manganese compounds in humans or animals.
97
MANGANESE
3. HEALTH EFFECTS
3.2.1.6 Developmental Effects
Very little information is available on the developmental effects of inorganic manganese from inhalation
exposure. The incidences of neurological disorders, birth defects, and stillbirths were elevated in a small
population of people living on an island where there were rich manganese deposits (Kilburn 1987).
However, no conclusions could be reached on the causes of either the neurological effects or the
increased incidence of birth defects and stillbirths because there were insufficient exposure data. Control
data were not provided, and the study population was too small for meaningful statistical analysis.
Although inhalation exposure was not ruled out, the route of exposure was assumed to be primarily oral.
As discussed in Section 3.2.1.4, two studies reported statistically significant inverse relationships between
an index of exposure to manganese in air (manganese concentration in hair) and intellectual function in
children living in communities near manganese industries (Menezes-Filho et al. 2011; Riojas-Rodríguez
et al. 2010). Additionally, Hernández-Bonilla et al. (2011) reported that children living in a manganese
mining area had higher manganese hair concentrations than children from a non-mining area, but did not
show clear performance deficits on several tests of motor skills (grooved pegboard, finger tapping, and
Santa Ana test), compared with the control group of children. No statistically significant associations
were found for increasing performance deficits with increasing hair concentrations, but a statistically
significant association was found for finger tapping deficits with increasing manganese blood
concentrations. The results provide suggestive evidence of an association between environmental
exposure of children to manganese and impaired cognitive abilities, but are inadequate to establish causal
relationships due to the cross-sectional design and inability to control for possible confounding factors.
The study of motor function did not find clear and consistent evidence for motor function deficits in these
children.
Lown et al. (1984) evaluated the developmental effects of inhaled manganese in mice. The study
involved exposing dams and non-pregnant female mice to either filtered air or manganese at an average
concentration of 61 mg/m
3
(as manganese dioxide) 7 hours/day, 5 days/week, for 16 weeks prior to
conception. The authors then exposed the mice to either air or manganese post-conception, irrespective
of preconception exposure. Once delivered, six pups (three of each sex) were distributed to foster
mothers and then nursed in the absence of exposure to manganese. The pups were then evaluated on
postpartum day 7 for weight gain and gross locomotor activity and on day 45 for different behavioral
parameters and learning performance. The authors observed that pups raised by foster mothers that had
been exposed to manganese preconception and filtered air postconception had reduced weights compared
98
MANGANESE
3. HEALTH EFFECTS
to pups raised by foster mothers exposed only to filtered air. The activity data indicated that there were
no observable differences in activity between pups who had been exposed to manganese in utero and
those that had not. Therefore, the data did not provide evidence that manganese exposure resulted in
adverse neurological developmental effects.
No studies were located concerning developmental effects in humans or animals following inhalation
exposure to organic manganese.
3.2.1.7 Cancer
No studies were located regarding carcinogenic effects in humans or animals after inhalation exposure to
inorganic or organic manganese.
3.2.2 Oral Exposure
Although humans are often exposed to significant quantities of inorganic manganese compounds in food
and water (see Sections 6.4 and 6.5), reports of adverse effects in humans from ingestion of excess
manganese are limited. Most information on the effects of oral exposure to inorganic manganese is
derived from studies in animals. These studies are summarized in Table 3-2 and Figure 3-2, and the
findings are discussed below. All doses are expressed as mg manganese/kg/day.
Health effects following oral exposure to the organic manganese compound, MMT, were observed in
animals. Studies involving oral exposure of animals to MMT are summarized in Table 3-3 and
Figure 3-3. As discussed previously, because inhalation, oral, and dermal pathways are not a concern
regarding exposure to mangafodipir, this compound’s studies are not presented in an LSE table or figure;
instead, they are discussed in Section 3.2.4.
3.2.2.1 Death
Three studies have been located in which death in humans may have been caused by the ingestion of
manganese-contaminated water (Hafeman et al. 2007; Kawamura et al. 1941; Spangler and Spangler
2009). Kawamura et al. (1941) reported death from "emaciation" in two adults who ingested drinking
water contaminated with high levels of manganese. Hafeman et al. (2007) reported high mortality among
infants <1 year of age in a Bangladesh population where the drinking water supplied by certain local
wells contained high levels of manganese. As discussed in detail in Sections 3.2.2.4 (Kawamura et al.

139
412
146
351
227
342
331
275
1039
642
1040
782
77
1082
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral
Exposure/
LOAEL
Duration/
a
Key to
Figure
Species
(Strain)
Frequency
(Route)
ACUTE EXPOSURE
Death
1
Rat
(Sprague-
Dawley)
once
(GW)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
412 M (LD50)
2
Rat
(albino)
once
(GW)
351 M (LD50)
3
Rat
(Wistar)
once
(GW)
342 M (LD50)
331 F (LD50)
275 (LD50 - pups)
4
Rat
(Swiss albino)
once
(G)
642 M (LD50)
5
Rat
(Swiss albino)
once
(G)
782 M (LD50)
6
Rat
(Wistar)
once
(GW)
1082 (LD50)
Reference
Chemical Form
Comments
Holbrook et al. 1975
MnCl2
Kostial et al. 1978
MnCl2
Kostial et al. 1989
MnCl2
Singh and Junnarkar 1991
MnCl2
Singh and Junnarkar 1991
MnSO4
Smyth et al. 1969
MnOAc
3. HEALTH EFFECTS
MANGANESE
99

234
1300
1300
650
1300
1300
650
1300
1300
1300
1300
650
1300
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
Systemic
7
Rat
(F344/N)
14 d
(F)
Resp 1300
NTP 1993
MnSO4
Cardio 1300
Hemato 650 M
1300 F
1300 M (decreased leukocyte
and neutrophil counts)
Hepatic 650 M 1300 M (reduced liver weight)
1300 F
Renal 1300
Endocr 1300
Bd Wt 650 1300 (57% decreased body
weight in males; 20% in
females)
3. HEALTH EFFECTS
MANGANESE
100

1002
2600
3900
2600
3900
2600
3900
2600
3900
2600
3900
2600
3900
375
22
8.8
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
LOAEL
Less Serious Serious
(mg/kg/day) (mg/kg/day)
a
Key to Species
Figure (Strain)
8
Mouse
(B6C3F1)
Neurological
9
Rat
(Wistar)
10
Rat
(Wistar)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
14 d
(F)
Resp 2600 M
3900 F
Cardio
Hemato
Hepatic
Renal
Endocr
2600 M
3900 F
2600 M
3900 F
2600 M
3900 F
2600 M
3900 F
2600 M
3900 F
6 d
(GW)
2 x/d
6 d
1 x (d 7)
(GW)
22 M (increase in
dihydroxyphenylacetic
acid and uric acid in
striatum)
8.8 M (decrased concentrations
of dopamine in
brainstem; glutathione
depletion potentiated Mn
effects on dopamine as
well as concentrations of
DOPAC and HVA)
Reference
Chemical Form
Comments
NTP 1993
MnSO4
Desole et al. 1994
MnCl2
Desole et al. 1997
MnCl2
1058
3. HEALTH EFFECTS
MANGANESE
101

235
1300
1067
13.9
1156
4.4
1157
4.4
13.1
384
2200
1003
1300
383
2200
11
12
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
Rat 14 d
1300
NTP 1993
(F344/N)
(F)
MnSO4
Rat 1 d
13.9 (decreased acquisition of
Shukakidze et al. 2003
(albino)
(GW)
an avoidance reaction)
MnCl2*4H2O
13
Mouse
(C57BL/6N)
Pnd 20-34
1 x/d
(G)
4.4 M (increased novelty
seeking behavior in open
field)
Moreno et al. 2009
(MnCl2*4H2O)
No difference in the
total number of
movements, total
distance traveled, or in
rearing frequency in
open field
14
Mouse
(C57BL/6N)
Pnd 20-34
1 x/d
(G)
4.4
13.1 (increased dopamine,
decreased dopamine
metabolite DOPAC, and
increased serotonin
metabolite 5HIAA in
striatum)
Moreno et al. 2009
(MnCl2*4H2O)
Reproductive
15
Rat
(Sprague-
Dawley)
Gd 6-17
(GW)
2200 F
Grant et al. 1997a
MnCl2
16
Rat
(Fischer- 344)
14 d
(F)
Developmental
17
Rat
(Sprague-
Dawley)
Gd 6-17
(GW)
1300 M
2200
NTP 1993
MnSO4
Grant et al. 1997a
MnCl2
MANGANESE
3. HEALTH EFFECTS
102

147
225
1143
1730
1730
760
44
180
449
6
1155
910
21
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
INTERMEDIATE EXPOSURE
Death
18
Rat
(Long- Evans)
21 d
(GW)
225 (LD50 - 21 days)
Rehnberg et al. 1980
Mn3O4
Systemic
19
Rat
(Wistar)
30 days
ad lib
(W)
Hepatic 1730
Avila et al 2008
(MnCl2)
Renal
Bd Wt
1730
760 (50% decrease in body
weight gain)
20
Rat
(Long- Evans)
224 d
(F)
Hemato 180 M
Carter et al. 1980
Mn3O4
Rat 1 x/d
Bd Wt 6 M (rats gained only 44% of
Exon and Koller 1975
28 d
(Wistar)
amount gained by control
Mn3O4
(F)
rats with normal food
consumption)
22
Rat Gd 1 - pnd 24
Bd Wt 910 F (30% decrease in
Molina et al. 2011
Effect dose is an
(Sprague-
Dawley)
(W)
maternal weight)
(MnCl2*4H2O)
average of reported
daily Mn intake during
gestation (565
mg/kg/day) and
lactation (1256
mg/kg/day).
MANGANESE
3. HEALTH EFFECTS
103

237
40
520
618
520
618
33
155
33
618
520
618
520
618
77
155
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
Duration/
a
Key to Species
Frequency
Figure (Strain)
(Route)
System
23
Rat
(F344/N)
13 wk
(F)
Resp
Cardio
Gastro
Hemato
Hepatic
Renal
Endocr
Bd Wt
NOAEL
(mg/kg/day)
520 M
618 F
520 M
618 F
520 M
618 F
520 M
618 F
77 F
LOAEL
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
40 F (reduced lung weight)
NTP 1993
MnSO4
33 M (increased neutrophil
count)
155 F (decreased leukocyte
count)
33 M (decreased liver weight)
618 F (decreased liver weight)
155 F (11% decrease in body
weight)
3. HEALTH EFFECTS
MANGANESE
104

1051
87
406
33
11
80
12
1050
277
56
205
205
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
24
Rat
(Sprague-
Dawley)
63 d
(GW)
Renal 87 M (increased incidence of
glomerulosclerosis/
glomerulonephritis or
urolithiasis [i.e., bile
stone formation] in
males)
Ponnapakkam et al. 2003b
MnOAc
Rats sacrificed
immediately after last
day of dosing. No
urolithiasis observed in
females of any
treatment group.
25
Rat
(Sprague-
Dawley)
Gd 0-21
(GW)
Endocr 33 F
Szakmary et al. 1995
MnCl2
No effect on secretion
or peripheral blood
levels of progesterone
or 17b-estradiol.
Metab 11 F (increased cytochrome
P450)
26
Rat
(white)
10 wk
(W)
Hepatic 12 M
Wassermann and
Wassermann 1977
MnCl2
Only ultrastructural
changes in liver cells
were noted.
27
28
Mouse
Swiss
Mouse
(CD-1)
12 wk
(W)
90 d
(F)
Bd Wt
Hepatic
277 F
205 M
Elbetieha et al. 2001
MnCl2
Gray and Laskey 1980
Mn3O4
No clinical signs or
changes in body,
kidney or liver weights.
Renal 205 M
MANGANESE
3. HEALTH EFFECTS
105

1004
284
284
1005
284
1006
284
220
284
284
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
29
Mouse
(ddY)
30
31
Mouse
(ddY)
Mouse
(ddY)
32
Mouse
(ddY)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
100 d
(F)
Hemato 284 M (decreased red blood cell
count and white blood
cell count)
Komura and Sakamoto 1991
MnOAc
Bd Wt 284 M (10% decrease in body
weight gain)
100 d
(F)
Hemato 284 M (decreased hematocrit)
Komura and Sakamoto 1991
MnCO3
100 d
(F)
Hemato 284 M (decreased white blood
cell count)
Komura and Sakamoto 1991
MnO2
100 d
(F)
Hemato 284 M (decreased red blood cell
count and white blood
cell count)
Komura and Sakamoto 1991
MnCl2
Bd Wt 284 M (10% decrease in body
weight gain)
3. HEALTH EFFECTS
MANGANESE
106

248
1950
1950
975
1950
1950
975
1950
975
1950
1950
1950
1950
975
1950
1950
361
4.4
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
33
Mouse
(B6C3F1)
34
Gn Pig
Exposure/
Duration/
Frequency
(Route)
13 wk
(F)
30 d; 1 d
(G)
System
Resp
Cardio
Gastro
Hemato
Hepatic
Renal
Endocr
Bd Wt
Gastro
NOAEL
(mg/kg/day)
1950
1950
975 M
1950 F
975
975 M
1950 F
1950
1950
975 M
1950 F
LOAEL
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
NTP 1993
MnSO4
1950 M (mild hyperplasia and
hyperkeratosis of the
forestomach)
1950 (decreased hematocrit,
hemoglobin, and
erythrocyte count)
1950 M (reduced liver weight)
1950 M (13% lower body weight
compared to controls)
4.4 M (patchy necrosis,
decreased ATPase,
GTPase in stomach and
small intestine)
Chandra and Imam 1973
MnCl2
3. HEALTH EFFECTS
MANGANESE
107

240
33
155
1084
0.3
1061
107.5
319
240
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Immuno/ Lymphoret
35
Rat
(F344/N)
13 wk
(F)
33 M (increased neutrophil
count)
NTP 1993
MnSO4
155 F (decreased leukocyte
count)
Neurological
36
Human 1 x/d
8 wk
varying dose
(IN)
0.3 F
Finley et al. 2003
MnSO4
37
Monkey
(Rhesus)
4 mo during
infancy
(F)
107.5 M (minimally adverse
behavioral effects in soy
and soy+Mn groups:
decreased activity during
sleep at 4 months and
decreased play activity
between 1-1.5 months)
Golub et al. 2005
MnCl2
38
Rat
(ITRC)
1 generation
(W)
240 (delayed air righting
reflex in F1 pups)
Ali et al. 1983a
MnCl2
Comments
The high Mn diet did
not influence
neuropsychological
variables (interpersonal
behavior survey and
state-trait anger
expression) or
handsteadiness.
No marked differences
from controls in gross
motor maturation,
growth, or cognitive
tests. No effect of Mn
on CSF DA, HVA or
5-HIAA.
No significant
alterations in the age of
eye opening or
development of
auditory startle
3. HEALTH EFFECTS
MANGANESE
108

1071
71.1
1142
68.3
1144
760
1145
760
1730
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
39
Rat
(Sprague-
Dawley)
40
Rat
(Sprague-
Dawley)
41
Rat
(Wistar)
42
Rat
(Wistar)
Exposure/
Duration/
Frequency
(Route)
System
LOAEL
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
6 wk
(W)
71.1 (decreased Fe levels in
caudate putamen and
substantia nigra;
decreased GABA uptake
activity in striatal
synaptosomes)
Anderson et al. 2007a
MnCl2
6 wks
ad lib
(W)
68.3 M (decreased
norepinephrine and iron
levels, decreased
norepinephrine uptake,
and decreased protein
and mRNA levels of
norepinephrine and
alpha-2 adrenergic
receptors in brain)
Anderson et al. 2009
(MnCl2)
30 days
ad lib
(W)
760 (decreased locomotor
activity, decreased
tongue protrusion
frequency)
Avila et al 2008
(MnCl2)
30 days
ad lib
(W)
760
1730 (increased oxidative
stress in striatum)
Avila et al 2008
(MnCl2)
Comments
Calcium influx in
striatal slices was also
decreased at the
LOAEL. There were no
differences in rearing
frequency.
No evidence of
increased oxidative
stress in the
hippocampus
3. HEALTH EFFECTS
MANGANESE
109

151
594
372
392.5
43
13
850
11
22
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
43
Rat
(Sprague-
Dawley)
44
Rat
(Sprague-
Dawley)
45
Rat
(Sprague-
Dawley)
46
Rat
(CD)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
2 mo
(W)
594 M (increased gamma-
aminobutyric acid levels)
Bonilla 1978b
MnCl2
8 mo
(W)
392.5 M (increased L-tyrosine
hydroxylase activity in
neostriatum, midbrain,
hippocampus, and
hypothalamus)
Bonilla 1980
MnCl2
8 mo
(W)
13 M (decreased
norepinephrine levels)
Bonilla and Prasad 1984
MnCl2
pnd 1-49
(GW)
11
22 (increased spontaneous
motor activity)
Brenneman et al. 1999
MnCl2
3. HEALTH EFFECTS
MANGANESE
110

1072
1310
1062
1310
45
146.7
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
47
Rat
(Wistar)
10 wk
(W)
1310 M (significantly increased
open field activity,
significantly elevated,
continued interest in a
novel object and
increased fear; enhanced
dopaminergic inhibitory
control of corticostriatal
excitatory transmission)
Calabresi et al. 2001
MnCl2
No effects on radial
maze performance,
neuronal numbers in
striatum, levels of
GFAP and TH in
striatum, or membrane
properties of striatal
neurons.
48
Rat
(Wistar)
10 wk
(W)
1310 M (increased frequency and
amplitude of
spontaneous excitatory
membrane potentials in
corticostriatal slices from
Mn-treated rats
compared with control
rats)
Centonze et al. 2001
MnCl2
49
Rat
(albino)
30 d
(W)
146.7 M (increased activity and
aggression, turnover of
striatal dopamine,
tyrosine and homovanillic
acid, altered
neurotransmitter levels)
Chandra 1983
MnCl2
MANGANESE
3. HEALTH EFFECTS
111

180
0.31
370
40
181
1
10
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
LOAEL
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
50
Rat
(ITRC albino)
60 d
(GW)
Chandra and Shukla 1978
MnCl2*4H2O
0.31 M (increased monoamine
oxidase activity in the
brain, neuronal
degeneration in cerebral
and cerebellar cortex and
caudate nucleus)
No evidence of
behavioral changes or
locomotor
disturbances; exposure
started at 21 days of
age.
51
Rat
(ITRC albino)
360 d
(W)
Chandra and Shukla 1981
MnCl2
40 M (increase of dopamine,
norepinephrine, and
homovanillic acid above
control levels in striatum
observed at 15-60 days
of treatment, followed by
a decrease of all three
compounds below control
levels at 300-360 days of
treatment)
52
Rat
(CD Neonatal)
24 d
(GW)
1 M
Deskin et al. 1980
MnCl2
10 M (decreased dopamine
levels in the
hypothalamus, significant
decrease in hypothalamic
tyrosine hydroxylase
activity, significant
increase in hypothalamic
monoamine oxidase
activity)
3. HEALTH EFFECTS
MANGANESE
112

360
15
20
830
11
48
390
1085
8
1183
25
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
53
Rat
(CD)
54
Rat
55
Rat
56
Rat
(Sprague-
Dawley)
57
Rat
(Sprague-
Dawley)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg) (mg/kg) (mg/kg)
Chemical Form
Comments
pnd 0-24
(GW)
15
20 M (increased serotonin in
hypothalamus,
decreased
acetylcholinesterase in
striatum)
Deskin et al. 1981
MnCl2
21 d
1 x/d
(GW)
11 (significant increase in
pulse elicited startle
reflex at pnd 21)
Dorman et al. 2000
MnCl2
100-265 d
(W)
390 M (increased dopamine and
dopamine metabolite
levels)
Eriksson et al. 1987a
MnCl2
Gd 7- pnd 21
(F)
8 (hematological changes
indicative of Fe
deficiency in dams and
pups; increased levels of
the inhibitory
neurotransmitter, GABA,
in pup brains)
Garcia et al. 2006
NS
pnd 1-21
1 x/d
(G)
25 M (increased GFAP protein
levels in weanling and
adult brains)
Kern and Smith 2011
(MnCl2)
3. HEALTH EFFECTS
MANGANESE
113

1184
25
50
1148
25
1149
25
50
1089
1248
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
58
Rat
(Sprague-
Dawley)
59
Rat
(Sprague-
Dawley)
60
Rat
(Sprague-
Dawley)
61
Rat
(Long- Evans)
pnd 1-21
1 x/d
(G)
pnd 1-21
1x/day
(G)
pnd 1-21
1x/day
(G)
20 d
Gd 0-20
(W)
25 M
25 M
1248
50 M (increased dopamine D2
receptor in adult
prefrontal cortex)
25 M (increased stereotypic
behavior during radial
arm test)
50 M (increased open field
activity, impaired spatial
learning during radial arm
maze, increased protein
expressoin of D1 and D2
dopamine receptors and
dopamine transporter in
multiple brain regions)
Kern and Smith 2011
(MnCl2*4H2O)
Kern et al. 2010
(MnCl2)
Kern et al. 2010
(MnCl2*4H2O)
Kontur and Fechter 1985
MnCl2
No alteration in open
field behavior in
preweaning exposed
adults.
No differences milk
intake, body weight
gain, hematocrit, or
behavior during
elevated plus maze.
No effect on dopamine
or norepinephrine
turnover in the
forebrain or hiindbrain
and no effect on
development of
acoustic startle
response.
MANGANESE
3. HEALTH EFFECTS
114

62
13.8
65
150
1076
2.2
1099
10
20
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
62
Rat
(Long- Evans)
14-21 d
(GO)
13.8
Kontur and Fechter 1988
MnCl2
No effect on
monoamine levels or
their metabolites in the
striatum, hypothalamus
or nucleus accumbens.
63
Rat 44 d
(GW)
150 (ataxia)
Kristensson et al. 1986
MnCl2
64
Rat
(Sprague-
Dawley)
30 d
(GW)
2.2 M (redistribution of iron in
body fluids associated
with upregulation of
transferritin receptor
mRNA and
downregulation of ferritin
mRNA from the choroid
plexus and striatum)
Li et al. 2006
MnCl2
Observed effects likely
to be marginally to
minimally adverse.
65
Rat
(Sprague-
Dawley)
30 d
(GO)
10 M
20 M (significant [p < 0.05]
body weight decrease
[~9%] and significant [p <
0.05] increase in
aspartate, glutamate,
glutamine, taurine and
GABA in the cerebellum
[~20-50%, depending
upon the amino acid] of
adult rats)
Lipe et al. 1999
MnCl2
MANGANESE
3. HEALTH EFFECTS
115

1074
15.1
26.7
1057
611
238
520
618
1090
120
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
66
Rat
(Wistar)
67
Rat
(Wistar)
68
Rat
(F344/N)
69
Rat
(Sprague-
Dawley)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
4 wk
(W)
15.1 M
26.7 M (increases in striatal Mn
levels in cirrhotic rats,
striatal neurotransmitter
[dopamine or
homovanillic acid]
increased with or without
Montes et al. 2001
MnCl2*4H2O
No effect on bilirubins,
alanine
aminotransferase or
collagen at either dose
with or without bile duct
ligation.
cirrhosis)
13 wk
(W)
611 M (33% reduction in
immunoreactive cells
Morello et al. 2007
MnCl2*4H2O
with glutamine
synthetase in the globus
pallidus)
13 wk
(F)
520 M
618 F
NTP 1993
MnSO4
Gd 1- pnd 30
(W)
120 M (significant decrease in
cortical thickness; with
Pappas et al. 1997
MnCl2
high dose rats
demonstrating evidence
of hyperactivity
[significantly increased
locomotor activity and
increased rearing in an
open field] on pnd 17)
3. HEALTH EFFECTS
MANGANESE
116

Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Frequency
Key to Species
NOAEL Less Serious Serious
Reference
(Route)
Figure (Strain)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
70
Rat
50 d
74.9 M (increased serum levels
Ranasinghe et al. 2000
(Sprague-
(NS)
of dopamine sulfate,
MnSO4
Dawley)
L-Dopa, and L-p-tyrosine
and decreased levels of
dopamine)
74.9
1064
71
Rat
21 d
No change in negative
4.4 M
13.1 M (subtle behavioral effects
Reichel et al. 2006
(Sprague-
(NS)
4.4
geotaxis performance;
[altered balance in the
MnCl2
Dawley)
no change in motor
neonatal period and
activity, coordination, or
diminished locomotor
olfactory orientation
response to cocaine in
tasks.
adulthood] and
neurochemical effects in
adulthood [decreased
dopamine binding sites in
the striatum])
13.1
1065
72
Rat
30 d
5.6 (severely impaired
Shukakidze et al. 2003
(albino)
(F)
cognitive performance in
MnCl2*4H2O
maze)
5.6
1068
73
Rat
13 wk
3311 M (impaired ability of globus
Spadoni et al. 2000
No neuronal loss or
(Wistar)
(W) gliosis (GFAP
pallidus neurons to
(NS)
accumulation) was
survive mechanical
evident in globus
dissociation)
pallidus by either
3311
histological or
immunohistochemical
examination).
1077
3. HEALTH EFFECTS
MANGANESE
117

282
11.8
1054
76
153
1069
3.8
7.5
7.5
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
74
Rat
(albino)
75
Rat
(Sprague-
Dawley)
76
Rat
(Sprague-
Dawley)
77
Rat
(Sprague-
Dawley)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
90 d
(W)
11.8 M (altered brain regional
dopamine and serotonin
levels and monoamine
Subhash and Padmashree
1991
MnCl2
oxidase activity)
21 wk
(GW, W)
76 M
153 M (significantly decreased
open field activity among
restrained rats, impaired
spatial learning with or
without restraint in a
Torrente et al. 2005
MnCl2*4H2O
water maze)
20 d
(GO)
3.8
7.5
(decreased performance
in the olfactory
discrimination [homing
test] and passive
avoidance task; striatal
Tran et al. 2002a
MnCl2
dopamine concentrations
were about 50% lowe
r
than control values)
20 d
(GO)
7.5 M
Tran et al. 2002b
MnCl2
Comments
All MnCl2*4H2O rats
received 38 mg
Mn/kg/d for the first 2
weeks. Other groups
at these doses were
restrained 2 hours/day.
No significant (p <0.05)
exposure-related
effects on righting test
conducted on pnd 6.
No significant effects in
either burrowing detour
task (pnd 50-56) or
passive avoidance task
(pnd 60-69).
1070
3. HEALTH EFFECTS
MANGANESE
118

1082
6.5
1078
13.8
52
2250.7
54
205
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
78
Rat
(Wistar)
79
Rat
(CD)
80
Mouse
(CD-1)
81
Mouse
(CD-1)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
22 wk
(GW)
6.5 M (significant decreases in
spatial memory
performance, open field
locomotor activity and
acoustic startle
responses; increased
latency of sensory
evoked potentials)
Vezér et al. 2005, 2007
MnCl2*4H2O
21 d
(IN)
13.8
Weber et al. 2002
MnCl2*4H2O
6 mo
2250.7 M (decreased dopamine
Gianutsos and Murray 1982
(F)
levels)
MnCl2
90 d
205 M (decreased locomotor
Gray and Laskey 1980
(F)
activity)
Mn3O4
Comments
Impairment of spatial
memory performance
and acoustic startle
response persisted
through 5-7 weeks
without exposure.
No obvious effect of
oral exposure during
pnd 1-21 on
biochemical measures
related to oxidative
stress in
cerebrocortical or
cerebellar regions.
3. HEALTH EFFECTS
MANGANESE
119

221
284
1086
43.7
1159
4.4
13.1
1181
4.4
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
82
Mouse
(ddY)
83
Mouse
(C57BL/6N)
84
Mouse
(C57BL/6N)
85
Mouse
(C57BL/6N)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
100 d
(F)
284 M (decreased motor
activity)
Komura and Sakamoto 1991
MnCl2, MnOAc, MnCO3,
MnO2
1 x/d
8 wk
(GW)
43.7 F (increased locomotor
activity in Mn-treated
mice; increased Mn
Liu et al. 2006
MnCl2
content of striatum and
substantia nigra;
decreased striatal
dopamine; increased
apoptotic neurons
expressing nitric oxide
synthase, choline
acetyltransferase and
enkephalin in striatum
and globus pallidus;
increased astrocytes
expressing evidence of
nitric oxide formation)
8 wks
Moreno et al. 2009
No alteration in levels
4.4
13.1 (decreased dopamine
1 x/d
of serotonin or its
and dopamine metabolite
(MnCl2*4H2O)
(G)
metabolite 5-HIAA. No
DOPAC levels in
differences in open
striatum)
field behavior.
Pnd 20-34, wk
Moreno et al. 2009
No alteration in levels
4.4 (decreased dopamine
12-20
of serotonin or its
and dopamine metabolite
1 x/d
(MnCl2*4H2O)
metabolite 5-HIAA.
DOPAC levels in
(G)
striatum)
3. HEALTH EFFECTS
MANGANESE
120

249
1950
64
624 1248
67
20
55
55
180
380
620
1063
68.6
137.2
404
22
33
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
LOAEL
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
86
Mouse
(B6C3F1)
13 wk
(F)
Reproductive
87
Rat
(Long- Evans)
20 d
Gd 0-20
(W)
NTP 1993
MnSO4
1950
Kontur and Fechter 1985
MnCl2
624 F
1248 F (decreased litter weight)
88
Rat
(Long- Evans)
100-224 d
(F)
Laskey et al. 1982
Mn3O4
20 M
55 F
55 M (significantly decreased
testicular weight with
low-Fe diet)
180 F (significantly decreased
[~25%] pregnancy rate)
No effect on litter size,
ovulations, resorptions,
preimplantation deaths
or mean fetal weights.
No effect on
testosterone or LH
levels.
89
Rat
(Sprague-
Dawley)
Gd 1- pnd 30
(W)
Pappas et al. 1997
MnCl2
620 F
Mn exposure of
pregnant dams did not
affect litter sizes or sex
ratios of pups at
delivery.
90
Rat
(Sprague-
Dawley)
63 d
(GW)
Ponnapakkam et al. 2003c
MnOAc*4H20
68.6 M
137.2 M (increased incidences of
testicular degeneration in
male rats)
91
Rat
(Sprague-
Dawley)
Gd 0-21
(GW)
Szakmary et al. 1995
MnCl2
22 F (increase in relative
weight of liver, thymus,
and brain)
33 F (post implantation loss)
3. HEALTH EFFECTS
MANGANESE
121

1044
154
309
1048
44
277
55
205
250
1950
1041
2.4
4.8
1043
9.6
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
92
Mouse
Swiss
93
Mouse
Swiss
94
Mouse
(CD-1)
95
96
Mouse
(B6C3F1)
Mouse
(CD-1)
97
Mouse
(CD-1)
Exposure/
LOAEL
Duration/
Reference
Chemical Form
Elbetieha et al. 2001
MnCl2
Elbetieha et al. 2001
MnCl2
Gray and Laskey 1980
Mn3O4
NTP 1993
MnSO4
Ponnapakkam et al. 2003a
MnOAc
Ponnapakkam et al. 2003a
MnOAc
Comments
No effects on fertility at
9.6 mg/kg/day when
treated males were
mated with unexposed
females.
Fertility endpoints were
not affected at 9.6 mg
Mn/kg/day. Fertility
was not affected when
exposed males mated
with nonexposed
females.
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
12 wk
(W)
154 M
12 wk
(W)
44 F (increased uterine
weights relative to body
weight)
90 d
(F)
205 M (delayed growth of testes
and sex accessory
glands)
13 wk
(F)
1 x/d
43 d
(GW)
1950
2.4 M
4.8 M (decreased sperm
motility and sperm
counts)
1 x/d
43 d
(GW)
9.6 M
Serious
(mg/kg/day)
309 M (statistically significantly
impaired male fertility)
277 F (implantation number
reduced by 17% and the
number of viable fetuses
reduced by 19% from the
control value)
MANGANESE
3. HEALTH EFFECTS
122

409
33
1087
107.5
1188
240
1000
11
22
850
11
22
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
98
Rabbit
(New
Zealand)
Developmental
99
Monkey
(Rhesus)
100
Rat
(ITRC)
101
Rat
(CD)
102
Rat
(CD)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg) (mg/kg) (mg/kg)
Chemical Form
Gd 6-20
(GW)
4 mo
(F)
1 generation
(W)
pnd 1-49
(W)
pnd 1-49
(GW)
33 F
Szakmary et al. 1995
MnCl2
107.5 M (minimally adverse
effects in soy and
soy+Mn groups:
decreased activity during
sleep at 4 months and
decreased play activity
between 1-1.5 months)
Golub et al. 2005
MnCl2
240 (delayed air righting
reflex in F1 pups)
Ali et al. 1983a
MnCl2
11
22 (~20% decrease in body
weight at pnd 49)
Brenneman et al. 1999
MnCl2
11
22 (increased spontaneous
motor activity)
Brenneman et al. 1999
MnCl2
Comments
No marked differences
from controls in gross
motor maturation,
growth, or cognitive
tests. No effect of Mn
on CSF DA, HVA or
5-HIAA.
No significant
alterations in the age of
eye opening or
development of
auditory startle
3. HEALTH EFFECTS
MANGANESE
123

1185
1
10
1186
15
20
830
11
1059
8
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
103
Rat
(CD Neonatal)
24 d
(GW)
1 M
10 M (decreased dopamine
levels in the
hypothalamus, significant
decrease in hypothalamic
tyrosine hydroxylase
activity, significant
increase in hypothalamic
monoamine oxidase
activity)
Deskin et al. 1980
MnCl2
104
Rat
(CD)
pnd 0-24
(GW)
15 M
20 M (increased serotonin in
hypothalamus,
decreased
acetylcholinesterase in
striatum)
Deskin et al. 1981
MnCl2
105
Rat 21 d
1 x/d
(GW)
11 (significant increase in
pulse elicited startle
reflex at pnd 21)
Dorman et al. 2000
MnCl2
106
Rat
(Sprague-
Dawley)
Gd 7- pnd 21
(F)
8 (hematological changes
indicative of Fe
deficiency in dams and
pups; increased levels of
the inhibitory
neurotrnasmitter, GABA,
Garcia et al. 2006
NS
in pup brains)
MANGANESE
3. HEALTH EFFECTS
124

Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Frequency
Key to Species
NOAEL Less Serious Serious
Reference
(Route)
Figure (Strain)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
107
Rat pnd 1-21
25 M (increased GFAP protein
Kern and Smith 2011
(Sprague-
1 x/d
levels in weanling and
(MnCl2)
Dawley)
(G)
adult brains)
25
1150
108
Rat pnd 1-21
Kern and Smith 2011
No alteration in open
25 M
50 M (increased dopamine D2
(Sprague-
1 x/d
25
field behavior in
receptor in adult
(MnCl2*4H2O)
Dawley)
(G)
preweaning exposed
prefrontal cortex)
adults.
50
1151
109
Rat 14-21 d
13.8
Kontur and Fechter 1988
No effect on
(Long- Evans)
(GO)
13.8
monoamine levels or
MnCl2
their metabolites in the
striatum, hypothalamus
or nucleus accumbens.
1187
110
Rat 44 d
150 (ataxia)
Kristensson et al. 1986
(GW)
150
430
MnCl2
111
Rat 15-20 days
4.4 (gliosis)
Lazrishvili et al 2009
No change in the
(NS)
before
4.4
number of neurons.
pregnancy,
(MnCl2*4H2O)
during
pregnancy, 1
mo postnat.
(F)
1152
3. HEALTH EFFECTS
MANGANESE
125

1153
910
1154
910
376
120
620
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
112
Rat
(Sprague-
Dawley)
113
Rat
(Sprague-
Dawley)
114
Rat
(Sprague-
Dawley)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
Gd 1 - pnd 24
(W)
910 (decreased anxiety
behavior on elevated
plus apparatus; altered
iron pharmacokinetics -
decreased tissue uptake
of iron, increased levels
of zinc protoporphyrin
levels)
Molina et al. 2011
(MnCl2*4H2O)
Effect dose is an
average of reported
daily Mn intake during
gestation (565
mg/kg/day) and
lactation (1256
mg/kg/day).
Gd 1 - pnd 24
(W)
910 (decreased tissue uptake
of iron, increased levels
of zinc protoporphyrin
levels)
Molina et al. 2011
(MnCl2*4H2O)
No change in intestinal
absorption of iron,
expression of duodenal
divalent metal
transporter 1,
hematocrit, or
non-heme iron levels)
Gd 1- pnd 30
(W)
120 M
620 M (transient decrease
(~20%) in pup body
weight on pnd 9-24;
difference not apparent
on pnd 90)
Pappas et al. 1997
MnCl2
No maternal toxicity
from Mn; brain Mn not
significantly elevated at
120 mg/kg/day; no
effects on brain levels
of serotonin or 5-HIAA.
3. HEALTH EFFECTS
MANGANESE
126

1088
4.4
13.1
405
33
1091
3.8
7.5
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
115
Rat
(Sprague-
Dawley)
21 d
(NS)
4.4 M
13.1 M (subtle behavioral effects
[altered balance in the
neonatal period and
diminished locomotor
response to cocaine in
adulthood] and
neurochemical effects in
adulthood [decreased
dopamine binding sites in
the striatum])
Reichel et al. 2006
MnCl2
No change in negative
geotaxis performance;
no change in motor
activity, coordination, or
olfactory orientation
tasks.
116
Rat
(Sprague-
Dawley)
Gd 0-21
(GW)
33 (increased retardation in
skeletal/organ
development)
Szakmary et al. 1995
MnCl2
117
Rat
(Sprague-
Dawley)
20 d
(GO)
3.8
7.5
(decreased performance
in the olfactory
discrimination [homing
test] and passive
avoidance task; striatal
dopamine concentrations
were about 50% lowe
r
than control values)
Tran et al. 2002a
MnCl2
MANGANESE
3. HEALTH EFFECTS
127

1092
7.5
1093
13.8
408
33
1096
0.26
241
200
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Frequency
Key to Species
NOAEL Less Serious
(Route)
Figure (Strain)
System
(mg/kg/day) (mg/kg/day)
118
Rat 20 d
7.5 M
(Sprague-
(GO)
Dawley)
119
Rat 21 d
13.8
(CD)
(IN)
120
Rabbit Gd 6-20
33
(New
(GW)
Zealand)
CHRONIC EXPOSURE
Death
121
Human </= 1 yr
(W)
122
Rat 2 yr
(F344/N)
(F)
Serious
(mg/kg/day)
0.26 (increased fatality among
children <1 year of age)
200 M (14% survival compared
to 49% in controls)
Reference
Chemical Form
Tran et al. 2002b
MnCl2
Weber et al. 2002
MnCl2*4H2O
Szakmary et al. 1995
MnCl2
Hafeman et al. 2007
NS
NTP 1993
MnSO4
Comments
No statistically
significant (p < 0.05)
effects in either
burrowing detour task
pnd 50-56) or passive
avoidance task (pnd
60-69).
No obvious effect of
oral exposure during
pnd 1-21 on
biochemical measures
related to oxidative
stress in
cerebrocortical or
cerebellar regions.
No effect on fetal body
weights or skeletal
anomalies in fetuses.
MANGANESE
3. HEALTH EFFECTS
128

242
200
232
65
200
232
65
200
200
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
Systemic
123
Rat
(F344/N)
2 yr
(F)
Resp 200 M
232 F
NTP 1993
MnSO4
Cardio 65 M
Gastro 200 M
232 F
Hemato 65 M
Renal 200 M (increased severity of
chronic progressive
nephropathy)
Bd Wt 200 M (body weight 10% lower
than controls)
MANGANESE
3. HEALTH EFFECTS
129

253
585
731
585
731
177
226
585
732
177
731
585
585
731
585
731
585
731
585
64
584
732
584
223
732
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to
Figure
Species
(Strain)
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
124
Mouse
(B6C3F1)
2 yr
(F)
Resp 585 M
731 F
Cardio 585 M
731 F
Gastro 177 M
226 F
585 M (hyperplasia, erosion)
Hemato 177 M
731 F
585 M (increased hematocrit,
hemoglobin, and
erythrocyte counts)
Musc/skel 585 M
731 F
Hepatic 585 M
731 F
Renal 585 M
731 F
Endocr 585 M (thyroid follicular
hyperplasia and
dilatation)
64 F (thyroid follicular
hyperplasia)
Dermal 584 M
732 F
Bd Wt 584 M
223 F
732 F (13% lower body weight
than controls)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
NTP 1993
MnSO4
732 F (ulceration and
inflammation of the
forestomach)
3. HEALTH EFFECTS
MANGANESE
130

245
200
232
1007
585
731
1168
0.104
171
0.0048 0.059
1097
0.103
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to
Figure
Species
(Strain)
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Immuno/ Lymphoret
125
Rat
(F344/N)
2 yr
(F)
200 M
232 F
126
Mouse
(B6C3F1)
2 yr
(F)
585 M
731 F
Neurological
127
Human ~68 d
intermittently x
5 yr
(W)
0.104 F (pica, emotional lability,
personality changes,
speech impairments, loss
of balance and
coordination, inability to
walk)
128
Human 50 yr
(W)
0.0048
0.059 (mild neurological signs)
129
Human ~68 d
intermittently x
5 yr
(W)
0.103 F (pica, emotional lability,
personality changes,
speech impairments, loss
of balance and
coordination, inability to
walk)
Reference
Chemical Form
Comments
NTP 1993
MnSO4
NTP 1993
MnSO4
Brna et al 2011
NS
Kondakis et al. 1989
NS
Sahni et al. 2007
NS
3. HEALTH EFFECTS
MANGANESE
131

353
0.009
1098
0.04
0.07
1169
0.015
0.081
1095
0.06
152
6.9
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
130
Human 10 yr or more
0.009
Vieregge et al. 1995
(W)
NS
131
Human 10 yr
0.04
0.07 (significantly reduced
Wasserman et al. 2006
No statistically
(W)
significant effects on
performance on
NS
Full-Scale IQ testing,
Full-Scale IQ test,
performance or verbal
performance and verbal
tests.
tests in children)
132
Human 8 yr or more
(W)
0.015
0.081
(significantly reduced
performance on
perceptual reasoning and
working memor
y
subscales in children)
Wasserman et al. 2011
(NS)
133
Human 5 yr
(W)
0.06 M (Mn possibly producing
deficit in free retrieval
skills, affecting general,
verbal and visual
memory and learning
skills; inattentiveness;
lack of focus in
classroom)
Woolf et al. 2002
NS
134
Monkey
(Rhesus)
18 mo
(GW)
6.9 M (weakness, rigidity,
neuronal loss and
depigmentation of the
substantia niagra)
Gupta et al. 1980
MnCl2
MANGANESE
3. HEALTH EFFECTS
132

66
40
71
40
213
10.6
1008
275
1009
275
1010
275
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
135
Rat
(Wistar)
136
Rat
(Sprague-
Dawley)
137
Mouse
(ddY)
138
Mouse
(ddY)
139
Mouse
(ddY)
140
Mouse
(ddY)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
LOAEL
Reference
Chemical Form
Comments
2 yr
(W)
40 (altered neurotransmitter
uptake)
Lai et al. 1984
MnCl2
65 wk
(W)
40 M (increased activity)
Nachtman et al. 1986
MnCl2
3 gen
(W)
10.6 (altered gait)
Ishizuka et al. 1991
MnCl2*4H2O
12 mo
(F)
275 M (decreased locomotor
activity)
Komura and Sakamoto 1992a
MnOAc
12 mo
(F)
275 M (decreased locomotor
activity)
Komura and Sakamoto 1992a
MnCO3
12 mo
(F)
275 M (decreased dopamine
and increased
homovanilic acid in brain;
decreased
norepinephrine and
epinephrine; decreased
locomotor activity)
Komura and Sakamoto 1992a
MnO2
3. HEALTH EFFECTS
MANGANESE
133

223
275
689
45
254
585
731
244
200
232
255
585
731
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
a
Key to Species
Figure (Strain)
141
Mouse
(ddY)
142
Mouse
(ddY)
143
Mouse
(B6C3F1)
Reproductive
144
Rat
(F344/N)
145
Mouse
(B6C3F1)
Exposure/
LOAEL
Duration/
Frequency
NOAEL Less Serious Serious
Reference
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
12 mo
(F)
275 M (decreased locomotor
activity)
Komura and Sakamoto 1992a
MnCl2
12 mo
(F)
45 M (significant [p < 0.05]
decreases in dopamine
and homovanillic acid
Komura and Sakamoto 1994
MnCl2
levels in the corpus
striatum)
2 yr
(F)
585 M
731 F
NTP 1993
MnSO4
2 yr
(F)
200 M
232 F
NTP 1993
MnSO4
2 yr
(F)
585 M
731 F
NTP 1993
MnSO4
3. HEALTH EFFECTS
MANGANESE
134

1011
420
Table 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
Figure (Strain)
(Route)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
Developmental
146
Rat 1 gen
420 M (altered neurotransmitter
Ali et al. 1985
(ITRC)
(W)
levels)
MnCl2*4H2O
MANGANESE
a The number corresponds to entries in Figure 3-2.
A
TPase = adenosine triphosphatase; Bd Wt = body weight; Cardio = cardiovascular; CSF = cerebrospinal fluid; d = day(s); DA = dopamine; DOPAC = dihydroxyphenylacetic acid;
Endocr = endocrine; F = Female; (F) = feed; (G) = gavage; GABA = gamma-aminobutyric acid; Gastro = gastrointestinal; Gd = gestational day; GFAP = glial fibrillary acidic protein;
Gn pig = guinea pig; (GO) = gavage in oil; (GW) = gavage in water; GTPase = glucose-6-phosphatase; Hemato = hematological; 5-HIAA = 5-hydroxy-indoleacetic acid; HVA =
homovanillic acid; Immuno/Lymphoret = immunological/lymphoreticular; (IN) = ingestion; LD50 = lethal dose, 50% kill; LH = luteinizing hormone; LOAEL =
lowest-observed-adverse-effect level; M = male; Metab = metabolic; mo = month(s); Musc/skel = musculoskeletal; NOAEL = no-observed-adverse-effect level; NS = not specified;
Resp = respiratory; TH = tyrosine hydroxylase (W) = drinking water; wk = week(s); x = time(s); yr = year(s)
3. HEALTH EFFECTS
135

Death
Respiratory
Cardiovascular
Hematological
Hepatic
Renal
Endocrine
Body Weight
Neurological
Reproductive
Developmental
Figure 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral
Acute (≤14 days)
Systemic
mg/kg/day
10000
8m 8m 8m 8m 8m 8m
8m 8m 8m 8m 8m 8m
15r 17r
7r 7r 7r 7r 7r 7r 7r 7r 7r 11r 16r
6r
1000
5r
4r
7r 7r 7r
1r
2r
3r
3r
3r
100
9r
12r
14m
10
10r
13m 14m
1
LD50/LC50
c-Cat -Human f-Ferret n-Mink
Cancer Effect Level-Humans Minimal Risk Level
d-Dog k-Monkey j-Pigeon o-Other Cancer Effect Level-Animals LOAEL, More Serious-Humans for effects
r-Rat m-Mouse e-Gerbil LOAEL, More Serious-Animals LOAEL, Less Serious-Humans other than
p-Pig h-Rabbit s-Hamster LOAEL, Less Serious-Animals NOAEL - Humans Cancer
q-Cow a-Sheep g-Guinea Pig NOAEL - Animals
MANGANESE
3. HEALTH EFFECTS
136
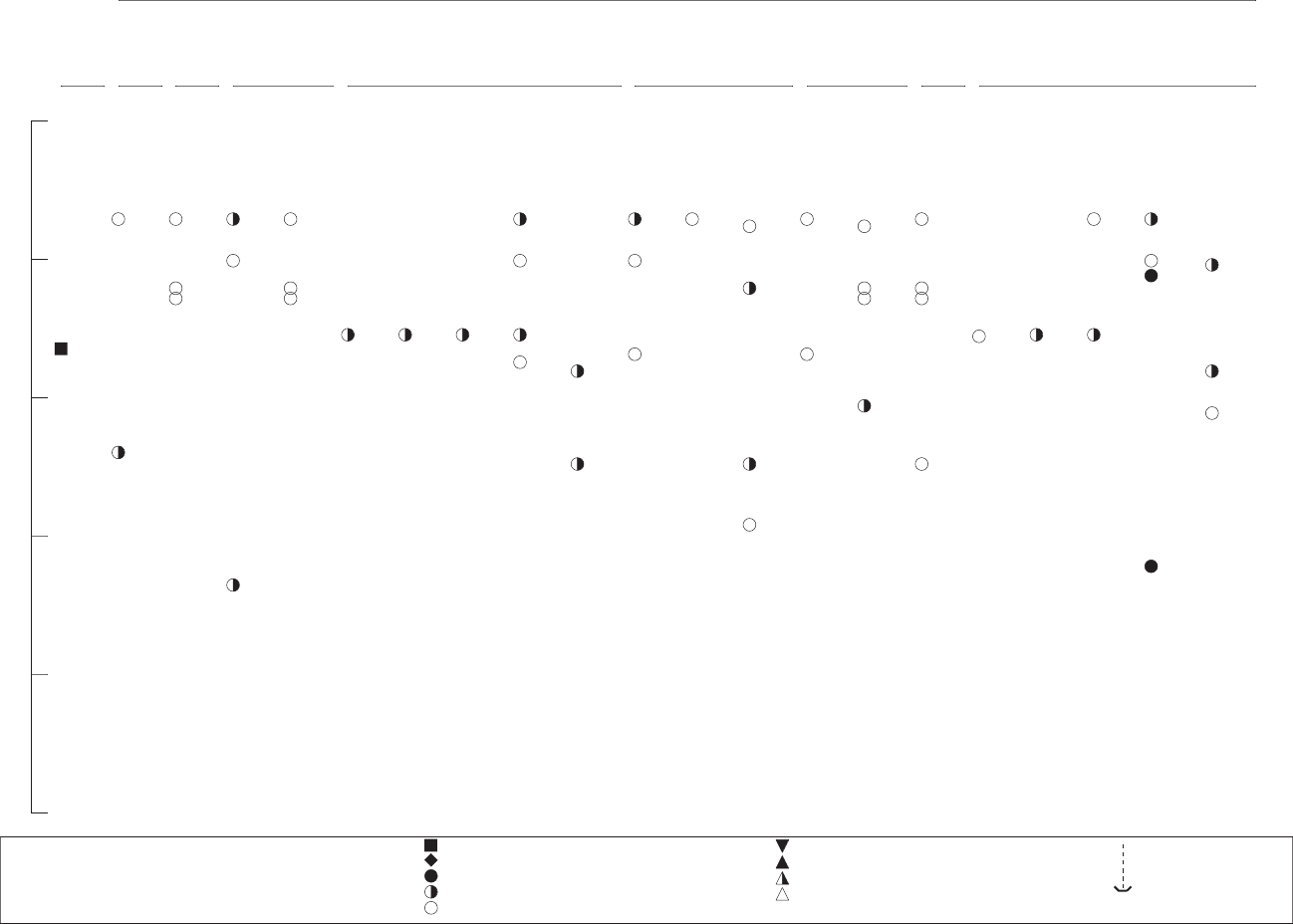
Death
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Hepatic
Renal
Endocrine
Body Weight
Systemic
mg/kg/day
10000
33m 33m 33m 33m 33m 33m 33m 33m 33m 33m 33m
19r 19r
1000
33m 33m 33m 33m
22r
19r
23r 23r
23r 23r 23r
23r 23r 23r 23r
29m 30m 31m 32m 29m 32m
27m
18r
28m 28m
20r
23r 23r
100
24r
23r
23r
23r 23r 25r
26r
10
21r
34g
1
0.1
LD50/LC50
c-Cat -Human f-Ferret n-Mink
Cancer Effect Level-Humans Minimal Risk Level
Cancer Effect Level-Animals
d-Dog k-Monkey j-Pigeon o-Other LOAEL, More Serious-Humans for effects
LOAEL, More Serious-Animals
r-Rat m-Mouse e-Gerbil LOAEL, Less Serious-Humans other than
LOAEL, Less Serious-Animals
p-Pig h-Rabbit s-Hamster NOAEL - Humans Cancer
NOAEL - Animals
q-Cow a-Sheep g-Guinea Pig
Figure 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (Continued)
Intermediate (15-364 days)
MANGANESE
3. HEALTH EFFECTS
137

Metabolic
Immuno/Lymphor
Neurological
10000
Figure 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (Continued)
Intermediate (15-364 days)
Systemic
mg/kg/day
73r
80m
86m
42r
47r
48r
61r
1000
41r 42r
43r
68r
67r
68r
44r
55r
82m
38r
81m
35r
75r
69r
63r
49r
37k
100
75r
70r
39r
40r
58r 60r
83m
51r
35r
57r
66r
58r 59r 60r
46r
53r 65r
53r
66r
62r 79r
25r
84m 71r
45r
74r
46r 54r
10 52r 65r
56r
76r 77r
78r
72r
84m 85m 71r
76r
64r
1
52r
50r
36
0.1
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
-Human
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
Minimal Risk Level
for effects
other than
Cancer
LD50/LC50
MANGANESE
3. HEALTH EFFECTS
138

0.1
1
10
100
1000
10000
88r
89r
90r
90r
91r
91r
98h
Developmental
99k
100r
101r
101r
102r
102r
103r
103r
104r
104r
105r
106r
107r 108r
108r
109r
110r
111r
112r 113r
114r
114r
115r
115r
116r
117r
117r 118r
119r
120h
mg/kg/day
Figure 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (Continued)
Intermediate (15-364 days)
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
-Human
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
Minimal Risk Level
for effects
other than
Cancer
Reproductive
92m
92m
93m
93m
94m
95m
96m
96m
97m
87r
87r
88r
88r
88r
LD50/LC50
3. HEALTH EFFECTS
MANGANESE
139
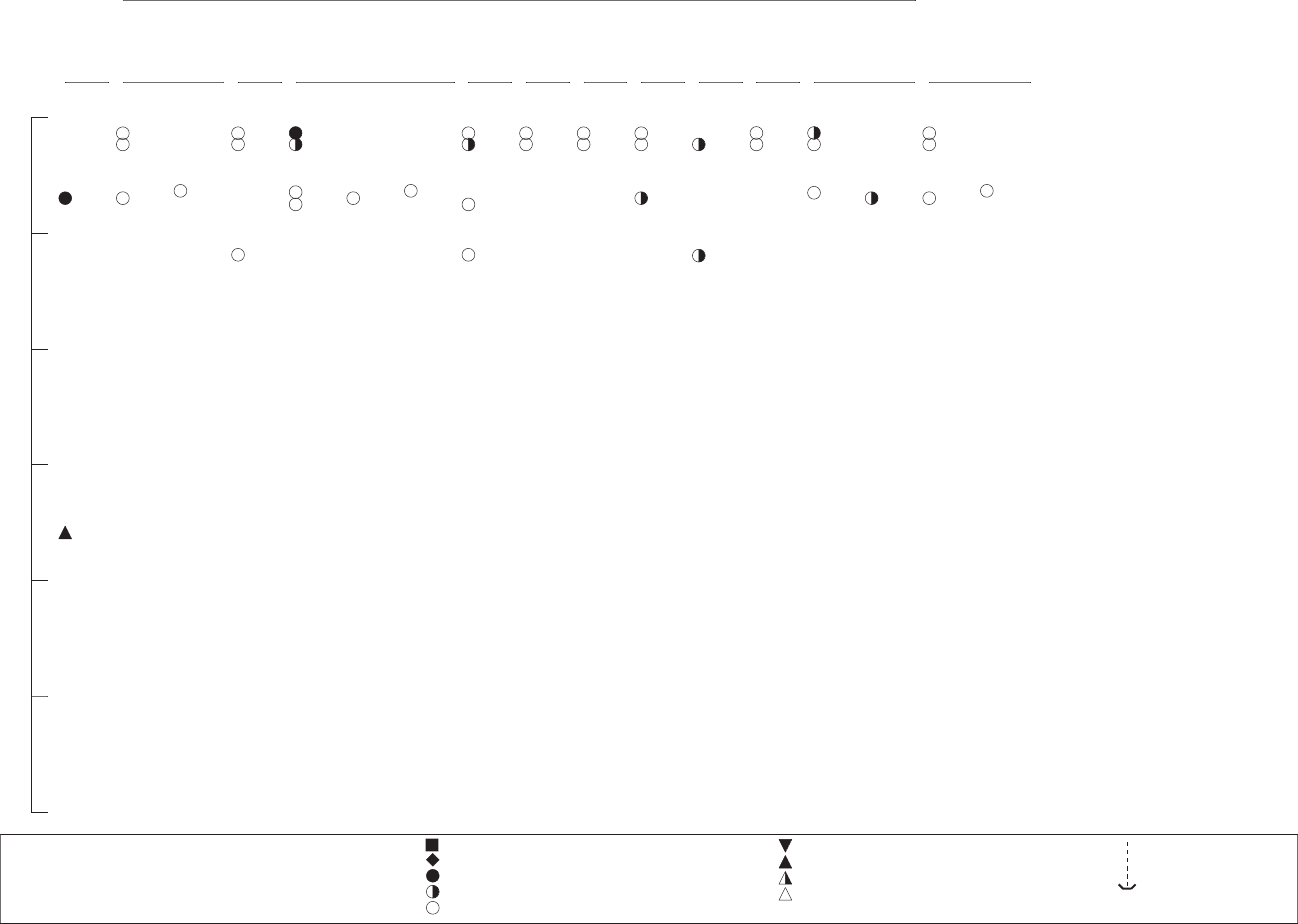
Death
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Endocrine
Dermal
Body Weight
Immuno/Lymphor
1000
Figure 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (Continued)
Chronic (≥365 days)
Systemic
mg/kg/day
124m 124m 124m
124m 124m
124m 124m 124m 124m 126m
124m 124m 124m 124m 124m 124m 124m 124m
124m 124m 126m
123r 123r
125r
124m
124m
122r 123r 123r 123r 123r 125r
124m 124m
100
123r 123r
124m
10
1
121
0.1
0.01
0.001
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
-Human
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
Minimal Risk Level
for effects
other than
Cancer
LD50/LC50
MANGANESE
3. HEALTH EFFECTS
140

Reproductive
Developmental
Neurological
Figure 3-2 Levels of Significant Exposure to Inorganic Manganese - Oral (Continued)
Chronic (≥365 days)
mg/kg/day
1000
143m 145m
143m
145m
146r
138m 139m
140m 141m
144r
144r
100
142m
135r 136r
137m
134k
10
1
127
129
0.1
132
131
133
131
132
128
0.01
130
128
0.001
c-Cat -Human f-Ferret n-Mink Cancer Effect Level-Humans Minimal Risk Level
LD50/LC50
d-Dog k-Monkey j-Pigeon o-Other Cancer Effect Level-Animals LOAEL, More Serious-Humans for effects
r-Rat m-Mouse e-Gerbil LOAEL, More Serious-Animals LOAEL, Less Serious-Humans other than
p-Pig h-Rabbit s-Hamster LOAEL, Less Serious-Animals NOAEL - Humans Cancer
q-Cow a-Sheep g-Guinea Pig NOAEL - Animals
MANGANESE
3. HEALTH EFFECTS
141

581
12.5
1137
15
1094
14.6
435
58
580
30
451
7.6
11.3
7.6
11.3
Table 3-3 Levels of Significant Exposure to MMT
-
Oral
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg)
Less Serious
(mg/kg)
LOAEL
Serious
(mg/kg)
Reference
Chemical Form
Comments
ACUTE EXPOSURE
Death
1
Rat
(Sprague-
Dawley)
once
(GO)
12.5 M (increase in mortality,
LD50=50 mg MMT/kg or
13 mg Mn/kg)
Hanzlik et al. 1980a
2
Rat
(Sprague-
Dawley)
1 x
15 (LD50)
Hinderer 1979
3
Rat
(COBS)
1 x
(GO)
14.6 (LD50)
Hysell et al. 1974
4
Mouse
(CD-1)
Systemic
5
Rat
(Sprague-
Dawley)
1 x
(GO)
once
(GO)
Resp
58 F (LD50)
30 M (distended lungs with
bloody fluid, hemorrhage,
perivascular and alveolar
edema)
Hinderer 1979
Hanzlik et al. 1980a
6
Rat
(COBS)
1 x
(GO)
Resp 7.6
11.3 (severe fibrinopurulent
pneumonia with
prominent macrophage
infiltrate of lungs)
Hysell et al. 1974
All rats from 3.8 and
7.6 mg Mn/kg bw/d
groups survived and
appeared normal 14
days post-exposure.
Hepatic 7.6
11.3 (hepatic parenchymal
necrosis and leukocytic
infiltration)
MANGANESE
3. HEALTH EFFECTS
142
441
11
442
11
688
11
Table 3-3 Levels of Significant Exposure to MMT
-
Oral (continued)
Exposure/
Duration/
a
Key to Species
Frequency
(Route)
Figure (Strain)
CHRONIC EXPOSURE
Systemic
7
Mouse 1 x/d
12 mo
(ddY)
(F)
System
Bd Wt
NOAEL
(mg/kg)
LOAEL
Less Serious Serious
(mg/kg) (mg/kg)
11 M (>10% decrease in body
weight in exposed group)
Reference
Chemical Form
Komura and Sakamoto 1992b
Comments
Neurological
8
Mouse
(ddY)
1 x/d
12 mo
(F)
11 M (increase in spontaneous
motor activity on day 80)
Komura and Sakamoto 1992b
9
Mouse
(ddY)
12 mo
(F)
11 M (changes in brain
neurochemistry)
Komura and Sakamoto 1994
a The number corresponds to entries in Figure 3-3.
Bd Wt = body weight; d = day(s); (F) = feed; F = Female; GO) = gavage in oil; LD50 = lethal dose, 50% kill; LOAEL = lowest-observed-adverse-effect level; M = male; mo = month(s);
NOAEL = no-observed-adverse-effect level; pnd = post-natal day; Resp = respiratory; x = time(s)
3. HEALTH EFFECTS
MANGANESE
143

Death
Respiratory
Hepatic
Figure 3-3 Levels of Significant Exposure to MMT - Oral
Acute (≤14 days)
Systemic
mg/kg/day
100
4m
5r
2r
3r
1r
6r 6r
10
6r 6r
1
c-Cat -Human f-Ferret n-Mink Cancer Effect Level-Humans
LD50/LC50
Minimal Risk Level
d-Dog k-Monkey j-Pigeon o-Other LOAEL, More Serious-Humans
Cancer Effect Level-Animals for effects
r-Rat m-Mouse e-Gerbil LOAEL, Less Serious-Humans
LOAEL, More Serious-Animals other than
p-Pig h-Rabbit s-Hamster NOAEL - Humans
LOAEL, Less Serious-Animals Cancer
q-Cow a-Sheep g-Guinea Pig
NOAEL - Animals
MANGANESE
3. HEALTH EFFECTS
144
Body Weight
Neurological
100
Figure 3-3 Levels of Significant Exposure to MMT - Oral (Continued)
Chronic (≥365 days)
Systemic
mg/kg/day
7m 8m 9m
c-Cat -Human f-Ferret n-Mink Cancer Effect Level-Humans
LD50/LC50
Minimal Risk Level
d-Dog k-Monkey j-Pigeon o-Other LOAEL, More Serious-Humans
Cancer Effect Level-Animals for effects
r-Rat m-Mouse e-Gerbil LOAEL, Less Serious-Humans
LOAEL, More Serious-Animals other than
p-Pig h-Rabbit s-Hamster NOAEL - Humans
LOAEL, Less Serious-Animals Cancer
q-Cow a-Sheep g-Guinea Pig
NOAEL - Animals
10
MANGANESE
3. HEALTH EFFECTS
145
MANGANESE
146
3. HEALTH EFFECTS
1941) and 3.2.2.6 (Hafeman et al. 2007; Spangler and Spangler 2009), several aspects of these three
reports suggest that factors other than, or in addition to, high levels of manganese in drinking water may
have been responsible for the deaths.
In animals, most studies indicate that manganese compounds have low acute oral toxicity when provided
in feed. In rats, daily doses of 1,300 mg manganese/kg/day (as manganese sulfate in the feed) for 14 days
did not affect survival (NTP 1993). Survival was decreased in male rats fed 200 mg manganese/kg/day
(as manganese sulfate) for 2 years (NTP 1993). The cause of death was attributed to increased severity of
nephropathy and renal failure; however, female rats fed 232 mg manganese/kg/day (as manganese sulfate)
for 2 years were not affected in this manner (NTP 1993). Similarly, doses as high as 2,251 mg
manganese/kg/day (as manganese chloride) in the diet were tolerated by male mice (females were not
tested) for 6 months without lethality (Gianutsos and Murray 1982). The survival of both male and
female mice was also unaffected by feeding as much as 731 mg manganese/kg/day (as manganese sulfate)
for 2 years (NTP 1993).
In contrast to these studies, when exposure is by gavage (usually as highly concentrated solutions of
manganese chloride in water), measured LD
50
values for 1–21 days of exposure range from 225 to
1,082 mg manganese/kg/day in mice and rats (Holbrook et al. 1975; Kostial et al. 1978, 1989; Rehnberg
et al. 1980; Singh and Junnarkar 1991; Smyth et al. 1969). These results suggest that gavage dosing with
a bolus of a concentrated soluble manganese compound in water may not be a good model for
determining the toxic effects of manganese ingested by humans from environmental sources. Bolus
dosing produced death in animals at concentrations near the daily dose levels tolerated in food or drinking
water by the same strains and species of animals subjected to longer durations of exposure. It is possible
that bolus dosing circumvents the homeostatic control of manganese absorption. It should be noted that
the concentrations used in the bolus dosing studies are much higher than even excess levels to which
certain humans are typically exposed.
In a study where young pigs were fed a diet moderately high (1.7 mg manganese/kg/day) in manganese
but deficient in magnesium, all eight pigs consuming the high manganese diet died within 5 weeks
following convulsive seizures; only two of the pigs in a group without supplemental manganese died
(Miller et al. 2000). Further studies suggested that high dietary manganese could exacerbate magnesium
deficiency in heart muscle, thus creating a complicating factor in the deaths of the magnesium-deficient
pigs (Miller et al. 2000).
MANGANESE
147
3. HEALTH EFFECTS
In conclusion, route of exposure and animal species and strain differences, as well as sex, may account for
some of the observed variations in the lethality of manganese. In addition, deficiencies in certain
essential nutrients, such as magnesium, may increase the lethal potential of excess manganese.
No studies were located concerning death in humans following ingestion of MMT.
MMT, dissolved in oil and administered by gavage, was found to have LD
50
values of 15 mg
manganese/kg in the male and female Sprague-Dawley rat and 58 mg manganese/kg in the adult female
CD-1 mouse (Hinderer 1979).
Hysell et al. (1974) administered via gavage increasing amounts of MMT (dissolved in oil) to adult
COBS rats, 10 animals/group. No lethality was observed at the lowest two doses of 3.8 and 7.5 mg
manganese/kg, but 5/10 rats died within 2–6 days postdosing at a dose of 11.3 mg manganese/kg.
Increasing numbers of rats died at higher doses, with decreasing times of death post-dosing; complete
mortality occurred at the highest dose of 37.5 mg manganese/kg. The survivors appeared normal by
14 days. The LD
50
(14-day) was estimated at 14.6 mg manganese/kg.
Hanzlik et al. (1980a) determined the 14-day LD
50
for purified MMT administered in corn oil via gavage
to adult male Sprague-Dawley rats to be 12.5 mg manganese/kg (95% confidence interval, 9.5–16.8 mg
manganese/kg). The animals survived similar times post-dosing as those in the Hysell et al. (1974) study.
All LD
50
values from each reliable study for death in each species and duration category are recorded in
Table 3-3 and plotted in Figure 3-3.
3.2.2.2 Systemic Effects
In general, there is a lack of data concerning systemic toxic effects in humans who have ingested
manganese. This is likely due to the strong homeostatic control the body exerts on the amount of
manganese absorbed following oral exposure; this control protects the body from the toxic effects of
excess manganese. Studies in humans and animals provide limited data regarding the effects of
manganese ingestion on systemic target tissues. This information is discussed below and is organized by
target tissue. Table 3-3 and Figure 3-3 present the highest NOAEL and all LOAEL values from each
reliable study for these effects for each species and each duration category.
MANGANESE
148
3. HEALTH EFFECTS
Respiratory Effects. No studies were located regarding respiratory effects in humans after oral
exposure to inorganic manganese.
No respiratory effects were reported in mice fed up to 3,900 mg manganese/kg/day (as manganese
sulfate) or rats fed 1,300 mg manganese/kg/day (as manganese sulfate) for 14 days (NTP 1993). Male
rats fed manganese sulfate for 13 weeks showed no respiratory effects at 520 mg manganese/kg/day;
however, females exhibited decreased lung weight at 40–618 mg manganese/kg/day (NTP 1993). No
respiratory effects were noted in mice of either sex fed 122–1,950 mg manganese/kg/day (as manganese
sulfate) for 13 weeks (NTP 1993), in rats fed up to 232 mg manganese/kg/day (as manganese sulfate), or
in mice fed up to 731 mg manganese/kg/day (as manganese sulfate) for 2 years (NTP 1993).
The lungs of adult male Sprague-Dawley rats administered one dose of MMT via gavage in corn oil
(31.25 mg manganese/kg) showed signs of hemorrhage and alveolar and perivascular edema, with an
accumulation of proteinaceous material in the alveoli. As early as 12 hours following gavage
administration of this same dose, the lung/body weight ratio increased to 2.5 times the control value
(Hanzlik et al. 1980). Hinderer (1979) observed dark red lungs in Sprague-Dawley rats and CD-1 mice
administered sublethal doses (values unspecified) of MMT in an acute toxicity study. Gross necropsy of
the lungs of COBS rats administered one dose of MMT in Wesson oil (dose range, 20–37.5 mg
manganese/kg) revealed severe congestion and the release of a serosanguinous fluid upon sectioning;
histopathology of lungs from rats dying within 24 hours post-exposure showed severe congestion,
perivascular and alveolar edema, and alveolar hemorrhage (Hysell et al. 1974). Sections of lungs from
rats surviving until 14 days post-exposure revealed extensive areas of consolidation, thickened alveolar
septa and focal areas of alveolar macrophage activity.
Cardiovascular Effects. No studies were located regarding cardiovascular effects in humans after
oral exposure to inorganic manganese.
In a 1993 National Toxicology Program (NTP) study, no cardiovascular effects (pathological lesions)
were observed in mice or rats fed 3,900 or 1,300 mg manganese/kg/day, respectively, for 14 days. No
cardiovascular effects were observed in rats or mice exposed for 13 weeks to doses as high as 1,950 mg
manganese/kg/day (as manganese sulfate) or for 2 years to doses as high as 731 mg manganese/kg/day (as
manganese sulfate) (NTP 1993).
MANGANESE
149
3. HEALTH EFFECTS
In a study of weanling male Sprague-Dawley rats provided with a diet supplemented with 55 mg
manganese/kg/day for 14 weeks, Kalea et al. (2006) found that the level of uronic acid in aortas of the
manganese-supplemented group was significantly (p<0.05) higher than in a group of rats fed a diet with
adequate manganese (5.5 mg manganese/kg/day). Among heparan sulfate glycosaminoglycans, aortas
from manganese-supplemented rats contained higher concentrations of total galactosaminoglycans and
decreased concentration of hyaluronan and heparan sulfate (50% less heparan sulfate) when compared to
aortas from rats consuming diets with adequate manganese. Heparan sulfate chains of aortas from
manganese-supplemented rats contained 41% higher concentration of non-sulfated units compared to
those of rats fed the adequate manganese diet (Kalea et al. 2006). These results raise concern about the
potential for manganese to influence vascular chemistry in deleterious ways, creating increased
vulnerability to cardiovascular events.
In the course of investigating a mechanism to explain the sudden deaths in pigs from high doses of
manganese (Miller et al. 2000), studies were conducted in which pigs were fed either low (3.4 mg/kg/day)
or adequate dietary magnesium (6.8 mg/kg/day) along with high (55 mg/kg/day) or low doses
(5.5 mg/kg/day) of manganese (Miller et al. 2004). No differences in heart muscle ultrastructure were
observed; however, marked myocardial necrosis and mitochrondrial swelling were observed in pigs fed
high dietary manganese in combination with low magnesium (13.9 mg magnesium/kg/day; Miller et al.
2004). In pigs fed high manganese and adequate magnesium, no swelling of myocardial mitrochondria
was observed. These results suggest that high manganese, when fed in combination with low magnesium,
disrupts mitochondrial ultrastructure (Miller et al. 2004). In another related study, when rats were
provided with high dietary manganese (13.8 mg manganese/kg/day as manganese carbonate) for 8 weeks,
heart muscle oxygen consumption was depressed, although no effects of manganese on hematologic
variables were observed (Miller et al. 2006). No effects of manganese were observed on heart muscle
activities for Ca
+2
ATPase, liver glutathione peroxidase, or brain glutathione peroxidase at doses as high
as 55 mg manganese/kg/day (Miller et al. 2006). The depression in heart muscle oxygen consumption
produced by high dietary manganese presents yet another possible mechanism by which high doses of
manganese can produce adverse cardiovascular events.
No studies were located regarding the cardiotoxic effects of MMT in either humans or animals following
oral exposure.
Gastrointestinal Effects. No studies were located regarding gastrointestinal effects in humans after
oral exposure to manganese, except for one case report of a child who accidentally ingested some
MANGANESE
150
3. HEALTH EFFECTS
potassium permanganate (Southwood et al. 1987). This led to severe local corrosion of the mouth,
esophagus, and stomach due to the caustic effects of potassium permanganate on the tissue, but there was
no evidence of systemic toxicity.
Adverse gastrointestinal effects have been reported in guinea pigs and mice but not in rats. Guinea pigs
administered 4.4 mg manganese/kg/day (as manganese chloride by gavage) did not suffer any gross
abnormalities in either the stomach or small or large intestines as a result of treatment but did have patchy
necrosis and decreased adenosine triphosphatase and glucose 6-phosphatase levels in both the stomach
and small intestine (Chandra and Imam 1973). This study differs from the others in its delivery of
manganese (by gavage); the gavage treatment may have partially or completely contributed to the adverse
effects seen in the stomach and small intestine of the guinea pigs. No gastrointestinal effects were
observed in female mice fed 1,950 mg manganese/kg/day (as manganese sulfate in food) or rats fed up to
618 mg manganese/kg/day (as manganese sulfate in food) for 13 weeks, but male mice exhibited mild
hyperplasia and hyperkeratosis of the forestomach at 1,950 mg manganese/kg/day, also in food (NTP
1993).
In a 1993 NTP study, rats fed as much as 232 mg manganese/kg/day (as manganese sulfate) for 2 years
showed no gastrointestinal effects; however, mice treated with manganese sulfate for 2 years exhibited
hyperplasia, erosion, and inflammation of the forestomach at 585 mg manganese/kg/day for males and
731 mg manganese/kg/day for females. The acanthosis was judged by the authors to be a result of direct
irritation of the gastrointestinal epithelium and to be of minor consequence.
No studies were located concerning gastrointestinal effects following oral exposure to MMT in humans.
Hinderer (1979) observed discolored intestinal tracts in Sprague-Dawley rats and fluid-filled intestines
and spotting of the intestine in CD-1 mice dosed by gavage with high concentrations (values not
provided) of MMT in a 14-day toxicity study. Hysell et al. (1974) observed that single lethal doses of
20–37.5 mg manganese/kg (as MMT, given by gavage) produced small intestines that were distended
with clear watery contents and thin, friable walls.
Hematological Effects. In a dietary study with female subjects (Davis and Greger 1992), no changes
in hematocrit, serum transferrin, or serum ferritin were reported following supplementation with
0.25 mg manganese/kg/day for 119 days. Vieregge et al. (1995) found no effects on hemoglobin,
ceruloplasmin, or copper and iron levels in serum for a population of 40-year-old people who had
MANGANESE
151
3. HEALTH EFFECTS
ingested at least 0.3 mg manganese/L in drinking water for a minimum of 10 years. These data indicate
that exposure to increased manganese in water did not result in observable hematological toxicity.
Alterations in hematological parameters have been reported in rats and mice, although they were found to
vary depending on species, duration, and the form of manganese administered. No conclusive evidence
regarding a significant functional deficit has been reported. In mice fed 284 mg manganese/kg/day for
100 days, red blood cell count was decreased by manganese acetate and manganese chloride; white blood
cell count was decreased by manganese acetate, manganese chloride, and manganese dioxide; and
hematocrit was decreased by manganese carbonate (Komura and Sakamoto 1991). However, manganese
carbonate had no effect on red blood cells or white blood cells, manganese dioxide had no effect on red
blood cells or total hematocrit, and manganese acetate and manganese chloride had no effect on total
hematocrit. It has been suggested that the manganese-related effects on red blood cells may be related to
the displacement of iron by manganese. The significance of the other hematological effects was not
noted. In a study in rats and mice dosed with manganese sulfate for 14 days, 13 weeks, or 2 years, minor
changes in hematology parameters were reported; these changes varied depending on species, dose, and
duration, and the study authors did not consider them to be clearly related to compound administration
(NTP 1993). No significant hematological effects were observed in mice exposed to 180 mg
manganese/kg/day (as manganese tetroxide) for 224 days (Carter et al. 1980). In a study where male
Sprague-Dawley rats were fed 55 mg manganese/kg/day as manganese carbonate for 8 weeks,
significantly decreased hematocrit and hemoglobin levels were observed (Miller et al. 2006). However,
an even lower level of dietary manganese carbonate (35.8 mg manganese/kg/day) fed to male Sprague-
Dawley rats in a diet containing a relatively low concentration of magnesium (200 mg magnesium/kg
feed/day) for 4 weeks also produced significantly decreased hematocrit and hemoglobin levels (Miller et
al. 2006). Thus, the potential for dietary manganese to produce adverse effects on red blood cells may be
further modulated by the relative availability of magnesium in the diet.
No studies were located concerning hematological effects following oral exposure to MMT in humans or
animals.
Musculoskeletal Effects. No studies were located regarding musculoskeletal effects in humans after
oral exposure to inorganic manganese.
In young rats, high concentrations of manganese chloride in the diet (218–437 mg manganese/kg/day) led
to rickets (Svensson et al. 1985, 1987); however, this was found to be due to a phosphate deficiency
MANGANESE
152
3. HEALTH EFFECTS
stemming from precipitation of manganese phosphate salt (MnHPO
4
) in the intestine rather than to a
direct biological effect of manganese on bone formation. No significant musculoskeletal effects were
observed in mice or rats fed up to 731 mg manganese/kg/day for 2 years (NTP 1993).
No studies were located concerning musculoskeletal effects following oral exposure to MMT in humans
or animals.
Hepatic Effects. A single study of human oral exposure of manganese investigated potential
hepatotoxicity by analyzing liver enzymes in serum. Vieregge et al. (1995) reported no effects on
bilirubin, alkaline phosphatase, glutamic pyruvic transaminase, glutamic oxalacetic transaminase, or
gamma glutamyl transferase in humans, ≥40 years old, who had ingested well water containing
≥0.30 mg/L for at least 10 years. These limited data indicate that chronic exposure to elevated levels of
manganese did not result in observable liver toxicity in this population.
In animals, a variety of histological changes in subcellular organelles (e.g., rough and smooth
endoplasmic reticulum, Golgi apparatus) were observed in the livers of rats exposed to 12 mg
manganese/kg/day for 10 weeks (as manganese chloride) (Wassermann and Wassermann 1977).
However, these changes were not considered to be adverse but to be adaptive, possibly in response to
increased manganese excretion in the bile (see Section 3.4.4). Reductions in liver weight have also been
reported in male Fischer 344 rats fed 1,300 mg manganese/kg/day (as manganese sulfate)
for 14 days.
However, these effects were not seen in B6C3F
1
mice fed dosages up to 3,900 mg manganese/kg/day (as
manganese sulfate) for 14 days (NTP 1993). Similarly, no treatment-related evidence of liver damage
based upon organ weight, histology, or liver function tests were found in male Wistar rats dosed with
271 mg manganese/kg/day (as manganese chloride) in drinking water for 2 or 4 weeks (Rivera-Mancía et
al. 2009). In rats fed up to 618 mg manganese/kg/day (as manganese sulfate) for 13 weeks, decreased
liver weights were reported in males at ≥33 mg manganese/kg/day and females at 618 mg
manganese/kg/day (NTP 1993). When mice were fed 122–1,950 mg manganese/kg/day (as manganese
sulfate) for 13 weeks, the females showed no hepatic effects; however, the males exhibited both relative
and absolute reduced liver weights at 1,950 mg manganese/kg/day (NTP 1993). In CD-1 mice, no hepatic
changes were seen in males fed 205 mg manganese/kg/day (as manganese tetroxide) (Gray and Laskey
1980). No significant hepatic histological changes were observed in either mice or rats exposed for
2 years, with rats fed up to 232 mg manganese/kg/day (as manganese sulfate), and mice fed up to 731 mg
manganese/kg/day (as manganese sulfate) (NTP 1993). Additionally, Avila et al. (2008) reported no
evidence of increased oxidative stress in the liver in Wistar rats, as measured by thiobarbituric acid
MANGANESE
153
3. HEALTH EFFECTS
reactive substance (TBARS) production, δ-aminolevulinate-dehydratase (δ-ALA-D) activity, and protein
carbonylation, at doses up to 1,730 mg manganese/kg/day (as manganese chloride in drinking water) for
30 days.
There are no studies concerning hepatic effects following oral exposure to MMT in humans.
Hinderer (1979) observed mottling of the liver in CD-1 mice administered high doses (unspecified) of
MMT via gavage in a 14-day acute toxicity study. Histological evaluation of livers of adult male
Sprague-Dawley rats administered 31.3 mg manganese/kg/day (as MMT) revealed scattered hepatocytes
throughout the lobule that contained cytoplasmic vacuoles (Hanzlik et al. 1980b). Twelve hours after
administration of the same dose, no changes in plasma glutamic pyruvic transaminase (GPT) or liver
glucose 6-phosphatase (G6P) activities were observed. After the death of 8/14 animals at this dose level
(24 hours post-dosing), there were still no changes in plasma GPT, liver G6P, or hepatic triglycerides
(Hanzlik et al. 1980b). Hysell et al. (1974) observed that COBS rats that were gavage-dosed with 20–
37.5 mg manganese/kg (as MMT) once and died within 24 hours post-dosing had livers with acute
centrolobular passive congestion. This damage progressed to hepatic parenchymal necrosis and
leukocytic infiltration in those rats surviving 48–72 hours (15–37.5 mg manganese/kg/day), and extensive
cytoplasmic vacuolar change in rats surviving to 14 days.
Renal Effects. No studies were located regarding renal effects in humans after oral exposure to
inorganic manganese.
In animal studies, no significant renal histopathological changes were observed in any of the following:
mice and rats fed up to 3,900 or 1,300 mg manganese/kg/day (as manganese sulfate) for 14 days (NTP
1993); mice exposed to 205 mg manganese/kg/day (as manganese tetroxide) in their diet for 90 days
(Gray and Laskey 1980); mice or rats fed up to 1,950 mg manganese/kg/day for 13 weeks (NTP 1993); or
mice fed up to 731 mg manganese/kg/day for 2 years and female rats fed 232 mg manganese/kg/day (as
manganese sulfate) (NTP 1993). Additionally, Avila et al. (2008) reported no evidence of increased
oxidative stress in the kidney in Wistar rats, as measured by TBARS production, δ-ALA-D activity, and
protein carbonylation, at doses up to 1,730 mg manganese/kg/day (as manganese chloride in drinking
water) for 30 days. Contrary to these findings, increased severity of chronic progressive nephropathy was
noted in male rats fed 200 mg manganese/kg/day (as manganese sulfate) for 2 years (NTP 1993). In
addition, glomerulosclerosis/nephritis and urolithiasis (kidney stones) were observed in male, but not
MANGANESE
154
3. HEALTH EFFECTS
female, Sprague-Dawley rats exposed to dietary doses ≥87 mg manganese/kg/day for 63 days
(Ponnapakkam et al. 2003b).
No studies were located concerning renal effects in humans following oral exposure to MMT.
Hanzlik et al. (1980b) observed occasional vacuolar degeneration of proximal convoluted tubules of the
kidney in Sprague-Dawley rats administered a single gavage dose of 31.3 mg manganese/kg (as MMT).
Histopathologic renal effects observed within 24 hours of a gavage dose of 20–37.5 mg manganese/kg
(Hysell et al. 1974) included hyaline droplet change, cytoplasmic vacuolation of the proximal convoluted
tubules, and distention of the glomerular space and tubule lumens with a finely granular material that
stained lightly basophilic. Within 48 hours post-dosing, there was severe tubular degeneration in the form
of nuclear pyknosis and cell lysis. Animals surviving the administration of 3.75–25 mg manganese/kg
did not have any adverse renal effects.
Endocrine Effects. No studies were located regarding endocrine effects in humans after oral
exposure to inorganic manganese; however, other elements of endocrine function (e.g., reproductive
effects) following oral exposure to inorganic manganese are discussed elsewhere.
In mice fed up to 3,900 mg manganese/kg/day (as manganese sulfate) and rats fed 1,300 mg
manganese/kg/day (as manganese sulfate) for 14 days, no endocrine effects (pathological lesions) were
observed (NTP 1993). The adrenal gland was assessed for atypical cells and hyperplasia. In the pituitary
gland, the pars distalis was assessed for cyst, hyperplasia, and hypertrophy. The pars intermedia was
checked for cysts. C-cells and hyperplasia were examined in the thyroid gland. No endocrine effects
were observed in mice or rats fed up to 1,950 mg manganese/kg/day (as manganese sulfate) for 13 weeks.
A 2-year study in rats fed up to 232 mg manganese/kg/day (as manganese sulfate) reported no endocrine
effects (NTP 1993). However, in a 2-year mouse study, thyroid follicular hyperplasia and dilatation were
observed in males fed 584 mg manganese/kg/day, and thyroid follicular hyperplasia was observed in
females fed 64 mg manganese/kg/day (NTP 1993).
No studies were located regarding endocrine effects in humans or animals following oral exposure to
MMT.
Dermal Effects. No studies were located regarding dermal effects in humans after oral exposure to
inorganic manganese.
MANGANESE
155
3. HEALTH EFFECTS
In animals, no significant dermal histopathological changes were observed in mice or rats exposed for
2 years to doses up to 731 or 232 mg manganese/kg/day, respectively, (NTP 1993).
No studies were located regarding dermal effects following oral exposure to organic manganese.
Ocular Effects. No studies were located regarding ocular effects in humans after oral exposure to
inorganic manganese.
In animals, no significant ocular histopathological changes were observed in mice or rats exposed for
2 years to average oral doses of 731 or 232 mg manganese/kg/day (as manganese sulfate), respectively
(NTP 1993).
No studies were located regarding ocular effects in humans or animals after oral exposure to organic
manganese.
Body Weight Effects. No studies were located regarding body weight effects in humans after oral
exposure to inorganic manganese.
In some animal studies, lower body weights were observed in rats and mice in manganese-dosed groups.
For example, an NTP study (1993) reported decreases in body weight gain of 57% in male rats and 20%
in female rats fed 1,300 mg manganese/kg/day (as manganese sulfate in food) for 14 days. Similarly,
Avila et al. (2008) reported decreases in body weight gain of 50% (sex unspecified) in Wistar rats fed
760 mg manganese/kg/day (as manganese chloride in drinking water). Exon and Koller (1975) reported
that rats fed daily doses of manganese tetroxide as low as 6 mg manganese/kg/day (mean ingestion value
over the duration of the experiment) for 28 days gained only 44% as much weight over the course of the
study as control rats. No changes in eating habits in this lowest dose group were observed, although rats
in the highest dose group at 4,820 mg manganese/kg/day did exhibit decreased weight gain due to
starvation and the effects of the manganese. No histopathological changes were reported in the exposed
animals. The authors suggested that the decrease in weight gain might have been due to manganese
interference in metabolism of calcium, phosphorous, and iron.
In chronic studies, a similar sex-related difference in the response to this effect was reported. By the end
of a 2-year exposure to the maximum daily dose of 200 mg manganese/kg/day (as manganese sulfate in
MANGANESE
156
3. HEALTH EFFECTS
food), male rats had a final mean body weight that was 10% lower than that of controls; however,
females’ mean body weights were not significantly different from those of controls throughout the study
at all dose levels (232 mg manganese/kg/day was the maximum dose for female rats) (NTP 1993). Food
intake (as mg/kg/day) was similar for exposed groups and control groups and for males and females (NTP
1993).
Laskey et al. (1982) investigated body weight changes in a study of adverse reproductive toxicity in male
and female Long-Evans rats exposed to manganese. Pregnant dams were fed 0, 350, 1,050, and 3,500 mg
manganese/kg/day (in conjunction with a low-iron diet [20 mg iron/kg/day] or a diet adequate in iron
[200 mg iron/kg/day]); the pups were continued on their respective diets from day 14 to 15 postpartum to
the end of the study (224 days). Manganese treatment did not have any effect on body weight, in either
sex fed adequate iron. In iron-deficient male rats, however, body weights were significantly decreased
from controls at 24 days postpartum in the 1,050 mg manganese/kg/day diet and at all doses at 40- and
60-day time points. Interestingly, body weight was not significantly different in iron-deficient male rats
fed manganese at 350 mg/kg/day at 100 days and at 224 days (no dose group had weight values
significantly different from control at day 224). Female body weights were only significantly different in
the highest dose at day 24 and in the remaining two manganese doses at day 60. Body weights were not
significantly different from controls for the remainder of the study. Significant mortality in both sexes
from the highest manganese group fed an iron-deficient diet limited the available data.
In a study designed to evaluate developmental effects of manganese exposure, groups of pregnant
Sprague-Dawley rats were exposed to 4.79 mg manganese/mL (as manganese chloride in drinking water)
from GD 1 through PND 24 (Molina et al. 2011). Mean body weights of exposed dams were decreased
by 15 and 28% at the end of gestation and lactation, respectively, compared with controls; the difference
was statistically significant (p<0.05) at the end of lactation, but not at the end of gestation. Water
consumption was significantly decreased in treated animals during gestation and lactation (24 and 29%
lower than controls, respectively). Based on body weight and water intake, the study authors calculated
daily manganese doses during gestation and lactation as 565 and 1,256 mg manganese/kg/day,
respectively.
No studies were located concerning body weight effects following oral exposure to MMT in humans.
Hanzlik et al. (1980b) observed no significant differences in acutely exposed rats at a dose of 31.3 mg
manganese/kg as MMT. Hinderer (1979) also observed normal weight gain in surviving Sprague-Dawley
MANGANESE
157
3. HEALTH EFFECTS
rats and CD-1 mice administered doses of MMT ranging from 7 to 159 mg manganese/kg in a one-dose
14-day lethality study.
In a chronic study, Komura and Sakamoto (1992b) administered 11 mg manganese/kg/day (as MMT) in
chow to male ddY mice for 12 months. A 12% decrease in weight gain was observed at 9 months
between exposed mice and mice fed unmodified chow, increasing to a 17% difference at 12 months. All
differences in these time points were statistically significant. There was no observed difference in food
intake between the exposed and control groups.
Metabolic Effects. No studies were located regarding metabolic effects following oral exposure to
inorganic manganese in humans or animals.
No studies were located regarding metabolic effects following oral exposure to MMT in humans or
animals.
3.2.2.3 Immunological and Lymphoreticular Effects
No studies were located regarding immunological or lymphoreticular effects in humans after oral
exposure to inorganic manganese.
Alterations in white blood cell counts have been reported in rats and mice following oral exposure to
manganese. One NTP study reported immunological effects in rodents treated for 13 weeks, but not in
those treated for 2 years (NTP 1993). Mice were fed 122–1,950 mg manganese/kg/day (as manganese
sulfate) for 13 weeks. Males exhibited decreased leukocyte counts at ≥975 mg manganese/kg/day;
however, these effects may not have been treatment-related; females were unaffected. For 13 weeks, rats
were fed 33–520 mg manganese/kg/day (males) and 40–618 mg manganese/kg/day (females); neutrophil
counts were increased in males at ≥33 mg manganese/kg/day, lymphocytes were decreased in males at
≥130 mg manganese/kg/day, and total leukocytes were decreased in females at ≥155 mg manganese/
kg/day (NTP 1993). Rats fed up to 232 mg manganese/kg/day (as manganese sulfate) and mice fed up to
731 mg manganese/kg/day (as manganese sulfate) for 2 years exhibited no gross or histopathological
changes or organ weight changes in the lymph nodes, pancreas, thymus, or spleen (NTP 1993). Komura
and Sakamoto (1991) reported decreased white blood cell counts in mice following dosing at 284 mg
manganese/kg/day with manganese acetate, manganese chloride, or manganese dioxide for 100 days. It is
not known if any of these changes are associated with significant impairment of immune system function.
MANGANESE
158
3. HEALTH EFFECTS
No studies were located regarding immunological or lymphoreticular effects following oral exposure to
MMT in humans or animals.
3.2.2.4 Neurological Effects
Manganism Effects in Humans—Oral Exposure to Inorganic Manganese. Although inhalation
exposure to high levels of manganese is known to result in a syndrome of profound neurological effects in
humans (see Section 3.2.1.4, above), there is only limited evidence that oral exposure leads to the severe
neurological effects associated with high-level occupational exposure to manganese.
An outbreak of a disease with manganism-like symptoms was reported in a group of six Japanese families
(about 25 people) exposed to high levels of manganese in their drinking water (Kawamura et al. 1941).
Noted symptoms included a masklike face, muscle rigidity and tremors, and mental disturbance. Five
people were severely affected (2 died), 2 were moderately affected, 8 were mildly affected, and 10 were
not affected. These effects were postulated to be due to the contamination of well water with manganese
(14 mg/L) that leached from batteries buried near the well. Although many of the symptoms reported
were characteristic of manganese toxicity, several aspects of this outbreak suggest that factors in addition
to manganese may have contributed to the course of the disease. First, symptoms appeared to have
developed very quickly. For example, two adults who came to tend the members of one family developed
symptoms within 2–3 weeks. Second, the course of the disease was very rapid, in one case progressing
from initial symptoms to death in 3 days. Third, all survivors recovered from the symptoms even before
the manganese content of the well had decreased significantly after removal of the batteries. Thus, while
there is no doubt that these people were exposed to manganese, there is considerable doubt that all of the
features of this outbreak (particularly the deaths) were due to manganese alone.
A manganism-like neurological syndrome has been noted in an aboriginal population living on an island
near Australia where environmental levels of manganese are high (Kilburn 1987). Symptoms included
weakness, abnormal gait, ataxia, muscular hypotonicity, and a fixed emotionless face. Although it seems
likely that excess manganese exposure is an etiologic factor in this disease (based on occupational
exposure data from a study where exposure was assumed to be primarily by inhalation although oral
exposure was not ruled out), absence of data on dose-response correlations and absence of data from a
suitable control group preclude a firm conclusion on the precise role of manganese (Cawte et al. 1987). It
is possible that other factors besides manganese exposure may have contributed to the neurological
MANGANESE
159
3. HEALTH EFFECTS
effects, including genetic factors, dietary deficiencies in antioxidants and calcium, and excess alcohol
consumption (Cawte et al. 1989). Also, it should be noted that if manganese intake is a causal factor for
neurological damage, exposure of the population evaluated in this study could occur not only through the
oral route (e.g., food, water, soil), but also by inhaling manganese-containing dusts in environmental or
workplace air (Cawte et al. 1987).
Other Neurologic Effects in Adults—Oral Exposure to Inorganic Manganese. Kondakis et al. (1989)
reported that chronic intake of drinking water containing elevated levels of manganese (1.8–2.3 mg/L) led
to an increased prevalence of neurological signs in the elderly residents (average age, 67 years) of
two small towns in Greece. Effects in these residents were compared with effects in similarly aged
residents in a town where manganese levels were 0.004–0.015 and 0.082–0.25 mg/L. These levels are
within and slightly above levels found in U.S. drinking water, respectively (see Section 6.4.2). Over
30 different neurological signs and symptoms were evaluated, each being weighted according to its
diagnostic value for Parkinsonism. Based on this system, the average neurological scores for the
residents of the control town (0.004–0.015 mg manganese/L), the town with mid-range levels (0.08–
0.25 mg manganese/L), and the town with elevated manganese (1.8–2.3 mg manganese/L) were 2.7, 3.9,
and 5.2, respectively. Results from this study suggest that higher-than-usual oral exposure to manganese
might contribute to an increased prevalence of neurological effects in the aged population.
However, there are a number of limitations to this study that make this conclusion uncertain. First, no
details were reported regarding which neurological signs or symptoms were increased, so it is difficult to
judge if the differences were due to effects characteristic of manganism or to nonspecific parameters.
Second, the weighting factors assigned to each neurological symptom were based on the symptom’s
diagnostic value for Parkinsonism; however, there are clinically significant differences between
manganism and Parkinsonism. Therefore, the weighting scheme should have placed more weight on
those symptoms (e.g., sleep disorders, emotional lability, weakness, fatigue, and irritability) reported in
humans with manganism, such as manganese-exposed miners. The report does not indicate whether
efforts were made to avoid bias in the examiner or in the study populations. Nonetheless, the use of the
weighting scheme does strengthen the authors' assertion of an association between elevated manganese
concentration in the water source and increased susceptibility to neurological symptoms in older
populations. Although the subjective parameters included in this scoring are indicative of alterations in
mood or emotional state, and affective disorders often accompany other more objective nervous system
effects, the authors did not state whether individuals who experienced neurological signs did, in fact,
ingest higher levels of manganese than unaffected individuals. The authors reported that the populations
MANGANESE
160
3. HEALTH EFFECTS
in the towns were very similar to each other, but they provided few data to substantiate this. In this
regard, even small differences in age, occupational exposures, or general health status could account for
the small differences observed. Thus, this study suggests, but does not prove, that chronic oral intake of
high levels of manganese can lead to neurological changes in humans.
A study by Vieregge et al. (1995) reported no difference in performance on neurological function studies
by people who had ingested well water with high concentrations of manganese. These individuals
(high-exposure group), ages ≥40 years, were exposed to manganese at a minimum concentration of
300 μg manganese/L in water for at least 10 years. The controls consisted of a matched group of people
who ingested well water with a manganese concentration no higher than 0.05 mg/L. Mean blood
manganese concentrations in the high-concentration group were 8.5±2.3 μg/L compared to the control
value of 7.7±2.0 μg/L. Performance on motor coordination tests in the ‘high-exposure’ group was no
different than the performance of the control group. The authors noted that they could not control for the
ingestion of water from sources other than the wells described. Ingestion of manganese in food is also a
major contributor, but the authors did not report an estimate of manganese levels ingested from
foodstuffs. However, these possible confounders were considered negligible because no differences
between groups were revealed in a risk factor analysis for nutritional factors performed by the authors and
because manganese concentrations in the blood were not statistically different between the two groups.
Manganese drinking water levels for the ‘control group’ in this study were within the range of levels
reported in U.S. drinking water (see Section 6.4.2). As with the report by Kondakis et al. (1989), a
limitation of this study is the use of a neurological assessment scale for ‘Parkinsonian signs’ rather than
an evaluation of symptoms associated with manganism, though the authors observed no ‘detectable’
neurological impairment.
Goldsmith et al. (1990) investigated a cluster of Parkinson's disease in the southern region of Israel. They
reported an increased prevalence of Parkinsonism particularly among those 50–59 years old, which
suggested early onset of the disease. The authors believed that a potential environmental cause was the
water source common to residents in the region where the cluster of Parkinson’s disease was observed.
Although the authors reported that the water samples examined showed a “substantial excess of aluminum
and a smaller excess of iron and manganese,” the concentrations were not reported. Soil samples were
reported to contain excess concentrations of manganese as well as beryllium, chromium, europium, and
ytterbium, though no quantitative values were provided. The residents were connected to a national water
system, so it could not be determined when the water supply may have become contaminated with excess
levels of manganese and other metals. Moreover, there was no clear evidence that persons living in the
MANGANESE
161
3. HEALTH EFFECTS
region were actually exposed to a contaminated water supply. Although identified as a cluster of
Parkinson’s disease rather than manganism, the authors suggested that the disease cluster might be related
to an environmental source. However, the limitations in this study make it difficult to make any clear
association between chronic oral intake of excess levels of manganese and the prevalence of neurological
disease.
Iwami et al. (1994) studied the metal concentrations in rice, drinking water, and soils in Hohara, a small
town on the Kii peninsula of Japan. This town reportedly had a high incidence of motor neuron disease.
The researchers observed that a significantly increased manganese content in local rice and a decreased
concentration of magnesium in drinking water were positively correlated with the incidence of motor
neuron disease in Hohara (r
2
=0.99).
Evidence of neurological effects following oral manganese exposure has been noted in case studies of
adults, as well. For example, in a case report of a man who accidentally ingested low doses of potassium
permanganate (about 1.8 mg manganese/kg/day) for 4 weeks, the man began to notice weakness and
impaired mental capacity after several weeks (Holzgraefe et al. 1986). Although exposure was stopped
after 4 weeks, the authors reported that a syndrome similar to Parkinson's disease developed after about
9 months. Though suggested by the appearance of a syndrome resembling Parkinsonism, it is difficult to
prove that these neurological effects were only caused by exposure to the manganese compound. The
authors speculated that the ingested MnO
4
–
was reduced to Mn(II) or Mn(III); however, while this would
be expected, it was not measured. Since MnO
4
–
is a corrosive agent, it seems likely that it may have
caused significant injury to the gastrointestinal tract (the patient did experience marked stomach pain),
perhaps leading to a larger-than-normal gastrointestinal absorption of manganese.
In another study, Banta and Markesbery (1977) reported on a case involving a 59-year-old man with no
occupational or environmental exposure to manganese. The man exhibited dementia and neuromuscular
deficiencies including bradykinesia, shuffling gait, retropulsion, and rigidity in the upper extremities.
Masked faces with infrequent blinking and stooped posture were also observed. Manganese
concentrations were significantly elevated in serum, urine, hair, feces, and cerebrum. Although the
authors posit that the man may have had Alzheimer’s disease as well as manganese toxicity, they question
how the individual could build up significant body stores of manganese in the absence of occupational
exposure or any other known source of excess manganese. The authors suggest that the manganese
overload may have been caused by abuse of vitamins and minerals.
MANGANESE
162
3. HEALTH EFFECTS
An association has been suggested between violent behavior and excess manganese exposure; this was
investigated by measuring the correlation between the manganese content in hair and violent behavior in
prison subjects and controls (Gottschalk et al. 1991). The prisoners did have significantly higher hair
manganese content than controls, but further research was indicated to determine whether manganese was
a causative factor in violent behavior. The highest concentrations of manganese demonstrated in the hair
samples (1.8–2.5 ppm) were, however, within the control ranges reported by Kondakis et al. (1989) (0–
13 ppm) and Huang et al. (1989) (0.1–2.2 ppm for scalp and 0.3–9.8 ppm for pubic hair). Another factor
to be considered in the interpretation of these results is the hair color composition within the samples
evaluated. At least one study (Cotzias et al. 1964) has reported that manganese content was greater in
dark hair when compared to that found in lighter colored hair. Another study showed that manganese
accumulated in melanin-containing tissues including the melanin from human hair (Lydén et al. 1984). In
their study of inhabitants living in Angurugu on Groote Eylandt, Australia, Stauber et al. (1987) found
that samples of grey hair from one elderly Aborigine participant had the same manganese content as the
individual’s black hair. The white hairs of a local dog also had the same manganese content as the dog’s
black hairs. Based on this evidence, these investigators stated that there was no evidence to support
previous reports that dark colored hair concentrated more manganese than light hair. The average
manganese content in scalp hair among male and female Aborigine residents was 3.5–5-fold greater than
the average scalp hair manganese in male and female Caucasian residents, respectively. The authors
cautioned that interpretation of data on manganese content in scalp hair should take into consideration
endogenous as well as potential exogenous sources. Moreover, long-term manganese exposure that may
be associated with adverse effects may not be represented by manganese content in hair growth from only
a few months (Stauber et al. 1987). Thus, further investigations are needed to determine whether
manganese content can vary significantly due to hair color pigment alone.
Manganese has also been associated with amyotrophic lateral sclerosis (ALS). In a human study, spinal
cord samples from ALS patients were found to have higher manganese concentrations in the lateral
fasciculus and anterior horn than in the posterior horn (Kihira et al. 1990). Also, ALS patients exhibited a
positive correlation between manganese and calcium spinal cord content, whereas controls exhibited a
negative correlation. It was suggested that an imbalance between manganese and calcium in ALS patients
plays a role in functional disability and neuronal death. There was also some indication from previous
studies that an excess intake of manganese in drinking water may have caused this imbalance, although
data to support this were not presented. While this is suggestive of an association between manganese
and ALS, it is equally plausible that ALS leads to an imbalance in manganese-calcium metabolism.
MANGANESE
163
3. HEALTH EFFECTS
No neuropsychological effects were found in a study by Finley et al. (2003) of healthy, nonsmoking,
premenopausal women were studied in a research project using a crossover design to determine the
combined effects of very low or high dietary manganese with foods containing either saturated or
unsaturated fats on measures of neuropsychological and basic metabolic function. Women were fed for
8 weeks at one of two doses of manganese (0.01 or 0.3 mg manganese/kg/day), with one-half of the
subjects receiving 15% energy as cocoa butter and the other half receiving 15% energy as corn oil. Blood
draws and neuropsychological tests (involving tests of steadiness and ability to control muscular tremor,
signs of Parkinson's and related neurologic diseases, as well as tests to determine a range of components
related to hostility and anger) were given at regular intervals during the dietary periods. Manganese
intake did not affect any neurological measures and only marginally affected psychologic variables.
Neurologic Effects in Children—Oral Exposure to Inorganic Manganese. A number of studies have
examined the potential for adverse neurological outcomes from childhood exposure to manganese-
contaminated drinking water and/or food (Bouchard et al. 2007c, 2011; Claus Henn et al. 2010, 2011;
Farias et al. 2010; He et al. 1994; Kim et al. 2009; Wasserman et al. 2006, 2011; Zhang et al. 1995).
Two early studies (He et al. 1994; Zhang et al. 1995) reported adverse neurological effects in children
(aged 11–13) who were exposed to excess manganese in well water and in foods fertilized with sewage
water. However, these two studies have several flaws that preclude their use as substantial support for the
link between ingestion of excess manganese and the incidence of preclinical neurological effects in
children. These studies utilized a group of 92 children pair-matched to 92 controls who lived in a nearby
region. The pairs were matched for age, sex, grade, family income level, and parental education level; in
addition, all children lived on farms. Although the groups were well matched, the duration and amount of
manganese uptake from the flour (from wheat fertilized with sewage) and drinking water containing
excess levels was not well characterized. Moreover, the studies did not indicate if nutritional status, such
as low iron or calcium intake, which could greatly enhance manganese uptake, were evaluated as
potential confounding factors.
The exposed population drank water with average manganese levels of 0.241 mg/L (He et al. 1994; Zhang
et al. 1995). The control group drank water containing 0.04 mg manganese/L. These values were
measured over 3 years, although it was not stated if the children were exposed during the entire 3 years,
or what the children’s daily manganese intakes were. The exposed children performed significantly more
poorly (p<0.01) in school and on neurobehavioral exams than control students. School performance was
measured as mastery of the native language and other subjects; neurobehavioral performance was
MANGANESE
164
3. HEALTH EFFECTS
measured using the WHO core test battery. However, the report did not state what measures, if any, were
taken to ensure that the individuals administering the tests were blind to the exposure status of the subject.
Such safeguards would be necessary to prevent the introduction of bias in measurement and analysis of
the performance data of the subjects. The exposed children’s hair, blood, and urine manganese levels
were significantly increased relative to controls. A simple correlation analysis indicated the performance
of exposed children on five of the six of the neurobehavioral tests administered (digit span, Santa Ana
manual dexterity, digit symbol, Benton visual retention test, and pursuit aiming test) was inversely
correlated with hair manganese levels. Although the authors reported that iron, copper, and zinc were
measured in blood and hair, no other metals were measured in these tissues. Because the exposed group
presumably ingested food from sources irrigated with sewage, the children may have been exposed to
increased levels of other metals, such as lead or mercury. The authors indicate that the children were
exposed to increased manganese in their diet from excess levels in foodstuffs and drinking water. Of the
foodstuffs evaluated (cabbage, spinach, potatoes, eggplant, sorghum, and flour), only wheat flour
contained excess manganese compared to that from the control area. Although the total amount of
manganese ingested from the wheat flour and drinking water was not estimated, the authors suggest that
the elevated manganese level in drinking water was the key factor contributing to the observed effects.
The authors report that children ingesting food and water containing elevated manganese showed poor
performance in neurobehavioral tests and poorer school performance when compared to children from a
control area. Because exposure levels and duration were not well defined, these studies as reported are
not rigorous enough to establish causality between ingestion of excess manganese and preclinical
neurological effects in children. Nonetheless, these studies are strongly suggestive that subclinical
neurobehavioral effects often seen in industrial workers exposed to excess manganese via inhalation are
observed in children.
Wasserman et al. (2006) conducted a cross-sectional investigation of intellectual function on 142 10-year-
old children in Araihazar, Bangladesh, who had consumed tube-well water with an average concentration
of 793 µg manganese/L and 3 µg arsenic/L. The children received a medical examination and their
weight, height, and head circumferences were measured. Intellectual function was assessed on tests
drawn from the Wechsler Intelligence Scale for Children, version III, by summing weighted items across
domains to create verbal, performance, and full scale raw scores (the tests were adapted for use in this
particular population). Maternal intelligence was assessed with Raven's Standard Progressive Matricies, a
non-verbal test considered relatively free of cultural influences. Children provided urine specimens for
measuring urinary arsenic and creatinine and provided blood samples for measuring blood lead, arsenic,
manganese, and hemoglobin concentrations. To assess the dose-response relationship between
MANGANESE
165
3. HEALTH EFFECTS
manganese in well water and intellectual function, children were stratified into four approximately equal
sized groups, based on well water manganese levels. The results of the intelligence tests are displayed in
Table 3-4.
The results indicated that, unadjusted for other contributors, children in group 1 (i.e., those with estimated
mean dose of 0.006 mg manganese/kg bw/day), when compared with the other three groups, had higher
full scale scores; groups 2 (estimated mean dose of 0.02 mg manganese/kg bw/day) and 4 (estimated
mean dose of 0.07 mg manganese/kg bw/day) were significantly different. The unadjusted result for
performance scores revealed that group 2 had a significantly lower score than group 1. In the verbal test,
group 4 had a significantly lower unadjusted score than group 1.
After adjustment for sociodemographic factors, groups 1 and 4 were significantly different on all three
tests, with group 4 performing more poorly (Table 3-4). Although groups 2 and 3 (estimated mean dose
of 0.04 mg manganese/kg bw/day) performed more poorly on average than group 1, the averages from
groups 2 and 3 were not statistically significantly different from group 1. Therefore, children consuming
the largest amounts of manganese from well water, estimated to be on average 0.07 mg manganese/kg
bw/day, are considered to have shown significant decrements in all forms of intellectual performance
tested.
Wasserman et al. (2011) conducted a similar epidemiological study in Araihazar, Bangladesh, evaluating
the intellectual function of 151 8–11-year-old children using the updated 4
th
edition of the Intelligence
Scale for Children, from which raw scores for verbal comprehension, perceptual reasoning, working
memory, processing speed indices, and full scale were calculated (the tests were adapted for use in this
particular population). Maternal intelligence was measured on a population-adapted Wechsler
Abbreviated Scale of Intelligence. The weight, height, and head circumferences of each child was
measured, as were concentrations of lead, manganese, arsenic, and selenium in blood samples. To assess
the relationship between manganese in well water and intellectual function, children were divided into
two approximately equal sized groups, with either "low" or "high" (>500 μg/L) well water manganese
levels (estimated daily manganese doses from water consumption are 0.015 and 0.081 mg/kg/day for the
“low” and “high” groups, respectively). Average manganese concentrations in blood for the low and high
groups were 14.58 and 15.49 μg/L, respectively. Before adjustment for other confounders, blood
concentrations of manganese and arsenic were significant (p<0.05) explanatory variables for deficits in
full scale scores, verbal comprehension scores, working memory scores, and perceptual reasoning scores
(the latter only for manganese), but not in processing speed scores; the magnitudes of these effects were

MANGANESE
166
3. HEALTH EFFECTS
Table 3-4. Scores on Intelligence Tests
Quartiles by mean calculated dose of manganese (mg/kg bw/day)
a
Test type
0.006
0.02
0.04
0.07
Full-scale
81.7±3.1
73.0±4.1
74.0±3.7
60.7±5.2
b
Performance
64.6±2.7
56.4±3.2
56.9±2.8
45.6±4.8
b
Verbal
17.6±0.8
16.6±0.9
17.0±1.0
14.3±1.3
b
a
Adjusted scores by four groups of water manganese for full-scale, performance, and verbal raw scores. In each
case, adjustments were made for maternal education and intelligence, type of housing, child height, head
circumference, and access to TV. Scores represent mean ± standard error on the mean.
b
Adjusted score significantly different from lowest dose group, p<0.05.
Source: Wasserman et al. 2006
MANGANESE
167
3. HEALTH EFFECTS
small, reflected by the finding that blood concentrations of arsenic and manganese together explained
<5% of the variances in test scores. After adjustment for sociodemographic factors, negative associations
between blood manganese concentration and both working memory and perceptual reasoning domains
remained statistically significant.
A pilot study conducted by Bouchard et al. (2007c) found significant associations between hair levels of
manganese and certain behavioral end points. The study involved a group of children (24 boys and
22 girls) from Quebec, Canada whose homes received drinking water from one of two wells; one well
provided water with a relatively high level of manganese (610 μg/L; W1) and the second well provided
water with a much lower level of manganese (160 μg/L; W2). The children, aged 9–13, had estimated
average exposure levels of 0.02 mg manganese/kg/day (W1) and 0.007 mg manganese/kg/day (W2). The
children with exposure to water from the high-manganese well had significantly higher (p<0.05) levels of
manganese in their hair than those children exposed to water from the low-manganese well. Moreover,
the children with high concentrations of manganese in their hair demonstrated significantly more (p<0.05)
oppositional behaviors (e.g., breaking rules, getting annoyed or angered) and more hyperactivity than
children with lower manganese hair concentrations (after adjustment of scores for age, sex, and income).
No manganese-related differences were observed for tests related to cognitive problems (disorganization,
slow learning, lack of concentration). Although this report is a pilot study, it nonetheless suggests the
possibility that exposure to relatively high levels of manganese in water can influence behavior in
children.
More recently, Bouchard et al. (2011) conducted a cross-sectional study assessing the intellectual function
of 362 school-aged children (6–13 years old) from eight municipalities in southern Quebec exposed to 1–
2,700 μg/L of manganese in their home tap water (median value, 34 μg/L). For each child, total
manganese intake was estimated from both the diet and water consumption (including direct water
ingestion and for water incorporated in food preparations) using a food frequency questionnaire orally
administered to the mother. Estimated manganese intakes from water consumption ranged from 0 to
0.03 mg/kg/day (50
th
percentile, 0.0003 mg/kg/day), while estimated manganese intakes from dietary
sources were >2 orders of magnitude higher than estimated water intakes ranging from 0.01 to
0.44 mg/kg/day (50
th
percentile, 0.08 mg/kg/day). Mothers also provided information on covariates,
including socioeconomic status indicators, home cognitive stimulation, and maternal depression.
Cognitive abilities in children were assessed with the Wechsler Abbreviated Scale of Intelligence and
maternal nonverbal intelligence was assessed with the Raven’s Progressive Matrices Test. Hair samples
were collected from each child for measurement of manganese concentration. In unadjusted analyses,
MANGANESE
168
3. HEALTH EFFECTS
there were significant negative associations between full scale, verbal, and performance scores, and both
tap water and hair manganese concentrations. The estimated dietary manganese intake was not
significantly (p>0.05) correlated with intellectual performance scores. After adjusting for covariates,
negative associations remained statistically significant (p<0.05) between tap water manganese
concentrations or estimated manganese water intake and full scale and performance scores (not verbal
scores) and between hair manganese concentration and full scale scores. Using a fully adjusted regression
model, predicted IQ scores for children with 1 and 216 µg manganese/L tap water concentrations differed
by 6.2 Full Scale IQ points. The results demonstrated associations between manganese concentrations in
tap water or estimated manganese intakes from water and intellectual impairment in children, but no
associations between estimated manganese intakes from diet and intelligence scores. Bouchard et al.
(2011) concluded that the findings support the hypothesis that low-level, chronic exposure in drinking
water is associated with intellectual impairments in children, acknowledged that inferences that can be
drawn from the study are limited due to the cross-sectional design, and suggested that the findings should
be replicated in another study.
Other studies have evaluated possible associations between manganese blood levels and cognitive
function in school-aged children (Kim et al. 2009), attention-deficit/hyperactivity disorder (ADHD) in
school-aged children (Farias et al. 2010), and mental and psychomotor development scores in children
from 12 to 36 months of age (Claus Henn et al. 2010). The children in these studies were not known to
have been exposed to any particularly high levels of manganese in the environment, and were expected to
have been exposed to manganese principally via the oral route, as expected for the general population.
In a cross-sectional study, Kim et al. (2009) evaluated the intellectual function of 261 school-aged Korean
children (mean age 9.7 years) with mean blood manganese and lead concentrations of 14.3 μg/L (range
5.3–29.02 μg/dL) and 1.73 μg/dL (range 0.42–4.91 μg/dL), respectively. Children included in the study
were recruited from four different Korean cities. Cognitive function was assessed with the abbreviated
form of the Korean Educational Development Institute-Wechsler Intelligence Scales, which individually
scores vocabulary and, arithmetic (verbal IQ), picture arrangement and block design (performance IQ),
and full scale IQ. Caregivers (e.g., mothers or fathers) completed an extensive questionnaire about
demographics and other potential covariates for cognitive development. Linear regression analysis, both
before and after adjustment for covariates, showed a significant (p<0.05) inverse association between
blood manganese and full scale and verbal IQs. The same results were found for blood lead levels. The
effect was small, as the blood manganese and blood lead levels explained only 4% of the variances in the
full scale IQ and 5% of the variances in the verbal IQ. However, there was an increase in the percentages
MANGANESE
169
3. HEALTH EFFECTS
of the variances explained when the blood levels of both metals were entered as predictive variables,
suggesting a joint action of lead and manganese concentrations on full scale and verbal IQs in the
children. Further analysis separated the children into two groups: low manganese (blood concentrations
<14 μg/L, n=131) and high manganese (blood concentrations >14 μg/L, n=130). There was no difference
in mean blood lead concentration between the high and low manganese groups. Linear regression
analysis showed lead to be a significant predictive variable for full scale and verbal IQ scores in the high
manganese group, but not in the low manganese group. The results are consistent with joint toxic action
of lead and manganese on full scale and verbal IQ scores in these children, but the design of the
experiment is inadequate to conclude whether the joint action is additive, greater than additive, or less
than additive.
Another study in school-aged children (ages 7–15 years) investigated the potential relationship between
ADHD and manganese exposure by comparing blood manganese levels in 96 students diagnosed with
ADHD and 35 controls (Farias et al. 2010). Treatment-naive students diagnosed either ADHD-combined
type (n=50) or ADHD-inattentive type (n=24) had significantly (p<0.05) elevated mean serum manganese
levels (4.5 and 5.2 μg/L, respectively), compared with controls (3.5 μg/L). Students with ADHD-
combined type (n=21) or ADHD-inattentive type (n=11) who were currently being treated with stimulants
had manganese levels that were significantly lower than treatment-naïve students with ADHD (2.9 and
2.8 μg/L, respectively), and not significantly different from controls. No differences were noted in
whole-blood iron, magnesium, calcium, or potassium between groups. The results provide evidence for
an association between increased manganese blood levels and ADHD, but provide inadequate evidence to
establish a causal relationship with this disorder.
In a prospective study, Claus Henn et al. (2010) examined possible associations between early postnatal
manganese blood levels and developmental scores in 486 infants from Mexico City. Blood samples were
obtained at 12 months (n= 296) and 24 months (n=475), and analyzed for manganese and lead
concentrations. Child neurodevelopment was assessed at 6-month intervals from 12 to 36 months using
the Mental and Psychomotor Development Indices (MDI, PDI) from the Bayley Scales of Infant
Development-II, Spanish version. Twelve- and 24-month manganese concentrations were correlated and
declined over time (24.3 and 20.3 μg/L, respectively), and 24-month blood lead concentration was
positively associated with 24-month manganese blood concentration. A statistically significant
association was found between 12-month MDI scores and 12-month blood manganese concentrations,
adjusting for potential confounding variables including blood lead, gender, and maternal IQ and
education. The data were consistent with an inverted U-shaped regression model: 12-month MDI scores
MANGANESE
170
3. HEALTH EFFECTS
increased with 12-month manganese concentrations up to about 25 μg/L and decreased at higher
concentrations. The highest 12-month MDI score of 97.7 points was predicted to occur at 24.4 μg/L;
lower scores of 93.9 and 94.2 points were predicted for the 5
th
and 95
th
percentile values of 18.1 and
32.5 μg/L, respectively. No statistically significant associations were found between MDI scores at 24 or
36 months and 24-month manganese blood concentrations or between PDI scores and blood manganese
concentrations at any time point. The results suggest that the mental development of infants at 12 months
of age is more susceptible to either deficient or excessive intakes of manganese than at 24 or 36 months.
In a companion study, Claus Henn et al. (2011) evaluated manganese-lead interactions in the same group
of children. At 12 months, but not 24 months, there was a significant manganese-blood interaction
among children in the highest manganese exposure group (5
th
quintile). In this quintile, MDI scores and
PDI scores were decreased 2.23 and 1.24 points per μg/dL increase in lead, respectively, compared with
0.07 and 0.27 points per μg/dL increase in lead in the lower four quintiles of manganese exposure. Claus
Henn et al. (2011) concluded that the results suggest a possible synergism between lead and excessive
manganese to impair development of mental and psychomotor skills during the first year of life. The
study design, however, is inadequate to discern if the possible interaction is additive or greater than
additive.
There are also individual case reports that supply further evidence of potential neurological effects in
children from exposure to drinking water contaminated with high levels of manganese. Sahni et al.
(2007) report a case history of a previously healthy Canadian 6-year-old girl who lived with her family in
an urban center in Canada. Since 2000, the child’s family had spent summers at their nearby cottage,
characterized as weekend visits in June, followed by full-time residence in July and August. While the
municipal water used at the primary residence of the family had non-detectable levels of manganese, the
cottage well used between 2000 and 2003 was found to have manganese concentrations of 1.7–2.4 mg/L.
A neighboring cottage well used in 2004 had 1.7–2.2 mg manganese/L, while spring water used in 2004
had non-detectable levels. The child’s estimated intake from well water exposure was 0.103 mg
manganese/kg/day. In 2005, municipal water was brought to the cottage for drinking, but well water was
used for washing and cooking. A food history demonstrated that the family consumed more manganese-
rich foods, such as pineapples and leafy green vegetables, than a typical Canadian family. However, the
family was not vegetarian. The patient and her 7-year-old, asymptomatic sister had very similar diets,
with the exception that the sister consumed soy milk due to lactose intolerance. No inhalation exposures
to manganese were identified. No industrial releases of manganese were reported in the vicinity of either
residence. No other possible source of manganese involving occupational exposures, hobbies among
family members, etc., was identified. The patient presented with pica and emotional lability in August
MANGANESE
171
3. HEALTH EFFECTS
2004. Over the following months, she developed progressive behavioral and neurologic symptoms. She
became withdrawn and less verbal, with stuttered and slurred speech. Her balance, coordination, and fine
motor skills declined; eventually (in November 2004), she could no longer stand independently, tended to
fall backward, and demonstrated a high steppage "cock-like" gait. An MRI indicated hyperintensity in
the basal ganglia, indicative of high manganese accumulation. The patient demonstrated high levels of
manganese in whole blood (39.7 µg/L). The patient also had severe iron deficiency and polycythemia,
which was attributed to elevated cobalt. Her blood levels of lead were normal. While her liver
manganese was elevated, her liver function was normal, as was her blood copper level. Other members of
the family had elevated blood levels of manganese (1.9–2.8 µg/L) when tested between March and June
2005. The patient's symptoms abated to a large degree when she was treated with phlebotomies for the
polycythemia and ethylene-diamine tetraacetic acid chelation for the manganese overload and iron
therapy. These treatments occurred from November 2004 through July 2005, when her iron
supplementation stopped. By August 2005, the patient’s condition had deteriorated, with her pica
returning; she fell frequently and needed assistance where she was previously independent. Phlebotomies
and oral iron therapy were resumed in October 2005. The authors concluded that a metabolic disorder
involving divalent metals (manganese, iron, and cobalt) interacting with environmental exposures was the
most likely explanation for the patient's symptoms.
Brna et al. (2011) describe a very similar case in a previously healthy 5-year-old girl, whose primary
exposure was also determined to be from elevated well-water manganese levels (1.7–2.4 mg/L) at her
family’s country vacation home. The child’s estimated intake from well water exposure was 0.104 mg
manganese/kg/day. The child presented with a recent history of intermittent urinary incontinence, pica,
behavioral changes, speech difficulties, social withdrawal, and gross and fine motor incoordination.
Upon admission, she could not walk independently. Neurological examination revealed a narrow-based,
high stepping gait, retropulsion with preserved strength, deep tendon reflexes, and sensation. She had
mild truncal ataxia and subtle action tremor. Clinical chemistry revealed polycythemia without
abnormalities in the bone marrow. She had elevated hemoglobin, decreased mean cell volume, increased
red blood cell distribution, decreased serum iron, and elevated total iron binding capacity. She also had
elevated serum cobalt levels and profoundly elevated whole-blood and serum manganese levels (723 and
38 nmol/L, respectively). Cranial MRI revealed bilateral symmetric hyperintense signals in the basal
ganglia, brainstem, and cerebellum on T1-weighted imaging, consistent with a diagnosis of
hypermanganism. Like the previous case, her parents also had elevated serum manganese levels (29.5–
42.8 nmol/L), but their whole-blood levels were normal and they were clinically well. Her 7-year-old
sister had normal manganese levels and no symptoms. Treatment with phlebotomy (for polycethemia),
MANGANESE
172
3. HEALTH EFFECTS
iron infusions, a manganese-free diet, and calcium ethylenediaminetetraacetic acid (EDTA) chelation
therapy improved her condition somewhat. At age 10, she was able to walk 40 m unaided with an
improved stepping gait, but regularly used an electric wheelchair for mobility. Her neurological status
was stable, with improved speech, behavioral, and fine motor skills.
Woolf et al. (2002) describe the case of a 10-year-old boy whose sole source of drinking water at home
over a 5-year period was from a well on the family's property in a Boston, Massachusetts suburb. The
well water tested after 5 years of use had a manganese concentration of 1.21 ppm (estimated intake:
0.06 mg manganese/kg/day). The child had elevated blood levels of manganese (serum concentration of
0.90 µg/100 mL, compared to reference normal of <0.265 µg/100 mL) and whole-blood manganese
concentration of 3.82 µg/100 mL (reference normal: <1.4 µg/100 mL). The child’s urinary excretion of
manganese was found to be 8.5 µg/L over a 24-hour period (reference normal: <1.07 µg/L). Although no
other member of the family exhibited elevated blood concentrations of manganese, the child and his
brother each had elevated manganese levels in hair samples (the patient's level was 3,091 ppb; the
brother's was 1,988 ppb; reference normal: <260 ppb hair). At this time, the family switched to bottled
drinking water, but continued to use the well water for other purposes (bathing, etc.). The child exhibited
no evidence of illness or tremors. A detailed neurologic examination was normal. His balance with his
eyes closed was good, but he did not coordinate rapid alternating motor movement well. His fine motor
skills were normal and he had no sensory deficits. A battery of neuropsychologic tests revealed that while
the child's global cognitive skills were intact, he had striking difficulties in both visual and verbal memory
(14
th
and 19
th
percentiles, respectively), suggesting a deficit in free retrieval skills, and had a general
memory index at the 13th percentile and learning index at the 19th percentile. The child was in 5
th
grade
at the time of testing and had no history of learning problems, although teachers had persistently reported
difficulties with listening skills and following directions. The authors report that the findings from the
neuropsychological testing are consistent with the toxic effects of manganese, although the authors
indicate that a causal relationship cannot be inferred in this case.
Though limited, these case reports also provide further evidence for a link between ingestion of elevated
levels of manganese and learning or behavioral problems in children. Other studies have found that
manganese levels in hair are higher in learning-disabled children than in normal-functioning children
(Collipp et al. 1983; Pihl and Parkes 1977). The route of excess exposure is not known, but is presumed
to be mainly oral. These observations are consistent with the possibility that excess manganese ingestion
could lead to learning or behavioral impairment in children as suggested by the results from other
epidemiological studies (Bouchard et al. 2007c, 2011; Claus Henn et al. 2010; Farias et al. 2010; He et al.
MANGANESE
173
3. HEALTH EFFECTS
1994; Kim et al. 2009; Wasserman et al. 2006, 2011; Zhang et al. 1995). However, an association of this
sort is not sufficient to establish a cause-effect relationship because a number of other agents, including
lead, might also be involved (Pihl and Parkes 1977). Moreover, other potentially confounding factors
(e.g., health and nutritional status) must be taken into consideration in interpreting such studies.
Neurologic Effects in Humans with Liver Dysfunction—Oral Exposure to Inorganic Manganese.
Several studies report the link between hepatic encephalopathy and an increased manganese body burden
following chronic liver disease in adults (Hauser et al. 1994; Pomier-Layrargues et al. 1998; Spahr et al.
1996) and children (Devenyi et al. 1994) and in individuals with surgically-induced portacaval shunts
(PCS) (Hauser et al. 1994). The manganese exposure in these studies was assumed to originate from a
normal diet. Hepatic encephalopathy comprises a spectrum of neurological symptoms commonly
occurring in individuals with chronic liver disease; these symptoms include varying degrees of mental
dysfunction, although extrapyramidal symptoms may also be identified during a clinical examination
(Spahr et al. 1996).
In the Hauser et al. (1994) study, two men aged 49 and 65 years, both with chronic liver disease, and one
56-year-old man with cirrhosis of the liver and a portacaval shunt, showed a variety of neurological
symptoms including bradykinesia, postural tremor of the upper extremities, and gait disturbances, as well
as a decrease in cognitive function. These men all had significant elevations (p<0.05) in blood
manganese as compared to healthy male and female controls, and had hyperintense signals in the basal
ganglia bilaterally as measured by T1-weighted MRI. Similar elevations of blood manganese were
reported in a population of 57 cirrhotic patients with an absence of clinical encephalopathy (Spahr et al.
1996). Blood manganese was elevated in 67% of the patients and was significantly higher in those
patients with previous portacaval anastomoses or transjugular intrahepatic portosystemic shunt. MRI
signal hyper intensity was observed in the globus pallidus; the elevated blood manganese levels were
significantly correlated with the intensity of the signal in affected patients. Neurological evaluation of
extrapyramidal symptoms using the Columbia rating scale indicated a significant incidence of tremor,
rigidity, or akinesia in ~89% of the patients, although there was no significant correlation between blood
manganese level and these symptoms.
Similar results were observed in a young girl with Alagille’s syndrome (involving neonatal cholestasis
and intrahepatic bile duct paucity) with end-stage cholestatic liver disease who exhibited several
neurological dysfunctions including dystonia, dysmetria, propulsion, retropulsion, and poor check
response bilaterally (Devenyi et al. 1994). The girl had elevated blood manganese (27 μg/L compared to
MANGANESE
174
3. HEALTH EFFECTS
normal value of ~9.03 μg/L) and exhibited hyperintense MRI signal in the basal ganglia. After a liver
transplant, the MRI signal abated and the blood manganese level returned to normal. This study and
those in adults indicate that the increased manganese body burden (as evidenced by increased manganese
blood and brain levels) may contribute to the resultant neurological symptoms and encephalopathy in
individuals with cirrhosis or chronic liver disease.
Rose et al. (1999) evaluated brain manganese levels in 12 autopsied cirrhotic individuals who died from
hepatic coma and 12 control subjects with no history of hepatic, neurological, or psychiatric disorders at
time of death. Neutron activation analysis of the brain tissue revealed an increase in manganese content
in the cirrhotic individuals, particularly in the globus pallidus, which had 186% more manganese than that
of controls (significant at a level of p<0.001). Significant, although less extreme, increases in manganese
were also found in the putamen and caudate nucleus from cirrhotic patients. However, the increased brain
manganese did not correlate with patient age, the etiology of the cirrhosis, or the history of recurrent
hepatic encephalopathy (reported in 6 patients).
Neurologic Effects in Adult Animals—Oral Exposure to Inorganic Manganese. A few animal studies
have observed effects that are comparable to clinical signs seen in people with manganism. Gupta et al.
(1980) reported that monkeys given 25 mg manganese/kg/day (as manganese chloride) for 18 months
developed weakness and muscular rigidity (however, no data were provided to support these
observations). Rats dosed with 150 mg manganese/kg/day (as manganese chloride) developed a rigid and
unsteady gait after 2–3 weeks, but this was a transient condition that was not apparent by 7 weeks
(Kristensson et al. 1986). In addition, in two separate studies, the authors reported a decrease in
spontaneous activity, alertness, muscle tone, and respiration in mice dosed once with 58 mg
manganese/kg/day by gavage (Singh and Junnarkar 1991) and staggered gait and histochemical changes
in two third-generation mice treated with 10.6 mg manganese/kg/day (as manganese chloride) in drinking
water (Ishizuka et al. 1991).
Most other early studies in animals reported changes in brain chemical end points, including
concentrations of neurotransmitters or alterations in motor activity with both hypo- and hyperactivity
reported. As shown in Table 3-3 and Figure 3-3, changes of this sort have been reported at oral exposure
levels that ranged from about 1 to >2,000 mg manganese/kg/day (as manganese chloride, manganese
acetate, or manganese tetroxide) (e.g., Bonilla 1978b; Bonilla and Prasad 1984; Chandra 1983; Eriksson
et al. 1987a; Gianutsos and Murray 1982; Gray and Laskey 1980; Komura and Sakamoto 1991, 1992b;
Lai et al. 1984; Nachtman et al. 1986; Subhash and Padmashree 1991).
MANGANESE
175
3. HEALTH EFFECTS
More recent studies have continued investigations of brain chemistry alterations in animals following
acute- to intermediate-duration oral exposure to manganese (Avila et al. 2008; Calabresi et al. 2001;
Desole et al. 1997; Lipe et al. 1999; Liu et al. 2006; Morello et al. 2007; Ranasinghe et al. 2000). In
particular, studies have focused on the dopaminergic system due to observed motor dysfunction following
manganese exposure and similarities between manganism and parkinsonism (Calabresi et al. 2001;
Desole et al. 1997; Ranasinghe et al. 2000). Additionally, a few studies have reported neuropathology
following manganese exposure, as evidenced by neuronal damage and/or increased oxidative stress (Avila
et al. 2008; Liu et al. 2006; Spadoni et al. 2000). Studies of the effects of manganese on a variety of
behavioral assessments in rats also have been conducted; these studies have found changes in measures
related to fear, locomotor activity, and cognitive performance (Calabresi et al. 2001; Shukakidze et al.
2003; Torrente et al. 2005; Vezér et al. 2005, 2007). In some of these studies, electrophysiological
changes in the brain were associated with behavioral changes (Calabresi et al. 2001; Vezér et al. 2005,
2007).
In a study by Lipe et al. (1999), groups of 30-day-old and 90-day-old male Sprague-Dawley rats were
exposed to10 or 20 mg manganese/kg/day as manganese chloride for 30 days. A dose-dependent
decrease in body weight gain was found in the adult, but not the weanling rats. Significant (p<0.05)
increases were observed in concentrations of aspartate, glutamate, glutamine, taurine, and
gamma-aminobutyric acid (GABA) in the cerebellum of the adult rats dosed with 20 mg
manganese/kg/day; this increase also appeared to be dose dependent. A significant (p<0.05) decrease in
the concentration of glutamine was observed in caudate nucleus and hippocampus of weanling rats dosed
with 10 mg manganese/kg/day. A significant (p<0.05) increase in GABA concentration in the caudate
nucleus of weanlings was observed in the 20 mg manganese/kg/day group. A significant (p<0.05)
decrease in the concentration of glutamine in the caudate nucleus and hippocampus was found in
weanlings of the 10 mg manganese/kg group.
In a study by Morello et al. (2007), groups of adult male Wistar rats had free access to either normal
drinking water or to a water solution providing 611 mg manganese/kg/day as manganese chloride, with
treatment lasting for 13 weeks. A significant reduction in the number of immunoreactive cells for
glutamine synthetase was observed in the globus pallidus for manganese-treated animals compared with
controls (33% reduction). No effect of manganese was observed in the sensorimotor cortex or striatum,
nor was there any effect observed for other manganoproteins tested.
MANGANESE
176
3. HEALTH EFFECTS
In a study by Ranasinghe et al. (2000), groups of male Sprague-Dawley rats were provided daily
with 0 (n=2), 74.9 (n=4), or 149.8 mg (n=4) mg manganese/kg/day, administered as manganese sulfate;
another control group of two rats received 20 mg sodium/day. All animals were treated for 50 days.
Mean manganese concentrations in liver, brain, heart, and kidney were elevated in the low- and high-dose
groups, compared with untreated sodium controls, but statistical analyses of these data were not
performed. A decrease was observed in dopamine serum levels in manganese-treated rats compared to
controls; the sulfated form was increased in both dose groups compared to controls (12–13 times; from
0.014 nmol/mL in controls to 0.179 nmol/mL in the 20 mg manganese group). Increases were also
observed in L-dopa and L-dopa sulfate in both treatment groups. No treatment-related differences were
observed in serum levels of L-P tyrosine or its L-P tyrosine sulfate.
In a study by Desole et al. (1997), groups of 3-month-old male Wistar rats were given gavage doses of
0 or 8.8 mg manganese/kg/day as manganese chloride in water for 6 days. Other groups of control or
manganese-treated rats received 20 mg/kg buthionine (S,r) sulfoximine0ethyl ester (BSO-E) by
intraperitoneal injection twice daily (1 hour before gavage treatment) on days 4, 5, 6, and 7. Rats were
sacrificed on day 7, and brainstem samples were extracted for determination of concentrations of
dopamine, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and noradrenaline (NA), as
well as concentrations of reduced glutathione, ascorbic acid, dehydroascorbic acid, and uric acid (the
latter being indicators of oxidative stress potential). Compared with controls, manganese treatment alone
increased concentrations of GSH (10–14%) and uric acid (28–45%) in striatum and brainstem, without
affecting ascorbic acid concentrations, increased concentrations of DOPAC and HVA in striatum, without
affecting dopamine, and decreased brainstem concentrations of dopamine. As expected, BSO-E treatment
alone decreased GSH concentrations in striatum (23%) and brainstem (35%), without affecting striatal or
brainstem concentrations of ascorbic acid, dehydroasocrbic acid, or uric acid or striatal concentrations of
dopamine, DOPAC, or HVA; however, brainstem concentrations of dopamine were decreased by this
treatment. Compared with controls, manganese plus BSO-E treatment decreased concentrations of GSH
and ascorbic acid in striatum (42 and 22%, respectively) and brainstem (23 and 22%, respectively) and
increased concentrations of dihydroxyascorbic acid and uric acid; these results are indicative of a
heightened oxidative stress condition. In addition, manganese plus BSO-E treatment decreased striatal
concentrations of dopamine, DOPAC, and HVA and brainstem concentrations of dopamine and
noradrenaline. The magnitude of the manganese plus BSO-E treatment changes were mostly larger than
changes seen in all other experimental groups. The results indicate that the manganese treatment
decreased brainstem concentrations of dopamine without affecting neurochemical indicators of oxidative
MANGANESE
177
3. HEALTH EFFECTS
stress and that a glutathione depleted condition potentiated the effects of manganese on brainstem and
striatal concentrations of dopamine, DOPAC, and HVA.
Calabresi et al. (2001) measured locomotor activity, fear, and learning and memory in male Wistar rats
treated with either tap water as drinking water or a solution of manganese chloride (1,310 mg
manganese/kg/day) as drinking water for 10 weeks. Brain manganese levels ranged from 3 to
approximately 4 times higher than controls. Manganese-treated rats were significantly (p<0.001) more
active than control rats in the open field. Manganese-treated rats showed progressively and significantly
more interest in the "novel" object over three trials than the control rats (p<0.001; an average of four
contacts for manganese-treated animals compared to an average of <2 for controls on the third trial).
Manganese-treated animals also produced significantly (p<0.05) more fecal boluses (indicative of
heightened fearfulness) in the open field than control rats over the three trials. No major differences were
observed between treatment groups in the eight-arm radial maze test, with the manganese-treated animals
taking significantly (p<0.01) more 45 degree angle turns than the control rats. An enhanced
dopaminergic inhibitory control of the corticostriatal excitatory transmission via presynaptic D2-like
dopamine receptors in corticostriatal slices obtained from the manganese-treated rats was observed. The
use of agonists acting on presynaptic purinergic, muscarinic, and glutamatergic metabotropic receptors
revealed normal sensitivity. Membrane responses recorded from single dopaminergic neurons following
activation of D2 dopamine autoreceptors were also unchanged following manganese intoxication. The
authors suggest that the behavioral symptoms described in the "early" clinical phase of manganism may
be produced by an abnormal dopaminergic inhibitory control on corticostriatal inputs (Calabresi et al.
2001).
Spadoni et al. (2000) studied groups of male, PND 20 Wister rats provided with either access to drinking
water or 3311 mg manganese/kg/day in drinking water, with treatment lasting for 13 weeks. No neuronal
loss or gliosis was detected in the globus pallidus with either treatment. However, the majority of GP
neurons from manganese-treated rats died following brief incubation in standard dissociation media.
Patch-clamp recordings in the whole-cell configuration were not tolerated by surviving GP neurons from
manganese-treated rats. Manganese-treated GP cells, but not striatal cells, demonstrated an unusual
response to glutamate, since repeated applications appeared to produce irreversible cell damage.
Liu et al. (2006) studied 12-week-old female C57Bl/6 mice, paired as littermates from timed pregnant
dams, that received by gavage either water or 43.7 mg manganese/kg/day as manganese chloride for
8 weeks prior to sacrifice. Manganese-treated mice had significantly (p<0.05) increased levels of
MANGANESE
178
3. HEALTH EFFECTS
manganese in the striatum and decreased locomotor activity and striatal dopamine content. Neuronal
injury in the striatum and globus pallidus was observed, especially in regions proximal to the
microvasculature. Neuropathological assessment revealed marked perivascular edema, with hypertrophic
endothelial cells and diffusion of serum albumin into the perivascular space. Immunofluorescence studies
revealed the presence of apoptotic neurons expressing neuronal NOS choline acetyltransferase, and
enkephalin in both the striatum and globus pallidus. Soma and terminals of dopaminergic neurons were
morphologically unaltered in either the substantia nigra or striatum. Regions with neuronal injury
contained increased numbers of reactive astrocytes that coexpressed inducible NOS2 and localized with
areas of increased neuronal staining for 3-nitrotyrosine protein adducts, a marker of NO formation. The
data suggest a possible role for astrocyte-derived NO in injury to striatal-pallidal interneurons from
manganese intoxication.
Avila et al. (2008) investigated open field behaviors and orofacial dyskinesia in adult Wistar rats (5/group
of unspecified gender) exposed to drinking water containing 0, 2.8, or 6.9 mg manganese/mL (0, 10, or
25 mg MnCl
2
/mL) for 30 days. Using an allometric equation for drinking water consumption (EPA 1988)
and averages of mean body weights reported for exposure day 0 and 30, estimated doses are: 0, 760, or
1,730 mg manganese/kg/day. Behavioral tests (open field, orofacial dyskinesia measures) were
performed on days 0 and 30. On day 30, animals were sacrificed and the striatum and hippocampus were
dissected for slice preparation. Some striatum slices were used to measure calcium influx. The remaining
striatum tissue and the hippocampus were homogenized for biochemical analyses measuring oxidative
stress indices (TBARS production, δ-ALA-D activity, protein carbonylation). In both exposed groups,
animals exhibited significantly (p<0.05) decreased motor activity in the open-field test and decreased
tongue protrusion frequency. Frequency of vacuous chewing movement was significantly (p<0.05)
decreased only in the high-dose group. There were no differences in rearing frequency in either treated
group compared with controls. Calcium influx in the striatum was significantly (p<0.05) decreased in
both treatment groups compared with controls. TBARS levels were significantly elevated and ALA
activity was significantly decreased in the striatum, but not hippocampus, of animals in the high-dose
group. Protein carbonylation in exposed groups did not differ from controls in either region. The results
indicate an association between manganese-induced decreases in motor activity in rats and increased
markers of oxidative stress in the striatum.
Vezér et al. (2005, 2007) evaluated multiple neurobehavioral end points in young adult male Wistar rats
treated by water gavage with 0, 6.5, or 25.9 mg manganese/kg/day for 10 weeks. Rats were tested in an
eight-arm radial maze test (spatial learning and memory test) and an open field test (locomotor ability).
MANGANESE
179
3. HEALTH EFFECTS
Rats were also tested for amphetamine-induced locomotor activity, acoustic startle response, and prepulse
inhibition. At 5 and 10 weeks of treatment, as well as at the end of the post-treatment period 8 weeks
later, electrophysiological testing was performed, including recording of cortical evoked potentials as well
as spontaneous electrical activity in the hippocampus. Immunohistochemistry was performed to detect
changes in density of glial fibrillary acid protein (GFAP) immunoreactive structures in the hippocampal
CA1region. Blood and tissue samples (from the cortex and hippocampus) were collected in the 5
th
and
10
th
treatment and 12 post-treatment week. Blood and tissue levels of manganese were determined.
Manganese accumulation was first seen in blood and then in brain of high-dose rats. Decreased short-
and long-term spatial memory performance (at least p<0.05) and decreased spontaneous open field
activity (p<0.05) were observed in both low- and high-dose groups, compared with controls. The number
of acoustic startle responses, as well as their associated prepulse inhibition of the acoustic startle
responses, were decreased in manganese-treated animals. The latency of sensory evoked potentials
increased and their duration decreased. Manganese levels returned to normal at the end of the post-
treatment period, but impairment of long-term spatial memory remained, as well as the decrease in
number of acoustic startle responses in high-dose rats. Prepulse inhibition responses returned to normal.
Open field activity returned to normal at the end of post-treatment, but a residual effect could be observed
under the influence of D-amphetamine. The electrophysiological effects partially returned to normal
during post-treatment. Significantly (p<0.05) high percentages of area showing GFAP immunoreactivity
were observed in the dentate gyrus (but not in the striatum radiatum or striatal oriens) in the low- and
high-dose groups, compared with controls.
Another factor that could potentiate the neurotoxicity of manganese was explored by Torrente et al.
(2005), with rats subjected to restraint stress along with manganese exposure. Groups of 15 adult male
Sprague-Dawley rats (250–300 g) were dosed for 2 weeks with either plain drinking water or drinking
water providing 38.2 mg manganese/kg/day as manganese chloride. The manganese chloride group was
then split into two groups, with drinking water doses of 76 and 153 mg manganese/kg/day provided for
another 19 weeks. One-half of the animals in each group were subjected to restraint stress for 2 hours
daily by placing them in metacrilate cylindrical holders. Animals treated with 153 mg manganese/kg/day
with restraint traveled a significantly shorter distance than control restraint animals (38% decrease;
p<0.05). Manganese concentrations in brain and cerebellum were significantly elevated in exposed
groups, compared with controls. Body weight and food consumption were significantly decreased
(p<0.05) in the exposed groups, compared with control values. Terminal body weights were 86 and 51%
of control values in the low- and high-dose unrestrained groups and 90 and 56% in the respective
restrained groups. Open field activity was significantly decreased (p<0.05) in the high-dose restrained
MANGANESE
180
3. HEALTH EFFECTS
groups. Spatial learning was also impaired in high-dose rats with or without restraint); for example,
unrestrained high-dose rats showed significantly (p<0.05) increased latency to find a hidden platform in
the water maze test on days 1, 2, 3, 4, and 5 of testing.
In a study by Shukakidze et al. (2003), groups of white rats were tested for cognitive performance in a
multipath maze. Group I served as a control group, which was trained in the maze for 10 days, fed
normal feed for 30 days, and then retested. Groups II and III, instead of receiving normal feed, received
dosed feed at 5.6 or 13.9 mg manganese/kg/day (as manganese chloride). Groups IV and V were dosed
the same doses as Groups II and III, respectively, but received the doses for 30 days prior to maze
training. Groups II and III received normal feed for the next 90 days prior to retesting for 10 days. An
additional group of animals received a single dose (undefined route) prior to 10 days of training in the
maze. Both groups of rats dosed after training (Groups II and III) showed moderate disruption of their
acquired skill in the maze compared to controls. Group III also demonstrated increased "aggressivity".
Both groups that were exposed prior to training (Groups IV and V) were entirely unable to learn the
maze. When these rats were reassessed after a 3-month period without excess manganese, they remained
unable to learn the maze. After training, 8/12 rats in the group with the single dose (Group VI) mastered
the maze; 4/12 required assistance from the experimenter to orient themselves. Groups of 9 (control) and
10 (manganese-treated) rats were tested in an active avoidance of conditioned and unconditioned stimuli
paradigm. Manganese-treated rats received by mouth 13.9 mg manganese/kg/day (as manganese
chloride) in water 1 hour prior to the experiment on day 1. Rats were tested over 16–17 days. Manganese
treatment resulted in significant and reversible behavioral change, with manganese exposure leading to
worsened acquisition of the avoidance reaction in response to unconditioned and conditioned stimuli,
increased latent period of conditioned reflex activity, and increased numbers of errors and time taken to
navigate a maze, beginning on day 5 of the experimental period and lasting until day 10–15, depending on
the end point.
Neurological Effects in Young Animals—Oral Exposure to Inorganic Manganese. Numerous studies
have evaluated the effect of early postnatal and juvenile manganese exposure on neurodevelopment in
animals. Several have reported biochemical changes in the brain, including alterations in the
dopaminergic, noradrenergic, serotonergic, or gabaergic systems; increased monoamine oxidase; and
decreased iron levels (Anderson et al. 2007a, 2009; Chandra and Shukla 1978; Deskin et al. 1981;
Dorman et al. 2000; Kern et al. 2010; Kern and Smith 2011; Kristensson et al. 1986; Moreno et al. 2009;
Reichel et al. 2006; Tran et al. 2002a, 2002b). Additionally, many studies have reported altered behavior
following developmental manganese exposure, including hyperactivity, altered social interactions,
MANGANESE
181
3. HEALTH EFFECTS
transient ataxia, altered acoustic startle, impaired learning, and increased stereotypic behaviors (Dorman
et al. 2000; Golub et al. 2005; Kern and Smith 2011; Kern et al. 2010; Kristensson et al. 1986; Moreno et
al. 2009; Tran et al. 2002a, 2002b). While results from these studies are varied, taken together, they
indicate that excess manganese exposure during early postnatal development can lead to alterations in
brain chemistry and behavioral development.
Studies of manganese in Rhesus monkeys by Golub et al. (2005) were prompted by the observation that
soy-based formulas provided to human infants contain relatively high levels of manganese and thus may
pose a potentially toxic hazard to early neurological development. Groups of eight male infant Rhesus
monkeys were fed a commercial cow's milk based formula (Similac containing 50 µg manganese/L as
control, providing 17.5 mg manganese/kg/day), a commercial soy protein based formula (soy containing
300 µg manganese/L, providing 107.5 mg manganese/kg/day), or the same soy formula with added
manganese chloride for a final concentration of 1,000 µg manganese/L (soy plus manganese, providing
328 mg manganese/kg/day). Formulas were exclusively fed to infants starting on the day of birth and
extending through 4 months of age, at which time monkeys were transitioned to standard laboratory diet.
A behavioral test battery was administered over an 18-month period. The battery included measures of
motor, cognitive, and social skills, as well as tests related to the dopamine system (reward delay, fixed
interval dopamine drug response). Infants that did not generate sufficient data in each test to permit
evaluation were excluded from data analyses. Growth and levels of the dopamine metabolite HVA and
the serotonin metabolite 5-hydroxyindolacetic acid (5-HIAA) in CSF at 4, 10, and 12 months of age were
also measured. No significant differences between groups were observed for body weights and levels of
dopamine and serotonin metabolites in cerebrospinal fluid.
Monkeys fed soy supplemented with manganese were consistently more active during 12 weekly
7-minute observation periods, compared with control and soy monkeys. "Motor behaviors" were
observed in seven of eight soy plus manganese monkeys, compared with three of eight in soy monkeys
and three of eight in control monkeys. Assessment of gross motor maturation during these observation
periods did not detect clear differences between the groups. Both soy and soy plus manganese groups
showed some changes in activity/sleep patterns. Compared with controls at 4 months, the 4-month
monkeys fed soy plus manganese showed 50% less activity (p<0.05) during the sleep portion of the
sleep/wake cycle (this change was not seen at 8 months). At 8 months (but not at 4 months), both soy and
soy plus manganese monkeys showed significantly (p<0.05) longer sleep periods and shorter longest time
inactive during awake periods than controls. Social interactions were assessed during 16 sessions in
which each monkey was paired with another monkey in the study. In these sessions, both soy and soy
MANGANESE
182
3. HEALTH EFFECTS
plus manganese monkeys demonstrated ~66% less time (p<0.05) in chase or rough play and more time in
clinging activity compared with control monkeys.
Significant group differences were not consistently observed in more highly structured tests to assess
cognitive functions including learning, memory, and attention than controls (p<0.05 for 328 mg
manganese/kg/day and p<0.01 for 107.5 mg manganese/kg/day). For example, a response latency
decrease was observed in a reward delay response task in the soy group by 50% compared to control, but
no significant difference (although a 20% reduction) was observed in the soy plus manganese group. The
authors noted that more formal tests of cognitive functions would be most appropriately administered at
more mature ages.
Other studies in neonatal animals have detected neurostructural and neurochemical changes at
supplementary doses similar to or slightly above dietary levels (1–10 mg manganese/kg/day) (Chandra
and Shukla 1978; Deskin et al. 1980), suggesting that young animals might be more susceptible to
manganese than adults.
Kristensson et al. (1986) investigated the developmental effects of manganese chloride on 3-day-old male
rat pups. The authors dosed the pups with 150 mg manganese/kg/day by gavage in water for 41 days.
The pups developed a transient ataxia on days 15–22, which was resolved by the end of the dosing period.
The exposed pups also had increased levels of manganese in the blood and the brain (7–40-fold increase
in 15- and 20-day-old rats, with cortex and striatum concentrations being relatively equal). In 43-day-old
rats, the increases in brain manganese levels were less than those observed in younger rats (i.e.,
approximately 3 times the control levels), but the striatal levels were higher than in the cortex.
Manganese treatment decreased the concentration of homovanillic acid (metabolite of dopamine) in the
striatum and the hypothalamus, but not in other brain regions. No other monoamines and metabolites
were affected. In a similar study, neonatal rats given bolus doses of manganese chloride in water of
0.31 mg manganese/kg/day for 60 days suffered neuronal degeneration and increased brain monoamine
oxidase on days 15 and 30 of the study, but did not show any clinical or behavioral signs of neurotoxicity
(Chandra and Shukla 1978).
Deskin et al. (1980, 1981) also found changes in brain chemistry in rat pups dosed with manganese. In
the first study, male rat pups were administered 0, 1, 10, or 20 mg manganese/kg/day (as manganese
chloride) via gavage in 5% sucrose solution for 24 days postnatal. The authors observed that the two
highest doses resulted in decreased dopamine levels in the hypothalamus, while the highest dose resulted
MANGANESE
183
3. HEALTH EFFECTS
in a significant decrease in brain tyrosine hydroxylase activity and a significant increase in monoamine
oxidase activity in the hypothalamus. Hypothalamic norepinephrine was unaffected by any manganese
dose, and no significant changes in neurochemistry were noted in the corpus striatum. The authors
suggested that the observed effects were probably due to decreased activity of tyrosine hydroxylase and
increased levels of monoamine oxidase.
The second study (Deskin et al. 1981) involved dosing male rat pups with 0, 10, 15, or 20 mg
manganese/kg/day (as manganese chloride) via gavage in 5% sucrose solution, for 24 days starting at
birth. The authors performed neurochemical analyses of hypothalamus and corpus striatum as before and
observed that serotonin was increased in the hypothalamus at the highest dose, but was not elevated
significantly in the striatum. Acetylcholinesterase levels were significantly decreased in the striatum at
the highest dose, but were unchanged in the hypothalamus. The authors believed that the decrease in
acetylcholinesterase to be of minor functional significance given that other mechanisms can also regulate
acetylcholine metabolism.
A study by Kontur and Fechter (1988) reported no difference in levels of monoamines and related
metabolites in neonatal rats at 22 mg manganese/kg/day as manganese chloride (14–21 days), although
Dorman et al. (2000) reported elevated striatal DA and DOPAC in 21-day-old rats administered the same
high daily dose used by Kontur and Fechter (1988) from PND 1 to 21. Effect of manganese treatment on
neurobehavior was also evaluated in this study. There was a significant decrease in body weight gain in
pups at the highest manganese exposure dose. Although there were no statistically significant effects on
motor activity or performance in the passive avoidance task in the neonates, manganese treatment induced
a significant increase in amplitude of the acoustic startle reflex at PND 21. However, in adult rats, the
amplitude of the acoustic startle reflex was significantly decreased compared to the control at the lowest
dose tested.
Reichel et al. (2006) studied the effects of manganese in male Sprague-Dawley rats that were born and
dosed daily with an oral dose of 0, 4.4 or 13.1 mg manganese/kg/day as manganese chloride on
postpartum days 1–21. Locomotor activity was assessed (distance traveled horizontally; PNDs 10–14), as
was olfactory orientation (PNDs 9–13), negative geotaxis (PNDs 8–12) and balance and coordination
(PND 90). Day of eye opening, pinna detachment, and incisor eruptions was also evaluated. Mean body
weights at PND 21 were decreased by about 2 and 3% in the low- and high-dose groups, respectively,
compared with controls. Manganese concentrations in striatum were elevated in the high dose group,
compared with control, at PND 14 (~4-fold) and PND 21 (~2-fold), but not at PND 90. Manganese levels
MANGANESE
184
3. HEALTH EFFECTS
were not measured in the low-dose group. No exposure-related effects were noted on developmental
landmarks (eye opening, pinna detachment, incisor eruption), basal motor activity during the neonatal
period (PNDs 10–14) and adulthood (PND 90), or olfactory discrimination of home cage bedding during
the neonatal period. The only behavioral end point affected during the neonatal period was a significant
(p<0.05) increase in mean latencies to rotate 180° on the inclined plane of a negative geotaxis task. At
PND 90, dopamine transporter binding sites in the striatum were decreased by about 20 and 60% in the
low- and high-dose groups, respectively; only the high-dose value was significantly different (p<0.05)
from the control. At PND 90, the locomotor activating effects of 20 mg/kg cocaine were significantly
(p<0.05) decreased in the neonatally exposed manganese high dose group, compared with controls. The
results indicate that neonatal exposure of rats to excess manganese caused subtle behavioral effects
(altered balance in the neonatal period and diminished locomotor response to cocaine in adulthood) and
neurochemical effects in adulthood (decreased dopamine binding sites in the striatum).
In a study by Tran et al. (2002a), Sprague-Dawley PND 1 litters were culled to 10–12 pups per dam and
then were supplemented from PNDs 1–20 with 0, 0.7, 3.8, or 7.5 mg manganese/kg/day as manganese
chloride provided by mouth. Male and female pups were used. Righting test (PND 6), homing test
(olfactory discrimination; PND 10), and passive avoidance (PND 32) were performed. Striatal dopamine
levels were also determined after sacrifice on PND 40. Brain tissue analyses for iron, copper, zinc, and
manganese content were performed on animals sacrificed on PNDs 14, 21, and 40. Animals were not
dosed after PND 20. The two highest dose groups of rats took approximately twice as long (2 seconds) as
control and 0.7 mg manganese/kg/d (approximately 1 second) to right themselves; this result was not
statistically significant. In the homing test of olfactory discrimination, the 7.5 mg manganese/kg/day
group took significantly longer to reach their goal compared to controls and the 3.8 mg manganese/kg/day
group (the 0.7 mg manganese/kg/day group performed similarly to the control). The control group
required approximately 40 seconds; the high-dose group required 75 seconds (an 88% increase in the
high-dose group over the control). In the passive avoidance task, there was a positive linear trend, with
the highest dose group showing a 3-fold increase in the number of footshocks received over the control.
The 3.8 mg manganese/kg/d group showed a 2-fold increase in the number of footshocks over the control.
A negative linear relationship was also observed in striatal dopamine concentrations, with the high-dose
group having approximately half the dopamine concentration of the control. No dose-related trends over
time points were observed in manganese content of tissues. The highest dose group showed some
statistically significant (p<0.05) increases in manganese in brain tissue. No changes were seen in iron,
copper, or zinc tissue concentration. Both males and females were used in behavioral tests since
ANOVAs showed no interactive effects of treatment or sex.
MANGANESE
185
3. HEALTH EFFECTS
In a companion study, Tran et al. (2002b) explored whether there were lasting behavioral and
neurochemical changes following manganese exposure. Again, Sprague-Dawley rat pups received
dietary supplementation in the form of 0, 0.7, 3.8, or 7.5 mg manganese/kg/day (as manganese chloride).
Male and female pups were sacrificed during infancy and at weaning (18–24 per treatment group) for
tissue analyses of trace elements. Twenty-four rats were sacrificed at PND 35 for dopamine analysis
(Tran et al. 2002a). The 32 remaining rats, all males, no longer received treatment. Behavioral testing
began with a burrowing detour test (PNDs 50–56) and ended with a passive avoidance test (PNDs 60–64).
No statistically significant results for any individual treatment group for any behavioral task or striatal
dopamine levels. A statistically significant positive trend was observed for passive avoidance
(approximately 50% more footshocks in highest dose group, compared with control). The control had
approximately 2 times the striatal dopamine levels of the two highest dose groups on animals sacrificed
on PND 65.
Kern et al. (2010) evaluated Sprague-Dawley rat pups administered 0, 25, or 50 mg manganese/kg/day as
manganese chloride in 25% sucrose vehicle via micropipette from PND 1 to 21. Dose groups were
balanced across sex within each of 26 litters, each culled to 10 pups with an approximate 2:1 male to
female ratio. On PND 23, 15– 20 and 7 males per group were tested in open area and the elevated plus
maze activities, respectively. Groups of 15–20 males were evaluated on PNDs 27–46 on the radial arm
maze. Blood and tissue samples were collected on PND 24 (8–12/sex/group) and PND 36 (females only,
number unspecified) for measurement of hematocrit and blood and brain manganese levels. On PND 24,
an additional 4–7 males/group were sacrificed for immunohistochemical analysis of dopamine transporter
(DAT) and dopamine D1 and D2 receptors in several brain regions. Following treatment, manganese
blood levels were increased 2–3-fold at PND 24 (p<0.05), but hematocrit levels were not altered. Males
in the 50 mg/kg/day group had significantly (p<0.05) increased activity in the open field and significantly
impaired spatial learning in the radial arm maze. All treated males had increased stereotypical behavior
in the radial arm maze (p<0.05). There were no differences in behavior during the elevated plus maze
test. Additionally, there were significant decreases in dopamine D1 and D2 receptors and dopamine
transporter in multiple brain regions from males from the 50 mg/kg/day group.
In a follow-up study, Kern and Smith (2011) evaluated the effects of preweaning manganese exposure on
the adult dopaminergic system, behavior, and astrocytic activation. Sprague-Dawley rat pups were
exposed to 0, 25, or 50 mg manganese/kg/day using the experimental design described above. On
PNDs 97–98, 15–20 adult males per group were evaluated in the open area test. Blood and tissue samples
MANGANESE
186
3. HEALTH EFFECTS
were collected on PND 24 (8–12/sex/group) and PND 107 (10 males/group) for measurement of blood
and brain manganese levels. Additionally, 4–7 males per group were sacrificed for immunohistochemical
analysis of dopamine transporter, dopamine D1 and D2 receptors, and GFAP levels in the brain were
sacrificed on PNDs 24 and 107. Preweaning exposure to manganese did not lead to significantly (p>0.05)
elevated manganese levels in the blood or brains of adult rats. However, exposure at 50 mg/kg/day led to
significantly (p≤0.05) increased densities of dopamine D2 receptors in the prefrontal cortex of adult
brains (in contrast to decreased dopamine receptor and transporter densities in weanling brains).
Astrocyte activation was significantly (p<0.05) increased in both weanling (prefrontal cortex) and adult
rats (medial striatum) with preweaning exposure at 25 or 50 mg/kg/day. At 50 mg/kg/day, additional
regions in the adult brain had significantly increased atrocytic activation (prefrontal cortex, nucleus
accumbens). In the open field, there were no measureable differences in activity in adults (compared to
increased activity in weanlings reported in the previous study). However, a residual effect could be
observed under the influence of D-amphetamine. Kern et al. (2011) concluded that preweaning
manganese exposure leads to lasting molecular and functional impacts in multiple brain regions of adult
animals, long after brain manganese levels return to normal. However, it appears that behavioral effects
may be reversible.
In order to determine if developing animals are more susceptible to the neurochemical and
neurobehavioral effects of manganese exposure, Moreno et al. (2009) exposed mice as juveniles, as
adults, or as both juveniles and adults. Littermates from timed-pregnant C57Bl/6 mice were paired in
control and manganese-exposed groups, receiving 0, 4.4, or 13.1 mg manganese/kg/day as manganese
chloride via gavage from PND 20 to 24 and again from week 12 to 20. Additional animal groups were
exposed only from PND 20 to 24 or from week 12 to 20. Open field activity was measured every other
day during early exposure (11–18 animal/group), and every other week thereafter (8–10 animals/group).
Following treatment, animals were sacrificed. Brain levels of dopamine and its metabolite DOPAC, and
serotonin and its metabolite 5-HIAA, were measured in the striatum of 3–4 animals per group. Brain
levels of manganese, iron, and copper were also measured in three animals per group. In all treated
animals, brain regions with the highest levels of manganese were striatum, substantia nigra, and cortex.
Only modest increases in copper and iron brain levels were detected. In the open field, juvenile-only
exposed males, but not females, spent significantly (p<0.05) less time in the margins of the open field
(increased novelty-seeking behavior). Adult-only exposed mice did not display any open field behavioral
alteration. However, male mice exposed at both ages spent significantly (p<0.05) more time in the
margins of the open field (decreased novelty seeking behavior). Additionally, males exposed to
13.1 mg/kg/day at both ages made significantly (p<0.05) fewer movements. Several alterations in the
MANGANESE
187
3. HEALTH EFFECTS
dopaminergic system were reported in all groups. In juveniles exposed to 13.1 mg/kg/day, dopamine
levels were significantly (p<0.05) increased, but DOPAC levels were significantly decreased. Therefore,
the DOPAC:dopamine ratio (an indicator of dopamine turnover) was increased in this group. In contrast,
mice with adult-only exposure at 13.1 mg/kg/day and all manganese-treated mice exposed at both ages
had significantly (p<0.05) decreased dopamine and DOPAC levels. In the serotonergic system, the only
significant (p<0.05) finding was increased 5-H1AA in juvenile-only exposed mice at 13.1 mg/kg/day.
These results indicate that developing mice are more sensitive to neurobehavioral and neurochemical
effects of manganese exposure than adult animals, and that previous juvenile exposure increases
susceptibility to these effects from manganese exposure in adults.
Weber et al. (2002) evaluated indicators of oxidative stress in Charles River CD rat pups that were dosed
(by mouth with micropipette) according to average pup weight for each litter starting on PND 1 and
continuing until PND 21 at doses of 0 (nanopure water vehicle), 6.9, or 138 mg manganese/kg/day. Pups
were sacrificed on PND 21, and samples of cerebellum and cerebral cortex were collected and frozen in
liquid nitrogen, with manganese concentrations evaluated in brain tissue. Also evaluated were
cerebrocortical and cerebellar metallothionein (MT) mRNA levels, glutamine synthetase (GS) activity,
GS protein levels, and total glutathione (GSH) levels. High-dose manganese exposure significantly
increased (p<0.05) total cerebrocortical GSH when compared to control without changes observed in any
of the other measures. The same change was apparent with the high-dose manganese exposure on
cerebellar GSH, although slight differences in the standard error of the mean prevented reaching
statistical significance. However, it should be noted that these measures actually decreased with respect
to the control in the low dose manganese group. Overall, data do not appear to support an effect of
manganese exposure on measured biochemical variables indicative of oxidative stress.
Neurologic Effects in Animals with Liver Dysfunction—Oral Exposure to Inorganic Manganese.
Several animal studies have evaluated the potential for hepatic dysfunction to enhance the neurotoxicity
of manganese (Montes et al. 2001, 2006; Rivera-Mancia et al. 2009; Rose et al. 1999).
Rose et al. (1999) reported the effects on manganese body burden (exclusively from the diet) in rats with
either induced cirrhosis of the liver, acute liver failure (induced by portacaval anastomosis followed by
hepatic artery ligation), or a surgically-administered portacaval shunt (PCS). Brain manganese levels in
these three groups of rats were compared to control rats and sham-operated rats. PCS and sham-operated
rats were evaluated 4 weeks following surgery, while cirrhotic rats were studied 6 weeks following
surgery. Rats with acute liver failure were studied 15–18 hours following devascularization at coma stage
MANGANESE
188
3. HEALTH EFFECTS
of encephalopathy. Manganese levels were statistically significantly increased as compared to non-
treated controls and sham-operated controls in both cirrhotic and PCS rats in the frontal cortex, globus
pallidus, and caudate/putamen; manganese levels were highest in the globus pallidus. For example, in the
globus pallidus, brain manganese was increased 57% in the PCS rats as compared to the control rats
(p<0.0001). However, the level of manganese in the globus pallidus in the PCS rats was significantly
elevated as compared to cirrhotic rats, indicating that shunting is a strong determinant of manganese
deposition in the brain.
Montes et al. (2001) also explored the potential for hepatic disease to potentiate the toxic effects of
manganese by observing effects on levels of specific neurotransmitters. Groups of male Wistar rats were
assigned to one of six treatments: (1) sham operated; (2) bile duct ligated (BDL); (3) sham operated with
15.1 mg manganese/kg/day supplied as manganese chloride in drinking water; (4) BDL with 15.1 mg
manganese/kg/day in drinking water; (5) sham operated with 26.7 mg manganese/kg/day in drinking
water; or (6) BDL with 26.7 mg manganese/kg/day in drinking water. The BDL condition models a
cirrhotic-type condition in the rats. Rats received this treatment for 4 weeks beginning at surgery. At the
end of treatment, rats were weighed and killed. Total bilirubins (as well as conjugated and unconjugated
forms) increased over control in all BDL groups, but there was no significant effect of manganese
treatment. There was also no effect of manganese on alanine aminotransferase levels or on collagen,
although these measures were significantly increased by BDL. However, the combination of BDL and
manganese exposure produced 2- and 4-fold increases (p<0.001) of striatal manganese content at the
15.1 and 26.7 mg manganese/kg/day doses, respectively, while BDL alone did not produce changes.
Striatal DA content was significantly decreased compared to control in BDL rats; the addition of 26.7 mg
manganese/kg/day to BDL produced an approximate 33% increase in dopamine (DA) content over BDL
alone. The highest dose of manganese produced 2-fold striatal HVA increases over control in both sham-
operated and BDL rats. BDL and manganese treatment at 15.1 mg manganese/kg/day each individually
produced 2-fold increases over control levels in striatal DA turnover, measured as HVA/DA; the
combination of BDL with manganese at 15.1 mg manganese/kg/day produced the same result as each
condition individually. The sham-operated and BDL high dose rats each had HVA/DA levels of nearly
3 times the control level; all of these differences were significant (p<0.05). These results suggest that
hepatic dysfunction can, indeed, potentiate the neurotoxicity of manganese.
In another study, Montes et al. (2006) explored the potential role of hepatic dysfunction as a potentiator of
the toxic effects of manganese on neuronal damage produced by oxidative stress. Groups of male Wistar
rats were assigned to one of four treatments (n=6–9 in each group): (1) sham operated; (2) BDL;
MANGANESE
189
3. HEALTH EFFECTS
(3) sham operated with 26.7 mg manganese/kg/day (as manganese chloride) in drinking water; or
(4) BDL with 26.7 mg manganese/kg/day in drinking water. Rats received this treatment for 4 weeks
beginning at time of surgery. Compared with sham-operated controls, BDL treatment with or without
manganese caused significant (p<0.05) increases (>2-fold) in gamma glutamyltranspeptidase and alanine
aminotransferase activities, collagen, and glycogen levels, but manganese alone did not increase these
indices of liver damage. Manganese or BDL treatments alone caused moderate, statistically significant
(p<0.05) increases (~20%) in manganese content in the striatum and globus pallidus. Manganese contents
in both regions were further and markedly increased by the BDL and manganese treatment (300–400%
increase). Levels of nitric oxide (NO) were not consistently changed in either brain region in manganese-
alone or BDL plus manganese-treated rats compared with sham-operated controls, with the exception that
the NO levels in the globus pallidus were decreased (p<0.05) by ~25% in BDL and BDL plus manganese
rats. Constitutive nitric oxide synthetase (NOS) activities in the globus pallidus were decreased (but not
to a statistically significant degree) in BDL and BDL plus manganese-treated rats.
In a similar study, Rivera-Mancía et al. (2009) investigated alterations in brain astocytes in manganese-
exposed male Wistar rats with and without liver damage. Similar experimental groups were used
(number of rats/group not specified: (1) sham operated; (2) BDL; (3) sham operated with 1 mg
manganese/mL (as manganese chloride) in drinking water; or (4) BDL with 1 mg manganese/mL (as
manganese chloride) in drinking water. Rats received this treatment for 2 or 4 weeks beginning at time of
surgery. Using an allometric equation for drinking water consumption (EPA 1988) and average reported
body weight, the estimated dose in manganese-exposed groups is 271 mg/kg/day. Brain levels of
manganese were measured in the cortex, striatum, and globus pallidus. Altered and normal astrocytes
were counted in the same regions. Manganese or BDL treatment, alone or in concert, led to significantly
(p<0.05) elevated manganese levels in all three brain regions tested. The number of damaged astrocytes
was significantly (p<0.05) increased in animals from both BDL groups compared to the sham-operated
control. However, manganese exposure in either sham operated or BDL animals did not increase the
extent of astrocyte damage. This indicates that short-term manganese exposure alone, either with or
without liver damage, did not induce gliosis in the cortex, striatum, or globus pallidus in rats.
Neurological Effects in Iron-Deficient Animals—Oral Exposure to Inorganic Manganese. Studies
reporting competition between iron and manganese in absorption indicate the impact an iron-poor diet
will have on manganese uptake in the human (Chandra and Tandon 1973; Davis et al. 1992a, 1992b;
Diez-Ewald et al. 1968; Mena et al. 1969; Rehnberg et al. 1982; Thomson et al. 1971). Further,
MANGANESE
190
3. HEALTH EFFECTS
competition between manganese and iron at the blood-brain barrier has been reported (Aschner and
Aschner 1990), indicating that excesses of either metal will affect the brain distribution of the other.
A recent study in rats has been conducted to determine the mechanism by which iron is regulated at the
blood-brain barrier and the blood-cerebrospinal fluid (B-CSF) barrier and how manganese may alter these
processes (Li et al. 2006). Additionally, two studies by Anderson et al. (2007a, 2009) have explored the
interplay between iron deficiency and manganese supplementation and its ultimate potential for
modulating neurotransmission in the neonatal rat brain. These studies, together with gestational exposure
studies evaluating altered iron metabolism (Garcia et al. 2006, 2007; Jarvinen and Ahlström 1975; Molina
et al. 2011; see Section 3.2.2.6), suggest that manganese-mediated alterations in iron pharmacokinetics
may, at least in part, underlie some of the observed adverse neurological effects associated with elevated
manganese exposure.
Li et al. (2006) dosed groups of 7–8-week-old male Sprague-Dawley rats were with sterile saline (control)
or manganese chloride dissolved in sterile saline at 2.2 or 6.6 mg manganese/kg/day; rats were dosed
daily via gavage for 5 consecutive days/week (weekdays only) for 30 days. Serum iron concentrations
were found to be significantly decreased (p<0.05) at 2.2 and 6.6 mg manganese/kg/day (50 and 66% of
control value, respectively. In contrast, iron concentrations in the cerebrospinal fluid (CSF) were
significantly (p<0.05) increased at 2.2 and 6.6 mg manganese/kg/day (136 and 167% of control values).
Manganese produced a dose-dependent increase of binding of IRP1 to iron-responsive element-containing
RNA in (percentage increase of high-dose group over control indicated in parentheses): the choroid
plexus (+70%); in capillaries of striatum (+39%), hippocampus (+56%), and frontal cortex (+49%); and
in brain parenchyma of striatum (+67%), hippocampus (+39%), and cerebellum (+28%). Manganese
exposure significantly increased the expression of TfR mRNA in choroid plexus and striatum with a
reduction in the expression of Ft mRNA. The results indicate that intermediate-duration oral exposure to
excess manganese decreased serum iron concentrations and increased iron concentrations in the CSF.
These changes were associated with: (1) increased binding of iron regulatory proteins and mRNA
containing iron responsive element in several brain regions and (2) upregulation of transferritin receptor
mRNA and down-regulation of ferritin mRNA in choroid plexus and striatum.
In a study by Anderson et al. (2007a), male and female PND 1 Sprague-Dawley rats were divided into
groups receiving either a control diet (35 mg iron/kg, 10 mg manganese/kg diet and drinking water) or a
diet with manganese supplementation (same as control diet with 1 g/L of manganese chloride added to
drinking water for a final dose of 71.1 mg manganese/kg/day). Rats were sacrificed after 6 weeks of
MANGANESE
191
3. HEALTH EFFECTS
treatment. Additional females and males (n=6 per group) were provided with an iron-deficient diet
(4 mg/kg iron, 10 mg manganese/kg diet and drinking water) and an iron deficient/manganese
supplemented diet (same iron-deficient diet plus 1 g manganese chloride/L water). Manganese exposure
significantly (p<0.05) reduced iron concentrations in the caudate putamen and the substantia nigra from
male and female rats. In female rats, manganese exposure also significantly reduced iron levels in the
caudate putamen. The largest decrease was seen in the female caudate putamen, where iron levels
dropped by approximately 66% compared to controls and the female substantia nigra, where iron levels
dropped by approximately 75% compared to controls. Manganese concentrations in the brain were seen
to increase over controls most prominently in the female globus pallidus (approximately 60%). A
significant negative correlation (p<0.05) was observed between synaptosomal manganese concentration
and 3H-GABA uptake in rats of both sexes. 3H-GABA levels were significantly reduced from controls in
both males and females (by approximately 50%). In rats provided with an iron-deficient diet, few
differences were observed between the iron-deficiency condition and the iron-deficiency plus manganese
condition. In males, iron levels were approximately 10 times higher in the caudate putamen of iron-
deficient animals than in the animals that were iron-deficient and manganese-supplemented.
In a more recent study by Anderson et al. (2009), a similar paradigm was used to evaluate the effect of
manganese exposure and iron deficiency on the noradrenergic system. Groups of male weanling
Sprague-Dawley rats were divided into the four groups described above (24/group), with the exception
that a final dose of 68.3 mg manganese/kg/day was attained in manganese-exposed animals (the previous
study used an average dose between males and females). Rats were sacrificed after 6 weeks of treatment.
Again, manganese exposure significantly (p<0.01) reduced extracellular iron concentrations and
increased extracellular manganese concentrations in the caudate putamen. A significant negative
correlation (p<0.01) was observed between synaptosomal manganese concentration and
3
H-NE uptake in
the locus coeruleus. In manganese-treated animals, extracellular NE concentrations were significantly
(p<0.01) decreased, and, in general, NE transporter and receptor proteins and mRNA levels were
decreased across the brain. Again, in rats provided with an iron-deficient diet, few differences were
observed between the iron-deficiency condition and the iron-deficiency plus manganese condition.
Neurological Effects—Oral Exposure to MMT. No studies regarding neurological effects following oral
exposure to MMT by humans were identified.
Komura and Sakamoto (1992b) administered 11 mg manganese/kg/day (as MMT) to ddY mice in food
for 12 months. To measure differences in behavior between exposed and control mice that were fed
MANGANESE
192
3. HEALTH EFFECTS
normal chow, spontaneous motor activity was measured at regular intervals during exposure to determine
differences in behavior between exposed and control mice fed normal chow. The authors observed a
significant increase in spontaneous activity at day 80; no other significant differences were noted. In a
separate study (Komura and Sakamoto 1994), the authors analyzed brain levels of different neurotrans-
mitters and metabolites after identical MMT treatment. MMT resulted in a 66% decrease in dopamine
(DA; p<0.05) and a 95% decrease in normetanephrine (NMN; p<0.01) in the hypothalamus; in the
hippocampus, DA was unchanged, while the level of DOPAC was reduced 41% (p<0.05), and the
3-methoxytyramine (3MT) level increased 3.5-fold (p<0.01). In the midbrain, the only significant
changes noted were an almost 6-fold increase in 3MT (p<0.01) and a 1.75-fold increase of HVA, a
metabolite of DOPAC via conjugation by catechol-o-methyl transferase (p<0.05). In the cerebral cortex,
HVA was decreased by 61%, norepinephrine (NE) by 64%, and epinephrine by 43% (all were p<0.05)
due to MMT administration. In the cerebellum, DOPAC was decreased 51% (p<0.05), while NMN was
increased 7.7-fold (p<0.01). Finally, in the medulla oblongata, DOPAC was decreased by 45% (p<0.05),
HVA was decreased by 55% (p<0.01), and serotonin (5HT) was decreased 81% (p<0.01); metanephrine
was increased approximately 2.75-fold in the medulla (p<0.05).
Through analysis of the distribution of manganese in the different brain regions of the mice, the authors
observed relationships between manganese content and neurotransmitter levels. For example, a weak
relationship was found between the manganese level in the corpus striatum and the level of NE. There
was no relationship between the increase in HVA and the manganese levels in this same region. The
relationship between the increase in 3MT and manganese levels in the midbrain was weak, as was the
relationship between DOPAC and manganese levels in the cerebellum. There were no relationships
between amines and manganese levels in the hippocampus, cerebral cortex, or medulla oblongata,
although some changes were found. A significant correlation was found between the level of NMN and
manganese in the cerebellum. As discussed more fully in Section 3.4.2, the cerebellum contained the
most manganese of any brain region following MMT administration (Komura and Sakamoto 1994).
3.2.2.5 Reproductive Effects
There are no available studies evaluating reproductive effects in humans following oral manganese
exposure.
In a 14-day study in rats, no changes in testicular weight were reported at 1,300 mg manganese/kg/day
(NTP 1993). However, several intermediate-duration studies in rats and mice indicate that manganese
MANGANESE
193
3. HEALTH EFFECTS
ingestion can lead to delayed maturation of the reproductive system in males. One study investigated the
effect of 1,050 mg manganese (as manganese tetroxide)/kg/day, provided to weanling mice and their
dams starting when the pups were 15 days old (Gray and Laskey 1980). On day 30, the mice were
weaned and maintained on the high-manganese diet until killed for analysis at 58, 73, or 90 days old. The
growth and general appearance of the weanling rats appeared normal. At time of death, preputial gland,
seminal vesicle, testes, and body weights were measured. The high-manganese diet resulted in a
significant decrease in growth of these reproductive tissues but no growth retardation of the body and no
change in liver or kidney weights.
A later study by Laskey et al. (1982) evaluated the reproductive functioning of male and female Long-
Evans rats that had been exposed to 0, 350, 1,050, and 3,500 mg manganese/kg/day (in conjunction with a
low-iron diet [20 mg iron/kg/day] or a diet adequate in iron [200 mg iron/kg/day]) while in utero (dams
were fed the described diets during gestation) and from day 14 to 15 postpartum. The rats were
maintained on the diet throughout the remainder of the study (224 days). The rats were mated at 100 days
postpartum and the reproductive success of these matings was evaluated.
In males, manganese treatment resulted in decreased testes weights (testes weights analyzed with body
weight as a covariable) observed at 40 days (at the 1,050 and 3,500 mg manganese/kg/day dose levels)
and 100 days (at the 1,050 mg manganese/kg/day dose level) of age, only when administered with the
low-iron diet. Hormone levels in male rats were also evaluated. No treatment-related effect was seen in
40-day-old males. At 60 and 100 days of age, however, dose-related decreases in serum testosterone
were observed, while serum LH (luteinizing hormone) levels remained relatively unchanged. Luteinizing
hormone (LH) is secreted by the pituitary to stimulate testosterone production in the Leydig cells.
Testosterone levels control LH production through a negative feedback loop. An increase in testosterone
would normally be associated with a subsequent decrease in LH. The decrease in testosterone
simultaneous with a stable LH levels suggests that manganese is targeting the Leydig cells. Manganese
treatment in both iron regimens prevented the normal decrease in serum follicle-stimulating hormone
(FSH) from 60 to 100 days. In addition, manganese only negatively affected epididymal sperm counts at
100 days in the iron-deficient group. When serum concentrations of LH, FSH, and testosterone and
epididymal sperm counts from the 60- and 100-day-old rats were used to predict the reproductive age of
the males, the 60-day-old animals were predicted correctly. Of the 100-day-old animals, 2/12 controls,
7/12 at 350 mg manganese/kg, and 12/12 at 1,050 mg manganese/kg were classified as 60 days old.
These data indicate that manganese induced a significant maturational delay in the reproductive organs of
the male rat (Laskey et al. 1982).
MANGANESE
194
3. HEALTH EFFECTS
To further assess the mechanism of toxicity of manganese in the pre-weanling rat, Laskey et al. (1985)
dosed rats from birth to 21 days of age with particulate manganese tetroxide in 50% sucrose solution by
gavage at doses of 0, 71, or 214 mg manganese/kg/day. They then assessed the hypothalamic, pituitary,
and testicular functions in the rat by measuring the endogenous or stimulated serum concentrations of
FSH, LH, and testosterone at 21 or 28 days of age. LH-releasing hormone (LH-RH) was used to
stimulate the pituitary-testicular axis to secrete FSH, LH, and subsequently testosterone; human chorionic
gonadotropin (hCG) was used to stimulate acutely (2-hour time period) the testicular secretion of
testosterone and repeatedly (7-day time period) to assess the ability of the Leydig cells to maintain
maximal testosterone synthesis and secretion. Some rats from both control and manganese-dosed groups
were castrated to determine the effect this would have on the study end points. Manganese treatment had
only a slight effect on body and testes weights, while no effects were observed on unstimulated or
stimulated FSH or LH serum levels. In addition, manganese did not affect endogenous or acute
hCG-stimulated serum testosterone concentrations, but did decrease serum testosterone level following
repeated hCG stimulation. Liver manganese at the 71 mg/kg/day manganese dose was significantly
elevated over controls in both castrated (8.42±7.23 mg/kg for treated vs. 1.96±0.22 mg/kg for controls)
and noncastrated (3.36±0.91 mg/kg for treated vs. 1.81±0.11 mg/kg for controls) rats. In addition,
hypothalamic manganese concentrations were significantly increased at the 71 mg/kg/day dose in both
castrated (6.10±3.0 mg/kg in treated vs. 0.59±0.11 mg/kg in controls) and noncastrated (3.73±1.18 mg/kg
in treated vs. 0.65±0.057 mg/kg in controls) rats. The authors speculate that since their earlier results had
shown changes in male reproductive development in postpubertal animals with minimal manganese
concentrations in tissues (Gray and Laskey 1980; Laskey et al. 1982), it seemed likely that the changes in
this later study (Laskey et al. 1985) would result from high manganese concentrations in the
hypothalamus, pituitary, or testes, with the tissue with the highest manganese concentration being the site
of the toxic reproductive effect. However, the results from this latest study reveal that manganese had no
effect on the hypothalamus or pituitary to produce LH or FSH in pre-weanling rats, despite the increased
manganese concentrations. Rather, the data indicate that it is delayed production of testosterone, shown
by the inability of the Leydig cells to maintain maximum serum concentrations of the hormone, which
results in the delayed sexual maturation. This delay in testosterone was not significant enough, however,
to impair rodent fertility at manganese doses as high as 1,050 mg/kg/day (Laskey et al. 1982).
A slight decrease in pregnancy rate was observed in rats exposed to 3,500 mg manganese/kg/day (as
manganese tetroxide) in the diet for 90–100 days prior to breeding (Laskey et al. 1982). Since both sexes
were exposed, it is not possible to conclude whether the effect was in males, females, or both. However,
MANGANESE
195
3. HEALTH EFFECTS
this exposure regimen did not have significant effects on female reproductive parameters such as ovary
weight, litter size, ovulations, or resorptions (Laskey et al. 1982).
Manganese was found to affect sperm formation and male reproductive performance in other
intermediate-duration oral studies (Elbetieha et al. 2001; Joardar and Sharma 1990; Ponnapakkam et al.
2003a, 2003c). Joardar and Sharma (1990) administered manganese to mice, as potassium permanganate
or manganese sulfate, at 23–198 mg/kg/day by gavage for 21 days. The treatment resulted in sperm head
abnormalities, and the percentage of abnormal sperm was significantly elevated in all exposed mice as
compared to controls. Increased incidences of testicular degeneration occurred in male Sprague-Dawley
rats exposed for 63 days to doses ≥137.2 mg manganese/kg/day as manganese acetate, but not at
68.6 mg/kg/day (Ponnapakkam et al. 2003c). Impaired male fertility was observed in male mice exposed
to manganese chloride in drinking water for 12 weeks before mating with unexposed females at a daily
dose level of 309 mg manganese/kg/day, but not at doses ≤154 mg manganese/kg/day (Elbetieha et al.
2001). In the 309-mg/kg/day group, 17 pregnancies occurred in 28 mated females, compared with
26 pregnancies out of 28 females mated with controls. At lower dose levels in another study, decreased
sperm motility and sperm counts were observed in male CD-1 mice after 43 days of exposure to
manganese acetate at doses of 4.6 or 9.6 mg manganese/kg/day, but these doses did not impair the ability
of these males to impregnate unexposed females (Ponnapakkam et al. 2003a).
In another intermediate feeding study, Jarvinen and Ahlström (1975) administered varying doses of
manganese sulfate, from nutritionally deficient levels to excess amounts, to Sprague-Dawley female rats
for 8 weeks prior to mating. The rats were continued on manganese diet (0.75, 4.5, 10, 29, 94, or 187 mg
manganese/kg/day) until GD 21. The authors found no effect of manganese on maternal weight gain,
implantation number, resorptions, or percentage of dead fetuses. The authors did observe that manganese
doses of 94 mg manganese/kg/day and higher resulted in significant increases in liver manganese
concentrations, whereas nonpregnant females had liver manganese concentrations that were unchanged,
irrespective of dose. These data suggest that pregnancy allows the female to develop significant liver
manganese stores, and it is possible these stores may be mobilized during gestation or at a future time.
The authors also noted that pregnant rats had consistent liver iron concentrations, whereas nonpregnant
rats developed a dose-dependent decrease in liver iron concentrations. Further, the highest dose in dams
caused a significant increase in fetal manganese content.
Szakmáry et al. (1995) studied the reproductive effects of manganese chloride, administered by gavage to
pregnant rabbits and rats at concentrations of 0, 11, 22, and 33 mg manganese/kg/day on GDs 6–20 in the
MANGANESE
196
3. HEALTH EFFECTS
rabbit and throughout gestation in the rat. Manganese did not result in any reproductive effect in the
rabbit, but the highest manganese dose did cause an increase in postimplantation loss in the rat. In
13-week dietary studies, no gross or histopathological lesions or organ weight changes were observed in
reproductive organs of rats fed up to 618 mg manganese/kg/day or mice fed 1,950 mg manganese/kg/day,
but the reproductive function was not evaluated (NTP 1993).
More recent oral studies indicate that ingested manganese does not result in female reproductive toxicity
when rat dams were exposed during pregnancy, but impaired female fertility was observed when female
mice were exposed to manganese in drinking water for 12 weeks before mating with unexposed males.
The first study involved a dose of 22 mg manganese/kg/day administered as manganese chloride by
gavage to female rats on days 6–17 of gestation (Grant et al. 1997a). No treatment-related mortality,
clinical signs, changes in food or water intake, or body weights were observed in the dams. In the second
study (Pappas et al. 1997), manganese chloride was provided to pregnant rats in drinking water at doses
up to 620 mg manganese/kg/day throughout gestation. The manganese did not adversely affect the health
of the dams, litter size, or sex ratios of the pups. More extensive analyses of female reproductive organs
were not performed. Similarly, Kontur and Fechter (1985) found no significant effect on litter size in
female rats exposed to manganese chloride in drinking water except at concentrations so high (1,240 mg
manganese/kg/day) that water intake by the dams was severely reduced. In contrast, Elbetieha et al.
(2001) reported that decreased numbers of implantations and viable fetuses were observed in female
Swiss mice exposed to manganese chloride in drinking water at a dose level of 277 mg manganese/kg/day
for 12 weeks before mating with unexposed males.
In a 2-year NTP study, no adverse reproductive effects (lesions in reproductive organs) from manganese
sulfate exposure were reported for rats at up to 232 mg manganese/kg/day or mice at up to 731 mg
manganese/kg/day (NTP 1993).
The highest NOAEL values and all LOAEL values from each reliable study for reproductive effects in
each species and duration category are recorded in Table 3-3 and plotted in Figure 3-3.
No studies were located regarding reproductive effects in humans or animals following oral exposure to
MMT.
MANGANESE
197
3. HEALTH EFFECTS
3.2.2.6 Developmental Effects
Developmental Effects in Humans—Oral Exposure to Inorganic Manganese. Very little information is
available on the developmental effects of manganese in humans. The incidences of neurological
disorders and the incidences of birth defects and stillbirths were elevated in a small population of people
living on an island where there were rich manganese deposits (Kilburn 1987); however, the lack of
exposure data, the small sample sizes, and the absence of a suitable control group preclude ascribing these
effects to manganese. The route of exposure was assumed to be primarily oral, but inhalation exposure
was not ruled out.
Potential developmental effects of manganese were suggested by the results of a study by Hafeman et al.
(2007), where high infant mortality in a Bangladesh community was reported in conjunction with the
presence of a local drinking water supply containing high levels of manganese. The Health Effects of
Arsenic Longitudinal Study (HEALS) was conducted on 11,749 participants 18–70 years of age living in
Araihazar, Bangladesh. Data on the reproductive history of the 6,707 women in this population were
collected and samples were taken of drinking water from all of the wells in the study region. Manganese
concentrations were determined for a total of 1,299 wells, representing the drinking water supply of
3,824 infants <1 year old. Eight-four percent of infants were exposed, directly or through maternal
intake, to water manganese levels above 0.4 mg/L with manganese concentrations ranging from 0 to
8.61 mg/L, for an average calculated daily intake of 0.26 mg manganese/kg/day. Of the 3,837 children
born to women who reported to drink from the same well for most of their childbearing years, 335 of
them died before reaching 1 year of age. Infants exposed to greater than or equal to the 0.4 mg/L WHO
(2004b) standard for manganese in drinking water had an elevated mortality risk during the first year of
life compared to unexposed infants (OR=1.8; 95% CI, 1.2–2.6). Adjustment for water arsenic indicators
of social class and other variables and potential confounders did not appreciably alter the results. When
the population was restricted to infants born to recently married parents (marriage year 1991 or after), the
elevation was larger (OR=3.4; 95% CI, 1.5–7.9). Although the results of the study suggest that the
presence of high levels of manganese in the water may be responsible for the high infant mortality
observed here, information provided by the authors on mechanism of manganese exposure suggests that
infant exposure to the high levels of manganese in the water may be complex (i.e., would likely require
direct rather than indirect or fractionated exposure, such as that occurring through breast milk or by
in utero exposure). The authors also indicate that it is not possible to infer that the manganese is solely
responsible for the high rate of infant mortality documented in this study
MANGANESE
198
3. HEALTH EFFECTS
Similarly, results from a pilot ecologic study in North Carolina suggest an association between increased
risk for infant mortality and increased groundwater manganese concentration (Spangler and Spangler
2009). County-level infant mortality rates and the percent of low birth weight births were obtained from
the North Carolina Center for State Health Statistics database, combined for the years 1997–2001.
Groundwater concentration values ranging from 0.003 to 0.346 mg/L (mean, 0.078 mg/L) were obtained
from the North Carolina Geological Survey groundwater database, which contains 5,778 samples from all
100 counties in North Carolina from 1973 and 1979. Analysis revealed that county-level infant mortality
rates were significantly (p<0.05) associated with logarithmically transformed groundwater manganese
concentration. With every log increase in concentration, there was a 2.074 increase in county level infant
deaths per 1,000 live births. Percent of babies born with low birth weight was not associated with
manganese groundwater concentration. However, as in Hafeman et al. (2007), potential sources of
manganese exposure (dietary, inhalation) and potential confounders were not examined. Taken together,
these two studies provide inadequate evidence to establish a causal relationship between elevated
manganese exposure in drinking water and increased infant mortality rates.
As discussed in Section 3.2.2.4, several studies have evaluated adverse neurological results in children
with increased oral exposure to manganese and/or elevated hair or blood concentration of manganese
(where the route of exposure is presumed to be mainly oral). Several studies have reported an inverse
relationship between manganese exposure in school-aged children and intellectual function (Bouchard et
al. 2011; Kim et al. 2009; Wasserman et al. 2006, 2011). Elevated manganese exposure has also been
associated with poor performance on the WHO neurobehavioral core tests (the emotional status test was
omitted) (He et al. 1994; Zhang et al. 1995), increased oppositional behavior and hyperactivity (Bouchard
et al. 2007c), and ADHD (Farias 2010). Additionally, elevated manganese levels in 12-month-old infants
were associated with decreased mental development scores (Claus Henn et al. 2010). Although observed
effects in these studies cannot be causally linked to manganese exposure exclusively, taken together, they
support the hypothesis that oral exposure to elevated manganese may be detrimental to
neurodevelopment.
Standard Developmental Studies in Animals—Oral Exposure to Inorganic Manganese. In animals,
standard developmental toxicity studies have not found distinct effects on fetal survival, gross fetal
malformations, or skeletal or visceral malformations or alterations. For example, acute administration of
manganese chloride by gavage to pregnant rats at a dose of 22 mg manganese/kg/day on GDs 6–
17 resulted in no adverse fetal developmental effects, measured as weight gain, gross malformations, or
skeletal malformations (Grant et al. 1997a). In another study, Szakmáry et al. (1995) studied the
MANGANESE
199
3. HEALTH EFFECTS
developmental toxicity of manganese in the rabbit and rat. The metal, as manganese chloride, was
administered by gavage during the whole period of gestation in the rat, and during organogenesis (day 6–
20) in the rabbit at concentrations of 0, 11, 22, and 33 mg/kg/day. In the rabbit, manganese treatments did
not result in decreases in fetal weights, skeletal retardation, or extra ribs, or in an increase in fetuses
afflicted with major anomalies. In the rat, the highest dose resulted in retardation of development of the
skeleton and internal organs. In addition, manganese at the highest dose caused a significant increase in
external malformations, such as clubfoot. However, when pups from dams treated at the same dose were
allowed to grow for 100 days after birth, no external malformations were observed, indicating that these
effects were self-corrected. No significant differences were found in any of the groups concerning the
development of the ears, teeth, eyes, forward motion, clinging ability, body posture correction reflex, or
negative geotaxis reflex.
Reproductive Development Studies in Animals—Oral Exposure to Inorganic Manganese. Several
animal studies of the effects of manganese on reproductive development show developmental effects
(Gray and Laskey 1980; Laskey et al. 1985, 1982).
One study involved pre-weanling mice (Gray and Laskey 1980) that were fed 1,050 mg manganese/
kg/day (as manganese tetroxide) beginning on PND 15. On days 58, 73, and 90, mice were sacrificed and
reproductive organ (preputial gland, seminal vesicle, and testes) weights and body weights were
measured. The manganese decreased the growth of these reproductive organs, but had no effect on body
growth or liver or kidney weights.
In another study, Laskey et al. (1982) evaluated the effect of dietary manganese exposure on rats during
gestation and continued during nursing and after weaning at doses of 0, 350, 1,050 or 3,500 mg
manganese/kg/day. The manganese was given in combination with either 20 or 200 mg iron/kg/day (the
former is deficient in iron, the latter is adequate). Manganese treatment was lethal at the highest dose in
the iron-deficient diet, but had no effect on male or female body weight at any age in animals receiving an
iron-sufficient diet. In the iron-poor diet, body weights of males were significantly depressed (p<0.05)
through day 100 of the study, whereas the females’ body weights were depressed only through day 60.
Select females and males were mated at day 90–100 of the study and the reproductive outcomes were
analyzed. The manganese treatment did not have any significant adverse effects at any dose except to
significantly decrease the number of pregnancies at the highest dose (p<0.05). Litter size, ovulations,
resorptions, preimplantation deaths, and fetal weights were unaffected by the metal. Testes weights in
males were significantly decreased from controls only when administered manganese in conjunction with
MANGANESE
200
3. HEALTH EFFECTS
an iron-poor diet: at day 40 at 1,050 and 3,500 mg manganese/kg/day and at day 100 at 1,050 mg/kg/day.
Hormone levels in male rats were also evaluated. No effect was seen from manganese treatment in
40-day-old male rats. At 60–100 days of age, however, dose-related decreases in serum testosterone were
observed, when age-related increases were expected and no increase in serum LH was observed.
Manganese given in both iron regimens prevented the normal decrease in serum follicle-stimulating
hormone (FSH) from 60 to 100 days. Manganese decreased epididymal sperm count only when given
with the iron-poor diet as measured at 100 days.
A third study involved gavage administration of 0, 71, or 214 mg manganese/kg/day (as manganese
tetroxide) to pre-weanling rats from birth to 21 days of age (Laskey et al. 1985). Functioning of the
hypothalamus, pituitary, and testicular tissues were measured by assaying endogenous or stimulated
serum concentrations of FSH, LH, and testosterone at days 21 or 28. No manganese-related effects were
observed on unstimulated or stimulated FSH or LH serum levels. In addition, manganese did not affect
endogenous or acute hCG-stimulated serum testosterone concentrations but did decrease serum
testosterone level following chronic hCG stimulation. Liver and hypothalamic manganese concentrations
were significantly increased in treated rats given the 71 mg/kg/day dose over controls. The authors
hypothesized that the manganese had an unknown affect on the testicular Leydig cell that resulted in the
delayed production of testosterone. This delayed production was presumably causing the delayed
reproductive maturation seen in the earlier study (Gray and Laskey 1980), but was not enough to affect
fertility outcomes at doses as high as 1,050 mg/kg/day (Laskey et al. 1982).
Neurodevelopmental Studies in Animals—Oral Exposure to Inorganic Manganese. As discussed in
Section 3.2.2.4, numerous studies have reported altered neurochemistry and/or neurobehavior following
neonatal or juvenile manganese exposure (Anderson et al. 2007a, 2009; Chandra and Shukla 1978;
Deskin et al. 1981; Dorman et al. 2000; Golub et al. 2005; Kern and Smith 2011; Kern et al. 2010;
Kristensson et al. 1986; Moreno et al. 2009; Reichel et al. 2006; Tran et al. 2002a, 2002b). Similarly,
many animal studies have examined neurological end points in animals repeatedly exposed during
gestation or through gestation into early postnatal development. End points evaluated include
neurochemistry (Lai et al. 1984), neurobehavior (Ali et al. 1983a; Pappas et al. 1997), and neuropathology
(Lazrishvilli et al. 2009; Pappas et al. 1997). Additionally, two studies have evaluated the potential
relationship between altered iron metabolism following manganese exposure and changes in
neurochemistry (Garcia et al. 2006) and neurobehavior (Molina et al. 2011). While results from these
studies are varied and inconsistent, taken together, the weight-of-evidence suggests that excess
MANGANESE
201
3. HEALTH EFFECTS
manganese exposure during development can lead to alterations in brain chemistry and behavioral
development.
Lai et al. (1984) studied the effect of chronic dosing of 40 mg manganese/kg/day (as manganese tetroxide
given in drinking water) to neonatal rats that were exposed from conception, throughout gestation, and up
to 2 years of age. The authors found that manganese treatment led to small decreases in choline
acetyltransferase activities in cerebellum and midbrain of 2-month-old rats. The regional distribution of
glutamic acid decarboxylase or acetylcholinesterase was unchanged.
Ali et al. (1983a) conducted a gestational study investigating the neurological effects of excess
manganese in drinking water on rats maintained on either a normal or low-protein diet. Manganese
exposure originated 90 days prior to mating and continued throughout gestation and nursing. The
offspring of rats who drank the equivalent of 240 mg manganese as manganese chloride/kg/day had pups
with delayed air righting reflexes. No treatment-related effects were observed in body weight or brain
weight in pups from dams fed the normal amount of protein. Significant delays in age of eye opening and
development of auditory startle were observed only in the pups of dams fed protein-deficient diets.
An intermediate drinking water study in pregnant rats (Pappas et al. 1997) investigated the developmental
neurotoxicity of manganese chloride doses of either 120 or 620 mg manganese/kg/day given on GDs 1–
21. Following birth, the dams were continued on manganese until weaning at PND 22. When the dams
were removed, the pups were continued on the same manganese doses until PND 30. Male pups were
observed on several days subsequent to exposure in a number of behavioral tests that measured
spontaneous motor activity, memory, and cognitive ability. The manganese-treated rats’ performance
was not significantly different from control rats. Pups from the highest-dose group exhibited a
significantly decreased weight gain on several days post-dosing, as well as an increased activity level on
PND 17 that was no longer evident by PND 30. The high-dose rats were not overactive on other days,
and the decreased weight gain was resolved by PND 90. Neurochemical analyses of the brains from
treated pups indicated that brain manganese concentrations were significantly elevated in the high-dose
group, as compared to controls. Brain enzyme and dopamine concentrations were not significantly
different between groups, but cortical manganese concentrations were significantly elevated in the high-
dose group. Cortical thickness was significantly different in several areas of the brains of pups in the
high-dose group but was only found to be significantly different in one area of the low-dose group. The
significance of the cortical thinning is not clear.
MANGANESE
202
3. HEALTH EFFECTS
Lazrishvilli et al. (2009) reports gliosis following developmental exposure to manganese in three groups
of rat pups (12/group) from mothers receiving 0, 10, or 20 mg manganese chloride/kg/day (0, 4.4, or
8.7 mg manganese/kg/day) in feed for 15–20 days before pregnancy, during pregnancy, and for 1 month
after parturition. Pups were sacrificed on postnatal day 40, and brains were removed and fixed for
histological and morphological assessment and estimations of manganese content. Manganese was
significantly elevated in cerebral cortex in both treatment groups, but not other brain regions (striatum,
diencephalon, mesencephalon, or medulla oblongata). There was no difference in the number of neurons,
and only a small proportion (7–10%) of neurons showed significant damage (pyknotic and swollen cells).
However, there was a significant (p<0.05), dose-related increase in the number of glial cells and the glial
index throughout the brain (gliosis). This is in contrast to negative findings for gliosis in adult rats
following exposure to 147 mg manganese/kg/day for 2 or 4 weeks (Rivera-Mancía et al. 2009, see
Section 3.2.2.4).
Developmental Effects on Iron Metabolism in Animals—Oral Exposure to Inorganic Manganese. In a
longer-duration intermediate study, Jarvinen and Ahlström (1975) fed female rats up to 187 mg manga-
nese/kg/day (as manganese sulfate) for 8 weeks prior to conception. The rats were continued on
manganese treatment until the 21st day of gestation. The unborn pups from dams administered 94 mg
manganese/kg/day had significantly decreased weights as compared to the other groups. No gross
malformations were observed in the fetuses, and alizarin-stained bone preparations revealed no
abnormalities in any dose group. However, fetuses from dams fed the highest manganese dose had
significantly higher concentrations of manganese in their bodies than fetuses from the other groups.
These data indicate that a level of 187 mg manganese/kg/day overwhelmed the rat’s homeostatic control
of manganese and the metal accumulated in the fetus. The highest manganese dose also resulted in a
significant decrease in the iron content of the fetuses.
Garcia et al. (2006, 2007) studied the relationship between dietary manganese and dietary iron on brain
chemistry and neurotransmission. In one study, groups of 5–7 dams were fed diets containing 35 ppm
iron (control) or 8 mg manganese/kg/day and 35 ppm iron (manganese-supplemented) from GD 7 through
PND 7 (Garcia et al. 2006). On PND 4, pups born to control dams were pooled and randomly cross-
fostered to dams fed one of the two diets such that initial mean litter weights were equivalent. Pups were
exposed to each of these diets via maternal milk from PND 4 to 21 as well as via direct ingestion of chow
(beginning around PND 11) and were euthanized on PND 21. In the dams, the high manganese diet
induced changes in hematological parameters similar to those seen with iron-deficiency: 50% decrease in
plasma iron (without significant decreases in hemoglobin) and increased plasma transferrin and total iron
MANGANESE
203
3. HEALTH EFFECTS
binding capacity. Compared with controls, manganese-exposed pups showed decreased hemoglobin
(about 20%), decreased plasma levels of iron (about 70%), increased plasma tranferrin and total iron
binding capacity (about 10%), increased brain concentrations of manganese, chromium, and zinc,
decreased brain iron levels, increased protein expression of divalent metal transporter-1 (DMT-1) and
transferrin receptor (TfR) in all brain regions, increased GABA concentrations, and increased ratios of
GABA to glutamate concentrations. Because GABA is an inhibitory amino acid and glutamate is an
excitatory amino acid, the authors suggested that the manganese treatment induced enhanced inhibitory
transmission in the brain of the pups. The results indicate that manganese treatment altered transport and
distribution of iron in developing rat pups and induced perturbations in brain levels of the
neurotransmitter, GABA.
In a further study by Garcia et al. (2007), groups of 5–7 GD 7 timed-pregnant Sprague-Dawley rats were
fed one of three experimental diets: control (35 mg Fe/kg diet; 10 mg manganese/kg diet), low iron (3 mg
Fe/kg diet; 10 mg manganese/kg diet), or low iron with supplemented manganese (3 mg Fe/kg diet,
100 mg manganese/kg diet). On PND 4, pups born to the control dams were pooled and randomly
cross-fostered to dams fed one of the two iron-deficient diets, such that initial mean litter weights were
approximately equivalent. The pups received these diets via maternal milk from PND 4 to 21, at which
time the pups were sacrificed. Levels of essential metals in the brain were measured (in cerebellum,
cortex, hippocampus, striatum, and midbrain) by inductively coupled plasma-mass spectrometry.
Increases in brain levels in low iron/manganese-treated rats (compared to control and low iron) were seen
for the following metals: copper, manganese (~50%), chromium (~150%), cobalt (~150%), molybdenum
(~25%), zinc (~130%), aluminum (~130), and vanadium (~150%). A decrease in brain iron levels was
observed for low iron animals; low iron/manganese-treated rats had iron levels significantly higher than
the low iron animals.
Molina et al. (2011) investigated the effect of manganese exposure during development on iron
metabolism. Pregnant Sprague-Dawley rats (3/group) were exposed to 0 or 4.79 mg manganese/mL (as
manganese chloride in drinking water) from GD 1 through PND 24. Based on body weight and water
intake, study authors calculated daily manganese doses in the exposed group to be 565 and
1,256 mg/kg/day during gestation and lactation, respectively. Offspring were culled to 12 per dam at
PND 2, with approximate equal male to female ratio. Iron pharmacokinetics were measured in all pups
on PND 25, after which they were sacrificed to evaluate the intestinal expression of divalent metal
transporter 1 (DMT1), blood and brain levels of manganese, liver and brain levels of non-heme iron, and
blood zinc protoporphyrin levels. Overall tissue uptake of iron was lower and zinc protoporphyrin levels
MANGANESE
204
3. HEALTH EFFECTS
were significantly decreased in manganese-exposed pups, compared with controls. Intestinal absorption
of iron was not altered, nor was expression of duodenal DMT1 by manganese exposure. Hematocrit and
non-heme iron levels were not altered in exposed pups. Additionally, before sacrifice, 4 pups/sex/dam
were tested on the elevated plus apparatus on PND 24. Pups demonstrated significantly (p<0.05) lower
anxiety-related behavior on several measures from this paradigm. These finding indicate that
developmental exposure to manganese leads to alterations in anxiety-like behaviors, which may be
mediated through manganese-induced changes in iron metabolism.
Developmental Studies in Animals—Oral Exposure to MMT. No studies of developmental effects
following oral exposure to MMT in humans or animals were located.
3.2.2.7 Cancer
No studies were located regarding carcinogenic effects in humans after oral exposure to inorganic
manganese.
Chronic (2-year) feeding studies in rats and mice have yielded equivocal evidence for the carcinogenic
potential of manganese. For example, rats exposed to up to 232 mg manganese/kg/day as manganese
sulfate for 2 years showed no increases in tumor incidence (NTP 1993). Mice fed up to 731 mg
manganese/kg/day as manganese sulfate for 2 years had a marginally increased incidence of thyroid gland
follicular cell adenomas (high-dose animals) and a significantly increased incidence of follicular cell
hyperplasia (NTP 1993); this was considered by NTP to be "equivocal evidence of carcinogenic activity
of Mn(II) sulfate monohydrate in male and female B6C3F
1
mice" (there was "no evidence of carcinogenic
activity" in rats in this study).
No studies were located regarding carcinogenic effects in humans or animals following oral exposure to
MMT.
3.2.3 Dermal Exposure
For inorganic manganese compounds, dermal exposure is not a typical pathway of exposure because
manganese does not penetrate the skin readily. For organic manganese, dermal exposure is a possibility
with all compounds discussed in this profile. This exposure pathway is most likely, however, with MMT,
where occupational workers (mechanics, workers in the gasoline industry, pesticide manufacturers and
sprayers) are likely to handle large quantities of these compounds.
MANGANESE
205
3. HEALTH EFFECTS
No studies were located regarding the any health effects in humans or animals after dermal exposure to
inorganic manganese.
3.2.3.1 Death
No studies were located regarding death in humans from dermal exposure to MMT.
Hinderer (1979) reported LD
50
values for rabbits (strain and sex were unreported) that were administered
varying doses of “neat” commercial MMT on abraded skin in the trunk area for 24 hours. These values,
generated by four different laboratories, ranged from 140 to 795 mg/kg. Although this dose range is
wide, the author reported that it was analogous to the wide oral LD
50
range given for the compound in
other reports.
3.2.3.2 Systemic Effects
Respiratory Effects. No studies were located regarding respiratory effects in humans or animals
following dermal exposure to MMT.
Cardiovascular Effects. No studies concerning cardiovascular effects following dermal exposure to
MMT in humans or animals were located.
Gastrointestinal Effects. No studies were located regarding gastrointestinal effects in humans
following dermal exposure to MMT. Hinderer (1979) observed bloody diarrhea in rabbits exposed
dermally to MMT; the compound was obtained as commercial grade, “neat,” and applied to shaved skin
for 24 hours. No histopathology was performed to ascertain the presence of lesions on the gastrointestinal
tract.
Hematological Effects. No studies were located regarding hematological effects in humans or
animals following dermal exposure to MMT.
Musculoskeletal Effects. No studies regarding musculoskeletal effects in humans or animals
following dermal exposure to MMT were located.
MANGANESE
206
3. HEALTH EFFECTS
Hepatic Effects. Hinderer (1979) observed that rabbits that underwent dermal application of a
commercial “neat” solution of MMT for 24 hours on shaved skin had discoloration of the liver and
swollen liver. No histopathology was performed.
Renal Effects. Hinderer (1979) observed that rabbits that underwent dermal application of a
commercial “neat” solution of MMT for 24 hours on shaved skin had discoloration of the kidneys and
swollen and congested kidneys. No histopathology was performed.
Endocrine Effects. No studies were located regarding endocrine effects in humans or animals
following dermal exposure to MMT.
Dermal Effects. No studies were located regarding dermal effects in humans following dermal
exposure to MMT. Hinderer (1979) observed that rabbits exposed dermally to commercial “neat” MMT
on shaved skin for 24 hours developed edema and erythema. Further dermal irritation tests performed
showed that MMT is a moderate skin irritant. Campbell et al. (1975) exposed male albino rats dermally
to MMT for 24 hours on closely clipped dorsolateral aspects of the trunk that were either abraded or
allowed to remain intact; skin reactions were evaluated and scored at 24 hours and again 48 hours later.
By comparing skin reactions following exposure to a test rating that categorized irritancy levels, MMT
was determined to be safe for intact or abraded skin contact. However, the authors note that MMT in
concentrated form is absorbed through the skin, and dermal absorption or interactions with other materials
or factors were not incorporated into their study.
Ocular Effects. No studies were located regarding ocular effects in humans or animals following
dermal exposure to inorganic manganese.
Hinderer (1979) performed a standard Draize irritation test with commercial “neat” MMT in rabbits and
found the compound not to be an eye irritant.
Body Weight Effects. No studies were located regarding body weight effects in humans or animals
following dermal exposure to inorganic manganese.
Rabbits exposed dermally to commercial “neat” MMT exhibited slight body weight loss, although the
actual amount was not reported (Hinderer 1979).
MANGANESE
207
3. HEALTH EFFECTS
Metabolic Effects. No studies were located regarding metabolic effects in humans or animals
following dermal exposure to inorganic manganese.
No studies were located regarding metabolic effects in humans or animals following dermal exposure to
MMT.
3.2.3.3 Immunological and Lymphoreticular Effects
No studies were located regarding immunological and lymphoreticular effects following dermal exposure
to inorganic manganese in either humans or animals.
No studies regarding immunological and lymphoreticular effects following dermal exposure to MMT in
humans or animals were located.
3.2.3.4 Neurological Effects
No studies were located regarding neurological effects following dermal exposure to inorganic
manganese in either humans or animals.
Rabbits exposed to “neat” commercial grade MMT on shaved areas of their trunks for 24 hours
experienced the following reported symptoms: polypnea, vocalization, excitation, ataxia, tremors,
cyanosis, and convulsions (Hinderer 1979).
3.2.3.5 Reproductive Effects
No studies were located regarding reproductive effects in humans or animals following dermal exposure
to inorganic manganese.
No studies were located regarding reproductive effects in humans or animals following dermal exposure
to organic manganese.
3.2.3.6 Developmental Effects
No studies were located regarding reproductive effects in humans or animals after dermal exposure to
inorganic manganese.
MANGANESE
208
3. HEALTH EFFECTS
No studies were located in humans or animals concerning developmental effects following dermal
exposure to MMT.
3.2.3.7 Cancer
No studies were located regarding carcinogenic effects in humans or animals after dermal exposure to
inorganic manganese.
No studies were located regarding carcinogenic effects in humans or animals after dermal exposure to
MMT.
3.2.4 Diagnostic Uses
Manganese is a paramagnetic element that can contain up to five unpaired electrons in its ionic form. The
unpaired electrons can facilitate T1 relaxation (in MRI) by interacting with hydrogen nuclei of water
molecules (Earls and Bluemke 1999). This T1 relaxation provides a contrast in signal during MRI from
normal cells and tumor cells because normal cells will take up the metal, whereas the cancerous cells take
up little or no manganese (Toft et al. 1997a). The Mn
2+
ion is the ion of choice because it is most readily
found in the body. However, because increased amounts of other sources of Mn
2+
, especially manganese
chloride, were found to have a high acute toxicity (as discussed in the previous sections), it is necessary to
chelate the Mn
2+
ion with another molecule that might decrease the toxic nature of the free ion. One such
chelate is the fodipir molecule, or dipyridoxal diphosphate. The result is mangafodipir, Mn(II)-N,N’-di-
pyridoxylethylendiamino-N,N;-diacetate-5,5'-bis(phosphate), or manganese dipyridoxal diphosphate
(MnDPDP). This clinical imaging agent is primarily used in the detection of hepatobiliary tumors, as it is
preferentially taken up by parenchymatous cells. However, as other organs have parenchymatous cells,
the compound is also useful in the detection of kidney, pancreas, and adrenal gland tumors (Earls and
Bluemke 1999).
This section will discuss the adverse effects of administration of mangafodipir. This section will not
discuss the efficacy of mangafodipir as a contrast agent in the identification of abdominal cancer.
Because this compound is used primarily in the detection of liver and other parenchymatous tumors, it is
found exclusively in hospitals and other clinical settings. It is only administered intravenously; therefore,
all subsequent studies discussed entail an intravenous exposure route. Because the toxicity of
MANGANESE
209
3. HEALTH EFFECTS
mangafodipir is mediated by manganese, the doses will be in mg manganese/kg body weight, rather than
in terms of the parent compound.
3.2.4.1 Death
There are no reports of lethality in humans following administration of mangafodipir.
Administration of mangafodipir can occur either all at once (bolus) or over a specific timed period
necessary to give the entire amount of a precalculated dose (slow infusion). The latter method has been
found to be better tolerated in a clinical setting (Bernardino et al. 1992; Lim et al. 1991; Padovani et al.
1996).
Mangafodipir was found to cause lethality in both sexes of Swiss-Webster mice with an LD
50
of 2,916 mg
manganese/kg after slow infusion of 15 seconds (Larsen and Grant 1997). The compound had an LD
50
of
103 mg/kg in both sexes of the same rodent when administered in a bolus dose (Larsen and Grant 1997),
showing the increased toxicity in the bolus administration. When given as a slow infusion over 5 minutes
in both sexes of the CD-1 mouse, the compound had an LD
50
value of 157 mg/kg, and when given at a
rate of 1.2 mL/second in BOM:NMRI male mice, the LD
50
was 211 mg/kg. In another study, the LD
50
in
both sexes of the Swiss-Webster mouse was found to be 290 mg/kg, when given as a slow infusion over
approximately 2.5 minutes (Elizondo et al. 1991). One male and one female beagle dog given a single
slow infusion (lasting ~110 seconds) of 160 mg/kg mangafodipir, as well as the one male given
120 mg/kg, died prior to the second day of the experiment; the remaining female given 120 mg/kg was
sacrificed due to a moribund condition on day 3 of the experiment (Larsen and Grant 1997). Dogs of both
sexes given 83 or 99 mg/kg survived the 14-day observation period. A single slow infusion (lasting
5 minutes) at a dose of 160 mg/kg did not result in lethality in the Sprague-Dawley rat (Larsen and Grant
1997).
Death was not observed in Sprague-Dawley rats administered nine doses of 16 mg manganese/kg/day (as
mangafodipir) given over 3 weeks (Elizondo et al. 1991; Larsen and Grant 1997). Moribund condition
prompted the sacrifice of one male and one female beagle dog on days 12 and 21, respectively, of a
21-day exposure period in which the animals were administered 5.4 mg/kg/day manganese (as
mangafodipir), whereas a lower dose of 1.6 mg/kg/day did not result in death or sacrifice of any treated
dogs (Larsen and Grant 1997). Moribund condition also prompted the sacrifice of a single male
Cynomolgus monkey on day 18 of a mangafodipir-dosing regimen involving 16 mg manganese/kg/day
MANGANESE
210
3. HEALTH EFFECTS
doses also given 3 times/week for 3 weeks (Larsen and Grant 1997). The authors did not indicate the
precise cause of lethality in the sacrificed dogs; however, they noted the dogs’ livers showed histological
signs of cholangiohepatitis, fibroplasia, bile duct proliferation, and hepatocyte necrosis, with cortical
tubular necrosis in the kidneys. The sacrificed monkey had a serum chemistry profile indicative of renal
failure and associated liver toxicity.
3.2.4.2 Systemic Effects
Respiratory Effects. No reports were located concerning respiratory effects in humans following
dosing with mangafodipir.
A single dose of 160 mg manganese/kg as mangafodipir in Sprague-Dawley rats of both sexes resulted in
dyspnea (Larsen and Grant 1997).
Cardiovascular Effects. Mangafodipir, when administered to humans in timed doses in clinical
studies has resulted in transient facial flushing and increased blood pressure at doses as low as 0.2 mg
manganese/kg (facial flushing) (Bernardino et al. 1992; Lim et al. 1991; Padovani et al. 1996; Wang et al.
1997).
Slow infusion of mangafodipir at doses of 16.5 mg manganese/kg resulted in no cardiotoxicity in mongrel
dogs of either sex (Karlsson et al. 1997). The dogs suffered from medically-induced acute ischaemic
heart failure; cardiotoxicity was measured as the depression of cardiovascular function, with specific
measured end points being aortic pressure, pulmonary artery pressure, right atrial pressure, cardiac output,
and heart rate (Karlsson et al. 1997). Sprague-Dawley rats suffered no cardiotoxicity (as measured by
histomorphological evaluation) after a single administration of mangafodipir at doses as high as 63 mg/kg
(Larsen and Grant 1997).
Rats administered nine doses (3 times/week for 3 weeks) of 16 mg manganese/kg did not suffer any
adverse cardiovascular effects as measured by histomorphological analyses (Larsen and Grant 1997).
Twenty-one days of daily administration of 5.4 mg manganese/kg in beagle dogs resulted in reduced heart
rate by the end of the treatment (Larsen and Grant 1997). Cynomolgus monkeys administered 16 mg/kg
for 3 days/week for 3 weeks resulted in flushing of the face, but no other measured cardiovascular effects
(Larsen and Grant 1997).
MANGANESE
211
3. HEALTH EFFECTS
Gastrointestinal Effects. Incidences of gastrointestinal effects in humans following injection with
mangafodipir have been limited to rare complaints of nausea or vomiting that are short-lived (15 seconds
to 5 minutes in length) and not dose- or administration rate-dependent (bolus vs. infusion) (Bernardino et
al. 1992; Lim et al. 1991; Padovani et al. 1996; Wang et al. 1997). A dose of 81 mg manganese/kg as
mangafodipir in beagle dogs of both sexes resulted in vomiting, diarrhea, and decreased food
consumption (Larsen and Grant 1997).
Vomiting was observed in Cynomolgus monkeys of both sexes after administration of nine doses of
16 mg manganese/kg, given 3 times/week for 3 weeks (Larsen and Grant 1997). No other gastrointestinal
effects in animals were reported.
Hematological Effects. No hematological changes (versus pretreatment values) were noted in three
different studies that included 13 healthy males (Wang et al. 1997), 54 healthy males (Lim et al. 1991), or
96 human volunteers of both sexes with known or suspected focal liver tumors (Bernardino et al. 1992)
administered up to 1.4 mg manganese/kg as mangafodipir (either via bolus or slow infusion).
A single dose of 63 mg manganese/kg as mangafodipir in both sexes of Sprague-Dawley rats resulted in
no adverse hematological effects (Larsen and Grant 1997). Intermediate studies of adverse effects were
also negative. Doses as high as 16 mg/kg given 3 times/week for 3 weeks to Sprague-Dawley rats
(Elizondo et al. 1991; Larsen and Grant 1997) or Cynomolgus monkeys, or 5.4 mg/kg in beagle dogs
dosed daily for 21 days, failed to induce any adverse hematological effects (Larsen and Grant 1997).
Musculoskeletal Effects. No reports of musculoskeletal effects in humans or animals following
mangafodipir administration were located.
Hepatic Effects. Blood chemistry analyses revealed no significant changes in liver enzymes in
several volunteers, either with or without tumors, given mangafodipir at doses up to 1.4 mg
manganese/kg (Bernardino et al. 1992; Lim et al. 1991; Wang et al. 1997). Three individuals dosed with
0.55 mg manganese/kg and one dosed with 1.4 mg/kg had increased serum alanine aminotransferase;
however, there was no dose response with these results and the maximum increase in the enzyme was to
70 International Units (IU)/l (the upper limit of the normal range is 45 IU/l) (Lim et al. 1991).
A single dose of up to 63 mg manganese/kg administered to both sexes of Sprague-Dawley rats did not
produce any adverse hepatic effects as observed by histomorphological analyses (Larsen and Grant 1997).
MANGANESE
212
3. HEALTH EFFECTS
The administration of nine total doses of mangafodipir, three per week, at 16 mg manganese/kg/day per
dose, resulted in an increased incidence (relative amount unreported) in hepatic microgranulomas in
female Sprague-Dawley rats, but no effect on liver enzymes as measured by serum chemistry (Elizondo et
al. 1991; Larsen and Grant 1997). Twenty-one daily doses of 1.6 mg/kg/day resulted in an increase in
serum enzymes (alanine aminotransferase, ornithine carbamyl transferase, glutamine dehydrogenase,
alkaline phosphatase, gamma-glutamyl transferase), as well as bilirubin and cholesterol, in both sexes of
beagle dogs, while a higher dose of 5.5 mg/kg/day resulted in increased liver enzymes and liver weight
and changes in liver pathology (cholangiohepatitis, fibroplasia, bile duct proliferation, and hepatocyte
necrosis) (Larsen and Grant 1997). The authors noted that altered serum albumin:globulin ratios and
increased prothrombin time were indicative of decreased liver protein synthesis. When dogs at this high
dose were allowed a 4-week recovery period, healing of the liver was observed; specific measures of
healing were not provided, although resolution of lesions in other affected organs, such as the kidneys,
was mentioned. The authors also noted that increased serum levels of liver enzymes and decreased liver
protein synthesis were reversible effects in dogs allowed a recovery period. Doses of 0.54 mg/kg/day did
not have any effect on the liver (Larsen and Grant 1997). In both sexes of the Cynomolgus monkey, nine
total doses of 16 mg/kg/day given 3 times/week for 3 weeks, resulted in increases in liver enzymes
(alanine aminotransferase, gamma-glutamyl transferase), as well as increases in bilirubin and relative
liver weights in males, and focal hepatitis/cholangiolitis in one male at the end of the dosing period.
When the monkeys were given a 2-week recovery period following a 3-week administration of the
highest dose, only one male had a liver lesion, which was in the process of healing. Doses of
1.6 mg/kg/day in this primate did not cause any adverse hepatic effects (Larsen and Grant 1997).
Renal Effects. Administration of mangafodipir at up to 1.4 mg manganese/kg in a few human studies
has not resulted in any adverse renal effects as measured by blood chemistry or urinalysis (Bernardino et
al. 1992; Wang et al. 1997).
Single doses of mangafodipir up to 63 mg manganese/kg given to Sprague-Dawley rats did not cause
renal effects as measured by blood chemistry, urinalysis, gross necropsy, and histopathology (Larsen and
Grant 1997). Sprague-Dawley rats of both sexes given nine doses (thrice weekly for 3 weeks) of
16 mg/kg manganese did not show any adverse renal effects as measured by urinalysis, blood chemistry,
and histomorphological analysis (Elizondo et al. 1991; Larsen and Grant 1997). Daily administration of
mangafodipir over 21 days in both sexes of the beagle dog at concentrations up to 6 mg/kg resulted in
cortical tubular necrosis of the kidneys at this highest dose, as well as decreased glomerular filtration rate,
as indicated by high serum carbamide and creatinine levels. There were no measurable effects at
MANGANESE
213
3. HEALTH EFFECTS
≤1.6 mg/kg (Larsen and Grant 1997). Administration of nine doses of mangafodipir, also given thrice
weekly for 3 weeks, at individual concentrations of 16 mg manganese/kg to Cynomolgus monkeys of
both sexes resulted in increased kidney weights and enzymes, as well as creatinine, urea, and other
inorganic ions. Doses of 1.6 mg/kg over the same time period did not result in any adverse effect (Larsen
and Grant 1997).
Endocrine Effects. No studies were located regarding endocrine effects in humans or animals
following administration of mangafodipir.
Dermal Effects. No studies were located regarding dermal effects in humans or animals following
intravenous administration of mangafodipir.
Ocular Effects. No studies were located concerning ocular effects in humans following
administration of mangafodipir.
Cynomolgus monkeys administered nine individual doses at 16 mg/kg over 3 weeks and beagle dogs
given up to 6 mg/kg daily for 21 days did not have any adverse ocular effects from the mangafodipir
treatment (Larsen and Grant 1997).
Body Weight Effects. No reports were located concerning body weight effects in humans following
mangafodipir dosing.
Mice given acute doses of mangafodipir as high as 275 mg manganese/kg and rats administered a dose of
160 mg/kg did not suffer any body weight effects (Larsen and Grant 1997).
Rats (Elizondo et al. 1991; Larsen and Grant 1997) and monkeys (Larsen and Grant 1997) administered
nine doses of mangafodipir over 3 weeks at doses as high as 16 mg manganese/kg did not have any
treatment-related effects on body weight. Dogs administered 21 daily doses of the compound suffered
decreased body weight (unspecified decrease) at 5.4 mg/kg, but no effect at 1.6 mg/kg (Larsen and Grant
1997). There were no significant treatment-related adverse effects on body weight of male and female
rats or female rabbits used in reproductive studies with mangafodipir (Blazak et al. 1996; Grant et al.
1997a; Treinen et al. 1995), except for a transient decrease in body weight during weeks 2–5, 9, and 10 in
male rats administered 6 mg manganese/kg/day for 85 days (Grant et al. 1997a). The authors noted that
the decrease was significant when compared to controls, but did not report actual data.
MANGANESE
214
3. HEALTH EFFECTS
Metabolic Effects. No studies were located regarding metabolic effects in humans or animals
following administration of mangafodipir.
3.2.4.3 Immunological and Lymphoreticular Effects
No studies were located regarding immunological or lymphoreticular effects in humans following
exposure to mangafodipir.
Injection of mangafodipir 3 times/week for 3 weeks in Sprague-Dawley rats at doses of 1.6, 6.3, or 16 mg
manganese/kg resulted in eosinophilia in females only at the highest dose, but had no effect in males. The
authors stated they are unsure of the clinical importance of this effect as it was only seen at repeated high
doses (Larsen and Grant 1997). Daily dosing of mangafodipir in beagle dogs of both sexes at doses of
1.6 mg manganese/kg for 21 days resulted in a decrease in eosinophils and an increase in toxic
neutrophils (absolute amounts not reported) (Larsen and Grant 1997). A lower dose of 0.54 mg/kg had no
immunological effect.
3.2.4.4 Neurological Effects
No statistically significant increases in adverse neurological effects in humans following mangafodipir
administration were reported. In one study, four subjects given doses ranging from a low of 0.17 mg/kg
to a high of 1.4 mg/kg complained of light-headedness or dizziness (Lim et al. 1991). Five of 96 patients
administered mangafodipir complained of a headache following dosing; only two of these five, given
varying doses of mangafodipir ranging from 0.17 to 1.4 mg manganese/kg, could be attributed to the
contrast agent (Bernardino et al. 1992). No other neurological effects were reported in human studies.
Single doses of mangafodipir ranging from 8.3 to 275 mg manganese/kg in mice and a single dose of
160 mg/kg in rats, resulted in decreased activity and abnormal gait and stance (Larsen and Grant 1997).
Mongrel dogs infused once with mangafodipir at doses of 0.55, 3.3, or 16.5 mg manganese/kg did not
have any treatment-related changes in plasma catecholamines or physiological signs of sympathetic
activation as compared to the undosed controls (Karlsson et al. 1997). In a separate study, beagle dogs
receiving either single doses ranging from 83 to 160 mg/kg or 21 daily doses at 5.4 mg manganese/kg
suffered decreased appetite as measured by decreased food consumption; when the dogs were allowed a
recovery period following the repeated dosing, the food consumption normalized within the first 2–3 days
(Larsen and Grant 1997).
MANGANESE
215
3. HEALTH EFFECTS
Rats and monkeys administered nine doses of up to 16 mg/kg each did not have any observable
neurotoxic effects (Larsen and Grant 1997).
Grant et al. (1997a) did observe behavioral changes in the pups of Sprague-Dawley dams exposed to 0,
0.6, 1.1, or 2.2 mg manganese/kg on GDs 6–17. Although no significant effects were observed at the
lowest dose, the exposed pups suffered a significant decrease in grasp/holding time and a 10–11%
decrease in body weight at PNDs 4 and 7 at the 1.1 mg/kg dose. At the highest dose, pup weight was
significantly decreased at PNDs 4, 7, 14, and 21; performance on grasp/holding, negative geotaxis, and
surface righting tests was also significantly impaired. In addition, postnatal survival was decreased on
days 0–4 (56 vs. 95.9% in the control group) and 4–21 (78.9 vs. 100% in the control group) at the highest
dose (Grant et al. 1997a).
Current studies do not provide evidence on the potential for neurotoxicity following clinical exposure to
mangafodipir. In general, studies on neurological effects in humans or animals following mangafodipir
exposure did not involve a long observation period. Because deposition of manganese in the brain can be
significantly delayed following exposure, it is possible that the studies to date were terminated prior to the
onset of potential neurotoxicity. However, neurotoxicity in humans or animals has not been reported
following single exposures to manganese, even at high doses. Studies on toxicokinetics of other
manganese compounds also indicate that a single exposure is not likely to result in significant
neurological effects. For further information on distribution, refer to Section 3.4 Toxicokinetics.
3.2.4.5 Reproductive Effects
No studies were located regarding reproductive effects in humans following administration of
mangafodipir.
A single dose of 160 mg/kg in male Sprague-Dawley rats resulted in no adverse effects in testes as
measured by organ weight and histomorphological analysis (Larsen and Grant 1997).
Male Sprague-Dawley rats dosed nine times in 3 weeks with 16 mg manganese/kg as mangafodipir
suffered a decrease in absolute testes weights, but no relative decrease in weight and no
histomorphological effects (Larsen and Grant 1997).
MANGANESE
216
3. HEALTH EFFECTS
Injection of pregnant Sprague-Dawley rats with up to 4.4 mg manganese/kg as mangafodipir, on GDs 6–
8, 9–11, 12–14, or 15–17 (all during organogenesis) resulted in no evidence of reproductive toxicity as
measured by pregnancy rate, numbers of corpora lutea, implantations or resorptions (Treinen et al. 1995).
Further, daily intravenous administration of doses up to 2.2 mg manganese/kg throughout GDs 6–17 did
not result in any significant changes in pregnancy rate, corpora lutea, implantations, or resorptions
(Treinen et al. 1995). However, Grant et al. (1997a) observed a >50% rate of post-implantation loss in
pregnant Sprague-Dawley rats administered 2.2 mg manganese/kg as mangafodipir during GDs 6–17.
Doses of 0.6 and 1.1 mg/kg resulted in postimplantation loss rates that were similar to that of the control
group. There were no obvious differences in compound administration or animal husbandry between the
two studies that would indicate why such disparate results would occur. Intravenous dosing of New
Zealand white rabbits with up to 1.1 mg manganese/kg/day on GDs 6–17 did not cause reproductive
toxicity in one study (Grant et al. 1997a), but a dose of 3.3 mg manganese/kg/day during GDs 6–18 in the
same species resulted in a significant increase (3-fold) in post-implantation loss (Blazak et al. 1996). This
latter dose corresponds to a 12-fold increase over the one-time human clinical dose (Earls and Bluemke
1999).
Mangafodipir dosing in female Sprague-Dawley rats for 22 total days, starting prior to conception and
ending on the 7
th
day of gestation at a dose of up to 6 mg manganese/kg, did not result in any adverse
reproductive effects (Grant et al. 1997a).
Male Sprague-Dawley rats dosed for 84–85 days with 0, 0.6, 2, or 6 mg manganese/kg as mangafodipir
did not show any signs of reproductive toxicity as measured by histomorphological analyses. Although
absolute testes weights in the intermediate dose group were reduced compared to controls, relative
weights were not, and in the absence of histopathological findings, this reduction is not considered an
adverse effect. The treated rats were bred with females to determine if mangafodipir dosing had any
effect on fertility. Pregnancy rates, and the number of corpora lutea, implantations, or resorptions were
unaffected by parental treatment (Grant et al. 1997a).
3.2.4.6 Developmental Effects
No studies were located regarding developmental effects in humans following intravenous exposure to
mangafodipir.
MANGANESE
217
3. HEALTH EFFECTS
Treinen et al. (1995) tested the sensitivity of different gestational periods to the administration of
mangafodipir in Sprague-Dawley rats. Pregnant rats were dosed with 0, 1.1, 2.2, or 4.4 mg manganese/kg
on 3 consecutive days: GDs 6–8, 9–11, 12–14, or 15–17. The 1.1 mg/kg dose given on days 15–17
resulted in a significant increase in skeletal malformations in fetuses (10/113 fetuses vs. 0/106 in the
control group; p<0.05). A higher dose of 2.2 mg/kg also caused a significant increase in malformations
when given on GDs 12–14 (10 out of 104 fetuses affected) and days 15–17 (21/143) (both p<0.05), and
the 4.4 mg/kg dose caused increases in malformations when given on days 9–11(5/83), 12–14 (45/128),
and 15–17 (98/129) (all p<0.05). The malformations seen in this study included angulated or irregularly
shaped clavicle, femur, fibula, humerus, ilium, radius, tibia, ulna, and/or scapula (Treinen et al. 1995).
The offspring of Sprague-Dawley rats dosed with 0, 0.1, 0.3, or 1 mg manganese/kg as mangafodipir
daily throughout GDs 6–17 had a significant increase (p<0.05) in abnormal limb flexures (38/270 fetuses
affected) and skeletal malformations (141/270 fetuses affected) only at the highest dose (Treinen et al.
1995). These malformations included the same ones listed for the segmented teratology study above. In
a separate experiment evaluating the teratology of mangafodipir administration on GDs 6–17 in pregnant
Sprague-Dawley rats, Treinen et al. (1995) observed a significant increase (p<0.05) in skeletal
malformations in offspring of rats dosed with 2.2 mg manganese/kg (86/92 fetuses affected) compared to
controls. In both the segmented and continuous teratology studies, no maternal toxicity was observed.
Fetuses from Sprague-Dawley females dosed with 0, 0.6, 1.1, or 2.2 mg manganese/kg on GDs 6–17
exhibited a statistically significant increase in wavy ribs at 0.6 mg/kg (20.5% of the viable fetuses
impacted vs. 0.7% at the control dose; p<0.05). At the intermediate dose, there was a statistically
significant increase in the number of fetuses with abnormalities (20 out of 159 viable fetuses) including
distortion or misshaping of one or more of the following bones: humerus, radius, ulna, scapula, clavicle,
femur, tibia, and fibula; in addition, 56.6% of the viable fetuses had wavy ribs and the fetuses weighed
14% less than controls (p<0.05). At 2.2 mg/kg, there was a significant decrease in fetal viability
(56% decrease; p<0.05), a greater increase in fetuses with abnormalities (45 out of 64 viable fetuses,) and
a greater percentage (85.9%) with wavy ribs (Grant et al. 1997a). These effects were observed in the
absence of maternal toxicity. By contrast, when the mangafodipir was administered for 22 days prior to
conception and up to GD 7 in the same species at doses of 0, 0.6, 2, and 6 mg manganese/kg/day, no
adverse effects on the number of viable fetuses, fetal weight, or the number of fetuses with abnormalities
were reported (Grant et al. 1997a). These teratogenic studies indicate that developmental toxicity
resulting from mangafodipir dosing is highly dependent on the time-frame of administration.
MANGANESE
218
3. HEALTH EFFECTS
Grant et al. (1997a) also observed behavioral changes in the offspring of Sprague-Dawley dams
administered 0, 0.6, 1.1, or 2.2 mg manganese/kg on GDs 6–17. The exposed pups suffered a significant
decrease in grasp/holding time and a 10–11% decrease in body weight at PNDs 4 and 7 at the 1.1 mg/kg
dose, but no significant effects at the lower dose (Grant et al. 1997a). At the highest dose, pup weight
was significantly decreased at PNDs 4, 7, 14, and 21, and performance on grasp/holding, negative
geotaxis, and surface righting tests was significantly impaired. In addition, postnatal survival was
decreased on days 0–4 (56 vs. 95.9% in the control group) and 4–21 (78.9 vs. 100% in the control group)
at the highest dose (Grant et al. 1997a). These effects occurred at doses that did not cause observable
maternal toxicity.
Mangafodipir administration in New Zealand white rabbits at doses of 0, 0.3, 0.55, or 1.1 mg
manganese/kg on GDs 6–18 resulted in incomplete ossification of the sternebrae at 1.1 mg/kg in one
study (Grant et al. 1997a), but no significant effects on fetotoxicity or fetal weight; this dose did not result
in any maternal toxicity. In a separate study, mangafodipir at doses as high as 3.3 mg manganese/kg in
the same strain of rabbit for the same period of exposure did not result in any significant increases in
external, skeletal, or visceral malformations in a separate teratology study (Blazak et al. 1996). This dose
did result in an 11% decrease in fetal weight (although this value was not statistically significant in the
study, it is considered a significant developmental effect) and a 20% decrease in the number of viable
fetuses (also not statistically significant). It is not readily apparent why two studies with similar dosing
regimens would obtain such conflicting results. A comparison between rat and rabbit gestational studies
indicates that the rabbit is a much less sensitive model for reproductive and developmental toxicity
induced by mangafodipir.
3.3 GENOTOXICITY
There is some evidence from a study on occupationally exposed welders that manganese may cause
chromosomal aberrations; the welders were exposed to other potentially toxic compounds including
nickel (known to cause chromosomal aberrations) and iron; therefore, the observed increase in
chromosomal aberrations cannot be attributed solely to manganese (Elias et al. 1989). Mutagenicity
studies in both bacteria and mammalian strains are equivocal. While manganese sulfate was shown to not
be mutagenic to Salmonella typhimurium strains TA97, TA98, TA100, TA1535, or TA1537 either in the
presence or absence of S9 from Aroclor 1254-induced liver from rats or Syrian hamsters (Mortelmans et
al. 1986), it was shown to be mutagenic to strain TA97 elsewhere (Pagano and Zeiger 1992). In yeast
(Saccharomyces cerevisiae strain D7), a fungal gene conversion/reverse mutation assay indicated that
MANGANESE
219
3. HEALTH EFFECTS
manganese sulfate was mutagenic (Singh 1984). Manganese chloride was reportedly not mutagenic in
S. typhimurium strains TA98, TA100, and TA1535, but it was mutagenic in strain TA1537, and
conflicting results were obtained for TA102 (De Méo et al. 1991; Wong 1988).
In vitro assays in mammalian cells also gave conflicting results concerning manganese mutagenicity.
Manganese chloride produced gene mutations in cultured mouse lymphoma cells (Oberly et al. 1982).
Manganese chloride caused DNA damage in vitro using human lymphocytes at a concentration of 25 µm
without metabolic activation, but not at the lower tested concentrations of 15 and 20 µm (Lima et al.
2008). The compound also caused DNA damage in human lymphocytes using the single-cell gel assay
technique in the absence of metabolic activation, but caused no DNA damage when S9 was present
(De Méo et al. 1991). Manganese sulfate induced sister chromatic exchange in Chinese hamster ovary
(CHO) cells in both the presence and absence of S9 from Aroclor 1254-induced rat liver (Galloway et al.
1987). In a separate assay, manganese sulfate also induced chromosomal aberrations in CHO cells in the
absence of S9 but not in its presence (Galloway et al. 1987). Manganese chloride caused chromosome
aberrations in human lymphocytes without metabolic activation, but only when treated in the G2 phase of
the cell cycle; treatment in the G1, G1/S, and S1 phases of the cell cycle did not result in chromosome
aberrations (Lima et al. 2008). The compound was also found to be clastogenic in root tip cells of Vicia
faba (Glass 1955, 1956), but not in cultured FM3A cells in the absence of metabolic activation (Umeda
and Nishimura 1979). Potassium permanganate caused chromosomal aberrations in FM3A cells (Umeda
and Nishimura 1979), but not in a primary culture of cells from Syrian hamster embryos when tested in
the absence of metabolic activation (Tsuda and Kato 1977). Manganese chloride caused cell
transformation in Syrian hamster embryo cells (Casto et al. 1979). A list of in vitro study results is given
in Table 3-5.
Manganese chloride did not produce somatic mutations in Drosophila melanogaster fruit flies in one
study (Rasmuson 1985), and manganese sulfate did not induce sex-linked recessive lethal mutations in
germ cells of male D. melanogaster (Valencia et al. 1985).
In vivo assays in mice showed that oral doses of manganese sulfate or potassium permanganate caused
micronuclei and chromosomal aberrations in bone marrow (Joardar and Sharma 1990). In contrast, oral
doses of manganese chloride did not cause chromosomal aberrations in the bone marrow or
spermatogonia of rats (Dikshith and Chandra 1978). A list of in vivo study results is given in Table 3-6.

MANGANESE
220
3. HEALTH EFFECTS
Table 3-5. Genotoxicity of Manganese In Vitro
Results
Species (test
system)
Compound
End point
Strain
With
activation
Without
activation
Reference
Inorganic manganese compounds
Prokaryotic
organisms:
Salmonella
typhimurium
(plate
incorporation
assay)
MnCl
2
Gene mutation
TA98 TA 102
TA1535
TA1537
–
–
–
–
–
–
–
+
Wong 1988
MnSO
4
Gene mutation
TA98, TA100,
TA1535,
TA1537, TA97
–
–
Mortelmans
et al. 1986
S. typhimurium
(preincubation
assay)
MnSO
4
Gene mutation
TA97
No data
+
Pagano and
Zeiger 1992
MnCl
2
Gene mutation
TA102
No data
+
DeMéo et al.
1991
TA100
No data
DeMéo et al.
–
1991
MnCl
2
Gene mutation
TA102
No data
+
DeMéo et al.
1991
TA100
No data
DeMéo et al.
–
1991
Photobacterium
fischeri
(bioluminescence
test)
MnCl
2
Gene mutation
(restored
luminecence)
Pf-13 (dark
mutant)
No data
+
Ulitzur and
Barak 1988
Escherichia coli
MnCl
2
Gene mutation
KMBL 3835
No data
+
Zakour and
Glickman
1984
Bacteriophage
(E. coli lysis)
MnSO
4
Gene mutation
T4
No data
+
Orgel and
Orgel 1965
Bacillus subtilis
(recombination
assay)
MnCl
2
Mn(NO
3
)
2
MnSO
4
Mn(CH
3
C00)
2
KmnO
4
Inhibition of
growth in
recombination
deficient mutant
(Rec
-
) compared
to wild type
(Rec
+
)
M45 (Rec
-
)
No data
+
+
+
+
–
Nishioka
1975
B. subtilis
(recombination
assay)
MnCl
2
Mn(NO
3
)
2
Mn(CH
3
C00)
2
Inhibition of
growth in
recombination
deficient mutant
M45 (Rec
-
)
No data
–
–
–
Kanematsu
et al. 1980
(Rec
-
) compared
to wild type
(Rec
+
)

MANGANESE
221
3. HEALTH EFFECTS
Table 3-5. Genotoxicity of Manganese In Vitro
Results
Species (test
With
Without
system)
Compound
End point
Strain
activation
activation
Reference
Eukaryotic organisms:
Fungi:
Saccharomeyces
MnSO
4
Gene
D7
No data
+
Singh 1984
cervisiae conversion,
reverse mutation
Mammalian cells:
Mouse lymphoma
cells
MnCl
2
Gene mutation
L5178Y TK+/-
No data
+
Oberly et al.
1982
Syrian hamster
MnCl
2
Enhancement of
No data
+
Casto et al.
embryo cells SA7 1979
transformation
Human
MnCl
2
DNA damage
lymphocyte
–
+
DeMéo et al.
lymphocytes 1991
(Single-cell gel
assay)
Chinese hamster
MnSO
4
Chromosomal
+
+
NTP 1993
ovary cells aberrations/
sister chromatid
exchange
Human
MnCl
2
Chromosomal
No data
+
Lima et al.
lymphocytes aberrations (G2 2008
phase)
Human
MnCl
2
DNA damage
No data
+
Lima et al.
lymphocytes
2008
Organic manganese compounds
Prokaryotic organisms:
E. coli and S.
MnDPDP
Gene mutation
E. coli:
–
–
Larsen and
typhimurium WP
2
uvrA
-
Grant 1997
S. typhimurium:
TA100,
TA1535, TA98,
TA1537
Eukaryotic organisms:
CHO cells
MnDPDP
Forward
–
–
Larsen and
mutation
Grant 1997
MnDPDP
Chromosomal
–
–
Larsen and
aberration
Grant 1997
– = negative result; + = positive result; ± = weakly positive result; CHO = Chinese hamster ovary;
DNA = deoxyribonucleic acid; Mn(CH
3
COO)
2
= manganese acetate; MnCI
2
= manganese chloride;
MnDPDP = mangafodipir; Mn(NO
3
)
2
= manganese nitrate; MnSO
4
= manganese sulfate; Rec = recombination
MANGANESE
222
3. HEALTH EFFECTS
Table 3-6. Genotoxicity of Manganese In Vivo
Species (test system)
Compound
End point
Route
Results
Reference
Inorganic manganese compounds
Nonmammalian systems:
Drosophila
melanogaster
MnSO
4
Sex-linked
recessive lethal
Feeding
injection
–
Valencia et al. 1985
D.melanogaster
MnCl
2
Somatic mutation
Soaking
larvae
–
Rasmuson 1985
Mammalian systems:
Albino rat
(bone marrow cells)
(spermatogonial cells)
MnCl
2
Chromosomal
aberrations
Oral
–
Dikshith and Chandra
1978
Albino mouse
MnSO
4
Chromosomal
aberrations
Oral
+
Joardar and Sharma
1990
Albino mouse
KMnO
4
Chromosomal
aberrations
Oral
+
Joardar and Sharma
1990
– = negative result; + = positive result; KMnO
4
= potassium permanganate; MnCl
2
= manganese chloride;
MnSO
4
= manganese sulfate
MANGANESE
223
3. HEALTH EFFECTS
The results of in vitro studies show that at least some chemical forms of manganese have mutagenic
potential. However, as the results of in vivo studies in mammals are inconsistent, no overall conclusion
can be made about the possible genotoxic hazard to humans from exposure to manganese compounds.
Genotoxicity data concerning MMT was not available.
One study was located regarding genotoxic effects in humans following inhalation exposure to
manganese. In this study, the incidences of chromosomal aberrations in three groups of welders with
occupational exposures (10–24 years) to metals including manganese, nickel, and chromium were
examined (Elias et al. 1989). An increase in chromosomal aberrations was found in the group working
with the metal active gas welding process; however, since their exposures included nickel as well as
manganese, the authors could not attribute the results to any one metal exposure (nickel is known to cause
chromosomal aberrations by the inhalation route). The median manganese concentrations during the
survey were 0.18 mg/m
3
for respirable dust and 0.71 mg/m
3
for total dust. No information was available
regarding the genotoxicity of manganese alone.
No studies were located regarding genotoxic effects in humans after oral exposure to inorganic
manganese.
In male Swiss albino mice, manganese sulfate and potassium permanganate have both been found to be
clastogenic, and their effects were found to be dependent primarily on the concentration (not duration) of
exposure (Joardar and Sharma 1990). In this in vivo study, oral doses were administered at varying levels
over a 3-week period. The manganese sulfate doses were 10.25, 20.25, and 61 mg/100 g body weight,
and the potassium permanganate doses were 6.5, 13, and 38 mg/100 g body weight. Sperm head
abnormalities and the frequency of chromosomal aberrations in bone marrow cells and micronuclei were
significantly increased. In male rats, repeated oral doses of 0.014 mg manganese/kg/day (as manganese
chloride) for 180 days did not produce any significant chromosomal damage in either bone marrow or
spermatogonial cells (Dikshith and Chandra 1978).
No studies were located regarding genotoxic effects in animals after inhalation exposure to inorganic
manganese.
No studies were located concerning genotoxic effects in humans or animals following inhalation or
exposure to MMT.
MANGANESE
224
3. HEALTH EFFECTS
3.4 TOXICOKINETICS
Manganese is required by the body and is found in virtually all diets. As discussed in Chapter 6, adults
consume between 0.7 and 10.9 mg of manganese per day in the diet, with higher intakes for vegetarians
who may consume a larger proportion of manganese-rich nuts, grains, and legumes than non-vegetarians
(WHO 2004b). Manganese intake from drinking water is substantially lower than intake from food.
Exposure to manganese from air is considered negligible as compared to intake from diet, although
persons in certain occupations may be exposed to much higher levels than the general public (see
Section 6.7).
Even though daily dietary intake of manganese can vary substantially, adult humans generally maintain
stable tissue levels of manganese through the regulation of gastrointestinal absorption and hepatobiliary
excretion (Andersen et al. 1999; Aschner and Aschner 2005; Aschner et al. 2005; Roth 2006). Following
inhalation exposure, manganese can be transported into olfactory or trigeminal presynaptic nerve endings
in the nasal mucosa with subsequent delivery to the brain, across pulmonary epithelial linings into blood
or lymph fluids, or across gastrointestinal epithelial linings into blood after mucociliary elevator clearance
from the respiratory tract (Aschner and Dorman 2006; Dorman et al. 2006a; Roth 2006). Manganese is
found in the brain and all other mammalian tissues, with some tissues showing higher accumulations of
manganese than others. For example, liver, pancreas, and kidney usually have higher manganese
concentrations than other tissues (Dorman et al. 2006a). The principal route of elimination of manganese
from the body is fecal elimination via hepatobiliary excretion; contributions from pancreatic, urinary, and
lactational elimination are expected to be small (Dorman et al. 2006a). Excess manganese is expected to
be eliminated from the body rapidly. For example, following the intravenous bolus injection of
manganese chloride in rats, manganese concentrations in plasma return to normal levels within 12 hours
(Zheng et al. 2000).
3.4.1 Absorption
3.4.1.1 Inhalation Exposure
No studies were located regarding the absolute amount of manganese that is absorbed by humans or
animals after inhalation exposure to manganese dusts.
MANGANESE
225
3. HEALTH EFFECTS
In general, the extent of inhalation absorption is a function of particle size, because size determines the
extent and location of particle deposition in the respiratory tract. Manganese from smaller particles that
are deposited in the lower airway is mainly absorbed into blood and lymph fluids, while manganese from
larger particles or nanosized particles deposited in the nasal mucosa may be directly transported to the
brain via olfactory or trigeminal nerves. Alternatively, particles deposited in the upper or lower
respiratory tract may be moved by mucociliary transport to the throat, where they are swallowed and enter
the stomach. The latter process is thought to account for clearance of a significant fraction of manganese-
containing particles initially deposited in the lung. Thus, manganese may be absorbed in the nasal
mucosa, in the lung, and in the gastrointestinal tract following inhalation of manganese dust. However,
the relative amounts absorbed from each site are not accurately known.
Absorption of manganese deposited in the lung is expected to be higher for soluble forms of manganese
compared with relatively insoluble forms of manganese (Aschner et al. 2005). Evidence in support of this
hypothesis comes from studies in which 3-month-old male Sprague-Dawley rats were given intratracheal
doses (1.22 mg manganese/kg) of relatively soluble (manganese chloride) or insoluble (manganese
dioxide) forms of manganese (Roels et al. 1997). Peak concentrations of manganese in blood were
observed earlier after manganese chloride intratracheal administration (0.5 hour) compared with
manganese dioxide (168 hours after administration). Peak concentration of manganese in blood were
about 4-fold higher in rats exposed to manganese chloride than in rats exposed to manganese dioxide
(Roels et al. 1997). Confirmatory evidence has been presented by Dorman et al. (2001a, 2004b). For
example, rats exposed to manganese sulfate (0.1 mg manganese/m
3
, 6 hours/day, 5 days/week for
13 weeks) showed higher olfactory bulb and striatum manganese concentrations than rats exposed to
0.1 mg manganese/m
3
manganese phosphate (hureaulite) (Dorman et al. 2004b).
Results consistent with nasal uptake of manganese and transport to the brain along neuronal tracts have
been obtained in several animal studies (Brenneman et al. 2000; Dorman et al. 2001a, 2002a; Elder et al.
2006; Fechter et al. 2002; Henriksson et al. 1999; Lewis et al. 2005; Normandin et al. 2004; Thompson et
al. 2011; Tjälve and Henriksson 1999; Tjälve et al. 1996; Vitarella et al. 2000). For example, following
intranasal administration of 4 μg/kg
54
Mn (as manganese chloride) to weanling Sprague-Dawley rats,
whole-body autoradiography showed that the olfactory bulb contained the vast majority of measured
manganese at 1, 3, and 7 days post-dosing (90, 69, and 47%, respectively) with values decreasing to a low
of 16% at 12 weeks (Tjälve et al. 1996). Significant uptake of manganese by other brain regions was not
observed until the third day, when the basal forebrain, cerebral cortex, hypothalamus, and striatum had
21, 2, 3, and 1% of the measured label, respectively (Tjälve et al. 1996). Subsequent experiments with
MANGANESE
226
3. HEALTH EFFECTS
varying doses of manganese chloride showed that the uptake of manganese into the olfactory epithelium
and the transfer to the brain olfactory bulb leveled off at the highest doses, indicating that these are
saturable processes (Henriksson et al. 1999). Following single, 90-minute, nose-only inhalation
exposures of 8-week old male CD rats to aerosols of manganese chloride (0.54 mg
54
Mn/m
3
; Brenneman
et al. 2000) or manganese phosphate (0.39 mg
54
Mn/m
3
; Dorman et al. 2002a), peak concentrations of
radioactivity in the brain olfactory bulb (at 1–3 days after exposure) were about 20- or 4-fold higher,
respectively, than peak concentrations in the striatum at 21 days after exposure. Results consistent with
transport of manganese to the brain along olfactory neurons have also been obtained in rats exposed to
manganese phosphate aerosols in inhalation chambers (0, 0.03, 0.3, or 3 mg manganese/m
3
) 6 hours/day
for up to 14 days (Vitarella et al. 2000). Elevated concentrations of manganese were observed in the
olfactory bulb, striatum, and cerebellum at the 0.3 and 3 mg manganese/m
3
exposure levels, compared
with control levels, and concentrations in the olfactory bulb were about 1.4–2.4-fold higher than
concentrations in the striatum (Vitarella et al. 2000). Elevated manganese concentrations were also found
in the olfactory bulb, striatum, and cerebellum, following 90 days of inhalation chamber exposure
(6 hours/day, 5 days/week) of young (6 weeks old at start) male or female CD rats or aged (16 months old
at start) male CD to aerosols of either manganese sulfate or manganese phosphate (“hureaulite”) at an
exposure concentration of 0.1 mg manganese/m
3
(Dorman et al. 2004b). Regardless of age or gender, the
olfactory bulb showed the highest elevation in manganese concentration, compared with other brain
tissues, and concentrations in the olfactory bulb were higher in rats exposed to soluble manganese than in
rats exposed to relatively insoluble manganese phosphate (Dorman et al. 2004a). Following 12 days of
inhalation exposure of rats to ultrafine manganese oxide particles (30 nm diameter; about 0.5 mg
manganese dioxide/m
3
), Elder et al. (2006) reported that manganese concentrations in the olfactory bulb
were increased by 3.5-fold over controls, compared with 2-fold increased concentrations in lungs. Lung
lavage analysis showed no signs of pulmonary inflammation following 11 days of exposure, but several
markers of inflammation were noted in the olfactory bulb including increase tumor necrosis factor-α
mRNA and protein. Elder (2006) argued that these results are consistent with the direct transport of the
nanosized particles from the nasal mucosa via the olfactory neuronal tract to the olfactory bulb, noting
that when the right nares were occluded, manganese only accumulated in the left olfactory bulb.
Elevated concentrations of manganese have also been observed in the trigeminal ganglia of rats and mice
at 0, 7, and 14 days following nose-only inhalation exposure to aerosols of manganese chloride at a
concentration of about 2 mg manganese/m
3
, 6 hours/day, 5 days/week for 2 weeks (Lewis et al. 2005).
The latter results are consistent with uptake of manganese in the nasal respiratory epithelium and
subsequent transport to the brain via trigeminal neurons. In Rhesus monkeys exposed to 1.5 mg
MANGANESE
227
3. HEALTH EFFECTS
manganese/m
3
manganese sulfate for 65 days, olfactory epithelium, olfactory bulb, and trigeminal nerve
manganese concentrations were increased by about 17-, 8-, and 2-fold over concentrations in air control
monkeys (Dorman et al. 2006a). These results are consistent with the hypothesis that the nasal olfactory
transport route may be more important than the trigeminal neuron transport route. Support for this
hypothesis comes from studies showing that in rats with more than 90% of neurons and supporting cells
destroyed in the olfactory epithelium, transport of radiolabelled manganese to the brain was markedly
inhibited, compared with normal rats, 7 days following intranasal instillation of a single dose of
54
MnCl
2
(7.5 µCi/kg); brain concentrations of radiolabel in rats with damaged olfactory epithelium were about
90% lower than concentrations in normal rats (57.22 versus 5.58 nCi/g; Thompson et al. 2011). The
relative importance of the nasal route of manganese absorption (and delivery to the brain) in humans
(versus lung absorption and transport across the blood-brain barrier) has not been quantified, but it may
be less important in humans than in rats because the olfactory bulb accounts for a larger part of the central
nervous system and the olfactory epithelium accounts for a larger proportion of the nasal mucosa in rats
compared with humans (Aschner et al. 2005; Dorman et al. 2002a). Using a pharmacokinetic model
describing the olfactory transport and blood delivery of manganese in rats, Leavens et al. (2007)
calculated that 21 days or 8 days following acute inhalation exposure of rats to
54
MnCl
2
or
54
MnHPO
4
,
respectively, direct olfactory transport accounted for the majority of label in the olfactory bulb, but only a
small percentage (≤3%) of the label in the striatum. In normal rats 7 days after nasal instillation of a
single dose of
54
MnCl
2
, the mean concentrations of radiolabel in the olfactory bulb were about 10-, 12-,
9-, 25-, 25-, and 41-fold higher than concentrations in the cortex, hippocampus, basal ganglia, substantia
nigra, brain stem, and cerebellum, respectively (Thompson et al. 2011). These results indicate that the
olfactory bulb is the principal site of accumulation for manganese absorbed and transported to the brain
via the nasal route, and that distribution to other brain regions is restricted.
Absorption of manganese deposited in the lung or nasal mucosa of rats is expected to be influenced by
iron status, with enhanced absorption under iron-deficient conditions and diminished absorption under
iron-excess conditions. Following intratracheal administration of
54
Mn-manganese chloride,
54
Mn blood
concentrations were lower in male Sprague-Dawley rats fed a high-iron diet (about 10,000 ppm Fe),
compared with concentrations in rats fed a control iron (210 ppm Fe) diet (Thompson et al. 2006). These
results are consistent with diminished pulmonary absorption of manganese under iron-loaded conditions.
Supporting this interpretation, 4 hours after
54
Mn administration, levels of
54
Mn (expressed as a
percentage of the instilled dose) were higher in the lungs of high-iron rats, compared with control rats, but
generally lower in other tissues in high-iron versus control rats (Thompson et al. 2006). In rats fed the
high-iron diet, mRNA levels for divalent metal transporter 1 (DMT1—a transport protein that facilitates
MANGANESE
228
3. HEALTH EFFECTS
membrane transport of divalent iron and manganese) were decreased in the bronchus-associated
lymphatic tissue of high-iron rats, compared with control rats (Thompson et al. 2006). In Belgrade rats,
homozygous (b/b) for a mutation in DMT1 that impairs transport function and fed 500 ppm Fe in the diet,
54
Mn blood levels following intranasal administrations of
54
Mn-manganese chloride were markedly (2–
5-fold) lower than those in blood of anemic heterozygous (+/b) rats fed a 20 ppm Fe diet (Thompson et al.
2007). For example, levels of
54
Mn remaining in the blood 4 hours after administration were 0.022 and
0.115% of the instilled dose in the homozygous (b/b) and anemic heterozygous (+/b) rats, respectively (a
5-fold difference). Intermediate levels of
54
Mn in blood were found in heterozygous (+/b) rats fed the
500 ppm Fe diet (Thompson et al. 2007). In Sprague-Dawley rats, levels of DMT1 protein in the
olfactory epithelium were 1.5- to 2.5-fold greater under anemic conditions (20 ppm Fe in diet for
3 weeks), compared with iron-sufficient conditions, 200 ppm Fe in diet for 3 weeks (Thompson et al.
2007). These results are consistent with the hypothesis that up- and down-regulation of DMT1 plays a
role in enhanced nasal absorption of manganese under iron-deficient conditions and diminished
absorption under iron-excess conditions, respectively.
No studies were located regarding the absorption of organic manganese compounds following inhalation
exposure in either humans or animals.
3.4.1.2 Oral Exposure
The amount of manganese absorbed across the gastrointestinal tract in humans is variable, but typically
averages about 3–5% (Davidsson et al. 1988, 1989a; Mena et al. 1969). Data were not located on the
relative absorption fraction for different manganese compounds, but there does not appear to be a marked
difference between retention of manganese ingested in food (5% at day 10) or water (2.9% at day 10)
(Davidsson et al. 1988, 1989a; Ruoff 1995). In humans, manganese absorption tends to be greater from
manganese chloride (in demineralized water) than from foods (labeled intrinsically or extrinsically with
54
Mn); however, the biological half-life of manganese from either manganese chloride or food is the same
(EPA 1995b; Johnson et al. 1991). In human adults, supplementation of the diet with manganese sulfate
for 12–35 weeks at a level approximately 2 times the normal dietary intake caused a 30–50% decrease in
absorption of a tracer dose of
54
MnCl
2
(Sandstrom et al. 1990).
Results from animal studies indicate that the gastrointestinal absorption of manganese is rapid and
expected to be higher for soluble forms of manganese compared with relatively insoluble forms of
manganese. Following a single gavage dose of 6 mg manganese/kg as manganese chloride to rats,
MANGANESE
229
3. HEALTH EFFECTS
maximal plasma concentrations were attained rapidly (T
max
=15 minutes) (Zheng et al. 2000). From
analysis of time course of plasma concentrations following oral and intravenous administration, the oral
bioavailability for manganese was calculated to be 13.9% (Zheng et al. 2000). Roels et al. (1997) noted
that in 3-month-old male rats, gavage administered manganese chloride (24.3 mg manganese/kg) reached
a maximal level in blood, 7.05 μg/100 mL, within the first 30 minutes post-dosing (first time point
measured), whereas manganese from manganese dioxide, administered in the same fashion, did not reach
a maximal level in blood of 900 ng/100 mL until 144 hours (6 days) post-dosing. Following four weekly
gavage doses of manganese chloride at 24.3 mg manganese/kg per dose, significant increases in
manganese concentration were observed in blood and the cerebral cortex, but not cerebellum or striatum,
as compared to controls; for identical doses of manganese dioxide, manganese levels were significantly
increased only in blood. The lack of significant increase in manganese levels in any brain region
following administration of the dioxide is likely due to the delayed uptake of manganese in the blood.
One study showed that, in full-term infants, manganese is absorbed from breast milk and cow’s milk
formulas that were either unsupplemented or supplemented with iron, copper, zinc, and iodine (Dorner et
al. 1989). Manganese intake was greater in the formula-fed infants than in the breast-fed infants due to
the higher manganese content of the formula. However, breast-fed infants retained more of their daily
intake of manganese (40%) than did the formula-fed infants (20%). It must be noted that the full-term
infants evaluated in this study were 2–18 weeks old, and the data did not stratify intake and retention
amounts by age. Further, the data did not indicate if there were similar proportions of manganese taken
up from breast milk as compared to the formulas. A study by Davidson and Lönnerdal (1989)
demonstrated the in vitro receptor-mediated uptake of manganese from lactoferrin; the authors speculated
that this may lead to the absorption of manganese from breast milk in human infants.
There is some evidence to suggest that gastrointestinal absorption of manganese is age-dependent.
Dorner et al. (1989) have shown that infants, especially premature infants, retain a higher proportion of
manganese than adults. Animal studies also support this finding. For example, Rehnberg et al. (1980,
1981, 1982) dosed 1-day-old rat pups with up to 214 mg manganese/kg/day (as manganese tetroxide) for
up to 224 days, then measured manganese concentrations in tissues. The authors noted that intermediate
and chronic exposure of rats to manganese tetroxide in water or food resulted in much larger increases in
tissue levels in young rats (1–15 days in intermediate studies, 24–40 days in chronic study) than in older
rats. These increases in neonates were judged to be due to the neonates' greater absorption of manganese
as a result of a slower rate of transport through the gut (Rehnberg et al. 1985). Similar results have been
reported in rats exposed to manganese chloride (Kostial et al. 1978). However, such age-dependent
MANGANESE
230
3. HEALTH EFFECTS
differences in tissue retention of manganese could also be due to differences in excretory ability (Cotzias
et al. 1976; Miller et al. 1975) or to age-related changes in dietary intake levels of iron and manganese
(Ballatori et al. 1987). Dorner et al. (1989) found that both pre-term and full-term infants had active
excretion of manganese; in fact, some infants had negative manganese balances. Animal studies show
that absorption and/or retention of manganese is higher in neonates, but returns to the level of older
animals at approximately post-GD 17–18 (Kostial et al. 1978; Lönnerdal et al. 1987; Miller et al. 1975;
Rehnberg et al. 1981). Available studies (Dorner et al. 1989) do not provide adequate data to determine
when this transition takes place in human infants.
One of the key determinants of absorption appears to be dietary iron intake, with low iron levels leading
to increased manganese absorption. Mena et al. (1969) administered oral
54
Mn and
39
Fe to subjects with
iron-deficiency anemia (ranging in age from 13 to 44 years old) and measured manganese and iron uptake
with whole-body autoradiography. The uptake of manganese by anemic subjects was 7.5% while in non-
anemic subjects, it was 3.0%. This is probably because both iron and manganese are absorbed by the
same transport system in the gut. The activity of this system is inversely regulated by dietary iron and
manganese intake levels (Chandra and Tandon 1973; Diez-Ewald et al. 1968; Rehnberg et al. 1982;
Thomson et al. 1971). Interaction between iron and manganese occurs only between nonheme iron and
manganese. Davis et al. (1992a) demonstrated that increasing dietary intakes of nonheme iron, but not
heme iron, depressed biomarkers of manganese status (i.e., serum manganese concentrations and
lymphocyte manganese-dependent superoxide dismutase activity).
Studies of oral absorption of manganese in animals have yielded results that are generally similar to those
in humans. Manganese uptake in pigs, which have similar gastrointestinal tracts to humans, has been
measured using labeled manganese administered orally (Finley et al. 1997). The mean absorption rates
for different times post-dosing were 5% 1–6 hours post-dosing, 7% 6–12 hours post-dosing, and 3.8%
12–24 hours post-dosing. Gastrointestinal uptake of manganese chloride in rats has been estimated to be
2.5–8.2% (Davis et al. 1993; Pollack et al. 1965). Uptake is increased by iron deficiency (Pollack et al.
1965) and decreased by preexposure to high dietary levels of manganese (Abrams et al. 1976a; Davis et
al. 1992b). In a rat study, the intestinal transfer of the calcium ion and manganese ion was found to be
competitive, and the authors suggested that there is a common mechanism for their transfer in the
intestines (Dupuis et al. 1992). High dietary intakes of phosphorus (Wedekind et al. 1991) and calcium
(Wilgus and Patton 1939) have also been demonstrated to depress manganese uptake in chicks.
MANGANESE
231
3. HEALTH EFFECTS
Manganese absorption has also been found to vary according to manganese intake; in rats with
manganese-deficient diets, absorption was at least 2-fold higher than in rats whose diets contained an
adequate amount of manganese (as manganese carbonate) (Davis et al. 1992b).
Two studies in suckling rat pups found differing absorptions of manganese from different milks and
formulas. The first study (Lönnerdal et al. 1987) found that the percent of
54
Mn (added to the food source
as an extrinsic label) retained (measured as whole-body retention) in 14-day-old pups fed breast milk,
cow milk, cow milk formula, and soy formula, was 82, 90, 77, and 65%, respectively.
The latter study (Lönnerdal et al. 1994) found that 13-day-old rat pups fed
54
Mn (from manganese
chloride that was incubated with the food for at least 24 hours prior to feeding) in breast milk, cow milk,
and several different manufacturers’ cow milk formulas had similar absorption values. These pups
absorbed (measured as whole-body retention) 80% of the label from breast milk, 83% from cow milk, and
63–90% from the cow milk formulas, with the two lowest retention values being significantly lower than
the others. In this latter study, manganese absorption from soy formulas was significantly lower than the
other milks and formulas tested, ranging from 63 to 72%.
The inherent concentration of manganese in each of these food sources from the first study was 0.01,
0.04, 0.05, and 0.30 μg/mL, respectively (Lönnerdal et al. 1987). Therefore, when the retention of the
label was multiplied by the actual manganese concentration of the food, the total amounts of absorbed
manganese were 0.004, 0.018, 0.019, and 0.097 μg/dose fed, respectively. These data indicate that infants
fed cow milk formula may retain 5 times more manganese, and infants fed soy formula may retain
25 times more manganese than breast-fed infants. Although the latter results differ significantly from
those observed earlier, the researchers report that the similar relative values for manganese absorption
were indicative of significant efforts made to optimize both the relative concentrations and the
bioavailability of minerals and trace elements in the manufactured formulas.
No studies were located regarding absorption of manganese following oral exposure to MMT in humans.
Several studies (Hanzlik et al. 1980a, 1980b; Hinderer 1979; Hysell et al. 1974; Komura and Sakamoto
1992a, 1992b) indicate that absorption is occurring because toxicity is observed following MMT
exposure; however, no absorption rates or relative amounts were provided in these studies. The plasma
temporal pattern of manganese following oral administration of MMT has been studied in male Sprague-
Dawley rats (Zheng et al. 2000). Following oral gavage of 20 mg MMT/kg, manganese appears in the
plasma with a C
max
between 2 and 12 hours after dosing. When nearly equivalent oral doses of MMT
MANGANESE
232
3. HEALTH EFFECTS
(5.6 mg manganese/kg) or manganese chloride (6 mg manganese/kg) were administered, the C
max
(0.93 mg manganese/mL) following oral MMT was about 3-fold higher than that following oral
manganese chloride (0.30 mg manganese/mL) (Zheng et al. 2000).
3.4.1.3 Dermal Exposure
The only available human study regarding dermal exposure to manganese discussed a case report of a
man burned with a hot acid solution containing 6% manganese. The authors speculated that manganese
absorption had occurred across the burn area (Laitung and Mercer 1983) because the man had slightly
elevated urinary manganese levels (11–14 vs. 1–8 mg/L). In most cases, manganese uptake across intact
skin would be expected to be extremely limited.
No studies were located regarding absorption of organic manganese in humans or animals following
dermal exposure.
3.4.2 Distribution
Manganese is a normal component of human and animal tissues and fluids. In humans, most tissue
concentrations range between 0.1 and 1 μg manganese/g wet weight (Sumino et al. 1975; Tipton and
Cook 1963), with the highest levels in the liver, pancreas, and kidney and the lowest levels in bone and fat
(see Table 3-7). Manganese levels in the blood, urine, and serum of healthy, unexposed subjects living in
the Lombardy region of northern Italy were 8.8±0.2, 1.02±0.05, and 0.6±0.014 μg/L, respectively (Minoia
et al. 1990). Serum manganese concentrations in healthy males and females in Wisconsin were 1.06 and
0.86 μg/L, respectively (Davis and Greger 1992; Greger et al. 1990). Although precise inhalation
exposure data were not available for humans, chronic occupational exposure studies have shown that
higher levels of inhalation exposure generally correspond with higher blood or urine manganese levels for
groups, but that individual measurements may not correspond to individual exposure or be reliable
exposure predictors (Abdel-Hamid et al. 1990; Alessio et al. 1989; Jarvisalo et al. 1992; Roels et al. 1992;
Siqueira et al. 1991).
Studies investigating manganese levels in human fetal tissues or fluids are very few. Widdowson et al.
(1972) measured manganese in fetal livers from 29 unborn infants (ranging in gestational age from 20 to
41 weeks) and from 5 adults. The fetal manganese levels ranged from 0.09 to 0.23 mg/100 g wet weight
with a mean of 0.14 mg/100g wet weight, while the mean of the five adults was 0.18 mg/100 g wet
weight (range of values not reported). The highest fetal manganese value of 0.23 mg/100 g wet weight

MANGANESE
233
3. HEALTH EFFECTS
Table 3-7. Manganese Levels in Human and Animal Tissues
Tissue concentrations (μg manganese/g wet weight)
Humans
Rats
Rabbits
Tipton and
Sumino et al.
Rehnberg et al.
Fore and
Tissue
Cook (1963)
(1975)
(1982)
Morton (1952)
Liver
1.68
1.2
2.6–2.9
2.1
Pancreas
1.21
0.77
No data
1.6
Adrenals
0.20
0.69
2.9
0.67
Kidney
0.93
0.56
0.9–1.0
1.2
Brain
0.34
0.30
a
0.4
0.36
Lung
0.34
0.22
No data
0.01
Heart
0.23
0.21
No data
0.28
Testes
0.19
0.20
0.4
0.36
Ovary
0.19
0.19
No data
0.60
Muscle
0.09
0.09
No data
0.13
Spleen
0.22
0.08
0.3
0.22
Fat
No data
0.07
No data
No data
Bone (rib)
No data
0.06
No data
No data
Pituitary
No data
No data
0.5
2.4
a
Average of cerebrum and cerebellum
MANGANESE
234
3. HEALTH EFFECTS
was from one of the two infants at 41 gestational weeks of age when analyzed. The data indicate that
fetal liver manganese levels throughout the latter half of gestation are comparable to those in the adult.
Concentrations of manganese also have been measured in the blood of pregnant women, as well as in the
plasma of cord blood of preterm and full-term infants (Wilson et al. 1991). Manganese concentrations in
full-term (37–42 weeks gestation) infants were 5.5±1.5 μg/L, slightly higher than the preterm (27–
36 weeks gestation) infants’ values of 5.0±1.1 μg/L, but the difference was not statistically significant.
There were no correlations between the levels in infants and mothers. The higher manganese levels in
cord blood of gestationally older infants, along with the higher manganese level in the oldest fetus from
the Widdowson et al. (1972) study, suggest that manganese levels may rise slightly as the fetus
approaches birth; however, there are inadequate data points to make a strong argument for this possibility.
Serum manganese values of 180 healthy Venezuelan infants decreased consistently from a high value of
0.45 μg/L (mean of 22 infants) at 5 days of age to a low value of 0.29 μg/L (mean of 40 infants) at
12 months of age (Alarcón et al. 1996). The level of manganese at 12 months was the only measurement
that was statistically different than the 5-day value. The values were not statistically different between
the sexes. Rükgauer et al. (1997) obtained very different results in their analyses of serum manganese
levels in German children, adolescents, and adults. The authors evaluated 137 children (aged 1 month–
18 years); the mean serum manganese level for all children was 1.4 μg/L (range 0.17–2.92 μg/L). When
the children were separated by age, the serum manganese values were found to decrease from a mean
value of 2.12 μg/L (age 0–1 year) to a minimum of 0.98 μg/L (age 14–18 years). Adults (age 22–
75 years) had a mean value of 0.79 μg/L. These data indicate that children had much higher manganese
levels in serum than those levels shown by the other studies. It is unknown why this latter study indicates
results that are vastly different from those reported in the earlier studies. Rükgauer et al. (1997) took
precautions to prevent manganese contamination of their experimental materials during sampling and
analysis. Also, the authors reported that the subjects were healthy and were not suffering from nutritional
diseases or metabolic disorders and were not taking medicines containing trace elements. However, the
children and adolescent subjects were chosen from a pediatric hospital after seeking medical attention on
non-nutrition related matters. Therefore, this population may not be a representative sample of the
general population. Animal studies, by contrast, suggest that distribution of manganese in the infant and
young child may be very different from the adult.
Levels in tissues from animals fed a normal diet are generally similar but, perhaps are slightly higher than
those in humans (Fore and Morton 1952; Rehnberg et al. 1982). Levels of manganese in the milk of rats
MANGANESE
235
3. HEALTH EFFECTS
fed a normal diet averaged 0.054 μg/g (Miller et al. 1975). Data on changes in tissue levels following
acute exposures to excess manganese are presented in exposure-specific subsections later in this chapter.
Manganese is also found in breast milk for the continuing metabolic nutrition of the infant. One study
reported manganese concentrations from 82 normal, healthy French women of 12±5.6 μg/L at postpartum
day 2 in human colostrum decreasing to 3.4±1.6 μg/L at postpartum day 6 in breast milk (Arnaud and
Favier 1995). Another study reported an average manganese concentration in breast milk of 6.2 μg/L
using 2,339 samples from mothers of 20 full-term and 6 preterm infants (Dorner et al. 1989). Collipp et
al. (1983) have reported concentrations of manganese in breast milk of 10 μg/L. These reports, however,
did not address the dietary manganese intake of the nursing mothers. It is unknown whether mothers
exposed to increased concentrations of manganese have higher-than-usual levels of the metal in breast
milk.
Manganese is distributed throughout all cells in the body; therefore, it is present in germ cells. However,
existing studies in humans and animals are not sufficient to predict if distribution of excess manganese
into germ cells might result in heritable genetic changes. Manganese is constantly present in human
tissues and, therefore, is able to enter germ cells. One human study involving inhalation exposure to
nickel and manganese observed chromosomal aberrations in welders working with these metals (Elias et
al. 1989). However, the presence of nickel is a confounding factor, as it is known for causing
chromosomal changes. Studies in animals are equivocal; there are not enough data to make predictions as
to the likelihood for excess exposures of manganese to cause heritable genetic changes.
Concentrations of manganese in select human and animal tissues are presented in Table 3-7 and
concentrations of manganese in plasma and serum in infants of differing ages and adults are presented in
Table 3-8.
3.4.2.1 Inhalation Exposure
Following inhalation exposure of mice to manganese dust, for a short period of time the concentration of
manganese in the lung is approximately proportional to the concentration of manganese in the air (Adkins
et al. 1980c). However, as noted earlier, some of the particles that are deposited in the lung are
transported to the gastrointestinal tract (Mena et al. 1969). The rate of particle transport from the lungs
has not been quantified in humans, but half-times of elimination in animals range from 3 hours to 1 day
(Adkins et al. 1980c; Bergstrom 1977; Newland et al. 1987).

Concentration (μg/L) (mean±2 standard deviations)
Age
Serum
c
Plasma
5 Days
a
0.45±0.12 (22)
1 Month
0.41±0.11 (20)
3 Months
0.39±0.13 (22)
5 Months
0.39±0.10 (14)
7 Months
0.38±0.09 (20)
10 Months
0.37±0.11 (20)
11 Months
0.36±0.12 (22)
12 Months
0.29±0.10 (40)
1 Month–18 years
b
1.4±1.25
22–75 Years
0.79±0.63
a
Data from infants 5 days–12 months in age are from Alarcón et al. (1996). Data are from mixed-sex groups. No
statistically significant differences in manganese concentrations were found between sexes.
b
Data from Rükgauer et al. (1997).
c
Value in parentheses is the number of subjects.
MANGANESE
236
3. HEALTH EFFECTS
Table 3-8. Manganese Levels in Human Serum/Plasma
MANGANESE
237
3. HEALTH EFFECTS
The relative increases in tissue levels of manganese following inhalation exposure to inorganic forms of
manganese have received considerable investigation in animals.
Increases of 20–60% in manganese levels in the kidney and spleen were noted in mice 24–48 hours after
exposure to manganese dioxide (Adkins et al. 1980c). Rats exposed to an aerosol containing 0.0003 mg
54
Mn/m
3
for 1 hour had manganese levels in the liver, lung, kidney, and brain of 0.0495, 0.1366, 0.0141,
and 0.0014 ng
54
Mn/organ, respectively, 5 days after exposure (Wieczorek and Oberdörster 1989b).
Sheep exposed to welding fumes for 3 hours exhibited a 40-fold increase in lung manganese content
(Naslund et al. 1990). Preferential accumulation of manganese in specific locations of the brain
(including the caudate nucleus, globus pallidus, and substantia nigra) was noted in one monkey exposed
to an aerosol of manganese chloride (20–40 mg/m
3
) several hours/day for 3–5 months (Newland et al.
1989). This preferential uptake could play a role in the characteristic neurological effects of manganese
(see Section 3.5).
Roels et al. (1997) investigated the distributional differences in rats exposed to manganese in two forms
(manganese chloride and manganese dioxide) administered via intratracheal injection (intended to
simulate inhalation), by gavage (oral administration) and via intraperitoneal injection. When
administered intratracheally once a week for 4 weeks, 1.22 mg manganese/kg as manganese chloride
resulted in a 68% steady-state increase in blood manganese concentration after the dosing period. This
dose also resulted in significantly increased concentrations of manganese in the rat cerebellum (27%
increase that approached statistical significance), striatum (205% increase), and cortex (48% increase),
compared with control rats.
When rats were administered the same amount of manganese under the same dosing regimen, with
manganese in the form of manganese dioxide, similar, but less striking, results were observed (Roels et al.
1997). Manganese concentrations in the blood were increased by 41%, and in the cerebellum, striatum,
and cortex by 31, 48, and 34%, respectively, over the control rats.
Tjälve et al. (1996) investigated the distribution of manganese in brain tissues, liver, and kidneys of
young male rats following intranasal injection of
54
MnCl
2
. Radiography data indicated that 1 day after
dosing, the olfactory bulb contained 90% of the manganese (measured as μg/100g wet weight) in the
measured tissues, while the basal forebrain contained 6% of the manganese. Concentrations of
manganese in the basal forebrain increased to 21 and 28% of the measured total at 3 and 7 days post-
MANGANESE
238
3. HEALTH EFFECTS
dosing, respectively. Manganese in the cerebral cortex, hypothalamus, striatum, and hippocampus were
also maximal at 7 days post-dosing. Manganese values in liver and kidneys were approximately 1% of
the total measured for the first 7 days, and then decreased steadily until 12 weeks. These results were
compared to distribution of manganese following intraperitoneal injection, in which no brain region
showed preferential distribution at 1, 7, or 21 days post-dosing (Tjälve et al. 1996). In another study,
Gianutsos et al. (1997) found a dose-dependent accumulation of manganese in the olfactory bulb and
tubercle following intranasal injection of manganese chloride into one nostril. Injection of 200 μg
manganese resulted in maximally elevated levels in the olfactory bulb (400% higher than the uninjected
side), with levels in the tubercle half that in the bulb within 12 hours post-exposure; these levels remained
elevated for 3 days. Two injections of 200 μg manganese doubled the level of manganese in the striatum
compared to saline-injected controls; single doses did not increase tissue manganese levels. No other
brain regions were noted and blood manganese levels were not changed with any treatment. These data
indicate that the olfactory mucosa is an important pathway for distribution of manganese into the brain.
Vitarella et al. (2000) exposed adult rats to airborne doses of particulate manganese, as manganese
phosphate, at 0, 0.03, 0.3, 3 mg manganese/m
3
. The particles had a mean diameter of 1.5 μm. Exposures
lasted for 6 hours/day for either 5 days/week (10 exposures) or 7 days/week (14 exposures). The
following tissues were analyzed for manganese content using neutron activation analysis: plasma,
erythrocytes, olfactory bulb, striatum, cerebellum, lung, liver, femur, and skeletal muscle. Increased
manganese concentrations were reported in olfactory bulb, lung, femur, and skeletal muscle following
exposure to 3 mg/m
3
(after either dosing regiment); a lower dose of 0.3 mg/m
3
resulted in increased
manganese concentrations in olfactory bulb, and lung (14-day dose regimen only). Striatal manganese
levels were increased at the two highest doses only after 14 days of exposure. However, concentrations in
the cerebellum were similarly elevated, which was interpreted by the authors to indicate that
accumulation of manganese was not selective for the striatum. Red blood cell and plasma manganese
levels were increased only in rats exposed to the highest dose for the 10-day exposure period. These data
indicate that even at lower doses manganese can accumulate in the olfactory bulb and that the neuronal
pathway to the brain is significant for inhaled manganese in rodents.
Thompson et al. (2011) reported that in normal rats 7 days after nasal instillation of a single dose of
54
MnCl
2
, the mean concentration of radiolabel in the brain olfactory bulb was about 9–41-fold higher than
concentrations in other brain regions. As discussed in Section 3.4.1, these results indicate that the
olfactory bulb is the principal site of accumulation for manganese absorbed and transported to the brain
via the nasal route, and that distribution from the olfactory bulb to other brain regions may be restricted.
MANGANESE
239
3. HEALTH EFFECTS
Although the results from the studies by Tjälve et al. (1996), Vitarella et al. (2000), and Thompson (2011)
indicate that manganese can be transported via the olfactory neural pathway from the nasal mucosa to the
olfactory bulb of the brain and, to a limited degree, to other brain regions in rodents, the relative
importance of this pathway to the delivery of manganese to basal ganglia sites of neurotoxicity is
uncertain. Statistical mapping of functional olfactory connections in rat brains using MRI following nasal
administration of manganese chloride could readily detect connections to the olfactory bulb, but could not
detect connections to other brain regions (Cross et al. 2004). Mainstream manganese entry into the brain
from blood occurs through capillary endothelial cells of the blood-brain barrier and through the cerebral
spinal fluid via the choroid plexuses (Bock et al. 2008; Crossgrove and Yokel 2005). A number of
transport mechanisms (including facilitated diffusion, active transport, transferrin-mediated transport,
divalent metal transporter-1 mediation, store-operated calcium channels) have been proposed to transport
manganese across the blood barrier, but current understanding is inadequate to determine the predominant
mechanism of transport (Aschner et al. 2005; 2007; Crossgrove and Yokel 2004, 2005; Roth 2006).
A concern that inhaled manganese, compared with ingested manganese, may more readily result in
manganese accumulation in the brain, a principal toxicity target of manganese, has led to recent detailed
investigations of manganese concentrations in various brain regions and in other tissues following
inhalation exposure of animals to environmentally relevant forms of manganese. These studies have
investigated manganese concentrations in tissues of young male and female CD rats exposed by
inhalation to manganese sulfate or manganese tetroxide for 14 days at concentrations of 0, 0.03, 0.3, or
3 mg manganese/m
3
(Dorman et al. 2001a), young male CD rats given low- (2 ppm), sufficient- (10 ppm),
or high-manganese (100 ppm) diets for 67 days, followed by inhalation exposure to manganese sulfate for
14 days at concentrations of 0, 0.092, or 0.92 mg manganese/m
3
(Dorman et al. 2001b), young male and
female CD rats or aged male CD rats after 90 days of inhalation exposure to manganese sulfate at 0.01,
0.1, or 0.5 mg manganese/m
3
or manganese phosphate at 0.1 mg manganese/m
3
(Dorman et al. 2004a),
maternal CD rats and offspring after inhalation exposure to manganese sulfate at 0, 0.05, 0.5, or 1.0 mg
manganese/m
3
starting 28 days prior to breeding through PND 18 (Dorman et al. 2005a, 2005b), and
young male Rhesus monkeys after inhalation exposure to manganese sulfate at 0.06, 0.3 or 1.5 mg
manganese/m
3
for 15, 33, or 65 exposure days (Dorman et al. 2006a).
The results from these animal studies indicate that tissue manganese concentrations in the brain depended
on aerosol concentration, exposure duration, and brain region. Tissue manganese concentrations
generally increased with increasing air concentrations and durations of exposure. With repeated
MANGANESE
240
3. HEALTH EFFECTS
exposures at the highest air concentrations (≥0.92 mg manganese/m
3
), manganese concentrations in brain
regions were elevated, compared with control animals, showing the following order: olfactory
bulb>striatum>cerebellum. Illustrative data for maternal CD rats (Dorman et al. 2005a) and young
Rhesus monkeys (Dorman et al. 2006a) exposed to manganese sulfate are shown in Tables 3-9 and 3-10,
respectively. Comparison of manganese concentrations across tissues shows the following order in
exposed maternal rats: liver > pancreas > olfactory bulb > lung > striatum ≈ femur > milk > cerebellum
>> whole blood (Table 3-9). In young Rhesus monkeys after 65 days of exposure, the order was: bile >
olfactory epithelium > pituitary > liver > pancreas ≈ globus pallidus > olfactory bulb > kidney > putamen
> caudate > cerebellum > heart >skeletal muscle > frontal cortex > lung > parietal bone ≈ femur >> blood
(Table 3-10).
Brain tissues from the monkeys were dissected into more regions than the rat brains and, immediately
following 65 days of exposure to the highest exposure concentration, showed the following order of
elevated manganese concentrations: pituitary>globus pallidus>olfactory bulb>putamen>caudate>
cerebellum>frontal cortex>trigeminal nerve (see Table 3-10). These results are consistent with the
evidence that the human striatum, globus pallidus, and substantia nigra are the primary neurotoxicity
target for manganese (Aschner et al. 2005; Pal et al. 1999). Three- to 5-fold increases (over air control
values) in mean manganese tissue concentrations were found in the globus pallidus, putamen, and caudate
in monkeys exposed to 1.5 mg manganese/m
3
manganese sulfate for 65 days, but levels were <3-fold
increased in the frontal cortex and cerebellum, two brain regions not generally associated with manganese
neurotoxicity (Dorman et al. 2006a; Table 3-10).
Comparison with the rat results in Table 3-9 suggests that rodents do not accumulate manganese in the
basal ganglia (i.e., the collection of deep regions of the brain including the striatum [comprised of the
caudate and putamen]) to the same relative degree as primates, a difference that may be related to findings
that overt signs of manganese neurotoxicity are more readily detected in nonhuman primates than rodents
(Aschner et al. 2005; Bock et al. 2008; Newland 1999). Recent corroborative findings showed that
marmosets, a nonhuman primate, accumulated more manganese in the brain (especially in the basal
ganglia and the visual cortex) than rats following intravenous injection of equivalent mg/kg body weight
doses of manganese chloride (Bock et al. 2008). The mechanisms by which manganese accumulates in
the basal ganglia of primates are poorly understood (Aschner et al. 2005; Bock et al. 2008; Brenneman et
al. 1999; Dorman et al. 2006b), but Bock et al. (2008) have hypothesized that primates may accumulate
relatively more manganese in the basal ganglia than rodents because of a relatively larger cerebral spinal
fluid space in lateral ventricles adjacent to the basal ganglia.

MANGANESE
241
3. HEALTH EFFECTS
Table 3-9. Terminal Mean (±Standard Error on the Mean) Tissue Manganese
Concentrations (µg Manganese/g Tissue Wet Weight) in Maternal CD Rats
Exposed to Aerosols of Manganese Sulfate 6 Hours/Day, 7 Days/Week
Starting 28 Days Prior to Breeding Through Postnatal Day 18
Exposure concentration (mg manganese/m
3
)
Tissue
0
0.05
0.5
1.0
Whole blood
0.08±0.04
0.06±0.02
0.06±0.01
0.05±0.01
Olfactory bulb
0.56±0.05
0.71±0.04
a
1.40±0.07
a
1.73±0.07
a
Striatum
0.51±0.02
0.54±0.02
0.74±0.02
a
0.89±0.02
a
Cerebellum
0.50±0.02
0.52±0.02
0.60±0.01
a
0.61±0.03
a
Lung
0.22±0.03
0.37±0.02
0.86±0.07
a
1.05±0.06
a
Liver
3.21±0.15
3.04±0.09
3.37±0.15
4.28±0.76
a
Femur
0.62±0.07
0.61±0.04
077±0.05
0.89±0.06
a
Pancreas
1.66±0.13
1.80±0.19
1.29±0.28
1.91±0.23
Milk
0.21±0.08
0.20±0.06
0.47±0.06
0.77±0.10
a
Group size (n)
8
10
9
8
a
Significantly (p<0.05) different from control mean value.
Source: Dorman et al. 2005a

Exposure to air 1.5 mg manganese/m
3
Exposure (days) 65 15 33 65 65 (+45)
a
65 (+90)
a
Tissue
Olfactory tissues
Olfactory 0.49±0.01 6.10±0.39
b
7.34±0.70
b
7.10±2.01
b
0.65±0.04 0.69±0.11
epithelium
Olfactory bulb 0.31±0.01 2.19±0.44
b
2.29±0.26
b
2.40±0.18
b
0.35±0.02 0.31±0.02
Olfactory tract 0.30±0.06 0.77±0.19
b
0.84±0.11
b
1.12±0.08
b
0.18±0.02 0.22±0.02
Olfactory cortex 0.19±0.01 0.43±0.04
b
0.45±0.01
b
0.42±0.01
b
0.26±0.01 0.21±0.01
Brain
Globus pallidus 0.48±0.04 1.92±0.40
b
2.41±0.29
b
2.94±0.23
b
1.09±0.03
b
0.59±0.12
Putamen 0.36±0.01 1.01±0.08
b
1.50±0.14
b
1.81±0.14
b
0.58±0.03
b
0.44±0.02
Caudate 0.34±0.02 0.93±0.11
b
1.37±0.13
b
1.72±0.10
b
0.57±0.03 0.43±0.02
Frontal cortex 0.25±0.03 0.36±0.01
b
0.52±0.03
b
0.47±0.02
b
0.26±0.01 0.23±0.01
Cerebellum 0.44±0.01 0.85±0.06
b
0.96±0.05
b
1.10±0.11
b
0.66±0.04 0.61±0.10
Pituitary 0.84±0.12 3.79±0.38
b
5.60±0.33
b
6.19±0.61
b
3.01±0.91
b
1.54±0.18
Trigeminal 0.17±0.05 0.27±0.02 0.51±0.14
b
0.42±0.08
b
0.18±0.01 0.17±0.02
nerve
Organs
Femur 0.13±0.02 0.27±0.04
b
0.13±0.03 0.20±0.03 0.12±0.02 0.09±0.01
Heart 0.16±0.03 0.25±0.05 0.50±0.03
b
0.62±0.05
b
0.23±0.3 0.27±0.01
Kidney 1.14±0.12 2.65±0.14
b
3.04±0.09
b
2.61±0.30
b
1.38±0.13 1.27±0.14
Liver 2.49±0.09 2.96±0.34 3.28±0.22 3.52±0.45
b
2.88±0.27 2.04±0.06
Lung 0.15±0.03 0.39±0.06
b
0.35 ±0.02
b
0.33±0.04b 0.09±0.01 0.06±0.01
Pancreas 1.59±0.11 2.89±0.14
b
2.38±0.34
b
2.95±0.24
b
1.41±0.270. 1.53±0.10
Skeletal muscle 0.15±0.03 0.22±0.03 0.22±0.02 0.58±0.19
b
19±0.02 0.12±0.01
Parietal bone 0.08±0.04 0.48±0.16
b
0.56±0.18
b
0.25±0.04 0.17±0.03 0.16±0.04
Testis 0.26±0.03 0.41±0.06 0.50±0.04
b
0.39±0.07 0.36±0.04 0.31±0.02
Table 3-10. Mean (±Standard Error on the Mean) Tissue Manganese
Concentrations (µg Manganese/g Tissue Wet Weight) in Young
Male Rhesus Monkeys Exposed to Aerosols of Manganese
Sulfate (1.5 mg Manganese/m
3
) 6 Hours/
Day, 5 Days/Week for Up to 65 Days
MANGANESE
242
3. HEALTH EFFECTS

Table 3-10. Mean (±Standard Error on the Mean) Tissue Manganese
Concentrations (µg Manganese/g Tissue Wet Weight) in Young
Male Rhesus Monkeys Exposed to Aerosols of Manganese
Sulfate (1.5 mg Manganese/m
3
) 6 Hours/
Day, 5 Days/Week for Up to 65 Days
Exposure to air 1.5 mg manganese/m
3
Exposure (days) 65 15 33 65 65 (+45)
a
65 (+90)
a
Tissue
Fluids
Bile 0.89±.22 7.38±.78
b
4.40±.89
b
7.60±1.68
b
1.17±0.28 0.77±0.13
0.010±.001 0.016±.06 0.022±.002 0.026±0.00 0.021±0.002 0.013±.001
Blood
a
3
b b
0.000±.000 0.000±.000 0.001±.000 0.005±0.00 0.000±0.000 0.000±.000
Urine
1
b
Group size (n) 6 4 4 4 4 4
a
These monkeys were sacrificed 45 or 90 days after the 65-day exposure period.
b
Significantly (p<0.05) greater than mean value for air control rats.
Source: Dorman et al. 2006a
MANGANESE
243
3. HEALTH EFFECTS
MANGANESE
244
3. HEALTH EFFECTS
The high concentrations of manganese in bile sampled from manganese-exposed monkeys (compared
with air control values in Table 3-10) are reflective of the hepatobiliary excretion of manganese. It is
currently unknown whether or not the high manganese concentrations attained in the pituitary glands of
these monkeys has any effect on normal pituitary function; in this study, exposed monkeys showed no
difference in serum levels of luteinizing hormone (LH), a hormone that stimulates production of
testosterone by the Leydig cells of the testes (Dorman et al. 2006a).
In pregnant rats repeatedly exposed to inhaled manganese, the placenta appears to partially limit the
transport of manganese to the developing fetus (Dorman et al. 2005b). After inhalation exposure to
manganese sulfate at 0, 0.05, 0.5, or 1.0 mg manganese/m
3
starting 28 days prior to breeding through
PND 18, samples of maternal tissues (whole blood, lung, pancreas, liver, brain, femur, and placenta) and
fetal tissues (whole blood, lung, liver, brain, and skull cap) were collected and analyzed for manganese
concentrations. Elevated (p<0.05) manganese concentrations were observed in exposed maternal rats
(compared with air control rats) in the following tissues: brain and placenta at 0.5 and 1.0 mg
manganese/m
3
and lung at 0.05, 0.5, and 1.0 mg manganese/m
3
. In contrast, statistically significant
elevations of manganese concentrations in sampled fetal tissues were observed only in the liver at 0.5 and
1.0 mg manganese/m
3
. In pups born and allowed to live up to PND 19 (and sampled for tissue
evaluations at PNDs 1, 14, and 19), statistically significant (p<0.05) elevated manganese concentrations
(compared with air control values) were observed in blood, liver, and bone samples from exposed
neonatal rats at concentrations ≥0.05 mg manganese/m
3
, starting at PND 1 (Dorman et al. 2005a). As
shown in Table 3-11, elevated brain manganese concentrations were observed in exposed neonates
starting at PND 14 (but not at earlier time points); tissue concentrations increased with increasing
exposure concentration (Dorman et al. 2005a). At PND 19, mean manganese concentration in the
striatum was about 2.6-fold higher in offspring exposed to 1 mg manganese/m
3
, compared with air control
means (Table 3-11). In contrast, the mean striatum concentration at PND 19 in maternal rats exposed to
1 mg manganese/m
3
was about 1.7-fold increased, compared with controls (Table 3-11). At the lowest
concentration tested, 0.05 mg/m
3
(50 µg/m
3
), no statistically significant increase in manganese
concentrations in maternal striatum or cerebellum occurred, and increases in manganese concentrations in
brain regions of offspring at PND 19, were modest compared with controls (1.4–1.7-fold, Table 3-11).
The results from this study suggest that the brain in developing fetuses and neonates is partially protected
from excess manganese by the placenta, and that the neonatal period, compared with adulthood, is
relatively more susceptible to increased manganese concentration in brain tissues with inhalation
exposure to manganese sulfate aerosol concentrations between 0.05 and 1 mg manganese/m
3
.

MANGANESE
245
3. HEALTH EFFECTS
Table 3-11. Manganese Concentrations in Brain Tissues of Lactating CD Rats and
Offspring Exposed to Aerosols of Manganese Sulfate
Mean maternal concentrations at
Mean offspring concentrations
PND 18 (µg manganese/g)
b
(µg manganese/g)
c
Olfactory
Air level
a
Brain/striatum
Cerebellum
bulb
(mg Olfactory
manganese/m
3
)
Striatum
Cerebellum
bulb
PND 1
PND 14
PND 19
PND 19
PND 19
0
0.51±0.02
0.50±0.02
0.56±0.04
0.39
0.19
0.37
0.34
0.36
0.05
0.54±0.02
0.52±0.02
0.71±0.04
d
0.42
0.35
d
0.63
d
0.51
d
0.52
d
0.5
0.74±0.02
d
0.60±0.01
d
1.40±0.07
d
0.45
0.59
d
0.83
d
0.64
d
0.70
d
1
0.89±0.02
d
0.61±0.03
d
1.73±0.07
d
0.50
0.55
d
0.97
d
0.72
d
0.76
d
a
Rats were exposed for 6 hours/day starting 28 days prior to breeding through postnatal day (PND) 18 as reported by
Dorman et al. (2005a, 2005b).
b
Mean±SEM from Table 3 in Dorman et al. (2005a).
c
Means from Figure 4 in Dorman et al. (2005a). Bar graphs were digitized to obtain numerical estimates of means for
male and female offspring combined. At PNDs 1 and 14, whole brain tissues were analyzed. At PND 19, brains were
dissected into striatum, cerebellum, and olfactory bulb before analysis.
d
Significantly (p<0.05) different from air control mean.
PND = postnatal day; SEM = standard error of the mean
MANGANESE
246
3. HEALTH EFFECTS
Consistent with the empirical observations in Table 3-11, PBPK model predictions of manganese
concentrations in striatum, olfactory bulb, and cerebellum in PND 19 offspring of rat dams exposed by
inhalation under the exposure scenarios used by Dorman et al. (2005a, 2005b) indicated that brain
concentrations did not begin to increase in offspring until air concentrations exceeded 0.05–0.1 mg/m
3
(Yoon et al. 2009a, 2009b). A human PBPK model developed to predict average daily AUC for
manganese concentrations in the globus pallidus of the fetus, suckling neonate, and 3-year-old child from
airborne manganese concentrations indicated that globus pallidus concentrations progressively increased
beyond 10% of baseline concentrations in fetuses and 3-year-old children when air concentrations
exceeded 0.01 mg/m
3
(10 µg/m
3
) and in suckling neonates when air concentrations exceeded 0.001 mg/m
3
(Yoon et al. 2011).
In an examination of the distribution of manganese in young adult male and female CD rats (28 days at
start) and aged male CD rats (16 months at start) following 90-day inhalation exposure to manganese
sulfate or manganese phosphate, no evidence was found for a gender or age effect on delivery of
manganese to the striatum or on the order of manganese concentrations in tissues (pancreas> olfactory
bulb > femur > testes), but gender or age-related differences in tissue manganese concentrations in other
brain regions, as well as in the lung, pancreas, femur, and testis, were noted (Dorman et al. 2004a).
Following a 90-day inhalation exposure to 0.5 mg manganese/m
3
manganese sulfate, young adult male
rats had significantly (p<0.05) higher olfactory bulb, blood, femur, and pancreas manganese
concentrations than aged male rats, and aged male rats had significantly higher testis manganese
concentrations than young male rats. Young male rats exposed to 0.5 mg manganese/m
3
had significantly
higher olfactory bulb, blood, and lung manganese concentrations than similarly exposed female rats, and
female rats exposed to 0.5 mg manganese/m
3
had significantly higher cerebellum manganese
concentrations than control females. Young male and female rats exposed to 0.5 mg manganese/m
3
for
90 days had increased
54
Mn clearance rates than air-exposed controls, but similarly-exposed aged male
rats did not display increased
54
Mn clearance rates, compared with controls (Dorman et al. 2004a). No
age-related effects were observed on the order of manganese concentrations in the various tissue.
Manganese concentrations in striatum of young male rats exposed to 0.1 mg/m
3
manganese sulfate were
about 1.7-fold higher than concentrations in young male rats identically exposed to manganese phosphate
(Dorman et al. 2004a). These results are consistent with results from 14-day inhalation studies (Dorman
et al. 2001a) and intratracheal instillation studies (Roels et al. 1997) indicating that inhalation of more
soluble forms of manganese (e.g., manganese sulfate and manganese chloride) results in higher
MANGANESE
247
3. HEALTH EFFECTS
manganese concentrations in the brain than inhalation of less soluble forms, such as manganese
phosphate, manganese tetroxide, or manganese dioxide. Olfactory bulb and striatal concentrations were
about 2.5- and 3-fold higher, respectively, in rats exposed for 14 days by inhalation to 3 mg/m
3
manganese sulfate, compared with rats exposed identically to manganese phosphate (Dorman et al.
2001a).
No studies were located regarding distribution of manganese in human or animals following inhalation
exposure to MMT or mangafodipir.
3.4.2.2 Oral Exposure
Excess manganese uptake has occurred in humans following oral exposure, presumably via the diet, when
the individuals suffered from chronic liver disease or some other liver dysfunction (cirrhosis, portacaval
shunt, etc.). In these instances, excess manganese was shown to accumulate in certain regions of the
brain, as determined by T1-weighted MRI or neutron activation analysis (Devenyi et al. 1994; Fell et al.
1996; Hauser et al. 1994, 1996; Pomier-Layrargues et al. 1998; Rose et al. 1999; Spahr et al. 1996).
These studies show that manganese preferentially accumulates in the basal ganglia, especially the globus
pallidus, and the substantia nigra.
Rats given a single oral dose of 416 mg manganese/kg body weight (as manganese chloride tetrahydrate)
exhibited little tissue accumulation of manganese 14 days later (Holbrook et al. 1975). Studies in animals
indicate that prolonged oral exposure to manganese compounds results in increased manganese levels in
all tissues, but that the magnitude of the increase diminishes over time (Kristensson et al. 1986; Rehnberg
et al. 1980, 1981, 1982). Table 3-12 provides illustrative data based on rats exposed to 214 mg
manganese/kg(body weight)/day (as manganese tetroxide) for up to 224 days. As the data reveal, large
increases in tissue levels of manganese compared with the controls occurred in all tissues over the first
24 days, but levels tended to decrease toward the control levels as exposure was continued. This pattern
is thought to be due to a homeostatic mechanism that leads to decreased absorption and/or increased
excretion of manganese when manganese intake levels are high (Abrams et al. 1976a; Ballatori et al.
1987; Mena et al. 1967). Davis et al. (1992b) and Malecki et al. (1996b) demonstrated that rats fed
elevated levels of manganese for several weeks had increased tissue manganese concentrations, despite
increased gut endogenous losses of manganese, as biliary manganese. This reflected several factors.
Although the percentage of manganese absorbed decreased, the total amount of manganese absorbed
increased when higher levels of manganese were fed. Moreover, although the total amount of manganese

MANGANESE
248
3. HEALTH EFFECTS
Table 3-12. Manganese Levels in Rat Tissue After Oral Exposure
Tissue concentrations (percent of control)
a
Tissue
24 Days
60 Days
224 Days
Liver
810
137
138
Kidney
430
102
128
Brain
540
175
125
Testes
260
125
100
a
Values presented are the ratio (expressed as a percentage) of tissue levels of manganese in animals receiving
3,550 ppm manganese in the diet (as manganese tetroxide) compared to animals receiving a normal diet (50 ppm).
Source: Rehnberg et al. 1980
MANGANESE
249
3. HEALTH EFFECTS
lost in bile increased when manganese intake increased, the percentage of manganese intake lost in bile
remained constant at ~1% of manganese intake (Malecki et al. 1996b).
A study measuring the retention of a single oral dose of radiolabeled manganese in adult and neonatal rats
indicated that retention of the label 6 days after exposure was much greater in pups (67%) than in adults
(0.18%); the addition of manganese to the animals' drinking water decreased radiolabel retention in pups
and adults (Kostial et al. 1989).
The distributional differences in rats exposed to either manganese chloride or manganese dioxide by
gavage were investigated by Roels et al. (1997). After administration of 24.3 mg manganese/kg body
weight (as manganese chloride) once weekly for 4 weeks, the authors analyzed blood and brain
concentrations of the metal. Manganese concentrations were significantly elevated in the blood
(approximately 83% increase over controls) and the cortex of the brain (approximately 39% increase over
controls). Gavage administration of manganese dioxide, by contrast, did not significantly increase the
amount of manganese in blood or any section of the brain. In addition, administration of manganese as
manganese chloride by gavage caused roughly the same amount of increased manganese in the blood as
intratracheal administration of manganese in the same form; it did not cause as significant an increase of
manganese in the cortex (Roels et al. 1997). These data indicate that inhalation exposure to manganese in
the form of manganese chloride or manganese dioxide causes accumulation of manganese in the brain
more readily than oral exposure.
Acute manganese exposure in drinking water was found to alter brain regional manganese levels in
neonatal rats; after 5 days of exposure, the highest level was in the striatum (12.05 μg/g wet weight) and
the lowest level was in the cerebral cortex (0.85 μg/g wet weight) (Chan et al. 1992). After 10 days, the
highest concentrations were in the pons and medulla and the lowest were in the hypothalamus. Regional
manganese differences were less pronounced in weanling and adult rats. A study by Lai et al. (1991)
confirms that intermediate exposure to manganese in drinking water increases brain manganese
concentrations; rats exposed from conception to 120 days at 0.04 or 0.4 mg manganese/kg/day had mean
brain manganese levels of 0.36–0.72 μg/g in the low-dose animals and 0.62–1.35 μg/g in the high-dose
animals, compared to 0.21–0.38 μg/g in controls.
In a dietary study, elevated manganese levels were found in the organs of male mice fed manganese
chloride, manganese acetate, manganese carbonate or manganese dioxide at 284 mg manganese/kg/day
for 100 days; levels of manganese in the liver and kidney were significantly higher in the animals exposed
MANGANESE
250
3. HEALTH EFFECTS
to manganese acetate or manganese carbonate than in those exposed to manganese chloride or manganese
dioxide (Komura and Sakamoto 1991). In a 1993 NTP study, mice and rats chronically fed manganese
sulfate generally exhibited elevated tissue levels of manganese; the manganese levels in the liver and
kidney were higher than the levels in the brain.
No studies were located concerning disposition of manganese in humans or animals following oral
exposure to MMT or mangafodipir.
3.4.2.3 Dermal Exposure
No studies were located regarding tissue distribution of manganese in humans or animals after dermal
exposure to inorganic manganese.
No studies were located regarding tissue distribution of manganese in humans or animals after dermal
exposure to organic manganese.
3.4.2.4 Other Routes of Exposure
No studies were located regarding tissue distribution of inorganic manganese in humans after exposure
via other routes of exposure.
A number of studies have been conducted that investigated various facets of the distribution of inorganic
manganese in animal models. The studies utilized a number of routes of administration, and the results
suggested that route may play an important role in distribution. In an intraperitoneal study performed in
monkeys, manganese was reported in all tissues studied. The highest levels were found in the pancreas,
liver, and kidney, and the lowest levels were found in the blood; levels in the central nervous system were
found to decrease more slowly than those in other tissues (Dastur et al. 1971). Calves injected
intravenously with
54
Mn were found to have 3-fold higher liver manganese concentrations and 13-fold
higher pancreatic manganese concentrations than calves fed manganese (Carter et al. 1974). Davis et al.
(1993) observed that rats injected intraportally with free
54
Mn or
54
Mn complexed with transferrin and rats
injected intraperitoneally with free
54
Mn accumulated more manganese in the pancreatic tissue and less in
the liver than those rats that were either fed
54
Mn or injected intravenously in the portal vein with an
albumin-
54
Mn complex. The similarity in the distribution of the injected manganese-albumin complex
and the free manganese in the diet when compared to the distribution of manganese when it was
MANGANESE
251
3. HEALTH EFFECTS
administered by other routes or complexed with other proteins suggests that the route of administration
and type of complexed protein may cause differences in the transport of manganese in the sera.
Roels et al. (1997) studied the effect of intraperitoneal administration of manganese chloride and
manganese dioxide on distributional differences of manganese in rats. Doses of 1.22 mg manganese/kg
as manganese chloride given once per week for 4 weeks resulted in significant increases (when compared
to controls) in blood (approximately 60%), striatum (34%), and cortex (36%) concentrations of
manganese; no changes were observed in the cerebellum. Identical dosing of rats with manganese
dioxide resulted in significant increases in manganese levels in blood (79%), cerebellum (40%), striatum
(124%), and cortex (67%) over those in controls. These data indicate that administration of manganese
dioxide by this route resulted in greater accumulation of manganese in the brain than did manganese
chloride.
The distribution of manganese in the brain was investigated using Cebus (Newland and Weiss 1992;
Newland et al. 1989) and Macaque (Newland et al. 1989) monkeys given intravenous injections of
manganese chloride that reached a cumulative dose of 10–40 mg manganese/kg. Magnetic resonance
images indicated hyper-intensity of the globus pallidus and substantia nigra consistent with an
accumulation of manganese in these areas (Newland and Weiss 1992; Newland et al. 1989). Substantial
accumulation of manganese was also noted in the pituitary at low cumulative doses (Newland et al. 1989).
London et al. (1989) reported a rapid localization of manganese in the choroid plexus observed on MRI;
similarly, radiotracer studies of manganese injected into the intracerebroventricular space revealed that
radiolabeled manganese was located in the choroid plexus within 1 hour and was located in the rat dentate
gyrus and CA3 of the hippocampus 3 days post-dosing (Takeda et al. 1994).
No studies were located regarding disposition of MMT in humans following other routes of exposure, but
toxicokinetics of MMT following parenteral administration has received some research attention in
animals.
Young adult male rats were administered MMT dissolved in propylene glycol via subcutaenous injection
at a dose of 1 mg manganese/kg (McGinley et al. 1987). Control rats received vehicle alone. The rats
were sacrificed 1.5, 3, 6, 12, 24, 48, or 96 hours post-injection. Levels of manganese in the control
animals were measured in the blood (0.09±0.01 mg/kg), lung (1.51±0.22 mg/kg), liver
(2.49±0.36 mg/kg), kidney (1.29±0.23 mg/kg), and brain (0.45±0.01 mg/kg). These values were assumed
by the authors to originate from the feed given to the rats and were subtracted from similar values
MANGANESE
252
3. HEALTH EFFECTS
analyzed for MMT-treated rats to determine the amount of manganese in these tissues and fluids that
originated from MMT. Maximum accumulation of MMT-derived manganese was measured 3 hours after
dosing and was found primarily in the following four tissues: lung (~9 mg/kg); kidney (3.9 mg/kg); liver
(2.75 mg/kg); and blood (~0.75 mg/kg). Concentrations of manganese in these four tissues was still
elevated (~1 mg/kg) at 96 hours post-dosing. Brain manganese concentrations were not significantly
elevated over control levels in MMT-treated animals (McGinley et al. 1987).
Gianutsos et al. (1985) administered 0, 11, or 22 mg manganese/kg as MMT (dissolved in propylene
glycol) to male adult mice via subcutaneous injection to determine distribution of manganese. Control
mice received vehicle alone. Mice were sacrificed at different time points after dosing. The experiment
was divided into an acute study (one dose) or a “chronic study” (ten doses). The brain manganese level
24 hours after the single dose of MMT at 11 mg/kg was 0.93±0.07 μg/g; the value after 22 mg/kg was
1.35±.09 μg/g. Both values were significantly different from the control value of 0.61±0.08 μg/g. The
brain manganese level in the mice administered 10 doses of 11 mg/kg each was 1.37±0.27 μg/g; after
10 doses of 22 mg/kg, the value was 3.33±0.15 μg/g; both were significantly greater than the control
value of 0.64±0.06 μg/g, and were significantly different than the levels reported after the acute exposure.
Manganese levels in the brains of mice given a single dose of MMT at 22 mg manganese/kg were
compared with those following injection of the same manganese dose as manganese chloride; mice were
sacrificed at different time points from 1–24 hours post-dosing. The brain manganese levels following
MMT exposure increased from a low at 1 hour to a maximum at 24 hours of ~1.4 μg/g wet weight. The
manganese level in brain after manganese chloride exposure followed the same increasing trend over the
24 hour analysis period, but was higher at each time point, with a maximum value of >2.0 μg/g wet
weight (Gianutsos et al. 1985).
Clinical studies involving cancer patients or healthy volunteers have analyzed the usefulness of
mangafodipir as a contrast agent for the identification of certain abdominal tumors. Although these
studies do not necessarily quantify the amount of manganese, or mangafodipir, in particular tissues, they
are useful tools in identifying the location of the metal; also relative proportions of manganese among two
or more tissues that contain the metal can be observed by differences in signal from these imaging studies.
Several studies have shown the qualitative presence of manganese in the liver due to increased signal in
that organ following mangafodipir administration of 0.17–0.83 mg manganese/kg upon T1-weighted MRI
(Bernardino et al. 1992; Lim et al. 1991; Padovani et al. 1996; Wang et al. 1997). Two studies show that
the human liver takes up more of the manganese from mangafodipir than any other organ: the signal from
MANGANESE
253
3. HEALTH EFFECTS
the liver was roughly 2 times the amount from the spleen after dosing with 0.55 mg manganese/kg (Lim
et al. 1991); the liver signal after dosing with 0.55 mg manganese/kg had reached a 100% increase over
baseline signal by 20 minutes following post-dosing, whereas the maximal signal from other organs was
only 80% in the pancreas, ~30% in the spleen, ~90% in the renal medulla, and 50% in the choroid plexus,
all at the same dose. The renal cortex was the only other tissue to reach a 100% increase over baseline
signal at 0.55 mg manganese/kg. Dosing with 0.25 mg manganese/kg (the clinically used dose for current
MRI testing of patients) resulted in a similar distribution pattern, although the signal was decreased
compared to the higher dose. The signal from the renal cortex at the lower dose had a maximum of 80%
over baseline, whereas the signal in the liver at this dose was ~75% of the baseline value (Wang et al.
1997).
Several studies have determined the distribution of manganese in tissues of animals following intravenous
administration of mangafodipir. Grant et al. (1994) reported that in rats injected with 2 times the clinical
dose of [
54
Mn] mangafodipir (0.55 mg manganese/kg), the carcass retained 8% of the label and the tissues
retained 7% of the label; individual tissue concentrations of manganese were not reported.
Gallez et al. (1997) injected adult male mice once with 0.25 mg manganese/kg as [
54
Mn] mangafodipir
(clinical dose) and determined the tissue manganese content at time points ranging from 15 minutes to
3 months post-dosing. Brain concentration of
54
Mn did not reach a maximum value of 0.26±0.04 (value
is the percent of injected dose/g tissue) until 24 hours post-dosing; this value was not different than the
brain manganese content of mice injected with manganese chloride
.
This maximum value was still
observed in the brain 2 weeks post-dosing, but measurements taken at 1 and 3 months post-dosing were
below the detection limit. By contrast, manganese from manganese chloride was still detectable, although
not at maximal levels, at 3 months’ time. Liver manganese reached a maximum value of 7.5±1.4 (percent
dose/g tissue) 15 minutes post-dosing and then decreased to below the detection limit 1 month later.
Male and female Sprague-Dawley rats injected with [
54
Mn] mangafodipir at a dose of 5.5 mg
manganese/kg had the following distribution of labeled manganese 30 minutes post-dosing (values are
given in percent injected dose/g tissue): liver, 1.3; kidney, 1.2; heart, 0.25; spleen, 0.2; blood, 0.3; small
bowel, 1.3; large bowel, 0.5; muscle, 0.1; and brain, negligible. Distribution of manganese in tissues of
rats injected with labeled manganese chloride was compared to the previous results, and for all tissues, the
label was greater after administration with the chloride than from the mangafodipir, with the exception of
kidney and large bowel, but these differences were not significant (Elizondo et al. 1991).
MANGANESE
254
3. HEALTH EFFECTS
The distribution of label in male and female Sprague-Dawley rats injected with either [
54
Mn] or [
14
C]
mangafodipir at a dose of 0.39 or 0.55 mg manganese/kg, respectively, was studied by Hustvedt et al.
(1997). The plasma concentration of labeled manganese reached a peak of 10.2 μg/mL at 5 minutes post-
dosing and was quickly distributed into the following organs (values given as μg equivalents of
compound/g): pancreas, 10.2; liver, 4.0; kidneys, 3.6; testes/ovaries, 1.7; spleen, 1.0; heart, 0.9; and
brain, 0.69. When the bile duct was cannulated, the distribution of an equivalent dose of mangafodipir
showed an increased retention of labeled manganese in all organs but the brain (0.62): pancreas, 17.2;
liver, 12.3; kidneys, 10.1; testes/ovaries, 5.6; small intestine, large intestine and heart, 2.1; and spleen,
1.9. By contrast, tissue retention of
14
C from radiolabeled mangafodipir was very low: pancreas, 0.016;
liver, 0.045; kidneys, 0.067; testes/ovaries, 0.015; spleen, 0.023; small intestine, 0.012; large intestine,
0.019; heart, 0.017; and brain, 0.009. These data indicate that manganese dissociates from the fodipir
moiety after mangafodipir administration and partitions into the tissues listed above.
The tissue distribution of normal and bile-cannulated dogs following administration of [
54
Mn] or [
14
C]
mangafodipir was also studied (Hustvedt et al. 1997). Doses of 0.55 manganese/kg were used except for
the normal dogs when the manganese was labeled; the dose in this case was 0.38 mg/kg. The general
pattern of distribution of manganese and carbon was similar to that seen with rats, except the
concentrations were increased in the dog. The values for normal dogs were taken 168 hours post-dosing
for both forms of labeled mangafodipir; the bile-cannulated dogs were analyzed 24 hours post-dosing.
The maximum concentration of
54
Mn in the plasma following dosing was 13.1 μg/mL at the end of the
infusion period. The plasma concentrations declined rapidly with a terminal half-life of approximately
15 minutes. In the normal dog and bile-cannulated dog, the tissue distribution was as follows (the values
for the bile-cannulated dog are given in parentheses; all values are in μg equivalents of compound/g):
liver, 8.7 (79.8); pancreas, 8.1 (2.5); kidneys, 6.6 (37.5); bile, 5.9 (no sample); testes/ovaries, 2.2 (3.2);
brain, 0.79 (1.1); spleen, 0.65 (26.6); and heart, 0.62 (3.1). The distribution of labeled carbon in normal
(or bile-cannulated dogs) was the following: kidneys, 0.79 (4.1); liver, 0.13 (0.48); bile, 0.059 (no
sample); testes/ovaries, 0.05 (0.079); pancreas, 0.015 (0.11); heart, 0.015 (0.035); spleen, 0.007 (0.15);
and brain, not detected (not detected). These data indicate that in the dog, as in the rat, the manganese
cation is retained by the tissues, but the fodipir moiety is not.
Distribution of
54
Mn and
14
C following mangafodipir administration was also studied in the pregnant rat
(Hustvedt et al. 1997). Whole-body autoradiography of a section of the rat made at different time points
revealed that the kidney had retained the highest amount of labeled manganese; later time points showed a
distribution similar to those seen in the rat and dog studies mentioned previously with the pancreas and
MANGANESE
255
3. HEALTH EFFECTS
liver causing the most intense signal upon autoradiography. By 24 hours, fetal livers and bones were
clearly seen, but placental radioactivity had decreased substantially. Fat deposits also contained a
significant amount of the radioactivity at 24 hours. By contrast, radioactivity from labeled carbon in the
mangafodipir was relatively uniformly distributed throughout the pregnant rat at 5 minutes and 1 hour
post-dosing, with the highest levels in the kidneys. At 24 hours, virtually all tissues were
indistinguishable from background.
The human distribution studies have involved much shorter observation times than the animal studies,
with maximal increase in MRI signal in human studies observed in minutes following administration.
These studies have shown the liver to accumulate the highest amount of manganese from the administered
dose of mangafodipir. This is an important limitation since the brain, the primary target of manganese
neurotoxicity, may not accumulate a significant amount of manganese until much later, possibly after the
current experiments in humans and animals were truncated. Experiments in rats and dogs, both normal
and bile-cannulated, indicate that the brain does not accumulate a significant amount of manganese
following administration of mangafodipir at levels much higher than the recommended clinical dose of
the agent (Hustvedt et al. 1997), even at 168 hours post-dosing in the dog. Gallez et al. (1997) reported
that manganese accumulation in the brain of adult mice following injection of a clinical dose of
mangafodipir did not reach maximal levels until 24 hours post-dosing. This would indicate that the
human distribution studies were terminated prematurely. However, while brain accumulation of
manganese following mangafodipir administration is similar to that from manganese chloride, the
manganese is not present after 2 weeks, whereas manganese from the inorganic compound was present,
although at a decreased amount, 3 months following dosing (Gallez et al. 1997). These data indicate that
single, clinical doses of mangafodipir are not likely to cause persistent accumulation of manganese in the
brain.
3.4.3 Metabolism
Manganese is capable of existing in a number of oxidation states, and limited data suggest that inorganic
manganese may undergo changes in oxidation state within the body. Circumstantial support for this
hypothesis comes from the observation that the oxidation state of the manganese ion in several enzymes
appears to be Mn(III) (Leach and Lilburn 1978; Utter 1976), while most manganese intake from the
environment is either as Mn(II) or Mn(IV) (see Chapter 6). Another line of evidence is based on
measurements of manganese in tissues and fluids using electron spin resonance (ESR), which detects the
unpaired electrons in Mn(II), Mn(III), and Mn(IV). When animals were injected with manganese
MANGANESE
256
3. HEALTH EFFECTS
chloride, levels of manganese increased in bile and tissues, but only a small portion of this was in a form
that gave an ESR signal (Sakurai et al. 1985; Tichy and Cikrt 1972). This suggests that Mn(II) is
converted to another oxidation state (probably Mn(III)), but it is also possible that formation of complexes
between Mn(II) and biological molecules (bile salts, proteins, nucleotides, etc.) results in loss of the ESR
signal without oxidation of the manganese ion.
Evidence by Gibbons et al. (1976) suggests that oxidation of manganese occurs in the body. It was
observed that human ceruloplasmin led to the oxidation of Mn(II) to Mn(III) in vitro, and although the
process was not studied in vivo, it is a likely mechanism for manganese oxidation in the blood. These
authors also noted that manganese oxidation led to a shift in manganese binding in vitro from α
2
-macro-
globulin to transferrin and that in vivo clearance of Mn(II)-α
2
-macroglobulin from cows was much more
rapid than the clearance of Mn(III)-transferrin (Gibbons et al. 1976). This suggests that the rate and
extent of manganese reduction/oxidation reactions may be important determinants of manganese retention
and toxicity in the body.
As demonstrated in a study by Komura and Sakamoto (1991), tissue levels of manganese in rats were
affected by the form in which the manganese was administered in the diet; levels of manganese were
significantly higher in animals fed manganese acetate or manganese carbonate than in animals fed
manganese chloride or manganese dioxide.
Reaney et al. (2006) compared brain concentrations of manganese, dopamine, and gamma amino butyric
acid in female retired breeder Long Evans rats exposed to cumulative intraperitoneal doses of 0, 30, or
90 mg manganese/kg of Mn(II) chloride or Mn(III) pyrophosphate. Rats were given intraperitoneal doses
of 0, 2, or 6 mg manganese/kg, 3 times/week for 5 weeks. In Mn(III)-treated rats, brain manganese
concentrations (analyzed in the striatum, globus pallidus, thalamus, and cerebrum regions) and blood
concentrations were higher than brain concentrations in Mn(II)-treated rats. The only other marked
changes in end points between the two treatment groups was that the highest Mn(III) exposure group
showed a 60% increased dopamine level in the globus pallidus (compared with controls), whereas the
comparably treated Mn(II) rats showed a 40% decrease in globus pallidus dopamine level. These results
suggest that manganese valence state can influence tissue toxicokinetic behavior, and possibly toxicity.
MMT. Following intravenous administration in the male rat, MMT was metabolized to hydroxyl-
methylcyclopentadienyl manganese tricarbonyl (CMT-CH
2
OH) and carboxycyclopentadienyl manganese
tricarbonyl (CMT-COOH), both of which are present in urine (Hanzlik et al. 1980a). Metabolites are also
MANGANESE
257
3. HEALTH EFFECTS
present in the bile, as indicated by the fecal recovery of
3
H from the ring structure in MMT following
intravenous or intraperitoneal administration of radiolabeled compound to rats (Hanzlik et al. 1980a,
1980b). After intravenous dosing of MMT in rats, 11% of the radiolabel was recovered in feces within
30 minutes (Hanzlik et al. 1980b). These metabolites have not been characterized; however, the
administration of phenobarbitol to the rat doubled the biliary excretion of the metabolite (Hanzlik et al.
1980a).
In vitro studies showed that rat liver microsomes activated with NADPH and molecular oxygen
metabolized MMT (Hanzlik et al. 1980b). Preliminary studies with pooled liver microsomes from 5 to
6 normal or phenobarbital-induced rats showed that reaction rates of metabolism were linear for the first
20 minutes. MMT and aminopyrine, a positive control compound that is metabolized exclusively by
cytochrome P450, showed parallel responses to changes in incubation conditions (i.e., NADPH
dependence, inhibition by carbon monoxide, induction by phenobarbital). Liver microsomes metabolized
MMT with an estimated K
M
of 78 μM and a V
max
of 3.12 nmol/mg protein/minute. When the studies
were done with liver microsomes from phenobarbital-treated rats, the K
M
remained the same, but the V
max
doubled (Hanzlik et al. 1980b). Lung microsomes were equally capable of metabolizing MMT, but
phenobarbital induction did not enhance the response.
In humans, an infusion of the clinical dose of MnDPDP (5 μmol/kg or 0.25 mg/kg) is rapidly
dephosphorylated to manganese dipyridoxyl monophosphate (MnDPMP). This metabolite has been
measured in human blood as quickly as 18 minutes after the beginning of infusion of the contrast agent,
and is still measurable 1.3 hours after the start of the infusion (Toft et al. 1997a). MnDPMP was not
observed in the blood after the first 18 minutes. The monophosphate is then fully dephosphorylated to
manganese dipyridoxyl ethylenediamine (MnPLED); this compound has been isolated in blood from
18 minutes after the start of an infusion until 40 minutes after the start. Transmetallation of either
MnDPDP, MnDPMP, or MnPLED with zinc can occur, forming ZnDPDP, ZnDPMP, or ZnPLED.
ZnDPDP has been identified in the bloodstream during the first 18 minutes of an infusion of 0.25 mg
manganese/kg as MnDPDP. ZnDPMP has been detected in the blood from 18 to 40 minutes following
the start of the infusion, and ZnPLED has been measured in the blood from 18 minutes to 8.33 hours
following the start of the infusion. The major metabolite detected in urine was ZnPLED (Toft et al.
1997a). Figure 3-4 depicts the metabolism of mangafodipir in the human.
To study mangafodipir metabolism in the dog, Toft et al. (1997c) injected three male and female beagles
with 0.55, 1.7, or 5.5 mg manganese/kg and took timed blood samples post-dosing to analyze for the

Figure 3-4. Metabolism of MnDPDP
-
PO
4
PO
4
-
MnDPMP MnPLED
MnDPDP
PO
4
-
PO
4
-
H
2
O H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
-
PO
4
ZnDPDP ZnDPMP ZnPLED
-
-
PO
4
PO
4
Source: Toft et al. 1997c
MANGANESE
258
3. HEALTH EFFECTS
MANGANESE
259
3. HEALTH EFFECTS
presence of metabolites. Mangafodipir was rapidly metabolized by dephosphorylation and
transmetallation at all three doses. After infusion with 0.55 mg/kg, MnPLED was the primary metabolite
observed in the bloodstream 1 minute after the end of the infusion period, and MnDPDP was present at a
concentration lower than the five metabolites. At 30 minutes post-dosing, ZnPLED was the main
metabolite. However, at 5.5 mg/kg, MnPLED was the main metabolite at all sampling times (1, 5, and
30 minutes). The authors estimated that the ratios of manganese metabolites to zinc metabolites were 1,
2, and 3.5 at doses of 0.55, 1.7, or 5.5 mg manganese/kg, respectively; these data are consistent with the
authors’ hypothesis that the limited availability of free or loosely bound plasma zinc governs the initial
transmetallation reaction (Toft et al. 1997c).
In vitro experiments with radiolabeled MnDPDP and whole blood or plasma from human donors indicate
that mangafodipir undergoes a rapid transmetallation with zinc that is nearly complete within 1 minute
after the start of incubation, followed by a relatively slow dephosphorylation process. The primary
metabolite after a 90-minute incubation of whole blood with MnDPDP was MnDPMP, followed by
CaDPDP/DPDP, Mn(III)DPDP (suggested as an artifact due to high pH and oxygen), and MnPLED.
Experiments using
14
C-DPDP indicate that this chelate cannot enter red blood cells; therefore, the zinc
contained within the cells is unavailable for binding to this compound. Binding of manganese ion to
serum proteins was observed as well, indicating that dissociation of the metal from the chelate had
occurred during incubation (Toft et al. 1997b).
3.4.4 Elimination and Excretion
In humans, absorbed manganese is removed from the blood by the liver where it conjugates with bile and
is excreted into the intestine. Biliary secretion is the main pathway by which manganese reaches the
intestines where most of the element is excreted in the feces (Bertinchamps et al. 1965; Davis et al. 1993;
Malecki et al. 1996). However, some of the manganese in the intestine is reabsorbed through
enterohepatic circulation (Schroeder et al. 1966).
Small amounts of manganese can also be found in urine, sweat, and milk (EPA 1993b). Urinary excretion
of manganese by healthy males was 7.0 nmole/g creatinine (7.0 nmole=385 ng=0.385 μg) (Greger et al.
1990). Similarly, urinary manganese excretion by women was 9.3 nmole/day. Moreover, urinary
excretion of manganese was not responsive to oral intake of manganese (Davis and Greger 1992). Dorner
et al. (1989) showed that some infants fed breast milk and formula suffered negative manganese balances
due to high fecal excretion. However, animal studies indicate that in the young, excretion is not well-
MANGANESE
260
3. HEALTH EFFECTS
developed and may result in increased retention of the element. For example, in mice, rats, and kittens,
there is an almost complete absence of excretion during the neonatal period (Cotzias et al. 1976).
However, data in neonatal rats indicate that manganese retention rates decrease to rates observed in adult
animals. This is indirect evidence that excretion may mature during the end of the neonatal period though
the exact time frame across species is unknown.
3.4.4.1 Inhalation Exposure
In humans who inhaled manganese chloride or manganese tetroxide, about 60% of the material originally
deposited in the lung was excreted in the feces within 4 days (Mena et al. 1969). Chronically exposed
male workers were reported to have urine manganese levels that were significantly higher than unexposed
persons; for example, male foundry workers had a mean manganese level of 5.7 μg/L compared to
0.7 μg/L in unexposed controls (Alessio et al. 1989). Other studies have reported significantly increased
levels of urinary manganese in men occupationally exposed to airborne manganese dusts and fumes
(Lucchini et al. 1995; Roels et al. 1987a, 1992). Mergler et al. (1994) did not report a significant
difference in urinary manganese levels between the exposed and control groups in their occupational
study. The differences in urinary excretion may be due to differences in duration or extent of exposure.
A listing of these occupational studies that measured exposure levels of manganese and the resultant
levels of the metal in biological samples is provided in Table 3-13.
Rats exposed to either manganese chloride or manganese tetroxide by intratracheal instillation excreted
about 50% of the dose in the feces within 3–7 days (Drown et al. 1986). Monkeys exposed to an aerosol
of
54
MnCl
2
excreted most of the manganese, with a half-time of 0.2–0.36 days (Newland et al. 1987).
However, a portion of the compound was retained in the lung and brain. Clearance of this label was
slower, occurring with half-times of 12–250 days. These data do not provide information on how much
of the manganese excreted in the feces after inhalation exposure was first absorbed and then excreted via
the bile versus the amount simply transported directly from the lung to the gastrointestinal tract where it
may have been absorbed. In addition, because these investigators measured manganese using gamma
spectrometry techniques, the relatively long elimination half-times from the brain may have been
influenced by manganese present in skull bones. In monkeys exposed to 1.5 mg manganese/m
3
manganese sulfate for 65 days, manganese concentrations were elevated (compared with air control
values) in many brain regions and other tissues; 45 days following cessation of exposure, concentrations
remained elevated in the olfactory cortex, globus pallidus, putamen, pituitary gland, and blood, but
returned to air control values by 90 days after exposure (Dorman et al. 2006a). Based on these data,

MANGANESE
261
3. HEALTH EFFECTS
Table 3-13. Levels of Manganese in Exposed and Non-Exposed Workers
Biological samples
Occupational study
Mean age
(years)
Mn in air (mg/m
3
)
Mn-blood μg/100
mL
Mn-urine μg/g
creatinine
Roels et al. (1987b)
Exposed
34.3±9.6
0.97
a
(total dust)
1.36
b
±0.64 (1.22)
c
4.76
b
(0.4)
c
Non-exposed
38.4±11.3
0.57
b
±0.27 (1.59)
c
0.30
b
(0.15)
c
Roels et al. (1992)
Exposed
31.3±7.4
0.179
a
(respirable dust)
0.81
c
0.84
c
Non-exposed
29.3±8.0
0.68
c
0.09
c
Chia et al. (1993a)
Exposed
36.6±12.2
1.59
b
(total dust)
2.53
c
6.1
c
(μg/L)
Non-exposed
35.7±12.1
2.33
c
3.9
c
(μg/L)
Mergler et al. (1994)
Exposed
43.4±5.4
0.032
a
(respirable dust)
1.12
b
(1.03)
c
1.07
b
(0.73)
c
Non-exposed
43.2±5.6
0.72
b
(0.68)
c
1.05 (0.62)
c
Lucchini et al. (1999)
Exposed
42.1±8.3
0.0967 (respirable dust)
0.97
b
(0.92)
c
1.81
b
(1.53)
c
(CEI/years)
Non-exposed
42.6±8.8
0.6
b
(0.57)
c
0.67
b
(0.40)
c
a
Median
b
Arithmetic mean
c
Geometric mean
CEI = cumulative exposure index
MANGANESE
262
3. HEALTH EFFECTS
Dorman et al. (2006a) calculated elimination half-lives of about 15–16 days for the globus pallidus and
putamen, suspected neurotoxicity targets of manganese.
Rat studies have demonstrated that urinary excretion of manganese 1 day following inhalation exposure
was increased 200- and 30-fold when the animals were treated with the chelating agents
1,2-cyclohexylene-aminetetraacetic acid (CDTA) and diethylene triamine pentaacetic acid (DTPA),
respectively, but fecal excretion was not altered (Wieczorek and Oberdörster 1989b).
No studies were located regarding excretion of manganese in either humans or animals following
inhalation exposure to organic manganese.
3.4.4.2 Oral Exposure
Humans who ingested tracer levels of radioactive manganese (usually as manganese chloride) excreted
the manganese with whole-body retention half-times of 13–37 days (Davidsson et al. 1989a; Mena et al.
1969; Sandstrom et al. 1986). The route of manganese loss was not documented, but was presumed to be
mainly fecal after biliary excretion. Serum manganese concentrations in a group of healthy men and
women in Wisconsin were 1.06 and 0.86 μg/L, respectively (Davis and Greger 1992; Greger et al. 1990).
Urinary excretion of manganese by men was 7.0 nmole/g creatinine (Greger et al. 1990). Similarly,
urinary manganese excretion of women was 9.3 nmole/day. Moreover, urinary excretion of manganese
was not responsive to oral intake of manganese (Davis and Greger 1992).
In a more recent study, young rats fed 45 mg manganese/kg/day were found to absorb 8.2% of the
manganese ingested and to lose approximately 37% of the absorbed manganese through endogenous gut
secretions (Davis et al. 1993).
The daily excretion of manganese from mice ingesting 11 mg manganese/kg as MMT in their daily diet
was 5.4% of their daily intake (Komura and Sakamoto 1992b). In a comparison of plasma manganese
kinetics following oral administration of MMT or manganese chloride in male rats, MMT-derived
manganese was eliminated extremely slowly, having an average elimination half-time of 55.2 hours,
compared with 4.56 hours for manganese chloride (Zheng et al. 2000). Rats receiving MMT showed an
apparent oral clearance (CL/F) of 0.09 L.hours
-1
.kg
-1
, which was about 37-fold less than the oral clearance
of manganese chloride (CL/F = 3.2 L.hours
-1
.kg
-1
). Accordingly, the AUC in MMT rats was about
37-fold higher than that in manganese chloride rats who received equivalent dose of manganese. A
MANGANESE
263
3. HEALTH EFFECTS
gender difference in manganese toxicokinetics following oral MMT exposure was also observed; female
rats showed higher mean AUC and longer half times of plasma manganese than male rats (93.1 versus
51.8 mM hours and 68.4 versus 42.0 hours, respectively (Zheng et al. 2000).
No other studies were located regarding excretion of manganese from organic manganese compounds in
either humans or animals.
3.4.4.3 Dermal Exposure
No studies were located regarding excretion of inorganic or organic manganese in humans or animals
after dermal exposure to manganese.
3.4.4.4 Other Routes of Exposure
No studies were located regarding excretion of manganese by humans after exposure to inorganic
manganese via other routes of exposure.
Rats exposed to manganese chloride by intravenous injection excreted 50% of the dose in the feces within
1 day (Klaassen 1974) and 85% by day 23 (Dastur et al. 1971), indicating that biliary excretion is the
main route of manganese clearance. Only minimal levels were excreted in urine (<0.1% of the dose
within 5 days) (Klaassen 1974). Direct measurement of manganese levels in bile revealed concentrations
up to 150-fold higher than in plasma, indicating the existence of either an active transport system
(Klaassen 1974) or some sort of trapping mechanism (Tichy and Cikrt 1972). Based on the difference in
blood levels following portal or femoral injection, Thompson and Klaassen (1982) estimated that about
33% of the manganese burden in blood is removed in each pass through the liver. Apparently, some
manganese can cross directly from the blood to the bile (Bertinchamps et al. 1965; Thompson and
Klaassen 1982), but most appears to be secreted into the bile via the liver (Bertinchamps et al. 1965).
The chemical state of manganese in bile is not known, but a considerable fraction is bound to bile
components (Tichy and Cikrt 1972). This material is apparently subject to enterohepatic recirculation,
since biliary manganese is reabsorbed from the intestine more efficiently than free Mn(II) (Klaassen
1974). The amount of manganese that contributes to total body burden following reabsorption from
enterohepatic recirculation is not known.
MANGANESE
264
3. HEALTH EFFECTS
While biliary secretion appears to be the main pathway by which manganese is excreted into the
intestines, direct transport from blood across the intestinal wall may also occur (Bertinchamps et al. 1965;
Garcia-Aranda et al. 1984). The relative amount of total excretion attributable to this pathway was not
quantified by Bertinchamps, but it appears to be only a fraction of that attributable to biliary secretion
(Bertinchamps et al. 1965).
Manganese originating from mangafodipir administered at clinical (0.25 mg/kg) and more than twice the
clinical dose (0.55 mg/kg) is primarily excreted in the feces via the bile in both humans and animals
(Grant et al. 1994; Hustvedt et al. 1997; Toft et al. 1997a; Wang et al. 1997). In contrast to the chelate,
DPDP, manganese is incompletely cleared from the body 24 hours after administration, and roughly 7–
8% of a dose is still retained in the body after 1 week (Hustvedt et al. 1997).
3.4.5 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models
Physiologically based pharmacokinetic (PBPK) models use mathematical descriptions of the uptake and
disposition of chemical substances to quantitatively describe the relationships among critical biological
processes (Krishnan et al. 1994). PBPK models are also called biologically based tissue dosimetry
models. PBPK models are increasingly used in risk assessments, primarily to predict the concentration of
potentially toxic moieties of a chemical that will be delivered to any given target tissue following various
combinations of route, dose level, and test species (Clewell and Andersen 1985). Physiologically based
pharmacodynamic (PBPD) models use mathematical descriptions of the dose-response function to
quantitatively describe the relationship between target tissue dose and toxic end points.
PBPK/PD models refine our understanding of complex quantitative dose behaviors by helping to
delineate and characterize the relationships between: (1) the external/exposure concentration and target
tissue dose of the toxic moiety, and (2) the target tissue dose and observed responses (Andersen and
Krishnan 1994; Andersen et al. 1987). These models are biologically and mechanistically based and can
be used to extrapolate the pharmacokinetic behavior of chemical substances from high to low dose, from
route to route, between species, and between subpopulations within a species. The biological basis of
PBPK models results in more meaningful extrapolations than those generated with the more conventional
use of uncertainty factors.
The PBPK model for a chemical substance is developed in four interconnected steps: (1) model
representation, (2) model parameterization, (3) model simulation, and (4) model validation (Krishnan and
MANGANESE
265
3. HEALTH EFFECTS
Andersen 1994). In the early 1990s, validated PBPK models were developed for a number of
toxicologically important chemical substances, both volatile and nonvolatile (Krishnan and Andersen
1994; Leung 1993). PBPK models for a particular substance require estimates of the chemical substance-
specific physicochemical parameters, and species-specific physiological and biological parameters. The
numerical estimates of these model parameters are incorporated within a set of differential and algebraic
equations that describe the pharmacokinetic processes. Solving these differential and algebraic equations
provides the predictions of tissue dose. Computers then provide process simulations based on these
solutions.
The structure and mathematical expressions used in PBPK models significantly simplify the true
complexities of biological systems. If the uptake and disposition of the chemical substance(s) are
adequately described, however, this simplification is desirable because data are often unavailable for
many biological processes. A simplified scheme reduces the magnitude of cumulative uncertainty. The
adequacy of the model is, therefore, of great importance, and model validation is essential to the use of
PBPK models in risk assessment.
PBPK models improve the pharmacokinetic extrapolations used in risk assessments that identify the
maximal (i.e., the safe) levels for human exposure to chemical substances (Andersen and Krishnan 1994).
PBPK models provide a scientifically sound means to predict the target tissue dose of chemicals in
humans who are exposed to environmental levels (for example, levels that might occur at hazardous waste
sites) based on the results of studies where doses were higher or were administered in different species.
Figure 3-5 shows a conceptualized representation of a PBPK model.
If PBPK models for manganese exist, the overall results and individual models are discussed in this
section in terms of their use in risk assessment, tissue dosimetry, and dose, route, and species
extrapolations.
PBPK models for manganese are discussed below, including descriptions of an initial conceptual PBPK
model for manganese (Andersen et al. 1999) and the development of whole-body adult rat and monkey
PBPK models (Nong et al. 2008, 2009; Teeguarden et al. 2007a, 2007b, 2007c), a PBPK model for
manganese transport from the olfactory mucosa to the striatum (Leavens et al. 2007), a whole-body PBPK
model for gestation and lactation in the rat (Yoon et al. 2009a, 2009b), and human whole-body PBPK
models for adults and for fetal and neonatal exposures (Schroeter et al. 2011; Yoon et al. 2011).

Figure 3-5. Conceptual Representation of a Physiologically Based
Pharmacokinetic (PBPK) Model for a
Hypothetical Chemical Substance
Inhaled chemical Exhaled chemical
Ingestion
Lungs
Liver
Fat
Slowly
perfused
tissues
Richly
perfused
tissues
Kidney
Skin
A
R
T
E
R
I
A
L
B
L
O
O
D
V
max
K
m
GI
Tract
Feces
Urine
Chemicals
V
E
N
O
U
S
B
L
O
O
D
contacting skin
Note: This is a conceptual representation of a physiologically based pharmacokinetic (PBPK) model for a
hypothetical chemical substance. The chemical substance is shown to be absorbed via the skin, by inhalation, or by
ingestion, metabolized in the liver, and excreted in the urine or by exhalation.
Source: adapted from Krishnan and Andersen 1994
MANGANESE
266
3. HEALTH EFFECTS
MANGANESE
267
3. HEALTH EFFECTS
Initial Conceptual PBPK Model for Manganese (Andersen et al. 1999). A qualitative PBPK model for
manganese disposition in humans and animals was initially developed by Andersen et al. (1999). This
model represented the current understanding of manganese nutrition and toxicology, and because several
data gaps existed concerning manganese pharmacokinetics, this model was anticipated to change with
time (Andersen et al. 1999). The model, shown in Figure 3-6, was not designed to be quantitative in
nature. The authors indicated that several data gaps prevented such an evaluation of manganese uptake,
distribution, and excretion. For instance, there were inadequate data concerning oxidation rates for
manganese in blood, uptake rates of protein-bound forms by the liver, neuronal transfer rates within the
central nervous system, and quantitative data on systems controlling manganese uptake via the intestines
and liver (such as transport mechanism in the intestines) (Andersen et al. 1999). Andersen et al. (1999)
suggested that an approach to setting acceptable exposure levels for an essential, but neurotoxic, nutrient
such as manganese could be based on predicting exposure levels by any route that would increase brain
manganese concentrations to a small fraction (e.g., 10–25%) of the variation observed in the general
human population. Reliable and validated multiple-route PBPK models for multiple species, including
humans, are needed to take this approach to setting acceptable exposure levels. Efforts to develop such
models in rats, monkeys, and humans have been recently described (Leavens et al. 2007; Nong et al.
2009, 2008; Schroeter et al. 2011; Teeguarden et al. 2007a, 2007b, 2007c; Yoon et al. 2011, 2009a,
2009b).
Whole-Body Rat PBPK Models (Nong et al. 2008; Teeguarden et al. 2007a, 2007b, 2007c). Utilizing
pharmacokinetic and tissue manganese concentration data from several published studies of manganese in
rats and mice, recent efforts have developed PBPK models for manganese in rats that include processes
involved in homeostatic regulation of tissue levels of manganese taken up by ingestion and by inhalation
(Nong et al. 2008; Teeguarden et al. 2007a, 2007b, 2007c). Two PBPK model structures were developed
and evaluated for their ability to account for kinetics of manganese in the liver and brain striatum
following inhalation and dietary administration of soluble forms of inorganic manganese. The data sets
used to evaluate the models were: (1) tissue manganese concentrations in rats receiving diets containing 2,
10, or 100 ppm manganese for 13 weeks and elimination kinetics for an intravenous tracer dose of
54
Mn-manganese chloride (Dorman et al. 2001b); (2) tissue manganese concentrations and tracer kinetics
in rats fed a 100-ppm diet and exposed to 0, 0.03, 0.3 or 3 mg manganese/m
3
manganese sulfate
6 hours/day for 14 consecutive days (Dorman et al. 2001a); and (3) tissue manganese concentrations
(sampled at 0, 45, and 90 days after exposure) in rats fed a 10-ppm diet and exposed to 0, 0.1, or 0.5 mg
manganese/m
3
for 6 hours/day, 5 days/week for 90 days (Dorman et al. 2004b).

MANGANESE
268
3. HEALTH EFFECTS
Figure 3-6. Qualitative PBPK Model for Manganese
Liver
Intestine
[Mn in Diet] Intestinal Lumen
[Airborne Mn]
Lung Deposition
Arterial Blood
Matrix
Bone
Tissues
CNS Targets
Blood-Brain Barrier
Capillary Blood
Feces
bile
Mn
3+
Mn
3+
Inhaled Mn
Nose
Source: Andersen et al. 1999
MANGANESE
269
3. HEALTH EFFECTS
Structures of the models are shown in Figure 3-7. Model A is based on regulation of tissue
concentrations by simple partitioning with slow inter-compartmental transfer from free manganese in
tissues to deeper tissue stores of manganese (“diffusion-controlled tissue partitioning”; Nong et al. 2008;
Teeguarden et al. 2007a, 2007b, 2007c). Model B features saturable binding of manganese in liver and
brain with equilibrium binding constants defined by slow association and dissociation rate constants
(Nong et al. 2008). Both models contain a submodel for deposition and absorption in the nose and lung
shown schematically in Figure 3-8 (Teeguarden et al. 2007c).
Nong et al. (2008) Model A Description and Development. Model A contains six compartments: the
respiratory tract, brain striatum, liver, kidneys, bone, and slowly perfused tissues (Figure 3-7). The
respiratory tract is divided into two subcompartments: nasopharyngeal tissues and lung (Figure 3-8).
Table 3-14 lists parameters of Model A as described by Teeguarden et al. (2007c). Each of the six
compartments is subdivided into a conventional flow-limited compartment connected to the blood and
tissue stores that are not readily equilibrated with blood moving through the tissue compartment. First-
order clearance rate constants (e.g., kInBrnC and koutBrnC) determine the transfer of manganese from
the flow-limited compartment to the deep compartment of each tissue. The clearance rate constants,
together with the blood flow to the tissue (e.g., QBrnC) and the tissue partition coefficients (e.g., PBrn),
determine the steady-state concentrations and the rate of change manganese in each of the tissues,
according to differential equations that are described in detail by Teeguarden et al. (2007c).
Physiological parameters were taken from the literature and included values for blood flows, organ
volumes, and food intake rate (Table 3-14). The initial (basal) concentrations of manganese in the tissues
(Table 3-14) were taken from literature values as described by Teeguarden et al. (2007c). Remaining
model parameters were estimated by fitting the model to experimental data. Fractions of manganese in
the shallow versus deep compartments of each tissue (e.g., fBrn and FDBrn, Table 3-14) were calibrated
to obtain the best fit to intraperitoneal
54
Mn clearance data collected by Furchner et al. (1966). Partition
coefficients (e.g., PBrn, Table 3-14) and clearance rate constants into and out of deep compartments (e.g.,
kInBrnC, kOutBrnC) were calibrated with
54
Mn kinetic data collected by Furchner et al. (1966) and
steady-state tissue manganese concentration data collected by Wieczorek and Oberdörster (1989c). The
fraction of manganese absorbed from the gut (F
DietUp
) was assumed to be 0.8%. The rate of biliary
excretion from liver (kBile0C) was determined by matching the rate of manganese excreted from liver
against the amount of manganese taken up from the diet, while maintaining steady-state levels of
Figure 3-7. Schematic Structures of Nong et al. (2008) PBPK Models A and B for
Manganese in CD Rats*
Model A
Model B
Inhaled Mn
Inhaled Mn
Lung and nose
Brain
Bone
Kidney
Rest of body
Liver
Deep Brain
(striatum)
Deep Bone
Deep Kidney
Deep Body
Deep Liver
QLiv
QBody
QKid
QBone
QBrn
QC
klnBone
kOutBone
kOutBrn kInBrn
kInKid kOutKid
kOutBody kInBody
kOutLiv
kInLiv
Lung and nose
Brain blood
PA
12
PA
21
Brain tissue (striatum)
Kd
brain
Mn
bound
Mn
free
ka
brain
Bone
Kidney
Rest of body
Liver
Kd
liver
Mn
bound
Mn
free
ka
liver
Deep Bone
Deep Kidney
Deep Body
QLiv
QBody
QKid
QBone
QBrn
QC
klnBone
kOutBone
kInKid kOutKid
kOutBody kInBody
Venous blood
Arterial blood
Venous blood
Arterial blood
Diet Bile
Diet Bile
*Values and descriptions of model parameters are in Tables 3-14, 3-15, and 3-16.
Source: Nong et al. 2008
MANGANESE
270
3. HEALTH EFFECTS

Figure 3-8. Schematic of Models for Nasopharyngeal and Lung Deposition of
Manganese and Transport to Blood in the Nong et al. (2008) PBPK
Models A and B for Manganese in CD Rats
Inhaled Mn
(klnh)
Nasopharyngeal
Deposited
Deep NP
Shallow NP
Deposited
Deep Lung
Shallow Lung
kOutLung
kInLung
kDepDLc kDepSLc
fdepLu
Lung
kInNP kOutNP
fdepNP
kDepSNc
kNPBIc
venous
kdepDNc
arterial
MANGANESE
271
3. HEALTH EFFECTS
Source: Teeguarden et al. 2007c

MANGANESE
272
3. HEALTH EFFECTS
Table 3-14. Parameter Values in the Teeguarden et al. (2007c) PBPK Model for
Manganese in CD Rats (Nong et al. 2008) Model A
Parameter
Value
a
BW
Body weight (kg)
0.325
b
QCC
Cardiac output (L/hour for 1-kg animal)
14.6
QPC
Alveolar ventilation (L/hour for 1-kg animal)
30.0
Blood flows (fraction of cardiac output)
QSlowC
Slow
0.534
QBoneC
Bone
0.122
QBrnC
Brain
0.02
QKidC
Kidneys
0.141
QLivC
Liver
0.183
Tissue volumes (fraction of body weight)
VArtC
Arterial blood
0.0224
VBldC
Blood
0.0676
VSlowC
Slow
0.738
VBoneC
Bone
0.021
VDBoneC
Bone deep compartment
0.052
VBrnC
Brain
0.006
VKidC
Kidneys
0.007
VLivC
Liver
0.034
VLungC
Lung
0.007
VNasPhaC
c
Nasopharyngeal
0.0038
VTraBroC
c
Tracheobronchial
0.01107
VPulmonC
Pulmonary
0.01107
VVenC
Venous blood
0.0452
Partition coefficients
Pslow
Slow
0.4
PBone
Bone
30
PBrn
Brain
0.1
PKid
Kidneys
1.25
PLiv
Liver
5.0
PLung
Lung
0.3
Pnaspha
Nasopharyngeal
0.3
Clearance rates
kBile0C
Biliary excretion (L/hour-kg body weight)
2.0
kFeces
Loss in feces (L/hour-kg body weight)
0.0001
Clearance rates (/h)
kInSlowC
Into deep slow compartment
0.017
kInBoneC
Into deep bone compartment
0.105

Parameter
a
Value
kInBrnC
Into deep brain compartment
0.011
kInKidC
Into deep kidney compartment
0.146
kInLivC
Into deep liver compartment
0.621
kInNPC
Into deep nose compartment
0.035
kInLungC
Into deep lung compartment
0.035
kOutSlowC
Out of deep slow
0.0035
kOutBoneC
Out of deep bone
0.00085
kOutBrnC
Out of deep brain
0.00056
kOutKidC
Out of deep kidneys
0.0034
kOutLivC
Out of deep liver
0.007
kOutNPC
Out of deep nose
0.0035
kOutLungC
Out of deep lung
0.0035
Initial concentrations of manganese (μg/L)
CArt0
Arterial blood
10.0
CBld0
Blood
10.0
CSlow0
Slow
110.0
CDSlow0
Deep slow
110.0
CBone0
Bone
650.0
CDBone0
Deep bone compartment
650.0
CBrn0
Brain
450.0
CDBrn0
Deep brain
450.0
CKid0
Kidneys
1000.0
CDKid0
Deep kidneys
1000.0
CLiv0
Liver
2600.0
CDLiv0
Deep liver
2600.0
CLung0
Lung
250.0
CDLung0
Deep lung
250.0
CNose0
Nose
0.0
CDNose
Deep nose
0.0
CVen0
Venous blood
10.0
Fractional coefficients
fDepNP
c
Particles deposited nasopharyngeal
0.2
fDepTB
c
Particles deposited tracheobronchial
0.21
c
fDepPu
Particles deposited pulmonary
0.07
d
Fraction of manganese in shallow versus deep tissue
(ratios of volumes; not separately estimated model parameters)
fSlow
Slow
0.5
fDSlow
Deep slow
0.5
MANGANESE
273
3. HEALTH EFFECTS
Table 3-14. Parameter Values in the Teeguarden et al. (2007c) PBPK Model for
Manganese in CD Rats (Nong et al. 2008) Model A

MANGANESE
274
3. HEALTH EFFECTS
Table 3-14. Parameter Values in the Teeguarden et al. (2007c) PBPK Model for
Manganese in CD Rats (Nong et al. 2008) Model A
Parameter
Value
a
fBrn
Brain
0.05
fDBrn
Deep brain
0.95
fKid
Kidneys
0.25
fDKid
Deep kidneys
0.75
fLiv
Liver
0.4
fDLiv
Deep liver
0.6
fLung
Lung
0.1
fDLung
Deep lung
0.9
FDNose
Deep nose
0.9
fDBody
Body
0.5
Dosing parameters
InFac1
Dietary intake factor for first diet
0.05
FDietUp
Fraction of manganese in diet that is absorbed
0.008
a
Physiological parameters are consistent with those reported by Brown et al. (1997). Rate constants were fit to
available experimental data on the kinetics of Mn in the various tissues. Rate constants fitted to the control steady-
state Mn tissue concentrations reported by Furchner et al. (1966) and used to simulate ip and inhalation experiments
are shown.
b
Default body weight. Some body weights were lower (0.25) to represent study conditions.
c
The deposition lung region of the lung is the sum of the tracheobronchial and pulmonary tissue
(fDepLu=fDepTB+fDepPu; VDepLuC=VTraBroC+VPulmonC).
d
This fraction is not an independently estimated variable. Instead, the fraction represents the ratio of the two rate
constants, kin and kout, for each tissue.
Source: Teeguarden et al. 2007c
MANGANESE
275
3. HEALTH EFFECTS
manganese in all tissues and matching the turnover of
54
Mn for each tissue (Teeguarden et al. 2007c). For
inhaled manganese, fractional depositions in the nasopharyngeal (fDepNP = 0.2), tracheobronchial
(fDepTB = 0.21), and pulmonary (fDeppu = 0.07) regions were taken from the EPA (1994a) respiratory
tract deposition model for 1.1-µm aerosols. The model assumed that deposited aerosols dissolved
immediately and that there was no clearance from the airway lumen to the gut via mucociliary transport;
this assumption is valid for soluble manganese forms such as manganese chloride and manganese sulfate,
but would not be valid for less-soluble forms of manganese such as manganese phosphate (Nong et al.
2008; Teeguarden et al. 2007c).
Nong et al. (2008) described further refinements to model A parameters shown in Table 3-15. Daily
manganese dietary intake (F
DietUp
) and biliary elimination rate constants (k
BileC
) were first calibrated for
different levels of manganese in the diet (2, 10, 100, and 125 ppm; Table 3-15) by fitting the model to the
observed steady-state tissue manganese concentration data for rats exposed to 2, 10, or 100 ppm
manganese in the diet for 13 weeks (Dorman et al. 2001b). After this refinement, clearance rates for the
liver and brain striatum (kIn and kOut values shown in Table 3-15) were refined by fitting the model to
tissue manganese concentration data from the 14-day inhalation study by Dorman et al. (2001a).
Nong et al. (2008) Model B Description and Development. Model B contains a similar structure to
Model A, except that manganese concentrations in the liver and brain striatum are dependent on capacity-
limited binding of manganese (Figure 3-7). In addition, uptake from striatal blood to striatal tissues is
described with diffusion terms (PA
12
and PA
21
, Figure 3-7). The diffusion terms were included to account
for observations of preferential increases in some brain regions compared with other tissues, such as liver
or blood, following inhalation exposure to manganese (see Dorman et al. 2006a for review). The
diffusion terms are thought to reflect movement of manganese across the blood-brain barrier (Nong et al.
2008). In Model B, the total amounts of manganese in the liver and brain striatum tissues are dependent
on concentrations of free circulating manganese, the binding capacity of the tissue, and the concentrations
of bound manganese in tissue stored (Nong et al. 2008). Differential equations to describe changes (with
time) in amounts of free or bound manganese in the liver and the brain striatum are described in detail by
Nong et al. (2008). Table 3-16 lists binding rate constants (e.g., kaBrnC, kdBrnC), binding capacities
(B
max,Brain
, B
max.Liver
), brain diffusion constants (PA
12
and PA
21
), and partition coefficients in Model B.
Liver and brain striatum binding capacity levels were first determined by fitting the model to steady-state
tissue concentration data from the 13-week dietary study by Dorman et al. (2001b), using starting values
for the tissue binding parameters that were estimated based on clearance rate values (kIn and kout) for
liver and brain striatum in Model A. Tissue binding parameters (e.g., kaBrnC, kdBrnC) and brain

Table 3-15. Refined Parameter Values in Nong et al. (2008) Model A
Parameter
a
Manganese level in diet Biliary excretion (/h/kg)
k
BileC
2 ppm manganese 0.19
10 ppm manganese 0.28
100 ppm manganese 0.60
125 ppm manganese 0.60
Tissue clearance rates (/h/kg)
kInLivC Into deep liver compartment 0.621
kInBrnC Into deep brain compartment 0.011
kOutLivC Out of deep liver compartment 0.007
kOutBrnC Out of deep brain compartment 0.00039
Dosing parameters: diet level Fraction of manganese in diet that is
of manganese absorbed
F
DietUp
2 ppm manganese 0.044
10 ppm manganese 0.018
100 ppm manganese 0.004
125 ppm manganese 0.003
MANGANESE
276
3. HEALTH EFFECTS
a
The remaining parameters are described in Teeguarden et al. (2007c). Clearance rates are scaled to the body
weight (BW
−0.25
).
Source: Nong et al. 2008

MANGANESE
277
3. HEALTH EFFECTS
Table 3-16. Parameter Values in Nong et al. (2008) Model B
Parameters
a
Values
Tissue binding rate constants
a
kaBrnC
Association striatum constant (/h/μg/kg)
0.000176
kaLivC
Association liver constant (/h/μg/kg)
0.06772
kdBrnC
Dissociation striatum constant (/h/kg)
0.00002
kdLivC
Dissociation liver constant (/h/kg)
0.0054196
Tissue binding constants (μg/kg)
B
max,brain
Maximal binding striatum constant
3,300
B
max,liver
Maximal binding liver constant
1,000
Brain diffusion constants (/hour/kg)
PA
12
Influx brain tissue constant
1
PA
21
Efflux brain tissue constant
0.16
Partition coefficient
P
brain
Brain (striatum):blood
1.0
P
liver
Liver:blood
1.08
a
The remaining parameters are described in Teeguarden et al. (2007c).
b
Rate constants are scaled to the BW
-0.25
and maximal binding capacities are scaled to BW
-0.75
.
Source: Nong et al. 2008
MANGANESE
278
3. HEALTH EFFECTS
diffusion constants (PA
12
and PA
21
) were then refined by fitting the model to the 14-day-inhalation tissue
concentration data from Dorman et al. (2001a).
Evaluation of Nong et al. (2008) Models A and B. Nong et al. (2008) compared the abilities of Models
A and B to predict: (1) whole-body elimination kinetics of
54
Mn in rats fed a 100-ppm diet for 13 weeks
(data from Dorman et al. 2001b); (2) liver and brain striatum manganese concentration data in rats
exposed to 0.03, 0.3, or 3 mg manganese/m
3
for 6 hours/day for 14 consecutive days (Dorman et al.
2001a); (3) whole-body elimination kinetics of
54
Mn in rats following 14-day inhalation exposure to 3 mg
manganese/m
3
; and (4) liver and brain striatum manganese concentrations in rats during and following a
90-day inhalation exposure period to 0.1 or 0.5 mg manganese/m
3
(Dorman et al. 2004b). Both models
adequately predicted observed
54
Mn elimination kinetics data, but Model B much more accurately
predicted liver and brain striatum manganese concentration data during and following 14- or 90-day
inhalation exposures. Model A consistently overestimated liver and brain striatum manganese
concentration, particularly at concentrations of 0.1, 0.3, or 0.5 mg manganese/m
3
(as shown in Figures 4
and 7 of Nong et al. 2008). Nong et al. (2008) concluded that the evaluation of the models “highlighted
the importance of tissue binding in maintaining relatively constant tissue concentrations across a wide
range of inhaled concentrations.” Nong et al. (2008) mentioned that the next steps in model development
would be to extend tissue binding in Model B to all other tissues in the models for which appropriate data
are available for calibrating tissue-specific binding rate constants.
PBPK Model for Manganese Transport from the Olfactory Mucosa to Striatum (Leavens et al. 2007).
Leavens et al. (2007) developed a pharmacokinetic model describing the olfactory transport and blood
delivery of manganese to the striatum in rats following acute inhalation exposure to manganese chloride
or manganese phosphate. Figure 3-9 shows the structure of the model, which presumes that manganese
undergoes axonal transport from the olfactory mucosa (OM) to the olfactory bulb (OB), followed by
serial transport to the olfactory tract and tubercle (OTT) and then to the striatum (S). Tables 3-17 and
3-18 list values of the model parameters for soluble manganese chloride and relatively insoluble
manganese phosphate, respectively. Each of the compartments in the model (containing a left and right
nasal cavity) is connected by blood and each is comprised of pools of free and bound manganese. The
rates of transport between tissue compartments and between bound and free pools are modeled as first-
order transport processes. Tables 3-17 and 3-18 show measured values for compartment volumes, values
for blood clearance into olfactory compartments (e.g., Cl
OM/blood
), values for rate constants for efflux from
compartments to blood (e.g., k
blood/OM
), values for transport rate constants between compartments (e.g.,
k
OM/el
), and binding rate constants in the olfactory compartments (e.g., OM free to bound, k
OM/f.b
and

Figure 3-9. Schematic of the Leavens et al. (2007) Model to Describe Olfactory
and Blood Delivery of Manganese to the Left Side of the Brain Isilateral to the
Olfactory Mucosa (OM) in the Left Nasal Cavity*
Blood
Bound
Bound
Bound Bound
Left Muscosa Left Bulb
Left Tract &
Tubercule Left Striatum
Free
Free
Cl
OM
blood
k
blood
OM
Cl
OB
blood
k
blood
OB
Cl
OTT
blood
k
blood
OTT
Cl
s
blood
k
s
blood
k
OM
f:b
k
OM
b:f
k
OM
el
k
OB
f:b
k
OB
b:f
k
OB
el
k
OTT
f:b
k
OTT
b:f
f
s
· k
OTT
el
(
1- f
s
)
· k
OTT
el
Free
Free
k
S
f:b
k
S
b:f
MANGANESE
279
3. HEALTH EFFECTS
*The model structure for the right side is identical. Values and descriptions of model parameters are in Tables 3-16,
3-17, and 3-18.
Source: Leavens et al. 2007

MANGANESE
280
3. HEALTH EFFECTS
Table 3-17. Parameter Values for Manganese Chloride in the Leavens et al. (2007)
PBPK Model for Olfactory Transport of Manganese in Rats
Parameter
Description
Value
Units
Source
Compartment volumes
V
Left OM
L
OM
0.059
mL
Measured
a
V
Left OB
L
OB
0.031
mL
Measured
a
V
Left OTT
OTT
L
0.030
mL
Measured
a
V
Left striatum
L
S
0.032
mL
Measured
a
V
Right OM
R
OM
0.065
mL
Measured
a
V
Right OB
R
OB
0.038
mL
Measured
a
V
Right OTT
OTT
R
0.046
mL
Measured
a
V
Right striatum
R
S
0.042
mL
Measured
a
Blood clearance into olfactory compartments
Cl
Influx to OM
OM
blood
4x10
-4
mL/hour
Estimated
Cl
Influx to OB
OB
blood
1x10
-5
mL/hour
Estimated
Cl
Influx to OTT
OTT
blood
6x10
-4
mL/hour
Estimated
Cl
Influx to striatum
blood
S
3x10
-5
mL/hour
Estimated
Rate constants for olfactory compartments efflux to blood
k
Efflux from OM to blood
blood
OM
1x10
-6
hour
-1
Estimated
k
Efflux from OB to blood
blood
OB
1x10
-6
hour
-1
Estimated
k
Efflux from OTT to blood
blood
OTT
0.0
hour
-1
Estimated
k
Efflux from striatum to blood
S
blood
1x10
-6
hour
-1
Estimated
Olfactory transport rate constants
k
OM to OB
OM
el
0.022
hour
-1
Estimated
k
OB to OTT
OB
el
0.037
hour
-1
Estimated
k
OTT to striatum
OTT
el
0.094
hour
-1
Estimated
f
Fraction of OTT loss rate to striatum
S
0.001
Unitless
Estimated
Binding rate constants in olfactory compartments
k
OM free to bound
OM
f:b
0.006
hour
-1
Estimated
k
OB free to bound
OB
f:b
0.0047
hour
-1
Estimated
k
OTT free to bound
OTT
f:b
0.0043
hour
-1
Estimated
k
Striatum free to bound
S
f:b
0.0026
hour
-1
Estimated
k
OM bound to free
OM
b:f
1x10
-6
hour
-1
Constant
b
k
OB bound to free
OB
b:f
1x10
-6
hour
-1
Constant
b
k
OTT bound to free
OTT
b:f
1x10
-6
hour
-1
Constant
b
k
Striatum bound to free
S
b:f
1x10
-6
hour
-1
Constant
b
a
Unpublished results measured in CD rats used in Brenneman et al. (2000) study. Plugge
d and unplugged exposure
data were averaged together because they were not significantly different.
b
Not possible to estimate both constants for the binding; therefore, the rate constants for the bound to free
manganese were set to a low rate to allow slow removal of manganese tracer from the bound compartment.
Source: Leavens et al. 2007

MANGANESE
281
3. HEALTH EFFECTS
Table 3-18. Parameter Values for Manganese Phosphate in the Leavens et al.
(2007) PBPK Model for Olfactory Transport of Manganese in Rats
Parameter
Description
Value
Units
Source
Compartment volumes
V
Left OM
L
OM
0.085
mL
Measured
a
V
Left OB
L
OB
0.038
mL
Measured
a
V
Left OTT
OTT
L
0.025
mL
Measured
a
V
Left striatum
L
S
0.05
mL
Measured
a
V
Right OM
R
OM
0.074
mL
Measured
a
V
Right OB
R
OB
0.038
mL
Measured
a
V
Right OTT
OTT
R
0.04
mL
Measured
a
V
Right striatum
R
S
0.035
mL
Measured
a
Blood clearance into olfactory compartments
Cl
Influx to OM
OM
blood
0.0017
mL/hour
Estimated
Cl
Influx to OB
OB
blood
0.0018
mL/hour
Estimated
Cl
Influx to OTT
OTT
blood
0.0016
mL/hour
Estimated
Cl
Influx to striatum
blood
S
1.8x10
-5
mL/hour
Estimated
Rate constants for olfactory compartments efflux to blood
k
Efflux from OM to blood
blood
OM
3x10
-6
hour
-1
Estimated
k
Efflux from OB to blood
blood
OB
0.0
hour
-1
Estimated
k
Efflux from OTT to blood
blood
OTT
1x10
-6
hour
-1
Estimated
k
Efflux from striatum to blood
S
blood
1.5x10
-5
hour
-1
Estimated
Olfactory transport rate constants
k
OM to OB
OM
el
0.011
hour
-1
Estimated
k
OB to OTT
OB
el
0.036
hour
-1
Estimated
k
OTT to striatum
OTT
el
0.099
hour
-1
Estimated
f
Fraction of OTT loss rate to striatum
S
0.033
Unitless
Estimated
Binding rate constants in olfactory compartments
k
OM free to bound
OM
f:b
0.00086
hour
-1
Estimated
k
OB free to bound
OB
f:b
0.0014
hour
-1
Estimated
k
OTT free to bound
OTT
f:b
0.0031
hour
-1
Estimated
k
Striatum free to bound
S
f:b
0.024
hour
-1
Estimated
k
OM bound to free
OM
b:f
1x10
-6
hour
-1
Constant
b
k
OB bound to free
OB
b:f
1x10
-6
hour
-1
Constant
b
k
OTT bound to free
OTT
b:f
1x10
-6
hour
-1
Constant
b
k
Striatum bound to free
S
b:f
1x10
-6
hour
-1
Constant
b
a
Unpublished results measured in CD rats used in Dorman et al. (2000) study. Plugged and unplugged exposure
data were averaged together because they were not significantly different.
b
Not possible to estimate both constants for the binding; therefore, the rate constants for the bound to free
manganese were set to a low rate to allow slow removal of manganese tracer from the bound compartment.
Source: Leavens et al. 2007
MANGANESE
282
3. HEALTH EFFECTS
OM bound to free, k
OM/b.f
). Equations for mass balance, clearance, and free concentrations of manganese
for each of the compartments are described in detail by Leavens et al. (2007).
Model parameters were estimated by optimization procedures using kinetics data from rats exposed nose-
only for 90 minutes to
54
Mn-manganese chloride (Brenneman et al. 2000) or
54
Mn-manganese phosphate
(Dorman et al. 2002a). In each experiment, one group was exposed with both nostrils unplugged, while a
second group was exposed with the right nostril plugged. Blood concentrations were not measured in
either of these studies, but
54
Mn concentrations in the kidney, liver, and pancreas were measured and
reported. The mean concentration in these three organs is used to represent blood concentration in the
model, and the data were used to obtain parameters for equations describing first-order absorption and
elimination into a single compartment; values for the parameters under plugged and unplugged
conditions, obtained through model optimization procedures, are listed in Table 3-19. The optimized
model was used to predict the percentage of
54
Mn that was transported into each compartment either via
direct olfactory transport or blood delivery. For manganese chloride, olfactory transport was predicted to
deliver >97–99% of the tracer in the left or right olfactory bulbs, 40–76% of the tracer in the left or right
olfactory tract and tubercle, and only 4–8% of the tracer in the left or right striatum under plugged or
unplugged conditions. For manganese phosphate, the respective predictions were 38–59% in the
olfactory bulbs, 86–90% in the olfactory tract and tubercle and 77–83% in the striatum. Leavens et al.
(2007) cautioned against the predictions for the striatum, since the model overpredicted striatum
concentrations at the later time points for the plugged exposures to manganese chloride or manganese
phosphate and the unplugged exposures to manganese phosphate (Figures 4–7 in Leavens et al. 2007).
Whole-body Rat and Monkey PBPK Models (Nong et al. 2009). Nong et al. (2009) modified the Nong
et al. (2008) rat Model B by adding: (1) saturable binding to all tissues with association and dissociation
rate constants; (2) preferential accumulation of manganese in brain regions, such as the striatum and
globus pallidus; (3) respiratory and olfactory uptake based on regional particle deposition within the
respiratory tract; (4) inducible biliary excretion; and (5) variable dietary absorption depending on the
manganese content in food substances. The model structure contains compartments for liver, bone, lung,
nasal cavity, blood, and brain (cerebellum, olfactory bulb, striatum and pituitary and manganese intakes
from the diet and by inhalation. In the model, inhaled manganese is absorbed following deposition of
particles on the nasal and lung epithelium. In the nose, absorbed free manganese is largely absorbed into
the systemic blood and a smaller portion is transported directly into the olfactory bulb. Free manganese is
transported in the blood and stored as bound manganese in each tissue, as determined by a binding
capacity (B
max
) and association and dissociation rate constants for each tissue (k
a
, k
d
; Figure 3-10). Each
a
Parameter
Description
Units
Source
Plugged
Unplugged
Manganese chloride exposures
b
C
Ө
Initial deposited concentration
261
791
ng/g
Estimated
k
a
First-order absorption
0.0068
0.005
hour
-1
Estimated
K
First-order elimination rate
0.057
0.063
hour
-1
Estimated
constant
Manganese phosphate exposures
b
C
Ө
Initial deposited concentration
171
376
ng/g
Estimated
k
a
First-order absorption
0.0035
0.0034
hour
-1
Estimated
K
First-order elimination rate
0.083
0.124
hour
-1
Estimated
constant
MANGANESE
283
3. HEALTH EFFECTS
Table 3-19. Parameter Values for Describing Blood Concentrations
in the Leavens et al. (2007) PBPK Model for
Olfactory Transport of Manganese in Rats
Value
a
Estimated pharmacokinetic parameters for mean of liver, kidney, and pancreas concentration reported in
Brenneman et al. (2000). See text for equation and details.
b
Equal to FX
0
/V
b
, where X
0
is initial dose, F is fraction dose bioavailable for absorption, and V
b
is the blood volume.
Source: Leavens et al. 2007
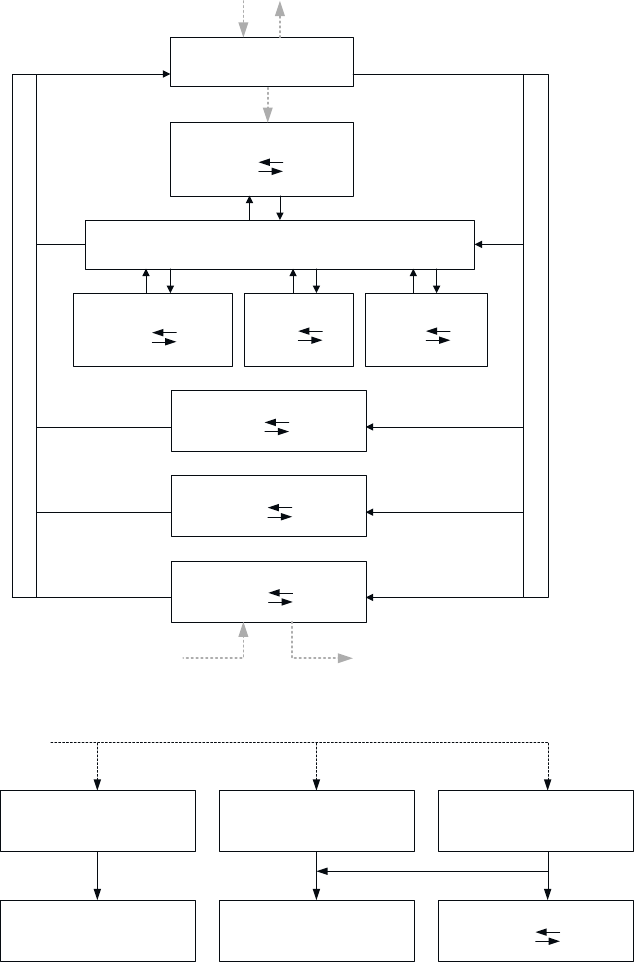
Figure 3-10. Physiologically Based Pharmacokinetic Model Structure Describing
Tissue Manganese Kinetics in Adult Rats*
Inhaled manganese
A
Lung and nose
Olfactory
ka
B + Mn
f
Mn
b
kd
Brain blood
Striatum-globus pallidus
ka
B + Mn
f
Mn
b
kd
Cerebellum
ka
B + Mn
f
Mn
b
kd
Bone
ka
B + Mn
f
Mn
b
kd
Rest of body
ka
B + Mn
f
Mn
b
kd
Liver
ka
B + Mn
f
Mn
b
kd
V
e
n
o
u
s
b
l
o
o
d
A
r
t
e
r
i
a
l
b
l
o
o
d
QC
QBrn
Qbone
Qbody
QLiv
k
in
Diet Bile
k
in
k
out
k
in
k
out
k
out
Pituitary
ka
B + Mn
f
Mn
b
kd
k
in
k
out
QP
B
Inhaled manganese
Lung tissue
ka
B + Mn
f
Mn
b
kd
Lung respiratory
Nasal respiratory
Venous blood
Nasal olfactory
Olfactory bulb
MANGANESE
284
3. HEALTH EFFECTS
*Schematic A is the structure of the full model. Schematic B describes substructures for deposition and absorption in
the nose and lung. Manganese in the nose is absorbed largely into the systemic blood and a small portion moves
directly to the olfactory bulb. Every tissue has a binding capacity, B
max
, with affinity defined by association and
dissociation rate constants (Ka, Kd). Free manganese (Mn
f
) moves in the blood throughout the body and is stored in
each tissue as bound manganese (Mn
b
). Influx and efflux diffusion rate constants (K
in
, k
out
) allow for differential
increases in manganese levels for different tissues. QP, QC, and Qtissue refer to pulmonary ventilation, cardiac
output, and tissue blood flows.
Source: Nong et al. 2009
MANGANESE
285
3. HEALTH EFFECTS
compartment contains influx and efflux diffusion rate constants (k
in
and k
out
) allowing for differential
increases for different tissues (Figure 3-10). Differential equations to describe changes in amounts of free
or bound manganese in the tissue compartments, as well as numerical values of final model parameters,
are described in detail by Nong et al. (2009). Model parameters were first calibrated with steady-state
tissue concentrations measured by Dorman et al. (2001b) in rats fed two diets differing in manganese
concentrations (10 and 125 ppm) to arrive at dose-dependent fractional gastrointestinal absorption and
biliary excretion. Model parameters (including tissue:blood partition coefficients, binding rate constants
and influx and efflux diffusional rate constants) were then refined by fitting the model to the 14-day
inhalation tissue concentration data from Dorman et al. (2001a), including data for striatum, cerebellum,
and olfactory bulb. Model simulations were consistent with empirical observations of brain tissue
concentrations following 14-day inhalation exposures to 0.03, 0.3, or 3 mg manganese/m
3
collected by
Dorman et al. (2001a). The model was used to predict tissue concentrations following 90-day exposures
of rats and compared with empirical tissue concentrations measured in two different 90-day inhalation
studies (Dorman et al. 2004; Tapin et al. 2006). Model predictions for the highest exposure concentration
(3 mg/m
3
) overestimated measured tissue concentrations, but when the rate constant for biliary excretion
was increased about 2-fold, better fit to the 90-day high-concentration data was obtained.
To develop the monkey model, physiological parameters were scaled to adult monkey values and model
parameters were adjusted to fit the model to manganese tissue concentrations collected by Dorman et al.
(2006a) from monkeys exposed by inhalation to manganese sulfate aerosols at concentrations up to
1.5 mg/m
3
for 90 days. Simulations from the final monkey model for 90-day inhalation exposure to
1.5 mg/m
3
followed by 80 days after exposure were consistent with time-dependent rises in measured
concentrations in liver, pituitary, globus pallidus during exposure, and post-exposure declines in pituitary
and globus pallidus measured concentrations, but the model overestimated measured concentrations in the
lung (during and after exposure) and in the liver after exposure.
Whole-body PBPK Model in Pregnant Rats and Fetuses (Yoon et al. 2009a). The adult rat model
developed by Nong et al. (2009) was extended to develop a PBPK model that would predict fetal
manganese dose and manganese disposition in rat dams and fetuses following maternal exposures (dietary
and inhalation) to manganese. The tissue concentration data used to parameterize the model were those
for rats exposed to 10 ppm in the diet and exposed by inhalation to manganese sulfate aerosols at 0, 0.05,
0.5, or 1 mg manganese/m
3
, starting from 28 days before breeding and continuing through a 14-day
mating period until GD 20 (Dorman et al. 2005a, 2005b). The model structure for the dams contains
compartments as in the Nong et al. (2009) adult rat model, plus a placenta through which fetal exposure
MANGANESE
286
3. HEALTH EFFECTS
occurs via two separate pathways operating simultaneously: a bidirectional diffusion process described
by a maternal to fetal diffusion rate (ktrans1) and a fetal blood to placenta diffusion rate constant
(ktrans 2C) and a saturable, active transport, maternal to fetal process described by a Vmax and
Michaelis-Menten constant (Km) (see Figure 3-11). Compartments in the fetus included the lung, liver,
brain blood, whole brain, bone, and rest of body, each with association and dissociation rate constants
(Figure 3-11). Differential equations to describe changes in amounts of free or bound manganese in the
tissue compartments, as well as numerical values of final model parameters, are described in detail by
Yoon et al. (2009a). In general, parameters from the original adult model were modified to accommodate
different life stages (i.e., pregnancy and fetal development), first using control group tissue concentration
data to estimate gestation-specific parameters for dietary only exposure, and then refined by fitting to
inhalation-exposure data for pregnancy and fetal development periods. Model simulations of maternal
and fetal tissue concentrations on GD 20 were visually similar to empirical measurements made by
Dorman et al. (2005a).
Whole-body PBPK Model in Lactating Rat Dams and Fetuses (Yoon et al. 2009b). This model was
developed in parallel to the development of the gestation and fetal rat model (Yoon et al. 2009a). The
lactating maternal model had the same compartments as the Nong et al. (2009) adult rat model, plus a
mammary gland compartment partitioned from the “rest of body” compartment in the adult model. The
mammary gland compartment was assigned the same tissue binding parameters, maximum binding
capacity, and partition coefficient as the “rest of body” compartment (see Figure 3-12). The model
included a milk compartment described as a mass of manganese transferred from the dam to the nursing
pup, with a variable rate of milk production over the lactational period (Figure 3-12). Manganese transfer
from the mammary gland to milk was described as a first-order clearance process. In pups, daily dose
was determined by three intake sources: diet, milk, and inhalation, and compartments were the same as
those in the original adult rat model (see Figure 3-12). As described in more detail by Yoon et al.
(2009b), the model incorporated time- and dose-dependent changes in the dams and offspring in
manganese-specific kinetic parameters for specific tissues (e.g., maternal gastrointestinal uptake and
biliary excretion, maximal binding capacities in growing pup compartments, and developmental changes
in gastrointestinal uptake and biliary elimination), as well as in physiological parameters, such as
maternal and fetal body weight, tissue volumes, and cardiac outputs. Manganese-specific kinetic
parameters were calibrated using tissue concentration data collected by Dorman et al. (2005a, 2005b)
from lactating rat dams and offspring exposed to 10 ppm manganese in the diet, and 0, 0.05, 0.5, or 1 mg
manganese/m
3
from PND 1 to 18 for dams and PND 19 for pups. The calibration process was described
in more detail by Yoon et al. (2009b). Model simulations of tissue concentrations in rat dams (at the end

MANGANESE
287
3. HEALTH EFFECTS
Figure 3-11. Model Structure for Simulating Manganese Exposure During
Gestation in the Rat*
Lung ka
B + Mn
b
Mn
f
kd
Respiratory nose Mn
f
Olfactory nose Mn
f
Olfactory bulb
ka
B + Mn
b
Mn
f
kd
Brain blood Mn
f
Striatum
ka
B + Mn
b
Mn
f
kd
Cerebellum
ka
B + Mn
b
Mn
f
kd
Placenta ka
B + Mn
b
Mn
f
kd
Bone ka
B + Mn
b
Mn
f
kd
Rest of body ka
B + Mn
b
Mn
f
kd
Liver ka
B + Mn
b
Mn
f
kd
V
e
n
o
u
s
b
l
o
o
d
A
r
t
e
r
i
a
l
b
l
o
o
d
A
r
t
e
r
i
a
l
b
l
o
o
d
V
e
n
o
u
s
b
l
o
o
d
Lung ka_F
B_F + Mn
b
Mn
f
kd_F
Brain blood Mn
f
Brain ka_F
B_F + Mn
b
Mn
f
kd_F
Bone ka_F
B_F + Mn
b
Mn
f
kd_F
Rest of body ka_F
B_F + Mn
b
Mn
f
kd_F
Liver ka_F
B_F + Mn
b
Mn
f
kd_F
DAM
Inhalation
FETUSES
QP
Maternal to
fetal transfer
fdep
LU
fdep
NR
fdep
NO
Vmax
Km
ktrans1
Ktrans2
QC
QNose
QBrn
QPla
QBone
QOth
QLiv
QC_f
QLung_F
QBrn_F
QBone_F
QOth_F
QLiv_F
k
out
Diet
Bile
k
out
k
out
k
in
k
in
k
in
K
in_F
K
out_F
Fetal to maternal
transfer
K
diet
K
bile
*Manganese is present in the body as either free (Mn
f
) or bound form (Mn
b
). Every tissue (dam and fetus) has a
binding capacity (B) and dissociation rate constants (ka, kd). “_F” is used to distinguish fetal parameters from those
of the dam. Maternal manganese is transferred to the fetuses through the placenta in the free form. QP, QC, and
Qtissue refer to pulmonary ventilation, cardiac output, and tissue blood flows.
Source: Yoon et al. 2009a

Figure 3-12. Model Structure for Predicting Manganese Tissue Levels in
Lactating Rat Dams and Pups*
QLiv_p
Diet Bile
Diet
Bile
K
diet
K
bile
K
diet_p
K
bile_p
Lung
B + Mn
b
Mn
f
kd
Respiratory nose Mn
f
Olfactory nose Mn
f
Olfactory bulb
ka
B + Mn
b
Mn
f
kd
Brain blood Mn
f
Striatum
ka
B + Mn
b
Mn
f
kd
Cerebellum
ka
B + Mn
b
Mn
f
kd
Mammary gland ka
B + Mn
b
Mn
f
kd
Bone ka
B + Mn
b
Mn
f
kd
Rest of body ka
B + Mn
b
Mn
f
kd
Liver ka
B + Mn
b
Mn
f
kd
V
e
n
o
u
s
b
l
o
o
d
A
r
t
e
r
i
a
l
b
l
o
o
d
V
e
n
o
u
s
b
l
o
o
d
Lung ka_p
B_p + Mn
b
Mn
f
kd_p
Respiratory nose Mn
f
Olfactory nose Mn
f
Olfactory bulb
ka_p
B_p + Mn
b
Mn
f
kd_p
Brain blood Mn
f
Cerebellum
ka_p
B_p + Mn
b
kd_p
Bone ka_p
B_p + Mn
b
Mn
f
kd_p
Rest of body ka_p
B_p + Mn
b
Mn
f
kd_p
Striatum
ka_p
B_p + Mn
b
Mn
f
kd_p
Liver ka_p
B_p + Mn
b
Mn
f
kd_p
Milk
Mn
f
fdep
LU
fdep
NR
fdep
NO
fdep
LU_p
fdep
NR_p
fdep
NO_p
QNose
QBrn
QM
QBone
QOth
QLiv
k
out
k
out
k
out
k
in
k
in
k
in
K
out_p
K
in_p
K
in_p
K
in_p
K
out_p
K
out_p
Loss from gut
K
loss
Inhalation
Inhalation
PUPDAM
QC_p
QP_p
QP QC
ka
QNose_p
QBrn_p
A
r
t
e
r
i
a
l
b
l
o
o
d
Mn
f
QBone_p
QOth_p
MANGANESE
288
3. HEALTH EFFECTS
*The diagram describes manganese kinetics in rat dams and pups. Manganese is present in the body as either free
(Mn
f
) or bound form (Mn
b
). Every tissue (dams and pups) has a binding capacity (B) and dissociation rate constant
(ka, kd). Parameters for the pups are distinguished from those of the dam using “_p.” Maternal manganese is
transferred to the pups through milk in the free form. QP, QC, and Qtissue refer to pulmonary ventilation, cardiac
output, and tissue blood flow. Fdep
tissue
refers to fractional depositions in respiratory tissues.
Source: Yoon et al. 2009b
MANGANESE
289
3. HEALTH EFFECTS
of lactation) and offspring (at PND 19 at the end of exposure, and at PNDs 45 and 63) were visually
similar to tissue concentrations reported by Dorman et al. (2005b). The model simulations and empirical
results indicated that at the end of the inhalation exposure period (PND 19), concentrations in the striatum
and olfactory bulb of offspring began to increase (compared with control values) when air concentrations
exceeded 0.05–0.1 mg/m
3
; maternal concentrations in these brain regions began to increase at somewhat
higher air concentrations between 0.1 and 0.3 mg/m
3
. These results indicate that at given air
concentrations above about 0.05–0.1 mg/m
3
, brain concentrations in neonates may be elevated, compared
with controls, to a greater degree than in lactating dams, but the age-specific difference in the tested air
concentration range does not appear to be large. Yoon et al. (2009b) concluded that these results indicate
that neonates are not at an especially increased risk to striatal manganese due to differences in
pharmacokinetic factors.
Whole-body PBPK Model in Adult Monkeys and Humans (Schroeter et al. 2011). The PBPK model
developed for monkeys (Nong et al. 2009) was scaled to humans to predict inhalation exposure conditions
associated with increased brain manganese concentrations and extended to include intravenous,
intraperitioneal, and subcutaneous exposure routes and a series of gastrointestinal compartments
consistent with the physiology of manganese absorption and elimination (see Figure 3-13 for model
structure). The models were extended to allow comparative analysis of kinetic data from studies of
nonhuman primates and humans exposed by these routes to soluble, carrier-free, radiolabeled
54
Mn.
Modifications from the Nong et al. (2009) monkey model included adjustments of dissociation rate
constants and maximal binding capacities so that each brain region contained about 60% bound
manganese under diet-only exposure, refinement of diffusion parameters to simulate the rise in brain
manganese concentrations during inhalation exposure and subsequent decline during post-exposure
displayed by the data collected by Dorman et al. (2006a), and refinement of a dose-dependent influx term
for the globus pallidus and pituitary regions. Physiological parameters in the human model were either
scaled from the monkey model or obtained from the literature. Dietary absorption and biliary excretion
were calibrated using human whole-body elimination kinetic data from earlier studies of humans given
intravenous tracer amounts of
54
Mn. Initially, diffusion rate constants and tissue binding capacities were
scaled from monkey values, and association and dissociation rate constants were adjusted to attain 80%
bound manganese in brain regions. Additional refinements to model parameters were necessary to
maintain tissue levels within expected values with only dietary manganese exposure. More details
concerning final parameters for the human and monkey models are provided by Schroeter et al. (2011).
Model simulations adequately described whole-body elimination kinetic data for monkeys given
54
Mn by
intraperitoneal (Dastur et al. 1971), intravenous (Furchner et al. 1966), or oral administration (Furchner et

Figure 3-13. Physiologically Based Pharmacokinetic Model Structure Describing
Manganese Tissue Kinetics in Adult Monkeys and Humans*
Inhalation
Lung and nose
Olfactory bulb
ka
B + Mn
f
Mn
b
kd
Brain blood
Globus pallidus
ka
B + Mn
f
Mn
b
kd
Pituitary
ka
B + Mn
f
Mn
b
kd
Bone
ka
B + Mn
f
Mn
b
kd
Rest of body
ka
B + Mn
f
Mn
b
kd
Liver
ka
B + Mn
f
Mn
b
kd
V
e
n
o
u
s
b
l
o
o
d
A
r
t
e
r
i
a
l
b
l
o
o
d
QC
Qbrn
Qbone
Qbody
Qliv
k
in
Bile
k
in
k
out
k
in
k
out
k
out
Cerebellum
ka
B + Mn
f
Mn
b
kd
k
in
k
out
QP
1-Fdietup
Peritoneal cavity
Lower gastrointestinal
tract lumen
Gut lumen
Gut epithelium
Fdietup
Feces
(1-F
ent
)*k
GI
F
ent
*k
GI
k
ent
k
feces
k
IP
F
IP
1-F
IP
Diet Intraperitoneal
MANGANESE
290
3. HEALTH EFFECTS
*In each tissue department, the amount of bound manganese is in equilibrium with the assumed binding capacity
(B
tissue
) and free manganese. Tissue-binding processes are controlled by association and dissociation rate constants
(ka, kd). Free manganese (Mn
f
) moves in the blood throughout the body and is stored in each tissue as bound
manganese (Mn
b
). Influx and efflux diffusion rate constants (K
in
and k
out
) control preferential increases in free
manganese in brain regions. QP, QC, and Qtissue refer to pulmonary ventilation, cardiac output, and tissue blood
flows.
Source: Schroeter et al. 2011
MANGANESE
291
3. HEALTH EFFECTS
al. 1966), fecal excretion data for monkeys following subcutaneous or inhalation exposures to
54
Mn
(Newland et al. 1987), and whole-body retention data in humans following intravenous injection
(Mahoney and Small, 1968; Mena et al. 1967) or ingestion of
54
Mn (Davidsson et al. 1988; Mahoney and
Small 1968). Model simulations of manganese concentrations in the globus pallidus in adult humans with
a normal diet following 90 days of inhalation exposure to air concentrations ranging from 0.0001 to
>10 mg manganese/m
3
indicated that concentrations increased slightly by about 5% over background
levels at 0.1 mg/m
3
and dramatically increased at higher air concentrations (see Figure 3-14).
Whole-body PBPK Model in Humans during Gestation and Neonatal Periods (Yoon et al. 2011). Yoon
et al. (2011) developed a series of PBPK models to describe manganese kinetics during fetal and neonatal
development in humans and to predict internal manganese concentrations in the developing brain. The
models were based on the basic structure of the rat gestation and lactation model developed by Yoon et al.
(2009a, 2009b), with modifications based on cross-species extrapolations in developing monkey (Nong et
al. 2009) and adult human models (Schroeter et al. 2011). The human models incorporated:
(1) pertinent physiological parameters in human females during gestation and lactation from
previously published human pregnancy and lactation PBPK models;
(2) female-specific functional residual capacity, breathing frequency, and tidal volume in the adult
human male model developed by Schroeter et al. (2011) describing manganese particle deposition;
(3) higher basal levels of absorption and biliary excretion of manganese in adult females compared
with males;
(4) characteristics of the placental transfer of manganese in the rat model with parameter
modifications based on observations of placental and fetal manganese concentrations in humans;
(5) a diffusional clearance from free manganese in mammary tissues to describe manganese milk
secretion as in the Yoon et al. (2009b) rat model, with adjustment of the diffusion rate constant
with data for human manganese milk concentrations during lactation from several published
studies;
(6) higher fractional gut absorption of manganese in suckling neonates, compared with adults;
(7) inducible biliary excretion of manganese in neonates, at rates lower than in adults;
(8) transitions of neonatal characteristics of gut absorption and biliary excretion to those of adults;
(9) enhanced brain uptake of manganese during fetal and postnatal development; and
(10) adjustment of tissue binding parameters in fetal tissue to be consistent with published manganese
concentration data from human fetal autopsy tissues and in neonatal tissues to be consistent with
observed neonatal and adult human tissue concentration data.

Figure 3-14. Simulated End-of-Exposure Tissue Total Manganese Levels in Rat
Striatum and Monkey and Human Globus Pallidus*
Rat striatum data: Dorman et al. (2004a); Tapin et al. (2006)
Monkey globus pallidus data: Dorman et al. (2006a)
Rat striatum: simulation, Nong et al. (2009)
Monkey globus pallidus: simulation, Schroeter et al. (2011)
Human globus pallidus: simulation, Schroeter et al. (2011)
MANGANESE
292
3. HEALTH EFFECTS
The simulated rat striatal manganese levels are from Nong et al. (2009) and are compared with data
(mean ± standard error) from Dorman et al. (2004a) and Tapin et al. (2006). The simulated monkey globus
pallidus manganese levels are compared with data from Dorman et al. (2006a). Rats and monkeys were
exposed 6 hours/day, 5 days/week for 90 days. Humans were exposed 8 hours/day, 5 days/week for
90 days.
Source: Schroeter et al. 2011
MANGANESE
293
3. HEALTH EFFECTS
More details on model structure, model equations, and model parameters and their development are
provided by Yoon et al. (2011).
Model simulations of placental and fetal tissue manganese concentrations in the absence of airborne
manganese were consistent with published data for humans with background air manganese exposure;
with increasing air concentrations of manganese, placental and fetal globus pallidus concentrations began
to rise at 0.01 mg/m
3
(18 and 11% increase, respectively, over 0 mg/m
3
values). Model simulations of
human milk manganese concentrations at various lactation stages were consistent with published data for
humans with background air manganese exposure; simulated milk concentrations rose with increasing air
concentration, showing <10% increase at 0.01 mg/m
3
. Simulated average daily AUC for manganese
concentrations in the globus pallidus of the fetus, suckling neonate, and 3-year-old child from manganese
air concentrations increased beyond 10% of background concentrations in fetuses and 3-year-old children
when air concentrations exceeded 0.01 mg/m
3
(10 µg/m
3
) and in suckling neonates when air
concentrations exceeded 0.001 mg/m
3
(1 µg/m
3
) (Yoon et al. 2011).
3.5 MECHANISMS OF ACTION
3.5.1 Pharmacokinetic Mechanisms
Absorption. Manganese absorption occurs primarily through the diet; however, absorption via the
lungs can be significant for occupationally exposed persons or for those exposed to excess levels of
airborne manganese, such as downwind of a manganese point source. Manganese absorption through the
gut may occur through a nonsaturable simple diffusion process through the mucosal layer of brush border
membranes (Bell et al. 1989) or via an active-transport mechanism that is high-affinity, low-capacity, and
rapidly saturable (Garcia-Aranda et al. 1983). Manganese particles that are too large to enter the alveoli
(>10 microns in diameter) remain in the upper respiratory tract, where they are coughed up by
mucociliary transport and swallowed. Differences in solubility of manganese compounds deposited in the
alveolar regions may impact the rate at which manganese will be absorbed, but manganese is bioavailable
when deposited in these regions (Drown et al. 1986).
Diets high in iron have been shown to suppress manganese absorption, and conversely, iron-poor diets
increase manganese uptake (Lönnerdal 1997, Lönnerdal et al. 1994). Phosphorus (Wedekind et al. 1991)
and calcium (Wilgus and Patton 1939) have also been found to decrease manganese uptake.
MANGANESE
294
3. HEALTH EFFECTS
Distribution. Review articles by Aschner and Aschner (1991) and Aschner et al. (2005, 2007)
summarize some of the available data regarding the distribution of manganese. Dietary manganese,
thought to be absorbed as Mn(II), enters portal circulation from the gastrointestinal tract and is bound to
α
2
-macroglobulin or albumin in the plasma. After delivery to the liver, the major portion of Mn(II) is
secreted in the bile, but some may be oxidized by ceruloplasmin to Mn(III). The Mn(III) enters systemic
circulation conjugated with plasma transferrin; once this complex enters a neuron, it dissociates, and from
there, the manganese is transported to axon terminals. For example, Sloot and Gramsbergen (1994)
observed that radiolabeled manganese injected into the striatum or substantia nigra of rat brain is
transported in an anterograde direction through both γ-amino-butyric acid-producing striato-nigral and
dopaminergic nigro-striatal fibers.
Other studies, however, argue for the transport of Mn(II) into the brain. For example, Murphy et al.
(1991) measured the kinetics of manganese transport in the brains of adult male rats using a perfusion
technique. The rats were infused with increasing concentrations of [
54
Mn]Cl
2
; blood and brain samples
were analyzed for manganese at varying time points. The data indicated a saturable mechanism for
transporting Mn(II) into the choroid plexus, and influx into the cerebral cortex was also near saturation at
the highest plasma concentration of manganese used. Influx into other brain regions (e.g., caudate
nucleus, hippocampus, hypothalamus) and cerebrospinal fluid (CSF) showed non-saturable transport of
the cation. The authors suggested that the non-saturable transport into these brain regions resulted from
passive diffusion of manganese down a concentration gradient from ventricular cerebrospinal fluid
because some of these brain regions have components adjacent to the ventricles and manganese
concentrations in these regions were below levels in the CSF. The authors also noted that at all plasma
manganese concentrations tested (from 0.8 to 78 nmol/mL), the transfer coefficient for manganese uptake
into the choroid plexus was significantly higher than in any other area of the central nervous system. For
example, at 0.08 nmol/mL, the transfer coefficients for the CSF and the choroid plexus were
16.2±2.43x10
-6
mL/second*g and 23,800±2,910x10
-6
mL/second*g, respectively. Even after correcting
for differences in compartment size, influx of manganese into the choroid plexus was an order of
magnitude greater than influx into CSF.
Rabin et al. (1993) also measured transport of [
54
Mn]Cl
2
in adult rats using a similar technique. In this
study, the authors used three perfusates (whole blood, plasma/serum, and saline) to determine brain
uptake in environments that facilitated or prevented protein binding of the metal. The authors reported
that uptake of manganese into the cortex, hippocampus, caudate nucleus, and choroid plexus was greater
and more rapid when saline was used rather than with whole blood. When EDTA-saline was used as the
MANGANESE
295
3. HEALTH EFFECTS
perfusate, uptake was not significantly different than zero, indicating that divalent manganese was the
form taken up by the brain. The transfer coefficients of Mn(II) from saline in the different regions of the
brain (frontal, parietal, and occipital cortex regions; hippocampus; caudate nucleus; and thalamus-
hypothalamus) ranged from 5 to 10x10
-5
mL/second*g, whereas that of the choroid plexus was
727x10
-3
mL/second*g. The authors noted that the transfer coefficients were greater than that expected
for passive diffusion and suggested a facilitated blood-brain barrier transport by a channel or carrier
mechanism (Rabin et al. 1993). These findings of a rapid uptake mechanism and concentrated uptake into
the choroid plexus are consistent with results reported by Murphy et al. (1991). Separate binding studies
performed by the authors determined that albumin, transferrin, α
2
-macroglobulin added to the manganese
during perfusion significantly decreased brain uptake of the cation in all brain regions. The authors were
uncertain whether Mn(II) in the form of low-molecular mass solutes was taken up at the blood-brain
barrier. However, based on other literature and their own unpublished results, they suggest that the free
ion is the species transported.
Other studies have also revealed the rapid appearance of manganese in the choroid plexus. Ingersoll et al.
(1995) demonstrated that manganese levels in the lateral choroid plexus were 44 and 24 times higher than
levels in CSF, and blood, respectively, 4 hours after intraperitoneal injection of 10 mg manganese/kg.
However, manganese concentration in the choroid plexus did not change significantly following
intrathecal administration of this same dose. This demonstrated that manganese in the blood could be
sequestered by the choroid plexus, whereas little to no transfer of manganese from CSF to the choroid
plexus occurred. Intrathecal administration of manganese increased manganese concentrations in all
brain regions examined while there were only slight changes in brain manganese concentrations after
intraperitoneal administration. Moreover, intrathecal administration of manganese decreased spontaneous
motor activity with no effect on motor activity following intraperitoneal dosing. The authors suggested
that these results indicated that the brain is protected from high concentrations of manganese through
sequestering in the choroid plexus, but this mechanism could become overwhelmed with rising levels of
blood manganese such that manganese could then “leak’ from the choroid plexus into CSF and thereby
enter the brain. This interpretation appears to be consistent with the findings of London et al. (1989). In
these studies, 50 and 100 mg/kg manganese was administered intraperitoneal doses 5 and 10 times that
used by Ingersoll et al. (1995). Using MRI images, these doses were shown to concentrate in the
ventricles, the pineal gland, and the pituitary gland and the authors indicated that this high concentration
of manganese appeared in the ventricular CSF because it crossed the barrier of the choroid plexus.
Takeda et al. (1994) used autoradiography to also show that manganese in selected brain regions was
taken up via the CSF from the choroid plexus. Moreover, Zheng et al. (1998) observed that, in a
MANGANESE
296
3. HEALTH EFFECTS
subchronic manganese intoxication rat model, the increases in manganese concentrations observed in
targeted brain regions were closely related in magnitude to that of CSF manganese, but not to that of
serum manganese. The observations of Takeda et al. (1994) and Zheng et al. (1998) support the view that
manganese in the CSF serves as the main source for manganese distribution in brain tissues.
Recent reviews of the state of the science have emphasized that manganese can enter the brain via three
pathways: (1) from the nasal mucosa to the brain olfactory bulb via olfactory neural connections;
(2) from the blood through capillary endothelial cells of the blood-brain barrier; and (3) from the blood
through the cerebral spinal fluid via the choroid plexuses (Aschner et al. 2005; Bock et al. 2008;
Crossgrove and Yokel 2005). Current understanding is inadequate to determine which of these pathways
may predominate in cases of severe manganism or cases of subtle neurological impairment in nonhuman
primates or humans. A number of transport mechanisms (including facilitated diffusion, active transport,
transferrin-mediated transport, divalent metal transporter-1 mediation, store-operated calcium channels)
have been proposed to transport manganese across the blood barrier or into the choroid plexus, but current
understanding is inadequate to determine the predominant molecular mechanism of transport in either of
the pathways (Aschner et al. 2005, 2007; Crossgrove and Yokel 2004, 2005; Roth 2006).
3.5.2 Mechanisms of Toxicity
T
he central nervous system is the primary target of manganese toxicity. Although it is known that
manganese is a cellular toxicant that can impair transport systems, enzyme activities, and receptor
functions, the principal manner in which manganese neurotoxicity occurs has not been clearly established
(Aschner and Aschner 1991; Aschner et al. 2007).
Mn(III) has been found to be more cytotoxic to human neural cells as a manganese pyrophosphate
complex (MnPPi) than as a manganese-transferrin complex (MnTf) (Suarez et al. 1995). Specifically,
human neuroblastoma cells (cell line SH-SY5Y) grown in culture showed effects of cytotoxicity from
30 μM MnPPi but did not show the same signs of cytoxicity from MnTf (membrane damage and cell
granulation and aggregation) until concentrations of 60 μM were reached (Suarez et al. 1995). Both
manganese complexes inhibited mitochondrial enzyme activity, but MnTf was slightly more toxic than
MnPPi in this respect (Suarez et al. 1995).
Neuropathological changes are detectable in the basal ganglia of humans with manganism, and the
specific area of injury appears to be primarily in the globus pallidus; the substantia nigra is sometimes
MANGANESE
297
3. HEALTH EFFECTS
affected, but generally to a lesser extent (Katsuragi et al. 1996; Yamada et al. 1986). Studies in
nonhuman primates have produced similar findings (Newland and Weiss 1992; Newland et al. 1989).
Limited evidence suggests that dopamine levels in the caudate nucleus and putamen are decreased in
manganism patients (Bernheimer et al. 1973). Similarities in the behavior of manganism patients to those
with Parkinson’s disease have prompted some to refer to manganism as "manganese-induced
Parkinsonism" or "Parkinson-like disease." Further, the two diseases do affect functional related regions
of the brain, but Parkinsonism is believed to be due to the selective loss of subcortical neurons whose cell
bodies lie in the substantia nigra and whose axons terminate in the basal ganglia (which includes the
caudate nucleus, the putamen, the globus pallidus, and other structures). These nigral neurons use
dopamine as their neurotransmitter, and treatment of Parkinson patients with levo-dopa (the metabolic
precursor to dopamine) often relieves some of the symptoms of Parkinson's disease (Bernheimer et al.
1973). Some investigators have reported that oral levo-dopa can temporarily improve symptoms of
manganese-induced neurotoxicity (Barbeau 1984). However, most studies show that manganism patients
typically do not respond to levo-dopa treatment (Calne et al. 1994; Chu et al. 1995; Huang et al. 1989),
indicating that they have likely suffered degeneration of the receptors and neurons that normally respond
to this neurochemical (Chu et al. 1995).
The precise biochemical mechanism by which manganese leads to this selective destruction of
dopaminergic neurons is not known, but many researchers believe that the manganese ion, Mn(II),
enhances the autoxidation or turnover of various intracellular catecholamines, leading to increased
production of free radicals, reactive oxygen species, and other cytotoxic metabolites, along with a
depletion of cellular antioxidant defense mechanisms (Barbeau 1984; Donaldson 1987; Garner and
Nachtman 1989b; Graham 1984; Halliwell 1984; Liccione and Maines 1988; Parenti et al. 1988; Verity
1999). Oxidation of catechols is more efficient with Mn(III), than with Mn(II) or Mn(IV) (Archibald and
Tyree 1987). Formation of Mn(III) may occur by oxidation of Mn(II) by superoxide (O
2
-
) In cases of
exposure to Mn(VII), it is likely that a reduction to the Mn(II) or Mn (III) state occurs (Holzgraefe et al.
1986), but this has not been demonstrated.
Hussain et al. (1997) studied the effects of chronic exposure of manganese on antioxidant enzymes,
including manganese superoxide dismutase (MnSOD). MnSOD is an antioxidant enzyme located
primarily in the mitochondria that contains manganese as a functional component. MnSOD protects
against oxidative injury by catalyzing the dismutation of O
2
-
, Hussain et al. (1997) found that
administration of 0, 1.1, and 2.2 mg manganese/kg/day (as manganese chloride), 5 days/week for
3 months, resulted in increased MnSOD in the hippocampus, cerebellum, and brainstem. Other areas of
MANGANESE
298
3. HEALTH EFFECTS
the brain were not affected and other antioxidant enzymes, such as Cu,ZnSOD and glutathione peroxidase
(GPx), were not increased. The researchers suggest that since a critical role of MnSOD is to protect
against oxidative injury, the increase of this enzyme after manganese exposure may reduce the risk of
oxidative stress induced by that exposure. Thus, this protective mechanism would have to be
overwhelmed in cases of manganese toxicity. Additionally, the authors suggest that, since MnSOD was
altered while Cu,ZnSOD and Gpx were unchanged, manganese may not affect cytosolic enzymes like
Cu,ZnSOD. In support of this point, the authors also mention other reports that suggest that these
antioxidant enzymes are independently regulated (Mossman et al. 1996; Warner et al. 1993; Yen et al.
1996).
Supporting evidence for the hypothesis that high levels of manganese exert neurotoxicity through
oxidation is provided by Desole et al. (1994). The authors observed that 22 mg manganese/kg/day (as
manganese chloride) administered orally in 6-month-old rats resulted in increased concentrations of
DOPAC (an oxidation product of DA) and uric acid, but left DA levels unchanged. Daily doses of 44 or
66 mg manganese/kg/day resulted in significantly decreased concentrations of DA, glutathione, ascorbic
acid, and DOPAC, and increased concentrations of uric acid in the rat striatum when compared to
controls. The researchers also measured levels of these metabolites in the rat striatal synaptosomes,
which were used as a model for neuronal terminals. Here, DA levels were unchanged at 22 mg
manganese/kg/day but were decreased at the two highest doses. DOPAC levels remained constant at all
three dose levels. Thus, the DOPAC/DA ratio was significantly increased at 44 and 66 mg
manganese/kg/day in the synaptosomes. While the authors suggest that these data support other findings
that manganese oxidizes dopamine (Segura-Aguilar and Lind 1989), the decrease in DA could be the
result of decreased production or release of the chemical, rather than increased oxidation. Catabolism of
adenosine triphosphate (ATP) forms xanthine and hypoxanthine, both of which are metabolized by
xanthine oxidase. The products of this metabolism are uric acid and superoxide radical anion (Desole et
al. 1994). The increase in uric acid production in rat striatum following oral dosing with 44 or 66 mg
manganese/kg (as manganese chloride) suggests that manganese induces oxidative stress mediated by
xanthine oxidase. Desole et al. (1995) expanded their studies to investigate the protective effect of
allopurinol, a xanthine-oxidase inhibitor, to 3-month-old rats exposed to manganese. In this study,
allopurinol was administered by gavage at a dose of 300 mg/kg/day for 4 days. Manganese
(87 mg/kg/day) was also administered by gavage, for 7 days, either alone or with allopurinol; the
allopurinol decreased the striatal ratio of DOPAC and homovanillic acid (HVA) to dopamine. When
given in conjunction with manganese, allopurinol antagonized the manganese-induced increase in
DOPAC levels and the (DOPAC + HVA)/DA ratio. Together, the two studies suggest that manganese-
MANGANESE
299
3. HEALTH EFFECTS
induced oxidative stress through the formation of reactive oxygen species may be a mechanism for
manganese neurotoxicity, and allopurinol may protect against this oxidative stress in the striatum and
brainstem of young rats.
Experiments such as the one by Desole et al. (1994) indicate that overexposure of rats to manganese
results in increased dopamine turnover in the rat striatum. However, patients with basal ganglia
dysfunction caused by manganese had normal striatal fluorodopa uptake on PET scan, indicating that the
nigrostriatal pathway was intact (Wolters et al. 1989). Seven intravenous injections of manganese
chloride into Rhesus monkeys resulted in an extrapyramidal syndrome characterized by bradykinesia,
facial grimacing, and rigity, with gliosis of the globus pallidus and the substantia nigra par reticularis
(Olanow et al. 1996). These intravenous injections, however, would have resulted in a highly elevated
but transient increase in blood manganese levels. Striatal dopamine and homovanillic acid levels were
within normal ranges; yet, there was clear evidence of manganese-induced neurotoxicity. Interestingly,
none of the symptoms improved after levo-dopa administration, supporting findings in humans that
manganism does not respond to levo-dopa treatment (Chu et al. 1995; Huang et al. 1989).
While there are a number of studies that support the hypothesis that manganese exerts its neurotoxicity
through oxidation, a study by Sziráki et al. (1999) has demonstrated atypical antioxidative properties of
manganese in iron-induced brain lipid peroxidation and copper dependent low density lipoprotein
conjugation. However, the underlying mechanisms of the antioxidant effects are not clear. Brenneman et
al. (1999) measured reactive oxygen species (ROS) in the brains of neonatal rats administered up to
22 mg manganese/kg/day for up to 49 days (dosing was only 5 days/week from day 22 to 49). On PND
21, no increase in ROS was seen in the striatum, hippocampus, or hindbrain of exposed rats at any dose,
compared to controls administered water only. In the cerebellum, ROS levels were significantly
increased to the same extent at both dose levels, as compared to controls. Manganese levels were not
increased significantly in the cerebellum at any dose level, but were increased in the striatum, and the rest
of the brain at the high dose level, when measured at PND 49. Mitochondrial manganese was not
significantly elevated in the cerebellum or striatum, but was elevated in the rest of the brain at this high
dose level, also at PND 49. These data do not support the hypothesis that oxidative damage is a
mechanism of action in manganese-induced neurotoxicity in the rat.
As reviewed Taylor et al. (2006), the available literature contains results both in support of and
inconsistent with oxidative stress involvement in manganese neurotoxicity. Recent support for oxidative
stress involvement includes the finding that co-treatment of rats with the antioxidant, N-acetylcysteine,
MANGANESE
300
3. HEALTH EFFECTS
and intraperitoneal injections of high doses of manganese chloride (50 mg/kg, once or daily for 4 days)
prevented the development of pathological changes observed following injection of manganese chloride
alone (Hazell 2006). Likewise, mouse catecholaminergic cells (CATH.a) were protected from the
cytotoxicity of 50–1000 µM manganese by supplementation of the culture media with 5 mM glutathione
or 10 mM N-acetylcysteine (Stredrick et al. 2004). In contrast, in a series of studies of neonatal rats, adult
male and female rats, or senescent male rats exposed by inhalation to manganese sulfate or manganese
phosphate at concentrations up to 3 mg manganese/m
3
with acute exposure durations or 1 mg
manganese/m
3
with subchronic exposure durations (Dorman et al. 2001a, 2004a, 2005a), no consistent
exposure-related changes were found in the following markers of oxidative stress in various brain regions:
glutathione, metallothionein, and glutamine synthetase (Taylor et al. 2006).
Mn(II) may also be involved in neurotoxicity. The neurotoxicity of Mn(II) has been linked to its ability
to substitute for Ca(II) under physiological conditions (Aschner and Aschner 1991), and the intestinal
transfers of Ca(II) and Mn(II) have been shown to be competitive in vivo (Dupuis et al. 1992). Although
the mechanism for Mn(II) transport into brain cells is uncertain, Mn(II) preferentially accumulates in the
mitochondria in the areas of the brain that are associated with manganism and neurological symptoms.
Manganese is taken up into mitochondria via the calcium uniporter, and once there, Mn(II) inhibits
mitochondrial oxidative phosphorylation. Gavin et al. (1992) observed that Mn(II) can inhibit
mitochondrial oxidative phosphorylation when incubating isolated mitochondria with Mn(II) at
concentrations >1 μM. Recently, it has also been shown that intramitochondiral Mn(II) can inhibit the
efflux of Ca(II), which may result in a loss of mitochondrial membrane integrity (Gavin et al. 1999). At
the same time, intramitochondrial Mn(II) can also inhibit oxidative phosphorylation and decrease energy
production. However, Brouillet et al. (1993) has suggested that the impaired oxidative metabolism
induced by manganese is indirectly linked to an excitotoxic process that results in neuronal degeneration.
Because manganese accumulates in the mitochondria and is associated with impaired energy production,
these authors compared the effects of intrastriatal injection of manganese with effects produced by known
mitochodral toxins, aminooxyacetic acid and 1-methyl-4-phenylpyridinium. Lesions produced by these
compounds can be blocked through an inhibition of the glutamatergic N-methyl-D-aspartate (NMDA)
receptor or by the removal of the cortical glutamatergic input into the striatum by decortication. Thus,
these lesions are termed “excitotoxic lesions.” It was shown that decortication or pre-treatment with the
NMDA noncompetitive antagonist, MK-801, could reverse or ameliorate neurochemical changes induced
by intrastriatal injection of manganese. These authors also showed that intrastriatal manganese treatment
also interfered with energy metabolism, ATP concentrations were significantly reduced by 51% and
lactate levels were increased by 97%. There is additional evidence that the glutamatergic excitatory
MANGANESE
301
3. HEALTH EFFECTS
system may play a role in manganese toxicity. Recent studies in genetically epilepsy-prone rats have
suggested that there are abnormalities in manganese-dependent enzymes. Although the manganese-
dependent enzymes are believed to be unrelated to seizure activity in these animals, it is suggested that
there is a link between the low manganese concentrations in glial cells and elevated glutamate levels due
to low glutamine synthetase activity (Critchfield et al. 1993).
Mn(II) (from manganese chloride) has also been shown to inhibit mitochondrial aconitase activity to a
significant level in the frontal cortex of male rats dosed with 6 mg manganese/kg/day for 30 days (Zheng
et al. 1998). Aconitase levels in striatum, hippocampus, and substantia nigra were decreased in treated
rats, but not to a significant extent. Aconitase, which catalyzes the interconversion of L-citrate to
isocitrate, via cis-aconitate, requires iron as a cofactor at its active center (Zheng et al. 1998). When the
authors incubated brain mitochondrial fractions with Mn(II), aconitase activity was decreased; the
addition of excess iron [Fe(II)] revived the enzyme activity. These data suggest that the similarity of
manganese and iron facilitates their proposed interaction at the subcellular level; however, the data do not
prove that Mn(II) is the form of manganese that is exerting the inhibitory effect.
Conversely, Suarez et al. (1995) did not observe cytotoxicity in cultured cells exposed to 100 μM Mn(II).
The discrepancy noted in this study, and that of Gavin et al. (1992) may have occurred because of a
protective effect of the cell membrane; if the cell membrane protects the cytosol, which typically has a
low manganese concentration, then the Mn(II) concentration may be too low to affect the mitochondria
through uniport uptake (Suarez et al. 1995). Another explanation is that mitochondrial uptake of Mn(II)
occurs, but toxic effects require that cells be exposed much longer than isolated mitochondria (Suarez et
al. 1995). It has also been established that manganese accumulation in the brain varies between regions,
particularly in developing animals; this region-specific accumulation may alter the metabolism and
homeostasis of manganese (Chan et al. 1992). In addition, it has been demonstrated that the manganese
concentration in the central nervous system, in particular the ventral mesencephalon, can be reduced by
cocaine, a dopamine reuptake inhibitor, or by reserpine, a dopamine depleting agent (Ingersoll et al.
1999). This suggests that the dopamine reuptake carrier is linked to a transport mechanism for
manganese.
In vitro studies of rat brain mitochondria have demonstrated that there is no apparent mechanism for
Mn(II) clearance other than the slow Na
+
independent mechanism; it is suggested that Ca(II) and Mn(II)
may accumulate in the brain mitochondria during manganese intoxication (Gavin et al. 1990). Other
theories regarding the mode of neurotoxicity for manganese (and other metal ions) include toxicity caused
MANGANESE
302
3. HEALTH EFFECTS
by the formation of hydroxyl radicals during the manganese-catalyzed autooxidation of hydrazines (Ito et
al. 1992).
It has been suggested that the mechanism of manganese neurotoxicity may in part involve complex
interactions with other minerals (Lai et al. 1999). In a developmental rat model of chronic manganese
toxicity, administration of manganese in drinking water was associated with increased levels of iron,
copper, selenium, zinc, and calcium in various regions of the brain. Moreover, the subcellular distribution
of various minerals was differentially altered following manganese treatment. Iron deficiency is
associated with increased manganese burden in the central nervous system of rats, while administration of
excess iron significantly decreases manganese uptake (Aschner and Aschner 1990). The biochemical
mechanisms underlying the interactions between manganese and other minerals are unclear.
Subtle deficits in fine motor and cognitive function in chronically exposed young adult male
Cynomologus macaques monkeys have been associated with manganese impairment of in vivo
amphetamine-induced dopamine release in the striatum, without detectable changes in markers of striatial
dopamine terminal integrity, and with decreased cerebral cortex N-acetylaspartate/creatine ratio (Guilarte
et al. 2006a, 2006b; Schneider et al. 2006). In these studies, four monkeys (5–6 years old at the start)
were given intravenous injections of manganese sulfate, 10–15 mg/kg or 3.26–4.89 mg manganese/kg,
once per week for an average of 34.2 weeks. Three additional monkeys without excess manganese
exposure or behavioral evaluations were used as a control group for post-mortem analyses of the brain
(Guilarte et al. 2006a). Prior to manganese exposure, the monkeys were trained to perform tests for
cognitive and motor function; overall behavior was assessed by ratings and videotaped analysis
(Schneider et al. 2006). By the end of the exposure period, monkeys developed deficits in spatial working
memory, showed modest decreases in spontaneous activity and manual dexterity, and showed increased
frequency of compulsive-type behaviors such as compulsive grooming (Schneider et al. 2006). At study
termination, mean manganese concentrations were elevated in exposed monkeys, compared with control
monkeys, in the following brain regions: globus pallidus (3.30 versus 0.72 µg/g tissue); caudate
(1.18 versus 0.38 µg/g tissue); putamen (1.5 versus 0.48 µg/g tissue); and frontal white matter
(0.57 versus 0.17 µg/g tissue) (Guilarte et al. 2006b; Schneider et al. 2006). Positron emission
tomography (PET) analysis found changes in amphetamine-induced release of dopamine in the striatum
(up to 60% decrease compared with baseline values), but no significant changes in striatal dopamine
receptor binding potentials (Guilarte et al. 2006a). Post-mortem chemical and immunohistochemical
analysis of caudate and putamen tissue found no evidence for exposure-related changes to levels of
D2-dopamine receptor (D2-DAR), dopamine receptor (DAT), tyrosine hydroxylase, or dopamine and its
MANGANESE
303
3. HEALTH EFFECTS
metabolite, homovanillic acid (Guilarte et al. 2006a). Using
1
H-magnetic resonance spectroscopy,
concentrations of creatine (Cr), N-acetylaspartate (NAA), choline, and myo-inositol were measured.
Decreases (relative to baseline) in the NAA/Cr ratio were measured in the parietal cortex and frontal
white matter, but not in the striatum (Gulilarte et al. 2006b). Guilarte et al. (2006b) suggested that the
changes in the NAA/Cr ratio are indicative of neuronal degeneration or dysfunction in the parietal cortex
that may also be associated with the neurobehavioral changes noted in the monkeys. Subsequent gene
expression profiling in the frontal cortex of these monkeys found changes consistent with cellular stress
responses that the investigators proposed may help to explain the subtle cognitive effects noted (Guilarte
et al. 2008). The collective results from these studies suggest that subtle neurobehavioral changes noted
in epidemiological studies of chronically exposed workers (see Section 3.2.1.4 and Appendix A) may be
similar to those noted in these monkeys and may be related to manganese-induced functional changes and
gene expression changes noted in the striatum and the cerebral cortex.
As reviewed by Fitsanakis et al. (2006), most mechanistic research on manganese neurotoxicity has
focused on perturbations of the dopaminergic system, but there is evidence to suggest that early
consequences of manganese neurotoxicity may involve perturbations of other neurotransmitters including
GABA and glutamate in the basal ganglia and other brain regions. For example, there is evidence to
suggest that manganese decreases the ability of astrocytes to clear glutamate from extracellular space
(Erikson and Aschner 2002, 2003), increases the sensitivity of glutamate receptors to glutamate (see
Fitsanakis and Aschner 2005 and Fitsanakis et al. 2006 for review), and perturbs glutamine-glutamate-
GABA interconversions in frontal cortex and basal ganglia of rats (Zwingmann et al. 2004, 2007). When
rat striatum was perfused with artificial cerebrospinal fluid with 200 nM manganese, GABA levels in the
perfusate were decreased by about 60% compared with controls, but no effects on levels of glutamate,
aspartate or glycine in the perfusate were observed (Takeda et al. 2003). In the perfused rat hippocampus,
200 nM manganese caused a 50% decrease in the levels of GABA, glutamate, and aspartate in the
perfusate (Takeda et al. 2002). The results from the studies of Takeda et al. (2002, 2003) suggest that
there are differential regional effects of manganese on the release of different neurotransmitters.
Fitsanakis et al. (2006) concluded that additional research is needed to better understand the
interdependence of neurotransmitters, including dopamine, glutamate, and GABA and their relationships
to manganese neurotoxicity.
MANGANESE
304
3. HEALTH EFFECTS
3.5.3 Animal-to-Human Extrapolations
As discussed in Section 3.2, the available literature on toxicological analysis of manganese in humans and
animals is quite extensive. However, due to the wide dose ranges administered, the variety of responses,
and the differences in measured end points, comparisons of effects across species is not straightforward.
Rodent models have primarily been used to study manganese neurotoxicity. These studies have reported
mostly neurochemical, rather than neurobehavioral, effects (Brouillet et al. 1993; Chandra 1983; Chandra
and Shukla 1978, 1981; Daniels and Abarca 1991; Deskin et al. 1980, 1981; Eriksson et al. 1987a;
Gianutsos and Murray 1982; Parenti et al. 1986; Singh et al. 1979; Subhash and Padmashree 1991), as
very few studies investigated neurobehavioral effects. It has been suggested that this focus may reflect
difficulties in characterizing behavioral changes following basal ganglia damage in the rodent (Newland
1999). Other techniques, such as those used to identify basal ganglia damage as a result of exposure to
neuroleptics (Newland 1999), may be refined to further exploit the rodent model as a predictor of
neurobehavioral change in the human. The usefulness of the rat model for manganese neurotoxicity is
also limited because the distribution of manganese in brain regions is dissimilar to that of the human
(Chan et al. 1992; Brenneman et al. 1999; Kontur and Fechter 1988; Pappas et al. 1997). Studies to date
have used exposure routes such as inhalation, intravenous, intraperitoneal, or subcutaneous, with few
exceptions (Brenneman et al. 1999; Dorman et al. 2000, 2002, 2004a, 2005a, 2006b; Lown et al. 1984;
Morganti et al. 1985; Pappas et al. 1997).
The rabbit has also been used as a model for manganese toxicity in a few studies (Chandra 1972;
Szakmáry et al. 1995). The only available neurotoxicity study using the rabbit (Chandra 1972) reported
that the species, when dosed intratracheally with 253 mg manganese/kg body weight (inferred as a one-
time dose), developed hindlimb paralysis (a response not typically observed in humans exposed to excess
manganese) after an observation period of 18 months. The animals also exhibited wide-spread neuronal
degeneration in the brain. This study suggests that rabbits and humans may be qualitatively similar in the
manifestation of neurobehavioral effects. However, further studies are needed to determine if the two
species manifest comparable symptoms within the same dose range.
The nonhuman primate has been a useful model for predicting neurotoxicity in the human as the monkey
presents neurobehavioral responses to toxicants that are very similar to those observed in humans
(Eriksson et al. 1987b; Golub et al. 2005; Gupta et al. 1980; Newland and Weiss 1992; Olanow et al.
1996). Further, the monkey also undergoes neurochemical changes (Bird et al. 1984) as a result of
MANGANESE
305
3. HEALTH EFFECTS
manganese exposure. Studies have shown that monkeys exposed to manganese injected either
intravenously or subcutaneously exhibit symptoms very similar to those observed in miners and others
exposed to manganese, including ataxia, bradykinesia, unsteady gait, grimacing, and action tremor
(Eriksson et al. 1992a, 1992b; Newland and Weiss 1992; Olanow et al. 1996). In addition, monkeys
exhibiting these effects show accumulation of manganese in the basal ganglia as observed by MRI
(Eriksson et al. 1992b; Newland and Weiss 1992), as do humans who are either exposed to, or are unable
to clear, excess manganese (Devenyi et al. 1994; Fell et al. 1996; Hauser et al. 1994; Ono et al. 1995;
Pomier-Layrargues et al. 1998; Rose et al. 1999; Spahr et al. 1996). However, primate studies showing
these neurobehavioral effects have involved routes of administration that do not mimic environmental
exposures, and although the effects in monkeys are qualitatively similar, it is currently unknown whether
the effects are seen at the same dose metric as those in humans. Newland (1999) proposes using MRI
techniques to relate the administration of certain amounts of manganese with a corresponding MRI signal
in the brain and the resultant neurobehavioral effects. This technique might be very useful in developing
a true dose-response relationship for manganese neurotoxicity in both the monkey and human.
Mechanisms of manganese toxicity in vivo are likely to be comprised in part by unique characteristics of
the exposed individual, as well as by general physiology and exposure route. Multiple route PBPK
models have recently been developed for predicting manganese brain concentrations in adult rats,
monkeys, and humans and during gestation and lactation (Leavens et al. 2007; Nong et al. 2008;
Schroeter et al. 2011;Teeguarden et al. 2007a, 2007b, 2007c; Yoon et al. 2011, 2009a, 2009b). As
discussed by Yoon et al. (2011), confidence in predictions from the human models may improve with
more information on the normal range and fluctuation of human brain manganese concentrations during
early postnatal periods, the relationship between blood manganese concentrations and target tissue
dosimetry, and the extent of induction of neonatal biliary excretion. Further extension of the models to
other suspected susceptible populations, such as the elderly and individuals with liver dysfunction, also
would be useful.
3.6 TOXICITIES MEDIATED THROUGH THE NEUROENDOCRINE AXIS
The potential hazardous effects of certain chemicals on the endocrine system are of current concern
because of the ability of these chemicals to mimic or block endogenous hormones. Chemicals with this
type of activity are most commonly referred to as endocrine disruptors. However, appropriate
terminology to describe such effects remains controversial. The terminology endocrine disruptors,
initially used by Thomas and Colborn (1992), was also used in 1996 when Congress mandated the EPA to
MANGANESE
306
3. HEALTH EFFECTS
develop a screening program for “...certain substances [which] may have an effect produced by a
naturally occurring estrogen, or other such endocrine effect[s]...”. To meet this mandate, EPA convened a
panel called the Endocrine Disruptors Screening and Testing Advisory Committee (EDSTAC), and in
1998, the EDSTAC completed its deliberations and made recommendations to EPA concerning endocrine
disruptors. In 1999, the National Academy of Sciences released a report that referred to these same types
of chemicals as hormonally active agents. The terminology endocrine modulators has also been used to
convey the fact that effects caused by such chemicals may not necessarily be adverse. Many scientists
agree that chemicals with the ability to disrupt or modulate the endocrine system are a potential threat to
the health of humans, aquatic animals, and wildlife. However, others think that endocrine-active
chemicals do not pose a significant health risk, particularly in view of the fact that hormone mimics exist
in the natural environment. Examples of natural hormone mimics are the isoflavinoid phytoestrogens
(Adlercreutz 1995; Livingston 1978; Mayr et al. 1992). These chemicals are derived from plants and are
similar in structure and action to endogenous estrogen. Although the public health significance and
descriptive terminology of substances capable of affecting the endocrine system remains controversial,
scientists agree that these chemicals may affect the synthesis, secretion, transport, binding, action, or
elimination of natural hormones in the body responsible for maintaining homeostasis, reproduction,
development, and/or behavior (EPA 1997). Stated differently, such compounds may cause toxicities that
are mediated through the neuroendocrine axis. As a result, these chemicals may play a role in altering,
for example, metabolic, sexual, immune, and neurobehavioral function. Such chemicals are also thought
to be involved in inducing breast, testicular, and prostate cancers, as well as endometriosis (Berger 1994;
Giwercman et al. 1993; Hoel et al. 1992).
Studies of endocrine effects in humans following manganese exposure are very limited. Alessio et al.
(1989) reported the elevation of serum prolactin and cortisol in chronically-exposed workers, while no
changes in prolactin, FSH, or LH levels were observed in an occupational study involving shorter
exposure periods (Roels et al. 1992). Lucchini et al. (1995) reported elevated serum prolactin levels in
ferromanganese workers; 20 of those workers still showed elevated prolactin levels 5 years later after
exposure to consistent levels of airborne manganese (Smargiassi and Mutti 1999). In fact, the serum
prolactin levels had increased significantly over the previous values. Although these changes are minor,
changes in prolactin secretion may have effects on different physiological functions, including loss of
libido and impotence in men, and infertility and change in menstrual cycle in women.
No studies of endocrine effects in animals following airborne manganese exposure were located. Short-
term animal studies and some of the long-term animal studies were negative for endocrine effects
MANGANESE
307
3. HEALTH EFFECTS
following oral exposure to manganese (NTP 1993). One intermediate study reported a decrease in
circulating testosterone and a significant increase in substance P in the hypothalamus and neurotensin in
the pituitary in rats dosed intraperitoneally with 6.6 mg manganese/kg/day as manganese chloride (Hong
et al. 1984). Two other studies in rats reported that manganese tetroxide in food, given at a dose of
350 mg manganese/kg/day for 224 days (starting on day 1 of gestation and continuing for 224 days)
(Laskey et al. 1982) and 214 mg manganese/kg/day given up to 28 days (Laskey et al. 1985), resulted in
reduced testosterone levels in male rats. The biological significance of this effect is unknown because the
decrease had no result on fertility in the latter study (Laskey et al. 1985), and there were no observed
effects on the hypothalamus or pituitary.
A current interest in endocrine effects of manganese revolves around the possibility that developmental
manganese exposure may influence the timing of puberty. One study performed on 23-day-old female
rats in which manganese was provided by a single, intraventricular administration of 0, 0.01, 0.02, 0.04,
or 0.17 mg manganese/kg as manganese chloride found that, at the three highest doses, manganese
stimulated a dose-responsive increase in luteinizing hormone (LH) levels (Pine et al. 2005). A dose of
2 mg manganese/kg/day, provided to another group of female pups by daily gavage from PND 12 to 29
significantly advanced the average age of puberty (by approximately 1 day) as well as produced
significant increases in serum levels of LH, follicule stimulating hormone (FSH), and estradiol (E
2
) (Pine
et al. 2005). In a follow-up study by Hiney et al. (2011), a dose of 10 mg manganese chloride/kg/day
(4.4 mg manganese/kg/day) by gavage from PND 12 to 29 in another group of female pups resulted in
elevated gene expression levels of IGF-1, COX-2, and LHRH in the hypothalamus (genes involved in
neuroendocrine axis control of puberty onset). Additionally, the release of LHRH and prostaglandin E
2
was increased in the median eminence of treated females in vitro. Taken together, the results from these
two studies suggest a role for manganese in regulating the timing of puberty in female rats and suggest
that excess manganese exposure may accelerate the onset of puberty. Manganese also appears to have
pubertal effects in male rats; an oral gavage dose of 11 mg manganese/kg/day provided daily on PNDs
15–48 or 15–55 produced significantly increased LH, FSH, and testosterone at 55 days of age (Lee et al.
2006). Increases in both daily sperm production and efficiency of spermatogenesis were also observed,
suggesting that manganese may be a stimulator of prepubertal LHRH/LH secretion and thus facilitate the
onset of male puberty. In vitro experiments using medial basal hypothalamic implants from adult male
Sprague-Dawley rats showed that manganese at 500 µM increased LHRH release, nitric oxide synthase
activity, and the content of cyclic cGMP in the medial basal hypothalamus (Prestifilippo et al. 2007). The
inhibition of nitric oxide synthase with a competitive inhibitor prevented the manganese-induced increase
MANGANESE
308
3. HEALTH EFFECTS
in LHRH release. The results of these in vitro studies provide added evidence of the ability of manganese
to modulate levels of LHRH, even in adult animals (Prestifilippo et al. 2007).
3.7 CHILDREN’S SUSCEPTIBILITY
This section discusses potential health effects from exposures during the period from conception to
maturity at 18 years of age in humans, when all biological systems will have fully developed. Potential
effects on offspring resulting from exposures of parental germ cells are considered, as well as any indirect
effects on the fetus and neonate resulting from maternal exposure during gestation and lactation.
Relevant animal and in vitro models are also discussed.
Children are not small adults. They differ from adults in their exposures and may differ in their
susceptibility to hazardous chemicals. Children’s unique physiology and behavior can influence the
extent of their exposure. Exposures of children are discussed in Section 6.6, Exposures of Children.
Children sometimes differ from adults in their susceptibility to hazardous chemicals, but whether there is
a difference depends on the chemical (Guzelian et al. 1992; NRC 1993). Children may be more or less
susceptible than adults to health effects, and the relationship may change with developmental age
(Guzelian et al. 1992; NRC 1993). Vulnerability often depends on developmental stage. There are
critical periods of structural and functional development during both prenatal and postnatal life, and a
particular structure or function will be most sensitive to disruption during its critical period(s). Damage
may not be evident until a later stage of development. There are often differences in pharmacokinetics
and metabolism between children and adults. For example, absorption may be different in neonates
because of the immaturity of their gastrointestinal tract and their larger skin surface area in proportion to
body weight (Morselli et al. 1980; NRC 1993); the gastrointestinal absorption of lead is greatest in infants
and young children (Ziegler et al. 1978). Distribution of xenobiotics may be different; for example,
infants have a larger proportion of their bodies as extracellular water, and their brains and livers are
proportionately larger (Altman and Dittmer 1974; Fomon 1966; Fomon et al. 1982; Owen and Brozek
1966; Widdowson and Dickerson 1964). The infant also has an immature blood-brain barrier (Adinolfi
1985; Johanson 1980) and probably an immature blood-testis barrier (Setchell and Waites 1975). Many
xenobiotic metabolizing enzymes have distinctive developmental patterns. At various stages of growth
and development, levels of particular enzymes may be higher or lower than those of adults, and
sometimes unique enzymes may exist at particular developmental stages (Komori et al. 1990; Leeder and
Kearns 1997; NRC 1993; Vieira et al. 1996). Whether differences in xenobiotic metabolism make the
MANGANESE
309
3. HEALTH EFFECTS
child more or less susceptible also depends on whether the relevant enzymes are involved in activation of
the parent compound to its toxic form or in detoxification. There may also be differences in excretion,
particularly in newborns who all have a low glomerular filtration rate and have not developed efficient
tubular secretion and resorption capacities (Altman and Dittmer 1974; NRC 1993; West et al. 1948).
Children and adults may differ in their capacity to repair damage from chemical insults. Children also
have a longer remaining lifetime in which to express damage from chemicals; this potential is particularly
relevant to cancer.
Certain characteristics of the developing human may increase exposure or susceptibility, whereas others
may decrease susceptibility to the same chemical. For example, although infants breathe more air per
kilogram of body weight than adults breathe, this difference might be somewhat counterbalanced by their
alveoli being less developed, which results in a disproportionately smaller surface area for alveolar
absorption (NRC 1993).
Prenatal and early postnatal developmental effects of manganese have largely been unstudied in humans.
Potential developmental effects of manganese were suggested by the results of a study by Hafeman et al.
(2007) that reported high mortality among infants <1 year of age in a Bangladesh population where the
drinking water supplied by certain local wells contained high levels of manganese. Similarly, Spangler
and Spangler (2009) reported increased infant mortality rates in counties in North Carolina with higher
groundwater manganese concentrations after accounting for such confounders as low birth weight,
economic status, education, and ethnicity. However, it cannot be determined if the observed effects in
these studies were solely due to excess manganese alone or could have been influenced by other drinking
water or dietary components. An older study by Kilburn (1987) showed that a native population living on
an island with rich manganese deposits suffered increased neurological disorders and incidences of birth
defects. Manganese exposure was most likely via inhalation and oral routes. However, since this study
involved small sample sizes and lacked exposure concentrations and a suitable control group, these
effects cannot be ascribed to manganese alone.
Two early studies investigated increased respiratory complaints and symptoms at a junior high school
situated 100 m from a manganese alloy plant in Japan (manganese concentrations in total dust at a
200 meter perimeter around the plant were 0.004 mg/m
3
[3.7 μg/m
3
]) (Kagamimori et al. 1973; Nogawa et
al. 1973). The initial study showed that the incidences of self-reported respiratory illnesses among
children in the exposed school were much higher than those of a control school 7 km away from the plant
(Nogawa et al. 1973). Further, evaluations of respiratory fitness showed significant decreases in several
MANGANESE
310
3. HEALTH EFFECTS
parameters. When the installation of dust catchers resulted in a decreased manganese concentration in
total dust, complaints of illness decreased, and the test results improved (Kagamimori et al. 1973). These
respiratory effects were not unique from those observed in adults exposed to airborne manganese.
Further, it was not reported if other compounds were present in the dust generated by the plant, which
might have contributed to or caused the reported illnesses. It is possible that these effects might have
been triggered by the dust and were not specific to manganese.
The possibility of neurological effects in children environmentally exposed to manganese is a continuing
area of epidemiological research.
In early studies, children who have been exposed to elevated levels of inorganic manganese presumably
through diet (either a normally ingested diet or through total parenteral nutrition, TPN) have shown signs
of motor disorders (e.g., dystonia, dysmetria, propulsion, retropulsion, poor check response bilaterally)
similar to those observed in cases of frank manganism (Devenyi et al. 1994; Fell et al. 1996). In a few of
the cases, the presence of liver dysfunction indicated a decreased ability to clear excess manganese
(Devenyi et al. 1994; Fell et al. 1996), but some of the children with apparently normal livers also
exhibited motor disorders (Fell et al. 1996). Several children also exhibited hyperintense signals on MRI
resulting from increased exposure to manganese due to cholestatic end-stage liver disease (Devenyi et al.
1994) and from high concentrations of the element in TPN, either in the presence (Fell et al. 1996) or
absence (Fell et al. 1996; Ono et al. 1995) of liver disease. The Ono et al. (1995) study involved a child
on TPN for more than 2 years; although this child did have increased blood manganese and hyperintense
signals in the basal ganglia as shown by MRI, the authors did not report any observable signs of
neurotoxicity. A similar lack of observable neurotoxicity was reported in two siblings fed TPN with high
manganese concentrations (0.2 mmol manganese/kg/day) for several months (the brother for 63 months
total starting at age 4 months; the sister for 23 months total starting at age 1 month) (Kafritsa et al. 1998).
Both children had elevated blood manganese levels and showed hyperintense signals in the basal ganglia
(especially the globus pallidus and subthalamic nuclei) on MRI. Reduction of manganese concentration
in the TPN resulted in a gradual loss of signal on MRI analysis (becoming comparable to normal scans)
and a decrease in blood manganese levels as measured in three subsequent annual exams. These
equivocal results indicate that there are considerable differences in susceptibility to the neurotoxic effects
of excess manganese in children.
Two other earlier studies show that children who drank water containing manganese at average
concentrations of at least 0.241 mg/L (Zhang et al. 1995) and ate food with increased manganese content
MANGANESE
311
3. HEALTH EFFECTS
(He et al. 1994) for 3 years performed more poorly in school (as shown by mastery of their native
language, mathematics, and overall grade average) and on the WHO neurobehavioral core test battery
than those students who drank water with manganese ≤0.04 mg/L. These neurobehavioral tests are
among those administered to workers occupationally exposed to manganese to determine the presence of
early neurological deficit (Chia et al. 1993a; Iregren 1990; Lucchini et al. 1995; Mergler et al. 1994;
Roels et al. 1987a, 1992). These concentrations are much lower than the ones to which adults were
exposed in the Kondakis et al. (1989) study. In this study, ingestion of drinking water with excess
manganese (1.8–2.3 mg/L) was linked to the onset of unspecified neurological symptoms in an aged
population (average age, over 67 years). Though there are limitations, this and other environmental
studies in adults (Baldwin et al. 1999; Beuter et al. 1999; Goldsmith et al. 1990; Kawamura et al. 1941;
Kondakis et al. 1989; Mergler et al. 1999) and two studies in children (He et al. 1994; Zhang et al. 1995)
indicate that both adults and children can manifest similar neurological deficits that are potentially linked
to ingesting excess manganese. However, these reports are lacking well-characterized and quantitative
exposure data that would indicate whether children and adults experience neurological effects at the same
or different exposure levels. Existing studies do not allow estimations of the quantitative susceptibility of
children to the preclinical effects of excess manganese exposure. They do indicate, though, that children
can develop symptoms of neurotoxicity after oral exposure to manganese that are similar to those effects
seen in adults environmentally or occupationally exposed to the metal. Further, these studies indicate that
neurological effects may be a concern for children exposed to excess manganese from the environment or
from a hazardous waste site.
The investigations by He et al. (1994) and Zhang et al. (1995) showed that children with poorer school
performance had higher manganese hair content than children from the control area. Other studies have
found that manganese levels in hair are higher in learning disabled children than in normal children
(Collipp et al. 1983; Pihl and Parkes 1977). The route of excess exposure is not known, but it is
presumed to be mainly oral. These observations are consistent with the possibility that excess manganese
ingestion could lead to learning or behavioral impairment in children. However, an association of this
sort is not sufficient to establish a cause-effect relationship since a number of other agents, including lead,
might also be involved (Pihl and Parkes 1977).
Several recent reports continue to implicate elevated manganese exposure with impaired
neurodevelopment. Four epidemiological reports of manganese neurotoxicity in children resulting from
manganese exposure in drinking water have been published. In two separate cross-sectional studies,
Wasserman et al. (2006, 2011) report statistically significant relationships for decreasing intelligence
MANGANESE
312
3. HEALTH EFFECTS
scores with increasing manganese levels in drinking water in 142–151 children (ages 8–11 years) in
Bangladesh. Similarly, in a cross-sectional study conducted by Bouchard et al. (2011), a significant
negative association was found between manganese levels in the home tap water and intelligence scores
in 362 children from Quebec, Canada. In previous study by Bouchard et al. (2007c), a statistically
significant relationship between increased levels of oppositional behaviors and hyperactivity and
increased levels of manganese in drinking water in an epidemiological study of 46 children (ages 6–
15 years), also from Quebec, Canada.
The findings from Farias et al. (2010) support of the hyperactivity findings by Bouchard et al. (2007c).
This cross-sectional study of 96 students (ages 7–15 years) diagnosed with ADHD and 35 controls reports
that students diagnosed with, but not treated for, ADHD had significantly elevated serum manganese
levels. However, in students treated for ADHD with stimulants, manganese levels were not different
from controls and were significantly lower than untreated ADHD students. The source of manganese
exposure in this study was not determined, but is presumed to be primarily oral.
Additionally, three recent case studies suggest that certain children are particularly susceptible to
manganese neurotoxicity from high levels in drinking water, including: (1) severe neurotoxic symptoms
(inability to walk independently, tendency to fall backward, and development of a “cock-like” walk) and
MRI scan findings consistent with a diagnosis of hypermanganism in a previously healthy 5-year-old
female that were associated with elevated drinking water concentrations of manganese (1.7–2.4 mg
manganese/L), pica, emotional lability, polycythemia, iron deficiency, and elevated levels of plasma
manganese (Brna et al. 2011); (2) a similar case of severe manganism-like neurotoxic symptoms in a
previously healthy 6-year-old female that were associated with elevated drinking water concentrations of
manganese (1.7–2.4 mg manganese/L), pica, a diet high in manganese-rich foods, and elevated levels of
plasma manganese (Sahni et al. 2007); and (3) inattentiveness and lack of focus in the classroom and low-
percentile performance in tests of memory in a 10-year-old male with no history of learning problems
associated with elevated manganese in drinking water (1.21 mg manganese/L) (Woolf et al. 2002).
Increased exposure to elevated airborne manganese near industrial sites has also been associated with
altered neurodevelopment. Two studies evaluated 79 children (ages 7–11 years) from the Molango
mining district in Mexico exposed to an average manganese air concentration of 0.13 μg/m
3
for at least
5 years. Riojas-Rodríguez et al. (2010) reported a significant inverse relationship between manganese
exposure and full scale and verbal IQs, while Hernández-Bonilla et al. (2011) reported a subtle negative
association of manganese exposure on motor speed and coordination. Similarly, Menezes-Filho et al.
MANGANESE
313
3. HEALTH EFFECTS
(2011) evaluated cognitive performance in 83 children from 55 families living near a ferromanganese
alloy plant in Brazil that has been emitting high levels of manganese into the air for 4 decades. Elevated
manganese exposure was inversely associated with intellectual function in both children and adults.
However, direct correlations between air manganese concentrations and cognitive function were not
evaluated in these studies. Likewise, other sources of environmental exposure (i.e., dietary, water) were
not considered.
Recent evidence suggests that the critical time-point for adverse neurodevelopmental effects from
elevated manganese exposure is as early as 12 months of age. In a prospective study, Claus Henn et al.
(2010) reported a U-shaped nonlinear relationship between blood manganese levels and Mental
Development Index scores at 12 months in 486 infants from Mexico City. However, by 24 months,
manganese levels were not correlated with neurodevelopment using the Bayley Scales of Infant
Development-II, Spanish version. These findings are consistent with manganese as both an essential
nutrient and a toxicant, and identify 12 months as a potential critical developmental window for
manganese exposure.
Taken together, these recent studies provide added weight to the evidence for the neurotoxic potential of
excessive manganese in children, but one or more of the following uncertainties preclude the
characterization of causal and dose-response relationships between the observed effects and manganese
exposure: (1) whether or not the observed effects were solely due to excess manganese alone or could
have been influenced by other drinking water or dietary components; (2) the lack of quantitative
information about manganese levels from different environmental sources (food, water, and air); and
(3) the small sample sizes.
Developmental studies in animals following inhalation exposure to manganese are sparse. One study
exists (Lown et al. 1984) in which pregnant mice were exposed to a high concentration of airborne
manganese or filtered air for 17 days preconception and then exposed to either the same concentration of
manganese or filtered air postconception. Their pups were then fostered to adult females who had
experienced the same inhalation exposures as the mothers (no manganese exposure, pre- or post-
conception exposure, or both). The pups of exposed mothers had decreased body weight, but exhibited
no differences in activity compared to pups from mothers exposed to air, irrespective of exposure history.
In neonatal rats orally exposed to 25 or 50 mg manganese/kg/day from PNDs 1 through 21, manganese
concentrations in various brain regions were about 2-fold higher than brain manganese concentrations in
adult rats exposed to the same oral dose levels for 21 days (Dorman et al. 2000). At the highest dose
MANGANESE
314
3. HEALTH EFFECTS
level, neonatal rats showed an increased acoustic startle response, but exposure-related changes in other
neurological end points (clinical signs, motor activity, and passive avoidance) were not found (Dorman et
al. 2000). In another study, inhalation exposure of female CD rats to manganese sulfate, starting 28 days
prior to breeding through PND 18, caused elevated manganese concentrations in exposed maternal rats
(compared with air control rats) in the following tissues: brain and placenta at 0.5 and 1.0 mg
manganese/m
3
and lung at 0.05, 0.5, and 1.0 mg manganese/m
3
(Dorman et al. 2005a). In contrast,
statistically significant elevations of manganese concentrations in sampled fetal tissues were observed
only in the liver at 0.5 and 1.0 mg manganese/m
3
, and elevated brain manganese concentrations were only
observed in offspring after PND 14. The results from this study suggest that the brain in developing
fetuses is partially protected from excess manganese by the placenta, and that the neonatal period is
sensitive to increased manganese concentration in brain and other tissues under exposure to elevated
airborne manganese concentrations.
Oral studies in animal models, whether involving the dosing of pregnant dams or sucklings, reveal a
variety of neurochemical and physiological changes as a result of manganese exposure. The majority of
studies have involved manganese chloride. One study in rats reported that pups exposed in utero 11 days
during gestation to a relatively low concentration of manganese chloride (22 mg/kg; by gavage in water)
did not have any observable decrease in weight gain, nor any gross or skeletal malformations upon
necropsy (Grant et al. 1997a). Another study (Szakmáry et al. 1995) that also administered manganese
chloride in water by gavage to pregnant rats at the slightly higher concentration of the 33 mg
manganese/kg/day throughout the entire gestation period reported a delay of skeletal and organ
development as well as an increase in skeletal malformations, such as clubfoot, in unborn pups. These
malformations, however, were self-corrected in pups allowed to grow to 100 days of age. In addition, the
same dose and route did not result in any observable developmental toxicity in the rabbit (Szakmáry et al.
1995). Rat pups exposed during gestation and after birth to manganese at relatively high concentrations
of 120–620 mg/kg in drinking water suffered no observable adverse effects at the low dose and only
transient adverse effects (decrease in weight and hyperactivity) at the high dose (Pappas et al. 1997).
Similar transient body weight decreases and increases in motor activity were observed in neonatal rats
administered 22 mg manganese/kg/day (as manganese chloride), by mouth or gavage, for up to 49 days
(Brenneman et al. 1999; Dorman et al. 2000). Jarvinen and Ahlström (1975) fed pregnant rats varying
doses of manganese sulfate in food for 8 weeks prior to and during gestation. Fetuses taken at 21 days
did not show gross abnormalities, but did have significantly increased body burdens of manganese from
mothers fed 187 mg/kg/day.
MANGANESE
315
3. HEALTH EFFECTS
Rat pups from a generational study in which the male and female parents were exposed to 240–715 mg
manganese/kg/day (as manganese chloride in drinking water) in either a diet adequate or deficient in
protein (Ali et al. 1983a) suffered a delayed air righting reflex (independent of protein content of diet) and
showed significant alterations in the age of eye opening and development of auditory startle when
produced by parents fed low-protein diets with 240 mg manganese/kg/day in water. Kontur and Fechter
(1988) administered up to 1,240 mg manganese/kg/day as manganese chloride in drinking water to
pregnant rats during days 0–20 of gestation. Although the authors found increased manganese levels in
the fetus, there were no measurable effects on dopamine or norepinephrine turnover in the pup brain, or in
the development of a startle response. However, in a study with both gestational and postnatal exposure
(GD 1–PND 24), Molina et al. (2011) reported decreased anxiety behavior on the elevated plus apparatus
on PND 24 in rat pups from pregnant dams exposed to 4.79 mg manganese/mL (as manganese chloride in
drinking water). Based on body weight and water intake, the study authors calculated daily manganese
doses during gestation and lactation as 565 and 1,256 mg/kg/day, respectively. In a postnatal exposure
study, an increased amplitude in acoustic startle reflex was observed at PND 21 in neonatal rats
administered 22 mg manganese/mg/day (as manganese chloride) by mouth from PND 1 to 21 (Dorman et
al. 2000). Significant increases in brain dopamine and DOPAC concentrations in select brain regions in
these animals as well as increased brain manganese concentrations were reported. This study
demonstrated that neonates treated with manganese showed neurological changes, whereas no effects
were observed in the adult animals treated similarly. Lazrishvili et al. (2009) reported marked gliosis in
rat pups (PND 40) from dams exposed to 4.4 mg manganese/kg/day (as manganese chloride) before,
during, and for 1 month following pregnancy. This is in contrast to the lack of evidence for astrocytic
alterations in adult rats exposed to 147 mg manganese/kg/day (as manganese chloride in drinking water)
for up to 1 month (Rivera-Mancía et al. 2009).
Neonatal rats given manganese chloride in drinking water for 44 days at a dose of 150 mg manganese/
kg/day developed a transient ataxia on days 15–20 of the treatment and had decreased levels of
homovanillic acid in the hypothalamus and striatum on day 15 but not day 60 (Kristensson et al. 1986).
Neonatal rats given bolus doses of manganese chloride in water of 1 mg manganese/kg/day for 60 days
suffered neuronal degeneration and increased monoamine oxidase on days 15 and 30 of the study, but did
not show any clinical or behavioral signs of neurotoxicity (Chandra and Shukla 1978). Similarly,
neonatal rats given bolus doses of manganese chloride in 5% sucrose at doses of 0, 1, 10 or 20 mg
manganese/kg/day for 24 days after birth showed decreased levels of dopamine, but not norepinephrine,
in the hypothalamus (Deskin et al. 1980); doses of 20 mg/kg/day caused a decrease of tyrosine
hydroxylase activity and an increase in monoamine oxidase activity in the hypothalamus. In a follow-up
MANGANESE
316
3. HEALTH EFFECTS
study, Deskin et al. (1981) gave 0, 10, 15 and 20 mg manganese/kg/day (as manganese chloride in 5%
sucrose by gavage) to neonatal rats from birth to age 24 days. The authors found that the highest dose
resulted in increased serotonin in the hypothalamus and decreased acetylcholinesterase in the striatum.
However, the authors did not indicate that the acetylcholinesterase decrease was important given other
mechanisms involved in the metabolism of this neurochemical. Another neonatal study reported
increased locomotor activity when rats were dosed with 10 mg/kg cocaine in adulthood (but no increased
locomotor activity without cocaine challenge) following oral exposure to 13.1 mg manganese/kg/day (but
not 4.4 mg manganese/kg/day) on PNDs 1–21 (Reichel et al. 2006).
A growing area of research is lasting adverse effects from early exposure to manganese. Tran et al.
(2002a) reported an impaired olfactory-mediated homing ability and passive avoidance of footshocks and
decreased striatal dopamine levels in male Sprague-Dawley rats exposed to oral doses of 17.2 mg
manganese/kg/day (but not 8.6 mg manganese/kg/day) as manganese chloride on PNDs 1–20. Evidence
indicates that alterations in passive avoidance behavior and dopamine expression persist into adulthood,
long after manganese exposure has ceased (Tran et al. 2002b). Kern and colleagues (Kern and Smith
2011; Kern et al. 2010) reported increased open field activity, impaired spatial learning, increased brain
expression of dopamine receptors (D1, D2) and dopamine transporter proteins, and increased glial
activation in neonatal (PND 24) Sprague-Dawley rats exposed to oral doses of 50 mg manganese/kg/day
(but not 25 mg manganese/kg/day) as manganese chloride on PNDs 1–21. In rats tested as adults
(following cessation of exposure at PND 21), open field activity returned to baseline and the only change
in the dopaminergic system was increased dopamine D2 receptor in the prefrontal cortex; however,
increased glial activation remaine. In another study, Moreno et al. (2009) examined the differential
effects of juvenile-only exposure, adult-only exposure, and juvenile followed by adult exposure up to
13.1 mg/kg/day (as manganese chloride via gavage) on both neurochemical and behavioral end points in
C57Bl/6 mice. Open-field activity was altered in juvenile-only and juvenile+adult exposure, but not
adult-only exposure. All groups had dopaminergic system alterations, with the magnitude of changes
being the greatest in juveniles. Only juvenile-exposed mice had alterations in the serotonergic system.
Together, these studies suggest that developing mice may be more sensitive to manganese exposure, and
that developmental exposure has lasting effects on neurochemical and behavioral end points and later
susceptibility to exposure.
Several studies evaluated the effects of manganese in the diet on reproductive development in the pre-
weanling rodent. Gray and Laskey (1980) fed mice 1,050 mg manganese/kg/day (as manganese
tetroxide) in the diet beginning on PND 15 and continuing for 90 days. The manganese caused decreased
MANGANESE
317
3. HEALTH EFFECTS
growth of the testes, seminal vesicles, and preputial gland. Later studies evaluated the effect of excess
manganese via the diet and gavage on development of the rat (Laskey et al. 1982, 1985). These studies
reported that 350 mg manganese/kg/day (as manganese tetroxide in food fed to pregnant rats and resulting
male offspring for a total of 224 days) (Laskey et al. 1982) or 214 mg manganese/kg/day (as manganese
tetroxide by gavage in water given for 28 days) (Laskey et al. 1985) reduced testosterone levels in
developing rats.
Studies involving intravenous or subcutaneous exposure routes of pregnant dams indicate that doses of
manganese chloride as low as 1.1 mg manganese/kg/day administered on GDs 6–17 in the rat (Grant et al.
1997a; Treinen et al. 1995) and 14 mg/kg/day administered on GDs 9–12 in the mouse (Colomina et al.
1996) can result in decreased fetal body weight and skeletal abnormalities.
The data indicate that animals may suffer adverse developmental effects after inhalation, oral, and
intravenous exposures of their pregnant mothers, but results are mixed. Taken together, the evidence
from environmental studies in humans and studies in animals suggests that younger children can be
affected by exposures to excess manganese. Only one study is available that compared the incidence of
adverse neurological effects in neonates and adults exposed to excess manganese (Dorman et al. 2000).
Another recent study (Dorman et al. 2005b) showed that fetal brains were protected from excess
manganese when their mothers were exposed to air concentrations as high as 1 mg manganese/m
3
manganese sulfate for 28 days before mating through PND 18, but increased brain manganese
concentrations developed in the offspring by PND 14. Additional information may help to quantitatively
characterize the potential differences in susceptibility to manganese-induced effects in young and adult
animals.
No studies currently exist on the health effects arising in children as a result of exposure to organic
manganese. Therefore, predictions concerning potential effects must be made from extrapolations from
existing animal studies.
Weanling mice who ingested 11 mg manganese/kg/day as MMT for 12 months exhibited a significant
increase in spontaneous activity at day 80, but no other behavioral differences throughout the exposure
period (Komura and Sakamoto 1992b). Concentrations of certain neurotransmitters and dopamine
metabolites were modified in different brain regions, but the relationship to manganese levels in the
affected regions was weak to none (Komura and Sakamoto 1994).
MANGANESE
318
3. HEALTH EFFECTS
Developmental studies in rats involving intravenous exposure of pregnant dams to mangafodipir during
organogenesis (days 6–17) indicate that the compound targets the skeletal system, resulting in irregularly
shaped bones at doses as low as 1 mg manganese/kg/day (Grant et al. 1997a; Treinen et al. 1995).
Further, application of specific doses of the compound during segmented time periods in organogenesis
causes the same skeletal defects (Treinen et al. 1995). When the compound is administered from 22 days
prior to conception until GD 7, at up to 6 mg manganese/kg/day, no developmental effects were observed
(Grant et al. 1997a). These data further indicate that animals developing during organogenesis are
particularly susceptible to developmental toxicity from mangafodipir exposure. Further, behavioral
changes and significant decreases in body weight were observed in rat pups delivered from dams dosed
with 1.1 mg manganese/kg/day, while decreased survival was observed in pups from dams given 2.2 mg
manganese/kg/day on GDs 6–17.
In contrast to the rat, available studies suggest that the rabbit is far less susceptible to the developmental
effects of mangafodipir. One study reported only decreased ossification in fetal sternebrae at 1.1 mg
manganese/kg/day when given to dams on GDs 6–17 (Grant et al. 1997a); a similar study in the same
species reported no observable developmental toxicity at 2.2 mg manganese/kg/day, but a significant
decrease in fetal weight and viable fetuses, with no skeletal abnormalities, at a dose of 3.3 mg manganese/
kg/day also given during organogenesis (Blazak et al. 1996).
In total, these developmental studies indicate that organic manganese can induce adverse developmental
effects in the unborn and young, with effects ranging from slight biochemical changes in the brain to
structural changes to changes in functional development. However, the majority of studies have involved
very high exposure doses.
The developmental toxicity of elemental manganese has been shown in large part by comparison studies
between manganese chloride and mangafodipir (Blazak et al. 1996; Grant et al. 1997a; Treinen et al.
1995). While these studies have provided much information as to the targeted teratogenicity of
manganese during organogenesis, they have generally involved intravenous exposures, which are not
particularly relevant to the general population. Further, it is likely that the majority of women who may
be exposed to mangafodipir are beyond child-bearing age, since clinical subjects with suspected liver
tumors that merit use of the compound to assist in diagnosis are often over 50 years old (mean values;
Bernardino et al. 1992). Should child-bearing women be exposed to the compound in a clinic
environment, the doses required to induce developmental toxicity in animals greatly exceed the clinical
dose (Blazak et al. 1996; Grant et al. 1997a; Treinen et al. 1995).
MANGANESE
319
3. HEALTH EFFECTS
The pharmacokinetics of manganese in infants is known to be different than in adults. Balance studies,
although limited, show that there is high retention of manganese during the neonatal period (Dorner et al.
1989). Formula-fed infants had an apparent manganese absorption of around 20% (Davidsson et al. 1988;
1989b), compared to absorption in adults, which is shown to be around 3–5% (Mena et al. 1969). The
increased absorption may be a compensatory mechanism due to the low concentration of manganese in
mother’s milk (Collipp et al. 1983; Dorner et al. 1989; Lönnerdal et al. 1987) and to the increased
metabolic needs of infants as compared to adults, since manganese is required for adequate bone
mineralization, as well as for connective tissue synthesis (Hurley and Keen 1987). Alternatively, the
increased absorption may be due to decreased excretion in the very young (Kostial et al. 1978; Lönnerdal
et al. 1987; Miller et al. 1975; Rehnberg et al. 1981), although at least one study indicates that both pre-
term and full-term infants actively excrete manganese (Dorner et al. 1989). Some studies have indicated
that infants, who acquire all of their manganese in the first 4 months of life from human milk or milk
formulas, ingest very different amounts of manganese due to the differing manganese content of these
food sources. More specifically, studies showed that due to the low manganese concentration of human
milk (4–10 μg/L) and its higher concentration in cow’s milk formulas (30–75 μg/L) and soy formulas
(100–300 μg/L) (Dorner et al. 1989; Lönnerdal et al. 1987), more manganese was absorbed from the
formula (with absorption rate from all sources being roughly equal). Recent changes in nutritional status
of infant formulas have resulted in a more nutritionally balanced absorption of manganese when
compared to human milk and cow’s milk formulas (~80–90%), with absorption of manganese from soy
milk formulas being slightly lower (~70%; Lönnerdal et al. 1994). However, given the existing
differences in inherent manganese concentrations between the different food sources, reports still suggest
that infant intake of manganese from milk formulas is 10–50 times that of a breast-fed infant (Lönnerdal
1997). Animal studies show that absorption and/or retention of manganese is similar to that of older
animals at approximately post-gestational day 17–18 (Kostial et al. 1978; Lönnerdal et al. 1987; Miller et
al. 1975; Rehnberg et al. 1981). However, when this transition takes place in human infants has not been
clearly defined.
Animal studies also show increased absorption of manganese in the young. For example, Kostial et al.
(1989) found that rat pups retained a greater proportion (67%) of a single oral dose of radiolabeled
manganese than adult rats (0.18%). Bell et al. (1989) found that manganese absorption in rat pups (using
isolated brush border membrane vesicles from the intestine) is nonsaturable and appears to occur
primarily by diffusion. In the older rat, however, a high affinity, low capacity, active-transport
mechanism for manganese absorption appears to be present (Garcia-Aranda et al. 1983).
MANGANESE
320
3. HEALTH EFFECTS
Several elements, including iron (Davis et al. 1992a), phosphorus (Wedekind et al. 1991), and calcium
(Wilgus and Patton 1939) are known to decrease manganese absorption in adults and animals. Iron-poor
diets result in increased manganese absorption in humans (Mena et al. 1969) and in rats (Pollack et al.
1965). These interactions have not been studied in infants or children, but are expected to occur.
Manganese is known to cross the placenta and has been detected in cord blood in healthy full-term and
pre-term infants. It is unknown whether mothers exposed to increased concentrations of manganese will
pass on toxic amounts of the metal to their unborn children via the blood. However, as manganese is an
essential nutrient and is part of the human body at all times, it is expected to be found in all tissues and
fluids of the infant. Manganese is also naturally found in breast milk (typical concentrations in mature
milk range from 4 to 10 μg/L) (Collipp et al. 1983). No studies exist concerning breast milk
concentrations of mothers exposed to increased concentrations of manganese, but milk manganese
concentrations increased with increasing exposure levels in lactating female rats exposed by inhalation to
manganese sulfate at 0.05, 0.5, or 1 mg manganese/m
3
for 28 days before mating through PND 18
(Dorman et al. 2005a). The mean milk concentration was statistically significantly increased, compared
with air control levels, however, only at the highest exposure level. It is unclear if manganese stored in
the brain, bone, or in another depot, in excess amounts, could be mobilized to affect a developing fetus.
However, one study by Jarvinen and Ahlström (1975) showed that pregnant rats fed 94 mg manganese/
kg/day (as manganese sulfate) for 8 weeks accumulated the metal in their livers in contrast to non-
pregnant females. Further, at a daily dose of 187 mg/kg/day, increased manganese concentrations were
found in 21-day-old fetuses. These data suggest that homeostatic control of pregnant mothers regulated
the distribution of the metal at lower concentrations, but this control was circumvented at high daily
concentrations, resulting in liver excesses and distribution in the developing fetus. Although the fetuses
in this study showed no physical abnormalities, no neurochemical or neurobehavioral studies were
performed to determine potential adverse effects on these relevant end points.
Transferrin is one of the proteins responsible for binding and transporting both iron and manganese
throughout the body. One study (Vahlquist et al. 1975) reported no correlation between infant cord blood
and maternal blood transferrin levels. The same study reported an increase in plasma transferrin from
1.68±0.60 mg/mL in blood from infants at 6 weeks of age, to a peak of 2.60±0.27 mg/mL at 10 months,
with values stabilizing at these adult levels throughout 16 years of age. The authors did not comment as
to the statistical difference, if any, of these values.
MANGANESE
321
3. HEALTH EFFECTS
There are no established biomarkers consistently used as indicators for overexposure to manganese in
either adults or children. Elevated blood concentrations and hyperintense signals in the globus pallidus on
T1-weighted MRI have been observed in children with increased exposure to manganese (Devenyi et al.
1994; Fell et al. 1996; Kafritsa et al. 1998; Ono et al. 1995). However, the same limitations of these
indicators of overexposure in adults (wide range of blood manganese in normal populations, high cost
and, hence, low availability of MRI) apply to children. Blood manganese has generally been poorly
related to current levels of exposure or cumulative exposure index (Smargiassi and Mutti 1999). Elevated
blood manganese alone does not constitute an adequate indicator of manganese overexposure. There are
no pediatric-specific biomarkers of exposure or effect. See Section 3.8.1 for further information.
Studies suggest that children may differ from adults in their susceptibility to the toxic effects of
manganese due to toxicokinetic differences (i.e., increased absorption and/or retention). Qualitative
similarities exist between respiratory and neurological effects seen in adults and children suffering from
extreme manganese exposure. While infant and animal studies indicate that the young have an increased
uptake of manganese, and distribution of the element in certain tissues may differ with age, studies that
reveal quantitative levels of manganese associated with discrete frank effects in both adults and children
are lacking. The studies to date (namely absorption, distribution and excretion studies in animals) suggest
a pharmacokinetic susceptibility to manganese that is different in children than in adults.
3.8 BIOMARKERS OF EXPOSURE AND EFFECT
Biomarkers are broadly defined as indicators signaling events in biologic systems or samples. They have
been classified as markers of exposure, markers of effect, and markers of susceptibility (NAS/NRC
1989).
A biomarker of exposure is a xenobiotic substance or its metabolite(s) or the product of an interaction
between a xenobiotic agent and some target molecule(s) or cell(s) that is measured within a compartment
of an organism (NAS/NRC 1989). The preferred biomarkers of exposure are generally the substance
itself, substance-specific metabolites in readily obtainable body fluid(s), or excreta. However, several
factors can confound the use and interpretation of biomarkers of exposure. The body burden of a
substance may be the result of exposures from more than one source. The substance being measured may
be a metabolite of another xenobiotic substance (e.g., high urinary levels of phenol can result from
exposure to several different aromatic compounds). Depending on the properties of the substance (e.g.,
biologic half-life) and environmental conditions (e.g., duration and route of exposure), the substance and
MANGANESE
322
3. HEALTH EFFECTS
all of its metabolites may have left the body by the time samples can be taken. It may be difficult to
identify individuals exposed to hazardous substances that are commonly found in body tissues and fluids
(e.g., essential mineral nutrients such as copper, zinc, and selenium). Biomarkers of exposure to
manganese are discussed in Section 3.8.1.
Biomarkers of effect are defined as any measurable biochemical, physiologic, or other alteration within an
organism that, depending on magnitude, can be recognized as an established or potential health
impairment or disease (NAS/NRC 1989). This definition encompasses biochemical or cellular signals of
tissue dysfunction (e.g., increased liver enzyme activity or pathologic changes in female genital epithelial
cells), as well as physiologic signs of dysfunction such as increased blood pressure or decreased lung
capacity. Note that these markers are not often substance specific. They also may not be directly
adverse, but can indicate potential health impairment (e.g., DNA adducts). Biomarkers of effects caused
by manganese are discussed in Section 3.8.2.
A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism's ability
to respond to the challenge of exposure to a specific xenobiotic substance. It can be an intrinsic genetic or
other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the
biologically effective dose, or a target tissue response. If biomarkers of susceptibility exist, they are
discussed in Section 3.10, Populations That Are Unusually Susceptible.
3.8.1 Biomarkers Used to Identify or Quantify Exposure to Manganese
Manganese can be measured with good sensitivity in biological fluids and tissues (see Section 7.1), and
levels in blood, urine, feces, and hair have been investigated as possible biomarkers of exposure. As a
group, workers exposed to a mean concentration of 1 mg manganese/m
3
had higher levels of manganese
in the blood and the urine than unexposed controls (Roels et al. 1987b). The group average levels in
blood appeared to be related to manganese body burden, while average urinary excretion levels were
judged to be most indicative of recent exposures. A study by Lucchini et al. (1995) is the only evidence
that suggests that blood and urine levels were correlated with manganese exposure on an individual basis.
This study differed from others in that it involved exposure to manganese dioxide and measured adverse
effects in workers after exposure ceased, whereas other studies involved current exposures, and some, like
Roels et al. (1987b) involved exposure to numerous manganese compounds (salts and oxides). The
findings of Lucchini et al. (1995) suggest that blood and urine levels of manganese, on an individual
basis, are positively correlated with exposure levels in the few weeks following cessation of exposure. In
MANGANESE
323
3. HEALTH EFFECTS
a study of chronically exposed workers who were evaluated while exposure was ongoing, Lucchini et al.
(1999) found a positive correlation between manganese levels in total dust and in blood of exposed
workers. This correlation did not exist for cumulative exposure index and blood levels of the metal.
Other studies have indicated that on an individual basis, the correlation between the level of workplace
exposure and the levels in blood or urine is not a reliable predictor of exposure (Jarvisalo et al. 1992;
Roels et al. 1987b, 1992; Smyth et al. 1973). However, two studies (Jarvisalo et al. 1992; Roels et al.
1992) suggest that blood and urinary manganese levels may be used to monitor group exposure, such as
exposure in an occupational setting. Also, a study (Siqueira et al. 1991) of ferromanganese workers
indicated that exposed workers had elevated levels of plasma and urinary urea and decreased levels of
urinary calcium, HDL cholesterol, and plasma inorganic phosphate. The study authors concluded that
measurement of these parameters may be useful in the early detection of manganese poisoning. Although
manganese may play a role in a metabolic pathway or other biological function involving these products,
it is unclear what physiological significance these parameters have as related to manganese toxicity.
There was no significant correlation between fecal excretion of manganese and occupational exposure to
the metal (Valentin and Schiele 1983). A recent study on environmental exposure to manganese
(Baldwin et al. 1999) in southwest Quebec, Canada, indicates that significantly higher levels of blood
manganese are correlated with high levels of airborne manganese. In this study, air samples were taken in
four geographic areas around a former ferroalloy plant (point source for airborne manganese). The air
samples, which were for total dust and PM
10
levels, were taken for 3 consecutive days in the summer.
Using a geometric algorithm, 297 blood manganese values from nearby residents in seven postal zones
were separated into two geographical areas corresponding to the point source. Higher blood manganese
values in men and women were located in the geographic area with the higher airborne manganese values.
It is notable that the air samples taken were limited in number and were taken only in the summer.
However, the authors mentioned that the data were consistent with samples taken in an adjacent urban
area and were consistent with potential exposure sources. Further, at the time of sampling, the ferroalloy
plant was not in use and exposure data indicated that airborne levels of manganese decreased dramatically
at a point 25 km downwind of the plant after the plant closed (Zayed et al. 1994). Thus, manganese
exposure of the population in the Baldwin et al. (1999) study is likely to have been greater in the past;
current blood manganese levels may be analogous to those observed in occupational workers undergoing
a forced layoff (Lucchini et al. 1995). These data, combined with the occupational studies, indicate that
there may be a plateau level of homeostatic control of the metal. At low levels, blood manganese
concentrations would be related to intake from food, water, and air; large differences in individual blood
manganese levels would be observed. At high exposure levels, such as in occupational environments, a
MANGANESE
324
3. HEALTH EFFECTS
higher but still non-toxic level of blood manganese may be maintained by homeostatic control (i.e., a
plateau level is reached); alternatively, that level may be exceeded.
These data also indicate that blood manganese levels can be an indicator of exposure to environmental
manganese. These data indicate that manganese in blood or urine may be useful in detecting groups with
above-average current exposure, but that measurements of manganese in these body fluids in individuals
may only be related to exposure dose after the exposure has ceased.
In addition to individual variability, another factor that limits the usefulness of measuring manganese in
blood, urine, or feces as a measure of excess manganese exposure is the relatively rapid rate of
manganese clearance from the body. As discussed in Section 3.4, excess manganese in blood is rapidly
removed by the liver and excreted into the bile, with very little excretion in urine (Klaassen 1974;
Malecki et al. 1996b). Thus, levels of manganese in blood or urine are not expected to be the most
sensitive indicators of exposure.
Serum prolactin (PRL) has been shown to be a possible biomarker of manganese action of dopamine
neurotransmission (Smargiassi and Mutti 1999). Manganese acts on the tuberoinfundibular dopaminergic
system, which exerts tonic inhibition of PRL secretion. Serum PRL levels observed in workers
occupationally exposed to manganese were shown to be consistent with mechanistic studies as they were
distinctly higher than unexposed workers. It is still unclear whether or not serum PRL levels indicate
recent or cumulative exposure. The value of PRL as a biomarker is called into question by the Roels et al.
(1992) study in which serum PRL levels were not increased in workers chronically exposed to airborne
manganese.
Lymphocyte manganese-dependent superoxide dismutase activity increases with increased manganese
uptake (Yiin et al. 1996). It has been suggested that this enzyme, in conjunction with serum manganese
levels, may be helpful in assessing low and moderate levels of manganese exposure (Davis and Greger
1992; Greger 1999). MnSOD has been shown to be elevated in women ingesting 15 mg of supplemental
manganese/day, while levels have been shown to be depressed in the heart and liver of manganese
deficient animals. MnSOD is important as a possible biomarker because its levels can be related to
oxidative damage. Its sensitivity as a biomarker depends on factors that induce oxidative stress or effect
manganese bioavailability including diets high in polyunsaturated fatty acids and strenuous physical
exercise (Greger 1999).
MANGANESE
325
3. HEALTH EFFECTS
Brain MRI scans and a battery of specific neurobehavioral tests (Greger 1998) may be useful in assessing
excessive manganese exposure even among industrial workers exposed to airborne manganese (Nelson
et al. 1993). These scans also have been successfully used to identify accumulation of manganese in the
brains of children exposed to excess manganese (Devenyi et al. 1994; Fell et al. 1996; Ihara et al. 1999;
Kafritsa et al. 1998; Ono et al. 1995; Sahni et al. 2007). Levels in feces could be useful in evaluating
relatively recent high-level exposures but would not be expected to be helpful in detecting chronic low-
level exposures. These methods are potentially useful biomarkers, but require additional evaluation to
determine their validity.
While it is well established that exposure to excess manganese can result in increased tissue levels in
animals, the correlations among exposure levels, tissue burdens, and health effects have not been
thoroughly investigated in humans or animals. Also, since homeostatic mechanisms largely prevent
fluctuations of manganese concentration in whole blood and since manganese is mainly excreted by the
biliary route, it is not believed possible to identify a biological marker to assess the intensity of exposure
or concentration in the target organ (Lauwerys et al. 1992). As noted by Rehnberg et al. (1982),
manganese levels in tissues are subject to homeostatic regulation via changes in absorption and/or
excretion rates. While exposure to very high levels may overwhelm these mechanisms, continuous
exposure to moderate excesses of manganese does not appear to cause a continuous increase in tissue
levels (Rehnberg et al. 1982). Moreover, even if tissue levels are increased in response to above-average
exposure, levels are likely to decrease toward the normal level after exposure ceases. For example, the
level of manganese in the brain of a subject with severe manganism was not different from the normal
level (Yamada et al. 1986). For these reasons, measurement of tissue levels of manganese at autopsy or
possibly biopsy may be of some value in detecting current exposure levels but is not useful in detecting
past exposures. Evaluation of manganese exposure by analysis of tissue levels is also not readily
applicable to living persons except through the collection of biopsy samples.
MRI has been used to track manganese distribution in the brains of monkeys (Dorman et al. 2006b;
Newland and Weiss 1992; Newland et al. 1989) and humans (Kafritsa et al. 1998; Klos et al. 2005; Nolte
et al. 1998; Park et al. 2003; Rose et al. 1999; Uchino et al. 2007; Wolters et al. 1989). In addition, it has
been used to assay hyperintense signaling in the globus pallidus and other brain areas of individuals with
chronic liver disease (Devenyi et al. 1994; Hauser et al. 1994, 1996; Klos et al. 2005; Nolte et al. 1998;
Park et al. 2003; Pomier-Layrargues et al. 1998; Spahr et al. 1996; Uchino et al. 2007), individuals on
chronically-administered TPN (Kafritsa et al. 1998; Nagatomo et al. 1999; Ono et al. 1995), and
individuals with symptoms characteristic of manganism (Nelson et al. 1993). Although data addressing
MANGANESE
326
3. HEALTH EFFECTS
the sensitivity and specificity of MRI as an indicator for body burden or exposure are limited, the
technique is being used to identify individuals who are likely to have increased stores of manganese in
brain and potentially in other tissues, as well. For example, the hyperintense signaling in the brain is
typically coincident with elevated blood manganese levels (Devenyi et al. 1994; Hauser et al. 1994, 1996;
Kafritsa et al. 1998; Klos et al. 2005; Nagatomo et al. 1999; Nolte et al. 1998; Ono et al. 1995; Park et al.
2003; Pomier-Layrargues et al. 1998; Spahr et al. 1996; Uchino et al. 2007). Dorman et al. (2006b)
evaluated the use of the pallidal index (PI—ratio of hyperintensities in the globus pallidus and the
adjacent subcortical frontal white matter) and the T1 relaxation rate (R1) from MRI to reflect manganese
concentrations determined by analytical chemistry in brain regions of monkeys repeatedly exposed by
inhalation to aerosols of manganese sulfate at several concentrations ≥0.06 mg. Increases in the PI and
R1 were correlated with the pallidal manganese concentration, but increased manganese concentrations in
white matter confounded the PI measurements. Dorman et al. (2006b) suggested that R1 can be used to
estimate regional brain manganese concentrations and that this technique may be used as a reliable
biomarker of occupational manganese exposure.
Neutron activation has been shown to be a possible means of in vivo measurement of manganese in the
liver and possibly other tissues and organs, including the brain (Arnold et al. 1999; Rose et al. 1999).
Minimum detection levels are low enough to distinguish between normal and elevated concentrations.
Scalp hair has also been investigated as a possible biomarker of manganese exposure. While some
studies have found a correlation between exposure level and manganese concentration in hair (Collipp
et al. 1983), use of hair is problematic for several reasons. For example, exogenous contamination may
yield values that do not reflect absorbed doses, and hair growth and loss limit its usefulness to only a few
months after exposure (Stauber et al. 1987). Manganese has also been reported to have a strong affinity
for pigmented tissues (Lydén et al. 1984), and Hurley and Keen (1987) and Sturaro et al. (1994) have
reported that manganese concentrations in hair vary with hair color. Further, hair may be contaminated
by dye, bleaching, or other materials. Thus, it is not surprising that other studies have found no
correlation between individual hair levels and the severity of neurological effects in manganese-exposed
persons (Stauber et al. 1987). A study that investigated the correlation between potentially toxic metal
content in hair and violent behavior found an association between manganese and violent behavior, but it
was not conclusively established that manganese was the causative factor (Gottschalk et al. 1991). He
et al. (1994) observed that poor performance in school and on neurobehavioral tests was inversely
correlated with hair levels of manganese. The manganese exposure in this study was via drinking water
and certain foods. Several studies have found that manganese levels in hair are higher in learning
MANGANESE
327
3. HEALTH EFFECTS
disabled children than in nondisabled children (Collipp et al. 1983; Pihl and Parkes 1977). The route of
excess exposure is not known but is presumed to be mainly oral. However, an association of this sort is
not sufficient to establish a cause-effect relationship since a number of other agents, including lead, might
also be involved (Pihl and Parkes 1977). Other studies have found statistically significant associations
between hair manganese levels and behavioral deficits (Bouchard et al. 2007c; Wright et al. 2006), subtle
motor deficits (Hernández-Bonilla et al. 2011; Standridge et al. 2008) and decreased intellectual function
(Bouchard et al. 2011; Menezes-Filho et al. 2011; Riojas-Rodríguez et al. 2010). These studies suggest
that hair manganese levels can provide meaningful exposure assessments.
Clara cell protein CC16 is a potential biomarker for exposure to MMT, because the protein decreases in
both BALF and serum following MMT exposure (Bernard and Hermans 1997; Halatek et al. 1998),
possibly due to decreased synthesis and/or protein secretion due to loss of producing cells (Halatek et al.
1998). The protein can be quantified in serum or urine, but no dose-response studies on the potential
biomarker have been performed.
There are no known biomarkers of exposure that are specific for children; any biomarkers applicable for
use in adults should be applicable for children. For example, manganese-induced hyperintense signals on
MRI have been seen in children (Devenyi et al. 1994; Kafritsa et al. 1998; Ono et al. 1995; Sahni et al.
2007) as well as adults (Hauser et al. 1994, 1996; Nagatomo et al. 1999; Pomier-Layrargues et al. 1998;
Spahr et al. 1996).
3.8.2 Biomarkers Used to Characterize Effects Caused by Manganese
The principal adverse health effects associated with exposure to manganese are respiratory effects (lung
inflammation, pneumonia, reduced lung function, etc.) and the neurological syndrome of manganism and
preclinical neurological effects. Although the respiratory effects are similar in many different exposure
studies (Kagamimori et al. 1973; Lloyd Davies 1946; Nogawa et al. 1973), there are no specific
biomarkers of effect other than reduced lung function. The fully developed disease can be diagnosed by
the characteristic pattern of symptoms and neurological signs (Mena et al. 1967; Rodier 1955), but the
early signs and symptoms are not specific for manganese. Careful neurological and psychomotor
examination in conjunction with known exposure to manganese may be able to detect an increased
incidence of preclinical signs of neurological effects in apparently healthy people (Iregren 1990; Roels
et al. 1987a). However, these signs are not sufficiently specific for preclinical effects of manganese to
reliably identify whether an individual has been exposed to excess levels for a prolonged period. In
MANGANESE
328
3. HEALTH EFFECTS
addition, no biochemical indicator is currently available for the detection of the early neurotoxic effects of
manganese. There are no specific biomarkers that would clearly indicate long-term exposure to excess
manganese.
Idiopathic Parkinsonism and manganism can be difficult to distinguish due to some similarity in the
symptoms (Kim et al. 1999). Idiopathic Parkinsonism is marked by neurodegeneration in the
dopaminergic nigrostriatal pathway, while manganism induced damage occurs postsynaptic to the
nigrostriatal system. PET with
18
F-dopa afforded a differentiation between manganism and idiopathic
Parkinsonism in isolated patients with manganese exposure by indexing the integrity of the dopaminergic
nigrostriatal pathway.
Measurement of altered levels of dopamine and other neurotransmitters in the basal ganglia has proven to
be a useful means of evaluating central nervous system effects in animals (e.g., Bonilla and Prasad 1984;
Eriksson et al. 1987a, 1987b), and these changes are often observed before any behavioral or motor
effects are apparent (Bird et al. 1984). No noninvasive methods are currently available to determine
whether there are decreased dopamine levels in the brain of exposed humans, but decreased urinary
excretion of dopamine and its metabolites has been noted in groups of manganese-exposed workers
(Bernheimer et al. 1973; Siqueira and Moraes 1989). However, the relationship between manganese
effects on peripheral versus central dopamine levels has not been clearly defined, and given the lack of
change in dopamine content in substantia nigra of humans exposed to manganese, the relevance of the
animal studies to central nervous system disorder is questionable.
Smargiassi et al. (1995) evaluated platelet monoamine oxidase (MAO) and serum dopamine
β-hydroxylase (DBH) activities in 11 men occupationally exposed to manganese via inhalation in a
ferroalloy plant. Exposed workers, in general, had lower MAO activities, but similar DBH activities, in
comparison to 15 nonexposed control males. However, a positive dose-effect relationship was observed
in the exposed group between a Cumulative Exposure Index (CEI) and DBH activity (r
2
=0.40, p<0.05).
The CEI took into account the average annual respirable or total manganese concentrations in dust, the
ventilation characteristic of each working area, the number of years that each worker spent in a given
area, and all of the areas that a worker had been during his job history. The authors proposed that DBH,
which is an expression of catecholamine release, might be increasing dose-dependently in response to
reduced turnover of MAO. The authors cautioned however, that while the data appear interesting, they
should be investigated in a larger study population, with careful analysis of possible confounding factors
(Smargiassi et al. 1995).
MANGANESE
329
3. HEALTH EFFECTS
Reduced urinary excretion of 17-ketosteroids (perhaps as a consequence of decreased testosterone
production) has been noted in many patients with neurological signs of manganism (Rodier 1955), but it
has not been determined whether this change is detectable prior to the occurrence of neurological effects.
Although the urinary excretion of manganese is generally not related to oral manganese intake, Davis and
Greger (1992) have suggested that the concentration of manganese in serum, combined with lymphocyte
manganese-dependent superoxide dismutase activity, may be helpful in assessing low and moderate levels
of manganese exposure. Manganese superoxide dismutase is activated by manganese, thus it is sensitive
to the overall manganese balance. Therefore, increased manganese concentrations will affect an
increased manganese superoxide dismutase level. There is no clear link between activity of superoxide
dismutase and the harmful effects of manganese. Therefore, the potential usefulness of this technique as a
biomarker of effect requires further evaluation.
The Clara cell protein CC16 is a potential biomarker for pulmonary effects from exposure to MMT
(Bernard and Hermans 1997; Halatek et al. 1998). Damage of Clara cells by MMT causes a significant
reduction in the levels of this protein in the BALF, but does not affect its level in serum. The protein can
be quantified in serum or urine as well. However, no dose-response studies on the potential biomarker
have been performed. Further, the protein has only been studied following intraperitoneal administration
of MMT. It is unknown if CC16 levels will change following other exposure pathways.
For more information on biomarkers for renal and hepatic effects of chemicals see ATSDR/CDC
Subcommittee Report on Biological Indicators of Organ Damage (Agency for Toxic Substances and
Disease Registry 1990) and for information on biomarkers for neurological effects see OTA (1990).
3.9 INTERACTIONS WITH OTHER CHEMICALS
There is clear evidence from studies in animals that the gastrointestinal absorption (and hence the
toxicity) of manganese is inversely related to dietary iron concentrations. That is, high levels of nonheme
iron lead to decreased manganese absorption and toxicity, and low levels of iron lead to increased
manganese absorption and toxicity (Chandra and Tandon 1973; Davis et al. 1992a, 1992b; Diez-Ewald
et al. 1968; Rehnberg et al. 1982). Conversely, high levels of dietary manganese lead to decreased iron
absorption (Davis et al. 1992b; Diez-Ewald et al. 1968; Garcia et al. 2006, 2007; Li et al. 2006;
Rossander-Hulten et al. 1991; Thomson et al. 1971). Short-term effects of this sort are believed to be the
result of kinetic competition between iron and manganese for a limited number of binding sites on
MANGANESE
330
3. HEALTH EFFECTS
intestinal transport enzymes (Thomson et al. 1971), while longer-term effects of iron deficiency or excess
are thought to be due to adaptive changes in the level of intestinal transport capacity (Cotzias 1958). The
studies reporting competition between iron and manganese in absorption clearly indicate the impact an
iron-poor diet will have on manganese uptake in the human (Chandra and Tandon 1973; Davis et al.
1992a, 1992b; Diez-Ewald et al. 1968; Mena et al. 1969; Rehnberg et al. 1982; Thomson et al. 1971).
Further, competition between manganese and iron at the blood-brain barrier has been reported (Aschner
and Aschner 1990), indicating that excesses of either metal will affect the brain distribution of the other.
Johnson and Korynta (1992) found that, in rats, dietary copper can also decrease manganese absorption
and increase manganese turnover; dietary ascorbate supplementation had minimal effects on manganese
absorption. However, there is insufficient information to determine the significance of these observations
for health effects in humans exposed to copper and manganese by the oral route.
Mn(II) pretreatment reduces Cd(II)-induced lethality (Goering and Klaassen 1985). Cadmium has been
noted to have an inhibitory effect on manganese uptake (Gruden and Matausic 1989). In addition,
manganese appears to be capable of increasing the synthesis of the metal-binding protein metallothionine
(Waalkes and Klaassen 1985). Data from a study by Goering and Klaassen (1985) suggest that
manganese pretreatment increases the amount of Cd
+2
bound to metallothionine, thereby decreasing
hepatotoxicity due to unbound Cd
+2
. The significance of these observations for health effects in humans
exposed to cadmium and manganese by the oral or inhalation routes is not clear.
High dietary intakes of phosphorus (Wedekind et al. 1991) and calcium (Wilgus and Patton 1939) were
shown to depress manganese utilization in chicks. Low levels of calcium and iron may act synergistically
to affect manganese toxicity by increasing absorption, but it is not known whether ensuring iron plus
calcium sufficiency will reduce the toxic effects of manganese once it has been absorbed (Cawte et al.
1989). Thus, the importance of these observations to humans exposed to manganese by the oral or
inhalation routes is not clear.
Ethanol has been suspected of increasing the susceptibility of humans to manganese toxicity (e.g., Rodier
1955), but evidence to support this is limited. Singh et al. (1979) and Shukla et al. (1976) reported that
concomitant exposure of rats to ethanol and manganese (as manganese chloride in drinking water) led to
higher levels of manganese in the brain and liver than if manganese were given alone; the higher levels
were accompanied by increased effects as judged by various serum or tissue enzyme levels (Shukla et al.
1978). Although the authors referred to these effects as "synergistic," the data suggest that the effects
were more likely additive. Based on the report in humans and evidence in animals, the effects of
MANGANESE
331
3. HEALTH EFFECTS
manganese on humans may be enhanced by the consumption of ethanol, but additional investigation is
needed.
There is some evidence from a study in animals that chronic administration of drugs such as
chlorpromazine (an antipsychotic) results in increased levels of manganese in the brain, including the
caudate nucleus (Weiner et al. 1977). Chronic chlorpromazine treatment sometimes results in tardive
dyskinesia, and manganese deposition in the brain might contribute to this condition. It has not been
determined whether excess manganese exposure increases the risk of chlorpromazine-induced dyskinesia.
Intramuscular injection of animals with metallic nickel or nickel disulfide (Ni
3
S
2
) normally leads to a high
incidence of injection-site sarcomas, but this increased incidence is reduced when the nickel is injected
along with manganese dust (Sunderman et al. 1976). The mechanism of this effect is not clear, but
natural killer cell activity normally undergoes a large decrease following nickel injection, and this is
prevented by the manganese (Judde et al. 1987). However, the significance that these observations have
for human health effects resulting from exposure to nickel and/or manganese by the oral or inhalation
routes is not clear.
One study found that allopurinol, when administered orally to rats, antagonized the oxidative effects of
manganese in the striatum and brainstem (Desole et al. 1994). The authors suggest that allopurinol, a
xanthine oxidase inhibitor, may exert its protective effect by inhibiting both dopamine oxidative
metabolism and xanthine oxidase-mediated production of reactive oxygen species.
3.10 POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
A susceptible population will exhibit a different or enhanced response to manganese than will most
persons exposed to the same level of manganese in the environment. Reasons may include genetic
makeup, age, health and nutritional status, and exposure to other toxic substances (e.g., cigarette smoke).
These parameters result in reduced detoxification or excretion of manganese, or compromised function of
organs affected by manganese. Populations who are at greater risk due to their unusually high exposure
to manganese are discussed in Section 6.7, Populations with Potentially High Exposures.
A number of researchers have observed that there is a wide range in individual susceptibility to the
neurological effects of inhaled manganese dusts (Rodier 1955; Schuler et al. 1957; Smyth et al. 1973;
Tanaka and Lieben 1969). For example, Rodier (1955) reported that the majority of manganism cases in
MANGANESE
332
3. HEALTH EFFECTS
miners occurred after 1–2 years of exposure to the metal, with only six cases observed occurring with 1–
3 months exposure. Schuler et al. (1957) showed that in his group of miners, the average time for
manifestation of manganism was 8 years, 2 months, with a minimum exposure of 9 months required for
symptoms to present. However, the reason for this variable susceptibility is not clear. One likely factor is
a difference in work activities and level of exertion. Another is that rates of manganese absorption and/or
excretion can vary widely among individuals (Saric et al. 1977a). These toxicokinetic variations may be
due to differences in dietary levels of iron and differences in transferrin saturation (Chandra and Tandon
1973; Davis et al. 1992a, 1992b; Mena et al. 1969; Thomson et al. 1971), to differences in dietary levels
of other metals (Chowdhury and Chandra 1987; Gruden and Matausic 1989) or of calcium (Cawte et al.
1989), or to different levels of alcohol ingestion (Schafer et al. 1974). Another factor that might be
relevant is dietary protein intake: low-level protein intake appears to increase the effect of manganese on
brain neurotransmitter levels in exposed animals (Ali et al. 1983a, 1983b, 1985). However, a genetic
basis for the wide difference in susceptibility cannot be ruled out.
One group that has received special attention as a potentially susceptible population is the very young.
This is mainly because a number of studies indicate that neonates retain a much higher percentage of
ingested or injected manganese than adults, both in animals (Keen et al. 1986; Kostial et al. 1978;
Rehnberg et al. 1980) and in humans (Zlotkin and Buchanan 1986). The basis for high manganese
retention in neonates is not certain, but is presumably a consequence of increased absorption (Mena et al.
1974; Rehnberg et al. 1980) and/or decreased excretion (Kostial et al. 1978; Miller et al. 1975; Rehnberg
et al. 1981), possibly because maternal milk is low in manganese (Ballatori et al. 1987). Regardless of the
mechanism, the result of the high retention is increased levels of manganese in the tissue of exposed
neonatal animals (Miller et al. 1975; Rehnberg et al. 1980, 1981), especially in the brain (Kontur and
Fechter 1985, 1988; Kostial et al. 1978; Kristensson et al. 1986; Miller et al. 1975; Rehnberg et al. 1981).
This increase has caused several researchers to express concern over possible toxic effects in human
infants exposed to manganese in formula (Collipp et al. 1983; Keen et al. 1986; Zlotkin and Buchanan
1986). At least one recent report indicates that an infant’s rate of absorption of manganese from infant
formulas, cow’s milk, and breast milk is similar (Lönnerdal et al. 1994), resulting mainly from recent
modifications to formulas to optimize the bioavailability of several essential minerals. There is some
limited evidence that prenatal or neonatal exposure of animals to elevated levels of manganese can lead to
neurological changes in the newborn (Ali et al. 1983a; Chandra and Shukla 1978; Deskin et al. 1980,
1981; Dorman et al. 2000; Kristensson et al. 1986); other studies have either not observed any
neurochemical or neurophysiological effects in young animals exposed to excess manganese or the effects
have been transient (Kontur and Fechter 1988; Kostial et al. 1978; Pappas et al. 1997). Currently, there is
MANGANESE
333
3. HEALTH EFFECTS
only one report that indicates that neonatal animals showed adverse neurological effects at a dose of
manganese that had no effect on adults (Dorman et al. 2000). Brain concentrations of manganese were
elevated in the neonates, but not in the adult animals given comparable doses of manganese for similar
durations. The concern is that the young may be more susceptible due to increased absorption and/or
retention and the potential toxicity from higher circulating levels of the metal. A few studies have
reported increased blood and brain levels of the metal, either because of an inability to clear manganese
due to chronic liver disease (Devenyi et al. 1994) or to an excess in parenteral nutrition (Kafritsa et al.
1998; Ono et al. 1995). However, observable neurological signs associated with manganese toxicity were
only reported in the case of chronic liver disease (Devenyi et al. 1994). Although data suggest that
children, particularly infants, are potentially more susceptible to the toxic effects of manganese, available
evidence indicates that individual susceptibility varies greatly. Current information is not sufficient to
quantitatively assess how susceptibility in children might differ from adults.
Elderly people might also be somewhat more susceptible to manganese neurotoxicity than the general
population. Neurological effects were observed in older persons consuming manganese levels similar to
levels found in U.S. surface water and groundwater (Deverel and Millard 1988; EPA 1984; Kondakis et
al. 1989). The neurological effects observed in a group of families exposed to manganese in their
drinking water were reportedly more severe among the older persons, whereas there was little effect in the
youngest (Kawamura et al. 1941). Further, occupational studies indicate that older workers represent the
largest numbers of manganese poisoning cases (Rodier 1955; Tanaka and Lieben 1969). More recent
occupational (Crump and Rousseau 1999; Gibbs et al. 1999) and environmental (Mergler et al. 1999)
manganese exposure studies indicate that increasing age was a factor in poorer performance on certain
neurobehavioral tests. For example, Beuter et al. (1999) and Mergler et al. (1999) reported that
performance on tests that required regular, rapid, and precise pointing movements was significantly
decreased in exposed individuals, especially in those 50 years of age and over with high blood manganese
levels. These reports suggest that older persons may have a greater susceptibility to adverse effects from
inhaled or ingested manganese. One factor that could contribute to this increased susceptibility is a loss
of neuronal cells due to aging or to accumulated neurological damage from other environmental
neurotoxicants (Silbergeld 1982). Homeostatic mechanisms might become less effective in aged
populations, which leads to higher tissue levels of manganese following exposure (Silbergeld 1982).
Mena et al. (1969) noted that the oral absorption of manganese was increased in individuals with iron-
deficiency anemia. Altered nutritional status might be another predisposing factor. The inverse relation-
ship of manganese absorption and iron-status has also been reported in animal models (Davis et al. 1992a,
MANGANESE
334
3. HEALTH EFFECTS
1992b). It has been suggested that anemic persons may be more susceptible to the toxic effects of
manganese because of enhanced absorption of iron and manganese through similar uptake mechanisms
(Cotzias et al. 1968). Baldwin et al. (1999) reported an inverse relationship between serum iron and
blood manganese levels in individuals environmentally exposed to airborne manganese.
Another group of potential concern is people with liver disease. This is because the main route of
manganese excretion is via hepatobiliary transport (see Section 3.4.4), so individuals with impaired
biliary secretion capacity would be expected to have a diminished ability to handle manganese excesses.
In support of this hypothesis, Hambidge et al. (1989) reported that in a group of infants and children
receiving parenteral nutrition, children with liver disease had higher average plasma concentrations of
manganese than children without liver disease. Devenyi et al. (1994) also observed increased blood
manganese concentrations, abnormal MRI scans indicative of increased manganese in the brain, and
dystonia similar to that of patients with manganism, in an 8-year-old girl suffering from cholestatic liver
disease. Hauser et al. (1994) reported increased blood and brain manganese in two patients with chronic
liver disease and one with cirrhosis of the liver and a portacaval shunt. All three exhibited some form of
neuropathy, including postural tremor of the upper extremities and a general lack of alertness, along with
failure to concentrate and follow simple commands. In a later study, Hauser et al. (1996) did not observe
movement disorders, but did observe the increased blood manganese concentrations and abnormal MRI
scans in a group of adults with failing livers. Other studies have shown the link between increased
deposition of manganese in the blood and/or the brains of humans with cirrhosis of the liver or chronic
liver disease (Pomier-Layrargues et al. 1998; Rose et al. 1999; Spahr et al. 1996).
Patients on parenteral nutrition may be at risk for increased exposure to manganese. Forbes and Forbes
(1997) observed that 31 of 32 adults treated with total parenteral nutrition (TPN) due to intestinal failure
had increased manganese concentrations in their blood. Nagatomo et al. (1999) observed elevated blood
manganese levels and hyperintense signals in the basal ganglia upon T1-weighted MRI in two elderly
patients receiving TPN. Both patients exhibited severe symptoms associated with manganese exposure
(masked facies, marked rigidity, hypokinesia). When manganese supplementation in the TPN was
reduced, the blood and brain levels returned to normal.
Children receiving parenteral nutrition have also been shown to have increased blood manganese
concentrations with accompanying hyperintense signals in the globus pallidus as observed by MRI (Fell
et al. 1996; Kafritsa et al. 1998; Ono et al. 1995). Fell et al. (1996) studied a group of 57 children
receiving parenteral nutrition, 11 of whom had a combination of hypermanganesemia and cholestasis.
MANGANESE
335
3. HEALTH EFFECTS
Four of these 11 patients died; the 7 survivors had whole blood manganese concentrations ranging from
34–101 μg/L. Four months after reduction or removal of manganese from the supplementation, the blood
concentration of manganese decreased by a median of 35 μg/L. Two of the seven survivors had
movement disorders, one of whom survived to have a MRI scan. The scan revealed bilateral
symmetrically increased signal intensity in the globus pallidus and subthalamic nuclei. These signals
were also observed in five other children—one from the original group exhibiting cholestasis with
hypermanganesemia and five more given parenteral nutrition chronically with no liver disease. These
results indicate that the cholestatic condition is not necessary for manganese to accumulate in the brain.
A supporting study is provided by Ono et al. (1995) who observed increased blood manganese
concentrations and hyperintense signals on MRI in the brain of a 5-year-old child on chronic parenteral
nutrition due to a gastrointestinal failure. Five months after the manganese was removed from the
parenteral solution, blood manganese levels returned to normal, and the brain MRI scans were almost
completely free of abnormal signals. Further, the authors reported no neurological effects from exposure
to manganese. Kafritsa et al. (1998) reported results similar to those of Ono et al. (1995). In the latter
study, two siblings, one 9 years old and the other 2 years old, had been administered TPN chronically
since the ages of 4 and 1 month(s), respectively. While elevated blood and brain manganese levels were
reported (via laboratory analyses and MRI), no adverse neurological or developmental effects were
observed. Once the manganese supplementation was reduced, the MRI signals abated, and the blood
manganese levels returned to a normal range.
Although human interindividual variability is great concerning the ability to tolerate excess amounts of
manganese in the body, these data indicate that, in general, children and the elderly may be more
susceptible than young and middle-aged adults due to differential toxicokinetics and potential adverse
effects superimposed on normal decline in fine motor function with age.
With respect to the respiratory effects of inhaled manganese (e.g., bronchitis, pneumonitis), people with
lung disease or people who have exposure to other lung irritants may be especially susceptible. This is
supported by the finding that the inhalation of manganese dusts by manganese alloy workers caused an
increased incidence of respiratory symptoms (e.g., wheezing, bronchitis) in smokers, but not in
nonsmokers (Saric and Lucic-Palaic 1977b).
MANGANESE
336
3. HEALTH EFFECTS
3.11 METHODS FOR REDUCING TOXIC EFFECTS
This section will describe clinical practice and research concerning methods for reducing toxic effects of
exposure to manganese. However, because some of the treatments discussed may be experimental and
unproven, this section should not be used as a guide for treatment of exposures to manganese. When
specific exposures have occurred, poison control centers and medical toxicologists should be consulted
for medical advice. The following texts provide specific information about treatment following exposures
to manganese.
Leikin JB, Paloucek JB. 2002. Leikin and Paloucek's poisoning and toxicology handbook. Hudson, OH:
Lexi-Comp, Inc., 773-774.
Schonwald S. 2004. Manganese. In: Dart RC, eds. Medical toxicology. 3rd ed. Philadelphia, PA:
Lippicott Williams & Wilkins, 1433-1434.
WHO. 1999. Concise international chemical assessment document 12. Manganese and its compounds.
Geneva: United Nations Environment Programme. International Labour Organisation. World Health
Organization. http://whqlibdoc.who.int/publications/1999/924153012X.pdf. August 04, 2008.
3.11.1 Reducing Peak Absorption Following Exposure
There is substantial evidence to indicate that an interaction between iron and manganese occurs during
intestinal absorption (Chandra and Tandon 1973; Diez-Ewald et al. 1968; Keen and Zidenberg-Cher
1990; Mena et al. 1969; Rehnberg et al. 1982). Cawte et al. (1989) cite low levels of iron and calcium as
"synergistic factors" that impact on the toxic effects associated with manganese exposures. In a dietary
study investigating the effects of copper, iron, and ascorbate on manganese absorption in rats, these
substances were all found to influence manganese absorption, depending in part on their relative
concentrations (Johnson and Korynta 1992).
Evidence from these reports suggests that it may be possible to reduce the uptake of manganese and
thereby circumvent the potential for toxic effects caused by current and future exposure to excess
manganese through specific dietary supplementation. For example, sufficient iron or calcium stores, as
opposed to a deficiency in these or other minerals, may reduce manganese absorption, and thus reduce
potential toxicity. It is not known whether ensuring iron and calcium sufficiency will reduce the toxic
effects of manganese once it has been absorbed into the body because information on critical levels of
manganese at target sites is not available. No consistent clinical data are available documenting benefit
from ipecac or dilution after ingestion of metallic, inorganic, or organic manganese (Schonwald 2004).
MANGANESE
337
3. HEALTH EFFECTS
3.11.2 Reducing Body Burden
Inhaled manganese is readily absorbed by the lungs, although some may be retained there. Larger
particles of dust containing manganese may be transported by mucociliary transport from the throat to the
gut (Drown et al. 1986). Manganese in the gut may be directly absorbed either by a simple diffusion
process (Bell et al. 1989) or by a high-affinity, low-capacity, active-transport mechanism (Garcia-Aranda
et al. 1983). Once in the plasma, manganese is reportedly transported by transferrin; however,
information on the mechanism of uptake in extrahepatic tissues is limited (Keen and Zidenberg-Cher
1990).
In severe cases of manganese poisoning, chelation therapy may be recommended in order to reduce the
body burden of manganese and to help alleviate symptoms. Chelation therapy with agents such as EDTA
may alleviate some of the neurological signs of manganism, but in cases where it has been used, not all
patients have shown improvement, and some of the improvements have not always been permanent
(Cook et al. 1974; Schonwald 2004). Nagatomo et al. (1999) recently reported the use of Ca-EDTA
treatment to reduce the body burden of two elderly patients with increased blood and brain levels of
manganese. These patients exhibited masked faces, hypokinesia, and rigidity that are among the clinical
signs of manganese poisoning. The potential use of calcium disodium ethylenediaminetetracetate (CaNa
2
EDTA) for the management of heavy metal poisoning was investigated in dogs by Ibim et al. (1992).
CaNa
2
EDTA-treated dogs (without excess manganese exposure) were found to have decreased
manganese levels in their hair. It is possible that the decrease was partially associated with mobilization
and redistribution of this element from storage as well as from soft tissues. The authors, however,
cautioned that the use of CaNa
2
EDTA could adversely affect the metabolism of manganese.
In an attempt to treat seven welders with manganism, a solution of 20% CaNa
2
EDTA was administered
intravenously at the dose of 1.0 g daily for 3 days followed by a pause for 4 days. The therapy continued
for 2–4 courses of this treatment, depending upon the improvement of symptoms. The symptoms, as well
as blood manganese concentrations and urinary manganese concentrations, were monitored before and
after each course of treatment. EDTA treatment resulted in increased manganese excretion in urine and
decreased manganese concentrations in the blood; however, the patients did not show significant
improvement in their symptoms (Crossgrove and Zheng 2004). A lack of improvement after EDTA
chelation has also been observed in an additional case study of an adult worker (Jiang et al. 2006). It is
postulated that four carboxyl groups in the EDTA structure, which are essential to its chelating property,
render the molecule poorly lipophilic, thus preventing it from effectively crossing the blood-brain barrier.
MANGANESE
338
3. HEALTH EFFECTS
Thus, EDTA appears to successfully chelate and remove the extracellular manganese ions in the blood,
but with limited access to brain parenchyma, it cannot effectively chelate and remove manganese ions
from the brain. Because EDTA cannot significantly remove manganese from damaged neurons, it
appears to be of very limited therapeutic value for more advanced cases of manganism.
Cyclohexylene-aminotetraacetic acid (CDTA) and dimercaptol-1-propanesulphonic acid sodium salt
(DTPA) were shown to decrease tissue manganese content in rats following inhalation exposure, but it is
unknown whether the effects of manganese were alleviated (Wieczorek and Oberdörster 1989a, 1989b).
The use of the anti-tuberculosis drug para-aminosalicylic acid (PAS) to treat manganism has been
reported (Jiang et al. 2006). The patient in this case study had palpitations, hand tremor, lower limb
myalgia, hypermyotonia, and a distinct festinating gait. She received 6 g PAS per day through an
intravenous drip infusion for 4 days and rest for 3 days. Fifteen courses of this treatment were
administered to the patient. At the end of PAS treatment, the patient’s symptoms were reportedly
significantly alleviated, and handwriting recovered to normal. A reexamination at 17 years after PAS
therapy found a general normal presentation in clinical, neurologic, brain MRI, and handwriting
examinations. Her gait improved, and although it did not improve to an entirely normal status, it could be
described as passable. A literature survey of more than 90 cases using PAS (Jiang et al. 2006) indicates a
significant therapeutic benefit.
A study in monkeys reported a long half-life of manganese in the brain following inhalation exposure
(Newland et al. 1987). Given that neurotoxicity is of concern with manganese exposure, knowledge of
the mechanisms behind this longer half-life in the brain may be central to the development of mitigation
methods. Newland et al. (1987) reported that this long half-life reflected both redistribution of
manganese from other body depots and a slow rate of clearance from the brain. A later study reported
that elevated levels in the brain persisted after inhalation exposure (due to redistribution), whereas for
subcutaneous exposure, levels declined when administration was stopped (Newland et al. 1989). The
authors observed that the accumulation of manganese in the brain was preferential in specific regions, but
was unrelated to the route of exposure (Newland et al. 1989). They also reported that there are no known
mechanisms or "complexing agents" that have been shown to remove manganese from the brain.
Few data are available regarding the reversibility of the neurological injury produced by prolonged excess
manganese exposure. The effects are thought to be largely irreversible, and treatment for manganese
intoxication is mainly supportive (Schonwald 2004). However, some evidence indicates that recovery
MANGANESE
339
3. HEALTH EFFECTS
may occur when exposure ceases (Smyth et al. 1973). Anti-Parkinsonian drugs, such as levo-dopa, have
been shown to reverse some of the neuromuscular signs of manganism (Ejima et al. 1992; Rosenstock et
al. 1971), but these drugs can produce a variety of side effects, and reports have indicated that they are not
effective in improving the symptoms of neurotoxicity in manganism patients (Calne et al. 1994; Chu et al.
1995; Cook et al. 1974; Haddad et al. 1998; Huang et al. 1989; Schonwald 2004). Para-aminosalicylic
acid was used successfully to treat two patients who exhibited neurological signs of manganese
poisoning; one person made an almost complete recovery and the other was significantly improved. The
mechanism for this treatment is unknown (Shuqin et al. 1992). Parenti et al. (1988) has proposed the use
of antioxidants such as vitamin E, but the effectiveness of this treatment has not been further evaluated.
3.11.3 Interfering with the Mechanism of Action for Toxic Effects
The oxidation state of manganese may influence both its retention in the body (see Section 3.4.3) and its
toxicity (see Section 3.5). Therefore, it is possible that interference with the oxidation of manganese
could be a method for preventing manganese cellular uptake and toxicity. Regarding retention, one study
suggests that clearance is much more rapid for divalent manganese than for trivalent manganese (Gibbons
et al. 1976). Regarding neurotoxicity, Mn(III) appears to be more efficient in enhancing the oxidation of
catechols than either Mn(II) or Mn(IV) (Archibald and Tyree 1987). Thus, it is plausible that reducing
the formation of Mn(III) could possibly both enhance elimination and prevent neurotoxicity, but no
studies were located that evaluate this theory.
Ceruloplasmin is involved in the oxidation of iron and has also been involved in the oxidation of divalent
manganese ion to the trivalent state (Gibbons et al. 1976). Selective inhibition of this oxidative function
may be a method of mitigating the toxic effects of exposure to manganese. However, inhibition of the
oxidation of manganese might also result in adverse effects on transport and cellular uptake of other
essential metals, especially iron. Furthermore, it is not completely clear how the oxidation state of
manganese is related to its normal function in neural cells or how this role is altered in manganese
toxicity. Both Mn(II) and Mn(III) have been reported as components of metalloenzymes (Keen and
Zidenberg-Cher 1990; Leach and Lilburn 1978; Utter 1976).
Manganese has been shown to catalyze the oxidation of dopamine in vitro; Cawte et al. (1989) reported
that the toxicity induced by manganese resulted from the depletion of dopamine and the production of
dopamine quinone and hydrogen peroxide through this mechanism. Antioxidants were tested for their
ability to inhibit the dopamine oxidation induced by manganese, and it was found that ascorbic acid and
MANGANESE
340
3. HEALTH EFFECTS
thiamine completely inhibited dopamine oxidation both in the presence and absence of manganese. The
report did not include data on background oxidation levels nor on the extent of dopamine oxidation in the
absence of manganese. Results from treatment with antioxidants were viewed as evidence for their use in
mitigating the adverse effects of manganese. However, because dopamine oxidation was inhibited to
some degree in the absence of manganese, these data could alternately be interpreted as suggesting a more
complex mechanism than the direct action of manganese for inducing dopamine oxidation and subsequent
cell toxicity. Further investigation of the inhibition of manganese oxidation as a possible mitigation
method should be preceded by additional studies to elucidate the role of manganese in its various
oxidation states in normal neuronal cell metabolism and to determine whether oxidative stress is a
primary mechanism for neurotoxicity mediated by manganese exposure.
3.12 ADEQUACY OF THE DATABASE
Section 104(I)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of manganese is available. Where adequate information is not
available, ATSDR, in conjunction with the National Toxicology Program (NTP), is required to assure the
initiation of a program of research designed to determine the health effects (and techniques for developing
methods to determine such health effects) of manganese.
The following categories of possible data needs have been identified by a joint team of scientists from
ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would
reduce the uncertainties of human health assessment. This definition should not be interpreted to mean
that all data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
3.12.1 Existing Information on Health Effects of Manganese
The existing data on health effects of inhalation, oral, and dermal exposure of humans and animals to
inorganic manganese are summarized in Figure 3-15. The purpose of this figure is to illustrate the
existing information concerning the health effects of manganese. Each dot in the figure indicates that one
or more studies provide information associated with that particular effect. The dot does not necessarily
imply anything about the quality of the study or studies, nor should missing information in this figure be
interpreted as a “data need”. A data need, as defined in ATSDR’s Decision Guide for Identifying
Substance-Specific Data Needs Related to Toxicological Profiles (Agency for Toxic Substances and

Figure 3-15. Existing Information on Health Effects of Inorganic Manganese
Death
Acute
Intermediate
Chronic
Immunologic/Lymphoretic
Neurologic
Reproductive
Developmental
Genotoxic
Cancer
Systemic
Human
Inhalation
Oral
Dermal
Death
Acute
Intermediate
Chronic
Immunologic/Lymphoretic
Neurologic
Reproductive
Developmental
Genotoxic
Cancer
Systemic
Animal
Inhalation
Oral
Dermal
Existing Studies
MANGANESE
341
3. HEALTH EFFECTS
MANGANESE
342
3. HEALTH EFFECTS
Disease Registry 1989), is substance-specific information necessary to conduct comprehensive public
health assessments. Generally, ATSDR defines a data gap more broadly as any substance-specific
information missing from the scientific literature.
As the upper part of Figure 3-15 reveals, studies in humans exposed to inorganic manganese have focused
mainly on intermediate and chronic inhalation exposure and the resulting neurological effects. There are
several reports of humans exposed by ingestion and these too have focused on neurological effects.
Reproductive effects have been studied in men exposed to manganese by inhalation, but other effects
have generally not been formally investigated.
Inorganic manganese toxicity has been investigated in numerous animal studies, both by the oral and the
inhalation routes. These studies have included most end points of potential concern. The dermal route
for inorganic manganese has not been investigated, but there is no evidence that this exposure pathway is
a human health concern. Dermal contact to MMT is expected to occur mainly in occupational settings,
and no human dermal contact with mangafodipir is expected to occur. In addition, organic compounds
are degraded to some extent in the environment. Thus, dermal effects from organic manganese
compounds are not expected to be of great concern for the general population or to persons near
hazardous waste sites.
3.12.2 Identification of Data Needs
Presented below is a brief review of available information and a discussion of research needs. Although
data are lacking, dermal studies to inorganic manganese are not discussed since there is no evidence that
this exposure pathway is a human health concern.
Acute-Duration Exposure. Studies in animals and humans indicate that inorganic manganese
compounds have very low acute toxicity by any route of exposure. An exception is potassium
permanganate, which is an oxidant that can cause severe corrosion of skin or mucosa at the point of
contact (Southwood et al. 1987). Acute inhalation exposure to high concentrations of manganese dusts
(manganese dioxide, manganese tetroxide) can cause an inflammatory response in the lung, which can
lead to impaired lung function (Maigetter et al. 1976; Shiotsuka 1984). However, this response is
characteristic of nearly all inhalable particulate matter (EPA 1985d) and is not dependent on the
manganese content of the particle. Large oral doses of highly concentrated solutions of manganese salts
given by gavage can cause death in animals (Holbrook et al. 1975; Kostial et al. 1978; Smyth et al. 1969),
MANGANESE
343
3. HEALTH EFFECTS
but oral exposures via food or water have not been found to cause significant acute toxicity (Gianutsos
and Murray 1982; NTP 1987a, 1987b). Exposure of mice (Moreno et al. 2009) and rats (Shukakidze et al.
2003) to acute oral doses of about 13 mg manganese/kg as manganese chloride has been associated with
behavioral changes, but dietary intakes of manganese in these studies were not measured. Since the acute
database is incomplete and studies demonstrating a dose-response are not available, acute MRLs were not
derived. In order to derive acute MRL values, further studies may be helpful to define the threshold for
adverse effects following acute exposure to manganese. However, any MRL derived for the oral route
would have to take into consideration that manganese is an essential nutrient.
Acute-duration exposure studies in animals exposed to MMT via inhalation or via a dermal pathway are
lacking. The dermal pathway is very important, because MMT in gasoline that may be spilled on the skin
could penetrate and become absorbed. Although the photolability of the compound is an important
obstacle for any animal study, carefully planned and executed analyses of the toxicity of this compound to
animal models through these exposure pathways are needed.
The likelihood for exposure to mangafodipir is small and clinical trials in humans have shown a great
tolerance for a controlled exposure to the compound. Toxicity studies in several different animal species
have been performed, including reproductive and developmental studies (and more specifically,
teratogenic analysis). Although behavioral data in the young who have been exposed during gestation are
relatively limited, human gestational exposure to this compound is not believed to be very likely. Reports
of neurological effects have been limited to complaints of headaches in clinical trials. Further evaluation
of these effects relative to the distribution of manganese to the brain during clinical use is warranted.
Mangafodipir is administered intravenously, which bypasses homeostatic control of the compound.
Although animal studies indicate that a single, clinical dose does not cause accumulation of manganese in
the brain for longer than 2 weeks (Gallez et al. 1997), human studies have not monitored central nervous
system distribution of manganese following mangafodipir injection for longer than half an hour (Lim et
al. 1991). In addition, given the neurotoxic effects of excess manganese, evaluation of patients treated
with mangafodipir for neurological sequelae are needed.
Intermediate-Duration Exposure. Intermediate-duration inhalation exposure of humans to
manganese compounds can lead to central nervous system effects (Rodier 1955). However, reliable
estimates of intermediate-duration NOAELs or LOAELs for neurotoxicity in humans are not available.
Epidemiological studies in occupationally exposed human populations that help define the intermediate-
duration exposure levels (<356 days of exposure) that are associated with neurological effects would be
MANGANESE
344
3. HEALTH EFFECTS
valuable. In the interim, it is expected that the chronic MRL for inhaled inorganic manganese would
provide protection for intermediate-duration exposure scenarios. The MRL is based on an analysis of
dose-response data for subtle neurological deficits in groups of occupationally exposed workers with
average durations of employment from about 5 to 24 years (see Table A-3 in Appendix A); the average
duration of employment in workers in the principal study for the MRL was 5.3 years.
Intermediate-duration inhalation studies in animals have yielded NOAEL and LOAEL values for
biochemical and neurobehavioral effects (EPA 1977; Morganti et al. 1985; Ulrich et al. 1979a, 1979b),
but the range of exposure levels associated with these effects is too wide (an order of magnitude) to define
a threshold. Although neurological effects were observed in animals, symptoms characteristic of
manganese toxicity (e.g., ataxia, tremor, etc.) are not typically observed in rodent species (with the
exception of one study in which ataxia was seen only transiently) (Kristensson et al. 1986). Other rodent
studies indicated decreases in motor activity (Gray and Laskey 1980; Komura and Sakamoto 1991),
increased activity and aggression (Chandra 1983; Shukakidze et al. 2003), delayed reflexes (Ali et al.
1983a), or deficits in learning (Shukakidze et al. 2003; Vezér et al. 2005, 2007) the effects are not
consistent and are observed over a wide dose range. For these reasons, it is concluded that these data are
not sufficient to derive an intermediate-duration inhalation MRL. Other animal intermediate-duration
studies provide evidence for associations between decreased neuronal cell counts in the globus pallidus
and neurobehavioral changes (increased locomotor activity) in rats exposed by inhalation for 13 weeks to
a mixture of manganese phosphate/sulfate (at 1.05 mg manganese/m
3
) or manganese sulfate alone (at
concentrations between 0.009 and 0.9 mg manganese/m
3
), but not to manganese phosphate alone at
concentrations up to 1.1 mg manganese/m
3
(Normandin et al. 2002; Salehi et al. 2003, 2006; Tapin et al.
2006). Other 13-week rat inhalation exposure studies reported increased brain manganese concentrations
and increased locomotor activity after exposure to 3.75 mg manganese/m
3
as metallic manganese (St-
Pierre et al. 2001) and increased brain manganese concentrations with no increases in olfactory bulb,
cerebellar, or striatal concentrations of GFAP after exposure to 0.5 mg manganese/m
3
as manganese
sulfate or 0.1 mg manganese/m
3
as manganese phosphate (Dorman et al. 2004b). Other animal studies
have examined the influence of inhalation exposure to manganese sulfate on biochemical end points
associated with oxidative stress or inflammation in the brain of rats (Erikson et al. 2005, 2006; HaMai et
al. 2006; Taylor et al. 2006) and monkeys (Erikson et al. 2007, 2008). The results from these studies
indicate that acute- or intermediate-duration inhalation exposure to manganese sulfate concentrations
ranging from about 0.1 to 1.5 mg manganese/m
3
can differentially affect brain biochemical markers of
neurotoxicity, but understanding of the neurotoxic mechanism of manganese is inadequate to confidently
define any one of the observed changes as biologically adverse.
MANGANESE
345
3. HEALTH EFFECTS
Intermediate-duration oral exposure of humans to manganese has been reported to cause neurotoxicity in
two cases (Holzgraefe et al. 1986; Kawamura et al. 1941), but the data for quantitating exposure levels are
too limited to define the threshold or to judge whether these effects were due entirely to manganese
exposure. An epidemiological investigation of people who have ingested high levels of manganese may
provide valuable information on the health risk of intermediate-duration oral exposure and may provide
sufficient dose-response data from which to derive an MRL. Additional oral studies in animals including
rodents may be valuable in revealing cellular and molecular mechanisms of manganese neurotoxicity;
studies on nonhuman primates would probably be the most helpful in estimating a MRL because they
appear to be the most suitable animal model for manganese-induced neurological effects comparable to
effects observed in humans. However, any MRL derived for the oral route would have to take into
consideration that manganese is an essential nutrient and account for manganese intake from daily dietary
sources.
Intermediate-duration studies of inhalation and oral exposure to MMT in humans and animals are lacking.
Animal studies of this duration evaluating systemic toxicity from exposure to MMT and typical
environmental concentrations of its combustion products would be helpful to determine body burdens that
might be anticipated for the general population in areas that use this compound. Further, these studies
would be helpful in determining mechanisms of toxicity and expected adverse effects in exposed
populations.
Due to the nature of mangafodipir administration, which typically occurs only once in a subject, no
intermediate-duration studies in humans have been identified for this compound. Although there are a
few intermediate-duration studies in animals (Grant et al. 1997a; Larsen and Grant 1997; Treinen et al.
1995), they have focused primarily on reproductive and developmental effects. Studies of the potential
neurological effects of exposure to this compound are lacking, although the reason for this may be due to
the lack of evidence that the compound distributes in the central nervous system. As discussed
previously, the exposure to mangafodipir is expected to be very limited due to the compound’s clinical
use. There are no identified data needs for this compound.
Chronic-Duration Exposure and Cancer. As discussed in Sections 2.3 and 3.2.1.4, and
Appendix A, a number of epidemiological studies have used sensitive techniques to study the
psychological or neurological effects of exposure to low levels of manganese in the workplace (Bast-
Pettersen et al. 2004; Beuter et al. 1999; Blond and Netterstrom 2007; Blond et al. 2007; Bouchard et al.
MANGANESE
346
3. HEALTH EFFECTS
2003, 2005, 2007a, 2007b; Chia et al. 1993a, 1995; Crump and Rousseau 1999; Deschamps et al. 2001;
Gibbs et al. 1999, Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Myers et al. 2003a,
2003b; Roels et al. 1987a, 1992, 1999; Summers et al. 2011; Wennberg et al. 1991) or in environmental
media close to manganese-emitting industries (Hernández-Bonilla et al. 2011; Lucchini et al. 2007; Kim
et al. 2011; Menezes-Filho et al. 2011; Mergler et al. 1999; Riojas-Rodríguez et al. 2010; Rodríguez-
Agudelo et al. 2006; Solís-Vivanco et al. 2009; Standridge et al. 2008). Some of the occupational studies
have found statistically significant differences between exposed and non-exposed groups or significant
associations between exposure indices and neurological effects (Bast-Pettersen et al. 2004; Chia et al.
1993a; Iregren 1990; Lucchini et al. 1995, 1999; Mergler et al. 1994; Roels et al. 1987a, 1992; Wennberg
et al. 1991), whereas others have not found significant associations (Deschamps et al. 2001; Gibbs et al.
1999, Myers et al. 2003a, 2003b; Summers et al. 2011; Young et al. 2005). Analyses of dose-response
relationships for changes in neurobehavioral tests in several of these studies (Gibbs et al.1999; Iregren et
al. 1990; Lucchini et al. 1999; Mergler et al. 1994; Roels et al. 1992; Wennberg et al. 1991) provide the
basis of the current chronic inhalation MRL for inorganic manganese (as described in Chapter 2 and
Appendix A)
Additional studies involving follow-up evaluation of previously exposed occupational cohorts may be
useful to provide information on threshold levels that are correlated with observed preclinical effects.
Additional studies of populations living close to manganese-emitting industries that clearly quantify
exposure sources (dietary, water consumption, airborne) also may be useful to better describe
neurotoxicological potentials of low-level exposure to airborne manganese.
In early animal studies, intermediate or chronic inhalation exposure of monkeys and rats to manganese
dusts did not produce neurological signs similar to those seen in humans (Bird et al. 1984; EPA 1983c;
Ulrich et al. 1979a, 1979b). For example, Ulrich et al. (1979a) reported that monkeys continually
exposed for 9 months to aerosols of manganese dioxide at concentrations as high as 1.1 mg
manganese/m
3
showed no obvious clinical signs of neurotoxicity, no histopathological changes in brain
tissues, and no evidence for limb (leg) tremor or electromyographic effects on flexor and extensor
muscles in the arm. However, in a chronic study with Rhesus monkeys, decreased levels of dopamine
were found in several regions of the brain (caudate and globus pallidus) (Bird et al. 1984). Behavioral
tests detected signs of neurological effects in mice (increased open-field activity and decreased maternal
pup retrieval latency), although these are only seen at relatively high exposure levels (60–70 mg
manganese/m
3
) (Lown et al. 1984; Morganti et al. 1985). More recent animal intermediate-duration
inhalation studies provide evidence for decreased neuronal cell counts in the globus pallidus and
MANGANESE
347
3. HEALTH EFFECTS
neurobehavioral changes (Normandin et al. 2002; Salehi et al. 2003, 2006; Tapin et al. 2006); increased
brain manganese concentrations and increased locomotor activity (St-Pierre et al. 2001); increased brain
manganese concentrations without increases in GFAP (Dorman et al. 2004b); and increased biochemical
end points associated with oxidative stress or inflammation in the brain (Erikson et al. 2005, 2006; HaMai
et al. 2006; Taylor et al. 2006) and monkeys (Erikson et al. 2007, 2008). The results from these studies
indicate that acute- or intermediate-duration inhalation exposure to manganese sulfate concentrations
ranging from about 0.1 to 1.5 mg manganese/m
3
can differentially affect brain biochemical markers of
neurotoxicity, but understanding of the neurotoxic mechanism of manganese is inadequate to confidently
define any one of the observed changes as biologically adverse.
Chronic inhalation studies in animal models (Bird et al. 1984; EPA 1977; Newland et al. 1989; Olanow et
al. 1996) indicate that while non-human primates are very sensitive to the neurological effects of
manganese at very low doses (depending on exposure route), rodent models do not exhibit the same
neurological symptoms as humans and monkeys despite the administration of high doses through
inhalation, oral, and intravenous exposure routes. Although there is an apparent difference in
susceptibility, neurological effects have been observed in rodents treated with manganese. Additional
studies in animals could be valuable to increase our understanding of the mechanism of manganese-
induced disease and the basis for the differences between humans and animals.
Some data on neurological or other health effects in humans from repeated or chronic oral intake of
manganese exist (Bouchard et al. 2007c, 2011; Cawte et al. 1987; He et al. 1994; Holzgraefe et al. 1986;
Kawamura et al. 1941; Kilburn 1987; Kondakis et al. 1989; Vieregge et al. 1995; Wasserman et al. 2006,
2011; Wright et al. 2006; Zhang et al. 1995). The majority of these studies are limited by uncertainties in
the exposure routes, total exposure levels, duration of exposure, or the influence of other confounding
factors; none of these studies adequately assessed daily dietary manganese intake. Eight studies
(Bouchard et al. 2007c, 2011; Brna et al. 2011; He et al. 1994; Sahni et al. 2007; Wasserman et al. 2006,
2011; Zhang et al. 1995) indicate concentrations of manganese in drinking water that may be associated
with preclinical neurological effects in children, but the studies have several limitations.
As discussed in Section 2.3, no oral MRLs were derived for acute-, intermediate-, or chronic-duration oral
exposure to manganese, even though the limited human data and extensive animal data clearly identify
neurobehavioral changes as the most sensitive effect from intermediate- and chronic-duration oral
exposure to excess inorganic manganese. However, inconsistencies in the dose-response relationship
information across studies evaluating different neurological end points under different experimental
MANGANESE
348
3. HEALTH EFFECTS
conditions in different species, as well as a lack of information concerning all intakes of manganese (e.g.,
dietary intakes plus administered doses), make it difficult to derive intermediate- or chronic-duration
MRLs using standard MRL derivation methodology from the animal studies. An interim guidance value
of 0.16 mg manganese/kg/day is recommended for ATSDR public health assessments. The interim
guidance value is based on the Tolerable Upper Intake Level for adults of 11 mg manganese/day
established by the FNB/IOM (2001) based on a NOAEL for Western diets. The interim guidance value is
necessary because of the prevalence of manganese at hazardous waste sites and the fact that manganese is
an essential nutrient.
Additional chronic oral studies, especially epidemiological studies in populations exposed to high levels
of either inorganic and organic manganese in the environment, particularly the combustion products of
MMT in areas of high traffic density, would be valuable for evaluating the potential for adverse effects
from oral exposure to excess manganese from the environment in addition to that ingested through dietary
intake.
No studies or anecdotal reports were located that described cancer associated with exposure of humans to
inorganic manganese. Chronic oral exposure of rats and mice to high doses of manganese sulfate has
provided equivocal evidence of carcinogenic potential (NTP 1993); however, the lack of evidence for the
carcinogenic potential of manganese in humans and the equivocal evidence in animals suggest that the
potential for cancer may be low. Further animal studies are not needed at this time.
MMT has not been found to induce tumor formation in rodents (Witschi et al. 1981) and additional
studies measuring this end point would be useful to corroborate the limited database. Though no studies
of carcinogenesis involving mangafodipir exposure were identified, there are no data needs regarding this
end point with this compound.
Genotoxicity. One study was located regarding the genotoxic effects of inorganic manganese in
humans. An increase in chromosomal aberrations was observed in welders exposed to manganese;
however, the welders were also exposed to nickel (known to cause chromosomal aberrations) and iron, so
the observed increase could not be attributed solely to manganese (Elias et al. 1989). Some in vivo
studies in fruit flies and rats have been negative (Dikshith and Chandra 1978; Rasmuson 1985; Valencia
et al. 1985), but manganese has been found to be clastogenic in mice (Joardar and Sharma 1990). In vitro
studies in bacteria, yeast, and cultured mammalian cells have yielded mixed, but mainly positive, results
(Casto et al. 1979; De Méo et al. 1991; Joardar and Sharma 1990; Kanematsu et al. 1980; Nishioka 1975;
MANGANESE
349
3. HEALTH EFFECTS
NTP 1993; Oberly et al. 1982; Orgel and Orgel 1965; Singh 1984; Ulitzur and Barak 1988; Wong and
Goeddel 1988; Zakour and Glickman 1984). Additional studies, especially in cultured mammalian cells,
heritable cell types, or in lymphocytes from exposed humans, would be valuable in clarifying the
genotoxic potential of manganese. As for organic manganese, no genotoxicity studies were located
regarding MMT and studies measuring this end point are needed. Genotoxicity studies for mangafodipir
have shown negative effects (Grant et al. 1997a).
Reproductive Toxicity. Men who are exposed to manganese dust in workplace air report decreased
libido and impotency (Emara et al. 1971; Mena et al. 1967; Rodier 1955), and may suffer from sexual
dysfunction (Jiang et al. 1996b) and decreased sperm and semen quality (Wu et al. 1996). In addition,
studies in animals indicate that manganese can cause direct damage to the testes (Chandra et al. 1973;
Seth et al. 1973). While the Jiang et al. (1996b) study suggests testicular damage in occupationally
exposed men, additional epidemiological studies involving these subjects or other exposed groups to
more fully evaluate reproductive function would be valuable. Results from such studies may provide
definitive exposure-response data on reproductive function (e.g., impotence, libido, and number of
children).
Additional studies in animals are needed to determine whether the testes are damaged directly from
exposure to manganese. Information on adverse reproductive effects in women is not available. Data
from studies in female animals indicate that manganese can cause post-implantation loss when
administered through both oral and subcutaneous exposure routes in female mice and rats (Colomina et al.
1996; Sánchez et al. 1993; Szakmáry et al. 1995; Treinen et al. 1995). To establish more clearly whether
or not this is a human health concern, two types of studies would be valuable. First, single-generation
reproductive studies of female animals exposed by the inhalation route could be done. Then, if strong
evidence for concern is found in animals from these studies, epidemiological studies that included women
and men exposed in the workplace would be valuable to assess the effects of manganese on reproductive
function.
Developmental Toxicity. There is a growing body of human data on potential developmental effects
of excess manganese, although these studies are generally confined to studies of neurodevelopmental
effects as observed in children. The incidences of stillbirths and malformations have been studied in an
Australian aboriginal population living on an island where environmental levels of manganese are high
(Kilburn 1987), but small population size and lack of data from a suitable control group preclude
determining whether reported incidence of developmental abnormalities is higher than average. Hafeman
MANGANESE
350
3. HEALTH EFFECTS
et al. (2007) reported high mortality among infants <1 year of age in a Bangladesh population where the
drinking water supplied by certain local wells contained high levels of manganese. Similarly, Spangler
and Spangler (2009) reported increased infant mortality rates in counties in North Carolina with higher
groundwater manganese concentrations after accounting for such confounders as low birth weight,
economic status, education, and ethnicity. Two studies investigated neurobehavioral and school
performances (He et al. 1994; Zhang et al. 1995) of children exposed to excess levels of manganese in
water and food. However, these studies did not report data on either lengths of exposure to the metal or
on excess manganese intake compared to control areas. More recent investigations include
epidemiological studies that have detected altered behavioral and cognitive performance among children
exposed to excess levels of manganese in their local drinking water (Bouchard et al. 2007c, 2011;
Wasserman et al. 2006, 2011). These results suggest the neurotoxic potential of excessive manganese
exposure to children, but these studies have uncertainties that preclude the establishment of causal
relationships between the observed effects and manganese exposure. The studies are limited in their
ability to address several important concerns, such as whether manganese alone is responsible for the
observed effects and the contribution of dietary manganese levels as well as inhalation exposure levels
and small sample sizes. Studies evaluating developmental effects with clear analysis of exposure levels
and duration are needed to estimate dose-response relationships of manganese toxicity in children.
Several developmental studies have been performed in animals, but they are mainly limited to rodent
species and have measured limited developmental end points. One study in pregnant mice that inhaled
manganese resulted in decreased pup weight and a transient increase in activity (Lown et al. 1984). Other
studies have indicated that oral exposure to manganese adversely affects reproductive development in
male mice (Gray and Laskey 1980) and rats (Laskey et al. 1982, 1985). A single study on rats involving
oral exposure indicated that manganese caused a transient decrease in pup weight and increased activity
(Pappas et al. 1997). Another study involving gavage dosing reported skeletal abnormalities in unborn
pups, but these effects were resolved in pups allowed to grow to 100 days of age (Szakmáry et al. 1995).
Neurobehavioral effects have been shown in neonates given excess manganese orally from PND 1 to 21
(Dorman et al. 2000; Reichel et al. 2006; Tran et al. 2002a). Several studies have shown neurochemical
changes in offspring of dams exposed to increased manganese concentrations (Lai et al. 1991; Garcia et
al. 2006, 2007) or in neonatal animals dosed with excess manganese (Anderson et al. 2007a, 2009;
Chandra and Shukla 1978; Deskin et al. 1981; Dorman et al. 2000; Kern and Smith 2011; Kern et al.
2010; Kristensson et al. 1986; Moreno et al. 2009; Reichel et al. 2006; Tran et al. 2002a, 2002b). Also of
interest is the possibility that developmental manganese exposure may influence the timing of puberty;
such results have been observed in studies of both male and female rats (Lee et al. 2006; Pine et al. 2005).
MANGANESE
351
3. HEALTH EFFECTS
Studies conducted in infant Rhesus monkeys found that soy-based infant formulas (which contain higher
manganese levels than cow’s milk) and a soy-based infant formula supplemented with manganese
produced behavioral changes that may be comparable to those implicated in attention deficit-hyperactivity
disorders (Golub et al. 2005). Several studies have shown neurobehavioral changes in rodents as well
(Dorman et al. 2000; Kern and Smith 2011; Kern et al. 2010; Kristensson et al. 1986; Moreno et al. 2009;
Tran et al. 2002a, 2002b).
Other studies indicate that injected manganese is more toxic to a developing fetus than inhaled or ingested
manganese. Manganese injected subcutaneously or intravenously during the gestation period causes
serious effects on skeletal development and ossification, but studies to date using this exposure pathway
have not measured neurological deficits in pups or young rodents. The relevance to humans of results
from these injection studies is unclear.
The monkey is increasingly regarded as a more appropriate model for neurological end points; however,
monkey studies are extremely expensive and will be limited for this reason. Evaluation of appropriate
end points in rodent assays by the oral and inhalation route are needed so that these models can be used to
increase the body of knowledge of the developmental toxicity of manganese. Further, the one
developmental study involving inhalation exposure (Lown et al. 1984) had many complications;
additional studies involving neurobehavioral effects in animals following gestational and postnatal
exposure to airborne manganese are necessary. A few developmental studies have involved sectioning
fetuses to detect internal malformations (Blazak et al. 1996; Grant et al. 1997a; Szakmáry et al. 1995;
Treinen et al. 1995). However, these studies have primarily administered the manganese intravenously,
except for Szakmáry et al. (1995). Additional teratogenesis studies that assess bone malformations
following inhalation and oral exposures using a wide range of doses are needed given that manganese
overexposure affects the developing skeletal system (Blazak et al. 1996; Grant et al. 1997a; Szakmáry et
al. 1995; Treinen et al. 1995). In order to improve the accuracy of the development of an oral MRI for
manganese, additional developmental neurotoxicology studies using a functional observational battery
design and using a wide range of well-established measures in rodents and primates would be useful
(Moser 2000).
Immunotoxicity. Studies in animals indicate that injection or consumption of manganese compounds
can cause significant changes in the functioning of several cell types of the immune system (NTP 1993;
Rogers et al. 1983; Smialowicz et al. 1985, 1987). However, it is not known whether these changes are
associated with significant impairment of immune system function. Further studies are needed to
MANGANESE
352
3. HEALTH EFFECTS
determine whether these effects also occur after inhalation exposure in animals or humans. If so, a
battery of immune function tests would be valuable in determining if observed changes result in a
significant impairment of immune system function.
Neurotoxicity. Studies in humans exposed to high levels of manganese dust in the workplace provide
clear evidence that the chief health effect of concern following manganese exposure is injury to the
central nervous system (Emara et al. 1971; Mena et al. 1967; Rodier 1955; Schuler et al. 1957; Smyth
et al. 1973). As discussed previously, a number of epidemiological studies have used batteries of
neurobehavioral tests of neuromotor, cognition, and mood states to study the neurological effects of
exposure to low levels of manganese in the workplace. Analyses of dose-response relationships for
changes in neurobehavioral tests in several of these studies (Gibbs et al.1999; Iregren et al. 1990;
Lucchini et al. 1999; Mergler et al. 1994; Roels et al. 1992; Wennberg et al. 1991) provide the basis of the
current chronic inhalation MRL for inorganic manganese (as described in Chapter 2 and Appendix A).
Additional follow-up studies to further evaluate the reversibility of manganese-induced effects and define
threshold exposure levels above which manganese-induced neurological effects are irreversible may be
useful
Studies in communities surrounding manganese industries have also reported evidence for associations
between deficits in neurological end points (such as attention impairments, postural stability, and motor
impairments) and increasing biomarkers of manganese exposure in adults and children, but all potential
sources of exposure (e.g., air, diet, drinking water) could not be accounted for in these studies and they do
not provide useful dose-response data for deriving an MRL for inhaled manganese (Baldwin et al. 1999;
Beuter et al. 1999; Bowler et al. 1999; Hernández-Bonilla et al. 2011; Kim et al. 2011; Menezes-Filho et
al. 2011; Mergler et al. 1999; Solís-Vivano et al. 2009; Standridge et al. 2008; Riojas-Rodríguez et al.
2010; Rodríguez-Agudelo et al. 2006). More studies that include analyses of both sexes and assess the
relationship between environmental sources of excess manganese, altered manganese body burden, and
the potential for adverse effects may be useful. Further studies may be useful to determine whether
manganese from MMT and/or its unique combustion products contribute to airborne manganese
concentrations that can be associated with adverse effects (e.g., respiratory or neurological effects).
The evidence for neurotoxicity in humans following oral exposure to manganese is inconclusive due to
several limitations in the majority of these reports (Bouchard et al. 2007c, 2011; Holzgraefe et al. 1986;
Kawamura et al. 1941; Kilburn 1987; Kondakis et al. 1989; Wasserman et al. 2006, 2011). One report in
Japanese adults (Iwami et al. 1994) showed the link between eating food with concentrations of
MANGANESE
353
3. HEALTH EFFECTS
manganese on the high end of the normal range of a typical Western diet (5.79 mg manganese ingested
per day) and low intake concentrations of magnesium associated with an increased incidence of motor
neuron disease. Six studies in children (Bouchard et al. 2007c, 2011; He et al. 1994; Wasserman et al.
2006, 2011; Zhang et al. 1995) indicated that those who ingested drinking water and/or who ate food with
increased concentrations of manganese (≥0.241 mg/L) for at least 3 years had measurable deficits in
performance on certain tests. In addition, the children exposed to manganese performed more poorly in
school compared to non-exposed control students (who drank water with manganese concentrations no
higher than 0.04 mg/L), as measured in mastery of Chinese, performance in mathematics, and overall
grade average (Zhang et al. 1995). These studies show that both adults and children show adverse
neurological effects from oral exposure to excess manganese.
Studies in rodents and nonhuman primates indicate that oral intake of high doses of manganese can lead
to biochemical and behavioral changes indicative of nervous system effects (Bonilla and Prasad 1984;
Chandra 1983; Gupta et al. 1980; Kristensson et al. 1986; Lai et al. 1984; Nachtman et al. 1986), and this
is supported by intravenous studies in monkeys (Newland and Weiss 1992). Rodents do not appear to be
as susceptible to manganese neurotoxicity as humans; however, a study by Newland and Weiss (1992)
indicates that Cebus monkeys would be a reasonable animal model. More recent studies demonstrated
LOAEL values of 5.6 mg manganese/kg/day for severely impaired cognitive performance in a maze test
following 30-day dietary exposure of adult white rats (Shukakidze et al. 2003); 6.5 mg manganese/kg/day
for decreased open-field locomotor activity and acoustic startle response and impaired performance in
maze learning (a test of spatial memory) in male adult Wistar rats exposed for 10 weeks by gavage (Vezér
et al. 2005, 2007); 11 mg manganese/kg/day for increased pulse-initiated acoustic startle response in
Sprague-Dawley rats exposed on PNDs 1–21 (Dorman et al. 2000); and 328 mg manganese/kg/day (but
not 107.5 mg manganese/kg/day) for decreased activity during sleep and decreased play activity but no
effects on gross motor maturation or performance in cognitive tests in young monkeys (Golub et al.
2005). In contrast, hand steadiness or self-reported scales for assertiveness or anger were not different in
adult human female subjects following 8 weeks of exposure to dietary doses of 0.01 or 0.3 mg
manganese/kg/day (Finley et al. 2003). Further studies in animals may help determine the basis for the
apparent differences in route and species susceptibility.
Additional studies in animals concerning the cellular and biochemical basis of manganese neurotoxicity,
including a more detailed analysis of precisely which neuronal cell types are damaged and why, are
needed. For example, Lazrishvilli et al. (2009) observed gliosis in the brains of 40-day-old pups of rat
dams administered 4.4 or 8.7 mg manganese/kg/day in the diet before, during, and after pregnancy, but
MANGANESE
354
3. HEALTH EFFECTS
Rivera-Mancia et al. (2007) did not find gliosis in the brains of adult rats exposed to 147 mg
manganese/kg/day. Further studies may prove helpful in elucidating mechanism(s) of toxic action and
could potentially lead to developing methods for mitigating adverse effects induced by manganese.
Epidemiological and Human Dosimetry Studies. As already noted, there are numerous
epidemiological studies of workers exposed to manganese dusts in air, and the clinical signs and
symptoms of the resulting disease are well established. However, these studies have only involved males
and have mostly involved the inhalation route of exposure. Additional epidemiological studies on
populations exposed to manganese dust in the workplace or local environments (e.g., such as near
foundries, populations exposed to manganese emissions from MMT-burning automobiles, particularly
those living in areas of high-traffic density, and populations exposed to above-average oral intakes [either
through water and/or food]) may help to strengthen conclusions on dose-response relationships and no-
effect exposure levels. This would be helpful in evaluating potential risks to people who may be exposed
to above-average manganese levels near hazardous waste sites.
Biomarkers of Exposure and Effect.
Exposure. Studies in humans have shown that it is difficult to estimate past exposure to manganese by
analysis of manganese levels in blood, urine, feces, or tissues (Roels et al. 1987b; Smyth et al. 1973;
Valentin and Schiele 1983; Yamada et al. 1986). This is the result of several factors: (1) manganese is a
normal component of the diet and is present in all human tissues and fluids, so above average exposure
must be detected as an increase over a variable baseline; (2) manganese is rapidly cleared from the blood
and is excreted mainly in the feces, with very little in the urine; and (3) manganese absorption and
excretion rates are subject to homeostatic regulation, so above average exposures may result in only small
changes in fluid or tissue levels. Probably the most relevant indicator of current exposure is manganese
concentrations in tissues, but at present, this can only be measured in autopsy or biopsy samples. Studies
on new, noninvasive methods capable of measuring manganese levels in vivo, either in the whole body or
in specific organs (e.g., brain), would be very helpful in identifying persons with above average exposure.
Dorman et al. (2006b) evaluated the use of the pallidal index (PI—ratio of hyperintensities in the globus
pallidus and the adjacent subcortical frontal white matter) and the T1 relaxation rate (R1) from MRI to
reflect manganese concentrations determined by analytical chemistry in brain regions and concluded that
R1 can be used to estimate regional brain manganese concentrations and be used as a reliable biomarker
of occupational manganese exposure.
MANGANESE
355
3. HEALTH EFFECTS
Effect. The principal biological markers of toxic effects from manganese exposure are changes in the
levels of various neurotransmitters and related enzymes and receptors in the basal ganglia (Bird et al.
1984; Bonilla and Prasad 1984; Eriksson et al. 1987a, 1987b). Noninvasive methods to detect preclinical
changes in these biomarkers or in the functioning of the basal ganglia need to be developed to help
identify individuals in whom neurological effects might result. Research to determine the correlation
between urinary excretion levels of neurotransmitters, neurotransmitter metabolites, and/or
17-ketosteroids (Bernheimer et al. 1973; Rodier 1955; Siqueira and Moraes 1989) and the probability or
severity of neurological injury in exposed people is also needed. Measurements of MnSOD as a
biomarker of effect may also be helpful (Greger 1999), but there is a lack of information concerning the
relationship of this enzyme to manganese toxicity.
Research in the use of Clara cell protein CC16 may be useful in identifying populations at risk from
exposure to MMT; however, the majority of exposure to this compound is expected to arise from
inhalation and ingestion of its combustion products. Therefore, increased use of MMT in gasolines
necessitates the development of biomarkers of exposure to inorganic manganese compounds, as discussed
previously.
Absorption, Distribution, Metabolism, and Excretion. The toxicokinetics of manganese
absorption, distribution, and excretion have been studied in both humans and animals. The oral
absorption rate is about 3–5% in humans (Davidsson et al. 1988, 1989a; Mena et al. 1969), but the rate
may vary depending on age and dietary iron and manganese intake levels (Chandra and Tandon 1973;
Diez-Ewald et al. 1968; Rehnberg et al. 1982; Thomson et al. 1971). Information is needed on the
relative proportion of manganese that is absorbed via the gut following mucociliary transport of particles
from the lung to the stomach. The oral absorption rate may depend on the chemical form of manganese
ingested, but data on this are sparse. Data on the differences in uptake as a function of chemical species
(manganese dioxide, manganese tetroxide) and particle size would also be valuable in assessing human
health risk from different types of manganese dusts. Absorption of manganese deposited in the lung is
expected to be higher for soluble forms of manganese compared with relatively insoluble forms of
manganese (Aschner et al. 2005; Roels et al. 1997). Results consistent with nasal uptake of manganese
and transport to the brain along neuronal tracts have been obtained in several animal studies (Brenneman
et al. 2000; Dorman et al. 2001a, 2002a; Elder et al. 2006; Fechter et al. 2002; Henriksson et al. 1999;
Lewis et al. 2005; Normandin et al. 2004; Tjälve and Henriksson 1999; Tjälve et al. 1996; Vitarella et al.
2000). Following nasal instillation of solutions of manganese chloride or sonicated suspensions of
ultrafine insoluble manganese oxide particles to rats, similar manganese concentrations were found in the
MANGANESE
356
3. HEALTH EFFECTS
brain olfactory bulb (Elder et al. 2006). These results suggest that ultrafine particles can be distributed
from the nasal mucosa to the brain olfactory bulb. Absorption of manganese deposited in the lung or
nasal mucosa of rats is expected to be influenced by iron status, with enhanced absorption under iron-
deficient conditions and diminished absorption under iron-excess conditions (Thompson et al. 2006,
2007).
Manganese appears to be distributed to all tissues, including the brain (Aschner et al. 2005, 2007;
Kristensson et al. 1986; Rehnberg et al. 1980, 1981, 1982). Inhaled manganese appears to be distributed
more extensively to the brain than ingested manganese and there are differences in distribution between
different forms of manganese (manganese chloride compared with manganese dioxide or manganese
phosphate) (Dorman et al. 2001a, 2004b; Roels et al. 1997). Addtional research would be useful in
understanding how particle size and solubility of manganese forms influence distribution of manganese to
and within the brain. In addition, the metabolism of manganese (specifically, the degree and the rate of
oxidation state interconversions) has not been thoroughly investigated. Data on this topic are needed to
understand the mechanism of manganese toxicity and would help in evaluating the relative toxicity of
different manganese compounds. Excretion of manganese is primarily through the feces (Drown et al.
1986; Klaassen 1974; Mena et al. 1969); because the rate of excretion is an important determinant of
manganese levels in the body, further studies would be valuable on the biochemical and physiological
mechanisms that regulate manganese excretion.
Additional studies would be useful to more fully elucidate the pharmacokinetic mechanisms responsible
for uptake, distribution, and excretion in humans and animals, including studies to determine the
following: control rates and processes for uptake of ingested manganese by the intestines and liver,
including uptake rates of protein-bound forms by the liver; oxidation rates of manganese in the blood and
tissues; relative speciation of Mn(II vs. III) in blood transport mechanisms into the central nervous
system, including transfer rates; competition between manganese and iron in terms of transport processes;
and distribution following long-term exposures to assess potential storage depots.
Andersen et al. (1999) suggested that an approach to setting acceptable exposure levels for an essential,
but neurotoxic, nutrient such as manganese could be based on predicting exposure levels by any route that
would increase brain manganese concentrations to a small fraction (e.g., 10–25%) of the variation
observed in the general human population. Reliable and validated multiple-route PBPK models for
multiple species, including humans, are needed to take this approach to setting acceptable exposure
levels. Efforts to develop such models in rats, monkeys, and humans have been described, including the
MANGANESE
357
3. HEALTH EFFECTS
development of models for gestational and postnatal periods (Leavens et al. 2007; Nong et al. 2008;
Schroeter et al. 2011;Teeguarden et al. 2007a, 2007b, 2007c; Yoon et al. 2011, 2009a, 2009b). As
discussed by Yoon et al. (2011), confidence in predictions from the human models may improve with
more information on the normal range and fluctuation of human brain manganese concentrations during
early postnatal periods, the relationship between blood manganese concentrations and target tissue
dosimetry, and the extent of induction of neonatal biliary excretion.
Data on the pharmacokinetics of mangafodipir are sufficient for environmental assessment purposes.
Additional studies concerning absorption, distribution, metabolism, and excretion of MMT, via
inhalation, ingestion, and dermal exposures, would be very helpful.
Comparative Toxicokinetics. Several papers have reviewed the fairly extensive literature showing
differences in the expression of manganese neurotoxicity in humans, nonhuman primates, and rodents
(Aschner et al. 2005; Gwiazda et al. 2007; Newland et al. 1999). Aschner et al. (2005) concluded that
manganese-exposed monkeys show overlapping effects to those observed in patients with manganism
(e.g., retention of manganese in the basal ganglia and loss of dopamergic neurons), but similar changes in
regional brain manganese concentrations, neurochemical concentrations, and neuropathological effects
have been observed less consistently in rodents. Likewise, Gwiazda et al. (2007) concluded from their
analysis of estimated internal cumulative doses associated with neurobehavioral, histological, and
neurochemical changes in manganese-exposed animals that the range of adverse internal cumulative
doses extended more than 2 orders of magnitude above the lowest estimated doses associated with subtle
neurological deficits in manganese-exposed workers. The reasons for these differences are poorly
understood, but may be due to interspecies differences in toxicokinetics or toxicodynamics (i.e.,
differences in tissue sensitivities). As discussed in the previous section, recent extrapolations of animal
PBPK models to humans may be improved by more information on the normal range and fluctuation of
human brain manganese concentrations during early postnatal periods, the relationship between blood
manganese concentrations and target tissue dosimetry, and the extent of induction of neonatal biliary
excretion (Yoon et al. 2011).
Methods for Reducing Toxic Effects. In general, the methods which provide the greatest
likelihood of reducing toxic effects are the same as those aimed at reducing body burden (see
Section 3.11.2). The recommended methods for the mitigation of manganese toxicity (manganism) are
mainly supportive (Schonwald 2004). Administration of anti-Parkinson drugs, such as levo-dopa, is of
little use (Calne et al. 1994; Chu et al. 1995; Cook et al. 1974; Schonwald 2004; Huang et al. 1989;
MANGANESE
358
3. HEALTH EFFECTS
Leikin and Paloucek 2002). Chelation therapy with agents such as ethylenediaminetretraacetic acid
(EDTA) has reportedly been effective in reducing some of the symptoms (Schonwald 2004; Haddad and
Winchester 1990), but was not effective in all cases (Crossgrove and Zheng 2004; Jiang et al. 2006).
Studies on the efficacy of newly developed methods to reduce the toxic effects of manganese are needed.
The available data indicate that para-aminosalicylate has been successfully used to treat neurological
symptoms of manganese poisoning in several patients (Shuqin et al. 1992; Jiang et al. 2006). The use of
the antioxidant vitamin E has also been proposed to mitigate manganese-induced effects (Parenti et al.
1988). Additional studies on the efficacy of these treatments are needed. Further evaluation for the
mitigation of effects from excess exposure to manganese is also needed.
Methods for reducing toxic effects have not been identified for MMT.
Children’s Susceptibility. Data needs relating to both prenatal and childhood exposures, and
developmental effects expressed either prenatally or during childhood, are discussed in detail in the
Developmental Toxicity subsection above.
Children have been identified as a potentially susceptible population because of their high absorption
and/or retention of manganese as compared to adults. Although some available studies indicate that tissue
concentrations of human fetuses are comparable to adults, animal studies indicate that neonates retain
higher tissue concentrations than adult animals. Researchers hypothesize that this increased retention of
manganese may lead to neurotoxicity. Existing data indicate that the adverse neurological effects of
manganese overexposure from intravenous and oral sources are qualitatively similar in children and
adults. One study has reported that neonates are more susceptible to the effects of oral exposure to excess
manganese than adults (Dorman et al. 2000). Additional quantitative information on the levels of
manganese that result in adverse effects in children as compared to adults for inhalation, oral, and
intravenous exposures are needed. Further, analysis of existing data from effects observed in the clinical
setting might be helpful.
There are inadequate data on the pharmacokinetics of manganese in children. Although two studies
provided typical serum manganese levels in differing ages of healthy children (Alarcón et al. 1996;
Rükgauer et al. 1997), no studies have provided any data on the distribution of manganese in infants or
adolescents. Studies in animals, particularly nonhuman primates, are needed to clearly elucidate the
pharmacokinetic handling of manganese in neonates and the young (absorption, metabolism, distribution,
elimination). There are no PBPK models for children, embryos, fetuses and pregnant women, infants and
MANGANESE
359
3. HEALTH EFFECTS
lactating women, or adolescents. Such models would be very informative if they could assist in the
identification of depots for manganese storage under conditions of excess exposure, as well as the
nutritional needs of these age groups for the compound. One study was available that would provide
information on the concentrations of manganese that might be found in the developing fetus of a highly-
exposed mother (Jarvinen and Ahlström 1975). Further studies of this nature, especially those that
measure neurological end points in live offspring following excess exposure, are needed. Similarly, data
are needed to determine whether increased amounts of manganese might be present in the breast milk of a
mother with significantly elevated blood or tissue manganese concentrations.
There are likely to be multiple mechanisms of manganese toxicity and most of these have probably been
elucidated. However, there is a deficiency in our knowledge of how these mechanisms act singly or in
combination to explain the different functional deficits observed in children versus adults. There are
inadequate data to determine whether metabolism of manganese is different in children than in adults.
Manganese is necessary for normal functioning of certain enzymes. However, there are no definitive data
to indicate that children might need more manganese than adults for normal body processes. A few
studies suggest that children may have a higher need for manganese than adults, based on the increased
retention of manganese in the brains of certain neonatal animals, but this hypothesis has not been proven.
Additional studies are necessary to determine the nutritional requirements of children for manganese,
especially in infants for which FNB/IOM has not provided any guidelines.
Studies indicate that children exposed to increased concentrations of inorganic manganese, either via the
diet, due to inability to clear the compound from the body or through parenteral nutrition, develop
neurological dysfunction similar to that of adults (Devenyi et al. 1994; Fell et al. 1996; He et al. 1994;
Zhang et al. 1995). Other data exist that indicate that children may not be as susceptible as adults to the
adverse neurological effects of inorganic manganese (Kawamura et al. 1941), but the limitations in this
report make predictions about susceptibility inconclusive. Additional animal studies comparing the
potential for inorganic manganese to induce neurological effects in different age groups are needed to
help understand the susceptibility of the young compared to adults.
The mechanism of action of inorganic manganese toxicity has not been identified. Studies in humans
indicate that children and adults with increased manganese deposition in the globus pallidus and other
basal regions suffer neuromuscular deficits. It has been suggested that manganese accelerates the
autoxidation of catecholamines and contributes to oxidative stress in these affected regions of the brain.
Further research is needed to more completely elucidate the mechanism of inorganic manganese toxicity.
MANGANESE
360
3. HEALTH EFFECTS
There are no dependable biomarkers of exposure or effect that are consistently used in a clinical setting.
However, MRI scans have been used in both adults and children to determine whether manganese is
accumulating in certain brain regions. More data are needed to determine the sensitivity and specificity
of this method.
Available data do not indicate that there are any interactions of manganese with other compounds that
occur only in children. Interactions with compounds in adults are expected to also occur in children.
Data concerning the significance of any interactions of manganese with other compounds are needed.
Child health data needs relating to exposure are discussed in Section 6.8.1, Identification of Data Needs:
Exposures of Children.
3.12.3 Ongoing Studies
Ongoing studies pertaining to manganese have been identified and are shown in Table 3-20.

Investigator
Affiliation
Research description
Sponsor
Aschner, Judy L
Vanderbilt
Brain manganese deposition in
National Institute of
University
high risk neonates
Environmental Health Sciences
Aschner, Michael
Vanderbilt
Mechanisms of manganese
National Institute of
University
neurotoxicity
Environmental Health Sciences
Berkowitz, Bruce A
Wayne State
Manganese-enhanced MRI
National Eye Institute
University studies of retinal
neovascularization
Brain, Joseph D
Harvard
Manganese, iron, cadmium, and
National Institute of
University lead transport from the Environmental Health Sciences
environment to critical organs
Culotta, Valeria C
Johns Hopkins
Intracellular pathways of
National Institute of
University
manganese trafficking
Environmental Health Sciences
Dees, WL
Texas A&M
Actions of manganese on
National Institute of
University
neuroendocrine development
Environmental Health Sciences
Dietrich, Kim
University of
Early lead exposure, ADHD, and
National Institute of
Cincinnati persistent criminality: Role of Environmental Health Sciences
genes and environment
Erikson, Keith M
University of
Neurotoxicology of dietary
National Institute of
North Carolina iron/manganese interactions Environmental Health Sciences
Greensboro
Glasfeld, Arthur
Reed College
Mechanism and specificity in
National Institute of General
manganese homeostasis
Medical Sciences
Graziano, Joseph H,
Columbia
Research description: Health
National Institute of
Grazi University effects and geochemistry of Environmental Health Sciences
arsenic and manganese
Guilarte, Tomas R
Johns Hopkins
Molecular and behavioral effects
National Institute of
University
of low level Mn exposure
Environmental Health Sciences
Gunter, Thomas E
University of
Mitochondrial role in manganese
National Institute of
Rochester
toxicity
Environmental Health Sciences
Hu, Howard, MD
Brigham and
Gene-metal interactions and
National Institute of
Women's Parkinson's disease Environmental Health Sciences
Hospital
Kanthasamy,
Iowa State
Mechanisms of manganese
National Institute of
Anumantha University neurotoxicity Environmental Health Sciences
Gounder, G
Klimis-Zacas, D
University of
Manganese, arterial functional
Department of Agriculture
Maine properties, and metabolism as Hatch
related to cardiovascular disease
Klimis-Zacas, D
University of
Manganese, arterial functional
Department of Agriculture
Maine properties, and proteoglycan- Hatch
lipoprotein interactions
MANGANESE
361
3. HEALTH EFFECTS
Table 3-20. Ongoing Studies on Manganese

Investigator
Affiliation
Research description
Sponsor
Klimis-Zacas, D
University Of
Manganese, proteoglycan-
Department of Agriculture NRI
Maine lipoprotein interactions, and Competitive
arterial wall functional properties
Korrick, Susan A
Brigham and
Metal and organochlorines
National Institute of
Women's exposure: Impact on adolescent Environmental Health Sciences
Hospital
behavior and cognition
Liu, Bin
University of
Combined dopaminergic
National Institute of
Florida neurotoxicity of manganese and Environmental Health Sciences
LPS
Miller, Gary W
Emory
Neurotoxicity of nanomaterials:
National Institute of
University Evaluation of subcellular redox Environmental Health Sciences
state
Nass, Richard
Vanderbilt
Molecular genetics of manganese
National Institute of
Michael
University
induced dopamine neuron toxicity
Environmental Health Sciences
Oberley, Larry W
University of
Oxidative stress and metabolism
National Institute of
Iowa
research cluster
Environmental Health Sciences
Pecoraro, Vincent L
University of
Structural models for multinuclear
National Institute of General
Michigan at manganese enzymes Medical Sciences
Ann Arbor
Rao, Rajini
Johns Hopkins
Secretory pathway calcium and
National Institute of General
University
manganese pumps
Medical Sciences
Shine, James P
Harvard
Exposure assessment of children
National Institute of
University
and metals in mining waste
Environmental Health Sciences
Smith, Donald R
University of
Role of manganese in
National Institute of
California neurodegenerative disease Environmental Health Sciences
Santa Cruz
Srinivasan, Chandra
California State
Superoxide dismutases and ionic
National Institute on Aging
University manganese in aging
Fullerton
Tjalkens, Ronald B
Colorado State
Manganese and basal ganglia
National Institute of
University-Fort dysfunction: Role of NO Environmental Health Sciences
Collins
Weisskopf, Marc G
Harvard
Metal neurotoxicity
National Institute of
University
Environmental Health Sciences
Wessling-Resnick,
Harvard
Influence of iron status on the
National Institute of
Marianne University neurotoxicity of inhaled Environmental Health Sciences
manganese
Williams, Michael T
Children's
Effect of lead, manganese, and
National Institute of
Hospital stress during development Environmental Health Sciences
Medical
Center,
Cincinnati
MANGANESE
3.
HEALTH EFFECTS
362
Table 3-20. Ongoing Studies on Manganese

Investigator
Affiliation
Research description
Sponsor
Wright, Robert O,
Brigham and
Metal mixtures and
National Institute of
MD Women's neurodevelopment Environmental Health Sciences
Hospital
Zheng, Wei
Purdue
Choroid plexus as a target in
National Institute of
University metal-induced neurotoxicity Environmental Health Sciences
West Lafayette
MANGANESE
363
3. HEALTH EFFECTS
Table 3-20. Ongoing Studies on Manganese
Source: FEDRIP 2008
MANGANESE 365
4. CHEMICAL AND PHYSICAL INFORMATION
4.1 CHEMICAL IDENTITY
Table 4-1 lists common synonyms, trade names, and other relevant information regarding the chemical
identity of manganese and several of its most important compounds.
4.2 PHYSICAL AND CHEMICAL PROPERTIES
Information regarding the physical and chemical properties of manganese is located in Table 4-2.

Table 4-1. Chemical Identity of Manganese and Compounds
a
Characteristic Information
Chemical name Manganese Mn(II) chloride Manganese sulfate
Synonym(s) Elemental Manganese chloride
b
; Manganese sulfate
manganese
b
;
manganese dichloride
collodial
manganese
b
;
cutaval
b
Registered trade name(s) Cutaval
b
; Mangan
b
No data Sorba-spray manganese
b
Chemical formula Mn MnCl
2
MnSO
4
Chemical structure
Cl
O
O
2+
2+
Mn
S
Mn
Mn
O
Cl
O
Identification numbers:
CAS registry 7439-96-5 7773-01-5 7785-87-7
NIOSH RTECS 009275000
b
009625000
b
OP1050000
b
EPA hazardous waste No data No data No data
OHM/TADS No data No data No data
DOT/UN/NA/IMDG shipping No data No data No data
HSDB 00550
b
02154
b
02187
b
NCI No data No data No data
MANGANESE 366
4. CHEMICAL AND PHYSICAL INFORMATION

Table 4-1. Chemical Identity of Manganese and Compounds
a
Characteristic Information
Mn
O
Mn
O
Mn
O
O
Chemical name Manganese (II, III) Manganese dioxide Potassium permanganate
oxide
Synonym(s) Manganese Manganese peroxide; Permanganic acid;
tetroxide; mangano- manganese binoxide; potassium salt; chameleon
manganic oxide
c
manganese black;
mineral
c
battery manganese
Registered trade name(s) No data No data No data
Chemical formula Mn
3
O
4
MnO
2
KMnO
4
Chemical structure
O
O Mn O
K+ O Mn O
O
Identification numbers:
CAS registry 1317-35-7 1313-13-9 7722-64-7
NIOSH RTECS OP0900000
b
No data SD6475000
b
EPA hazardous waste No data No data No data
OHM/TADS No data No data No data
DOT/UN/NA/IMDG shipping No data No data UN1490
b
, IMDG 5.1
b
HSDB No data No data 01218
b
NCI No data No data No data
MANGANESE 367
4. CHEMICAL AND PHYSICAL INFORMATION

Table 4-1. Chemical Identity of Manganese and Compounds
a
Characteristic Information
Chemical name Mn(II) carbonate Mangafodipir Methylcyclopentadienyl
manganese tricarbonyl
(MMT)
Synonym(s) Carbonic acid; Mangafodipir trisodium
d
; MMT; manganese,
manganese (2+)
MnDPDP
d
tricarbonyl ([1,2,3,4,5-eta]-1-
salt; manganous methyl-2,4-cyclopentadien-
carbonate
b
;
1yl)-; methylcymantrene;
natural
tricarbonyl (2-
rhodochrosite
b
methylcyclopentadientyl)
b
manganese
Registered trade name(s) No data Teslascan
d
; Win 59010
d
AK-33X; Antiknock-33; CI-2;
Combustion Improver-2
b
Chemical formula MnCO
3
C
22
H
24
MnN
4
O
14
P
2
H
3
Na
3
C
9
H
7
MnO
3
Chemical structure
O
No data
Mn
O O
O
O
Mn
++
O
Identification numbers:
CAS registry 598-62-9 140678-14-4 12108-13-3
NIOSH RTECS No data OO9163250 48184
EPA hazardous waste No data No data No data
OHM/TADS No data No data No data
DOT/UN/NA/IMDG shipping No data No data No data
HSDB 00790
b
No data 2014
NCI No data No data No data
a
All information obtained from Sax and Lewis 1987, except where noted.
b
HSDB 2008.
c
O’Neil et al. 2006.
d
RTECS 2007.
CAS = Chemical Abstracts Service; DOT/UN/NA/IMDG = Department of Transportation/United Nations/North
America/International Maritime Dangerous Goods Code; EPA = Environmental Protection Agency;
HSDB = Hazardous Substances Data Bank; NCI = National Cancer Institute; NIOSH = National Institute for
Occupational Safety and Health; OHM/TADS = Oil and Hazardous Material Technical Assistance Data System;
RTECS = Registry of Toxic Effects of Chemical Substances
MANGANESE 368
4. CHEMICAL AND PHYSICAL INFORMATION

Property Manganese Mn(II) chloride Manganese sulfate
Molecular weight 54.94
b
125.85
b
151.00
b
Color Steel-gray
b
Pink Pale rose-red
Physical state Solid Solid Solid
Melting point 1,244 °C
c
650 °C 700 °C
Boiling point 2,095 °C
b
1,412 °C
b
850 °C (decomposes)
Density at 20 °C 7.26 g/cm
3 b
at 20 °C 2.325 g/cm
3
at 25 °C
b
3.25 g/cm
3 d
Odor No data No data Odorless
Odor threshold:
Water No data No data No data
Air No data No data No data
Solubility:
Water at 20 °C Decomposes No data No data
Acids Reacts with diluted mineral
acids with evolution of
hydrogen and formation of
divalent manganous salts
b
No data No data
Organic solvents No data Soluble in alcohol,
insoluble in ether
Soluble in alcohol,
insoluble in ether
Partition coefficients:
Log K
ow
No data No data Not applicable
Log K
oc
No data No data Not applicable
Vapor pressure at 20 °C 1 Pa at 955 °C
c
1,000 Pa at 760 °C
c
No data
Henry's law constant at 25 °C No data Not applicable Not applicable
Autoignition temperature No data Noncombustible No data
Flashpoint No data No data No data
Flammability limits Flammable and
moderately explosive in
dust form when exposed to
flame
d
No data No data
Conversion factors Not applicable Not applicable Not applicable
Explosive limits
Reactivity
Mixture of aluminum and
manganese dust may
explode in air. Mixtures
with ammonium nitrate
may explode when heated
d
No data
Hydrogen
d
; when heated
above 200 °C in presence
of nitrogen, forms nitrode;
violent reaction with NO
2
and oxidants;
incandescent reaction with
phosphorous, nitryl
fluoride, nitric acid
d
No data
No data
No data
Table 4-2. Physical and Chemical Properties of Manganese and Compounds
a
MANGANESE 369
4. CHEMICAL AND PHYSICAL INFORMATION

Table 4-2. Physical and Chemical Properties of Manganese and Compounds
a
Manganese (II, III) Potassium
Property oxide Manganese dioxide permanganate
Molecular weight 228.81
b
86.94
b
158.03
b
Color Brownish-black
b
Black Purple
Physical state Solid Solid Solid
Melting point 1,564 °C Loses oxygen at 535 °C
d
<240 °C (decomposes)
Boiling point No data No data No data
Density at 20 °C No data 5.0 g/cm
3 d
2.703 g/cm
3
Odor No data No data Odorless
Odor threshold:
Water No data No data No data
Air No data No data No data
Solubility:
Water at 20 °C Insoluble Insoluble No data
Acids Soluble in hydrochloric Soluble in hydrochloric Soluble in sulfuric acid
acid acid
Organic solvents No data No data Soluble in acetone
Partition coefficients:
Log K
ow
Not applicable No data No data
Log K
oc
Not applicable No data No data
Vapor pressure at 20 °C No data No data No data
Henry's law constant at 25 °C Not applicable No data No data
Autoignition temperature No data No data No data
Flashpoint No data No data No data
Flammability limits No data No data No data
Conversion factors Not applicable Not applicable Not applicable
Explosive limits No data No data No data
Reactivity No data No data Spontaneously
flammable on contact
with ethylene glycol
MANGANESE 370
4. CHEMICAL AND PHYSICAL INFORMATION

Table 4-2. Physical and Chemical Properties of Manganese and Compounds
a
Property Mn(II) carbonate
Mangafodipir
trisodium
Methylcyclopentadienyl
manganese tricarbonyl (MMT)
f
Molecular weight 114.95 757.4
e
218.1
Color Pink
c
No data Yellow to dark orange
Physical state hexagonal, crystals
c
Liquid (solution for Liquid, solid below 2 °C
infusion)
Melting point Decomposes No data 1.5 °C
d
Boiling point No data No data 232 °C
Density at 20 °C 3.70 g/cm
3 c
1.537 g/cm
3 b
1.39 g/cm
3
Odor No data No data Faint, pleasant
Odor threshold:
Water No data No data No data
Air No data No data No data
Solubility:
Water at 20 °C Insoluble 459.6 g/L
b
Insoluble
Acids Soluble in dilute acid
c
No data No data
Organic solvents No data 23 g/L (methanol); Readily soluble in hydrocarbons
0.8 g/L (ethanol); and the usual organic solvents
0.6 g/L (acetone);
1.1 g/L (chloroform)
b
including hexane, alcohols,
ethers, acetone, ethylene glycol,
lubricating oils, gasoline and
diesel fuel
b
Partition coefficients:
Log K
ow
No data -5.62
b
No data
Log K
oc
No data No data No data
Vapor pressure at 20 °C No data No data Ranges from 8 mm Hg at 100 °C
to 360.6 mm Hg at 200 °C
b
Henry's law constant at 25 °C No data No data No data
Autoignition temperature No data No data No data
Flashpoint No data No data 110 °C
Flammability limits No data No data No data
Conversion factors Not applicable No data No data
Explosive limits No data No data No data
Reactivity No data No data Light (decomposes)
MANGANESE 371
4. CHEMICAL AND PHYSICAL INFORMATION
a
All information obtained from Sax and Lewis 1987, except where noted.
b
O’Neil et al. 2006.
c
Lide 2000.
d
Lewis 2000.
e
RTECS 2007.
f
Data for MMT from NIOSH 2005 unless otherwise noted.
MANGANESE 373
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
5.1 PRODUCTION
Tables 5-1 and 5-2 list the facilities in each state that manufacture or process manganese, the intended
use, and the range of maximum amounts of manganese that are stored on site. The data listed in
Tables 5-1 and 5-2 are derived from the Toxics Release Inventory (TRI09 2011). Only certain types of
facilities were required to report. Therefore, this is not an exhaustive list.
Manganese is an abundant element comprising about 0.1% of the earth's crust (Graedel 1978). It does not
occur naturally as a base metal, but is a component of over 100 minerals, including various sulfides,
oxides, carbonates, silicates, phosphates, and borates (NAS 1973). The most commonly occurring
manganese-bearing minerals include pyrolusite (manganese dioxide), rhodocrosite (manganese
carbonate), and rhodanate (manganese silicate) (EPA 1984; NAS 1973; Windholz et al. 1983).
Most manganese ore is smelted in electric furnaces to produce ferromanganese, a manganese-iron alloy
widely used in the production of steel (EPA 1984; NAS 1973). Approximately 2 tons of manganese ore
are required to make 1 ton of ferromanganese (NAS 1973). Production of manganese metal is achieved
by aluminum reduction of low iron-content manganese ore, and electrolytically from sulfate or chloride
solution (Lewis 2001). Manganese with <0.1% metallic impurities can be produced electrolytically from
a manganese sulfate solution (EPA 1984; Lewis 2001).
Manganese compounds are produced either from manganese ores or from manganese metal. For
example, manganese chloride is produced by the reaction of hydrochloric acid with manganese oxide
(Pisarczyk 2005). Manganese carbonate and manganese sulfate are produced by dissolving manganese
carbonate ore (rhodochrosite) or Mn(II) oxide in sulfuric acid (Pisarczyk 2005). Potassium permanganate
may be manufactured by the one-step electrolytic conversion of ferromanganese to permanganate, or by a
two-step process involving the thermal oxidation of manganese(IV) dioxide of a naturally occurring ore
into potassium manganate(VI), followed by electrolytic oxidation to permanganate (Pisarczyk 2005).
Most manganese is mined in open pit or shallow underground mines (EPA 1984; NAS 1973). Manganese
ores were previously mined in the United States, but no appreciable quantity has been mined in the United
States since 1978 (USGS 2007). The only mine production of manganese in the United States consisted
of small amounts of manganiferous material having a natural manganese content of <5%. This type of

MANGANESE 374
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-1. Facilities that Produce, Process, or Use Manganese
Minimum
Maximum
Number of amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
AK
6
0
99,999
1, 5, 12, 13, 14
AL
113
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
AR
75
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
AZ
44
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
CA
123
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
CO
42
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14
CT
31
0
9,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
DE
6
100
999,999
1, 3, 4, 5, 8, 10
FL
50
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
GA
78
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 14
HI
7
0
99,999
1, 2, 3, 4, 5, 7, 8, 9, 12
IA
113
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ID
15
0
9,999,999
1, 3, 4, 5, 7, 8, 9, 12, 13
IL
194
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
IN
192
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
KS
61
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
KY
113
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
LA
70
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MA
42
0
49,999,999
1, 2, 3, 4, 5, 7, 8, 9, 11, 12, 14
MD
44
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ME
25
0
999,999
1, 2, 3, 5, 6, 8, 9, 11, 12, 13
MI
173
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MN
63
0
9,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MO
85
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MS
42
0
9,999,999
1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14
MT
11
10,000
999,999
1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12
NC
104
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ND
19
0
9,999,999
1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12
NE
49
0
49,999,999
1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14
NH
17
0
49,999,999
1, 2, 4, 5, 7, 8, 9, 11, 12, 13
NJ
73
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14
NM
9
1,000
9,999,999
1, 2, 3, 5, 6, 8, 9, 11, 12, 14
NV
39
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
NY
106
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OH
249
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OK
83
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14

MANGANESE 375
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-1. Facilities that Produce, Process, or Use Manganese
Minimum
Maximum
Number of amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
OR
65
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
PA
234
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
PR
14
0
999,999
2, 3, 4, 7, 8, 9, 11, 12
RI
16
0
999,999
2, 3, 4, 8, 9, 11, 12
SC
70
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14
SD
36
0
49,999,999
1, 2, 3, 5, 7, 8, 9, 10, 11, 12, 13, 14
TN
114
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
TX
175
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
UT
71
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
VA
61
0
9,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
VT
4
0
999,999
2, 4, 7, 11, 12
WA
73
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WI
143
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WV
49
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WY
12
0
999,999
1, 2, 3, 5, 8, 9, 11, 12, 13, 14
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce
6. Impurity
11. Chemical Processing Aid
2. Import 7. Reactant 12. Manufacturing Aid
3. Onsite use/processing 8. Formulation Component 13. Ancillary/Other Uses
4. Sale/Distribution 9. Article Component 14. Process Impurity
5. Byproduct
10. Repackaging
Source: TRI09 2011 (Data are from 2009)

Table 5-2. Facilities that Produce, Process, or Use Manganese Compounds
Minimum Maximum
Number of amount on site amount on site
State
a
facilities in pounds
b
in pounds
b
Activities and uses
c
AK 19 0 49,999,999 1, 2, 3, 5, 7, 8, 10, 11, 12, 13, 14
AL 155 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
AR 82 0 99,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
AZ 72 0 999,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14
CA 115 0 999,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
CO 72 0 499,999,999 1, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
CT 36 0 9,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
DC 3 1,000 99,999 12
DE 39 0 9,999,999 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14
FL 103 0 9,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
GA 109 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
HI 6 100 999,999 1, 5, 7, 9, 10
IA 101 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ID 38 0 49,999,999 1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
IL 204 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
IN 187 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
KS 75 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
KY 97 0 999,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14
LA 66 0 9,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MA 33 0 999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14
MD 76 0 99,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ME 19 0 9,999,999 1, 5, 6, 8, 12, 13, 14
MI 182 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MN 73 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MO 90 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MS 78 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MT 27 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 12, 13, 14
NC 131 0 99,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ND 24 1,000 9,999,999 1, 3, 5, 7, 8, 9, 10, 11, 12, 13, 14
NE 59 0 9,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14
NH 14 0 99,999 1, 2, 3, 5, 7, 8, 9, 12, 13
NJ 95 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
NM 36 0 10,000,000,000 1, 3, 4, 5, 7, 9, 12, 13, 14
NV 42 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
NY 123 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OH 292 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
MANGANESE 376
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL

Table 5-2. Facilities that Produce, Process, or Use Manganese Compounds
Minimum Maximum
Number of amount on site amount on site
State
a
facilities in pounds
b
in pounds
b
Activities and uses
c
OK 61 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OR 54 0 9,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
PA 265 0 99,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
PR 23 0 999,999 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12
RI 5 10,000 999,999 8, 11
SC 111 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
SD 17 0 9,999,999 1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
TN 151 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
TX 211 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
UT 88 0 999,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
VA 76 0 9,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
VT 5 0 99,999 1, 5, 7, 8
WA 80 0 49,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WI 113 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WV 72 0 499,999,999 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WY 26 0 999,999 1, 3, 4, 5, 7, 9, 12, 13, 14
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce 6. Impurity 11. Chemical Processing Aid
2. Import 7. Reactant 12. Manufacturing Aid
3. Onsite use/processing 8. Formulation Component 13. Ancillary/Other Uses
4. Sale/Distribution 9. Article Component 14. Process Impurity
5. Byproduct 10. Repackaging
Source: TRI09 2011 (Data are from 2009)
MANGANESE 377
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
MANGANESE 378
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
material was produced in South Carolina for use in coloring brick (USGS 2007). Essentially all
manganese ore used in manganese production in the United States is now imported (USGS 2007).
Currently, there are 3,703 facilities in the United States that indicate that they produce, process, or use
manganese (TRI09 2011). These facilities are scattered across the United States, with the largest numbers
in Ohio (249), Pennsylvania (234), and Illinois (194). Over 4,300 facilities are involved in the
distribution or use of manganese or manganese compounds (TRI09 2011). Tables 5-1 and 5-2 list the
number of facilities in each state, the ranges of the maximum amounts stored at each facility, and the uses
of the material (TRI09 2011).
The organomanganese compound methylcyclopentadienyl manganese tricarbonyl (MMT) is produced in
either of the following ways: via the reaction of manganous chloride, cyclopentadiene, and carbon
monoxide in the presence of manganese carbonyl and an element of group II or IIIA, or via the reaction of
methylcyclopentadiene with manganese carbonyl (EPA 1984; Sax and Lewis 1987). According to data
submitted to the EPA by the American Chemistry Council Petroleum Additives Panel, MMT is
manufactured by adding methylcyclopentadienyl dimer to a dispersion of sodium metal in diethylene
glycol dimethyl ether under a nitrogen environment (EPA 2006b). Keeping the mixture at elevated
temperature yields sodium-methylcyclopentadienyl, which is an intermediate in the reaction process.
Manganese chloride is added to the stirred mixture containing the sodium methylcyclopentadienyl
intermediate. The reaction eventually yields bis(methylcyclopentadienyl)manganese as a second
intermediate of the reaction process. The reaction vessel is then pressurized with carbon monoxide,
which results in the formation of MMT, which is separated from the reaction mixture via vacuum
distillation (EPA 2006b).
No production data from facilities that manufacture or process MMT were found. According to data from
the 2007 Directory of Chemical Producers, only one company located in Orangeburg, South Carolina
produces MMT in the United States (SRI 2007).
Mn(II) dipyridoxyl diphosphate (MnDPDP), or mangafodipir trisodium, is classified as a drug or
therapeutic agent, and no production data were found for it.
MANGANESE 379
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
5.2 IMPORT/EXPORT
The United States does not produce manganese and is 100% import reliant (USGS 2007). Import and
export data for manganese are provided in Table 5-3. Demand for manganese metal comes primarily
from the aluminum and steel industry (USGS 2007). Manganese consumption in 2007 was about 13%
lower than that of 2006, owing to constant demand by the domestic steel industry and reduction of
producer and consumer stocks. From January through August of 2007, domestic steel production was
1.4% lower than that for the same period in 2006 (USGS 2008). The United States imports the bulk of its
manganese ore from Gabon, 65%; South Africa, 19%; Australia, 7%; Ghana, 2%; and other nations, 7%
(USGS 2007). Ferromanganese is imported from South Africa, 51%; China, 14%; Mexico, 6%; Republic
of Korea, 5%; and other nations, 24% (USGS 2007).
There were no data located regarding the import or export of MMT or mangafodipir.
5.3 USE
Metallic manganese (ferromanganese) is used principally in steel production to improve hardness,
stiffness, and strength. It is used in carbon steel, stainless steel, high-temperature steel, and tool steel,
along with cast iron and superalloys (EPA 1984; NAS 1973). According to data obtained from the
U.S. Geological Society (USGS), manganese ore was consumed primarily by eight firms with plants
principally in the east and midwest United States (USGS 2008). The majority of ore consumed was
associated with steel production, directly in pig iron manufacture and indirectly through upgrading ore to
ferroalloys. Additional quantities of ore were used for nonmetallurgical purposes such as production of
dry cell batteries, in plant fertilizers and animal feed, and as a brick colorant. Manganese ferroalloys were
produced at two smelters, although one operated sporadically throughout the year (USGS 2008).
Construction, machinery, and transportation end uses accounted for approximately 24, 10, and 10%,
respectively, of manganese demand (USGS 2008). Most of the rest went to a variety of other iron and
steel applications. The value of domestic consumption, estimated from foreign trade data, was about
$730 million (USGS 2008).
Manganese compounds have a variety of uses. Manganese dioxide is commonly used in production of
dry-cell batteries, matches, fireworks, porcelain and glass-bonding materials, amethyst glass, and as the
starting material for production of other manganese compounds (EPA 1984; NAS 1973; Venugopal and
Luckey 1978). Manganese chloride is used as a precursor for other manganese compounds, as a catalyst
in the chlorination of organic compounds, in animal feed to supply essential trace minerals, and in

MANGANESE 380
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-3. Manganese Import/Export Data for 2003–2007
2003
2004
2005
2006
2007
Imports for consumption
a
Manganese ore
347
451
656
572
610
Ferromanganese
238
429
255
358
322
Silicomanganese
267
422
327
400
390
Exports
a
Manganese ore
18
123
13
2
2
Ferromanganese
11
9
14
22
33
a
Data in thousand metric tons gross weight
Source: USGS 2008
MANGANESE 381
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
dry-cell batteries (EPA 1984). Manganese sulfate is used primarily as a component of fertilizer (60% of
total consumption) and as a livestock supplement (30% of total consumption); it is also used in some
glazes, varnishes, ceramics, and fungicides (EPA 1984; Windholz et al. 1983). Potassium
permanganate’s oxidizing power allows it to be used as a disinfectant; an antialgal agent; for metal
cleaning, tanning, and bleaching; and as a water purification agent (Lewis 2001). Another common
source of manganese is found in the street drug “Bazooka”. It is a cocaine-based drug contaminated with
manganese-carbonate from free-base preparation methods (Ensing 1985).
MMT is a fuel additive developed in the 1950s to increase the octane level of gasoline and thus improve
the antiknock properties of the fuel (Davis 1998; EPA 1984; Lynam et al. 1990; NAS 1973). MMT was
introduced into Canada in 1976 and its use increased so substantially that it completely replaced tetraethyl
lead in gasoline in that country in 1990 (Zayed et al. 1999a). The major refiners in Canada have
voluntarily stopped using MMT, out of concern that its use may harm on-board diagnostic equipment
(OBD), which monitors the performance of emissions control devices in the vehicle (ICCT 2004). As a
result, as much as 95% of Canadian gasoline is now MMT-free (ICCT 2004). MMT was used as an
additive in leaded gasoline in the United States; however, EPA banned its use in unleaded gasoline in
1977 (EPA 1978, 1979a, 1981). In 1995, the ban on MMT use in unleaded gasoline was lifted, and a
court decision ordered EPA to register the product for use as a fuel additive (EPA 1995a). Recent data
suggest that MMT is currently used only sparsely in the developed world including the United States,
although exact quantities are not known (ICCT 2004). Historical data suggest that approximately
70 million pounds of MMT were sold for use in leaded gasoline in the United States between 1976 and
1990 (Veysseyre et al. 1998).
Mangafodipir trisodium (MnDPDP) is used as both a liver- and pancreas-specific contrast agent for
magnetic resonance imaging (MRI); it improves lesion detection in MRI of these organs by selectively
enhancing the normal parenchyma, but not lesions, so that the contrast between tumorous and normal
tissue is increased (Federle et al. 2000).
5.4 DISPOSAL
Manganese is listed as a toxic substance under Section 313 of the Emergency Planning and Community
Right to Know Act (EPCRA) under Title III of the Superfund Amendments and Reauthorization Act
(SARA) (EPA 1998). Disposal of wastes containing manganese is controlled by a number of federal
regulations (see Chapter 8).
MANGANESE 382
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Disposal of waste manganese into water requires a discharge permit from the EPA (see Chapter 8), but
disposal of solid wastes such as manganese metal or manganese compounds is not regulated under current
federal law. There are incomplete federal records of this disposal because most, but not all, solid
manganese wastes are disposed of by being deposited on land or by being trucked to off-site disposal
facilities (TRI09 2011). The total amount of waste manganese disposed of in this way in 2009 was
approximately 50 million pounds (TRI09 2011) (see Tables 6-1 and 6-2).
Manganese and other metals are commonly recycled for future use. In 1998, 218,000 metric tons of
manganese were estimated to have been recycled from old scrap, of which 96% was from iron and steel
scrap (USGS 2001). In 2007, the USGS reported that manganese was recycled incidentally as a minor
constituent of ferrous and nonferrous scrap; however, scrap recovery specifically for manganese was
negligible (USGS 2008). No quantitative statistics were provided regarding the amount recovered from
steel slag.
No information on disposal of MMT was located.
MANGANESE 383
6. POTENTIAL FOR HUMAN EXPOSURE
6.1 OVERVIEW
Manganese has been identified in at least 869 of the 1,699 hazardous waste sites that have been proposed
for inclusion on the EPA National Priorities List (NPL) (HazDat 2007). However, the number of sites
evaluated for manganese is not known. The frequency of these sites can be seen in Figure 6-1. Of these
sites, 861 are located within the United States, 5 are located in the Commonwealth of Puerto Rico, 2 are
located in the Virgin Islands, and 1 is located in Guam (not shown).
Manganese is ubiquitous in the environment, and human exposure arises from both natural and
anthropogenic activities. It occurs naturally in more than 100 minerals with background levels in soil
ranging from 40 to 900 mg/kg, with an estimated mean background concentration of 330 mg/kg
(Barceloux 1999). Manganese is released to the environment from industrial emissions, fossil fuel
combustion, and erosion of manganese-containing soils. Volcanic eruptions can also contribute to levels
of manganese in air. Almost 80% of industrial emissions of manganese are attributable to iron and steel
production facilities (EPA 2003a). Power plant and coke oven emissions contribute about 20% (EPA
2003a). Manganese may also be released to the environment through the use of MMT as a gasoline
additive. Thus, all humans are exposed to manganese, and manganese is a normal component of the
human body.
Background levels of manganese in the atmosphere vary widely depending on the proximity of point
sources, such as ferroalloy production facilities, coke ovens, and power plants. The estimated average
background concentration of manganese in urban areas is approximately 40 ng/m
3
, based on
measurements obtained in 102 U.S. cities (EPA 2003a; WHO 2004b). Concentrations near source
dominated areas were reported to range from 220 to 300 ng/m
3
(WHO 2004b) and rural/remote levels are
typically under 10 ng/m
3
(Sweet et al. 1993). Manganese occurs naturally in surface water and
groundwater. A median dissolved manganese concentration of 24 μg/L in samples from 286 U.S. rivers
and streams was reported (Smith et al. 1987). Natural concentrations of manganese in seawater
reportedly range from 0.4 to 10 μg/L (EPA 1984).
The general population is exposed to manganese primarily through food intake. The World Health
Organization (WHO) estimates that adults consume between 0.7 and 10.9 mg of manganese per day in the
diet, with higher intakes for vegetarians who may consume a larger proportion of manganese-rich nuts,
grains, and legumes in their diet as compared to non-vegetarians in the general population (WHO 2004b).
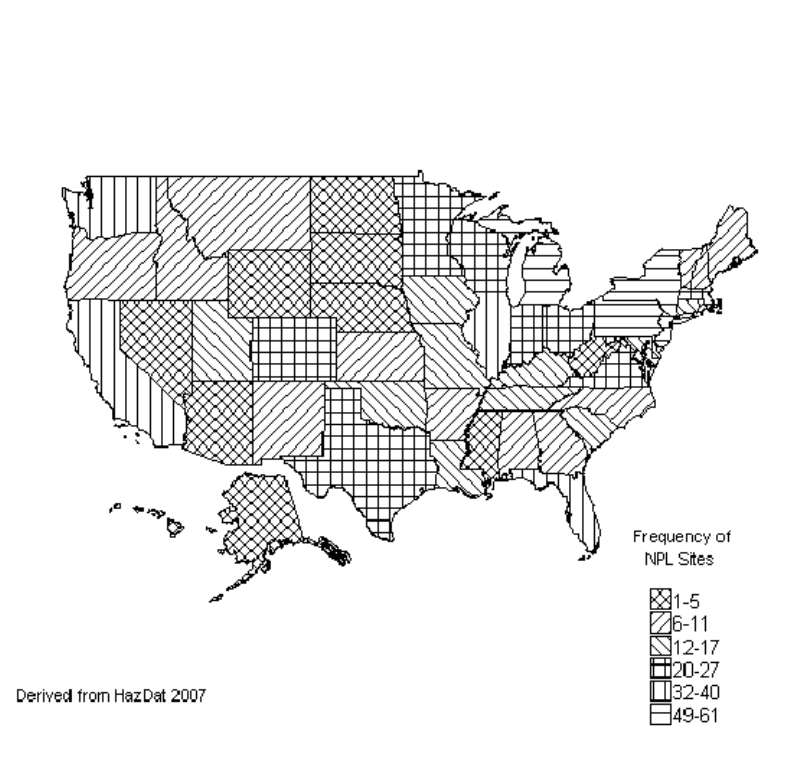
MANGANESE 384
6. POTENTIAL FOR HUMAN EXPOSURE
Figure 6-1. Frequency of NPL Sites with Manganese Contamination
MANGANESE 385
6. POTENTIAL FOR HUMAN EXPOSURE
Manganese intake from drinking water is substantially lower than intake from food. Using a median
drinking-water level of 10 μg/L and an assumption that the average adult drinks 2 L of water/day, an
average intake of approximately 0.020 mg/day was estimated (WHO 2004b). Exposure to manganese
from air is considered negligible as compared to intake from diet; however, persons in certain occupations
may be exposed to much higher levels than the general public (see Section 6.7).
Manganese adsorbed to particulate matter in air can be classified by the size of the particles. Air
concentrations can be reported as total suspended particulate matter (TSP), respirable particulates, and
fine particulates. In this document, manganese adsorbed to particulate matter <10 microns in
aerodynamic diameter is referred to as PM
10
. The EPA has further divided these tiny particles into "fine"
particles of ≤2.5 microns (PM
2.5
) and "coarse" particles of between 2.5 and 10 microns.
6.2 RELEASES TO THE ENVIRONMENT
The Toxics Release Inventory (TRI) data should be used with caution because only certain types of
facilities are required to report (EPA 2005). This is not an exhaustive list. Manufacturing and processing
facilities are required to report information to the TRI only if they employ 10 or more full-time
employees; if their facility is included in Standard Industrial Classification (SIC) Codes 10 (except 1011,
1081, and 1094), 12 (except 1241), 20–39, 4911 (limited to facilities that combust coal and/or oil for the
purpose of generating electricity for distribution in commerce), 4931 (limited to facilities that combust
coal and/or oil for the purpose of generating electricity for distribution in commerce), 4939 (limited to
facilities that combust coal and/or oil for the purpose of generating electricity for distribution in
commerce), 4953 (limited to facilities regulated under RCRA Subtitle C, 42 U.S.C. section 6921 et seq.),
5169, 5171, and 7389 (limited S.C. section 6921 et seq.), 5169, 5171, and 7389 (limited to facilities
primarily engaged in solvents recovery services on a contract or fee basis); and if their facility produces,
imports, or processes ≥25,000 pounds of any TRI chemical or otherwise uses >10,000 pounds of a TRI
chemical in a calendar year (EPA 2005).
According to the Toxics Release Inventory (TRI), in 2009, a total of 13,635,017 pounds (6,185 metric
tons) of manganese was released to the environment from 1,929 large processing facilities (TRI09 2011).
An additional 162,358,105 pounds (73,644 metric tons) of manganese compounds were released from
1,656 facilities. Tables 6-1, and 6-2 list the amount of manganese and manganese related compounds,
respectively, that were released from all of the facilities that manufacture or process manganese to each
medium within each state in 2009 (TRI09 2011). The TRI data should be used with caution because only

MANGANESE 386
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-1. Releases to the Environment from Facilities that Produce, Process, or
Use Manganese
a
Reported amounts released in pounds per year
b
Total release
On- and
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
off-site
AL
55
11,963
1,320
1
23,951
311
13,125
24,421
37,546
AR
33
5,802
271
0
37,088
164
6,044
37,281
43,325
AZ
16
1,360
22
0
906,639
0
907,918
103
908,021
CA
51
2,936
443
8
100,828
4,622
2,941
105,896
108,837
CO
16
957
50
0
24,347
0
962
24,392
25,354
CT
6
0
5
0
680
3
0
688
688
DE
1
0
0
0
8
0
0
8
8
FL
27
1,247
25
0
11,190
0
1,247
11,214
12,461
GA
46
2,375
910
0
19,308
106
2,668
20,032
22,699
IA
65
15,236
2,561
0
315,743
96,802
25,560
404,782
430,341
ID
1
0
0
0
0
0
0
0
0
IL
115
8,258
1,903
398
143,228
128,530
8,809
273,508
282,317
IN
128
31,067
10,708
0
210,384
7,914
31,725
228,349
260,074
KS
43
13,393
282
0
446,709
118,248
420,378
158,254
578,632
KY
57
19,213
3,923
0
66,913
17,385
21,772
85,661
107,433
LA
38
11,298
2,734
0
168,828
2,736
84,718
100,878
185,596
MA
14
858
1
0
1,308
1,518
858
2,827
3,686
MD
11
47
226
0
0
86
47
312
359
ME
4
258
14
0
16
242
258
272
530
MI
84
6,564
2,753
0
488,199
7,930
7,350
498,096
505,446
MN
36
3,321
56
0
30,877
8
3,326
30,936
34,262
MO
51
356,385
1,168
0
19,424
1
368,134
8,844
376,978
MS
28
8,949
193
0
21,617
0
11,805
18,954
30,759
MT
2
0
0
0
167,254
0
167,254
0
167,254
NC
50
1,627
190
0
6,455
7,618
1,643
14,247
15,890
ND
6
685
260
0
530
5
695
785
1,480
NE
17
1,504
258
0
42,241
240
1,511
42,732
44,242
NH
6
11
0
0
0
0
11
0
11
NJ
9
425
0
0
149,540
0
425
149,540
149,965
NM
1
0
0
0
0
0
.
0
0
NV
12
345
0
0
77,599
0
76,860
1,084
77,944
NY
33
1,269
4,379
0
72,506
662
3,870
74,946
78,816
OH
155
14,141
36,557
0
5,481,604
270,842
4,969,747
833,397
5,803,145
OK
72
3,691
18
0
39,556
0
3,692
39,573
43,265
OR
15
318
0
0
90,679
713
90,651
1,059
91,710

MANGANESE 387
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-1. Releases to the Environment from Facilities that Produce, Process, or
Use Manganese
a
Reported amounts released in pounds per year
b
Total release
On- and
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
off-site
PA
143
237,541
9,250
0
761,198
67,434
512,022
563,402
1,075,423
PR
1
0
0
0
0
0
0
0
0
RI
2
0
0
0
0
0
0
0
0
SC
44
3,457
1,797
0
70,141
44
36,297
39,142
75,439
SD
13
1,118
3
0
9,444
728
5,201
6,092
11,293
TN
58
5,908
576
0
148,202
1,259
49,857
106,088
155,945
TX
139
17,392
11,813
5,312
101,237
3,570
53,830
85,494
139,324
UT
20
1,764
10
0
30,054
0
29,506
2,322
31,828
VA
26
1,111
29
0
24,847
4,117
1,111
28,993
30,104
WA
23
2,604
3,155
0
166,537
479
6,548
166,228
172,776
WI
147
129,960
1,201
0
1,273,787
2,489
132,938
1,274,499
1,407,437
WV
5
2,020
1,288
0
1,566
40
2,052
2,862
4,915
WY
4
347
0
0
101,109
0
101,456
0
101,456
Total
1,929
928,727
100,353
5,719
11,853,373
746,845
8,166,823
5,468,193
13,635,017
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an
exhaustive list. Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, waste water treatment-(metals only), and publicly owned treatment works (
POTWs) (metal
and metal compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface
impoundments, other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-site management, transfers to waste broker for
disposal, unknown
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
RF = reporting facilities; UI = underground injection
Source: TRI09 2011 (Data are from 2009)

State
c
AL
RF
d
Air
e
69
26,726
b
Reported amounts released in pounds per year
Total release
On- and
f i j k
UI
g
Water
Land
h
Other
On-site
Off-site
off-site
785,399
0
4,807,610
696,690
4,618,951
1,697,473
6,316,424
AK
7
28,562
2,195
0
1,077,747
7,103
1,081,419
34,188
1,115,607
AZ
19
4,972
256
0
1,269,078
57,022
1,055,480
275,848
1,331,329
AR
45
18,356
436,835
0
1,501,825
430,133
1,906,316
480,834
2,387,150
CA
32
3,155
3,639
0
250,073
5,866
87,700
175,034
262,734
CO
24
6,502
7,921
0
1,647,793
187
1,327,931
334,472
1,662,403
CT
9
329
319
0
38,700
6,197
638
44,907
45,545
DE
8
571
34,677
0
1,795,738
0
89,415
1,741,571
1,830,986
DC
3
0
1,862
0
7,665
0
9,527
0
9,527
FL
33
9,512
183,588
0
1,856,695
7,957
1,639,719
418,033
2,057,752
GA
60
24,740
695,336
0
1,955,991
6,876
2,396,106
286,837
2,682,943
HI
1
57
0
0
37,976
0
57
37,976
38,033
ID
14
650
133,402
0
18,440,929
309
18,292,599
282,690
18,575,289
IL
81
70,640
17,478
0
6,925,832
1,114,421
1,487,271
6,641,100
8,128,371
IN
80
98,896
86,034
24,700
12,313,396
102,985
7,874,506
4,751,505
12,626,011
IA
46
40,933
4,540
0
1,388,935
897,867
311,337
2,020,939
2,332,275
KS
20
6,716
371
250
345,067
299
327,527
25,176
352,703
KY
46
79,582
127,043
0
2,467,702
40,720
2,022,163
692,884
2,715,047
LA
24
14,454
287,076
0
5,374,839
55,278
5,308,597
423,050
5,731,647
ME
10
11,053
341,643
0
552,090
92,185
732,267
264,704
996,971
MD
28
14,032
110,411
30,462
1,064,434
156,798
1,132,173
243,964
1,376,137
MA
6
488
10,819
0
17,806
9,564
1,987
36,690
38,677
MI
56
18,463
52,592
370
1,894,107
58,388
986,770
1,037,149
2,023,919
MN
36
11,395
58,458
0
1,369,277
17,384
1,370,866
85,648
1,456,514
MS
37
13,873
426,666
9,479,269
8,428,879
140,005
17,150,435
1,338,257
18,488,692
MO
45
10,884
17,685
0
719,070
14,005
363,708
397,936
761,643
MT
7
8,671
20,878
0
1,778,158
67,592
1,628,974
246,325
1,875,299
NE
19
13,635
264
0
268,939
48,505
280,654
50,689
331,343
NV
13
8,857
17
0
10,146,579
0
8,249,878
1,905,576
10,155,454
NH
4
1,023
597
0
24,082
4,130
5,721
24,112
29,833
NJ
12
1,081
11,936
0
122,998
0
13,017
122,998
136,015
NM
6
3,000
311
0
968,320
0
971,631
0
971,631
NY
21
4,215
76,763
0
611,484
22,150
243,143
471,469
714,613
NC
55
17,845
207,682
0
2,056,521
59,722
1,578,832
762,939
2,341,770
ND
9
11,456
16,308
0
1,952,569
5,164
1,033,517
951,980
1,985,497
MANGANESE 388
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-2. Releases to the Environment from Facilities that Produce, Process, or
Use Manganese Compounds
a
MANGANESE 389
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-2. Releases to the Environment from Facilities that Produce, Process, or
Use Manganese Compounds
a
Reported amounts released in pounds per year
b
Total release
On- and
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
off-site
OH
131
197,569
177,737
35,502
6,308,073
530,421
4,419,743
2,829,558
7,249,301
OK
28
9,552
57,795
0
707,517
112,652
602,049
285,467
887,516
OR
17
5,151
74,703
0
841,241
3
320,278
600,820
921,098
PA
133
54,376
211,669
0
8,526,704
221,617
4,342,438
4,671,927
9,014,365
PR
4
8,372
5
0
7,430
0
8,372
7,435
15,807
SC
37
20,380
237,802
0
3,048,315
74,168
1,995,186
1,385,479
3,380,665
SD
7
217
0
0
27,926
0
25,293
2,850
28,143
TN
52
42,279
160,029
0
12,144,425
11,702
11,659,763
698,672
12,358,434
TX
105
44,308
183,435
0
6,434,013
80,394
5,964,959
777,191
6,742,149
UT
18
5,829
1,000
0
1,029,393
854
921,556
115,520
1,037,076
VT
2
26
0
0
0
254
26
254
280
VA
33
12,555
195,010
0
1,621,846
15,981
650,455
1,194,937
1,845,392
WA
17
1,820
150,906
0
597,144
11,468
555,305
206,032
761,337
WV
22
158,447
12,263
0
1,430,843
3,600
1,187,721
417,432
1,605,153
WI
58
11,891
116,789
0
1,395,609
164,597
348,429
1,340,457
1,688,886
WY
7
8,266
1,052
0
926,764
636
880,588
56,130
936,718
Total
1,656
1,166,362
5,741,194
9,570,553
140,526,147
5,353,849
119,462,992
42,895,113
162,358,105
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an
exhaustive list. Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, waste water treatment-(metals only), and publicly owned treatment works (POTWs) (metal
and metal compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface
impoundments, other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-
site management, transfers to waste broker for disposal,
unknown
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
RF =
reporting facilities; UI = underground injection
Source: TRI09 2011 (Data are from 2009)
MANGANESE 390
6. POTENTIAL FOR HUMAN EXPOSURE
certain types of facilities are required to report. This is not an exhaustive list. Also, because these data
reflect past releases, they may not be representative of current releases at these facilities.
Manganese may also be emitted to the environment through the use of gasoline that contains MMT;
however, no data on the amount of MMT that is currently being used in gasoline in the United States were
located. No data for releases of mangafodipir to the environment were found. Because mangafodipir is a
compound used exclusively in a clinical environmental, it is not expected to be released to the
environment and will not be discussed in subsequent sections concerning fate and transport.
6.2.1 Air
Estimated releases of 928,727 pounds (421 metric tons) of manganese to the atmosphere from
1,929 domestic manufacturing and processing facilities in 2009, accounted for about 6.8% of the
estimated total environmental releases from facilities required to report to the TRI (TRI09 2011).
Estimated releases of 1,166,362 pounds (530 metric tons) of manganese compounds to the atmosphere
from 1,656 domestic manufacturing and processing facilities in 2009, accounted for about 0.7% of the
estimated total environmental releases from facilities required to report to the TRI (TRI09 2011). These
releases are summarized in Tables 6-1 and 6-2.
According to data from the National Pollutant Release Inventory (NPRI) maintained by Environment
Canada, approximately 273.9 metric tons of manganese were released to air in Canada in 2003 from
various industrial sources (Health Canada 2008). The major industrial sources for manganese emissions
in Canada were attributed to an iron-ore mine located in Labrador, iron- and steel-related industries,
pulp/paper/newsprint mills, fossil fuel electric power generation, and the manufacturing of heating and
commercial refrigeration equipment.
The amount of manganese compounds emitted to air in 2005 was estimated in the EPA's National
Emission Inventory (NEI) database. This database contains detailed information about sources that emit
criteria air pollutants and their precursors, and hazardous air pollutants for the 50 United States,
Washington DC, Puerto Rico, and the U.S. Virgin Islands. The NEI database derives emission data from
several sources including state and local environmental agencies, the TRI database, computer models for
on- and off-road emissions, and databases related to EPA's Maximum Achievable Control Technology
(MACT) programs to reduce emissions of hazardous air pollutants. Data are available as zipped
Microsoft Access database files that may be accessed directly from the EPA website (EPA 2011). For
MANGANESE 391
6. POTENTIAL FOR HUMAN EXPOSURE
2005, approximately 2,134 tons of manganese were released to air with the greatest contribution arising
from point sources involving industrial metals processing and combustion processes.
Manganese has been identified in air samples collected at 31 of the 869 NPL hazardous waste sites where
it was detected in some environmental media (HazDat 2007).
The main sources of manganese release to the air are industrial emissions, combustion of fossil fuels, and
reentrainment of manganese-containing soils (EPA 1983c, 1984, 1985c, 1985d, 1987a; Lioy 1983). The
principal sources of industrial emissions are ferroalloy production and iron and steel foundries, and the
principal sources of combustion emissions are power plants and coke ovens (EPA 1983c, 1985c, 1985d).
Atmospheric emissions of manganese and other trace metals from these industrial sources have declined
over the last 2 decades due to the use of advanced pollution control devices and increased government
regulations regarding these emissions (EPA 1984, 1985d).
Windblown erosion of dusts and soils is also an important atmospheric source of manganese. Wallace
and Slonecker (1997) estimated that the background contribution of windblown soil to fine particulate
atmospheric manganese levels was 1–2 ng/m
3
in the United States and Canada. Volcanic eruptions may
also release manganese to the atmosphere (Schroeder et al. 1987).
MMT is a manganese-containing compound used to enhance the octane rating in gasoline. MMT was
used as an additive in leaded gasoline until the phase-out of leaded gas in the United States in 1995. It
was also used in unleaded gasoline for a short period of time in the late 1970’s, but was banned as an
additive in unleaded gasoline by EPA in 1977 (EPA 1978, 1979a, 1981). In 1995, the ban on MMT use
in unleaded gasoline was lifted, and a court decision ordered EPA to register the product for use as a fuel
additive, although testing for health effects continues (EPA 1995a). Analysis of manganese levels in the
air indicates that vehicular emissions from MMT containing fuels contributed an average of 13 ng
manganese/m
3
in southern California, while vehicular emissions were only responsible for about 3 ng/m
3
in central and northern California (Davis et al. 1988). A survey of ambient air concentrations of fine
(PM
2.5
) manganese in rural sites in U.S. national parks and in urban sites in California indicated that from
1988 to 1993, ambient concentrations of manganese ranged from 1 ng/m
3
in rural sites to 3 ng/m
3
in urban
sites (Wallace and Slonecker 1997). Part of the increase in fine manganese during this period was
considered to be the result of the use of MMT in leaded gasoline. It was estimated that automobile
emissions from leaded gasoline were responsible for 37% of the fine manganese levels in California in
1992. In 1994, automobile emissions were estimated to contribute 12% of the fine manganese levels in
MANGANESE 392
6. POTENTIAL FOR HUMAN EXPOSURE
the atmosphere, as the use of leaded gasoline declined. It has been estimated that if MMT were used in
all gasoline, urban air manganese levels would be increased by about 50 ng/m
3
(Cooper 1984; Ter Haar
et al. 1975). Other authors have estimated that the increase in atmospheric manganese levels would be
<20 ng/m
3
(Lynam et al. 1994).
In Canada, where the use of MMT containing gasoline has been extensive, a 10% per year increase in
manganese emission rates from MMT in gasoline since 1981 was estimated (Loranger and Zayed 1994).
A positive correlation between atmospheric manganese concentration and traffic density has been
observed (Loranger and Zayed 1997a; Loranger et al. 1994a). The principal emission product of MMT
combustion is a fine particulate matter (0.1–0.4 μm diameter) consisting of manganese oxide (Egyed and
Wood 1996; Ter Haar et al. 1975), manganese phosphate, and some manganese sulfate (Lynam et al.
1999). The finding of soluble manganese (<0.4 μm) in snow samples obtained close to a highway in
Montreal, Canada suggested a possible contamination from mobile sources (Loranger and Zayed 1997a;
Loranger et al. 1995). However, it has been difficult to assess the exact contribution of mobile sources to
overall contamination from natural and industrial sources because of the physico-chemical characteristics
of manganese particulate, environmental factors affecting its dispersion, and the difficulties in
distinguishing between mobile sources of manganese and background manganese levels (Loranger and
Zayed 1997a; Veysseyre et al. 1998).
Despite the estimated 10% per year increase in manganese emission rates from the use of MMT in
gasoline in Canada, atmospheric manganese concentrations in Montreal have remained fairly constant
between 1981 and 1990, and have decreased markedly in 1991 and 1992 (Loranger and Zayed 1994).
The decline in manganese concentration after 1990 may have been due to a shutdown in 1991 of a
ferromanganese plant located near Montreal. Air concentrations are in general below the EPA reference
concentration (RfC) of 0.05 μg/m
3
for respirable manganese. However, in 1998, it was observed that
some atmospheric concentrations in specific microenvironments with important traffic density were
higher than the RfC (Zayed et al. 1999a).
6.2.2 Water
Estimated releases of 103,53 pounds (46 metric tons) of manganese to water from 1,929 domestic
manufacturing and processing facilities in 2009, accounted for about 0.1% of the estimated total
environmental releases from facilities required to report to the TRI (TRI09 2011). Estimated releases of
5,741,194 pounds (2,604 metric tons) of manganese compounds to water from 1,656 domestic
MANGANESE 393
6. POTENTIAL FOR HUMAN EXPOSURE
manufacturing and processing facilities in 2009, accounted for about 3.5% of the estimated total
environmental releases from facilities required to report to the TRI (TRI09 2011). These releases are
summarized in Tables 6-1 and 6-2.
Manganese has been identified in surface water and groundwater samples collected at 392 and 692,
respectively, of the 869 NPL hazardous waste sites where it was detected in some environmental media
(HazDat 2007).
Based on comparison to typical background levels of manganese in surface water or groundwater (see
Section 6.4.2), it seems likely that some waste sites where manganese is detected contain only natural
levels. Although ambient manganese levels are about 200 μg/L in a number of cases, high levels (in
excess of 1,000 μg/L) have been detected indicating that manganese wastes may lead to significant
contamination of water at some sites. For example, at one site in Ohio where "heavy metals" had been
disposed, manganese concentrations up to 1,900 μg/L were found in on-site wells (Cooper and Istok
1988). Levels in water at two NPL sites in Missouri ranged from 0.009 to 3.7 μg/L (MDNR 1990). No
information is available on the method used to determine these values, so it is not clear whether the data
refer to total or dissolved manganese.
6.2.3 Soil
Estimated releases of 11,853,373 pounds (5,377 metric tons) of manganese to soil from 1,929 domestic
manufacturing and processing facilities in 2009, accounted for about 87% of the estimated total
environmental releases from facilities required to report to the TRI (TRI09 2011). An additional 5,719
pounds (2.6 metric tons) were injected underground. Estimated releases of 140,526,147 pounds (63,742
metric tons) of manganese compounds to the soil from 1,656 domestic manufacturing and processing
facilities in 2009, accounted for about 87% of the estimated total environmental releases from facilities
required to report to the TRI (TRI09 2011). An additional 9,570,553pounds (4,341 metric tons) were
injected underground. These releases are summarized in Tables 6-1 and 6-2.
Manganese deposition to soils from the use of MMT in gasoline was estimated for two sites in Toronto,
Canada (Bhuie et al. 2005). Accounting for variables such as annual average daily traffic (AADT)
density, fuel consumption, distance traveled by automobiles, and a manganese content of 10 mg/L of
gasoline, the annual average manganese contribution to soils from MMT emissions were calculated as
MANGANESE 394
6. POTENTIAL FOR HUMAN EXPOSURE
5.73 and 2.47 mg/kg at two sites (Bhuie et al. 2005). These concentrations were considered insignificant
when compared to natural background manganese levels (541 and 557 mg/kg) in soil for these areas.
Manganese has been identified in soil and sediment, samples collected at 355 and 257, respectively, of the
869 NPL hazardous waste sites where it was detected in some environmental media (HazDat 2007).
6.3 ENVIRONMENTAL FATE
6.3.1 Transport and Partitioning
Manganese compounds have negligible vapor pressures (see Table 4-2), but may exist in air as suspended
particulate matter derived from industrial emissions or the erosion of soils. Manganese-containing
particles are mainly removed from the atmosphere by gravitational settling, with large particles tending to
fall out faster than small particles (EPA 1984). The half-life of airborne particles is usually on the order
of days, depending on the size of the particle and atmospheric conditions (Nriagu 1979). Some removal
by washout mechanisms such as rain may also occur, although it is of minor significance in comparison
to dry deposition (EPA 1984; Turner et al. 1985).
In a study completed by Evans (1989), there were two mechanisms involved in explaining the retention of
manganese and other metals in the environment by soil. First, through cation exchange reactions,
manganese ions and the charged surface of soil particles form manganese oxides, hydroxides, and
oxyhydroxides, which in turn form absorption sites for other metals. Secondly, manganese can be
adsorbed to other oxides, hydroxides, and oxyhydroxides through ligand exchange reactions. When the
soil solution becomes saturated, these manganese oxides, hydroxides, and oxyhydroxides can precipitate
into a new mineral phase and act as a new surface to which other substances can absorb (Evans 1989).
The behavior of heavy metals in the combustion gases of urban waste incinerators was studied by
Fernandez et al. (1992). Manganese was detected inside gaseous fly ash particles in the form of oxides
and chlorides. When these soluble oxides and chlorides reach environmental media, they can leach out
and become mobile (Fernandez et al. 1992).
The transport of manganese in air is largely determined by its particle size. About 80% of the manganese
in suspended particulate matter is associated with particles having a mass median aerodynamic diameter
(MMAD) of <5 μm (WHO 1981). The compound’s small particle size (approximately 80% with a
MANGANESE 395
6. POTENTIAL FOR HUMAN EXPOSURE
MMAD <5 μm and approximately 50% with an MMAD <2 μm) favors widespread airborne distribution
and is within the respirable range (WHO 1981).
The transport and partitioning of manganese in water is controlled by the solubility of the specific
chemical form present, which in turn is determined by pH, Eh (oxidation-reduction potential), and the
characteristics of the available anions. The metal may exist in water in any of four oxidation states;
however, Mn(II) predominates in most waters (pH 4–7), but may become oxidized under alkaline
conditions at pH >8 (EPA 1984). The principal anion associated with Mn(II) in water is usually
carbonate (CO
3
–2
), and the concentration of manganese is limited by the relatively low solubility
(65 mg/L) of manganese carbonate (Schaanning et al. 1988). Under oxidizing conditions, the solubility of
Mn(II) may be controlled by manganese oxide equilibria (Ponnamperuma et al. 1969), with manganese
being converted to the Mn(II) or Mn(IV) oxidation states (Rai et al. 1986). In extremely reduced water,
the fate of manganese tends to be controlled by formation of a poorly soluble sulfide (EPA 1984).
Manganese is often transported in rivers as suspended sediments. It has been reported that most of the
manganese in a South American river came from industrial sources and was bound to suspended particles
in the water (Malm et al. 1988).
In an aquifer studied in France, manganese was shown to originate from within the aquifer itself (Jaudon
et al. 1989). In the presence of decreased dissolved oxygen in the groundwater, Mn(IV) has been shown
to be reduced both chemically and bacterially into the Mn(II) form (Jaudon et al. 1989). This oxidation
state is water soluble and easily released into the groundwater.
Manganese in water may be significantly bioconcentrated at lower trophic levels. A bioconcentration
factor (BCF) relates the concentration of a chemical in plant and animal tissues to the concentration of the
chemical in the water in which they live. Folsom et al. (1963) estimated that the BCFs of manganese
were 2,500–6,300 for phytoplankton, 300–5,500 for marine algae, 800–830 for intertidal mussels, and
35–930 for coastal fish. Similarly, Thompson et al. (1972) estimated that the BCFs of manganese were
10,000–20,000 for marine and freshwater plants, 10,000–40,000 for invertebrates, and 100–600 for fish.
In general, these data indicate that lower organisms such as algae have larger BCFs than higher
organisms. In order to protect consumers from the risk of manganese bioaccumulation in marine
mollusks, EPA has set a criterion for manganese at 0.1 mg/L for marine waters (EPA 1993b).
MANGANESE 396
6. POTENTIAL FOR HUMAN EXPOSURE
The tendency of soluble manganese compounds to adsorb to soils and is dependent upon the cation
exchange capacity and the organic composition of the soil (Curtin et al. 1980; Hemstock and Low 1953;
Kabata-Pendias and Pendias 1984; McBride 1979; Schnitzer 1969). Baes and Sharp (1983) noted that
soil adsorption constants (the ratio of the concentration in soil to the concentration in water) for Mn(II)
span five orders of magnitude, ranging from 0.2 to 10,000 mL/g, increasing as a function of the organic
content and the ion exchange capacity of the soil; thus, adsorption may be highly variable. In some cases,
adsorption of manganese to soils may not be a readily reversible process. At low concentrations,
manganese may be "fixed" by clays and will not be released into solution readily (Reddy and Perkins
1976). At higher concentrations, manganese may be desorbed by ion exchange mechanisms with other
ions in solution (Rai et al. 1986). For example, the discharge of waste water effluent into estuarine
environments resulted in the mobilization of manganese from the bottom sediments (Helz et al. 1975;
Paulson et al. 1984). The metals in the effluent may have been preferentially adsorbed resulting in the
release of manganese.
6.3.2 Transformation and Degradation
6.3.2.1 Air
Very little information is available on atmospheric reactions of manganese (EPA 1984). Manganese can
react with sulfur dioxide and nitrogen dioxide, but the occurrence of such reactions in the atmosphere has
not been demonstrated.
MMT undergoes photolysis rapidly by sunlight in the atmosphere or in aqueous solutions with a very
short half-life (i.e., <2 minutes) (Ter Haar et al. 1975; Garrison et al. 1995). The photodegradation
products tentatively identified in aqueous photolysis experiments were methylcyclopentadiene,
cyclopentadiene, carbon monoxide, manganese carbonyl, and trimanganese tetroxide (Garrison et al.
1995). Undegraded MMT is not likely to be released directly to the atmosphere in significant quantities
from it intended use as a gasoline additive. Spectroscopic studies of the tailpipe emissions of
MMT-containing gasoline indicated that the manganese in MMT is converted to a mixture of solid
manganese oxides, sulfates, and phosphates. The manganese containing particulates were determined to
be Mn
3
O
4
, MnSO
4
·H
2
O and a divalent manganese phosphate, Mn
5
(PO
4
)[PO
3
(OH)]
2
·4H
2
O (Mölders et al.
2001; Ressler et al. 2000).
MANGANESE 397
6. POTENTIAL FOR HUMAN EXPOSURE
6.3.2.2 Water
Manganese in water may undergo oxidation at high pH or Eh (see Section 6.3.1) and is also subject to
microbial activity. For example, Mn(II) in a lake was oxidized during the summer months, but this was
inhibited by a microbial poison, indicating that the oxidation was mediated by bacteria (Johnston and
Kipphut 1988). The microbial metabolism of manganese is presumed to be a function of pH,
temperature, and other factors, but no data were located on this.
The rate of MMT degradation in natural aquifer and sediment systems was determined to be very slow
under anaerobic conditions (Garrison et al. 1995). Calculated half-lives ranged from approximately 0.2 to
1.5 years at 25 °C. However, MMT photolyzed rapidly in purified, distilled water exposed to sunlight.
The disappearance of MMT followed first-order kinetics, with a calculated half-life of 0.93 minutes.
Reaction products included methylcyclopentadiene, cyclopentadiene, carbon monoxide, and a manganese
carbonyl that readily oxidized to trimanganese tetroxide.
6.3.2.3 Sediment and Soil
The oxidation state of manganese in soils and sediments may be altered by microbial activity. Geering
et al. (1969) observed that Mn(II) in suspensions of silt or clay loams from several areas of the United
States was oxidized by microorganisms, leading to the precipitation of manganese minerals. Other
studies (Francis 1985) have shown that bacteria and microflora can increase the mobility of manganese in
coal-waste solids by increasing dissolution of manganese in subsurface environments.
MMT was found to be stable in a stream bottom sediment under anaerobic conditions. Photodegradation
of MMT is not likely to occur in sediments, and it may equilibrate between the sediment, sediment
porewater, and water column manganese (Garrison et al. 1995).
6.4 LEVELS MONITORED OR ESTIMATED IN THE ENVIRONMENT
Reliable evaluation of the potential for human exposure to manganese depends in part on the reliability of
supporting analytical data from environmental samples and biological specimens. Concentrations of
manganese in unpolluted atmospheres and in pristine surface waters are often so low as to be near the
limits of current analytical methods. In reviewing data on manganese levels monitored or estimated in the
environment, it should also be noted that the amount of chemical identified analytically is not necessarily
MANGANESE 398
6. POTENTIAL FOR HUMAN EXPOSURE
equivalent to the amount that is bioavailable. The analytical methods available for monitoring manganese
in a variety of environmental media are detailed in Chapter 7.
6.4.1 Air
Table 6-3 summarizes historic manganese air level data collected over a period of nearly 30 years from
numerous urban, nonurban, and source-dominated areas of the United States. Direct comparisons of data
from different time periods are complicated because of changes in sample collection and analytical
methodology. However, it is clear that manganese levels tend to be higher in source-dominated and urban
areas than in nonurban areas. These data also indicate that concentrations in all areas have tended to
decrease over the past three decades (EPA 1984; Kleinman et al. 1980). This decrease came as the result
of the installation of emission controls in the metals industry (EPA 1984, 1985d). A concurrent decrease
in total suspended particulates (TSP) was observed in most areas. Ambient air levels of manganese (PM
10
and PM
2.5
) in Canadian locations monitored from the late 1980s through the early 2000s were reported to
have a 13–77% reduction over that time period (Health Canada 2008). Annual averages of manganese in
urban and rural areas without significant manganese pollution are in the range of 10–70 ng/m
3
(0.01–
0.07 μg/m
3
) (WHO 1997). The daily intake of manganese in the air by the general population in areas
without manganese emitting industries was estimated to be <2 μg/day (WHO 1981). In areas with major
foundry facilities, intake may rise to 4–6 μg/day, and in areas associated with ferro- or silicomanganese
industries, it may be as high as 10 μg, with 24-hour peak values exceeding 200 μg/day (WHO 1981).
Data compiled for 2006 under the EPA Urban Air Toxics Monitoring Program, studied ambient air levels
of manganese and several other metals at 20 urban locations across the United States. Manganese (PM
10
)
was detected in 415 samples of urban air at levels ranging from 0.24 to 89.10 ng/m
3
(EPA 2007b). The
arithmetic mean, geometric mean, and median concentrations were 10.13, 6.68, and 6.29 ng/m
3
,
respectively. Manganese levels ranged from 0.85 to 614.00 ng/m
3
in 114 samples of total suspended
particulates (TSP) at these 20 urban locations. The arithmetic mean, geometric mean, and median
concentrations of manganese in TSP were 47.89, 22.39, and 23.98 ng/m
3
, respectively.
During 1988–1993, ambient concentration of fine (PM
2.5
) manganese ranged from 1 ng/m
3
(0.001 μg/m
3
)
in rural sites in U.S. National Parks to 3 ng/m
3
(0.003 μg/m
3
) in urban sites in California (Wallace and
Slonecker 1997). There is concern in Canada regarding the combustion of MMT as an important source
of manganese contamination in the urban environment, especially in areas of high traffic density. For
instance, Loranger and Zayed (1997a) reported significantly higher levels of both respirable and total
manganese levels at a high traffic density site (24 and 50 ng/m
3
, respectively) in Montreal in contrast to a
MANGANESE 399
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-3. Average Levels of Manganese in Ambient Air
a
Concentration (ng/m
3
)
Sampling location
1953–1957
1965–1967
1982
Nonurban
60
12
5
Urban
110
73
33
Source dominated
No data
250–8,300
130–140
a
Adapted from EPA 1984
MANGANESE 400
6. POTENTIAL FOR HUMAN EXPOSURE
low traffic density site (15 and 27 ng/m
3
, respectively). Temporal variation of respirable and total
manganese was similar for both sites, and atmospheric manganese concentrations reflected a positive
relationship with the traffic density. However, as discussed in Section 6.2.1, it has been difficult to assess
the exact contribution of the combustion of MMT by vehicles to manganese levels in the environment.
In Montreal, Canada, ambient atmospheric concentrations of MMT, and respirable and total manganese,
were measured in five microenvironments including a gas station, an underground car park, downtown
Montreal, near an expressway, and near an oil refinery (Zayed et al. 1999a). The overall mean
concentrations of respirable manganese, total manganese, and MMT measured for all the
microenvironments were 36, 103, and 5 ng/m
3
, respectively (0.036, 0.103, and 0.005 μg/m
3
); however,
the mean respirable manganese concentration 53 ng/m
3
(0.053 μg/m
3
) near the expressway was greater
than the EPA Reference Concentration (RfC) of 0.05 μg/m
3
.
The Canadian National Air Pollution Surveillance (NAPS) Program reported that average fine (PM
2.5
)
manganese levels from 2003 to 2005 in cities with industrial sources (Windsor and Hamilton) were 9–
15 ng/m
3
(Health Canada 2008). In Vancouver, Winnipeg, Montreal, Quebec, Toronto, and Edmonton,
the average levels were 4–14 ng/m
3
. In Saskatoon, Ottawa, Victoria, St. John, and background sites,
levels were <5 ng/m
3
. NAPS also reported manganese PM
10
levels were: 20–60 ng/m
3
in Hamilton and
Windsor; 8–25 ng/m
3
in Montreal, Toronto, Edmonton, Winnipeg, Quebec, Calgary, Vancouver, and
Victoria; and generally <10 ng/m
3
in Saskatoon, Ottawa, St. John, Yellowknife, and background sites
(Health Canada 2008).
Studies were conducted in Indianapolis, Indiana and Toronto, Canada to assess levels of PM
2.5
and PM
10
manganese in indoor, outdoor, and personal air samples (Pellizzari et al. 1999, 2001). The levels
observed in Toronto, where MMT had been used in gasoline for over 20 years, were approximately
2 times greater in indoor and outdoor air than in Indianapolis, where MMT was not being used as a
gasoline additive. The monitoring data from these studies are summarized in Table 6-4.
6.4.2 Water
Many factors, both environmental (e.g., the presence of high or low levels of other inorganics in drinking
water) and biological or host-related (e.g., age, nutritional status, and alcohol consumption) can
significantly influence the uptake of manganese by an individual (EPA 1993b). The determination of a

Table 6-4. Levels of PM
2.5
and PM
10
in Indoor and Outdoor Air in Toronto, Canada
and Indianapolis, Indiana
Median concentration 90
th
concentration
Location Number
(ng/m
3
) (ng/m
3
)
PM
10
Manganese
Toronto (indoor) 203 6.7 14
Indianapolis (indoor) 59 3.9 8.7
Toronto (outdoor) 203 17 28
Indianapolis (outdoor) 59 8.8 14
PM
2.5
Manganese
Toronto (indoor) 187 4.7 9.9
Indianapolis (indoor) 58 2.2 4.6
Toronto (outdoor) 185 8.6 16
Indianapolis (outdoor) 57 3.2 5.8
MANGANESE 401
6. POTENTIAL FOR HUMAN EXPOSURE
Sources: Pellizzari et al. 1999, 2001
MANGANESE 402
6. POTENTIAL FOR HUMAN EXPOSURE
single concentration of manganese in drinking water, then, must be recognized as a process that is limited
in its ability to reflect the variable nature of manganese toxicity (EPA 1993b).
Concentrations of manganese in surface water are usually reported as dissolved manganese. Although
total manganese may be a better indicator, since manganese adsorbed to suspended solids may exceed
dissolved manganese in many systems, the bioavailability of manganese in this form has not been
established (EPA 1984; NAS 1977). In a 1962–1967 survey of U.S. surface waters, dissolved manganese
was detected in 51% of 1,577 samples, at a mean concentration of 59 μg/L. Individual values ranged
from 0.3 to 3,230 μg/L. Mean concentrations for 15 different drainage basins in the United States ranged
from 2.3 μg/L in the western Great Lakes to 232 μg/L in the Ohio River drainage basin (Kopp and Kroner
1967). A later (1974–1981) survey of U.S. river waters reported a median dissolved manganese
concentration of 24 μg/L in samples from 286 locations, with values ranging from <11 μg/L (25th
percentile) to >51 μg/L (75th percentile) (Smith et al. 1987). Analyzing data available from the USGS
National Water Quality Assessment (NAWQA) database, the EPA reported that the median concentration
of manganese was 16 μg/L for surface water and 5 μg/L for groundwater from 20 watersheds and
16 drainage basins in the United States (EPA 2003a). The results of this analysis for all sites are
reproduced in Table 6-5. Reported mean groundwater concentrations of manganese were 20 and 90 μg/L
in an analysis of California shallow groundwater from two geologic zones (Deverel and Millard 1988).
Values up to 1,300 and 9,600 μg/L have been reported in neutral and acidic groundwater, respectively
(EPA 1984). Manganese levels of 9,500–18,600 μg/L have been reported in four private wells in
Connecticut (CDHS 1990). Natural concentrations of manganese in seawater reportedly range from
0.4 to 10 μg/L (EPA 1984).
A 1962 survey of public drinking water supplies in 100 large U.S. cities reported that 97% contained
<100 μg/L of manganese (USGS 1964). Similarly, a 1969 survey of 969 systems reported that 91%
contained <50 μg/L, with a mean concentration of 22 μg/L (U.S. DHEW 1970). Several other studies
reported similar manganese concentrations, with mean values ranging from 4 to 32 μg/L (EPA 1984;
NAS 1980a; WHO 1981). The EPA analyzed drinking water statistics from Alabama, California, Illinois,
New Jersey, and Oregon for occurrence and concentration data for manganese in public water supplies.
The data used contained >37,000 analytical results from about 4,000 public water supplies from 1993 to
1997, although some prior monitoring data were also employed in the analysis. The median manganese
level for all detections was 10 μg/L and the 99
th
percentile of the detections was 720 μg/L (EPA 2003a).

MANGANESE 403
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-5. Manganese Detections and Concentrations in Surface Water and
Groundwater in the United States
Detection frequency
Above the minimal
Above the health reference
reporting level (1 μg./L)
level
a
(300 μg/L)
Concentration (μg/L)
Samples
Sites
Samples
Sites
Median
99
th
Surface water
Urban
99.1%
99.6%
4.6%
13.0%
36
700
Mixed
92.4%
98.5%
1.3%
6.4%
12
400
Agricultural
96.3%
97.2%
3.7%
12.3%
19
700
Forest/rangeland
90.9%
96.4%
5.0%
6.6%
11
800
All sites
94.0%
96.9%
3.0%
10.2%
16
700
Groundwater
Urban
74.7%
85.3%
17.2%
21.0%
15
5,600
Mixed
56.9%
62.9%
8.9%
9.0%
2
1,300
Agricultural
61.4%
64.0%
11.9%
12.8%
4
1,600
Forest/rangeland
75.3%
81.3%
10.9%
13.8%
12
2,900
All sites
64.1%
70.1%
12.8%
13.8%
5
2,900
a
The Health Reference Level (HRL) is based on the dietary reference dose (RfD) and application of a modifying
factor (MF) of 3 for drinking water, and on an allocation of an assumed 20% relative source contribution from water
ingestion as opposed to total manganese exposure.
Source: EPA 2003a
MANGANESE 404
6. POTENTIAL FOR HUMAN EXPOSURE
6.4.3 Sediment and Soil
Manganese comprises about 0.1% of the earth's crust (Graedel 1978; NAS 1973), and manganese occurs
naturally in virtually all soils. Average natural ("background") levels of manganese in soils range from
around 40 to 900 mg/kg, with an estimated mean background concentration of 330 mg/kg (Barceloux
1999; Cooper 1984; Eckel and Langley 1988; EPA 1985c; Rope et al. 1988; Schroeder et al. 1987). The
maximum value reported was 7,000 mg/kg (Eckel and Langley 1988). Using data from 20 watersheds
and 16 drainage basins in the United States, manganese was detected at 100% of the National Water-
Quality Assessment Program (NAWQA) stream bed sediment sampling sites. The median and 99
th
percentile concentrations in bed sediments were reported as 1.1 mg/kg (dry weight) and 9.4 mg/kg (dry
weight), respectively (EPA 2003a). Manganese levels as high as 1,900 mg/kg were detected in sediment
samples obtained from the Tar Creek Superfund site (a site heavily contaminated with mining wastes) in
Ottawa County, Oklahoma (Wright et al. 2006).
Accumulation of manganese in soil usually occurs in the subsoil and not on the soil surface; 60–90% of
manganese is found in the sand fraction of the soil (WHO 1981). A preliminary survey was conducted in
Utah to provide an initial field measurement of the contamination by manganese oxides from exhaust in
roadside soil and plant species due to the addition of MMT to motor vehicle fuels. Soil (0–5 cm)
manganese concentrations were strongly correlated with distance from roadways with moderate and
moderately high traffic volumes (Lytle et al. 1994). In addition, exchangeable manganese was found to
be significantly higher in an organic soil located at stations with a high traffic density comparing to
another one with a low traffic density (Brault et al. 1994). The average soil manganese concentration
measured at 1 meter from a moderate to moderately-high traffic volume roadside was 3,046 μg/g dry
weight. At 15m, the average soil manganese concentration decreased to 254 μg/g dry weight.
6.4.4 Other Environmental Media
Manganese is a natural component of most foods. A summary of mean manganese concentrations in
foods analyzed by the Food and Drug Administration (FDA) Total Diet Study (TDS) 1991–1996 is
summarized in Table 6-6. TDS sampling is conducted 4 times annually, once in each of the major
geographical regions of the country (west, north central, south, and northeast). Each round of sampling is
referred to as an individual market basket survey and for each market basket survey, samples of
260 selected food and beverages were obtained from three cities within the region. The mean and median
concentration of manganese in all foods were 2.4 and 1.0 mg/kg, respectively (Capar and Cunningham

Table 6-6. Mean Concentrations of Manganese for FDA’s Total Diet Study Market
Baskets 1991 through 1997
a
Food product Range (mg/kg)
Milk and cheese Not detected–<2
Eggs <1
Meat, poultry, and fish Not detected–3.7
Legumes and nuts 3.4–23.2
Grain products <1–33.8
Fruit <1–10.0
Vegetables <1–5.9
Mixed dishes and meals <1–3.4
Desserts Not detected–4.9
Snacks 3.4–9.3
Condiments and sweeteners Not detected–4.1
Fats and dressings Not detected–<1
Beverages Not detected–2.9
Infant and junior foods Not detected–7.5
a
A < symbol indicates that manganese was detected, but at a level lower than the limit of quantification.
Source: Capar and Cunningham 2000
MANGANESE 405
6. POTENTIAL FOR HUMAN EXPOSURE
MANGANESE 406
6. POTENTIAL FOR HUMAN EXPOSURE
2000). The TDS results concluded that detectable levels of manganese were present in roughly 75% of all
foods, although approximately 24% of these detections were below the quantification limits used in the
study (Capar and Cunningham 2000). The highest manganese level was observed in a sample of
shredded wheat cereal (44.4 mg/kg). The five foods with the highest mean manganese levels were oat
ring cereal (33.8 mg/kg), raisin bran cereal (28.8 mg/kg), shredded wheat cereal (25.0 mg/kg), mixed nuts
(23.2 mg/kg), and granola cereal (20.1 mg/kg). These levels are similar to levels found in previous
market basket surveys (Pennington et al. 1986). Tea and leafy green vegetables were the major dietary
sources of manganese for young women taking part in a dietary study in Wisconsin (Davis et al. 1992a).
Bioaccumulation of manganese by plants was examined using oats (Avena nova) and beans (Phaseolus
vularis) (Brault et al. 1994). These plants were grown in sandy and organic soil at a control site
(greenhouse) and at two outdoor sites near <20,000 and 132,000 vehicles/day respectively. The highest
manganese accumulation was found in the fruits and stems of oats grown in the organic and sandy soils at
the station with the highest traffic density. Lönnerdal (1997) reported that infant formulas contain 30–
75 ppb (0.03–0.075 ppm) manganese, as compared to concentrations of 3–10 ppb (0.003–0.01 ppm) in
breast milk and 30 ppb (0.03 ppm) in cow's milk.
During a 1992 survey conducted by Canada’s Department of Fisheries and Oceans, concentrations of
manganese were detected in the muscle samples of bluefin tuna (Thunnus thynnus) (Hellou et al. 1992).
Concentrations of manganese in 14 samples of fish muscle ranged from 0.16 to 0.31 μg manganese/g dry
weight, with a mean of 0.22 μg/g. Although the analysis was administered with a high accuracy of 94%
using inductively coupled plasma-mass spectrometry (ICP-MS), the sample population was small.
In the field survey conducted by Lytle et al. (1994), terrestrial and aquatic plant samples were collected
along motorways and local urban roadways throughout Utah during 1992 and 1993. Manganese was
detected in the plant samples, with manganese concentrations ranging from 30.2 to 13,680 μg/g dry
weight. Manganese was detected in plants found nearest to the motorway. Loranger et al. (1994b)
evaluated the use of the pigeon as a monitor for manganese contamination from motor vehicles in urban
and rural areas of Canada, a country in which MMT has been used to replace lead in gasoline.
Manganese concentrations were similar in the two groups of pigeons for all tissues except the liver and
feces; urban pigeons had about 35% more manganese than rural ones. Loranger et al. (1994b) suggested
that although pigeon feces and liver may be good biomarkers of manganese contamination, it is premature
to associate the excess manganese with the combustion of MMT. Toxicokinetic studies of manganese in
both male and female rats suggested that MMT-derived manganese administered in oral doses resulted in
MANGANESE 407
6. POTENTIAL FOR HUMAN EXPOSURE
higher and more prolonged plasma concentration versus time profiles than inorganic (MnCl
2
) complexes,
leading to the conclusion that MMT-derived manganese was likely to accumulate following repeated
exposures (Zheng et al. 2000).
6.5 GENERAL POPULATION AND OCCUPATIONAL EXPOSURE
Since manganese is ubiquitous in the environment, the general population is exposed to manganese from
both natural and anthropogenic sources. The manganese concentration in blood of healthy adults is
reported to range from 4 to 15 μg/L with an average value of about 9 μg/L (Barceloux 1999). Typical
daily human exposure levels to manganese from water, air, and food are summarized in Table 6-7 (EPA
1984). As the table illustrates, the most significant exposure for the general population is from food. The
average daily intake for a 70-kg adult was estimated as 3,800 μg/day (EPA 1984). Other estimates of
daily intake for adults range from 2,000 to 8,800 μg (EPA 1984; NAS 1977; Patterson et al. 1984;
Pennington et al. 1986; WHO 1984a) and 700–10,900 μg/day (WHO 2004b). Even though
gastrointestinal absorption of manganese is low (3–5%), oral exposure is the primary source of absorbed
manganese.
Manganese intake among individuals varies greatly, depending upon dietary habits. For example, an
average cup of tea may contain 0.4–1.3 mg of manganese (Pennington et al. 1986; Schroeder et al. 1966).
Thus, an individual consuming three cups of tea per day might receive up to 4 mg/day from this source
alone, increasing the average intake from all dietary sources.
As part of the Third National Health and Nutrition Examination Survey (NHANES) conducted by the
Centers of Disease Control and Prevention (CDC), manganese was detected at quantifiable levels in urine
samples from 73% of 496 participants of the monitoring study (Paschal et al. 1998). The mean urinary
manganese concentration in these 496 individuals (aged 6–88 years of age) was 1.19 μg/L (Paschal et al.
1998).
The EPA Reference Dose (RfD)/RfC workgroup in June 1990 set an RfD for manganese in food of
0.14 mg manganese/kg/day, equivalent to about 10 mg/day for a 70-kg man based on chronic manganese
uptake (EPA 1993b). The Food and Nutrition Board of the National Research Council (NRC) estimated
the adequate and safe intake of manganese for adults at 2–5 mg/day (NAS 1980b). This level was chosen
because it includes an "extra margin of safety" of 5 mg/day below the level of 10 mg/day, which the NRC
considered to be safe for occasional intake (IRIS 2011).
Table 6-7. Summary of Typical Human Exposure to Manganese
a
Exposure medium
Parameter Water Air Food
Typical concentration in medium 4 μg/L 0.023 μg/m
3
1.28 μg/calories
Assumed daily intake of medium by 70-kg adult 2 L 20 m
3
3,000 calories
Estimated average daily intake by 70-kg adult 8 μg 0.46 μg
b
3,800 μg
Assumed absorption fraction 0.03
c
1
c
0.03
d
Approximate absorbed dose 0.24 μg 0.46 μg 114 μg
a
Adapted from EPA 1984
b
Assumes 100% deposition in the lungs
c
No data; assumed value
d
Vitarella et al. 2000
MANGANESE 408
6. POTENTIAL FOR HUMAN EXPOSURE
MANGANESE 409
6. POTENTIAL FOR HUMAN EXPOSURE
A large-scale population-based exposure study was conducted from June 1995 through August 1996 in
Toronto, Canada, a city with widespread use of MMT, to estimate personal exposures to particulate
matter (PM
2.5
and PM
10
) and manganese contained in particulates (Lynam et al. 1999; Pellizzari et al.
1999). In addition to personal samples, air samples were also collected at indoor and outdoor residential
sites as well as ambient levels at two fixed locations and a rooftop. Correlations between manganese in
personal samples and the other environmental samples were calculated. The correlation coefficients for
manganese in personal samples with residential indoor air and outdoor air were 0.56 and 0.49,
respectively (Pellizzari et al. 1999). Correlations at the fixed sites were lower, but considered statistically
significant. The estimated 3-day personal exposure distributions for the population (n=922) are
summarized in Table 6-8. Additional regression and correlation analysis of the Toronto study was
performed by Crump (2000) to further elucidate the role that MMT and other environmental factors play
in personal exposure to manganese in this population. Subgroups of the population were identified that
could have potentially significant manganese exposures from various sources. It was determined that
subway riders, metal workers, and persons exposed to tobacco smoke (smokers and those exposed to
environmental tobacco smoke) had the highest potential personal exposure to manganese. After
eliminating these groups from the population, the mean personal PM
2.5
manganese exposure declined
approximately 40%. The remaining personal exposure to manganese in the Toronto population study is
from a variety of naturally occurring and anthropogenic sources. Citing data suggesting that there are
several non-MMT-related sources of manganese in ambient air and that manganese levels in both
personal samples and fixed site samples were negatively correlated with MMT levels in gasoline, Crump
(2000) concluded that most of the personal exposure to manganese from the Toronto sample group was
from sources other than MMT in gasoline.
An exposure analysis similar in design to the Toronto study was conducted in Indianapolis, Indiana where
MMT was not being used as a gasoline additive at the time of the study (Pellizzari et al. 2001). A smaller
personal sample size (n=240) was obtained in the Indianapolis data set as compared to the Toronto study.
In general, manganese levels in indoor air, outdoor air, and personal samples were substantially lower in
Indianapolis as compared to Toronto. For example, the median and mean levels for personal manganese
exposure were 2.8 and 7.5 ng/m
3
, respectively, in Indianapolis and 8.0 and 13.1, ng/m
3
, respectively, in
Toronto. Similar to the Toronto study, tobacco smokers and workers occupationally exposed to
manganese tended to have higher personal exposure levels than nonsmokers and non-occupationally
exposed individuals. For non-occupationally exposed individuals in Toronto, the greatest correlation
between manganese personal exposure and environmental factors was the amount of time traveling by

Table 6-8. Estimated 3-Day PM
2.5
Manganese Exposure Distribution for a
Population (n=922) in Toronto, Canada
a
25
th
50
th
75
th
90
th
95
th
99
th
Geometric 10
th
Mean mean percentile percentile percentile percentile percentile percentile percentile
13.1 8.3 3.9 5.5 8.0 12.0 16.9 23.0 47.3
a
Mn concentration in ng/m
3
.
Source: Pellizzari et al. 1999
MANGANESE 410
6. POTENTIAL FOR HUMAN EXPOSURE
MANGANESE 411
6. POTENTIAL FOR HUMAN EXPOSURE
subway; however, Indianapolis does not have a subway system, so a similar comparison cannot be made
for this study. For the non-occupationally exposed subgroups with no exposure to tobacco smoke and no
subway riders, the median personal manganese exposure was 2.6 ng/m
3
in Indianapolis and 7.8 ng/m
3
in
Toronto.
Sierra et al. (1995) conducted a study using 35 automotive workers in Montreal, Canada suspected of
being exposed to high levels of manganese from MMT and 30 nonautomotive workers (control group).
Exposure to manganese was measured for 5 consecutive working days. In addition, the workers’
environmental exposure at home was measured on 2 days of the same week. Air sampling was performed
by portable personal pumps; for sampling at homes, workers were asked to wear the pumps as much as
possible. At the workplace, the mechanics were exposed to manganese concentrations ranging from
0.010 to 6.673 μg/m
3
(10–6,673 ng/m
3
) with a mean of 0.45 μg/m
3
(450 ng/m
3
), while nonautomotive
workers were exposed to manganese concentrations ranging from 0.011 to 1.862 μg/m
3
(11–1,862 ng/m
3
).
The mean concentration was 0.04 μg/m
3
(40 ng/m
3
). The average manganese concentrations in the indoor
air of the homes were 0.012 μg/m
3
(120 ng/m
3
) for the mechanics and 0.008 μg/m
3
(8 ng/m
3
) for the
nonautomotive workers (Sierra et al. 1995). Based on measurements of manganese particle size
distributions, Sierra et al. (1995) estimated that <10% of the manganese exposure of the garage mechanics
was due to MMT; however, the exact contribution of MMT could not be determined.
A similar study conducted in Montreal by these investigators, but involving taxi drivers and garage
mechanics, indicated that garage mechanics at work were exposed to an average of 0.250 μg/m
3
(250 ng/m
3
) and taxi drivers to 0.024 μg/m
3
(24 ng/m
3
) (Zayed et al. 1994). In another study, exposure of
office workers and taxi drivers to both respirable and total manganese was evaluated (Zayed et al. 1996).
Manganese concentrations measured for the office workers ranged from 0.001 to 0.034 μg/m
3
(1–
34 ng/m
3
respirable manganese) and from 0.002 to 0.044 μg/m
3
(2–44 ng/m
3
total manganese). For the
taxi drivers, the manganese concentrations ranged from 0.007 to 0.032 μg/m
3
(7–32 ng/m
3
respirable
manganese) and from 0.008 to 0.073 μg/m
3
(8–73 ng/m
3
total manganese). Zayed et al. (1996) concluded
that the higher exposure to atmospheric manganese in the outdoor urban environment may be at least
partly due to the use of MMT in cars. Nevertheless, these investigators indicated that the exposures of
taxi drivers to manganese were well below existing exposure and health guidelines.
In order to assess the potential health risks from MMT combustion, Loranger and Zayed (1995)
conducted a multi-media assessment (i.e., food, water, and ambient air) of manganese exposure in
two groups of workers (garage mechanics and blue-collar workers not involved in automotive repair)
MANGANESE 412
6. POTENTIAL FOR HUMAN EXPOSURE
potentially exposed to different levels of manganese from MMT. Garage mechanics were exposed to
higher air manganese concentrations (0.42 μg/m
3
) than nonautomotive blue-collar workers (0.04 μg/m
3
).
However, for the garage workers, exposure to atmospheric manganese represented only approximately
4% of the total absorbed dose, while ingestion of food represented 95.7% of the total multi-media dose.
For the blue collar workers, atmospheric manganese contributed only 0.3% to the total absorbed dose,
whereas ingestion of food represented 99.2% of the total multi-media dose. These results were consistent
with values of multi-media doses predicted by GADUS, an environmental fate/exposure model (Loranger
and Zayed 1997b). Based on governmental standards or criteria for occupational and environmental
exposures, Loranger and Zayed (1995) concluded that the manganese levels in food and air may not cause
any problems for these workers.
In the workplace, exposure to manganese is most likely to occur by inhalation of manganese fumes or
manganese-containing dusts. This is a concern mainly in the ferromanganese, iron and steel, dry-cell
battery, and welding industries (WHO 1986). Exposure may also occur during manganese mining and
ore processing; however, the most recent data indicate that only a very small amount of manganese is still
mined in the United States (USGS 2007). Excluding insignificant quantities of similar low-grade
manganiferous ore, the United States has not mined significant amounts of manganese since 1978 and
now relies on imports to fill its needs (USGS 2007). In 1980, it was estimated that in the United States
about 300 workers were exposed to pure manganese and about 630,000 workers were exposed to other
forms of manganese (NOES 1989). Concentrations as large as 1.5–450 mg manganese/m
3
have been
reported in U.S. manganese mines (EPA 1984), 0.30–20 mg manganese/m
3
in ferroalloy production
facilities (Saric et al. 1977), and 3–18 mg manganese/m
3
in a dry-cell battery facility (Emara et al. 1971).
Steel-manufacturing facilities are significant employers in the United States. There is a potential for
manganese exposure to workers in these facilities. Airborne manganese levels in a metal-producing plant
in the United States were reported as 0.066 mg/m
3
(mean), 0.051 mg/m
3
(median) as respirable dust, and
0.18 mg/m
3
in total dust (Gibbs et al. 1999). Exposure levels should not exceed the Occupational Safety
and Health Administration (OSHA) time-weighted average Permissible Exposure Limit (PEL) of 1 mg
total manganese/m
3
(see Table 8-1). Average airborne manganese levels during welding operations of
two factories located in China were 0.24 and 2.21 mg/m
3
(Wang et al. 2008). Manganese levels in
workplace air at a smelting facility in China ranged from 0.30 to 2.9 mg/m
3
in the furnace smelting area
and from about 0.2 to 0.8 mg/m
3
in a power control room (Jiang et al. 2007). The workplace air at this
facility contained mainly MnO (20%) and SiO
2
(22%), in addition to other trace metals including
Fe
2
O
3
(4%), CaO (4.5%), MgO (4%), and Al
2
O
3
(5%). Thus, for workers in industries using manganese,
the major route of exposure may be inhalation from workplace air rather than from ingestion of food.
MANGANESE 413
6. POTENTIAL FOR HUMAN EXPOSURE
6.6 EXPOSURES OF CHILDREN
This section focuses on exposures from conception to maturity at 18 years in humans. Differences from
adults in susceptibility to hazardous substances are discussed in Section 3.7, Children’s Susceptibility.
Children are not small adults. A child’s exposure may differ from an adult’s exposure in many ways.
Children drink more fluids, eat more food, breathe more air per kilogram of body weight, and have a
larger skin surface in proportion to their body volume. A child’s diet often differs from that of adults.
The developing human’s source of nutrition changes with age: from placental nourishment to breast milk
or formula to the diet of older children who eat more of certain types of foods than adults. A child’s
behavior and lifestyle also influence exposure. Children crawl on the floor, put things in their mouths,
sometimes eat inappropriate things (such as dirt or paint chips), and spend more time outdoors. Children
also are closer to the ground, and they do not use the judgment of adults to avoid hazards (NRC 1993).
Children would be exposed to manganese in the same manner as adults. The main source of exposure of
children to manganese is through food. Infants and young toddlers who are formula-fed may receive
higher daily intakes of manganese than breast-fed infants because of the increased levels of the element in
infant formulas as compared to breast milk (Collipp et al. 1983; Cook 1997; Dorner et al. 1989; Keen et
al. 1986; Lönnerdal et al. 1983, 1994). For example, a study of 2,339 breast milk samples obtained from
nursing mothers in Germany had a mean manganese level of 6.2 μg/L, while two different brands of
formula had levels of 77 and 99 μg/L (Dorner et al. 1989). It was concluded that the mean daily
manganese intake of formula-fed infants was approximately 13 times greater than that of breast-fed
infants.
Manganese concentrations in blood serum of children of different ages are provided in Section 3.4.2. The
data indicate that manganese concentrations decrease slightly from the time the infant is 5 days of age
until he or she is 12 months of age (Alarcón et al. 1996; Rükgauer et al. 1997). Manganese
concentrations increase after this time, and they have been measured as an average of 1.4±1.25 μg/L in
children aged 1 month to 18 years (Rükgauer et al. 1997).
Children are exposed in utero because manganese in maternal blood crosses the placenta to satisfy the
fetus’s need for manganese. The compound has been measured in cord blood plasma of premature and
full-term infants and their mothers (Wilson et al. 1991). Full-term babies had higher (but not statistically
MANGANESE 414
6. POTENTIAL FOR HUMAN EXPOSURE
significantly different) blood concentrations of manganese than premature babies, and pregnant women
had higher blood concentrations than nonpregnant women. The average manganese concentration in the
cord blood of full term babies was 5.5 μg/L, as compared to 5.0 μg/L for preterm babies (Wilson et al.
1991). No correlations were observed between maternal and infant concentrations of manganese. The
arithmetic mean (standard deviation) manganese concentrations in maternal and cord blood obtained from
female volunteers at a hospital in France were reported as 10.5±4.1 and 31.2±13.4 μg/L, respectively
(Abdelouahab et al. 2010). Monoamine oxidase activity (MAO), which is known to be influenced by
metals in experimental studies, was shown to be a useful biomarker in humans for manganese exposure.
MAO activity was significantly positively correlated with maternal and cord blood manganese
concentrations in subjects with high MAO activity.
Manganese in breast milk has been found to range from 3.4 to 10 μg/L (Arnaud and Favier 1995; Collipp
et al. 1983) depending on the maturity of the milk. The Food and Nutrition Board of the NRC based the
recommended manganese intake of infants on the analyses of pooled human milk samples. As discussed
above, manganese intakes of infants fed some formulas appear high, but no signs of toxicity have been
observed (Dorner et al. 1989; Lönnerdal et al. 1983). Differences in weight-adjusted intake are likely to
be caused by the type of diet that infants and small children receive. It is unknown whether nursing
mothers exposed to higher-than-average concentrations of manganese would excrete increased
concentrations of the metal in their breast milk.
Young children often eat dirt (exhibiting what is called soil pica, the ingestion of a material unfit for food)
and exhibit frequent hand-to-mouth activity; they can be exposed to manganese through this unique
pathway if the soils contain the metal. Current estimates indicate that soil pica may be more prevalent in
the general population than previously thought and that most children periodically ingest soil to varying
degrees; this may be a potential health concern (EPA 1986d; Stanek and Calabrese 1995). The predicted
oral average daily intake of manganese for children from soils in the vicinity of a municipal solid waste
incinerator was estimated to range from approximately 0.0021 to 0.0032 mg/kg/day (Mari et al. 2007).
However, no information was found concerning the bioavailability of manganese from soil and, therefore,
determining the actual risk posed to children from this exposure pathway is difficult. This behavior
should not pose an increased risk of exposure to manganese in most residential situations where the
manganese levels are in the normal or background range. If the soils are from a hazardous waste site that
contains high concentrations of manganese, then increased exposure to the compound may occur.
Manganese levels in hair samples of 32 children residing near a hazardous waste site (former mining
facility) in Northeast Oklahoma ranged from 89.1 to 2,145.3 ppb (471.5 ppb mean) (Wright et al. 2006).
MANGANESE 415
6. POTENTIAL FOR HUMAN EXPOSURE
The authors found that in school-aged children, higher manganese and arsenic levels in hair samples were
associated with significantly lower scores on a standardized test, as well as on tests of verbal learning and
memory.
Children who suffer from cholestatic liver disease or who have gastrointestinal disorders that mandate
they be given parenteral nutrition may be at increased risk from overexposure to manganese. Increased
manganese concentrations in blood and brain, and symptoms of neuromotor dysfunction were observed in
an 8-year-old girl with cholestatic liver failure (Devenyi et al. 1994). Children with or without chronic
liver disease and a 5-year-old boy who had gastrointestinal disorders, all of whom were administered
parenteral nutrition, had abnormal MRI scans indicative of manganese accumulation (Fell et al. 1996;
Ono et al. 1995) accompanied by motor disorders (Fell et al. 1996).
Because manganese is a trace element that is essential for normal human health and is predominantly
obtained from food, it is unlikely that toxic amounts of manganese will be absorbed from food. However,
diets vary and some are higher in manganese than others (diets high in grains and tea, for instance). One
case study suggested that a 59-year-old man developed manganism-like symptoms from abusing vitamins
and minerals. This man had very high manganese concentrations in blood, urine, feces, hair, and brain
(Banta and Markesbery 1977). Both manganese and iron are bound by transferrin and these elements
compete for the binding protein in the body. Therefore, diets that are low in iron allow transferrin to bind
more manganese. For this reason, it is important to provide children with a balanced diet to maintain
optimal iron and manganese stores in the body. Studies show that adults absorb only 3–5% of manganese
ingested from the diet (Davidsson et al. 1988, 1989a; Mena et al. 1969); infants have increased absorption
relative to adults (Dorner et al. 1989). Neonatal animals also exhibit increased absorption relative to older
animals (Ballatori et al. 1987; Miller et al. 1975; Rehnberg et al. 1981).
Children may be exposed to organic manganese compounds through a variety of routes. They may be
exposed to MMT combustion products via inhalation of these products in air, or ingestion of them after
deposition on the soil.
6.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES
As discussed in Section 6.5, workers in industries using or producing manganese are mostly likely to have
higher exposures to manganese, primarily by inhalation of manganese dusts in workplace air as compared
to the general population. In a year-long investigation of personal exposure to manganese fine particulate
MANGANESE 416
6. POTENTIAL FOR HUMAN EXPOSURE
matter (PM
2.5
) for residents of Toronto, Canada, it was determined that workers in the metal industry had
the highest personal exposures as compared to other groups. The mean concentration of manganese PM
2.5
in personal samples for 39 workers engaged in welding, soldering, or other metal working practices was
105 ng/m
3
, which was more than 10 times greater than the mean concentration (10 ng/m
3
) for 886 non-
metal workers (Crump 2000). Smokers and those nearby second-hand smoke were also shown to be
exposed to higher levels of fine particulate matter manganese as compared to nonsmokers. The mean
concentration of PM
2.5
manganese in 702 personal air samples of nonsmokers in Toronto, Canada was
10 ng/m
3
, while the mean concentration calculated from 223 personal samples obtained from smokers
was 27 ng/m
3
(Crump 2000). A positive correlation was observed between personal manganese exposure
and subway travel in Toronto, presumably due to the erosion of the steel wheels and subway tracks
(Crump 2000; Pellizzari et al. 1999).
Average airborne manganese levels (total dust) in the breathing zone of two factories located in China
were 0.24 and 2.21 mg/m
3
(Wang et al. 2008). The greatest levels were observed during welding
operations in enclosed spaces. The workers at these two factories had higher measurable manganese
levels in their saliva (3.47±1.42 and 5.55±2.31 μg/L), as compared to a control group of non-
occupationally exposed individuals (3.04±1.40 μg/L).
Workers in three manganese alloy production plants located in Norway were found to have slightly higher
manganese blood and urine levels when compared to a group of non-occupationally exposed individuals.
The arithmetic mean manganese level in the blood of workers at these plants was 189 nmol/L (10.3 μg/L)
versus 166 nmol/L (9.1 μg/L) for the reference group (Ellingsen et al. 2003c). The urinary arithmetic
mean concentrations were 3.9 nmol/mmol creatinine for the occupationally exposed workers and
0.9 nmol/mmol creatinine for the reference group (Ellingsen et al. 2003c). The arithmetic mean inhalable
and respirable concentrations of manganese in the air of these production plants were 0.769 and
0.064 mg/m
3
, respectively (Ellingsen et al. 2003c). Section 3.2.1.4 summarizes other studies that
compared noted health effects with urinary and blood manganese levels of occupationally exposed
individuals and reference populations. It has been demonstrated that levels in the blood and urine may
not be adequate biomarkers for high level manganese exposure since free manganese usually does not
accumulate within the circulatory system (Josephs et al. 2005).
Populations living in the vicinity of ferromanganese or iron and steel manufacturing facilities, coal-fired
power plants, or hazardous waste sites may also be exposed to elevated manganese particulate matter in
MANGANESE 417
6. POTENTIAL FOR HUMAN EXPOSURE
air or water, although this exposure is likely to be much lower than in the workplace. Populations living
in regions of natural manganese ore deposits may be exposed to above-average levels in soil, water, or air.
People ingesting large amounts of foods high in manganese also have a potential for higher-than-usual
exposure. Included in this group would be vegetarians, who ingest a larger proportion of grains, legumes,
and nuts in their diets than the average U.S. population, and heavy tea drinkers. While the intake of
manganese from vegetarians may exceed the estimates of daily dietary intake, the bioavailability of
manganese from vegetable sources is substantially decreased by dietary components such as fiber and
phytates (EPA 1993b). In addition to the population with these dietary habits, individuals with iron
deficiency show increased rates of manganese absorption (Mena et al. 1969, 1974); iron deficiency leads
to increased brain manganese concentrations in experimental animals (Aschner and Aschner 1990).
Manganese is eliminated from the body primarily through the bile. Interruption of the manufacture or
flow of bile can impair the body’s ability to clear manganese. Several studies have shown that adults and
children (Devenyi et al. 1994; Fell et al. 1996; Hauser et al. 1994, 1996; Pomier-Layrargues et al. 1998;
Rose et al. 1999; Spahr et al. 1996), as well as experimental animals (Rose et al. 1999), with cholestatic
liver disorders have increased manganese levels in their blood and brain and are at risk from potentially
increased exposure to manganese due to their decreased homeostatic control of the compound.
In addition to oral diets, people on partial and total parenteral nutrition may be exposed to increased
amounts of manganese. Forbes and Forbes (1997) found that of 32 patients receiving home parenteral
nutrition due to digestive problems, 31 had elevated serum manganese levels (0.5–2.4 mg/L). It is unclear
whether these levels reflected steady-state conditions due to the time the samples were taken. However,
these levels are much higher than other studies involving patients on TPN; thus, it is unlikely that these
levels represent steady-state conditions. Further, the normal range reported by these authors (0.275–
0.825 mg/L) is elevated compared to other studies, suggesting the possibility that the blood samples were
contaminated with exogenous manganese. The authors observed no clinical evidence of toxicity in the
patients. Fourteen of the patients suffered iron deficiency anemia; because low iron concentrations are
associated with increased manganese uptake, the anemia may have exacerbated the increased blood
manganese concentrations. Increased blood manganese levels and MRI scans indicative of increased
manganese in brains have been reported in children fed entirely on parenteral nutrition (Fell et al. 1996;
Ono et al. 1995). Only in the Fell et al. (1996) study were neurotoxic effects reported. Whole-blood
manganese in the children from this study ranged from 9.9 to 110 μg/L. Devenyi et al. (1994) found
hyperintense signals in the brain of an 8-year-old child who had cholestatic liver disease and exhibited
MANGANESE 418
6. POTENTIAL FOR HUMAN EXPOSURE
dystonia and other motor dysfunctions. Nagatomo et al. (1999) reported that two elderly patients who had
been administered TPN for 3–4 months exhibited clinical signs of manganism (including masked facies,
marked rigidity, hypokinesia) with associated elevated blood manganese levels and hyperintense signals
on MRI, localized to the basal ganglia, especially the globus pallidus. Signs of manganism abated upon
levodopa treatment and the administration of Ca-EDTA; the high intensity signals on MRI abated when
manganese supplementation ceased. In addition to patients on parenteral nutrition, uremic patients on
hemodialysis have been found to have increased manganese levels due to increased concentrations of
manganese in the dialysis solution (Lin et al. 1996). These studies indicate that while increased levels of
manganese in blood and brain are often associated with TPN administration, adverse neurological effects
are not always reported. Nagatomo et al. (1999) found increased serum concentrations of manganese and
brain abnormalities in two patients who showed Parkinsonism with psychiatric symptoms after 3–
4 months of total parenteral nutrition. Discontinuation of manganese supplementation in the parenteral
diet, coupled with levodopa treatment, gradually improved both the symptoms and brain abnormalities in
the patients.
In comparison to other groups within the general population, persons living close to high density traffic
areas, automotive workers, gas station attendants, and taxi drivers may be exposed to higher
concentrations of manganese arising from the combustion of MMT. Levels of respirable manganese, in
both indoor and outdoor air near an expressway with high traffic density were shown to be greater than
corresponding air samples obtained from a rural location in Montreal, Canada (Bolte et al. 2004). The
average concentration of respirable manganese (defined in this study as <5 μm diameter) in outdoor air
from the urban location of Montreal was 0.025 μg/m
3
, which is 5 times greater than the average of
0.005 μg/m
3
found in the rural location. The average indoor respirable manganese concentration was also
greater for the urban area (0.017 μg/m
3
) as compared to the rural area (0.007 μg/m
3
). However,
differences in exposure levels did not lead to significantly greater levels of manganese in blood for
residents of these areas. The mean manganese concentration in blood samples obtained from female
residents in the urban location (8.4 μg/L) was only slightly greater than the average level observed for
females living in the rural location (7.8 μg/L).
It is possible that medical workers may be exposed to higher concentrations of mangafodipir than the
general population, although exposure routes other than intravenous are not expected to pose a significant
risk.
MANGANESE 419
6. POTENTIAL FOR HUMAN EXPOSURE
6.8 ADEQUACY OF THE DATABASE
Section 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of manganese is available. Where adequate information is not
available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of research
designed to determine the health effects (and techniques for developing methods to determine such health
effects) of manganese.
The following categories of possible data needs have been identified by a joint team of scientists from
ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would
reduce the uncertainties of human health assessment. This definition should not be interpreted to mean
that all data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
6.8.1 Identification of Data Needs
Physical and Chemical Properties. The fundamental physical and chemical properties of
manganese and manganese compounds are known (see Table 4-2), and additional research does not
appear necessary.
Production, Import/Export, Use, Release, and Disposal. According to the Emergency
Planning and Community Right-to-Know Act of 1986, 42 U.S.C. Section 11023, industries are required
to submit substance release and off-site transfer information to the EPA. The TRI, which contains this
information for 2009, became available in March of 2011. This database is updated yearly and should
provide a list of industrial production facilities and emissions.
Information is available on U.S. import, export and production of manganese ore and related materials
(USGS 2007, 2008). It is clear that most manganese is used in steel production. Information regarding
the import, export, and use of MMT in U.S. fuels is a data need.
Data from the TRI database provide valuable information on the amounts of manganese released to
different environmental media (e.g., air, soil, and water) each year, although details on the chemical form
and physical state of the waste materials are not included. These disposal practices are not regulated
MANGANESE 420
6. POTENTIAL FOR HUMAN EXPOSURE
under current federal law. TRI data may not be complete estimates of total release. Also, because these
data reflect past releases, they may not be representative of current releases at these facilities.
Environmental Fate. The partitioning of manganese between water and soil can be fairly well
predicted using thermodynamic equilibrium concepts, if soil-specific information is available (Baes and
Sharp 1983; Rai et al. 1986). The fate of manganese particles released into the air is determined by the
particle size, and the direction and distance of particle transport at a site can be predicted from
meteorological data and particle size data (EPA 1984; Nriagu 1979). Transport of manganese in water is
determined mainly by the solubility of the manganese compounds present, although suspended particles
may also be transported in flowing waters (EPA 1984; Schaanning et al. 1988).
The primary transformations that manganese undergoes in the environment are oxidation/reduction
reactions (EPA 1984; Rai et al. 1986). Reactions of manganese with airborne oxidants have not been
studied. Information on the rate and extent of such reactions would be helpful in understanding the fate
of atmospheric releases. The transformation of manganese in water or soil is dependent mainly on Eh,
pH, and available counter ions (EPA 1984). In some soils, manganese may also be oxidized by bacteria
(Geering et al. 1969; Johnston and Kipphut 1988). More work is needed on the environmental factors,
such as soil composition and pH, which may determine the form in which manganese will appear and thus
impact manganese availability and absorption.
Modeling has also provided interesting insight into the contribution of the combustion of MMT to
atmospheric manganese (Loranger et al. 1995). According to the model estimations, the contribution of
direct emissions from motor vehicles to the atmospheric background manganese (as measured from
sampling stations) would be about 50% at <25 m and <8% at 250 m. These results are confirmed with an
in situ study using snow as the environmental indicator where the average deposition rates of manganese
for the top and bottom layers ranged from 0.01 to 0.21 mg/m
2
/day (Loranger et al. 1996). The average
concentrations of manganese decreased with distance from the road. However, it was impossible to
distinguish between directly-emitted manganese from automobiles, manganese enriched road dust, and
the naturally-occurring manganese in crustal materials. No study to date has provided the complete
answer to this question and this constitutes one of the major remaining data needs regarding the
environmental significance of manganese from MMT and the resulting potential for exposure.
Bioavailability from Environmental Media. Manganese is known to be absorbed following
inhalation or oral exposure (Mena et al. 1969; Pollack et al. 1965; Zheng et al. 2000), but dermal exposure
MANGANESE 421
6. POTENTIAL FOR HUMAN EXPOSURE
is not considered to be significant. The uptake of manganese from air, food, milk, and water has been
studied (Davidsson et al. 1988, 1989a). However, absorption from soil has not been investigated. In
view of the potential for tight binding of manganese to some soil types, studies on this subject would be
valuable in evaluating risk to humans, especially children who may ingest contaminated soils near
hazardous waste sites. Additional information would also be valuable on the relative bioavailability of
different manganese compounds across various environmental media.
Food Chain Bioaccumulation. It has been established that while lower organisms (e.g., plankton,
aquatic plants, and some fish) can significantly bioconcentrate manganese, higher organisms (including
humans) tend to maintain manganese homeostasis (EPA 1984; Folsom et al. 1963; Thompson et al.
1972). This indicates that the potential for biomagnification of manganese from lower trophic levels to
higher ones is low, and it does not appear that additional research in this area is essential at this time.
Exposure Levels in Environmental Media. Reliable monitoring data for the levels of manganese
in contaminated media at hazardous waste sites are needed so that the information obtained on levels of
manganese in the environment can be used in combination with the known body burden of manganese to
assess the potential risk of adverse health effects in populations living in the vicinity of hazardous waste
sites.
Manganese levels have been monitored in all environmental media, including air, water, soil, and food
(Capar and Cunningham 2000; EPA 1984; NAS 1980a; Pennington et al. 1986). Estimates are available
for the average human intake levels of manganese from water, air, and food (EPA 1984; WHO 2004b).
More specific data on levels in the environment around those particular sites where manganese is believed
to have been dumped would be helpful in determining the extent of exposure levels around such waste
sites. In particular, data on the concentration of manganese in the air around hazardous waste sites would
be valuable in assessing the potential significance of this exposure pathway.
Exposure Levels in Humans. This information is necessary for assessing the need to conduct
health studies on these populations. Manganese is a normal component of human tissues and fluids
(Sumino et al. 1975; Tipton and Cook 1963). Increased average levels of manganese have been detected
in blood and urine of populations exposed to high concentrations of manganese in the workplace (Roels
et al. 1987b). Manganese has been measured in hair samples of children residing near a hazardous waste
site (Wright et al. 2006); however, the absence of data on levels of manganese in the hair of U.S. children
MANGANESE 422
6. POTENTIAL FOR HUMAN EXPOSURE
in the general population makes it difficult to draw conclusions about whether the exposures of the
children at this site are unusually high. Surveys of manganese levels in the blood or urine of populations
living near waste sites could be useful in identifying groups with above-average levels of manganese
exposure. More information is also needed to determine whether iron-deficient populations have a higher
manganese body burden. Manganese and iron have many physico/chemical similarities and there is a
possibility of competition between these elements. Increased manganese concentrations have been shown
to inhibit the metabolic function of the iron-dependent enzyme, aconitase (Zheng et al. 1998). Iron
deficiency is the single most prevalent nutritional deficiency in the world, and so the potential health risk
associated with iron deficiencies exacerbating the brain manganese burden may represent a crucial issue
of exposure and susceptibility, and has yet to be evaluated. Air concentrations in areas with high traffic
density are sometimes higher than the guide level (Zayed et al. 1999a); therefore, some individuals could
be at risk. Research focusing on the environmental level of exposure of certain groups of the population,
such as those living near a major highway, is needed.
This information is necessary for assessing the need to conduct health studies on these populations.
Exposures of Children. Children are exposed daily to manganese. The compound is an essential
trace element vital for the body to function properly and body burden studies are available (Alarcón et al.
1996; Rükgauer et al. 1997). Although the primary pathway for exposure is the diet, studies involving
exposures to airborne manganese (e.g., in dust that may be present at a nearby hazardous waste site or
manganese-processing plant) would aid in understanding other pathways that may contribute significantly
to children’s total body burden of manganese
Soil ingestion is likely the only unique exposure pathway for children. Additional studies concerning
bioavailability of manganese from soil would provide important information concerning the proportion of
the total daily manganese intake that could originate from ingested soils.
Although infants differ in their weight-adjusted intake of manganese, it is unknown whether older
children differ in this parameter. Studies concerning this end point would be very valuable.
Studies involving inhalation or ingestion exposure to MMT in the young are very few (Komura and
Sakamoto 1992b, 1994). Although these studies indicate that MMT had very little measurable effect on
development, only one dose level was used. Although analytical data indicate that environmental MMT
is unlikely to persist (Lynam et al. 1999), it is unknown what typical body burdens of manganese might
MANGANESE 423
6. POTENTIAL FOR HUMAN EXPOSURE
be in children following long-term exposure to MMT combustion products. Additional studies measuring
these end points in the young would be helpful.
Child health data needs relating to susceptibility are discussed in Section 3.12.2, Identification of Data
Needs: Children’s Susceptibility.
Exposure Registries. No exposure registries for manganese were located. This substance is not
currently one of the compounds for which a sub-registry has been established in the National Exposure
Registry. The substance will be considered in the future when chemical selection is made for sub-
registries to be established. The information that is amassed in the National Exposure Registry facilitates
the epidemiological research needed to assess adverse health outcomes that may be related to exposure to
this substance.
6.8.2 Ongoing Studies
The Federal Research in Progress (FEDRIP 2008) database provides additional information obtainable
from a few ongoing studies that may fill in some of the data needs identified in Section 6.8.1.
Researchers at the University of Delaware (D.M. Di Toro, principal investigator) are conducting research
to develop models for predicting the toxicity and mobilization of individual metals (including manganese)
and metal mixtures in sediments. These predictions are critical in evaluating the risk associated with
contaminated sediments at Superfund sites.
Thomas R. Guilarte and co-workers at Johns Hopkins University are studying the behavioral and
neuropathological changes that occur as a result of chronic exposure to low levels of manganese. The
findings from the proposed studies will be used to aid in understanding the mechanism(s) of chronic, low-
level manganese neurotoxicity. Moreover, these data will identify sensitive markers for the early
detection of manganese neurotoxicity that can be used in vivo in humans.
Wei Zheng and co-workers at Purdue University are studying the biomarkers for early diagnosis of
manganese toxicity among Chinese smelting workers. They plan to combine exposure indices and
biological effects into one parameter for quick clinical assessment of manganese toxicity. They are also
conducting clinical trials to investigate the efficacy of para-aminosalicylic acid in treatment of severe
MANGANESE 424
6. POTENTIAL FOR HUMAN EXPOSURE
manganism. Advanced MRI and MRS techniques along with molecular biotechnology have been used in
these studies.
Donald Smith and co-workers at the University of California, Santa Cruz are studying the effect that early
manganese exposure in neonatal rats has on neurobehavioral and neurocognitive deficits and comparing
these data with epidemiological studies in children.
MANGANESE 425
7. ANALYTICAL METHODS
The purpose of this chapter is to describe the analytical methods that are available for detecting,
measuring, and/or monitoring manganese, its metabolites, and other biomarkers of exposure and effect to
manganese. The intent is not to provide an exhaustive list of analytical methods. Rather, the intention is
to identify well-established methods that are used as the standard methods of analysis. Many of the
analytical methods used for environmental samples are the methods approved by federal agencies and
organizations such as EPA and the National Institute for Occupational Safety and Health (NIOSH). Other
methods presented in this chapter are those that are approved by groups such as the Association of
Official Analytical Chemists (AOAC) and the American Public Health Association (APHA).
Additionally, analytical methods are included that modify previously used methods to obtain lower
detection limits and/or to improve accuracy and precision.
The most common analytical procedures for measuring manganese levels in biological and environmental
samples use the methods of atomic absorption spectroscopy (AAS) and atomic emission spectroscopy
(AES). In AAS analysis, the sample is aspirated into a flame or in a graphite furnace (GFAAS) until the
element atomizes (Tsalev 1983). The ground-state atomic vapor absorbs monochromatic radiation from a
source and a photoelectric detector measures the intensity of radiation absorbed at 279.5 nm (Tsalev
1983). Furnace atomic absorption analysis is often used for very low analyte levels and for the analysis
of solid samples or slurries (Baruthio et al. 1988). Inductively coupled plasma-atomic emission
spectrometry (ICP-AES) analysis is frequently employed for multianalyte analyses that include
manganese. Neutron activation analysis is also a very effective method for determining manganese
concentrations in different samples (Rose et al. 1999). This technique uses no reagents and a minimum of
sample handling; thus, potential contamination with exogenous sources of manganese can be avoided. In
addition, the technique has a low detection limit in biological tissues (4 ng/g) and high precision. Further,
the technique can be used for environmental samples as well as biological samples. Other methods for
measuring manganese include spectrophotometry, mass spectrometry, neutron activation analysis, and
x-ray fluorimetry.
It is important to note that none of these methods distinguish between different oxidation states of
manganese or between different manganese compounds. Thus, monitoring data on manganese are nearly
always available only as total manganese present.
MANGANESE 426
7. ANALYTICAL METHODS
Levels of organometallic species in environmental and toxicological samples are typically in ppb
concentrations, ng/mL in solution, or ng/g in solids (Walton et al. 1991). Therefore, methods of
determination must be both selective and sensitive, achieved usually by coupling liquid or gas
chromatography (GC) with detection via electrochemical, mass spectrometry, and atomic spectrometry
detectors. A number of analytical methods for quantifying MMT in gasoline have been described,
including simple determination of total elemental manganese by atomic absorption and gas
chromatography followed by flame-ionization detection (FID). These methods usually measure MMT by
detecting the metallic portion of the compound and reporting detection of MMT as manganese.
X-ray absorption near edge structure (XANES) and x-ray absorption fine structure (XAFS) spectroscopy
have been used for the analysis of manganese-containing particulates emitted from automobile exhaust
containing MMT (Mölders et al. 2001; Ressler et al. 2000). These methods are particularly useful in
determining the chemical speciation and valence state of manganese or other metal complexes attached to
particulate matter.
7.1 BIOLOGICAL MATERIALS
Normally, determination of manganese in biological materials requires digestion of the organic matrix
prior to analysis. For tissue samples or feces (detection limits ranging from 0.2 to <1 μg/g), this is usually
done by treatment with an oxidizing acid mixture such as 3:1:1 (v/v/v) nitric:perchloric:sulfuric acid
mixture (Kneip and Crable 1988a). Fluid samples such as blood, saliva, or urine may be digested in the
same way (blood, detection limits=1 μg/100 g, 10 μg/L), or manganese can be extracted by an ion
exchange resin (urine, detection limit=0.5–2 μg/L) or by chelating agents such as cupferon in
methylisobutylketone (urine, detection limit=<1 μg/L). A method for directly measuring concentrations
of trace elements in hair that does not require digestion prior to analysis has been developed (Stupar and
Dolinsek 1996). While the authors used their technique to determine chromium, lead, and cadmium
levels in hair, it is assumed that their slurry sampling or direct solid sampling technique might also work
for manganese determination. Table 7-1 summarizes some of the methods used for sample preparation
and analysis of manganese in biological materials. It is important to note that special care is needed to
avoid contamination of biological materials with exogenous manganese, especially for samples with low
levels of manganese (Tsalev 1983; Versieck et al. 1988).
GC-FID may be used to determine levels of MMT in biological tissues and fluids with a detection limit of
1–2 ppm and percent recovery of 93.5–102.7% (Hanzlik et al. 1979).

MANGANESE 427
7. ANALYTICAL METHODS
Table 7-1. Analytical Methods for Determining Manganese in Biological
Materials
a
Sample
Analytical
Sample
Percent
matrix
Preparation method
method
detection limit
recovery
Reference
Urine
Extraction into
AAS (furnace
<1 μg/L
b
No data
Baselt 1988
methylisobutyl-ketone as
technique)
the cupferon chelate
Urine
Extract with resin, ash resin
ICP-AES
<1 μg/L
b
100±10
NIOSH 1984d
Blood
Acid digestion
ICP-AES
1 μg/dL
98±2.1
Kneip and
Craple 1988a
Blood
Digestion in oxidizing acid
ICP-AES
1 μg/100 g
98±2.1
NIOSH 1984c
Tissue
Digestion in oxidizing acid
ICP-AES
0.2 μg/g
98±2.1
NIOSH 1984c
Tissue
Acid digestion
ICP-AES
0.2 μg/g
104±5.6
Kneip and
Craple 1988a
Feces
Dry at 110 °C, ash at
AAS (furnace
<1 μg/g
102±7
Friedman et al.
550 °C, dissolve in nitric
technique) 1987
acid
Hair
Digestion in concentrated
Flameless
<0.2 μg/g
No data
Collipp et al.
nitric:perchloric acid (3:1)
AAS 1983
mixture
Hair
(a) slurry sample
ETAAS
No data
No data
Stupar and
introduction technique (hair (furnace
Dolinsek 1996
c
powder added to twice
technique)
distilled water to measure
bulk hair trace elements, or
(b) direct introduction of
hair
segments to measure
longitudinal gradients
Methods for determination of MnDPDP
Human
Mix heparinized blood
Mixed-bed
0.8–2.3 μM
85–115
Toft et al. 1997a
plasma
samples of patients resin HPLC- (manganese
receiving MnDPDP via anion compounds)
injection with solid exchange 0.1–
0.8 μM (zinc
trisodium phosphate and reverse-
compounds) of
dodecahydrate pH
phase
50 μL injection
10.0±0.2; ultrafiltrate
volume
a
Magnetic resonance imaging (MRI) has been useful in determining brain accumulation of manganese, but is not a
quantitative method; therefore, it is not listed as an entry in this table.
b
Estimated from sensitivity and linearity data
c
Methods were used to determine levels of chromium, lead, and cadmium in hair. Manganese concentrations in hair
were evaluated for some, but not all, of the samples and tested one, but not both, new methods. However, it is
assumed that both techniques will work for the trace element manganese.
AAS = atomic absorption spectroscopy; HPLC = high performance liquid chromatography; ICP-AES
= inductively
coupled-plasma atomic emission spectroscopy; MnDPDP = mangafodipir; NIOSH = National Institute for
Occupational Safety and Health
MANGANESE 428
7. ANALYTICAL METHODS
Walton et al. (1991) have described high performance liquid chromatography (HPLC) coupled with laser-
excited atomic fluorescence spectrometry (LEAFS) to detect various species of MMT. The detection
limit for this GC-LEAFS method ranged from 8 to 20 pg of manganese for the various organomanganese
species; the detection limit for determining manganese in MMT was 0.4 ng/mL. This limit of detection
was several orders of magnitude better than those for HPLC with ultraviolet (UV) detection or
HPLC-atomic fluorescence spectrometry (AFC) (Walton et al. 1991), but was worse than detection by
GC-FID (DuPuis and Hill 1979). Walton et al. (1991) used their method to determine manganese species
present in rat urine after rats had been administered MMT prepared in propylene glycol via subcutaneous
injection.
Table 7-1 summarizes some common methods for the determination of manganese in various types of
biological materials.
7.2 ENVIRONMENTAL SAMPLES
Manganese in air exists as particulate matter, so sampling is done by drawing air through a filter in order
to collect the suspended particles. A variety of filter types (e.g., glass fibers and cellulose acetate) and
sampling devices (e.g., low volume, high volume, and dichotomous) are available, depending on the
particle sizes of concern and the concentration range of interest. In some cases, material on the filter may
be analyzed directly (e.g., by x-ray fluorescence), or the filter may be digested by ashing in acid prior to
analysis. In general, sensitivity is dependent on the volume of air drawn through the filter prior to
analysis, and typically, detection limits are 1–2 μg/sample.
Several analytical methods from the EPA Office of Solid Waste publication SW-846, entitled Test
Methods for Evaluating Solid Waste, Physical/Chemical Methods are applicable for analyzing manganese
in water, soil, and wastes. In addition, the EPA Emission Measurement Center (EMS) and Office of
Water (OW) have standardized methods for the measurement of manganese and other metals in
environmental media. Several of these methods, including the analytical instrumentation and detection
limits, are summarized in Table 7-2.
Water may either be analyzed directly, or, if the concentration of manganese is low, a concentration step
(e.g., evaporation, extraction, and binding to a resin) may be employed (detection limits ranging from
0.005–50 μg/L). In all cases, acid is added to the sample to prevent precipitation of manganese.

Table 7-2. Analytical Methods for Determining Manganese in Environmental
Samples
Sample
matrix Preparation method
Analytical
method
Sample
detection limit
Percent
recovery Reference
Air Collect sample on MCE
or PVC filter, followed
by nitric/perchloric acid
ashing
Method 7300
(ICP-AES)
0.2 mg/m
3
94.7–101
(MCE)
99.3–101.9
(PVC)
NIOSH 2003a
Air Collect sample on MCE
filter, followed by hot
block/HCl/HNO
3
digestion
Method 7303
(ICP-AES)
1.2 mg/m
3
No data NIOSH 2003b
Air Collect sample on MCE
or PVC filter, followed
by aqua regia ashing
Method 7301
(ICP-AES)
0.2 mg/m
3
91.2–103.5
(MCE)
77.4–93.4
(PVC)
NIOSH 2003c
Water Acidify with nitric acid AAS (furnace
technique)
0.2 μg/L No data EPA 1983b
Water Acidify with nitric acid AAS (flame)
AAS (furnace)
ICP-AES
2 μg/L
0.01 μg/L
1 μg/L
No data
No data
No data
Taylor 1982
Water Acidify with nitric acid Method 311
(AAS)
<10 μg/L No data APHA 1998a
Water Filter and acidify filtrate
with HNO
3
and analyze
Method 3113A
(AAS furnace
technique)
0.2 μg/L No data APHA 1998b
Water Digest sample with
HNO
3
/HCl and analyze
Method 3120B
(ICP-AES)
2 μg/L No data APHA 1998c
Water Acidify with nitric acid AAS (direct
aspiration)
10 μg/L 100±2
a
EPA 1983a
Water Acid digest and analyze Method 3125A
(ICP-MS)
0.002 μg/L 91.81–110 APHA 1998d
Water Preconcentration
manganese-containing
solution and
3,3’5,5’-tetramethyl-
benzidine (TMB) onto
filter paper; add oxidant
KIO
4
to catalyze
oxidation; measure
Catalytic
kinetic method
of analysis
0.005 μg/L No data Beklemishev et
al. 1997
absorbance
Water,
waste water,
sludge, and
soils
For dissolved
constituents: filter,
acidify filtrate, and
analyze; for samples
containing solids:
digestion with HNO
3
/HCl
prior to analysis
Method 200.8
(ICP-MS)
0.01–0.04 μg/L
(liquids);
0.05 mg/kg
(solids)
95.8–96.9
(water);
95.2–103.6
(wastes)
EPA 1994b
MANGANESE 429
7. ANALYTICAL METHODS

Table 7-2. Analytical Methods for Determining Manganese in Environmental
Samples
Sample
matrix Preparation method
Analytical
method
Sample
detection limit
Percent
recovery Reference
Water and
wastes
Acid digestion AAS 10 μg/L 100±2 EPA 1986c
Water,
solids,
sediment,
For dissolved Method 6010C 0.93 μg/L
constituents: filter,
(ICP-AES)
acidify filtrate, and
analyze; for samples
containing solids:
digestion with HNO
3
/HCl
prior to analysis
No data EPA 2007a
Foods Digest wet or dry foods
with HNO
3
–H
2
SO
4
mixture (12:2 mL)
AAS (flame) AAS (flame):
AAS (furnace)
0.15 mg/kg
AAS (furnace):
1.10 μg/kg
No data Tinggi et al.
1997
Foods Digestion with nitric,
sulfuric, perchloric acid
solution
ICP-AES 0.2 mg/kg 96.2–97 Capar and
Cunningham
2000
Methods for MMT determination
Air Draw known volume of
air through XAD-2
sampling tubes for
10–60 minutes
GC-ECD 0.001 mg/m
3
(in
10-L sample);
0.02 ng from a
2.0 μL injection
of a 0.01 μg/mL
MMT solution
No data Gaind et al.
1992
Gasoline Dilute gasoline in
acetone (1:10)
Capillary
GC-ACP
detector
62 pg/s No data Ombaba and
Barry 1994
Gasoline Dilute with hexane GC-ECD No data No data Gaind et al.
(1:99); direct injection 1992
Gasoline Inject sample GC-MED 0.25 pg/s No data Quimby et al.
1978
Gasoline Inject sample GC-FPD 0.6 ppm No data Aue et al. 1990
MANGANESE 430
7. ANALYTICAL METHODS
a
Percent recovery at manganese concentration >80 μg/L; at lower concentrations (10–20 μg/L), percent recoveries
were >120%.
AAS = atomic absorption spectrometry; ACP = alternating current plasma; AES = atomic emission spectroscopy;
APDC = ammonium pyrrolidine dithiocarbamate; APHA = American Public Health Association; ECD = electron-
capture detection; EPA = Environmental Protection Agency; FPD = flame photometric detection; GC = gas
chromatography; ICP = inductivity coupled plasma; MCE = mixed cellulose ester; MED = microwave emission
detector; MS = mass spectrometry; NIOSH = National Institute for Occupational Safety and Health; PVC = polyvinyl
chloride; XRF = x-ray fluorescence
MANGANESE 431
7. ANALYTICAL METHODS
Beklemishev et al. (1997) measured the concentrations of manganese in tap and river water. Their
analytical method relies on an indicator reaction that is catalyzed by Mn(II) (the oxidation of
3,3',5,5'-tetramethylbenzidine [TMB] by potassium periodate [KIO
4
]) and is carried out on the surface of
a paper-based sorbent. The advantages of this novel technique are that it has a much lower detection limit
(0.005 μg/L) than do established methods and is transportable, allowing it to be used for rapid tests in the
field (i.e., spot tests and similar procedures).
Determination of manganese levels in soils, sludges, or other solid wastes requires an acid extraction/
digestion step prior to analysis. The details vary with the specific characteristics of the sample, but
usually treatment will involve heating in nitric acid, oxidation with hydrogen peroxide, and filtration
and/or centrifugation to remove insoluble matter.
Manganese levels in foods have been determined in order to define more clearly human dietary
requirements or levels of absorption of manganese from the diet (Tinggi et al. 1997). Atomic absorption
spectrometry has been the most widely used analytical technique to determine manganese levels in a
broad range of foods, as well as other environmental and biological samples (Tinggi et al. 1997). Tinggi
et al. (1997) contributed a wet digestion technique using a 12:2 (v/v) nitric:sulfuric acid mixture for their
determination, and for food samples with low levels of manganese, they found that the more sensitive
graphite furnace atomic absorption analysis was required. Because manganese is often found at very low
levels in many foods, its measurement requires methods with similarly low detection limits; these
researchers identified detection limits of 0.15 mg/kg (ppm) and 1.10 μg/kg (ppb) for flame and graphite
furnace atomic absorption spectrometry, respectively (Tinggi et al. 1997). Neutron activation analysis is
an effective technique for measuring manganese in environmental samples; it provides a low detection
limit and high precision (Kennedy 1990).
A number of analytical methods for quantifying MMT in gasoline have been described including simple
determination of total elemental manganese by atomic absorption (Smith and Palmby 1959) and gas
chromatography followed by FID (DuPuis and Hill 1979). The former has measured manganese
concentrations from 0.1 to 4 g/gallon of gasoline after dilution of the sample with isooctane to minimize
the effects of differences in base stock composition and is accurate to about 3% of the amount of
manganese present. The latter has an absolute detection limit of 1.7x10
-14
g/sample (0.017 pg/s) and
could easily measure 6 mg/gallon of manganese in a gasoline sample; it is one of the most sensitive
approaches. Aue et al. (1990) described a method in which MMT is detected in gasolines by gas
chromatography coupled with flame photometric detection (FPD); the chemiluminescence of manganese
MANGANESE 432
7. ANALYTICAL METHODS
is measured to determine MMT levels in a method that uses simple, inexpensive, and commercially
available instrumentation. MMT levels can be determined down to 0.6 ppm (w/w) in gasoline (Aue et al.
1990). In another method showing excellent performance, Quimby et al. (1978) used GC followed by
atmospheric pressure helium microwave detection system (or microwave emission detector [MED]); this
method has a high degree of selectivity (1.9x10
6
) and a detection limit of 0.25 pg/s at a wavelength of
257.6 nm.
GC followed by electron-capture detection (ECD) (Gaind et al. 1992) or alternating current plasma (ACP)
emission detection (Ombaba and Barry 1994) (detection limit: 62 pg as manganese) has also been
described for determination of MMT in gasoline. GC followed by ACP emission detection has been
described for detecting MMT in air samples; airborne MMT concentrations as low as 0.001 mg/m
3
can be
measured (Ombaba and Barry 1994).
Table 7-2 summarizes some common methods for the determination of manganese in various types of
environmental media.
7.3 ADEQUACY OF THE DATABASE
Section 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of manganese is available. Where adequate information is not
available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of research
designed to determine the health effects (and techniques for developing methods to determine such health
effects) of manganese.
The following categories of possible data needs have been identified by a joint team of scientists from
ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would
reduce the uncertainties of human health assessment. This definition should not be interpreted to mean
that all data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
MANGANESE 433
7. ANALYTICAL METHODS
7.3.1 Identification of Data Needs
Methods for Determining Biomarkers of Exposure and Effect
Exposure. Sensitive and selective methods are available for the detection and quantitative measurement
of manganese in blood, urine, hair, feces, and tissues (Baselt 1988; Collipp et al. 1983; Friedman et al.
1987; Kneip and Crable 1988a; NIOSH 1984c, 1984d). Since levels in biological samples are generally
rather low, sample contamination with exogenous manganese can sometimes occur (Tsalev 1983;
Versieck et al. 1988). Development of standard methods for limiting this problem would be useful.
Measurement of average manganese concentrations in these materials has proved useful in comparing
groups of occupationally exposed people to nonexposed people (Roels et al. 1987b), but has not been
especially valuable in evaluating human exposure in individuals (Rehnberg et al. 1982). This is due to the
inherent variability in intake levels and toxicokinetics of manganese in humans, rather than a limitation in
the analytical methods for manganese. Smith et al. (2007) have discussed the limitations of using blood
and urine levels of manganese as biomarkers of exposure and have suggested further investigation of
using manganese levels in teeth and hair as exposure biomarkers. The use of tooth enamel as a potential
biomarker has been explored by Ericson et al. (2007). Josephs et al. (2005) have also discussed the
limitations of using manganese levels in serum or urine as a direct measure of exposure since free
manganese does not accumulate in the circulatory system. Magnetic resonance imaging (MRI) in
conjunction with analysis of manganese in whole blood (MnB), plasma (MnP), or red blood cells has
been used in the diagnosis of manganism in humans (Jiang et al. 2007). Abdelouahab et al. (2010) have
shown that MAO activity is a potentially useful biomarker for manganese exposure. Montes et al. (2008)
conducted a study supporting prolactin as a potential biomarker for manganese exposure. Development
of additional noninvasive methods for measuring whole-body or tissue-specific manganese burdens
would be valuable in estimating human exposure levels, but would be limited by the same considerations
of individual variability that limit existing methods.
There is a need to evaluate the accuracy and reproducibility of analytical measures of manganese in
biological media, so that analytical variability is not inappropriately incorporated into natural biological
variability in reported data, as may now be the case.
Effect. No reliable biomarkers of manganese effect are known. Biochemical changes such as altered
blood or urinary levels of steroids, neurotransmitters, or their metabolites are plausible biomarkers of
exposure, but this possibility has not been thoroughly investigated. Although methods exist for the
MANGANESE 434
7. ANALYTICAL METHODS
analysis of these biochemicals, further work to improve the analyses does not seem warranted unless the
utility of this approach is established.
Methods for Determining Parent Compounds and Degradation Products in Environmental
Media. All humans are exposed to manganese, primarily through food (EPA 1984). Near a hazardous
waste site that contains manganese or a factory that uses manganese, humans could receive above-average
exposure by inhalation of air or ingestion of water, soil, or food. Methods exist for the analysis of
manganese in air (NIOSH 2003a, 2003b, 2003c), water (APHA 1998a, 1998b, 1998c, 1998d; EPA 1994b,
2007a), and soils and sediment (EPA 2007a). Methods are also available to analyze manganese in food
(Capar and Cunningham 2000; Tinggi et al. 1997).
7.3.2 Ongoing Studies
The Federal Research in Progress (FEDRIP 2008) database provides additional information obtainable
from a few ongoing studies that may fill in some of the data needs pertinent to the analysis of manganese
in biological or environmental samples. Donald Smith and co-workers at the University of California,
Santa Cruz are studying the role of manganese in neurodegenrative disease using particle induced x-ray
emission (PIXE) analyses of in situ brain regional manganese levels of rodents (FEDRIP 2008). Carmen
Enid Martinez and co-workers at Pennsylvania State University are studying the elemental distribution in
soil particles using novel techniques that include synchrotron-based microprobe x-ray fluorescence (XRF)
and x-ray diffraction (XRD) in addition to scanning electron microscopy coupled to energy or wavelength
dispersive x-ray analysis (SEM/E-W-DS). Metal solubility measurements are to be studied by
inductively coupled plasma emission spectroscopy (ICP), anodic/cathodic stripping voltammetry
(A/C-SV), and ion-selective electrodes (ISE).
MANGANESE 435
8. REGULATIONS, ADVISORIES, AND GUIDELINES
MRLs are substance-specific estimates, which are intended to serve as screening levels, are used by
ATSDR health assessors and other responders to identify contaminants and potential health effects that
may be of concern at hazardous waste sites.
An MRL of 0.0003 mg manganese/m
3
(0.3 μg manganese/m
3
) in respirable dust has been derived for
chronic inhalation exposure to manganese. As discussed in Appendix A, dichotomous models in the EPA
BMDS were fit to the incidence data for abnormal eye-hand coordination scores in battery workers
exposed to respirable manganese (Roels et al. 1992). BMCL
10
estimates from the different models
showed an approximate 2-fold range from 73 µg/m
3
from a one-stage multistage model to 142 µg/m
3
from the logistic model. The logistic model was indicated as the best fitting model by the AIC measure
(Table A-2) and was used to provide the POD for the MRL.
The MRL of 0.3 µg respirable manganese/m
3
was derived by adjusting the POD to a continuous exposure
basis (142 x 5/7 x 8/24) and dividing by an uncertainty factor of 100. An uncertainty factor of 10 was
used for human variability including possibly enhanced susceptibility of the elderly, infants, and children;
individuals with chronic liver disease or parenteral nutrition; and females and individuals with iron
deficiency. The current assessment does not use an additional modifying factor of 5 for potentially
increased susceptibility in children based on differential kinetics in the young (which was used in the
Agency for Toxic Substances and Disease Registry [2000] assessment), because recent toxicokinetic
studies in lactating rats and their offspring exposed to manganese by the oral or inhalation routes suggest
that the human variability factor of 10 provides sufficient protection for differential kinetics in children
and adults. For example, in neonatal rats orally exposed to 25 or 50 mg manganese/kg/day manganese
chloride from PND 1 through 21, manganese concentrations in various brain regions were about 2-fold
higher than brain manganese concentrations in adult rats exposed to the same oral dose levels for 21 days
(Dorman et al. 2000). Similarly, 18-day-old neonatal rats exposed from birth to aerosols of manganese
sulfate at 1 mg manganese/m
3
, 6 hours/day showed a 2.6-fold increase in striatum manganese
concentrations, compared with controls, while lactating adults exposed to the same aerosol concentration
showed a 1.7-fold increase compared with controls (Dorman et al. 2005a). Likewise, simulations with
PBPK models for inhaled manganese in lactating rat dams and offspring indicate that manganese
concentrations in the striatum and olfactory bulb of the brains of PND 19 offspring begin to increase
when air concentrations exceed 50–100 µg manganese/m
3
, whereas maternal concentrations begin to
increase at air concentrations between 100 and 300 µg manganese/m
3
(Yoon et al. 2009b). These results
MANGANESE 436
8. REGULATIONS, ADVISORIES, AND GUIDELINES
indicate that at air concentrations above about 0.05–0.1 mg/m
3
, brain concentrations in neonates may be
elevated, compared with controls, to a greater degree than in lactating dams, but the age-specific
difference in the tested air concentration range does not appear to be large. Simulations from a human
PBPK model for inhaled manganese in lactating mothers and their offspring indicate that average daily
AUCs for manganese concentrations in the globus pallidus of the fetus, suckling neonate, and 3-year-old
child from manganese air concentrations increased beyond 10% of background concentrations in fetuses
and 3-year-old children when air concentrations exceeded 0.01 mg/m
3
(10 µg/m
3
) and in suckling
neonates when air concentrations exceeded 0.001 mg/m
3
(1 µg/m
3
) (Yoon et al. 2011). Thus, the
inhalation MRL derived herein, 0.3 µg/m
3
, is below air concentrations at which brain concentrations in
human fetuses (10 µg/m
3
) and nursing infants (1 µg/m
3
) are predicted to begin to rise under normal
dietary manganese exposure conditions.
An uncertainty factor of 10 was applied for limitations/uncertainties in the database including the lack of
epidemiological data for humans chronically exposed to soluble forms of manganese and the concern that
the general population may be exposed to more soluble forms of manganese than most of the manganese-
exposed workers in the principal and supporting studies. In addition, data on developmental toxicity for
this route and duration of exposure are lacking. There is limited information on reproductive effects in
females (one study in rat dams) and reported effects on male reproductive organs have not been clearly
associated with decreased reproductive function. Though it is clear that the neurological system is the
most sensitive identified target organ for effects from subchronic- to chronic-duration inhalation exposure
to manganese, data are lacking to fully characterize the potential risk for all organ systems from chronic
inhalation exposure.
No oral MRLs were derived for acute-, intermediate-, or chronic-duration oral exposure to manganese,
but an interim guidance value of 0.16 mg manganese/kg/day, based on the Tolerable Upper Intake Level
(UL) for adults of 11 mg manganese/day (established by FNB/IOM [2001]) is recommended to be used
for ATSDR public health assessments of oral exposure to inorganic forms of manganese. The interim
guidance value is necessary because of the prevalence of manganese at hazardous waste sites and the fact
that manganese is an essential nutrient. It is recommended to be used until more information on actual
intake levels across environmental media can be obtained.
The EPA derived a chronic inhalation RfC of 5x10
-5
mg/m
3
for respirable manganese (IRIS 2011). This
value is based on the LOAEL of 0.15 mg/m
3
from a study of battery workers exposed to manganese
dioxide (Roels et al. 1992). EPA verified this assessment in 1993. The LOAEL was calculated by
MANGANESE 437
8. REGULATIONS, ADVISORIES, AND GUIDELINES
dividing the geometric mean concentration of the lifetime-integrated respirable dust concentration for the
exposed workers by the average duration of employment in the facility. EPA calculated the RfC by
adjusting for continuous exposure and dividing by an uncertainty factor of 1,000 (10 for use of a LOAEL,
10 to protect sensitive individuals, and 10 for database limitations reflecting both the less-than-chronic
periods of exposure and the lack of developmental data, as well as potential, but unquantified, differences
in the toxicity of different forms of manganese). The estimated breathing rate in the exposed workers was
assumed to be 10 m
3
/workday.
The EPA (IRIS 2011) derived an oral reference dose (RfD) value of 0.14 mg/kg/day manganese from all
oral exposures. As of August 2008, this value was last updated in May 1996. The agency suggested
using a modifying factor of 1 if the manganese is ingested in food and a modifying factor of 3 if the
element is ingested in water or soil. The RfD was developed using a previous determination of the upper
range of total dietary intake of 10 mg/day. The modifying factor of 1 was based on composite data on
chronic human NOAELs from the World Health Organization (WHO 1973) (0.11–0.13 mg/kg/day), the
National Academy of Sciences/National Research Council (1989) “safe and adequate level” (0.04–
0.07 mg/kg/day), and a study by Freedland-Graves et al. (1994) concerning nutritional requirements for
manganese. The FNB/IOM (2001) re-established an Adequate Intake (AI) value for manganese for men
and women at 2.3 and 1.8 mg manganese/day, respectively (for 70-kg individuals, this would result in
exposures of 0.033 and 0.026 mg manganese/kg/day, respectively). The UL of 11 mg/day was also set by
the FNB/IOM (2001) for adults based on a NOAEL for Western diets (approximately 0.16 mg
manganese/kg/day assuming a 70-kg body weight).
The international and national regulations, advisories, and guidelines regarding manganese in air, water,
and other media are summarized in Table 8-1.

MANGANESE 438
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Manganese
Agency
Description
Information
Reference
INTERNATIONAL
Guidelines:
IARC
Carcinogenicity classification
No data
IARC 2008
WHO
Air quality guidelines
Manganese
a
0.15 µg/m
3
WHO 2000a
Drinking water quality guidelines
Manganese
b
0.4 mg/L
WHO 2004a
NATIONAL
Regulations and
Guidelines:
a. Air
ACGIH
TLV (8-hour TWA)
Manganese
MMT
c
0.2 mg/m
3
0.2 mg/m
3
ACGIH 2007
TLV basis (critical effects)
Manganese
Central nervous system
impairment
MMT
Central nervous system
impairment, lung, liver,
and kidney damage
EPA
Second list of AEGL priority chemicals
for guideline development
EPA 2008a
Manganese
Yes
MMT
Yes
NIOSH
Category of pesticides
NIOSH 1992
Potassium permanganate
Group 1 pesticide
REL (10-hour TWA)
Manganese
Manganese (II,III) oxide
d
MMT
e
1 mg/m
3
Not established
0.2 mg/m
3
NIOSH 2005
STEL (15-minute TWA)
Manganese
3 mg/m
3
IDLH
Manganese
500 mg/m
3
Target organs
Manganese
Respiratory system,
central nervous system,
blood, and kidneys

Table 8-1. Regulations, Advisories, and Guidelines Applicable to Manganese
Agency Description Information Reference
Manganese (II,III) oxide
NATIONAL (cont.)
NIOSH
Target organs (cont.)
MMT
OSHA PEL (8-hour TWA) for general industry
(ceiling limit)
Manganese (compounds and fume)
PEL (8-hour TWA) for shipyard industry
(ceiling limit)
Manganese (compounds and fume)
PEL (8-hour TWA) for construction
industry (ceiling limit)
Manganese (compounds and fume)
b. Water
EPA Designated as hazardous substances in
accordance with Section 311(b)(2)(A) of
the Clean Water Act
Potassium permanganate
Drinking water contaminant candidate
list
Manganese
Drinking water standards and health
advisories
Manganese
1-Day health advisory for a 10-kg
child
10-Day health advisory for a 10-kg
child
DWEL
Lifetime
National recommended water quality
criteria
Manganese
f
Human health for consumption of
water + organism
Human health for consumption of
organism only
National secondary drinking water
standards
Respiratory system,
central nervous system,
blood, and kidneys
Eyes, central nervous
system, liver, and
kidneys
5 mg/m
3
5 mg/m
3
5 mg/m
3
Yes
Yes
1 mg/L
1 mg/L
1.6 mg/L
0.3 mg/L
0.05 mg/L
0.1 mg/L
OSHA 2007c
29 CFR 1910.1000,
Table Z-2
OSHA 2007a
29 CFR 1915.1000
OSHA 2007b
29 CFR 1926.55,
Appendix A
EPA 2008b
40 CFR 116.4
EPA 1998
EPA 2006a
EPA 2006c
EPA 2003b
MANGANESE 439
8. REGULATIONS, ADVISORIES, AND GUIDELINES

Table 8-1. Regulations, Advisories, and Guidelines Applicable to Manganese
Agency Description Information Reference
Manganese
g
Reportable quantities of hazardous
substances designated pursuant to
Section 311 of the Clean Water Act
Potassium permanganate
NATIONAL (cont.)
c. Food
EPA Inert ingredients permitted for use in
nonfood use pesticide products
Mn(II) carbonate
Manganese dioxide
Manganese sulfate
Potassium permanganate
FDA Bottled drinking water
Manganese
EAFUS
h
Potassium permanganate
Indirect food additives: adhesives and
components of coatings
Potassium permanganate
d. Other
ACGIH Carcinogenicity classification
Manganese
MMT
DEA Records and reports of listed chemicals
Potassium permanganate
EPA Carcinogenicity classification
Manganese
RfC
Manganese
RfD
Manganese
Superfund, emergency planning, and
community right-to-know
Designated CERCLA hazardous
substance
Manganese
j
Potassium permanganate
k
Reportable quantity
Manganese
Potassium permanganate
0.05 mg/L
100 pounds
Yes
Yes
Yes
Yes
0.05 mg/L
Yes
Yes
No data
No data
List II chemical
Group D
i
5x10
-5
mg/m
3
0.14 mg/kg/day
Yes
Yes
None
l
100 pounds
EPA 2008d
40 CFR 117.3
EPA 2008e
FDA 2007a
21 CFR 165.110
FDA 2008
FDA 2007b
21 CFR 175.105
ACGIH 2007
DEA 2007
21 CFR 1310.02
IRIS 2011
EPA 2008c
40 CFR 302.4
MANGANESE 440
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Manganese
Agency Description Information Reference
NATIONAL (cont.)
Effective date of toxic chemical EPA 2008g
release reporting
40 CFR 372.65
Manganese 01/01/1987
EPA Superfund, emergency planning, and
community right-to-know
Extremely Hazardous Substances EPA 2008f
MMT
40 CFR 355,
Reportable quantity 100 pounds
Appendix A
Threshold planning quantity 100 pounds
NTP Carcinogenicity classification No data NTP 2005
MANGANESE 441
8. REGULATIONS, ADVISORIES, AND GUIDELINES
a
TWA based on effects other than cancer or odor/annoyance using an averaging time of 1 year.
b
Concentrations of the substance at or below the health-based guideline value may affect the appearance, taste, or
odor of the water, resulting in consumer complaints.
c
Skin designation refers to the potential significant contribution to the overall exposure by the cutaneous route,
including mucous membranes and the eyes, by contact with vapors, liquids, and solids.
d
NIOSH has not established a REL for magnesium oxide fume under the “Proposed Rule on Air Contaminants"
(29 CFR 1910, Docket No. H-020) in which NIOSH questioned whether the OSHA PEL for magnesium oxide fume
(1 mg/m
3
) was adequate enough to protect workers from potential health hazards (NIOSH 2005).
e
Skin designation indicates the potential for dermal absorption; skin exposure should be prevented as necessary
through the use of good work practices, gloves, coveralls, goggles, and other appropriate equipment.
f
The human health criteria are based on carcinogenicity of 10
-6
risk. This criterion for manganese is not based on
toxic effects, but rather is intended to minimize objectionable qualities such as laundry stains and objectionable tastes
in beverages.
g
National Secondary Drinking Water Standards are non-enforceable guidelines regulating contaminants that may
cause cosmetic effects (such as skin or tooth discoloration) or aesthetic effects (such as taste, odor, or color) in
drinking water.
h
The EAFUS list of substances contains ingredients added directly to food that FDA has either approved as food
additives or listed or affirmed as GRAS.
i
Group D: not classifiable as to human carcinogenicity.
j
Designated CERCLA hazardous substance pursuant to Section 112 of the Clean Air Act.
k
Designated CERCLA hazardous substance pursuant to Section 311(b)(2) of the Clean Water Act.
l
No reportable quantity is being assigned to the generic or broad class.
ACGIH = American Conference of Governmental Industrial Hygienists; AEGL = acute exposure guideline levels;
CERCLA = Comprehensive Environmental Response, Compensation, and Liability Act; CFR = Code of Federal
Regulations; DEA = Drug Enforcement Administration; DWEL = drinking water equivalent level; EAFUS = Everything
Added to Food in the United States; EPA = Environmental Protection Agency; FDA = Food and Drug Administration;
GRAS = Generally Recognized As Safe; IARC = International Agency for Research on Cancer; IDLH = immediately
dangerous to life or health; IRIS = Integrated Risk Information System; MMT = methylcyclopentadienyl manganese
tricarbonyl; NIOSH = National Institute for Occupational Safety and Health; NTP = National Toxicology Program;
OSHA = Occupational Safety and Health Administration; PEL = permissible exposure limit; REL = recommended
exposure limit; RfC = inhalation reference concentration; RfD = oral reference dose; STEL = short-term expsoure
limit; TLV = threshold limit values; TWA = time-weighted average; WHO = World Health Organization
_______________________
MANGANESE 443
9. REFERENCES
Abdel-Hamid MM, El-Desoky SA, Magdi SM. 1990. Estimation of manganese in blood between
exposed workers to different concentrations at industrial units. Egypt J Pharm Sci 31:143-150.
Abdelouahab N, Huel G, Suvorov A, et al. 2010. Monoamine oxidase activity in placenta in relation to
manganese, cadmium, lead, and mercury at delivery. Neurotoxicol Teratol 32(2):256-261.
Abrams E, Lassiter JW, Miller WJ, et al. 1976a. Effect of dietary manganese as a factor affecting 54Mn
absorption in rats. Nutr Rep Int 14:561-565.
ACGIH. 2007. Manganese. Threshold limit values for chemical substances and physical agents and
biological exposure indices. Cincinnati, OH: American Conference of Governmental Industrial
Hygienists, 37.
Adinolfi M. 1985. The development of the human blood-CSF-brain barrier. Dev Med Child Neurol
27(4):532-537.
*Adkins B, Luginbuhl GH, Gardner DE. 1980a. Biochemical changes in pulmonary cells following
manganese oxide inhalation. J Toxicol Environ Health 6:445-454.
Adkins B, Luginbuhl GH, Gardner DE. 1980b. Acute exposure of laboratory mice to manganese oxide.
Am Ind Hyg Assoc J 41:494-500.
Adkins B, Luginbuhl GH, Miller FJ, et al. 1980c. Increased pulmonary susceptibility to streptococcal
infection following inhalation of manganese oxide. Environ Res 23:110-120.
Adlercreutz H. 1995. Phytoestrogens: Epidemiology and a possible role in cancer protection. Environ
Health Perspect Suppl 103(7):103-112.
Agency for Toxic Substances and Disease Registry. 1989. Decision guide for identifying substance-
specific data needs related to toxicological profiles; Notice. Agency for Toxic Substances and Disease
Registry, Division of Toxicology. Fed Regist 54(174):37618-37634.
Agency for Toxic Substances and Disease Registry. 1990. Biomarkers of organ damage or dysfunction
for the renal, hepatobiliary, and immune systems. Subcommittee on Biomarkers of Organ Damage and
Dysfunction. Atlanta, GA: Agency for Toxic Substances and Disease Registry.
Agency for Toxic Substances and Disease Registry. 1997. Public health assessment. Tobyhanna army
depot Coolbaugh township, Monroe County, Pennsylvania. Agency for Toxic Substances and Disease
Registry. http://www.atsdr.cdc.gov/HAC/PHA/toby/tob_toc.html. August 07, 2008.
Agency for Toxic Substances and Disease Registry. 2000. Toxicological profile for manganese (update).
Atlanta, GA: Agency for Toxic Substances and Disease Registry. A-3 to A-5.
* Not cited in text
MANGANESE 444
9. REFERENCES
Agency for Toxic Substances and Disease Registry. 2003. Public health assessment. Fish and shellfish
evaluation Isla de Vieques bombing range. Vieques, Puerto Rico. Agency for Toxic Substances and
Disease Registry. http://www.atsdr.cdc.gov/hac/PHA/viequesfish/viequespr-toc.html. August 07, 2008.
Akbar-Khanzadeh F. 1993. Short-term respiratory function changes in relation to workshift welding
fume exposures. Int Arch Occup Environ Health 64:393-397.
Alarcón OM, Reinosa-Fuller JA, Silva T, et al. 1996. Manganese levels in serum of healthy Venezuelan
infants living in Mérida. J Trace Elem Med Biol 10:210-213.
Alessio L, Apostoli P, Ferioli A, et al. 1989. Interference of manganese on neuroendocrinal system in
exposed workers. Preliminary report. Biol Trace Elem Res 21:249-253.
Ali MM, Murthy RC, Mandal SK, et al. 1985. Effect of low protein diet on manganese neurotoxicity:
III. Brain neurotransmitter levels. Neurobehav Toxicol Teratol 7:427-431.
Ali MM, Murthy RC, Saxena DK, et al. 1983a. Effect of low protein diet on manganese neurotoxicity:
I. Developmental and biochemical changes. Neurobehav Toxicol Teratol 5:377-383.
Ali MM, Murthy RC, Saxena DK, et al. 1983b. Effect of low protein diet on manganese neurotoxicity:
II. Brain GABA and seizure susceptibility. Neurobehav Toxicol Teratol 5:385-389.
Altman PL, Dittmer DS. 1974. Biological handbooks: Biology data book. Vol. III. 2nd ed. Bethesda,
MD: Federation of American Societies for Experimental Biology, 1987-2008, 2041.
Andersen ME, Krishnan K. 1994. Relating in vitro to in vivo exposures with physiologically based
tissue dosimetry and tissue response models. In: Salem H, ed. Animal test alternatives: Refinement,
reduction, replacement. New York, NY: Marcel Dekker, Inc., 9-25.
Andersen ME, Clewell HJ, Gargas ML, et al. 1987. Physiologically based pharmacokinetics and the risk
assessment process for methylene chloride. Toxicol Appl Pharmacol 87(2):185-205.
Andersen ME, Gearhart JM, Clewell HJ. 1999. Pharmacokinetic data needs to support risk assessments
for inhaled and ingested manganese. Neurotoxicology 20:161-171.
Anderson JG, Cooney PT, Erikson KM. 2007a. Brain manganese accumulation is inversely related to γ-
amino butyric acid uptake in male and female rats. Toxicol Sci 95(1):188-195.
*Anderson JG, Cooney PT, Erikson KM. 2007b. Inhibition of DAT function attenuates manganese
accumulation in the globus pallidus. Environ Toxicol Pharmacol 23:179-184.
Anderson JG, Fordahl SC, Cooney PT, et al. 2009. Extracellular norepinephrine, norepinephrine
receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due
to dietary iron deficiency and manganese exposure. Brain Res 1281:1-14.
APHA. 1998a. Method 3111. Metals by flame atomic absorption spectrometry. In: Clesceri LS,
Greenberg AE, Eaton AD, et al., eds. Standard Methods for the Examination of Water and Wastewater.
20th ed. Washington, DC: American Public Health Association. American Water Works Association.
Water Environmental Federation, 3-13 to 3-18.
MANGANESE 445
9. REFERENCES
APHA. 1998b. Method 3113. Metals by electrothermal atomic absorption spectrometry. In: Clesceri
LS, Greenberg AE, Eaton AD, et al., eds. Standard methods for the examination of water and wastewater.
20th ed. Washington, DC: American Public Health Association. American Water Works Association.
Water Environmental Federation, 3-24 to 3-31.
APHA. 1998d. Method 3120 A. Introduction. Method 3120 B. Inductively coupled plasma (ICP)
method. In: Clesceri LS, Greenberg AE, Eaton AD, eds. Standard methods for the examination of water
and wastewater. 20th ed. Washington, DC: American Public Health Association. American Water
Works Association. Water Environmental Federation, 3-37 to 3-43.
APHA. 1998c. Method 3125. Metals by inductively coupled plasma/mass spectrometry. In: Clesceri
LS, Greenberg AE, Eaton AD, et al., eds. Standard Methods for the Examination of Water and
Wastewater. 20th ed. Washington, DC: American Public Health Association. American Water Works
Association. Water Environmental Federation, 3-44 to 3-52.
Archibald FS, Tyree C. 1987. Manganese poisoning and the attack of trivalent manganese upon
catecholamines. Arch Biochem Biophys 256:638-650.
Arnaud J, Favier A. 1995. Copper, iron, manganese and zinc contents in human colostrum and transitory
milk of French women. Sci Total Environ 159:9-15.
Arnold ML, McNeill FE, Chettle DR. 1999. The feasibility of measuring manganese concentrations in
human liver using neutron activation analysis. Neurotoxicology 20:407-412.
Aschner JL, Aschner M. 2005. Nutritional aspects of manganese homeostasis. Mol Aspects Med
26:353-362.
Aschner M, Aschner JL. 1990. Manganese transport across the blood-brain barrier: relationship to iron
homeostasis. Brain Res Bull 24:857-860.
Aschner M, Aschner JL. 1991. Manganese neurotoxicity: Cellular effects and blood-brain barrier
transport. Neurosci Biobehav Rev 15:333-340.
Aschner M, Dorman DC. 2006. Manganese: Pharmacokinetics and molecular mechanisms of brain
uptake. Toxicol Rev 25(3):147-154.
Aschner M, Erikson KM, Dorman DC. 2005. Manganese dosimetry: Species differences and
implications for neurotoxicity. Crit Rev Toxicol 35(1):1-32.
Aschner M, Guilarte TR, Schneider JS, et al. 2007. Manganese: Recent advances in understanding its
transport and neurotoxicity. Toxicol Appl Pharmacol 221:131-147.
Aue WA, Millier B, Sun XY. 1990. Determination of (methylcyclopentadienyl)manganese tricarbonyl in
gasolines by gas chromatography with flame photometric detection. Anal Chem 62:2453-2457.
Avila DS, Gubert P, Fachinetto R, et al. 2008. Involvement of striatal lipid peroxidation and inhibition
of calcium influx into brain slices in neurobehavioral alterations in a rat model of short-term oral
exposure to manganese. Neurotoxicology 29(6):1062-1068.
*Ayotte P, Plaa GL. 1985. Hepatic subcellular distribution of manganese in manganese and manganese-
bilirubin induced cholestasis. Biochem Pharmacol 34:3857-3865.
MANGANESE 446
9. REFERENCES
Baes CF, Sharp RD. 1983. A proposal for estimation of soil leaching and leaching constants for use in
assessment models. J Environ Qual 12:17-28.
*Bairati C, Goi G, Bollini D, et al. 1997. Effects of lead and manganese on the release of lysosomal
enzymes in vitro and in vivo. Clin Chim Acta 261(1):91-101.
*Baker DH, Halpin KM. 1991. Manganese and iron interrelationship in the chick. Poultry Sci 70:146-
152.
Baldwin M, Mergler D, Larribe F, et al. 1999. Bioindicator and exposure data for a population based
study of manganese. Neurotoxicology 20:343-354.
Ballatori N, Miles E, Clarkson TW. 1987. Homeostatic control of manganese excretion in the neonatal
rat. Am J Physiol 252:R842-R847.
Banta RG, Markesbery WR. 1977. Elevated manganese levels associated with dementia and
extrapyramidal signs. Neurology 27:213-216.
Barbeau A. 1984. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George
C. Cotzias). Neurotoxicology 5:13-35.
Barceloux DG. 1999. Manganese. Clin Toxicol 37(2):293-307.
Barnes DG, Dourson M. 1988. Reference dose (RfD): Description and use in health risk assessments.
Regul Toxicol Pharmacol 8(4):471-486.
Baruthio F, Guillard O, Arnaud J, et al. 1988. Determination of manganese in biological materials by
electrothermal atomic absorption spectrometry: A review. Clin Chem 34:227-234.
Baselt RC. 1988. Manganese. In: Biological monitoring methods for industrial chemicals. Littleton,
MA: PSG Publishing Company, Inc., 194-197.
Bast-Pettersen R, Ellingsen DG, Hetland SM, et al. 2004. Neuropsychological function in manganese
alloy plant workers. Int Arch Occup Environ Health 77:277-287.
*Baxter DJ, Smith WO, Klein GC. 1965. Some effects of acute manganese excess in rats. Proc Soc Exp
Biol Med 119:966-970.
Beklemishev MK, Stoyan TA, Dolmanova IF. 1997. Sorption-catalytic determination of manganese
directly on a paper-based chelating sorbent. Analyst 122:1161-1165.
Bell JG, Keen CL, Lönnerdal B. 1989. Higher retention of manganese in suckling than in adult rats is
not due to maturational differences in manganese uptake by rat small intestine. J Toxicol Environ Health
26:387-398.
Berger GS, ed. 1994. Epidemiology of endometriosis. In: Endometriosis: Advanced management and
surgical techniques. New York, NY: Springer-Verlag, 3-7.
Bergstrom R. 1977. Acute pulmonary toxicity of manganese dioxide. Scand J Work Environ Health
3(Suppl 1):1-40.
MANGANESE 447
9. REFERENCES
Bernard A, Hermans C. 1997. Biomonitoring of early effects on the kidney or the lung. Sci Total
Environ 199:205-211.
Bernardino ME, Young SW, Lee JKT, et al. 1992. Hepatic MR imaging with MnDPDP: Safety, image
quality, and sensitivity. Radiology 183:53-58.
Bernheimer H, Birkmayer W, Hornykiewicz O, et al. 1973. Brain dopamine and the syndromes of
Parkinson and Huntington: Clinical, morphological and neurochemical correlations. J Neurol Sci 20:
415-455.
Bertinchamps AJ, Miller ST, Cotzias GC. 1965. Interdependence of routes excreting manganese. Am J
Physiol 211:217-224.
Beuter A, Edwards R, de Geoffroy A, et al. 1999. Quantification of neuromotor function for detection of
the effects of manganese. Neurotoxicology 20:355-366.
*Bhargava HN. 1987. Effect of repeated administration of manganese on the striatal cholinergic and
dopaminergic receptors in the rat. Toxicol Lett 37:135-141.
Bhuie AK, Ogunseitan OA, White RR, et al. 2005. Modeling the environmental fate of manganese from
methylcyclopentadienyl manganese tricarbonyl in urban landscapes. Sci Total Environ 339:167-178.
Bird ED, Anton AH, Bullock B. 1984. The effect of manganese inhalation on basal ganglia dopamine
concentrations in rhesus monkey. Neurotoxicology 5:59-65.
Blazak WF, Brown GL, Gray TJB, et al. 1996. Developmental toxicity study of mangafodipir trisodium
injection (MnDPDP) in New Zealand white rabbits. Fundam Appl Toxicol 33:11-15.
Blond M, Netterstrom B. 2007. Neuromotor function in a cohort of Danish steel workers.
Neurotoxicology 28:336-344.
Blond M, Netterstrom B, Laursen P. 2007. Cognitive function in a cohort of Danish steel workers.
Neurotoxicology 28:328-335.
Bock NA, Paiva FF, Nascimento GC, et al. 2008. Cerebrospinal fluid to brain transport of manganese in
a non-human primate revealed by MRI. Brain Res 1198:160-170.
Bolte S, Normandin L, Kennedy G, et al. 2004. Human exposure to respirable manganese in outdoor and
indoor air in urban and rural areas. J Toxicol Environ Health A 67:459-467.
*Bona MA, Castellano M, Plaza L, et al. 1992. Determination of heavy metals in human liver. Hum
Exp Toxicol 11:311-313.
*Bonilla E. 1978a. Flameless atomic absorption spectrophotometric determination of manganese in rat
brain and other tissues. Clin Chem 24:471-474.
Bonilla E. 1978b. Increased GABA content in caudate nucleus of rats after chronic manganese chloride
administration. J Neurochem 31:551-552.
MANGANESE 448
9. REFERENCES
Bonilla E. 1980. L-tyrosine hydroxylase activity in the rat brain after chronic oral administration of
manganese chloride. Neurobehav Toxicol 2:37-41.
Bonilla E, Prasad AL. 1984. Effects of chronic manganese intake on the levels of biogenic amines in rat
brain regions. Neurobehav Toxicol Teratol 6:341-344.
Boojar MMA, Goodarzi F. 2002. A longitudinal follow-up of pulmonary function and respiratory
symptoms in workers exposed to manganese. J Occup Environ Med 44:282-290.
Boshnakova E, Divanyan H, Zlatarov I, et al. 1989. Immunological screening of welders. J Hyg
Epidemiol Microbiol Immunol 33:379-382.
Bouchard M, Mergler D, Baldwin M, et al. 2003. Blood manganese and alcohol consumption interact on
mood states among manganese alloy production workers. Neurotoxicology 24:641-647.
Bouchard M, Mergler D, Baldwin M. 2005. Manganese exposure and age: Neurobehavioral
performance among alloy production workers. Environ Toxicol Pharmacol 19(3):687-694.
Bouchard M, Mergler D, Baldwin M, et al. 2007b. Neurobehavioral functioning after cessation of
manganese exposure: A follow-up after 14 years. Am J Ind Med 50:831-840.
Bouchard M, Mergler D, Baldwin M, et al. 2007a. Neuropsychiatric symptoms and past manganese
exposure in a ferro-alloy plant. Neurotoxicology 28:290-297.
Bouchard M, Laforest F, Vandelac L, et al. 2007c. Hair manganese and hyperactive behaviors: Pilot
study of school-age children exposed through tap water. Environ Health Perspect 115:122-127.
Bouchard MF, Sauve S, Barbeau B, et al. 2011. Intellectual impairment in school-age children exposed
to manganese from drinking water. Environ Health Perspect 119(1):138-143.
Bowler RM, Mergler D, Sassine MP, et al. 1999. Neuropsychiatric effects of manganese on mood.
Neurotoxicology 20:367-378.
*Boyce W, Witzleben CL. 1973. Bilirubin as a cholestatic agent. II. Effect of variable doses of
bilirubin on the severity of manganese-bilirubin cholestasis. Am J Pathol 72:427-432.
Brault N, Loranger S, Courchesne F, et al. 1994. Bioaccumulation of manganese by plants: Influence of
MMT as a gasoline additive. Sci Total Environ 153:77-84.
Bredow S, Falgout MM, March TH, et al. 2007. Subchronic inhalatin of soluble manganese induces
expression of hypoxia-associated angiogenic genes in adult mouse lungs. Toxicol Appl Pharmacol
221:148-157.
Brenneman KA, Cattley RC, Ali SF, et al. 1999. Manganese-induced developmental neurotoxicity in the
CD rat: Is oxidative damage a mechanism of action? Neurotoxicology 20:477-488.
Brenneman KA, Wong BA, Buccellato MA, et al. 2000. Direct olfactory transport of inhaled manganese
(54MnCl2) to the rat brain: Toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol
Appl Pharmacol 169:238-248.
MANGANESE 449
9. REFERENCES
*Britton AA, Cotzias GC. 1966. Dependence of manganese turnover on intake. Am J Physiol 211:203-
206.
Brna P, Gordon K, Dooley JM, et al. 2011. Manganese toxicity in a child with iron deficiency and
polycythemia. J Child Neurol 26(7):891-894.
*Brock AA, Chapman SA, Ulman EA, et al. 1994. Dietary manganese deficiency decreases rat hepatic
arginase activity. J Nutr 124:340-344.
Brouillet EP, Shinobu L, McGarvey U, et al. 1993. Manganese injection into the rat striatum produces
excitotoxic lesions by impairing energy metabolism. Exp Neurol 120:89-94.
*Brown DSO, Wills CE, Yousefi V, et al. 1991. Neurotoxic effects of chronic exposure to manganese
dust. Neuropsychiatry Neuropsychol Behav Neurol 4(3):238-250.
Brown RP, Delp MD, Lindstedt SL, et al. 1997. Physiological parameter values for physiologically
based pharmacokinetic models. Toxicol Ind Health 13(4):407-484.
*Brurok H, Schjott J, Berg K, et al. 1997. Manganese and the heart: Acute cardiodepression and
myocardial accumulation of manganese. Acta Physiol Scand 159:33-40.
Calabresi P, Ammassari-Teule M, Gubellini P, et al. 2001. A synaptic mechanism underlying the
behavioral abnormalities induced by manganese intoxication. Neurobiol Dis 9:419-432.
Calne DB, Chu NS, Huang CC, et al. 1994. Manganism and idiopathic Parkinsonism: Similarities and
differences. Neurology 44:1583-1586.
Camner P, Curstedt T, Jarstrand C, et al. 1985. Rabbit lung after inhalation of manganese chloride: A
comparison with the effects of chlorides of nickel, cadmium, cobalt, and copper. Environ Res 38:301-
309.
Campbell KI, George EL, Hall LL, et al. 1975. Dermal irritancy of metal compounds: Studies with
palladium, platinum, lead, and manganese compounds. Arch Environ Health 30:168-170.
Capar SG, Cunningham WC. 2000. Element and radionuclide concentrations in food: FDA total diet
study 1991-1996. J AOAC Int 83(1):157-177.
Carl GF, Blackwell LK, Barnett FC, et al. 1993. Manganese and epilepsy: Brain glutamine synthetase
and liver arginase activities in genetically epilepsy prone and chronically seizured rats. Epilepsia 34:441-
446.
Carter JC, Miller WJ, Neathery MW, et al. 1974. Manganese metabolism with oral and intravenous
54Mn in young calves as influenced by supplemental manganese. J Animal Sci 38:1284-1290.
Carter SD, Hein JF, Rehnberg GL, et al. 1980. Chronic manganese oxide ingestion in rats:
Hematological effects. J Toxicol Environ Health 6:207-216.
Casarett W, Klaassen CD, Doull, J. 2001. Casarett and Doull's toxicology: The basic science of poisons.
6th ed. New York: McGraw-Hill, 844.
MANGANESE 450
9. REFERENCES
Casto BC, Meyers J, DiPaolo JA. 1979. Enhancement of viral transformation for evaluation of the
carcinogenic or mutagenic potential of inorganic metal salts. Cancer Res 39:193-198.
*Cawte J. 1991. Environmental manganese toxicity. Med J Austral 154:291-292.
Cawte J, Hams G, Kilburn C. 1987. Manganism in a neurological ethnic complex in nothern Australia
[Letter]. Lancet 1(8544):1257.
Cawte J, Kilburn C, Florence M. 1989. Motor neurone disease of the Western Pacific: Do the foci
extend to Australia? Neurotoxicity 10:263-270.
CDHS. 1990. Written communication regarding levels of manganese found in private wells. Hartford,
CT: Connecticut Department of Health Services.
Centonze D, Gubellini P, Bernardi G, et al. 2001. Impaired excitatory transmission in the striatum of rats
chronically intoxicated with manganese. Exp Neurol 172(2):469-476.
Chan AW, Minski MJ, Lim L, et al. 1992. Changes in brain regional manganese and magnesium levels
during postnatal development: Modulations by chronic manganese administration. Metab Brain Dis
7:21-33.
Chandra SV. 1972. Histological and histochemical changes in experimental manganese encephalopathy
in rabbits. Arch Toxicol 29:29-38.
Chandra SV. 1983. Psychiatric illness due to manganese poisoning. Acta Psychiatr Scand 67(Suppl
303):49-54.
Chandra SV, Imam Z. 1973. Manganese induced histochemical and histological alterations in
gastrointestinal mucosa of guinea pigs. Acta Pharmacol Toxicol 33:449-458.
Chandra SV, Shukla GS. 1978. Manganese encephalopathy in growing rats. Environ Res 15:28-37.
Chandra SV, Shukla GS. 1981. Concentrations of striatal catechloramines in rats given manganese
chloride through drinking water. J Neurochem 36:683-687.
Chandra SV, Tandon SK. 1973. Enhanced manganese toxicity in iron-deficient rats. Environ Physiol
Biochem 3:230-235.
Chandra SV, Ara R, Nagar N, et al. 1973. Sterility in experimental manganese toxicity. Acta Biol Med
Ger 30:857-862.
*Chandra SV, Saxena DK, Hasan MZ. 1975. Effect of zinc on manganese induced testicular injury in
rats. Ind Health 13:51-56.
*Chandra SV, Shukla GS, Srivastava RS. 1981. An exploratory study of manganese exposure to
welders. Clin Toxicol 18:407-416.
Chia SE, Foo SC, Gan SL, et al. 1993a. Neurobehavioral functions among workers exposed to
manganese ore. Scand J Work Environ Health 19:264-270.
MANGANESE 451
9. REFERENCES
Chia SE, Gan SL, Chua LH, et al. 1995. Postural stability among manganese exposed workers.
Neurotoxicology 16:519-526.
Chowdhury BA, Chandra RK. 1987. Biological and health implications of toxic heavy metal and
essential trace element interactions. Prog Food Nutr Sci 11:55-113.
Chu NS, Hochberg FH, Calne DB, et al. 1995. Neurotoxicity of manganese. In: Chang L, Dyyer R, eds.
Handbook of neurotoxicology. New York, NY: Marcel Dekker, Inc., 91-103.
Claus Henn B, Ettinger AS, Schwartz J, et al. 2010. Early postnatal blood manganese levels and
children's neurodevelopment. Epidemiology 21(4):433-439.
Claus Henn B, Schnaas L, Ettinger AS, et al. 2011. Associations of early childhood manganese and lead
co-exposure with neurodevelopment. Environ Health Perspect [Epub ahead of print].
*Clay RJ, Morris JB. 1989. Comparative pneumotoxicity of cyclopentadienyl manganese tricarbonyl
and methylcyclopentadienyl manganese tricarbonyl. Toxicol Appl Pharmacol 98:434-443.
Clewell HJ, Andersen ME. 1985. Risk assessment extrapolations and physiological modeling. Toxicol
Ind Health 1(4):111-131.
Clewell HJ, Crump KS. 1999. Benchmark dose analysis of the neurological effects of managanese in
smelter workers. Agency for Toxic Substances and Disease Registry
Clewell HJ, Lawrence GA, Calne DB, et al. 2003. Determination of an occupational exposure guideline
for manganese using the benchmark method. Risk Anal 23(5):1031-1046.
Cockell KA, Bonacci G, Belonje B. 2004. Manganese content of soy or rice beverages is high in
comparison to infant formulas. J Am Coll Nutr 23(2):134-130.
Collipp PJ, Chen SY, Maitinsky S. 1983. Manganese in infant formulas and learning disability. Ann
Nutr Metab 27:488-494.
Colomina MT, Domingo JL, Llobet JM, et al. 1996. Effect of day of exposure on the developmental
toxicity of manganese in mice. Vet Hum Toxicol 38:7-9.
Cook KK. 1997. Extension of dry ash atomic absorption and spectrophotometric methods to
determination of minerals and phosphorus in soy-based, whey-based, and enteral formulae (Modification
of AOAC official methods 985.35 and 986.24): Collaborative study. J AOAC Int 80:834-844.
Cook DG, Fahn S, Brait KA. 1974. Chronic manganese intoxication. Arch Neurol 30:59-64.
Cooper RM, Istok JD. 1988. Geostatistics applied to groundwater contamination. II: Application. J
Environ Eng 114:287-299.
Cooper WC. 1984. The health implications of increased manganese in the environment resulting from
the combustion of fuel additives: A review of the literature. J Toxicol Environ Health 14:23-46.
Cotzias GC. 1958. Manganese in health and disease. Physiol Rev 38:503-533.
MANGANESE 452
9. REFERENCES
Cotzias GC, Horiuchi K, Fuenzalida S, et al. 1968. Chronic manganese poisoning: Clearance of tissue
manganese concentrations with persistence of the neurological picture. Neurology 18:376-382.
Cotzias GC, Miller ST, Papavasiliou PS, et al. 1976. Interactions between manganese and brain
dopamine. Med Clin North Am 60:729-738.
Cotzias GC, Papavailiou PS, Miller ST. 1964. Manganese in melanin. Nature 201:1228-1229.
*Cox DN, Traiger GJ, Jacober SP, et al. 1987. Comparison of the toxicity of methylcyclopentadienyl
manganese tricarbonyl with that of its two major metabolites. Toxicol Lett 39:1-5.
*Critchfield JW, Keen CL. 1992. Manganese +2 exhibits dynamic binding to multiple ligands in human
plasma. Metabolism 41:1087-1092.
Critchfield JW, Carl GF, Keen CL. 1993. The influence of manganese supplementation on seizure onset
and severity, brain monoamines in the genetically epilepsy prone rat. Epilepsy Res 14:3-10.
Cross DJ, Minoshima S, Anzai Y, et al. 2004. Statistical mapping of functional olfactory connections of
the rat brain in vivo. Neuroimage 23:1326-1335.
Crossgrove J, Zheng W. 2004. Review article. Manganese toxicity upon overexposure. NMR Biomed
17:544-553.
Crossgrove JS, Yokel RA. 2004. Manganese distribution across the blood–brain barrier III. The divalent
metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology
25(3):451-460.
Crossgrove JS, Yokel RA. 2005. Manganese distribution across the blood-brain barrier IV. Evidence of
brain influx through store-operated calcium channels. Neurotoxicology 26:297-307.
Crump KS. 2000. Manganese exposure in Toronto during use of the gasonline additive,
methylcyclopentadienyl mangenese tricaronyl. J Expo Anal Environ Epidemiol 10(3):227-239.
Crump KS, Rousseau P. 1999. Results from eleven years of neurological health surveillance at a
manganese oxide and salt producing plant. Neurotoxicology 20:273-286.
Curtin D, Ryan J, Chaudhary RA. 1980. Manganese adsorption and desorption in calcareous Lebanese
soils. Soil Sci Soc Am J 44:947-950.
Daniels AJ, Abarca J. 1991. Effect of intranigral Mn2+ on striatal and nigral synthesis and levels of
dopamine and cofactor. Neurotoxicol Teratol 13:483-487.
*Dastur DK, Manghani DK, Raghavendran KV, et al. 1969. Distribution and fate of Mn54 in the rat,
with special reference to the C.N.S. Q J Exp Physiol 54:322-331.
Dastur DK, Manghani DK, Raghavendran KV. 1971. Distribution and fate of 54Mn in the monkey:
Studies of different parts of the central nervous system and other organs. J Clin Invest 50:9-20.
Davidson LA, Lönnerdal B. 1989. Fe-saturation and proteolysis of human lactoferrin: Effect on brush-
border receptor-mediated uptake of Fe and Mn. Am J Physiol 257(6Pt1):G930-934.
MANGANESE 453
9. REFERENCES
Davidsson L, Cederblad A, Hagebo E, et al. 1988. Intrinsic and extrinsic labeling for studies of
manganese absorption in humans. J Nutr 118:1517-1524.
Davidsson L, Cederblad A, Lönnerdal B, et al. 1989a. Manganese retention in man: A method for
estimating manganese absorption in man. Am J Clin Nutr 49:170-179.
Davidsson L, Cederblad A, Lönnerdal B, et al. 1989b. Manganese absorption from human milk, cow's
milk, and infant formulas in humans. Am J Dis Child 143:823-827.
Davis JM. 1998. Methylcyclopentadienyl manganese tricarbonyl: Health risk uncertainties and research
directions. Environ Health Perspect Suppl 106(1):191-201.
Davis CD, Greger JL. 1992. Longitudinal changes of manganese-dependent superoxide dismutase and
other indices of manganese and iron status in women. Am J Clin Nutr 55:747-752.
Davis CD, Malecki EA, Greger JL. 1992a. Interactions among dietary manganese, heme iron and non-
heme iron in women. Am J Clin Nutr 56:926-932.
*Davis CD, Ney DM, Greger JL. 1990. Manganese, iron and lipid interactions in rats. J Nutr 120:507-
513.
Davis CD, Wolf TL, Greger JL. 1992b. Varying levels of manganese and iron affect absorption and gut
endogenous losses of manganese by rats. J Nutr 122:1300-1308.
Davis CD, Zech L, Greger JL. 1993. Manganese metabolism in rats: An improved methodology for
assessing gut endogenous losses. Proc Soc Exp Biol Med 202:103-108.
Davis DW, Hsiao K, Ingels R, et al. 1988. Origins of manganese in air particulates in California. J Air
Pollut Control Assoc 38:1152-1157.
de Carvalho E, Faria V, Loureiro A, et al. 1989. Acute renal failure and nephrotic syndrome after maneb
exposure: A new case with light and electron microscopic study. Acta Med Port 1989 2:215-218.
*de Lamirande E, Tuchweber B, Plaa GL. 1982. Morphological aspects of manganese-bilirubin induced
cholestasis. Liver 2:22-27.
De Méo M, Laget M, Castegnaro M, et al. 1991. Genotoxic activity of potassium permanganate in acidic
solutions. Mutat Res 260:295-306.
Deschamps FJ, Guillamot M, Raux S. 2001. Neurological effects in workers exposed to manganese. J
Occup Environ Med 43(2):127-132.
DEA. 2007. Records and reports of listed chemicals and certain machines. U.S. Drug Enforcement
Administration. Code of Federal Regulations. 21 CFR 1310.02.
http://www.access.gpo.gov/nara/cfr/waisidx_07/21cfrv9_07.html. April 29, 2008.
Deschamps FJ, Guillamot M, Raux S. 2001. Neurological effects in workers exposed to manganese. J
Occup Environ Med 43(2):127-132.
Deskin R, Bursian SJ, Edens FW. 1980. Neurochemical alterations induced by manganese chloride in
neonatal rats. Neurotoxicology 2:65-73.
MANGANESE 454
9. REFERENCES
Deskin R, Bursian SJ, Edens FW. 1981. The effect of chronic manganese administration on some
neurochemical and physiological variables in neonatal rats. Gen Pharmacol 12:279-280.
Desole MS, Esposito G, Migheli R, et al. 1995. Allopurinol protects against manganese-induced
oxidative stress in the striatum and in the brainstem of the rat. Neurosci Lett 192:73-76.
Desole MS, Esposito G, Migheli R, et al. 1997. Glutathione deficiency potentiates manganese toxicity in
rat striatum and brainstem and in PC12 cells. Pharmacol Res 36(4):285-292.
Desole MS, Miele M, Esposito G, et al. 1994. Dopaminergic system activity and cellular defense
mechanisms in the striatum and striatal synaptosomes of the rat subchronically exposed to manganese.
Arch Toxicol 68:566-570.
Devenyi AG, Barron TF, Mamourian AC. 1994. Dystonia, hyperintense basal ganglia, and whole blood
manganese levels in Alagille’s syndrome. Gastroenterology 106:1068-1071.
Deverel SJ, Millard SP. 1988. Distribution and mobility of selenium and other trace elements in shallow
groundwater of the western San Joaquin Valley, California. Environ Sci Technol 22:697-702.
*Dieter HH, Rotard W, Simon J, et al. 1992. Manganese in natural mineral waters from Germany. Die
Nahrung 5:488-484.
Diez-Ewald M, Weintraub LR, Crosby WH. 1968. Interrelationship of iron and manganese metabolism.
Proc Soc Exp Biol Med 129:448-451.
Dikshith TS, Chandra SV. 1978. Cytological studies in albino rats after oral administration of
manganese chloride. Bull Environ Contam Toxicol 19:741-746.
Doisy EA. 1973. Effects of deficiency in manganese upon plasma levels of clotting proteins and
cholesterol in man. Trace element metabolism. In: Hoekstra WG, Suttie JW, Ganther AE, et al., eds.
Animals-2, 2nd Ed. Baltimore, MD: University Park Press, 668-670.
Donaldson J. 1987. The physiopathologic significance of manganese in brain: Its relation to
schizophrenia and neurodegenerative disorders. Neurotoxicology 8:451-462.
Dorman DC, Brenneman KA, McElveen AM, et al. 2002a. Olfactory transport: A direct route of
delivery of inhaled manganse phosphate to the rat brain. J Toxicol Environ Health 65(20):1493-1511.
Dorman DC, McElveen AM, Marshall MW, et al. 2005b. Maternal-fetal distribution of manganese in
the rat following inhalation exposure to manganese sulfate. NeuroToxicology 26:625-632.
Dorman DC, McElveen AM, Marshall MW, et al. 2005a. Tissue manganese concentrations in lactating
rats and their offspring following combined in utero and lactation exposure to inhaled manganese sulfate.
Toxicol Sci 84:12-21.
Dorman DC, McManus BE, Marshall MW, et al. 2004a. Old age and gender influence the
pharmacokinetics of inhales manganese sulfate and manganese phosphate in rats. Toxicol Appl
Pharmacol 197:113-124.
MANGANESE 455
9. REFERENCES
Dorman DC, McManus BE, Parkinson CU, et al. 2004b. Nasal toxicity of manganese sulfate and
manganese phosphate in young male rats following subchronic (13-week) inhalation exposure. Inhal
Toxicol 16(6-7):481-488.
Dorman DC, Struve MF, Gross EA, et al. 2005c. Sub-chronic inhalation of high concentrations of
manganese sulfate induces lower airway pathology in rhesus monkeys. Respir Res 6(1):121.
http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1283983&blobtype=pdf. May 5, 2008.
Dorman DC, Struve MF, James RA, et al. 2001b. Influence of dietary manganese on the
pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol Sci 60:242-251.
Dorman DC, Struve MF, James RA, et al. 2001a. Influence of particle solubility on the delivery of
inhaled manganese to the rat brain: Manganese sulfate and manganese tetroxide pharmacokinetics
following repeated (14-day) exposure. Toxicol Appl Pharmacol 170:79-87.
Dorman DC, Struve MF, Marshall MW, et al. 2006a. Tissue manganese concentrations in young male
Rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol Sci 92(1):201-210.
Dorman DC, Struve MF, Vitarella D, et al. 2000. Neurotoxicity of manganese chloride in neonatal and
adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Tox 20(3):179-187.
*Dorman DC, Struve MF, Wong BA. 2002b. Brain manganese concentrations in rats following
manganese tetroxide inhalation are unaffected by dietary manganese intake. Neurotoxicology 23(2):185-
195.
Dorman DC, Struve MF, Wong, et al. 2006b. Correlation of brain magnetic resonance imaging changes
with pallidal manganese concentrations in Rhesus monkeys following subchronic manganese inhalation.
Toxicol Sci 92(1):219-227.
Dorner K, Dziadzka S, Hohn A, et al. 1989. Longitudinal manganese and copper balances in young
infants and preterm infants fed on breast-milk and adapted cow's milk formulas. Br J Nutr 61:559-572.
*Droms KA, Malkinson AM. 1991. Mechanisms of glucocorticoid involvement in mouse lung
tumorigenesis. Exp Lung Res 17:359-370.
Drown DB, Oberg SG, Sharma RP. 1986. Pulmonary clearance of soluble and insoluble forms of
manganese. J Toxicol Environ Health 17:201-212.
DuPuis MD, Hill HH. 1979. Analysis of gasoline for antiknock agents with a hydrogen atmosphere
flame ionization detector. Anal Chem 51:292-295.
Dupuis Y, Porembska Z, Tardivel S, et al. 1992. Intestinal transfer of manganese: Resemblance to and
competition with calcium. Reprod Nutr Dev 32:453-460.
Earls JP, Bluemke DA. 1999. New MR imaging contrast agents. Magn Reson Imaging Clin N Am
7:255-273.
Eckel WP, Langley WD. 1988. A background-based ranking technique for assessment of elemental
enrichment in soils at hazardous waste sites. In: Superfund '88: Proceedings of the 9th National
Conference. Washington, DC, 282-286.
MANGANESE 456
9. REFERENCES
Egyed M, Wood GC. 1996. Risk assessment for combustion products of the gasoline additive MMT in
Canada. Sci Total Environ 189/190:11-20.
Ejima A, Imamura T, Nakamura S, et al. 1992. Manganese intoxication during total parenteral nutrition
[Letter]. Lancet 339:426.
Elbetieha A, Bataineh H, Darmani H, et al. 2001. Effects of long-term exposure to manganese chloride
on fertility of male and female mice. Toxicol Lett 119:193-201.
Elder A, Gelein R, Silva V, et al. 2006. Translocation of inhaled ultrafine manganese oxide particles to
the central nervous system. Environ Health Perspect 114(8):1172-1178.
Elias Z, Mur JM, Pierre F, et al. 1989. Chromosome aberrations in peripheral blood lymphocytes of
welders and characterization of their exposure by biological samples analysis. J Occup Med 31:477-483.
Elizondo G, Fretz CJ, Stark DD, et al. 1991. Preclinical evaluation of MnDPDP: New paramagnetic
hepatobiliary contrast agent for MR imaging. Radiology 178:73-78.
Ellingsen DG, Hetland SM, Thomassen Y. 2003c. Manganese air exposures assessment and biological
monitoring in the manganese alloy production industry. J Environ Monit 5(1):84-90.
*El-Rahman SS. 2004. Assessment of neuropathology, amino acid profile and bioaccumulation
following sub chronic inhalation of manganese phosphate (as one of gasoline combustion products) in
male sprague-dawley rats. Vet Med J 52(4):495-506.
Emara AM, El-Ghawabi SH, Madkour OI, et al. 1971. Chronic manganese poisoning in the dry battery
industry. Br J Ind Med 28:78-82.
Ensing JG. 1985. Bazooka: Cocaine-base and manganese carbonate. J Anal Toxicol 9:45-46.
EPA. 1977. Inhalation toxicology of airborne particulate manganese in rhesus monkeys. Research
Triangle Park, NC: U.S. Environmental Protection Agency. EPA600177026. PB268643.
EPA. 1978. U.S. Environmental Protection Agency. Fed Regist 43:41424-41429.
EPA. 1979a. Regulation of fuel and fuel additives MMT. Lifting of suspension of enforcement. U.S.
Environmental Protection Agency. Fed Regist 44:58952-58965.
*EPA. 1979b. Sources of toxic pollutants found in influents to sewage treatment plants. VI. Integrated
interpresentation. Washington, DC: U.S. Environmental Protection Agency, Office of Water Planning
and Standards. EPA 4404008. PB81219685.
EPA. 1981. Ethyl Corp: Denial of application for fuel wiaver; summary of decision. U.S.
Environmental Protection Agency. Fed Regist 46:58360.
EPA. 1983a. Manganese: Atomic-absorption, direct aspiration—method 243.1. In: Methods for
chemical analysis of water and wastes. Cincinnati, OH: U.S. Environmental Protection Agency, Office
of Research and Development. EPA600479020.
MANGANESE 457
9. REFERENCES
EPA. 1983b. Manganese. Method 243.2. Atomic absorption, furnace technique. In: Methods for
chemical analysis of water and wastes. Cincinnati, OH: U.S. Environmental Protection Agency, 243.2-1
to 243.2-2. EPA600479020.
EPA. 1983c. Human exposure to atmospheric concentrations of selected chemicals. Vol. II. Report to
U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Research Triangle
Park, NC, by Systems Applications, Incorporated, San Rafael, CA. PB83265249.
EPA. 1984. Health assessment document for manganese. Final draft. Cincinnati, OH: U.S.
Environmental Protection Agency, Office of Research and Development. EPA600883013F.
*EPA. 1985a. Chemical identity—manganese, tricarbonyl methylcyclopentadienyl. Cincinnati, OH:
U.S. Environmental Protection Agency, Office of Toxic Substances.
*EPA. 1985b. Chemical, physical and biological properties of compounds present at hazardous waste
sites. Report to U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response,
Washington, DC, by Clement Associates, Inc., Arlington, VA.
EPA. 1985c. Locating and emitting air emissions from sources of manganese. Research Triangle Park,
NC: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards.
EPA450484007h.
EPA. 1985d. Decision not to regulate manganese under the Clean Air Act. U.S. Environmental
Protection Agency. Fed Regist 50:32627-32628.
*EPA. 1986a. Acid digestion of sediments, sludges, and soils—method 3050. In: Test methods for
evaluating solid waste. 3rd ed. SW-846. Washington, DC: U.S. Environmental Protection Agency,
Office of Solid Waste and Emergency Response.
*EPA. 1986b. Inductively coupled plasma atomic emission spectroscopy—method 6010. In: Test
methods for evaluating solid waste. 3rd ed. SW-846. Washington, DC: U.S. Environmental Protection
Agency, Office of Solid Waste and Emergency Response.
EPA. 1986c. Manganese (atomic absorption, direct aspiration)—method 7460. In: Test methods for
evaluating solid waste. 3rd ed. SW-846. Washington, DC: U.S. Environmental Protection Agency,
Office of Solid Waste and Emergency Response.
EPA. 1986d. Air quality criteria for lead. Research Triangle Park, NC: U.S. Environmental Protection
Agency, Office of Research and Development, Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office. EPA600833028F.
EPA. 1987a. Toxic air pollutant/source crosswalk: A screening tool for locating possible sources
emitting toxic air pollutants. Research Triangle Park, NC: U.S. Environmental Protection Agency,
Office of Air Quality Planning and Standards. EPA450487023a.
*EPA. 1987b. U.S. Environmental Protection Agency: Part II. Fed Regist 52:13400.
EPA. 1988. Recommendations for the documentation of biological values for use in risk assessment.
Cincinnati, OH: U. S. Environmental Protection Agency. PB88179874.
MANGANESE 458
9. REFERENCES
EPA. 1990. Interim methods for development of inhalation reference concentrations. Washington, DC:
U.S. Environmental Protection Agency, Office of Health and Environmental Assessment, Office of
Research and Development, Environmental Criteria and Assessment Office. EPA600890066A.
*EPA. 1993a. U.S. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 302.4.
EPA. 1993b. Drinking water criteria document for manganese. Cincinnati, OH: U.S. Environmental
Protection Agency. ECAO-CIN-D008
EPA. 1994b. Method 200.8. Determination of trace elements in waters and wastes by inductively
coupled plasma-mass spectrometry. Revision 5.4. EMMC version. U.S. Environmental Protection
Agency. http://www.epa.gov/waterscience/methods/method/files/200_8.pdf. May 02, 2008.
EPA. 1994a. Methods for derivation of inhalation reference concentrations and application of inhalation
dosimetry. Washington, DC: U.S. Environmental Protection Agency, Office of Research and
Development. EPA600890066F.
EPA. 1994. Reevaluation of inhalation health risks associated with methylcyclopentadienyl manganese
tricarbonyl (MMT) in gasoline. U.S. Environmental Protection Agency. EPA600R94062.
*EPA. 1995a. Fuels and fuel additives; grant of waiver application. Fed Regist 60. U.S. Environmental
Protection Agency.:36414. http://frwebgate4.access.gpo.gov/cgi-
bin/PDFgate.cgi?WAISdocID=279770422119+5+1+0&WAISaction=retrieve. July 28, 2008.
EPA. 1995b. Proceedings: Workshop on the bioavailability and oral toxicity of manganese.
Washington, DC: Environmental Criteria and Assessment Office, Office of Research and Development,
Office of Science and Technology, Office of Water, U.S. Environmental Protection Agency.
EPA. 1997. Special report on environmental endocrine disruption: An effects assessment and analysis.
Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum. EPA630R96012.
EPA. 1998. Announcement of the drinking water contaminant candidate list. U.S. Environmental
Protection Agency. Fed Regist 63:10274-10287. http://frwebgate.access.gpo.gov/cgi-
bin/getdoc.cgi?IPaddress=frwais.access.gpo.gov&dbname=1998_register&docid=98-5313-filed.pdf.
May 5, 2008.
EPA. 2000. Benchmark dose technical guidance document. Washington, DC: U.S. Environmental
Protection Agency. EPA630R00001.
EPA. 2003a. Health effects support document for manganese. U.S. Environmental Protection Agency.
EPA822R03003.
http://www.epa.gov/safewater/ccl/pdfs/reg_determine1/support_cc1_magnese_healtheffects.pdf. April
07, 2008.
EPA. 2003b. National primary drinking water regulations. Washington, DC: U.S. Environmental
Protection Agency, Office of Ground Water and Drinking Water. EPA816F03016.
http://www.epa.gov/safewater/mcl.html. March 07, 2006.
EPA. 2004. Drinking water health advisory for manganese. U.S. Environmental Protection Agency.
http://www.epa.gov/safewater/ccl/pdfs/reg_determine1/support_cc1_magnese_dwreport.pdf. June 19,
2008.
MANGANESE 459
9. REFERENCES
EPA. 2005. Toxic chemical release inventory reporting forms and instructions: Revised 2004 version.
Section 313 of the Emergency Planning and Community Right-to-Know Act (Title III of the Superfund
Amendments and Reauthorization Act of 1986). U.S. Environmental Protection Agency. Office of
Environmental Information. EPA260B05001.
EPA. 2006a. 2006 Edition of the drinking water standards and health advisories. Washington, DC: U.S.
Environmental Protection Agency. EPA822R06013.
http://www.epa.gov/waterscience/criteria/drinking/dwstandards.pdf. April 11, 2007.
EPA. 2006b. High production volume (HPV) challenge program. Final submission for
methylcyclopentadienyl manganese tricarbonyl (MMTr). U.S. Environmental Protection Agency.
http://www.epa.gov/chemrtk/pubs/summaries/mthmntri/c14889rt.pdf. April 10, 2008.
EPA. 2006c. National recommended water quality criteria. Washington, DC: U.S. Environmental
Protection Agency, Office of Water, Office of Science and Technology.
http://www.epa.gov/waterscience/criteria/nrwqc-2006.pdf. January 08, 2008.
EPA. 2007a. Method 6010C. Inductively coupled plasma-atomic emission spectrometry. U.S.
Environmental Protection Agency. http://www.epa.gov/sw-846/pdfs/6010c.pdf. May 02, 2008.
EPA. 2007b. 2006 Urban air toxics monitoring program (UATMP) final report. U.S. Environmental
Protection Agency. EPA454R08001.
http://www.epa.gov/ttnamti1/files/ambient/airtox/2006_uatmp_final_report.pdf. May 02, 2008.
EPA. 2008a. Acute exposure guideline levels (AEGLs). Second AEGL chemical priority list. U.S.
Environmental Protection Agency. http://www.epa.gov/oppt/aegl/pubs/priority_2.htm. April 24, 2008.
EPA. 2008b. Designation of hazardous substances. U.S. Environmental Protection Agency. Code of
Federal Regulations.40 CFR 116.4. http://www.epa.gov/lawsregs/search/40cfr.html. April 24, 2008.
EPA. 2008c. Designation of hazardous substances. U.S. Environmental Protection Agency. Code of
Federal Regulations. 40 CFR 302.4. http://www.epa.gov/lawsregs/search/40cfr.html. April 24, 2008.
EPA. 2008d. Determination of reportable quanities. U.S. Environmental Protection Agency. Code of
Federal Regulations. 40 CFR 117.3. http://www.epa.gov/lawsregs/search/40cfr.html. April 24, 2008.
EPA. 2008e. Inert ingredients permitted for use in nonfood use pesticide products. Washington, DC:
U.S. Environmental Protection Agency. http://www.epa.gov/opprd001/inerts/lists.html. April 24, 2008.
EPA. 2008f. The list of extremely hazardous substances and their threshold planning quantities. U.S.
Environmental Protection Agency. Code of Federal Regulations. 40 CFR 355, Appendix A.
http://www.epa.gov/lawsregs/search/40cfr.html. April 24, 2008.
EPA. 2008g. Toxic chemical release reporting. Chemicals and chemical categories to which this part
applies. U.S. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 372.65.
http://www.epa.gov/lawsregs/search/40cfr.html. April 24, 2008.
EPA. 2011. Emissions inventory. U.S. Environmental Protection Agency.
http://www.epa.gov/ttn/chief/eiinformation.html. November 28, 2011.
MANGANESE 460
9. REFERENCES
Erikson K, Aschner M. 2002. Manganese causes differential regulation of glutamate transporter
(GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology 23(4-
5):595-602.
Erikson KM, Aschner M. 2003. Manganese neurotoxicity and glutamate-GABA interaction.
Neurochem Int 43:475-480.
Erikson KM, Dorman DC, Fitsanakis V, et al. 2006. Alterations of oxidative stress biomarkers due to in
utero and neonatal exposures of airborne manganese. Biol Trace Elem Res 111(1-3):199-215.
Erikson KM, Dorman DC, Lash LH, et al. 2007. Manganeses inhalation by Rhesus monkeys is
associated with brain regional changes in biomarkers of neurotoxicity. Toxicol Sci 97(2):459-466.
Erikson KM, Dorman DC, Lash LH, et al. 2008. Duration of airborne-manganese exposure in rhesus
monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Neurotoxicology
29(3):377-385.
Erikson KM, John CE, Jones SR, et al. 2005. Manganese accumulation in striatum of mice exposed to
toxic doses is dependent upon a functional dopamine transporter. Environ Toxicol Pharmacol 20:390-
394.
Eriksson H, Gillberg PG, Aquilonius SM, et al. 1992a. Receptor alterations in manganese intoxicated
monkeys. Arch Toxicol 66:359-364.
Eriksson H, Lenngren S, Heilbronn E. 1987a. Effect of long-term administration of manganese on
biogenic amine levels in discrete striatal regions of rat brain. Arch Toxicol 59:426-431.
Eriksson H, Magiste K, Plantin LO, et al. 1987b. Effects of manganese oxide on monkeys as revealed by
a combined neurochemical, histological and neurophysiological evaluation. Arch Toxicol 61:46-52.
Eriksson H, Tedroff J, Thuomas K, et al. 1992b. Manganese induced brain lesions in Macaca
fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol
66:403-407.
Evans LJ. 1989. Chemistry of metal retention by soils: Several processes are explained. Environ Sci
Technol 23:1046-1056.
Exon JH, Koller LD. 1975. Effects of feeding manganese antiknock gasoline additive exhaust residues
(Mn
3
O
4
) in rats. Bull Environ Contam Toxicol 14:370-373.
Farias AC, Cunha A, Benko CR, et al. 2010. Manganese in children with attention-deficit/hyperactivity
disorder: Relationship with methylphenidate exposure. J Child Adolesc Psychopharmacol 20(2):113-
118.
FDA. 2007a. Beverages. Bottled water. U.S. Food and Drug Administration. Code of Federal
Regulations. 21 CFR 165.110. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm.
April 24, 2008.
FDA. 2007b. Indirect food additives: Adhesives and components of coatings. U.S. Food and Drug
Administration. Code of Federal Regulations. 21 CFR 175. 105.
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm. April 24, 2008.
MANGANESE 461
9. REFERENCES
FDA. 2007c. Food ingredients and packaging. Summary of color additives listed for use in the United
States in food, drugs, cosmetics, and medical devices. U.S. Department of Health and Human Services.
U.S. Food and Drug Administration. Center for Food Safety and Applied Nutrition.
http://www.cfsan.fda.gov/~dms/opa-col2.html. June 17, 2008.
FDA. 2008. Everything added to food in the United States (EAFUS). U.S. Food and Drug
Administration. http://vm.cfsan.fda.gov/~dms/eafus.html. April 24, 2008.
Fechter LD, Johnson DL, Lynch RA. 2002. The relationship of particle size to Olfactory nerve uptake of
non-soluble form of manganese into brain. Neurotoxicology 23:177-183.
Federle MP, Chezmar JL, Rubin DL, et al. 2000. Safety and efficacy of mangafodipir trisodium
(MnDPDP) injection for hepatic MRI in adults: Results of the U.S. multicenter phase III clinical trials
(safety). J Magn Reson Imaging 12(1):186-197.
FEDRIP. 2008. Manganese. Federal Research in Progress database. Springfield, VA: National
Technical Information Service.
Fell JM, Reynolds AP, Meadows N, et al. 1996. Manganese toxicity in children receiving long-term
parenteral nutrition. Lancet 347:1218-1221.
Fernandez MA, Martinez L, Segarra M, et al. 1992. Behavior of heavy metals in the combustion gases of
urban waste incinerators. Environ Sci Technol 26:1040-1047.
Finley JW, Caton JS, Zhou Z, et al. 1997. A surgical model for determination of true adsorption and
biliary excretion of manganese in conscious swine fed commercial diets. J Nutr 127:2334-2341.
Finley JW, Penland JG, Pettit RE, et al. 2003. Dietary manganese intake and type of lipid do not affect
clinical or neuropsychological measures in healthy young women. J Nutr 133:2849-2856.
*Fishman BE, McGinley PA, Gianutsos G. 1987. Neurotoxic effects of methylcyclopentadienyl
manganese tricarbonyl (MMT) in the mouse: Basis of MMT-induced seizure activity. Toxicology
45:193-201.
Fitsanakis VA, Aschner M. 2005. The importance of glutamate, glycine, and γ-aminobutyric acid
transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol
204:343-354.
Fitsanakis VA, Au C, Erikson KM, et al. 2006. The effects of manganese on glutamate, dopamine and y-
aminobutyric acid regulation. Neurochem Int 48:426-433.
*Flaten TP, Bolviken B. 1991. Geographical associations between drinking water chemistry and the
mortality and morbidity of cancer and some other diseases in Norway. Sci Total Environ 102:75-100.
MANGANESE 462
9. REFERENCES
FNB/IOM. 2001. Manganese. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron,
chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc (2000). A
Report of the Panel on Micronutrients, subcommittees on upper reference levels of nutrients and of
interpretation and uses of dietary reference intakes, and the standing committee on the scientific
evaluation of dietary reference intakes. Washington, DC: Food and Nutrition Board. Institute of
Medicine. National Academy Press, 394-419.
http://books.nap.edu/openbook.php?record_id=10026&page=394. April 03, 2008.
Folsom TR, Young DR, Johnson JN, et al. 1963. Manganese-54 and zinc-65 in coastal organisms of
California. Nature 200:327-329.
Fomon SJ. 1966. Body composition of the infant: Part I: The male reference infant. In: Falkner F, ed.
Human development. Philadelphia, PA: WB Saunders, 239-246.
Fomon SJ, Haschke F, Ziegler EE, et al. 1982. Body composition of reference children from birth to age
10 years. Am J Clin Nutr 35(Suppl 5):1169-1175.
Forbes GM, Forbes A. 1997. Micronutrient status in patients receiving home parenteral nutrition.
Nutrition 13:941-944.
Fore H, Morton RA. 1952. Manganese in rabbit tissues. Biochem J 51:600-603.
Francis AJ. 1985. Anaerobic microbial dissolution of toxic metals in subsurface environments. Upton,
NY: Brookhaven National Laboratory. BNL-36571.
Freeland-Graves JH, Bales CW, Behmardi F. 1987. Manganese requirements of humans. Nutritional
bioavailability of manganese. American Chemical Society, 90-104.
Friedman BJ, Freeland-Graves JH, Bales CW, et al. 1987. Manganese balance and clinical observations
in young men fed a manganese-deficient diet. J Nutr 117:133-143.
Furchner JE, Richmond CR, Drake GA. 1966. Comparative metabolism of radionuclides in mammals
III. Health Phys 12:1415-1423.
Furst A. 1978. Tumorigenic effect of an organomanganese compound on F344 rats and Swiss albino
mice [Brief communication]. J Natl Cancer Inst 60:1171-1173.
Gaind VS, Vohra K, Chai F. 1992. Determination of tricarbonyl(2-methylcyclopentadienyl) manganese
in gasoline and air by gas chromatography with electron-capture detection. Analyst 117:161-164.
Gallez B, Baudelet C, Adline J, et al. 1997. Accumulation of manganese in the brain of mice after
intravenous injection of manganese-based contrast agents. Chem Res Toxicol 10:360-363.
Galloway SM, Armstrong MJ, Reuben C, et al. 1987. Chromo-some aberrations and sister chromatid
exchanges in Chinese hamster ovary cells: Evaluations of 108 chemicals. Environ Mol Mutagen 1
(Suppl. 10):1-175.
Garcia SJ, Gellein K, Syversen T, et al. 2006. A manganese-enchanced diet alters brain metals and
transporters in the developing rat. Toxicol Sci 92(2):516-525.
MANGANESE 463
9. REFERENCES
Garcia SJ, Gellein K, Syversen T, et al. 2007. Iron deficient and manganese supplemented diets alter
metals and transporters in the developing rat brain. Toxicol Sci 95(1):205-217.
Garcia-Aranda JA, Lifshitz F, Wapnir RA. 1984. Intestinal absorption of manganese in experimental
malnutrition. J Pediatr Gastroenterol Nutr 3:602-607.
Garcia-Aranda JA, Wapnir RA, Lifshitz F. 1983. In vivo intestinal absorption of manganese in the rat. J
Nutr 113:2601-2607.
Garner CD, Nachtman JP. 1989b. Manganese catalyzed auto-oxidation of dopamine to 6-
hydroxydopamine in vitro. (Erratum in: Chem Biol Interact 71(2-3):309). Chem Biol Interact 69:345-
351.
Garrison AW, Cipollone MG, Wolfe NL, et al. 1995. Environmental fate of methylcyclopentadienyl
manganese tricarbonyl. Environ Toxicol Chem 14(11):1859-1864.
Gavin CE, Gunter KK, Gunter TE. 1990. Manganese and calcium efflux kinetics in brain mitochondria.
Relevance to manganese toxicity. Biochem J 266:329-334.
Gavin CE, Gunter KK, Gunter TE. 1992. Mn2+ sequestration by mitochondria and inhibition of
oxidative phosphorylation. Toxicol Appl Pharmacol 115:1-5.
Gavin CE, Gunter KK, Gunter TE. 1999. Manganese and calcium transport in mitochondria:
Implications for manganese toxicity. Neurotoxicology 20:445-454.
Geering HR, Hodgson JF, Sdano C. 1969. Micronutrient cation complexes in soil solution: IV. The
chemical state of manganese in soil solution. Soil Sci Soc Amer Proc 33:81-85.
Gennart JP, Buchet JP, Roels H, et al. 1992. Fertility of male workers exposed to cadmium, lead, or
manganese. Am J Epidemiol 135:1208-1219.
*Gerdin B, McCann E, Lundberg C, et al. 1985. Selective tissue accumulation of manganese and its
effect on regional blood flow and hemodynamics after intravenous infusion of its chloride salt in the rat.
Int J Tissue React 7(5):373-380.
Gianutsos G, Murray MT. 1982. Alterations in brain dopamine and GABA following inorganic or
organic manganese administration. Neurotoxicology 3:75-81.
Gianutsos G, Morrow GR, Morris JB. 1997. Accumulation of manganese in rat brain following
intranasal administration. Fundam Appl Toxicol 37:102-105.
Gianutsos G, Seltzer MD, Saymeh R, et al. 1985. Brain manganese accumulation following systemic
administration of different forms. Arch Toxicol 57(4):272-275.
Gibbons RA, Dixon SN, Hallis K, et al. 1976. Manganese metabolism in cows and goats. Biochim
Biophys Acta 444:1-10.
Gibbs JP, Crump KS, Houck DP, et al. 1999. Focused medical surveillance: A search for subclinical
movement disorders in a cohort of U.S. workers exposed to low levels of manganese dust.
Neurotoxicology 20:299-313.
MANGANESE 464
9. REFERENCES
Giwercman A, Carlsen E, Keiding N, et al. 1993. Evidence for increasing incidence of abnormalities of
the human testis: A review. Environ Health Perspect Suppl 101(2):65-71.
Glass E. 1955. Untersuchungen uber die einwirkung von schwermetallsalzen auf dir
wurzelspitzenmitose von Vicia faba. Zeitschrift fuer Botanik 43:359-403.
Glass E. 1956. Untersuchungen uber die einwirkung von schwermetallsalzen auf dir
wurzelspitzenmitose von Vicia faba. Zeitschrift fuer Botanik 44:1-58.
Goering PL, Klaassen CD. 1985. Mechanism of manganese-induced tolerance to cadmium lethality and
hepatotoxicity. Biochem Pharmacol 34:1371-1379.
Goldsmith J, Herishanu Y, Abarbanel J, et al. 1990. Clustering of Parkinson's disease points to
environmental etiology. Arch Env Health 45(2):88-94.
Golub MS, Hogrefe CE, Germann SL, et al. 2005. Neurobehavioral evaluation of rhesus monkey infants
fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol
27(4):615-627.
*Gordon CJ, Fogelson L, Highfill JW. 1990. Hypothermia and hypometabolism: Sensitive indices of
whole-body toxicity following exposure to metallic salts in the mouse. J Toxicol Environ Health 29:185-
200.
*Gorell JM, Johnson CC, Rybicki BA, et al. 1997. Occupational exposures to metals as risk factors for
Parkinson's disease. Neurology 48:137-145.
Gorell JM, Johnson CC, Rybicki BA, et al. 1999. Occupational exposure to manganese, copper, lead,
iron, mercury, and zinc and the risk of Parkinson’s disease. Neurotoxicology 20:239-248.
Gottschalk LA, Rebello T, Buchsbaum MS, et al. 1991. Abnormalities in hair trace elements as
indicators of aberrant behavior. Comp Psych 32:229-237.
Graedel TE. 1978. Inorganic elements, hydrides, oxides, and carbonates. In: Chemical compounds in
the atmosphere. New York, NY: Academic Press, 35-41, 44-49.
Graham DG. 1984. Catecholamine toxicity: A proposal for the molecular pathogenesis of manganese
neurotoxicity and Parkinson's disease. Neurotoxicology 5:83-95.
Grant D, Blazak WF, Brown GL. 1997a. The reproductive toxicology of intravenously administered
MnDPDP in the rat and rabbit. Acta Radiol 38:759-769.
Grant D, Refsum H, Rummeny E, et al. 1997b. Editorial on MnDPDP. Acta Radiol 38:623-625.
Grant D, Zech K, Holtz E. 1994. Biodistribution and in vivo stability of manganese dipyridoxyl
diphosphate in relation to imaging efficacy. Invest Radiol 29:S249-S250.
Gray LE, Laskey JW. 1980. Multivariate analysis of the effects of manganese on the reproductive
physiology and behavior of the male house mouse. J Toxicol Environ Health 6:861-867.
Greger JL. 1998. Dietary standards for manganese: Overlap between nutritional and toxicological
studies. J Nutr 128(2 Suppl):368S-371S.
MANGANESE 465
9. REFERENCES
Greger JL. 1999. Nutrition versus toxicology of manganese in humans: Evaluation of potential
biomarkers. Neurotoxicology 20:205-212.
Greger JL, Davis CD, Suttie JW, Lyle BJ, et al. 1990. Intake, serum concentrations and urinary
excretion of manganese by adult males. Am J Clin Nutr 51(3):457-461.
Gruden N, Matausic S. 1989. Some factors influencing cadmium-manganese interaction in adult rats.
Bull Environ Contam Toxicol 43:101-106.
Guilarte TR, Burton NC, Verina T, et al. 2008. Increased APLP1 expression and neurodegeneration in
the frontal cortex of manganese-exposed non-human primates. J Neurochem [Epub ahead of print]:1-12.
Guilarte TR, Chen M, McGlothan JL. 2006a. Nigrostriatal dopamine system dysfunction and subtle
motor deficits in manganese-exposed non-human primates. Exp Neurol 2002:381-390.
Guilarte TR, McGlothan JL, Degaonkar M, et al. 2006b. Evidence for cortical dysfunction and
widespread manganese accumulation in the nonhuman primate brain following chronic manganese
exposure: A 1H-MRS and MRI study. Toxicol Sci 94(2):351-358.
Gupta SK, Murthy RC, Chandra SV. 1980. Neuromelanin in manganese-exposed primates. Toxicol Lett
6:17-20.
Guzelian PS, Henry CJ, Olin SS, eds. 1992. Similarities and differences between children and adults:
Implications for risk assessment. Washington, DC: International Life Sciences Institute Press.
Gwiazda R, Lucchini R, Smith D. 2007. Adequacy and consistency of animal studies to evaluate the
neurotoxicity of chronic low-levle manganese expsosure to humans. J Toxicol Environ Health 70(7):594-
605.
Haddad CM, Shannon MW, Winchester JF, eds. 1998. In: Clinical management of poisoning and drug
overdose. 3rd ed. Philadelphia, PA: WB Saunders, 796-797.
Hafeman D, Factor-Litvak P, Cheng Z, et al. 2007. Association between manganese exposure through
drinking water and infant mortality in Bangladesh. Environ Health Perspect 115:1107-1112.
Hafeman D, Factor-Litvak P, Cheng Z, et al. 2007. Association between manganese exposure through
drinking water and infant mortality in Bangladesh. Environ Health Perspect 115:1107-1112.
*Hakkinen PJ, Haschek WM. 1982. Pulmonary toxicity of methylcyclopentadienyl manganese
tricarbonyl: Nonciliated bronchiolar epithelial (Clara) cell necrosis and alveolar damage in the mouse,
rat, and hamster. Toxicol Appl Pharmacol 65:11-22.
Halatek T, Hermans C, Broeckaert F, et al. 1998. Quantification of Clara cell protein in rat and mouse
biological fluids using a sensitive immunoassay. Eur Respir J 11:726-733.
Halliwell B. 1984. Manganese ions, oxidation reactions and the superoxide radical. Neurotoxicology
5:113-118.
HaMai D, Rinderknecht AL, Guo-Sharman K, et al. 2006. Decreased expression of inflammation-related
genes following inhalation exposure to manganese. Neurotoxicology 27:395-401.
MANGANESE 466
9. REFERENCES
Hambidge KM, Sokol RJ, Fidanza SJ, et al. 1989. Plasma manganese concentrations in infants and
children receiving parenteral nutrition. J Parenter Enteral Nutr 13(2):168-171.
Hanzlik RP, Bhatia P, Stitt R, et al. 1980a. Biotransformation and excretion of methylcyclopentadienyl
manganese tricarbonyl in the rat. Drug Metab Dispos 8:428-433.
Hanzlik RP, Harkness CE, Arnoldi S. 1979. Gas chromatographic determination of
methylcyclopentadienyl manganese tricarbonyl in biological tissues and fluids. J Chromatogr 171:279-
283.
Hanzlik RP, Stitt R, Traiger GJ. 1980b. Toxic effects of methylcyclopentadienyl manganese tricarbonyl
(MMT) in rats: Role of metabolism. Toxicol Appl Pharmacol 56:353-360.
Hauser RA, Zesiewicz TA, Martinez C, et al. 1996. Blood manganese correlates with brain magnetic
resonance imaging changes in patients with liver disease. Can J Neurol Sci 23:95-98.
Hauser RA, Zesiewicz TA, Rosemurgy AS, et al. 1994. Manganese intoxication and chronic liver
failure. Ann Neurol 36:871-875.
HazDat. 2007. Manganese. HazDat Database: ATSDR’s Hazardous Substance Release and Health
Effects Database. Atlanta, GA: Agency for Toxic Substances and Disease Registry.
http://www.atsdr.cdc.gov/hazdat.html. May 1, 2008.
Hazell AS, Normandin L, Norenberg MD, et al. 2006. Alzheimer type II astrocytic changes following
sub-acute exposure to manganese and its prevention by antioxidant treatment. Neurosci Lett 396:167-
171.
He P, Liu D, Zhang G, et al. 1994. [Effects of high-level manganese sewage irrigation on children’s
neurobehavior.] Chung Hua Yu Fang I Hsueh Tsa Chih 28:216-218. (Chinese).
Health Canada. 2008. Human health risk assessment for inhaled manganese. Draft. Health Canada.
http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/air/out-
ext/_consult/draft_ebauche/manganese_e.pdf. May 07, 2008.
Health Canada. 2010. Human health risk assessment for inhaled manganese. Ottawa, Ontario: Health
Canada.
Hellou J, Fancey LL, Payne JF. 1992. Concentrations of twenty-four elements in bluefin tuna, Thunnus
thynnus from the Northwest Atlantic. Chemosphere 24:211-218.
Helz GR, Huggett RJ, Hill JM. 1975. Behavior of Mn, Fe, Cu, Zn, Cd and Pb discharged from a
wastewater treatment plant into an estuarine environment. Water Research 9:631-636.
Hemstock GA, Low PF. 1953. Mechanisms responsible for retention of manganese in the colloidal
fraction of soil. Soil Science 76:331-343.
Henriksson J, Tallkvist J, Tjälve H. 1999. Transport of manganese via the olfactory pathway in rats:
Dosage dependency of the uptake and subcellular distribution of the metal in the olfactory epithelium and
the brain. Toxicol Appl Pharmacol 156:119-128.
MANGANESE 467
9. REFERENCES
Hernandez-Bonilla D, Schilmann A, Montes S, et al. 2011. Environmental exposure to manganese and
motor function of children in Mexico. Neurotoxicology 32(5):615-621.
Hinderer RK. 1979. Toxicity studies of methylcyclopentadienyl manganese tricarbonyl (MMT). Am Ind
Hyg Assoc J 40:164-167.
Hiney JK, Srivastava VK, Dees WL. 2011. Manganese induces IGF-1 and cyclooxygenase-2 gene
expressions in the basal hypothalamus during prepubertal female development. Toxicol Sci 121(2):389-
396.
Hobbesland A, Kjuus H, Thelle DS. 1997a. Mortality from nonmalignant respiratory diseases among
male workers in Norwegian ferroalloy plants. Scand J Work Environ Health 23:342-350.
Hobbesland A, Kjuus H, Thelle DS. 1997b. Mortality from cardiovascular diseases and sudden death in
ferroalloy plants. Scand J Work Environ Health 23:334-341.
Hoel DG, Davis DL, Miller AB, et al. 1992. Trends in cancer mortality in 15 industrialized countries,
1969-1986. J Natl Cancer Inst 84(5):313-320.
Holbrook DJ Jr, Washington ME, Leake HB, et al. 1975. Studies on the evaluation of the toxicity of
various salts of lead, manganese, platinum, and palladium. Environ Health Perspect 10:95-101.
Holzgraefe M, Poser W, Kijewski H, et al. 1986. Chronic enteral poisoning caused by potassium
permanganate: A case report. J Toxicol Clin Toxicol 24:235-244.
Hong JS, Hung CR, Seth PK, et al. 1984. Effect of manganese treatment on the levels of
neurotransmitters, hormones, and neuropeptides: Modulation by stress. Environ Res 34:242-249.
HSDB. 2008. Manganese. Hazardous Substances Data Bank. National Library of Medicine.
http://toxnet.nlm.nih.gov. April 17, 2008.
*Hua MS, Huang CC. 1991. Chronic occupational exposure to manganese and neurobehavioral function.
J Clin Exp Neuropsychol 13:495-507.
Huang C, Chu N, Lu C, et al. 1989. Chronic manganese intoxication. Arch Neurol 46:1104-1106.
Huang C, Chu N, Lu C, et al. 1998. Long-term progression in chronic manganism. Ten years of follow-
up. Neurology 50:698-700.
Hurley LS, Keen CL. 1987. Manganese. In: Mertz W, ed. Trace elements in human and animal
nutrition, 5th Ed., Vol. 1. San Diego, CA: Academic Press, Inc., 185-223.
*Hurley LS, Keen CL, Baly DL. 1984. Manganese deficiency and toxicity: Effects on carbohydrate
metabolism in the rat. Neurotoxicology 5:97-104.
Hussain S, Lipe GW, Slikker W, et al. 1997. The effects of chronic exposure of manganese on
antioxidant enzymes in different regions of rat brain. Neurosci Res Commun 21:135-144.
Hustvedt SO, Grant D, Southon TE, et al. 1997. Plasma pharmacokinetics, tissue distribution, and
excretion of MnDPDP in the rat and dog after intravenous administration. Acta Radiologica 38:690-699.
MANGANESE 468
9. REFERENCES
Hysell DK, Moore W, Stara JF, et al. 1974. Oral toxicity of methylcyclopentadienyl manganese
tricarbonyl (MMT) in rats. Environ Res 7:158-168.
IARC. 2008. Agents reviewed by the IARC monographs: Volumes 1-99. Lyon, France: International
Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Classification/index.php. April 24,
2008.
Ibim SE, Trotman J, Musey PI, et al. 1992. Depletion of essential elements by calcium disodium EDTA
treatment in the dog. Toxicology 73:229-237.
ICCT. 2004. Status report concerning the use of MMT in gasoline. International Council on Clean
Transportation. http://www.theicct.org/documents/MMT_ICCT_2004.pdf. May 07, 2008.
Ihara K, Hijii T, Kuromaru R, et al. 1999. High-intensity basal ganglia lesions on T1-weighted images in
two toddlers with elevated blood manganese with portosystemic shunts. Neuroradiology 41(3):195-198.
*Imam Z, Chandra SV. 1975. Histochemical alterations in rabbit testis produced by manganese chloride.
Toxicol Appl Pharmacol 32:534-544.
Ingersoll RT, Montgomery EB, Aposhian HV. 1995. Central nervous system toxicity of manganese. I.
Inhibition of spontaneous motor activity in rats after intrathecal administration of manganese chloride.
Fundam Appl Toxicol 27:106-113.
Ingersoll RT, Montgomery EB, Aposhian HV. 1999. Central nervous system toxicity of manganese II:
Cocaine or reserpine inhibit manganese concentration in the rat brain. Neurotoxicology 20:467-476.
Iregren A. 1990. Psychological test performance in foundry workers exposed to low levels of
manganese. Neurotoxicol Teratol 12:673-675.
Iregren A. 1994. Using psychological tests for the early detection of neurotoxic effects of low level
manganese exposure. Neurotoxicology 15(3):671-677.
Iregren A. 1999. Manganese neurotoxicity in industrial exposures: Proof of effects, critical exposure
level, and sensitive tests. Neurotoxicology 20:315-324.
*IRIS. 2011. Manganese. Integrated Risk Information System. Washington, DC: U.S. Environmental
Protection Agency. http://www.epa.gov/iris/subst/index.html. November 30, 2011.
Ishizuka H, Nishida M, Kawada J. 1991. Changes in stainability observed by light microscopy in the
brains of ataxial mice subjected to three generations of manganese administration. Biochem Int 25:677-
687.
Ito K, Yamamoto K, Kawanishi S. 1992. Manganese-mediated oxidative damage of cellular and isolated
DNA by isoniazid and related hydrazines: Non-Fenton-type hydroxyl radical formation. Biochemistry
31(46):11606-11613.
Iwami O, Watanabe T, Moon CS, et al. 1994. Motor neuron disease on the Kii Peninsula of Japan:
Excess manganese intake from food coupled with low magnesium in drinking water as a risk factor. Sci
Total Environ 149:121-135.
MANGANESE 469
9. REFERENCES
Jarvinen R, Ahlström A. 1975. Effect of the dietary manganese level on tissue manganese, iron, copper
and zinc concentrations in female rats and their fetuses. Med Biol 53:93-99.
Jarvisalo J, Olkinuora M, Kiilunen M, et al. 1992. Urinary and blood manganese in occupationally
nonexposed populations and in manual metal arc welders of mild steel. Int Arch Occup Environ Health
63:495-501.
Jaudon P, Massiani C, Galea J, et al. 1989. Groundwater pollution by manganese. Manganese
speciation: Application to the selection and discussion of an in situ groundwater treatment. Sci Total
Environ 84:169-183.
Jiang Y, Lu J, Mai H, et al. 1996a. [Effects of manganese exposure on ECG and blood pressure.] Ind
Health Occup Dis 22:341-343. (Chinese).
Jiang Y, Lu J, Xie P, et al. 1996b. [Effects of manganese on the sexual function and reproductive
outcome of male exposed workers]. Chi J Ind Hyg Occup Dis 14:271-273. (Chinese).
Jiang Y, Mo X, Du F, et al. 2006. Effective treatment of manganese-induced occupational Parkinsonism
with p-aminosalicylic acid: A case of 17-year follow-up study. J Occup Environ Med 48:644-649.
Jiang Y, Zheng W, Long L, et al. 2007. Brain magnetic resonance imaging and manganese
concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure.
Neurotoxicology 28:126-135.
Joardar M, Sharma A. 1990. Comparison of clastogenicity of inorganic manganese administered in
cationic and anionic forms in vivo. Mutat Res 240:159-163.
Johanson CE. 1980. Permeability and vascularity of the developing brain: Cerebellum vs cerebral
cortex. Brain Res 190(1):3-16.
Johnson PE, Korynta ED. 1992. Effects of copper, iron, and ascorbic acid on manganese availability to
rats. Proc Soc Exp Biol Med 199:470-480.
Johnson PE, Lykken GI, Korynta ED. 1991. Absorption and biological half-life in humans of intrinsic
and extrinsic
54
Mn tracers from foods of plant origin. J Nutr 121(5):711-717.
Johnston CG, Kipphut GW. 1988. Microbially mediated Mn(II) oxidation in an oligotrophic arctic lake.
Appl Environ Microbiol 54:1440-1445.
Josephs KA, Ahlskog Je, Klos KJ, et al. 2005. Neurologic manifestations in welders with pallidal MRI
T1 hyperintensity. Neurology 64:2033-2039.
Judde JG, Breillout F, Clemenceau C, et al. 1987. Inhibition of rat natural killer cell function by
carcinogenic nickel compounds: Preventive action of manganese. J Natl Cancer Inst 78:1185-1190.
Kabata-Pendias A, Pendias H. 1984. Trace elements in soils and plants. Boca Raton, FL: CRC Press,
Inc.
Kafritsa Y, Fell J, Long S, et al. 1998. Long term outcome of brain manganese deposition in patients on
home parenteral nutrition. Arch Dis Child 79:263-265.
MANGANESE 470
9. REFERENCES
Kagamimori S, Makino T, Hiramaru Y, et al. 1973. [Studies of effects on the respiratory organs of air
pollution through dust consisting mainly of manganese.] Nipon Koshu Eisei Zasshi [Japanese Journal of
Public Health] 20:413-421. (Japanese).
Kalea AZ, Lamari FN, Theocharis AD, et al. 2006. Dietary manganese affects the concentration,
composition and sulfation pattern of heparan sulfate glycosaminoglycans in Sprague-Dawley rat aorta.
Biometals 19(5):535-546.
Kanematsu N, Hara M, Kada T. 1980. Rec assay and mutagenicity studies on metal compounds. Mutat
Res 77:109-116.
Karlsson JOG, Mortensen E, Pedersen HK, et al. 1997. Cardiovascular effects of MnDPDP and MnCl
2
in dogs with acute ischaemic heart failure. Acta Radiologica 38:750-758.
*Kato M. 1963. Distribution and excretion of radiomanganese administered to the mouse. Q J Exp
Physiol 48:355-369.
Katsuragi T, Takahashi T, Shibuya K, et al. 1996. [A Parkinsonism patient exhibiting high-signal
intensity in the globus pallidus on T1-weighted MRI of the head: The correlation with manganese
poisoning.] Clin Neurol 36:780-782. (Japanese).
Kawamura R, Ikuta H, Fukuzumi S, et al. 1941. Intoxication by manganese in well water. Kitasato Arch
Exp Med 18:145-171.
Keen CL, Zidenberg-Cher S. 1990. Manganese. In: Brown M, ed. Present knowledge in nutrition,
sixth edition. Washington, DC: International Life Sciences Institute Nutrition Foundation, 279-286.
Keen CL, Bell JG, Lönnerdal B. 1986. The effect of age on manganese uptake and retention from milk
and infant formulas in rats. J Nutr 116:395-402.
Kent C. 1998. Basics of toxicology. New York: John Wiley and Sons, 90.
Kern CH, Smith DR. 2011. Preweaning Mn exposure leads to prolonged astrocyte activation and lasting
effects on the dopaminergic system in adult male rats. Synapse 65(6):532-544.
Kern CH, Stanwood GD, Smith DR. 2010. Preweaning manganese exposure causes hyperactivity,
disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and
transporter levels. Synapse 64(5):363-378.
*Khan KN, Andress JM, Smith PF. 1997. Toxicity of subacute intravenous manganese chloride
administration in beagle dogs. Toxicol Pathol 25:344-350.
Kihira T, Mukoyama M, Ando K, et al. 1990. Determination of manganese concentrations in the spinal
cords from amyotrophic lateral sclerosis patients by inductively coupled plasma emission spectroscopy. J
Neurol Sci 98:251-258.
Kilburn CJ. 1987. Manganese, malformations and motor disorders: Findings in a manganese-exposed
population. Neurotoxicology 8:421-429.
Kim Y, Bowler RM, Abdelouahab N, et al. 2011. Motor function in adults of an Ohio community with
environmental manganese exposure. Neurotoxicology 32(5):606-614.
MANGANESE 471
9. REFERENCES
Kim Y, Kim BN, Hong YC, et al. 2009. Co-exposure to environmental lead and manganese affects the
intelligence of school-aged children. Neurotoxicology 30(4):564-571.
Kim Y, Kim JW, Ito K, et al. 1999. Idiopathic Parkinsonism with superimposed manganese exposure:
Utility of positron emission tomography. Neurotoxicology 20:249-252.
Klaassen CD. 1974. Biliary excretion of manganese in rats, rabbits, and dogs. Toxicol Appl Pharmacol
29:458-468.
Kleinman MT, Pasternack BS, Eisenbud M, et al. 1980. Identifying and estimating the relative
importance of airborne particulates. Environ Sci Technol 14:62-65.
Klos KJ, Ahlshog E, Josepshs KA, et al. 2005. Neurologic spectrum of chronic liver failure and basal
ganglia T1 hyperintensity on magnetic resonance imaging. Arch Neurol 62:1385-1390.
Klos KJ, Chandler M, Kumar N, et al. 2006. Neuropsychological profiles of manganese neurotoxicity.
Eur J Neurol 13(10):1139-1141.
Kneip TJ, Crable JV, eds. 1988a. Metals in blood or tissue - method 118. In: Methods for biological
monitoring. Washington, DC: American Public Health Association, 221-228.
*Kneip TJ, Crable JV, eds. 1988b. Metals in urine—method 119. In: Methods for biological
monitoring. Washington, DC: American Public Health Association, 229-235.
*Knudsen E, Sandstrom B, Andersen O. 1995. Zinc and manganese bioavailability from human milk
and infant formula used for very low birthweight infants, evaluated in a rat pup model. Biol Trace Elem
Res 49:53-65.
Komori M, Nishio K, Kitada M, et al. 1990. Fetus-specific expression of a form of cytochrome P-450 in
human livers. Biochemistry 29(18):4430-4433.
Komura J, Sakamoto M. 1991. Short-term oral administration of several manganese compounds in mice:
Physiological and behavioral alterations caused by different forms of manganese. Bull Environ Contam
Toxicol 46:921-928.
Komura J, Sakamoto M. 1992a. Disposition, behavior, and toxicity of methylcyclopentadienyl
manganese tricarbonyl in the mouse. Arch Environ Contam Toxicol 23:473-475.
Komura J, Sakamoto M. 1992b. Effects of manganese forms on biogenic amines in the brain and
behavioral alterations in the mouse: Long-term oral administration of several manganese compounds.
Environ Res 57:34-44.
Komura J, Sakamoto M. 1994. Chronic oral administration of methylcyclopentadienyl manganese
tricarbonyl altered brain biogenic amines in the mouse: Comparison with inorganic manganese. Toxicol
Lett 73:65-73.
Kondakis XG, Makris N, Leotsinidis M, et al. 1989. Possible health effects of high manganese
concentration in drinking water. Arch Environ Health 44:175-178.
MANGANESE 472
9. REFERENCES
Kontur PJ, Fechter LD. 1985. Brain manganese, catecholamine turnover, and the development of startle
in rats prenatally exposed to manganese. Teratology 32:1-11.
Kontur PJ, Fechter LD. 1988. Brain regional manganese levels and monoamine metabolism in
manganese-treated neonatal rats. Neurotoxicol Teratol 10:295-303.
Kopp JF, Kroner RC. 1967. Trace metals in waters of the United States. A five year summary of trace
metals in rivers and lakes of the United States (Oct. 1, 1962 - Sept. 30, 1967). Cincinnati, OH: U.S.
Department of the Interior, Federal Water Pollution Control Administration. NTIS No. PB-215680.
Kostial K, Blanusa M, Maljkovic T, et al. 1989. Effect of a metal mixture in diet on the toxicokinetics
and toxicity of cadmium, mercury and manganese in rats. Toxicol Ind Health 5:685-698.
Kostial K, Kello D, Jugo S, et al. 1978. Influence of age on metal metabolism and toxicity. Environ
Health Perspect 25:81-86.
Krishnan K, Andersen ME. 1994. Physiologically based pharmacokinetic modeling in toxicology. In:
Hayes AW, ed. Principles and methods of toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 149-
188.
Krishnan K, Andersen ME, Clewell HJ, et al. 1994. Physiologically based pharmacokinetic modeling of
chemical mixtures. In: Yang RSH, ed. Toxicology of chemical mixtures: Case studies, mechanisms,
and novel approaches. San Diego, CA: Academic Press, 399-437.
Kristensson K, Eriksson H, Lundh B, et al. 1986. Effects of manganese chloride on the rat developing
nervous system. Acta Pharmacol Toxicol 59:345-348.
Lai JC, Leung TK, Lim L. 1984. Differences in the neurotoxic effects of manganese during development
and aging: Some observations on brain regional neurotransmitter and non-neurotransmitter metabolism in
a developmental rat model of chronic manganese
Lai JC, Leung TK, Lim L, et al. 1991. Effects of chronic manganese treatment on rat brain regional
sodium-potassium-activated and magnesium-activated adenosine triphosphatase activities during
development. Metab Brain Dis 6:165-174.
*Lai JC, Minski MJ, Chan AW, et al. 1981. Brain regional manganese distribution after chronic
manganese treatment. Biochem Soc Trans 9:228.
Lai JCK, Minski MH, Chan AWK, et al. 1999. Manganese mineral interactions in brain.
Neurotoxicology 20:433-444.
Laitung JK, Mercer DM. 1983. Manganese absorption through a burn. Burns Incl Therm Inj 10:145-
146.
Larsen LE, Grant D. 1997. General toxicology of MnDPDP. Acta Radiol 38:770-779.
Laskey JW, Rehnberg GL, Hein JF, et al. 1985. Assessment of the male reproductive system in the pre-
weanling rat following Mn3O4 exposure. J Toxicol Environ Health 15:339-350.
Laskey JW, Rehnberg GL, Hein JF. 1982. Effects of chronic manganese (Mn3O4) exposure on selected
reproductive parameters in rats. J Toxicol Environ Health 9:677-687.
MANGANESE 473
9. REFERENCES
Lauwerys R, Roels H, Genet P, et al. 1985. Fertility of male workers exposed to mercury vapor or to
manganese dust: A questionnaire study. Am J Ind Med 7:171-176.
Lauwerys RR, Bernard A, Roels H, et al. 1992. Health risk assessment of long term exposure to
chemicals: Application to cadmium and manganese. Arch Toxicol Suppl 15:97-102.
Lazrishvili IL, Shukakidze AA, Chkhartishvili NN, et al. 2009. Morphological changes and manganese
content in the brains of rat pups subjected to subchronic poisoning with manganese chloride. Neurosci
Behav Physiol 39(1):7-12.
Leach RM, Lilburn MS. 1978. Manganese metabolism and its function. World Rev Nutr Diet 32:123-
134.
Leavens TL, Rao D, Andersen ME, et al. 2007. Evaluating transport of manganese from olfactory
mucosa to straitum by pharmacokinetic modeling. Toxicol Sci 97(2):265-278
Lee B, Pine M, Johnson L, et al. 2006. Manganese acts centrally to activate reproductive hormone
secretion and pubertal development in male rats. Reproductive Toxicology 22:580-585.
Leeder JS, Kearns GL. 1997. Pharmacogenetics in pediatrics: Implications for practice. Pediatr Clin
North Am 44(1):55-77.
Leikin JB, Paloucek JB. 2002. Leikin and Paloucek's poisoning and toxicology handbook. Hudson, OH:
Lexi-Comp, Inc., 773-774.
Leung HW. 1993. Physiologically-based pharmacokinetic modelling. In: Ballentyne B, Marrs T,
Turner P, eds. General and applied toxicology. Vol. 1. New York, NY: Stockton Press, 153-164.
*Leung TK, Lai JC, Lim L. 1981. The regional distribution of monoamine oxidase activities towards
different substrates: Effects in rat brain of chronic administration of manganese chloride and of ageing. J
Neurochem 36(6):2037-2043.
*Leung TK, Lai JC, Lim L. 1982. The effects of chronic manganese feeding on the activity of
monamine oxidase in various organs of the developing rat. Comp Biochem Physiol 71C:223-228.
Lewis RJ. 2000. Manganese. Sax's dangerous properties of industrial materials. 10th ed. New York,
NY: John Wiley & Sons, Inc., 2275-2276, 2278-2780.
Lewis RJ, ed. 2001. Hawley's condensed chemical dictionary. 14th ed. New York, NY: John Wiley &
Sons, Inc., 694-698.
Lewis J, Bench G, Myers O, et al. 2005. Trigeminal uptake and clearance of inhaled manganese chloride
in rats and mice. Neurotoxicology 26:113-123.
Li GJ, Choi B, Wang X, et al. 2006. Molecular mechanism of distorted iron regulation in the blood–CSF
barrier and regional blood–brain barrier following in vivo subchronic manganese exposure.
Neurotoxicology 27:737-744.
MANGANESE 474
9. REFERENCES
Li GJ, Zhang LL, Lu L, et al. 2004. Occupational exposure to welding fume among welders: Alterations
of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ
Med 46(3):241-248.
Liccione JJ, Maines MD. 1988. Selective vulnerability of glutathione metabolism and cellular defense
mechanisms in rat striatum to manganese. J Pharmacol Exp Ther 247:156-161.
Lide DR, ed. 2000. CRC Handbook of chemistry and physics. New York, NY: CRC Press LLC, 4-1,
6-66, 6-68.
Lim KO, Stark DD, Leese PT, et al. 1991. Hepatobiliary MR imaging: First human experience with
MnDPDP. Radiology 178:79-82.
Lima PDL, Vasconcellos MC, Bahia MO, et al. 2008. Genotoxic and cytotoxic effects of manganese
chloride in cultured human lymphocytes treated in different phases of cell cycle. Toxicol In Vitro
22(4):1032-1037.
Lin TH, Chen JG, Liaw JM, et al. 1996. Trace elements and lipid peroxidation in uremic patients on
hemodialysis. Biol Trace Elem Res 51:277-283.
Lioy PJ. 1983. Air pollution emission profiles of toxic and trace elements from energy related sources:
Status and needs. Neurotoxicology 4(3):103-112.
Lipe GW, Duhart H, Newport GD, et al. 1999. Effect of manganese on the concentration of amino acids
in different regions of the rat brain. J Environ Sci Health B 34(1):119-132.
Liu X, Sullivan KA, Madl JE, et al. 2006. Manganese-induced neurotoxicity: The role of astroglial-
derived nitric oxide in striatal interneuron degeneration. Toxicol Sci 91(2):521-531.
Livingston AL. 1978. Forage plant estrogens. J Toxicol Environ Health 4(2-3):301-324.
Lloyd Davies TA. 1946. Manganese pneumonitis. Br J Ind Med 3:111-135.
*Lloyd Davies TA, Harding HE. 1949. Manganese pneumonitis: Further clinical and experimental
observations. Br J Ind Med 6:82-90.
London RE, Toney G, Gabel SA, et al. 1989. Magnetic resonance imaging studies of the brains of
anesthetized rats treated with manganese chloride. Brain Res Bull 23:229-235.
Lönnerdal B. 1997. Effects of milk and milk components on calcium, magnesium, and trace element
absorption during infancy. Physiol Rev 77:643-669.
Lönnerdal B, Keen CL, Bell JG, et al. 1987. Manganese uptake and retention: Experimental animal and
human studies. In: Kies C, ed. Nutritional bioavailability of manganese: ACS Symposium Series 354,
Washington, DC: American Chemical Society, 9-20.
Lönnerdal B, Keen CL, Ohtake M, et al. 1983. Iron, zinc, copper, and manganese in infant formulas.
Am J Dis Child 137:433-437.
Lönnerdal B, Yuen M, Huang S. 1994. Calcium, iron, zinc, copper and manganese bioavailability from
infant formulas and weaning diets assessed in rat pups. Nutr Res 14:1535-1548.
MANGANESE 475
9. REFERENCES
Loranger S, Zayed J. 1994. Manganese and lead concentrations in ambient air and emission rates from
unleaded and leaded gasoline between 1981 and 1992 in Canada: A comparative study. Atmos Environ
28:1645-1651.
Loranger S, Zayed J. 1995. Environmental and occupational exposure to manganese: A multimedia
assessment. Int Arch Occup Environ Health 67(2):101-110.
Loranger S, Zayed J. 1997a. Environmental contamination and human exposure to airborne total and
respirable manganese in Montreal. J Air Waste Manag Assoc 47(9):983-989.
Loranger S, Zayed J. 1997b. Environmental contamination and human exposure assessment to
manganese in the St. Lawrence River ecozone (Quebec, Canada) using an environmental fate/exposure
model: Geotox. SAR QSAR Environ Res 6:105-119.
Loranger S, Demers G, Kennedy G, et al. 1994b. The pigeon (Columba livia) as a monitor for
manganese contamination from motor vehicles. Arch Environ Contam Toxicol 27:311-317.
Loranger S, Tetrault M, Kennedy G, et al. 1996. Manganese and other trace elements in urban snow near
an expressway. Environ Pollut 92(2):203-211.
Loranger S, Zayed J, Forget E. 1994a. Manganese contamination in Montreal in relation with traffic
density. Water Air Soil Pollut 74:385-396.
Loranger S, Zayed J, Kennedy G. 1995. Contribution of methylcyclopentadienyl manganese tricarbonyl
(MMT) to atmospheric manganese concentration near expressway: Dispersion modeling estimations.
Atmos Environ 29(5):591-599.
Lown BA, Morganti JB, D'Agostino R, et al. 1984. Effects on the postnatal development of the mouse of
preconception, postconception and/or suckling exposure to manganese via maternal inhalation exposure
to MnO2 dust. Neurotoxicology 5:119-129.
Lucchini RG, Albini E, Benedetti L, et al. 2007. High prevalence of parkinsonian disorders associated to
manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med 50:788-800.
Lucchini R, Apostoli P, Perrone C, et al. 1999. Long term exposure to “low levels” of manganese oxides
and neurofunctional changes in ferroalloy workers. Neurotoxicology 20:287-298.
Lucchini R, Selis L, Folli D, et al. 1995. Neurobehavioral effects of manganese in workers from a
ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health 21:143-149.
*Lustig S, Pitlik SD, Rosenfeld JB. 1982. Liver damage in acute self-induced hypermanganemia. Arch
Intern Med 142:405-406.
Lydén A, Larsson B, Lindquist NG. 1984. Melanin affinity of manganese. Acta Pharmacol Toxicol
55:133-138.
Lynam DR, Pfeifer GD, Fort BF, et al. 1990. Environmental assessment of MMT fuel additive. Sci
Total Environ 93:107-114.
MANGANESE 476
9. REFERENCES
Lynam DR, Pfeifer GD, Fort BF, et al. 1994. Atmospheric exposure to manganese from use of
methylcyclopentadienyl manganese tricarbonyl (MMT) performance additive. Sci Total Environ
146/147:103-109.
Lynam DR, Roos JW, Pfeifer GD, et al. 1999. Environmental effects and exposures to manganese from
use of methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline. Neurotoxicology 20:145-150.
Lytle CM, McKinnon CZ, Smith BN. 1994. Manganese accumulation in roadside soil and plants.
Naturwissenschaften 81:509-510.
*Mahoney JP, Small WJ. 1968. Studies on manganese: III. The biological half-life of radiomanganese
in man and factors which affect this half-life. J Clin Invest 47:643-653.
Maigetter RZ, Ehrlich R, Fenters JD, et al. 1976. Potentiating effects of manganese dioxide on
experimental respiratory infections. Environ Res 11:386-391.
Malecki EA, Radzanowski GM, Radzanowski TJ, et al. 1996. Biliary manganese excretion in conscious
rats is affected by acute and chronic manganese intake but not by dietary fat. J Nutr 126:489-498.
Malm O, Pfeiffer WC, Fiszman M, et al. 1988. Transport and availability of heavy metals in the Paraiba
do Sul-Guandu River system, Rio de Janeiro state, Brazil. Sci Total Environ 75:201-209.
Mari M, Ferre-Huguet N, Nadal M, et al. 2007. Temporal trends in metal concentrations in soils and
herbage collected near a municipal waste incinerator: Human health risks. Hum Ecol Risk Assess
13:457-472.
*Matrone G, Hartman RH, Clawson AJ. 1959. Studies of a manganese-iron antagonism in the nutrition
of rabbits and baby pigs. J Nutr 67:309-317.
Mayr U, Butsch A, Schneider S. 1992. Validation of two in vitro test systems for estrogenic activities
with zearalenone, phytoestrogens and cereal extracts. Toxicology 74(2-3):135-149.
McBride MB. 1979. Chemisorption and precipitation of Mn2+ at CaCO3 surfaces. Soil Sci Soc Am J
43:693-698.
McGinley PA, Morris JB, Clay RJ, et al. 1987. Disposition and toxicity of methylcyclopentadienyl
manganese tricarbonyl in the rat. Toxicol Lett 36:137-145.
MDNR. 1990. Written communication regarding contaminant levels in water at hazardous waste sites.
Jefferson City, MO: Missouri Department of Natural Resources.
*Mehta R, Reilly JJ. 1990. Manganese levels in a jaundiced long-term total parenteral nutrition patient:
Potentiation of haloperidol toxicity? Case report and literature review. JPEN J Parenter Enteral Nutr
14:428-430.
Mena I. 1979. Manganese poisoning. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical
Neurology. Amsterdam, the Netherlands: North-Holland Publishing Co., 217-237.
Mena I, Horiuchi K, Burke K, et al. 1969. Chronic manganese poisoning: Individual susceptibility and
absorption of iron. Neurology 19:1000-1006.
MANGANESE 477
9. REFERENCES
Mena I, Horiuchi K, Lopez G. 1974. Factors enhancing entrance of manganese into the brain: Iron
deficiency and age. J Nucl Med 15:516.
Mena I, Marin O, Fuenzalida S, et al. 1967. Chronic manganese poisoning: Clinical picture and
manganese turnover. Neurology 17:128-136.
Menezes-Filho JA, Novaes Cde O, Moreira JC, et al. 2011. Elevated manganese and cognitive
performance in school-aged children and their mothers. Environ Res 111(1):156-163.
Mergler D, Baldwin M, Bélanger S, et al. 1999. Manganese neurotoxicity, a continuum of dysfunction:
Results from a community based study. Neurotoxicology 20:327-342.
Mergler D, Huel G, Bowler R, et al. 1994. Nervous system dysfunction among workers with long-term
exposure to manganese. Environ Res 64:151-180.
Miller KB, Caton JS, Finley JW. 2006. Manganese depresses rat heart muscle respiration. Biofactors
28:33-46.
Miller KB, Caton JS, Schafer DM, et al. 2000. High dietary manganese lowers heart magnesium in pigs
fed a low-magnesium diet. J Nutr 130:2032-2035.
Miller KB, Newman SM, Caton JS, et al. 2004. Manganese alters mitochodrial integrity in the hearts of
swine marginally deficient in magnesium. Biofactors 20:86-96.
Miller ST, Cotzias GC, Evert HA. 1975. Control of tissue manganese: Initial absence and sudden
emergence of excretion in the neonatal mouse. Am J Physiol 229:1080-1084.
Minoia C, Sabbioni E, Apostoli P, et al. 1990. Trace element reference values in tissues from inhabitants
of the European community. I. A study of 46 elements in urine, blood and serum of Italian subjects. Sci
Total Environ 95:89-105.
Mölders N, Schilling PJ, Wong J, et al. 2001. X-ray fluorescence mapping and micro-XANES
spectroscopic characterization of exhaust particulates emitted from auto engines burning MMT-added
gasoline. Environ Sci Technol 35(15):3122-3129.
Molina RM, Phattanarudee S, Kim J, et al. 2011. Ingestion of Mn and Pb by rats during and after
pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology 32(4):413-422.
Montes S, Alcaraz-Zubeldia M, Muriel P, et al. 2001. Striatal manganese accumulation induces changes
in dopamine metabolism in the cirrhotic rat. Brain Res 891:123-129.
Montes S, Perez-Severiano F, Vergara P, et al. 2006. Nitric oxide production in striatum and pallidum of
cirrhotic rats. Neurochem Res 31(1):11-20.
Montes S, Riojas-Rodriquez H, Sabido-Pedraza E, et al. 2008. Biomarkers of manganese exposure in a
population living close to a mine and mineral processing plant in Mexico. Environ Res 106:89-95.
Moore W, Hysell D, Miller R, et al. 1975. Exposure of laboratory animals to atmospheric manganese
from automotive emissions. Environ Res 9:274-284.
MANGANESE 478
9. REFERENCES
Morello M, Zatta P, Zambenedetti P, et al. 2007. Manganese intoxication decreases the expression of
manganoproteins in the rat basal ganglia: An immunohistochemical study. Brain Res Bull 74:406-415.
Moreno JA, Yeomans EC, Streifel KM, et al. 2009. Age-dependent susceptibility to manganese-induced
neurological dysfunction. Toxicol Sci 112(2):394-404.
Morganti JB, Lown BA, Stineman CH, et al. 1985. Uptake, distribution and behavioral effects of
inhalation exposure to manganese (MnO2) in the adult mouse. Neurotoxicology 6:1-16.
Morselli PL, Franco-Morselli R, Bossi L. 1980. Clinical pharmacokinetics in newborns and infants:
Age-related differences and therapeutic implications. Clin Pharmacokin 5(6):485-527.
Mortelmans K, Haworth S, Lawlor T, et al. 1986. Salmonella mutagenicity tests: II. Results from
testing of 270 chemicals. Environ Mutagen 8:1-26.
Moser VC. 2000. The functional observational battery in adult and developing rat. Neurotoxicology
21(6):989-996.
Mossman BT, Surinrut P, Brinton BT, et al. 1996. Transfection of a manganese-containing superoxide
dismutase gene into hamster tracheal epithelial cells ameliorates asbestos-mediated cytotoxicity. Free
Radical Biol Med 21:125-131.
Murphy VA, Wadhwani KC, Smith QR, et al. 1991. Saturable transport of manganese (II) across the rat
blood-brain barrier. J Neurochem 57:948-954
Myers JE, teWaterNaude J, Fourie M, et al. 2003a. Nervous system effects of occupational manganese
exposure on South African manganese mineworkers. Neurotoxicology 24(4-5):649-656.
Myers JE, Thompson ML, Ramushu S, et al. 2003b. The nervous system effects of occupational
exposure on workers in a South African manganese smelter. Neurotoxicology 24:885-894.
Nachtman JP, Tubben RE, Commissaris RL. 1986. Behavioral effects of chronic manganese
administration in rats: Locomotor activity studies. Neurobehav Toxicol Teratol 8:711-715.
Nagatomo S, Umehara F, Hanada K, et al. 1999. Manganese intoxication during total parenteral
nutrition: report of two cases and review of the literature. J Neurol Sci 162:102-105.
NAS. 1973. Manganese in the ecosystem. In: Medical and biological effects of environmental
pollutants: Manganese. Washington, DC: National Academy of Sciences, 3-50.
NAS. 1977. Drinking water and health. Washington, DC: National Academy of Sciences, 214-215,
267-270, 311-312.
NAS. 1980a. Drinking water and health. Vol. 3. Washington, DC: National Academy Press, 331-337.
NAS. 1980b. Manganese. In: Recommended dietary allowances. 9th revised ed. Washington, DC:
National Academy of Sciences, 154-157.
MANGANESE 479
9. REFERENCES
NAS/NRC. 1989. Report of the oversight committee. In: Biologic markers in reproductive toxicology.
Washington, DC: National Academy of Sciences, National Research Council, National Academy Press,
15-35.
Naslund PE, Andreasson S, Bergstrom R, et al. 1990. Effects of exposure to welding fume: An
experimental study in sheep. Eur Respir J 3:800-806.
Nelson K, Golnick J, Korn T, et al. 1993. Manganese encephalopathy: Utility of early magnetic
resonance imaging. Br J Ind Med 50: 510-513.
Newland MC. 1999. Animal models of manganese’s neurotoxicity. Neurotoxicology 20:415-432.
Newland MC, Weiss B. 1992. Persistent effects of manganese on effortful responding and their
relationship to manganese accumulation in the primate globus pallidus. Toxicol Appl Pharmacol 113:87-
97.
Newland MC, Ceckler TL, Kordower JH, et al. 1989. Visualizing manganese in the primate basal
ganglia with magnetic resonance imaging. Exp Neurology 106:251-258.
Newland MC, Cox C, Hamada R, et al. 1987. The clearance of manganese chloride in the primate.
Fundam Appl Toxicol 9:314-328.
Ni Y, Petre C, Bosmans H, et al. 1997. Comparison of manganese biodistribution and MR contrast
enhancement in rats after intravenous injection of MnDPDP and MnCl2. Acta Radiol 38:700-707.
NIOSH. 1984c. Elements in blood or tissue-method 8005. In: NIOSH manual of analytical methods.
3rd ed. Vol. 2. Cincinnati, OH: National Institute for Occupational Safety and Health, DHHS (NIOSH)
Publication No. 84-100.
NIOSH. 1984d. Metals in urine-method 8310. In: NIOSH manual of analytical methods. 3rd ed. Vol.
2. Cincinnati, OH: National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication
No. 84-100.
NIOSH. 1992. NIOSH recommendations for occupational safety and health. Compendium of policy
documents and statements. Categories of pesticides. Atlanta, GA: National Institute for Occupational
Safety and Health, Centers for Disease Control and Prevention. http://www.cdc.gov/niosh/92-100.html.
April 29, 2008.
NIOSH. 2003a. Method 7300. Elements by ICP. (Nitric/perchloric acid ashing). NIOSH manual of
analytical methods (NMAM). 4th ed. National Institute for Occupational Safety and Health.
http://www.cdc.gov/niosh/nmam/pdfs/7300.pdf. April 30, 2008.
NIOSH. 2003b. Method 7303. Elements by ICP. (Hot block/HCL/HNO3 digestion). NIOSH manual
of analytical methods (NMAM). 4th ed. National Institute for Occupational Safety and Health.
http://www.cdc.gov/niosh/nmam/pdfs/7303.pdf. May 01, 2008.
NIOSH. 2003c. Method 7301. Elements by ICP. (Aqua regia ashing). NIOSH manual of analytical
methods (NMAM). 4th ed. National Institute for Occupational Safety and Health.
http://www.cdc.gov/niosh/nmam/pdfs/7301.pdf. May 01, 2008.
MANGANESE 480
9. REFERENCES
NIOSH. 2005. Manganese. NIOSH pocket guide to chemical hazards. Atlanta, GA: National Institute
for Occupational Safety and Health, Centers for Disease Control and Prevention.
http://www.cdc.gov/niosh/npg/ April 24, 2008.
*Nishida M, Ogata K, Sakurai H, et al. 1992. A binding profile of manganese to the nucleus of rat liver
cells, and manganese-induced aberrations in thyroid hormone content and RNA synthesis in the nucleus.
Biochem Int 27:209-219.
Nishioka H. 1975. Mutagenic activities of metal compounds in bacteria. Mutat Res 31:185-189.
NLM. 2008. Manganese violet. Household products database. Health and safety information on
household products. National Library of Medicine. http://householdproducts.nlm.nih.gov/cgi-
bin/household/brands?tbl=chem&id=1556. June 18, 2008.
NOES. 1989. National Occupational Exposure Survey. National Institute of Occupational Safety and
Health, Cincinnati, OH. October 18, 1989.
Nogawa K, Kobayashi E, Sakamoto M, et al. 1973. Epidemiological studies on disturbance of
respiratory system caused by manganese air pollution: (Report 1) Effects on respiratory system of junior
high school students. Nippon Koshu Eisei Zasshi 20(6):315-325.
Nolte W, Wiltfang J, Schindler CG, et al. 1998. Bright basal ganglia in T1-weighted magnetic resonance
images are frequent in patients with portal vein thrombosis without liver cirrhosis and not suggestive of
hepatic encephalopathy. J Hepatol 29:443-449.
Nong A, Taylor MD, Clewell HJ, et al. 2009. Manganese tissue dosimetry in rats and monkeys:
Accounting for dietary and inhaled Mn with physiologically based pharmacokinetic modeling. Toxicol
Sci 108(1):22-34.
Nong A, Teeguarden JG, Clewell HJ, et al. 2008. Pharmacokinetic modeling of manganese in the rat IV:
Assessing factors that contribute to brain accumulation during inhalation exposure. J Toxicol Environ
Health A 71:413-426.
Normandin L, Beaupre LA, Salehi F, et al. 2004. Manganese distribution in the brain and
neurobehavioral changes following inhalation exposure of rats to three chemical forms of manganese.
Neurotoxicology 25:433-441.
Normandin L, Carrier G, Gardiner PF, et al. 2002. Assessment of bioaccumulation, neuropathology, and
neurobehavior following subchronic (90 days) inhalation in Sprague-Dawley rats exposed to manganese
phosphate. Toxicol Appl Pharmacol 183:135-145.
NRC. 1993. National Research Council. Pesticides in the diets of infants and children. Washington,
DC: National Academy Press.
NRC. 1989. Recommended dietary allowances. Washington, DC: National Research Council. Tenth
Edition, 230-235.
Nriagu JO. 1979. Copper in the atmosphere and precipitation. In: Nriagu JO, ed. Copper in the
environment. Part I: Ecological cycling. New York, NY: John Wiley and Sons, Inc., 43-67.
MANGANESE 481
9. REFERENCES
NTP. 1987b. The chronic study of manganese sulfate monohydrate (CAS No. 10034-96-5) in B6C3F1
mice. Research Triangle Park, NC: National Toxicology Program.
NTP. 1987a. The chronic study of manganese sulfate monohydrate (CAS No. 10034-96-5) in F344 rats.
Research Triangle Park, NC: National Toxicology Program.
NTP. 1993. Toxicology and carcinogenesis studies of manganese (II) sulfate monohydrate in F344/N
rats and B6C3F1 mice (feed study). National Toxicology Program. Technical Report Series 428.
RISKLINE 94030007.
NTP. 2005. Report on carcinogens. 11th ed. Research Triangle Park, NC: U.S. Department of Health
and Human Services, Public Health Service, National Toxicology Program. http://ntp-
server.niehs.nih.gov/ntp/roc/toc11.html. April 24, 2008.
Oberly TJ, Piper CE, McDonald DS. 1982. Mutagenicity of metal salts in the L5178Y mouse lymphoma
assay. J Toxicol Environ Health 9:367-376.
Olanow CW, Good PF, Shinotoh H, et al. 1996. Manganese intoxication in the rhesus monkey: A
clinical, imaging, pathologic, and biochemical study. Neurology 46:492-498.
Ombaba JM, Barry EF. 1994. Determination of methylcyclopentadienyl manganese tricarbonyl in
gasoline by capillary gas chromatography with alternating current plasma emission detection. J
Chromatogr A 678:319-325.
O'Neil MJ, Heckelman PE, Koch CB, et al, eds. 2006. The Merck Index. 14th ed. Whitehouse Station,
NJ: Merck & Co., Inc., 990-991, 1074-1075.
Ono J, Harada K, Kodaka R. 1995. Manganese deposition in the brain during long-term total parenteral
nutrition. J Parent Enter Nutr 19:310-312.
*Onoda K, Hasegawa A, Sunouchi M, et al. 1978. Studies on the fate of poisonous metals in
experimental animal (VII): Distribution and transplacental passage of manganese in pregnant rat and
fetus. J Food Hyg Soc 19:208-215.
Orgel A, Orgel LE. 1965. Induction of mutations in bacteriophage T4 with divalent manganese. J Mol
Biol 14:453-457.
OSHA. 2007a. Air contaminants. Occupational Safety and Health Administration. Code of Federal
Regulations 29 CFR 1915.1000. http://www.osha.gov/comp-links.html. April 24, 2008.
OSHA. 2007b. Gases, vapors, fumes, dusts, and mists. Occupational Safety and Health Administration.
Code of Federal Regulations 29 CFR 1926.55, Appendix A. http://www.osha.gov/comp-links.html.
April 24, 2008.
OSHA. 2007c. Limits for air contaminants. Occupational Safety and Health Administration. Code of
Federal Regulations 29 CFR 1910.1000, Table Z 1. http://www.osha.gov/comp-links.html. April 24,
2008.
OTA. 1990. Neurotoxicity: Identifying and controlling poisons of the nervous system. Washington,
DC: Office of Technology Assessment. OTABA438.
MANGANESE 482
9. REFERENCES
Owen GM, Brozek J. 1966. Influence of age, sex and nutrition on body composition during childhood
and adolescence. In: Falkner F, ed. Human development. Philadelphia, PA: WB Saunders, 222-238.
Padovani B, Lecesne R, Raffaelli C. 1996. Tolerability and utility of mangafodipir trisodium injection
(MnDPDP) at the dose of 5 μmol/kg body weight in detecting focal liver tumors: Results of a phase III
trial using an infusion technique. Eur J Radiol 23(3):205-211.
Pagano DA, Zeiger E. 1992. Conditions for detecting the mutagenicity of divalent metals in Salmonella
typhimurium. Environ Mol Mutagen 19:139-146.
Pal PK, Samii A, Calne DB. 1999. Manganese neurotoxicity: A review of clinical features, imaging and
pathology. Neurotoxicology 20(2-3):227-238.
Pappas BA, Zhang D, Davidson CM, et al. 1997. Perinatal manganese exposure: Behavioral,
neurochemical, and histopathological effects in the rat. Neurotoxicol Teratol 19:17-25.
Parenti M, Flauto C, Parati E, et al. 1986. Manganese neurotoxicity: Effects of L-DOPA and pargyline
treatments. Brain Res 367:8-13.
Parenti M, Rusconi L, Cappabianca V, et al. 1988. Role of dopamine in manganese neurotoxicity. Brain
Res 473:236-240.
Park NH, Park JK, Choic Y, et al. 2003. Whole blood manganese correlates with high signal intensities
on T1-weighted MRI in patients with liver cirrhosis. Neurotoxicology 24:909-915.
Paschal DC, Ting BG, Morrow JC, et al. 1998. Trace metals in urine of United States residents:
Reference range concentrations. Environ Res 76(1):53-59.
Patterson KY, Holbrook JT, Bodner JE, et al. 1984. Zinc, copper, and manganese intake and balance for
adults consuming self-selected diets. Am J Clin Nutr 40:1397-1403.
Paulson AJ, Feely RA, Curl HC, et al. 1984. Behavior of Fe, Mn, Cu and Cd in the Duwamish River
estuary downstream of a sewage treatment plant. Water Research 18:633-641.
*Paynter DI. 1980. Changes in activity of the manganese superoxide dismutase enzyme in tissues of the
rat with changes in dietary manganese. J Nutr 110:437-447.
Pellizzari ED, Clayton CA, Rodes CE, et al. 1999. Particulate matter and manganese exposures in
Toronto, Canada. Atmos Environ 33:721-734.
Pellizzari ED, Clayton CA, Rodes CE, et al. 2001. Particulate matter and manganese exposures in
Indianapolis, Indiana. J Expo Anal Environ Epidemiol 11(6):423-440.
Pennington JAT, Young BE, Wilson DB, et al. 1986. Mineral content of foods and total diets: The
selected minerals in foods survey, 1982 to 1984. J Am Diet Assoc 86:876-891.
Perl DP, Olanow CW. 2007. The neuropathology of manganese-induced Parkinsonism. J Neuropathol
Exp Neurol 66(8):675-682.
MANGANESE 483
9. REFERENCES
Perocco P, Santucci MA, Campani AG, et al. 1989. Toxic and DNA-damaging activities of the
fungicides mancozeb and thiram (TMTD) on human lymphocytes in vitro. Teratog Carcinog Mutagen
9:75-81.
Pihl RO, Parkes M. 1977. Hair element contents in learning disabled children. Science 198:204-206.
Pine M, Lee B, Dearth R, et al. 2005. Manganese acts centrally to stimulate luteinizing hormone
secretion: A potential influence on female pubertal development. Toxicol Sci 85(2):880-885.
Pisarczyk K. 2005. Manganese compounds. Kirk-Othmer encyclopedia of chemical technology. Vol.
15. http://mrw.interscience.wiley.com/emrw/9780471238966/kirk/article/mangpisa.a01/current/pdf.
April 07, 2008.
*Plantin LO, Lying-Tunell U, Kristensson K. 1987. Trace elements in the human central nervous system
studied with neutron activation analysis. Biol Trace Elem Res 13:69-75.
Pollack S, George JN, Reba RC, et al. 1965. The absorption of nonferrous metals in iron deficiency. J
Clin Invest 44:1470-1473.
Pomier-Layrargues G, Rose C, Spahr L, et al. 1998. Role of manganese in the pathogenesis of portal-
systemic encephalopathy. Metabol Brain Dis 13:311-317.
Ponnamperuma FN, Loy TA, Tianco EM. 1969. Redox equilibria in flooded soils: II. The manganese
oxide systems. Soil Science 108:48-57.
Ponnapakkam TP, Bailey KS, Graves KA, et al. 2003a. Assessment of male reproductive system in the
CD-1 mice following oral manganese exposure. Reprod Toxicol 17(5):547-551.
Ponnapakkam T, Iszard M, Henry-Sam G. 2003b. Effects of oral administration of manganese on the
kidneys and urinary bladder of Sprague-Dawley rats. Int J Toxicol 22:227-232.
Ponnapakkam TP, Sam GH, Iszard MB. 2003c. Histopathological changes in the testis of the Sprague
Dawley rat following orally administered manganese. Bull Environ Contam Toxicol 71(6):1151-1157.
Prestifilippo JP, Fernandez-Solari J, Mohn C, et al. 2007. Effect of manganese on luteinizing hormone-
releasing hormone secretion in adult male rats. Toxicol Sci 97(1):75-80.
Quimby BD, Uden PC, Barnes RM. 1978. Atmospheric pressure helium microwave detection system for
gas chromatography. Anal Chem 50:2112-2118.
Rabin O, Hegedus L, Bourre J-M, et al. 1993. Rapid brain uptake of manganese(II) across the blood-
brain barrier. J Neurochem 61:509-517.
Racette BA, Antenor JA, McGee-Minnich L, et al. 2005. [
18
F]FDOPA PET and clinical features in
parkinsonism due to manganism. Mov Disord 20(4):492-496.
Rai D, Zachara JM, Schwab AP, et al. 1986. Manganese. In: Chemical attenuation rates, coefficients,
and constants in leachate migration. Volume 1: A critical review. Report to Electric Power Research
Institute, Palo Alto, CA, by Battelle, Pacific Northwest Laboratories, Richland, WA, 15-1-15-4.
MANGANESE 484
9. REFERENCES
Ranasinghe JGS, Liu M, Sakakibara Y, et al. 2000. Manganese administration induces the increased
production of dopamine sulfate and depletion of dopamine in Sprague-Dawley rats. J Biochem (Tokyo)
128:477-480.
Rasmuson A. 1985. Mutagenic effects of some water-soluble metal compounds in a somatic eye-color
test system in Drosophila melanogaster. Mutat Res 157:157-162.
Reaney SH, Bench G, Smith DR. 2006. Brain accumulation and toxicity of Mn(II) and Mn(III)
exposures. Toxicol Sci 93(1):114-124.
Reddy MR, Perkins HF. 1976. Fixation of manganese by clay minerals. Soil Science 121:21-24.
Rehnberg GL, Hein JF, Carter SD, et al. 1980. Chronic manganese oxide administration to pre-weanling
rats: Manganese accumulation and distribution. J Toxicol Environ Health 6:217-226.
Rehnberg GL, Hein JF, Carter SD, et al. 1981. Chronic ingestion of Mn3O4 by young rats: Tissue
accumulation, distribution, and depletion. J Toxicol Environ Health 7:263-272.
Rehnberg GL, Hein JF, Carter SD, et al. 1982. Chronic ingestion of Mn3O4 by rats: Tissue
accumulation and distribution of manganese in two generations. J Toxicol Environ Health 9:175-188.
Rehnberg GL, Hein JF, Carter SD, et al. 1985. Age-dependent changes in gastrointestinal transport and
retention of particulate manganese oxide in the rat. J Toxicol Environ Health 16:887-899.
Reichel CM, Wacan JJ, Farley CM, et al. 2006. Postnatal manganese exposure attenuates cocaine-
induced locomotor activity and reduces dopamine transporters in adult male rats. Neurotoxicol Teratol
28(3):323-332.
Ressler T, Wong J, Roos J, et al. 2000. Quantitative speciation of Mn-bearing particulates emitted from
autos burning (methylcyclopentadienyl) manganese tricarbonyl-added gasolines usine XANES
spectroscopy. Environ Sci Technol 34:950-958.
Rice RH, Cohen DE. 1996. Toxic responses of the skin. In: Klassen CD, Amdur MO, Doull J, eds.
Casarett and Doull’s toxicology: The basic science of poisons. 5th ed. New York, NY: McGraw-Hill,
529-544.
Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, et al. 2010. Intellectual function in Mexican
children living in a mining area and environmentally exposed to manganese. Environ Health Perspect
118(10):1465-1470.
Rivera-Mancia S, Montes S, Mendez-Armenta M, et al. 2009. Morphological changes of rat astrocytes
induced by liver damage but not by manganese chloride exposure. Metab Brain Dis 24(2):243-255.
Rodier J. 1955. Manganese poisoning in Moroccan miners. Br J Ind Med 12:21-35.
Rodríguez-Agudelo Y, Riojas-Rodriguez H, Rios C, et al. 2006. Motor alterations associated with
exposure to manganese in the environment in Mexico. Sci Total Environ 368(2-3):542-556.
Roels H, Lauwerys R, Buchet JP, et al. 1987a. Epidemiological survey among workers exposed to
manganese: Effects on lung, central nervous system, and some biological indices. (Erratum in: Am J Ind
Hyg 12:119-120). Am J Ind Med 11:307-327.
MANGANESE 485
9. REFERENCES
Roels H, Lauwerys R, Genet P, et al. 1987b. Relationship between external and internal parameters of
exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med
11:297-305.
Roels H, Meiers G, Delos M, et al. 1997. Influence of the route of administration and the chemical form
(MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol 71:223-
230.
*Roels H, Sarhan MJ, Hanotiau I, et al. 1985. Preclinical toxic effects of manganese in workers from a
manganese salts and oxides producing plant. Sci Total Environ 42:201-206.
Roels HA, Ghyselen P, Buchet JP, et al. 1992. Assessment of the permissible exposure level to
manganese in workers exposed to manganese dioxide dust. Br J Ind Med 49:25-34.
Roels HA, Ortega Eslava MI, Ceulemans E, et al. 1999. Prospective study on the reversibility of
neurobehavioral effects in workers exposed to manganese dioxide. Neurotoxicology 20:255-272.
Rogers RR, Garner RJ, Riddle MM, et al. 1983. Augmentation of murine natural killer cell activity by
manganese chloride. Toxicol Appl Pharmacol 70:7-17.
Rope SK, Arthur WJ, Craig TH, et al. 1988. Nutrient and trace elements in soil and desert vegetation of
southern Idaho. Environ Monit Assess 10:1-24.
Rose C, Butterworth RF, Zayed J, et al. 1999. Manganese deposition in basal ganglia structures results
from both portal-systemic shunting and liver dysfunction. Gastroenterology 117:640-644.
Rosenstock HA, Simons DG, Meyer JS. 1971. Chronic manganism: Neurologic and laboratory studies
during treatment with levodopa. J Am Med Assoc 217:1354-1358.
Rossander-Hulten L, Brune M, Sandstrom B, et al. 1991. Competitive inhibition of iron absorption by
manganese and zinc in humans. Am J Clin Nutr 54:152-156.
Roth JA. 2006. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and
elimination.39:45-57.
RTECS. 2007. Manganese. Registry of Toxic Effects on Chemical Substances. National Institute of
Occupational Safety and Health. MDL Information Systems, Inc. May 8, 2008.
Rükgauer M, Klein J, Kruse-Jarres JD. 1997. Reference values for the trace elements copper,
manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace
Elements Med Biol 11:92-98.
Ruoff W. 1995. Relative bioavailability of manganese ingested in food or water. In: Proceedings:
Workshop on the bioavailability and oral toxicity of manganese, Omni Netherland Plaza, August 30-31,
1994. Lexington, MA: Eastern Research Group, Inc., 65-75.
Sahni V, Leger Y, Panaro L, et al. 2007. Case report: A metabolic disorder presenting as pediatric
manganism. Environ Health Perspect 115:1776-1779.
MANGANESE 486
9. REFERENCES
Sakurai H, Nishida M, Yoshimura T, et al. 1985. Partition of divalent and total manganese in organs and
subcellular organelles of MnCl2-treated rats studied by ESR and neutron activation analysis. Biochim
Biophys Acta 841:208-214.
Salehi F, Krewski D, Mergler D, et al. 2003. Bioaccumulation and locomotor effects of manganese
phosphate/sulfate mixture in Sprague-Dawley rats following subchronic (90 day) inhalation exposure.
Toxicol Appl Pharmacol 191:264-271.
Salehi F, Normandin L, Krewski D, et al. 2006. Neuropathology, tremor and electromyogram in rats
exposed to manganese phosphate/sulfate mixture. J Appl Toxicol 26:419-426.
Sánchez DJ, Domingo JL, Llobet JM, et al. 1993. Maternal and developmental toxicity of manganese in
the mouse. Toxicol Lett 69:45-52.
Sandstrom B, Davidsson L, Cederblad A, et al. 1986. Manganese absorption and metabolism in man.
Acta Pharmacol Toxicol (Copenh) 59:60-62.
Sandstrom B, Davidsson L, Eriksson R, et al. 1990. Effect of long-term trace element supplementation
on blood trace element levels and absorption of (75Se), (54Mn) and (65Zn). J Trace Elem Electrolytes
Health Dis 4:65-72.
*Sarhan MJ, Roels H, Lauwerys R. 1986. Influence of manganese on the gastrointestinal absorption of
cadmium in rats. J Appl Toxicol 6313-6316
Saric M, Hrustic O. 1975. Exposure to airborne manganese and arterial blood pressure. Environ Res
10:314-318.
Saric M, Lucic-Palaic S. 1977. Possible synergism of exposure to airborne manganese and smoking
habit occurrence of respiratory symptoms. In: Walton WH, ed. Inhaled particles. IV. New York, NY:
Pergamon Press, 773-779.
Saric M, Markicevic A, Hrustic O. 1977. Occupational exposure to manganese. Br J Ind Med 34:114-
118.
Sax NI, Lewis RJ. 1987. Hawley's condensed chemical dictionary. 11th ed. New York, NY: Van
Nostrand Reinhold Company, 727-731.
Schaanning M, Naes K, Egeberg PK, et al. 1988. Cycling of manganese in the permanently anoxic
Drammens fjord. Marine Chemistry 23:365-382.
Schafer DF, Stephenson DV, Barak AJ, et al. 1974. Effects of ethanol on the transport of manganese by
small intestine of the rat. J Nutr 104:101-104.
*Scheuhammer AM. 1983. Chronic manganese exposure in rats: Histological changes in the pancreas.
J Toxicol Environ Health 12:353-360.
*Scheuhammer AM, Cherian MG. 1983. The influence of manganese on the distribution of essential
trace elements. II. The tissue distribution of manganese, magnesium, zinc, iron, and copper in rats after
chronic manganese exposure. J Toxicol Environ Health 12(2-3):361-370.
MANGANESE 487
9. REFERENCES
Schneider JS, Decamp E, Koser AJ, et al. 2006. Effects of chronic manganese exposure on cognitive and
motor functioning in non-human primates. Brain Res 1118(1):222-231.
Schnitzer M. 1969. Reactions between fulvic acid, a soil humic compound and inorganic soil
constituents. Soil Sci Soc Amer Proc 33:75-80.
Schonwald S. 2004. Manganese. In: Dart RC, ed. Medical toxicology. 3rd ed. Philadelphia, PA:
Lippicott Williams & Wilkins, 1433-1434.
Schroeder HA, Balassa JJ, Tipton IH. 1966. Essential trace metals in man: Manganese. A study in
homeostasis. J Chron Dis 19:545-571.
Schroeder WH, Dobson M, Kane DM, et al. 1987. Toxic trace elements associated with airborne
particulate matter: A review. J Air Pollut Control Assoc 37:1267-1285.
Schroeter JD, Nong A, Yoon M, et al. 2011. Analysis of manganese tracer kinetics and target tissue
dosimetry in monkeys and humans with multi-route physiologically based pharmacokinetic models.
Toxicol Sci 120(2):481-498.
Schuler P, Oyanguren H, Maturana V, et al. 1957. Manganese poisoning: Environmental and medical
study at a Chilean mine. Ind Med Surg 26:167-173.
Segura-Aguilar J, Lind C. 1989. On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine:
Prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol
Interact 72:309-324.
Setchell BP, Waites GMH. 1975. The blood-testis barrier. In: Creep RO, Astwood EB, Geiger SR, eds.
Handbook of physiology: Endocrinology V. Washington, DC: American Physiological Society, 143-
172.
*Seth PK, Hong JS, Kilts CD, et al. 1981. Alteration of cerebral neurotransmitter receptor function by
exposure of rats to manganese. Toxicol Lett 9:247-254.
Seth PK, Nagar N, Husain R, et al. 1973. Effects of manganese on rabbit testes. Environ Physiol
Biochem 3:263-267.
Shiotsuka RN. 1984. Inhalation toxicity of manganese dioxide and a magnesium oxide-manganese
dioxide mixture. Report to U.S. Army Medical Research and Developmental Command, Fort Detrick,
Frederick, MD, by Inhalation Toxicology Facility, Medical Department.
Shukakidze AA, Lazriev IL, Mitagvariya N. 2003. Behavioral impairments in acute and chronic
manganese poisoning in white rats. Neurosci Behav Physiol 33(3):263-267.
Shukla GS, Chandra SV, Seth KP. 1976. Effect of manganese on the levels of DNA, RNA, DNase and
RNase in cerebrum, cerebellum and rest of brain regions of rat. Acta Pharmacol Toxicol 39:562-569.
*Shukla GS, Dubey MP, Chandra SV. 1980. Manganese-induced biochemical changes in growing
versus adult rats. Arch Environ Contam Toxicol 9:383-391.
Shukla GS, Singh S, Chandra SV. 1978. The interaction between manganese and ethanol in rats. Acta
Pharmacol Toxicol 43:354-362.
MANGANESE 488
9. REFERENCES
Shuqin K, Haishang D, Peiyi X, et al. 1992. A report of two cases of chronic serious manganese
poisoning treated with sodium para-aminosalicyclic acid. Br J Ind Med 49:66-69.
*Sierra P, Chakrabarti S, Tounkara R, et al. 1998. Bioaccumulation of manganese and its toxicity in
feral pigeons (Columba livia) exposed to manganese oxide dust (Mn3O4). Environ Res 79:94-101.
Sierra P, Loranger S, Kennedy G, et al. 1995. Occupational and environmental exposure of automobile
mechanics and nonautomotive workers to airborne manganese arising from the combustion of
methylcyclopentadienyl manganese tricarbonyl (MMT). Am Ind Hyg Assoc J 56(7):713-716.
Silbergeld EK. 1982. Current status of neurotoxicology, basic and applied. Trends Neurosci 5:291-294.
Singh I. 1984. Induction of gene conversion and reverse mutation by manganese sulphate and nickel
sulphate in Saccharomyces cerevisiae. Mutat Res 137:47-49.
*Singh J, Husain R, Tandon SK, et al. 1974. Biochemical and histopathological alterations in early
manganese toxicity in rats. Environ Physiol Biochem 4:16-23.
*Singh J, Kaw JL, Zaidi SH. 1977. Early biochemical response of pulmonary tissue to manganese
dioxide. Toxicology 8:177-184.
Singh PP, Junnarkar AY. 1991. Behavioural and toxic profile of some essential trace metal salts in mice
and rats. Ind J Pharmacol 23:153-159.
Singh S, Shukla GS, Srivastava RS, et al. 1979. The interaction between ethanol and manganese in rat
brain. Arch Toxicol 41(4):307-316.
Siqueira ME, Moraes EC. 1989. Homovanillic acid (HVA) and manganese in urine of workers exposed
in a ferromanganese alloy plant. Med Lav 80:224-228.
Siqueira ME, Hirata MH, Adballa DS. 1991. Studies on some biochemical parameters in human
manganese exposure. Med Lav 82(6):504-509.
*Sitaramayya A, Nagar N, Chandra SV. 1974. Effect of manganese on enzymes in rat brain. Acta
Pharmacol Toxicol 35:185-190.
Sloot WN, Gramsbergen JP. 1994. Axonal transport of manganese and its relevance to selective
neurotoxicity in the rat basal ganglia. Brain Res 657:124-132.
Smargiassi A, Mutti A. 1999. Peripheral biomarkers of exposure to manganese. Neurotoxicology
20:401-406.
Smargiassi A, Mergler D, Bergamaschi E, et al. 1995. Peripheral markers of catecholamine metabolism
among workers occupationally exposed to manganese (Mn). Toxicol Lett 77:329-333.
Smialowicz RJ, Luebke RW, Rogers RR, et al. 1985. Manganese chloride enhances natural cell-
mediated immune effector cell function: Effects on macrophages. Immunopharmacology 9:1-11.
MANGANESE 489
9. REFERENCES
Smialowicz RJ, Rogers RR, Riddle MM, et al. 1987. Effects of manganese, calcium, magnesium, and
zinc on nickel-induced suppression of murine natural killer cell activity. J Toxicol Environ Health 20:67-
80.
Smith D, Gwiazka R, Bowler R, et al. 2007. Biomarkers of Mn exposure in humans. Am J Ind Med
50:801-811.
Smith GW, Palmby AK. 1959. Flame photometric determination of lead and manganese in gasoline.
Anal Chem 31:1798-1802.
Smith RA, Alexander RB, Wolman MG. 1987. Water-quality trends in the nation's rivers. Science
235:1607-1615.
*Smith SE, Medlicott M, Ellis GH. 1944. Manganese deficiency in the rabbit. Arch Biochem Biophys
4:281-289.
Smyth HF, Carpenter CP, Weil CS, et al. 1969. Range-finding toxicity data: List VII. Am Ind Hyg
Assoc J 30:470-476.
Smyth LT, Ruhf RC, Whitman NE, et al. 1973. Clinical manganism and exposure to manganese in the
production and processing of ferromanganese alloy. J Occup Med 15:101-109.
Solis-Vivanco R, Rodriguez-Agudelo Y, Riojas-Rodriguez H, et al. 2009. Cognitive impairment in an
adult Mexican population non-occupationally exposed to manganese. Environ Toxicol Pharmacol
8(2):172-178.
Southwood T, Lamb CM, Freeman J. 1987. Ingestion of potassium permanganate crystals by a 3-yr-old
boy. Med J Aust 146:639-640.
Spadoni F, Stefani A, Morello M, et al. 2000. Selective vulnerability of pallidal neurons in the early
phases of manganese intoxication. Exp Brain Res 138:544-551.
Spahr L, Butterworth RF, Fontaine S, et al. 1996. Increased blood manganese in cirrhotic patients:
Relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms.
Hepatology 24:1116-1120.
Spangler AH, Spangler JG. 2009. Groundwater manganese and infant mortality rate by county in North
Carolina: An ecological analysis. Ecohealth 6(4):596-600.
SRI. 2007. Methylcyclopentadienyl manganese tricarbonyl. 2007 Directory of chemical producers.
Menlo Park, CA: SRI Consulting. Access Intelligence, LLC., 739.
*Srisuchart B, Taylor MJ, Sharma RP. 1987. Alteration of humoral and cellular immunity in manganese
chloride-treated mice. J Toxicol Environ Health 22:91-99.
*Srivastava VK, Chauhan SS, Srivastava PK, et al. 1990. Placental transfer of metals of coal fly ash into
various fetal organs of rat. Arch Toxicol 64:153-156.
St-Pierre A, Normandin L, Carrier G, et al. 2001. Bioaccumulation and locomotor effect of manganese
dust in rats. Inhal Toxicol 13:623-632.
MANGANESE 490
9. REFERENCES
Standridge JS, Bhattacharya A, Succop P, et al. 2008. Effect of chronic low level manganese exposure
on postural balance: A pilot study of residents in southern Ohio. J Occup Environ Med
50(12):1421-1429.
Stanek EJ, Calabrese EJ. 1995. Daily estimates of soil ingestion in children. Environ Health Perspect
103:276-285.
Stauber JL, Florence TM, Webster WS. 1987. The use of scalp hair to monitor manganese in aborigines
from Groote Eylandt. Neurotoxicology 8:431-435.
*Stoner GD, Shimkin MB, Troxell MC, et al. 1976. Test for carcinogenicity of metallic compounds by
the pulmonary tumor response in strain A mice. Cancer Res 36:1744-1747.
*Strause LG, Hegenauer J, Saltman P, et al. 1986. Effects of long-term dietary manganese and copper
deficiency on rat skeleton. J Nutr 116:135-141.
Stredrick DL, Stokes AH, Worst TJ, et al. 2004. Manganese-induced cytotoxicity in dopamine-
producing cells. Neurotoxicology 25(4):543-553.
Stupar J, Dolinsek F. 1996. Detemination of chromium, manganese, lead, and cadmium in biological
samples including hair using direct electrothermal atomic absorption spectrometry. Spectrochim Acta B
51:665-683.
Sturaro A, Parvoli G, Doretti L, et al. 1994. The influence of color, age, and sex on the content of zinc,
copper, nickel, manganese, and lead in human hair. Biol Trace Elem Res 40:1-8.
Suarez N, Walum E, Eriksson H. 1995. Cellular neurotoxicity of trivalent manganese bound to
transferrin or pyrophosphate studied in human neuroblastoma (SH-SY5Y) cell cultures. Toxicol in Vitro
9:717-721.
Subhash MN, Padmashree TS. 1991. Effect of manganese on biogenic amine metabolism in regions of
the rat brain. Food Chem Toxicol 29:579-582.
Sumino K, Hayakawa K, Shibata T, et al. 1975. Heavy metals in normal Japanese tissues: Amounts of
15 heavy metals in 30 subjects. Arch Environ Health 30:487-494.
Summers MJ, Summers JJ, White TF, et al. 2011. The effect of occupational exposure to manganese
dust and fume on neuropsychological functioning in Australian smelter workers. J Clin Exp
Neuropsychol 33(6):692-703.
Sunderman FW, Kasprzak KS, Lau TJ, et al. 1976. Effects of manganese on carcinogenicity and
metabolism of nickel subsulfide. Cancer Res 36:1790-1800.
Suzuki Y, Fujii N, Yano H, et al. 1978. Effects of the inhalation of manganese dioxide dust on monkey
lungs. Tokushima J Exp Med 25(3-4):119-125.
Svensson O, Engfeldt B, Reinholt FP, et al. 1987. Manganese rickets: A biochemical and stereologic
study with special reference to the effect of phosphate. Clin Orthop (No. 218):302-311.
MANGANESE 491
9. REFERENCES
Svensson O, Hjerpe A, Reinholt FP, et al. 1985. The effect of manganese ingestion, phosphate depletion,
and starvation on the morphology of the epiphyseal growth plate: A stereologic study. Clin Orthop (No.
197):286-294.
Sweet CW, Vermette SJ, Landsberger S. 1993. Sources of toxic trace elements in urban air in Illinois.
Environ Sci Technol 27(12):2502-2510.
Szakmáry E, Ungvary G, Hudak A, et al. 1995. Developmental effect of manganese in rat and rabbit.
Cent Eur J Occup Environ Med 1:149-159.
*Sziráki I, Rauhala P, Chiueh CC. 1995. Novel protective effect of manganese against ferrous citrate-
induced lipid peroxidation and nigrostriatal neurodegeneration in vivo. Brain Res 698(1-2):285-287.
Sziráki I, Rauhala P, Kon Koh K, et al. 1999. Implications for atypical antioxidative properties of
manganese in iron-induced brain lipid peroxidation and copper-dependent low density lipoprotein
conjugation. Neurotoxicology 20:455-466.
Takeda A, Sawashita J, Okada S. 1994. Localization in rat brain of the trace metals, zinc and
manganese, after intracerebroventricular injection. Brain Res 658:252-254.
Takeda A, Sotogaku N, Oku N. 2002. Manganese influences the levels of neurotransmitters in synapses
in rat brain. Neuroscience 114(3):669-674.
Takeda A, Sotogaku N, Oku N. 2003. Influence of manganese on the release of neurotransmitters in rat
striatum. Brain Res 965:279-282.
Tanaka S, Lieben J. 1969. Manganese poisoning and exposure in Pennsylvania. Arch Environ Health
19:674-684.
Tapin D, Kennedy G, Lambert J, et al. 2006. Bioaccumulation and locomotor effects of manganese
sulfate in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl
Pharmacol 211(2):166-174.
Taylor HE. 1982. A summary of methods for water-quality analysis of specific species. In: Minear RA,
Keith LH, eds. Water analyis. Vol. 1. Inorganic Species. Part 1. New York, NY: Academic Press,
235-273.
Taylor MD, Erikson KM, Dobson AW, et al. 2006. Effects of inhaled manganese on biomarkers of
oxidative stress in the rat brain. Neurotoxicology 27(5):788-797.
Teeguarden JG, Dorman DC, Covington TR, et al. 2007a. Pharmacokinetic modeling of manganese. I.
Dose dependencies of uptake and elimination. J Toxicol Environ Health A 70:1493-1504.
Teeguarden JG, Dorman DC, Nong A, et al. 2007b. Pharmacokinetic modeling of manganese. II.
Hepatic processing after ingestion and inhalation. J Toxicol Environ Health A 70:1505-1514.
Teeguarden JG, Gearhart J, Clewell HJ, et al. 2007c. Pharmacokinetic modeling of manganese. III.
Physiological approaches accounting for background and tracer kinetics. J Toxicol Environ Health A
70:1515-1526.
MANGANESE 492
9. REFERENCES
Ter Haar GL, Griffing ME, Brandt M, et al. 1975. Methylcyclopentadienyl manganese tricarbonyl as an
antiknock: Composition and fate of manganese exhaust products. J Air Pollut Control Assoc 25:858-
860.
Thomas K, Colborn T. 1992. Organochlorine endocrine disruptors in human tissue. In: Colborn T,
Clement C, eds. Chemically induced alterations in sexual and functional development: The
wildlife/human connection. Princeton, NJ: Princeton Scientific Publishing, 365-394.
Thompson TN, Klaassen CD. 1982. Presystemic elimination of manganese in rats. Toxicol Appl
Pharmacol 64:236-243.
Thompson K, Molina RM, Donaghey T, et al. 2006. The influence of high iron diet on rat lung
manganese absorption. Toxicol Appl Pharmacol 210(1-2):17-23.
Thompson K, Molina RM, Donaghey T, et al. 2007. Olfactory uptake of manganese requires DMT1 and
is enhanced by anemia. FASEB J 21(1):223-230.
Thompson KJ, Molina RM, Donaghey T, et al. 2011. Manganese uptake and distribution in the brain
after methyl bromide-induced lesions in the olfactory epithelia. Toxicol Sci 120(1):163-172.
Thompson SE, Burton CA, Quinn DJ, et al. 1972. Concentration factors of chemical elements in edible
aquatic organisms. Lawrence Livermore Laboratory, Bio-Medical Division, University of California,
Livermore, CA.
Thomson AB, Olatunbosun D, Valberg LS, et al. 1971. Interrelation of intestinal transport system for
manganese and iron. J Lab Clin Med 78:642-655.
Tichy M, Cikrt M. 1972. Manganese transfer into the bile in rats. Arch Toxikol 29:51-58.
Tinggi U, Reilly C, Patterson C. 1997. Determination of manganese and chromium in food by atomic
absorption spectromety after wet digestion. Food Chem 60:123-128.
Tipton IH, Cook MJ. 1963. Trace elements in human tissue. Part II. Adult subjects from the United
States. Health Phys 9:103-145.
Tjälve H, Henriksson J. 1999. Uptake of metals in the brain via olfactory pathways. Neurotoxicology
20:181-195.
Tjälve H, Henriksson J, Tallkvist J, et al. 1996. Uptake of manganese and cadmium from the nasal
mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol 79:347-356.
Toft KG, Friisk GA, Skotland T. 1997a. Mangafodipir trisodium injection, a new contrast medium for
magnetic resonance imaging: Detection and quantification of the parent compound MnDPDP and
metabolites in human plasma by high performance liquid chromatography. J Pharm Biomed Anal
15:973-981.
Toft KG, Hustvedt SO, Grant D, et al. 1997b. Metabolism and pharmacokinetics of MnDPDP in man.
Acta Radiol 38:677-689.
MANGANESE 493
9. REFERENCES
Toft KG, Hustvedt SO, Grant D, et al. 1997c. Metabolism of mangafodipir trisodium (MnDPDP), a new
contrast medium for magnetic resonance imaging, in beagle dogs. Eur J Drug Metab Pharmacokinet
22:65-72.
Torrente M, Colomina MT, Domingo JL. 2005. Behavioral effects of adult rats concurrently exposed to
high doses of oral manganese and restraint stress. Toxicology 211(1-2):59-69.
Tran TT, Cowanadisai W, Crinella FM, et al. 2002b. Effect of high dietary manganese intake of neonatal
rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status.
Neurotoxicology 23:635-643.
Tran TT, Chowanadisai W, Lonnerdal B, et al. 2002a. Effects of neonatal dietary manganese exposure
on brain dopamine levels and neurocognitive functions. Neurotoxicology 23(4-5):645-651.
Treinen KA, Gray TJB, Blazak WF. 1995. Developmental toxicity of mangafodipir trisodium and
manganese chloride in Sprague-Dawley rats. Teratol 52:109-115.
TRI09. 2011. TRI explorer: Providing access to EPA’s toxics release inventory data. Washington, DC:
Office of Information Analysis and Access. Office of Environmental Information. U.S. Environmental
Protection Agency. Toxics Release Inventory. http://www.epa.gov/triexplorer/. September 15, 2011.
Tsalev DL. 1983. Manganese. In: Tsalev DL. Atomic absorption spectrometry in occupational and
environmental health practice. Vol. II. Determination of individual elements. Boca Raton, FL: CRC
Press, Inc.
Tsuda H, Kato K. 1977. Chromosomal aberrations and morphological transformation in hamster
embryonic cells treated with potassium dichromate in vitro. Mutat Res 46:87-94.
Turner RR, Lindberg SE, Coe JM. 1985. Comparative analysis of trace metal accumulation in forest
ecosystems. 5th International Conference on Heavy Metals in the Environment 1:356-358.
Uchino A, Noguchi T, Nomiyama K, et al. 2007. Manganese accumulation in the brain: MR imaging.
Neuroradiology 49:715-720.
Ulitzur S, Barak M. 1988. Detection of genotoxicity of metallic compounds by the bacterial
bioluminescence test. J Biol Chem 2:95-99.
Ulrich CE, Rinehart W, Brandt M. 1979a. Evaluation of the chronic inhalation toxicity of a manganese
oxide aerosol. III - Pulmonary function, electromyograms, limb tremor, and tissue manganese data. Am
Ind Hyg Assoc J 40:349-353.
Ulrich CE, Rinehart W, Busey W. 1979b. Evaluation of the chronic inhalation toxicity of a manganese
oxide aerosol. I. Introduction, experimental design, and aerosol generation methods. Am Ind Hyg Assoc
J 40:238-244.
Ulrich CE, Rinehart W, Busey W, et al. 1979c. Evaluation of the chronic inhalation toxicity of a
manganese oxide aerosol. II - Clinical observations, hematology, clinical chemistry and histopathology.
Am Ind Hyg Assoc J 40:322-329.
Umeda M, Nishimura M. 1979. Inducibility of chromosomal aberra-tions by metal compounds in
cultured mammalian cells. Mutat Res 67:221-229.
MANGANESE 494
9. REFERENCES
U.S. DHEW. 1970. Community water supply study. Analysis of national survey findings. Cincinnati,
OH: U.S. Department of Health, Education, and Welfare, Bureau of Water Hygiene. NTIS No. PB-
214982.
USGS. 1964. Public water supplies of the 100 largest cities in the United States, 1962. Washington,
DC: U.S. Geological Survey. Water-supply paper 1812.
USGS. 2001. Manganese recycling in the United States in 1998. U.S. Geological Survey. Open file
report 01-304. http://pubs.usgs.gov/of/2001/of01-304/of01-304.pdf. April 07, 2008.
USGS. 2007. 2005 Minerals yearbook. Manganese. U.S. Geological Survey.
http://minerals.usgs.gov/minerals/pubs/commodity/manganese/mangamyb05.pdf. April 07, 2008.
USGS. 2008. Manganese. Mineral commodity summaries. U.S. Geological Survey, 104-105.
http://minerals.usgs.gov/minerals/pubs/commodity/manganese/mcs-2008-manga.pdf. April 07, 2008.
Utter MF. 1976. The biochemistry of manganese. Med Clin North Am 60:713-727.
Vahlquist A, Rask L, Peterson PA, et al. 1975. The concentrations of retinol-binding protein,
prealbumin, and transferrin in the sera of newly delivered mothers and children of various ages. Scand J
Clin Lab Invest 35:569-375.
Valencia R, Mason JM, Woodruff RC, et al. 1985. Chemical mutagenesis testing in Drosophila. III.
Results of 48 coded compounds tested for the National Toxicology Program. Environ Mutagen 7:325-
348.
Valentin H, Schiele R. 1983. Manganese. In: Alessio L, et al. Human biological monitoring of
industrial chemicals series. Luxembourg: Commission of the European Communities. EUR-8476-EN.
NTIS No. PB86-217908.-gov doc
*van der Elst L, Colet JM, Muller RN. 1997. Spectroscopic and metabolic effects of MnCl2 and
MnDPDP on the isolated and perfused rat heart. Invest Radiol 32:581-588.
Venugopal B, Luckey TD. 1978. Toxicity of group VII metals. In: Metal toxicity in mammals. 2.
Chemical toxicity of metals and metalloids. New York, NY: Plenum Press, 262-268.
Verity MA. 1999. Manganese toxicity: A mechanistic hypothesis. Neurotoxicology 20:489-498.
Versieck J, Vanballenberghe L, De Kese A. 1988. More on determination of manganese in biological
materials [Letter]. Clin Chem 34:1659-1660.
*Vescovi A, Gebbia M, Cappelletti G, et al. 1989. Interactions of manganese with human brain
glutathione-S-transferase. Toxicology 57:183-191.
Veysseyre A, Vondevelde K, Ferrari C, Bourton C, et al. 1998. Searching for manganese pollution from
MMT anti-knock gasoline additives in snow from central Greenland. Sci Total Environ 221:149-158.
Vezér T, Kurunczi A, Naray M, et al. 2007. Behavioral effects of subchronic inorganic manganese
exposure in rats. Am J Ind Med 50:841-852.
MANGANESE 495
9. REFERENCES
Vezér T, Papp A, Hoyk Z, et al. 2005. Behavioral and neurotoxicological effects of subchronic
manganese exposure in rats. Environ Toxicol Pharmacol 19:797-810.
Vieira I, Sonnier M, Cresteil T. 1996. Developmental expression of CYP2E1 in the human liver:
Hypermethylation control of gene expression during the neonatal period. Eur J Biochem 238(2):476-483.
Vieregge P, Heinzow B, Korf G, et al. 1995. Long term exposure to manganese in rural well water has
no neurological effects. Can J Neurol Sci 22:286-289.
Vitarella D, Wong BA, Moss OR, et al. 2000. Pharmacokinetics of inhaled manganese phosphate in
male Sprague-Dawley rats following subacute (14-day) exposure. Toxicol Appl Pharmacol 163:279-285.
Waalkes MP, Klaassen CD. 1985. Concentration of metallothione in major organs of rats after
administration of various metals. Fundam Appl Toxicol 5:473-477.
Wallace L, Slonecker T. 1997. Ambient air concentrations of fine (PM2.5) manganese in U.S. national
parks and in California and Canadian cities: The possible impact of adding MMT to unleaded gasoline. J
Air Waste Manag Assoc 47:642-652.
Walton AP, Wei GT, Liang Z, et al. 1991. Laser-excited atomic fluorescence in a flame as a high-
sensitivity detector for organomanganese and organotin compounds following separation by high-
performance liquid chromatography. Anal Chem 63:232-240.
Wang C, Gordon PB, Hustvedt SO, et al. 1997. MR imaging properties and pharmacokinetics of
MnDPDP in healthy volunteers. Acta Radiologica 38:665-676.
Wang D, Du X, Zheng W. 2008. Alteration of saliva and serum concentrations of manganese, copper,
zinc, cadmium and lead among career welders. Toxicol Lett 176:40-47.
*Wang JD, Huang CC, Hwang YH, et al. 1989. Manganese induced Parkinsonism: An outbreak due to
an unrepaired ventilation control system in a ferromanganese smelter. Br J Ind Med 46:856-859.
Warner BB, Papes R, Heile M, et al. 1993. Expression of human MnSOD in Chinese hamster ovary cells
confers protection from oxidant injury. Am J Physiol 264:L598-L605.
Wassermann D, Wassermann M. 1977. The ultra structure of the liver cell in subacute manganese
administration. Environ Res 14:379-390.
Wasserman GA, Liu X, Parvez F, et al. 2006. Water manganese exposure and children's intellectual
function in Araihazar, Bangladesh. Environ Health Perspect 114(1):124-129.
Wasserman GA, Liu X, Parvez F, et al. 2011. Arsenic and manganese exposure and children's
intellectual function. Neurotoxicology 32(4):450-457.
Weber S, Dorman DC, Lash LH, et al. 2002. Effects of manganese (Mn) on the developing rat brain:
Oxidative-stress related endpoints. Neurotoxicology 23(2):169-175.
*Webster WS, Valois AA. 1987. Reproductive toxicology of manganese in rodents, including exposure
during the postnatal period. Neurotoxicology 8:437-444.
MANGANESE 496
9. REFERENCES
Wedekind KJ, Titgemeyer EC, Twardock AR, et al. 1991. Phosphorus, but not calcium, affects
manganese absorption and turnover in chicks. J Nutr 121:1776-1786.
Wedler FC. 1994. Biochemical and nutritional role of manganese: An overview. In: Klimis-Tavantzis
DJ, ed. Manganese in health and disease. Boca Raton, LA: CRC Press, 1-36.
Weiner WJ, Nausieda PA, Klawans HL. 1977. Effect of chlorpromazine on central nervous system
concentrations of manganese, iron, and copper. Life Sci 20:1181-1186.
Weiss B. 2006. Economic implications of manganese neurotoxicity. Neurotoxicology 27:362-368.
*Wennberg A, Hagman M, Johansson L. 1992. Preclinical neurophysiological signs of Parkinsonism in
occupational manganese exposure. Neurotoxicology 13:271-274.
Wennberg A, Iregren A, Struwe G, et al. 1991. Manganese exposure in steel smelters a health hazard to
the nervous system. Scand J Work Environ Health 17:255-262.
West JR, Smith HW, Chasis H. 1948. Glomerular filtration rate, effective renal blood flow, and maximal
tubular excretory capacity in infancy. J Pediatr 32:10-18.
Whitlock CM, Amuso SJ, Bittenbender JB. 1966. Chronic neurological disease in two manganese steel
workers. Am Ind Hyg Assoc J 27:454-459.
WHO. 1973. Manganese. Trace elements in human nutrition. Report of a WHO committee. Geneva,
Switzerland: World Health Organization, 34-36.
WHO. 1981. Environmental health criteria 17: Manganese. World Health Organization, Geneva,
Switzerland.
WHO. 1984a. Guidelines for drinking water quality. Vol. 1. Recommendations. World Health
Organization, Geneva, Switzerland, 7, 52, 79, 82.
*WHO. 1984b. Guidelines for drinking water quality. Vol. 2. Health criteria and other supporting
information. World Health Organization, Geneva, Switzerland, 275-278.
WHO. 1986. Diseases caused by manganese and its toxic compounds. Early detection of occupational
diseases, World Health Organization, Geneva, Switzerland, 69-73.
WHO. 1987. Manganese. In: Air quality guidelines for Europe. European Series No. 23. Copenhagen,
Denmark: World Health Organization Regional Office for Europe, 262-271.
WHO. 1999. Concise international chemical assessment document 12. Manganese and its compounds.
Geneva: United Nations Environment Programme. International Labour Organisation. World Health
Organization. http://whqlibdoc.who.int/publications/1999/924153012X.pdf. August 04, 2008.
WHO. 2000a. Air quality guidelines. 2nd ed. Geneva, Switzerland: World Health Organization.
http://www.euro.who.int/Document/AIQ/AirQualRepMtg.pdf. March 08, 2006.
WHO. 2000b. Air quality guidelines for Europe. 2nd ed. World Health Organization.
http://www.euro.who.int/document/e71922.pdf. August 02, 2008.
MANGANESE 497
9. REFERENCES
WHO. 2001. Manganese. In: Air quality guidelines. 2nd ed. World Health Organization.
http://www.euro.who.int/document/aiq/6_8manganese.pdf. August 02, 2008.
WHO. 2004a. Guidelines for drinking-water quality. Vol. 1. Recommendations. 3rd ed. Geneva,
Switzerland: World Health Organization. http://www.who.int/water_sanitation_health/dwq/gdwq3/en/.
March 08, 2006.
WHO. 2004b. Manganese in drinking-water. Background document for development of WHO
guidelines for drinking-water quality. World Health Organization. WHO/SDE/WSH/03.04/104.
http://www.who.int/water_sanitation_health/dwq/chemicals/manganese.pdf. April 07, 2008.
WHO/IPSC. 1999. Concise International Chemical Assessment Document 12: Manganese and its
compounds. World Health Organization/Inter-Organization Programme for the Sound Management of
Chemicals.
Widdowson EM, Dickerson JWT. 1964. Chemical composition of the body. In: Comar CL, Bronner F,
eds. Mineral metabolism: An advanced treatise. Volume II: The elements Part A. New York, NY:
Academic Press, 1-247.
Widdowson EM, Chan H, Harrison GE, et al. 1972. Accumulation of Cu, Zn, Mn, Cr and Co in the
human liver before birth. Biol Neonate 20:360-367.
Wieczorek H, Oberdörster G. 1989a. Effects of selected chelating agents on organ distribution and
excretion of manganese after inhalation exposure to 54MnCl2. I. Injection of chelating agents. Pol J
Occup Med 2:261-267.
Wieczorek H, Oberdörster G. 1989b. Effects of chelating on organ distribution and excretion of
manganese after inhalation exposure to 54MnCl2. II: Inhalation of chelating agents. Pol J Occup Med
2:389-396.
Wieczorek H, Oberdörster G. 1989c. Kinetics of inhaled
54
MnCl
2
aerosols: Influence of inhaled
concentrations. Polish J Occup Med 2(3):248-260.
Wilgus HS, Patton AR. 1939. Factors affecting manganese utilization in the chicken. J Nutr 18:35-45.
Wilson DC, Tubman R, Bell N, et al. 1991. Plasma manganese, selenium and glutathione peroxidase
levels in the mother and newborn infant. Early Hum Dev 26:223-226.
Windholz M, ed. 1983. The Merck index: An encyclopedia of chemicals, drugs and biologicals. 10th
ed. Rahway, NJ: Merck and Company, Inc., 816-818.
Witschi HP, Hakkinen PJ, Kehrer JP. 1981. Modification of lung tumor development in A/J mice.
Toxicology 21:37-45.
Wolters EC, Huang CC, Clark C, et al. 1989. Positron emission tomography in manganese intoxication.
Ann Neurol 26:647-651.
Wong GHW, Goeddel DV. 1988. Induction of manganous superoxide dismutase by tumor necrosis
factor: possible protective mechanism. Science 242:941-944.
Wong PK. 1988. Mutagenicity of heavy metals. Bull Environ Contam Toxicol 40:597-603.
MANGANESE 498
9. REFERENCES
Woolf A, Wright R, Amarasiriwardena C, et al. 2002. A child with chronic manganese exposure from
drinking water. Environ Health Perspect 110:613-616.
Wright RO, Amarasiriwardena C, Woolf AD, et al. 2006. Neuropsychological correlates of hair arsenic,
manganese, and cadmium levels in school-age children residing near a hazardous waste site.
Neurotoxicology 27(2):210-216.
Wu W, Zhang Y, Zhang F, et al. 1996. [Studies on the semen quality in workers exposed to manganese
and electric welding.] Chin J Prev Med 30:266-268. (Chinese)
Yamada M, Ohno S, Okayasu I, et al. 1986. Chronic manganese poisoning: A neuropathological study
with determination of manganese distribution in the brain. Acta Neuropathol (Berl) 70:273-278.
Yen HC, Oberley TD, Vichitbandha S, et al. 1996. The protective role of superoxide dismutase against
adriamycin-induced cardiac toxicity in transgenic mice. J Clin Invest 98:1253-1260.
Yiin SJ, Lin TH, Shih TS. 1996. Lipid peroxidation in workers exposed to manganese. Scand J Work
Environ Health 22:381-386.
Yokel RA. 2002. Brain uptake, retention, and efflux of aluminum and manganese. Environ Health
Perspect Suppl 110:699-704.
*Yong VW, Perry TL, Godolphin WJ, et al. 1986. Chronic organic manganese administration in the rat
does not damage dopaminergic nigrostriatal neurons. Neurotoxicology 7:19-24.
Yoon M, Nong A, Clewell HJ, et al. 2009a. Evaluating placental transfer and tissue concentrations of
manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol Sci
112(1):44-58.
Yoon M, Nong A, Clewell HJ, et al. 2009b. Lactational transfer of manganese in rats: Predicting
manganese tissue concentration in the dam and pups from inhalation exposure with a pharmacokinetic
model. Toxicol Sci 112(1):23-43.
Yoon M, Schroeter JD, Nong A, et al. 2011. Physiologically based pharmacokinetic modeling of fetal
and neonatal manganese exposure in humans: Describing manganese homeostasis during development.
Toxicol Sci 122(2):297-316.
Young T, Myers JE, Thompson ML. 2005. The nervous system effects of occupational exposure to
manganese--measured as respirable dust--in a South African manganese smelter. Neurotoxicology
26(6):993-1000.
*Zaidi SH, Dogra RK, Shanker R, et al. 1973. Experimental infective manganese pneumoconiosis in
guinea pigs. Environ Res 6:287-297.
Zakour RA, Glickman BW. 1984. Metal-induced mutagenesis in the lacI gene of Escherichia coli.
Mutat Res 126:9-18.
Zayed J, Gérin M, Loranger S, et al. 1994. Occupational and environmental exposure of garage workers
and taxi drivers to airborne manganese arising from the use of methylcyclopentadienyl manganese
tricarbonyl in unleaded gasoline. Am Ind Hyg Assoc J 55(1):53-58.
MANGANESE 499
9. REFERENCES
Zayed J, Mikhail M, Loranger S, et al. 1996. Exposure of taxi drivers and office workers to total
respirable manganese in an urban environment. Am Ind Hyg Assoc J 57(4):376-380.
Zayed J, Thibault C, Gareau L, et al. 1999a. Airborne manganese particulates and
methylcyclopentadienyl manganese tricarbonyl (MMT) at selected outdoor sites in Montreal.
Neurotoxicology 20:151-157.
Zayed J, Vyskocil A, Kennedy G. 1999b. Environmental contamination and human exposure to
manganese: Contribution of methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline. Int
Arch Occup Environ Health 72(1):7-13.
Zhang G, Liu D, He P. 1995. [Effects of manganese on learning abilities in school children.] Chung
Hua Yu Fang I Hsueh Tsa Chih 29:156-158.
Zheng W, Kim H, Zhao Q. 2000. Comparative toxicokinetics of manganese chloride and
methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats. Toxicol Sci 54:295-301.
Zheng W, Ren S, Graziano JH. 1998. Manganese inhibits mitochondrial aconitase: A mechanism of
manganese neurotoxicity. Brain Res 799:334-342.
Ziegler EE, Edwards BB, Jensen RL, et al. 1978. Absorption and retention of lead by infants. Pediatr
Res 12(1):29-34.
Zlotkin SH, Buchanan BE. 1986. Manganese intakes in intravenously fed infants: Dosages and toxicity
studies. Biol Trace Element Res 9:271-279.
Zwingmann C, Leibfritz D, Hazell AS. 2004. Brain energy metabolism in a sub-acute rat model of
manganese neurotoxicity: An ex vivo nuclear magnetic resonance study using [1-13C]glucose.
Neurotoxicology 25(4):573-587.
Zwingmann C, Leibfritz D, Hazell AS. 2007. NMR spectroscopic analysis of regional brain energy
metabolism in manganese neurotoxicity. Glia 55(15):1610-1617.
MANGANESE 501
10. GLOSSARY
Absorption—The taking up of liquids by solids, or of gases by solids or liquids.
Acute Exposure—Exposure to a chemical for a duration of 14 days or less, as specified in the
Toxicological Profiles.
Adsorption—The adhesion in an extremely thin layer of molecules (as of gases, solutes, or liquids) to the
surfaces of solid bodies or liquids with which they are in contact.
Adsorption Coefficient (K
oc
)—The ratio of the amount of a chemical adsorbed per unit weight of
organic carbon in the soil or sediment to the concentration of the chemical in solution at equilibrium.
Adsorption Ratio (Kd)—The amount of a chemical adsorbed by sediment or soil (i.e., the solid phase)
divided by the amount of chemical in the solution phase, which is in equilibrium with the solid phase, at a
fixed solid/solution ratio. It is generally expressed in micrograms of chemical sorbed per gram of soil or
sediment.
Benchmark Dose (BMD)—Usually defined as the lower confidence limit on the dose that produces a
specified magnitude of changes in a specified adverse response. For example, a BMD
10
would be the
dose at the 95% lower confidence limit on a 10% response, and the benchmark response (BMR) would be
10%. The BMD is determined by modeling the dose response curve in the region of the dose response
relationship where biologically observable data are feasible.
Benchmark Dose Model—A statistical dose-response model applied to either experimental toxicological
or epidemiological data to calculate a BMD.
Bioconcentration Factor (BCF)—The quotient of the concentration of a chemical in aquatic organisms
at a specific time or during a discrete time period of exposure divided by the concentration in the
surrounding water at the same time or during the same period.
Biomarkers—Broadly defined as indicators signaling events in biologic systems or samples. They have
been classified as markers of exposure, markers of effect, and markers of susceptibility.
Cancer Effect Level (CEL)—The lowest dose of chemical in a study, or group of studies, that produces
significant increases in the incidence of cancer (or tumors) between the exposed population and its
appropriate control.
Carcinogen—A chemical capable of inducing cancer.
Case-Control Study—A type of epidemiological study that examines the relationship between a
particular outcome (disease or condition) and a variety of potential causative agents (such as toxic
chemicals). In a case-controlled study, a group of people with a specified and well-defined outcome is
identified and compared to a similar group of people without outcome.
Case Report—Describes a single individual with a particular disease or exposure. These may suggest
some potential topics for scientific research, but are not actual research studies.
Case Series—Describes the experience of a small number of individuals with the same disease or
exposure. These may suggest potential topics for scientific research, but are not actual research studies.
MANGANESE 502
10. GLOSSARY
Ceiling Value—A concentration of a substance that should not be exceeded, even instantaneously.
Chronic Exposure—Exposure to a chemical for 365 days or more, as specified in the Toxicological
Profiles.
Cohort Study—A type of epidemiological study of a specific group or groups of people who have had a
common insult (e.g., exposure to an agent suspected of causing disease or a common disease) and are
followed forward from exposure to outcome. At least one exposed group is compared to one unexposed
group.
Cross-sectional Study—A type of epidemiological study of a group or groups of people that examines
the relationship between exposure and outcome to a chemical or to chemicals at one point in time.
Data Needs—Substance-specific informational needs that if met would reduce the uncertainties of human
health assessment.
Developmental Toxicity—The occurrence of adverse effects on the developing organism that may result
from exposure to a chemical prior to conception (either parent), during prenatal development, or
postnatally to the time of sexual maturation. Adverse developmental effects may be detected at any point
in the life span of the organism.
Dose-Response Relationship—The quantitative relationship between the amount of exposure to a
toxicant and the incidence of the adverse effects.
Embryotoxicity and Fetotoxicity—Any toxic effect on the conceptus as a result of prenatal exposure to
a chemical; the distinguishing feature between the two terms is the stage of development during which the
insult occurs. The terms, as used here, include malformations and variations, altered growth, and in utero
death.
Environmental Protection Agency (EPA) Health Advisory—An estimate of acceptable drinking water
levels for a chemical substance based on health effects information. A health advisory is not a legally
enforceable federal standard, but serves as technical guidance to assist federal, state, and local officials.
Epidemiology—Refers to the investigation of factors that determine the frequency and distribution of
disease or other health-related conditions within a defined human population during a specified period.
Genotoxicity—A specific adverse effect on the genome of living cells that, upon the duplication of
affected cells, can be expressed as a mutagenic, clastogenic, or carcinogenic event because of specific
alteration of the molecular structure of the genome.
Half-life—A measure of rate for the time required to eliminate one half of a quantity of a chemical from
the body or environmental media.
Immediately Dangerous to Life or Health (IDLH)—The maximum environmental concentration of a
contaminant from which one could escape within 30 minutes without any escape-impairing symptoms or
irreversible health effects.
Immunologic Toxicity—The occurrence of adverse effects on the immune system that may result from
exposure to environmental agents such as chemicals.
MANGANESE 503
10. GLOSSARY
Immunological Effects—Functional changes in the immune response.
Incidence—The ratio of individuals in a population who develop a specified condition to the total
number of individuals in that population who could have developed that condition in a specified time
period.
Intermediate Exposure—Exposure to a chemical for a duration of 15–364 days, as specified in the
Toxicological Profiles.
In Vitro—Isolated from the living organism and artificially maintained, as in a test tube.
In Vivo—Occurring within the living organism.
Lethal Concentration
(LO)
(LC
LO
)—The lowest concentration of a chemical in air that has been reported
to have caused death in humans or animals.
Lethal Concentration
(50)
(LC
50
)—A calculated concentration of a chemical in air to which exposure for
a specific length of time is expected to cause death in 50% of a defined experimental animal population.
Lethal Dose
(LO)
(LD
Lo
)—The lowest dose of a chemical introduced by a route other than inhalation that
has been reported to have caused death in humans or animals.
Lethal Dose
(50)
(LD
50
)—The dose of a chemical that has been calculated to cause death in 50% of a
defined experimental animal population.
Lethal Time
(50)
(LT
50
)—A calculated period of time within which a specific concentration of a chemical
is expected to cause death in 50% of a defined experimental animal population.
Lowest-Observed-Adverse-Effect Level (LOAEL)—The lowest exposure level of chemical in a study,
or group of studies, that produces statistically or biologically significant increases in frequency or severity
of adverse effects between the exposed population and its appropriate control.
Lymphoreticular Effects—Represent morphological effects involving lymphatic tissues such as the
lymph nodes, spleen, and thymus.
Malformations—Permanent structural changes that may adversely affect survival, development, or
function.
Minimal Risk Level (MRL)—An estimate of daily human exposure to a hazardous substance that is
likely to be without an appreciable risk of adverse noncancer health effects over a specified route and
duration of exposure.
Modifying Factor (MF)—A value (greater than zero) that is applied to the derivation of a Minimal Risk
Level (MRL) to reflect additional concerns about the database that are not covered by the uncertainty
factors. The default value for a MF is 1.
Morbidity—State of being diseased; morbidity rate is the incidence or prevalence of disease in a specific
population.
Mortality—Death; mortality rate is a measure of the number of deaths in a population during a specified
interval of time.
MANGANESE 504
10. GLOSSARY
Mutagen—A substance that causes mutations. A mutation is a change in the DNA sequence of a cell’s
DNA. Mutations can lead to birth defects, miscarriages, or cancer.
Necropsy—The gross examination of the organs and tissues of a dead body to determine the cause of
death or pathological conditions.
Neurotoxicity—The occurrence of adverse effects on the nervous system following exposure to a
chemical.
No-Observed-Adverse-Effect Level (NOAEL)—The dose of a chemical at which there were no
statistically or biologically significant increases in frequency or severity of adverse effects seen between
the exposed population and its appropriate control. Effects may be produced at this dose, but they are not
considered to be adverse.
Octanol-Water Partition Coefficient (K
ow
)—The equilibrium ratio of the concentrations of a chemical
in n-octanol and water, in dilute solution.
Odds Ratio (OR)—A means of measuring the association between an exposure (such as toxic substances
and a disease or condition) that represents the best estimate of relative risk (risk as a ratio of the incidence
among subjects exposed to a particular risk factor divided by the incidence among subjects who were not
exposed to the risk factor). An OR of greater than 1 is considered to indicate greater risk of disease in the
exposed group compared to the unexposed group.
Organophosphate or Organophosphorus Compound—A phosphorus-containing organic compound
and especially a pesticide that acts by inhibiting cholinesterase.
Permissible Exposure Limit (PEL)—An Occupational Safety and Health Administration (OSHA)
allowable exposure level in workplace air averaged over an 8-hour shift of a 40-hour workweek.
Pesticide—General classification of chemicals specifically developed and produced for use in the control
of agricultural and public health pests.
Pharmacokinetics—The dynamic behavior of a material in the body, used to predict the fate
(disposition) of an exogenous substance in an organism. Utilizing computational techniques, it provides
the means of studying the absorption, distribution, metabolism, and excretion of chemicals by the body.
Pharmacokinetic Model—A set of equations that can be used to describe the time course of a parent
chemical or metabolite in an animal system. There are two types of pharmacokinetic models: data-based
and physiologically-based. A data-based model divides the animal system into a series of compartments,
which, in general, do not represent real, identifiable anatomic regions of the body, whereas the
physiologically-based model compartments represent real anatomic regions of the body.
Physiologically Based Pharmacodynamic (PBPD) Model—A type of physiologically based dose-
response model that quantitatively describes the relationship between target tissue dose and toxic end
points. These models advance the importance of physiologically based models in that they clearly
describe the biological effect (response) produced by the system following exposure to an exogenous
substance.
Physiologically Based Pharmacokinetic (PBPK) Model—Comprised of a series of compartments
representing organs or tissue groups with realistic weights and blood flows. These models require a
MANGANESE 505
10. GLOSSARY
variety of physiological information: tissue volumes, blood flow rates to tissues, cardiac output, alveolar
ventilation rates, and possibly membrane permeabilities. The models also utilize biochemical
information, such as air/blood partition coefficients, and metabolic parameters. PBPK models are also
called biologically based tissue dosimetry models.
Prevalence—The number of cases of a disease or condition in a population at one point in time.
Prospective Study—A type of cohort study in which the pertinent observations are made on events
occurring after the start of the study. A group is followed over time.
q
1
*—The upper-bound estimate of the low-dose slope of the dose-response curve as determined by the
multistage procedure. The q
1
* can be used to calculate an estimate of carcinogenic potency, the
incremental excess cancer risk per unit of exposure (usually μg/L for water, mg/kg/day for food, and
μg/m
3
for air).
Recommended Exposure Limit (REL)—A National Institute for Occupational Safety and Health
(NIOSH) time-weighted average (TWA) concentration for up to a 10-hour workday during a 40-hour
workweek.
Reference Concentration (RfC)—An estimate (with uncertainty spanning perhaps an order of
magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups)
that is likely to be without an appreciable risk of deleterious noncancer health effects during a lifetime.
The inhalation reference concentration is for continuous inhalation exposures and is appropriately
expressed in units of mg/m
3
or ppm.
Reference Dose (RfD)—An estimate (with uncertainty spanning perhaps an order of magnitude) of the
daily exposure of the human population to a potential hazard that is likely to be without risk of deleterious
effects during a lifetime. The RfD is operationally derived from the no-observed-adverse-effect level
(NOAEL, from animal and human studies) by a consistent application of uncertainty factors that reflect
various types of data used to estimate RfDs and an additional modifying factor, which is based on a
professional judgment of the entire database on the chemical. The RfDs are not applicable to
nonthreshold effects such as cancer.
Reportable Quantity (RQ)—The quantity of a hazardous substance that is considered reportable under
the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). Reportable
quantities are (1) 1 pound or greater or (2) for selected substances, an amount established by regulation
either under CERCLA or under Section 311 of the Clean Water Act. Quantities are measured over a
24-hour period.
Reproductive Toxicity—The occurrence of adverse effects on the reproductive system that may result
from exposure to a chemical. The toxicity may be directed to the reproductive organs and/or the related
endocrine system. The manifestation of such toxicity may be noted as alterations in sexual behavior,
fertility, pregnancy outcomes, or modifications in other functions that are dependent on the integrity of
this system.
Retrospective Study—A type of cohort study based on a group of persons known to have been exposed
at some time in the past. Data are collected from routinely recorded events, up to the time the study is
undertaken. Retrospective studies are limited to causal factors that can be ascertained from existing
records and/or examining survivors of the cohort.
Risk—The possibility or chance that some adverse effect will result from a given exposure to a chemical.
MANGANESE 506
10. GLOSSARY
Risk Factor—An aspect of personal behavior or lifestyle, an environmental exposure, or an inborn or
inherited characteristic that is associated with an increased occurrence of disease or other health-related
event or condition.
Risk Ratio—The ratio of the risk among persons with specific risk factors compared to the risk among
persons without risk factors. A risk ratio greater than 1 indicates greater risk of disease in the exposed
group compared to the unexposed group.
Short-Term Exposure Limit (STEL)—The American Conference of Governmental Industrial
Hygienists (ACGIH) maximum concentration to which workers can be exposed for up to 15 minutes
continually. No more than four excursions are allowed per day, and there must be at least 60 minutes
between exposure periods. The daily Threshold Limit Value-Time Weighted Average (TLV-TWA) may
not be exceeded.
Standardized Mortality Ratio (SMR)—A ratio of the observed number of deaths and the expected
number of deaths in a specific standard population.
Target Organ Toxicity—This term covers a broad range of adverse effects on target organs or
physiological systems (e.g., renal, cardiovascular) extending from those arising through a single limited
exposure to those assumed over a lifetime of exposure to a chemical.
Teratogen—A chemical that causes structural defects that affect the development of an organism.
Threshold Limit Value (TLV)—An American Conference of Governmental Industrial Hygienists
(ACGIH) concentration of a substance to which most workers can be exposed without adverse effect.
The TLV may be expressed as a Time Weighted Average (TWA), as a Short-Term Exposure Limit
(STEL), or as a ceiling limit (CL).
Time-Weighted Average (TWA)—An allowable exposure concentration averaged over a normal 8-hour
workday or 40-hour workweek.
Toxic Dose
(50)
(TD
50
)—A calculated dose of a chemical, introduced by a route other than inhalation,
which is expected to cause a specific toxic effect in 50% of a defined experimental animal population.
Toxicokinetic—The absorption, distribution, and elimination of toxic compounds in the living organism.
Uncertainty Factor (UF)—A factor used in operationally deriving the Minimal Risk Level (MRL) or
Reference Dose (RfD) or Reference Concentration (RfC) from experimental data. UFs are intended to
account for (1) the variation in sensitivity among the members of the human population, (2) the
uncertainty in extrapolating animal data to the case of human, (3) the uncertainty in extrapolating from
data obtained in a study that is of less than lifetime exposure, and (4) the uncertainty in using lowest-
observed-adverse-effect level (LOAEL) data rather than no-observed-adverse-effect level (NOAEL) data.
A default for each individual UF is 10; if complete certainty in data exists, a value of 1 can be used;
however, a reduced UF of 3 may be used on a case-by-case basis, 3 being the approximate logarithmic
average of 10 and 1.
Xenobiotic—Any chemical that is foreign to the biological system.
A-1 MANGANESE
APPENDIX A. ATSDR MINIMAL RISK LEVELS AND WORKSHEETS
The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) [42 U.S.C.
9601 et seq.], as amended by the Superfund Amendments and Reauthorization Act (SARA) [Pub. L. 99–
499], requires that the Agency for Toxic Substances and Disease Registry (ATSDR) develop jointly with
the U.S. Environmental Protection Agency (EPA), in order of priority, a list of hazardous substances most
commonly found at facilities on the CERCLA National Priorities List (NPL); prepare toxicological
profiles for each substance included on the priority list of hazardous substances; and assure the initiation
of a research program to fill identified data needs associated with the substances.
The toxicological profiles include an examination, summary, and interpretation of available toxicological
information and epidemiologic evaluations of a hazardous substance. During the development of
toxicological profiles, Minimal Risk Levels (MRLs) are derived when reliable and sufficient data exist to
identify the target organ(s) of effect or the most sensitive health effect(s) for a specific duration for a
given route of exposure. An MRL is an estimate of the daily human exposure to a hazardous substance
that is likely to be without appreciable risk of adverse noncancer health effects over a specified duration
of exposure. MRLs are based on noncancer health effects only and are not based on a consideration of
cancer effects. These substance-specific estimates, which are intended to serve as screening levels, are
used by ATSDR health assessors to identify contaminants and potential health effects that may be of
concern at hazardous waste sites. It is important to note that MRLs are not intended to define clean-up or
action levels.
MRLs are derived for hazardous substances using the no-observed-adverse-effect level/uncertainty factor
approach. They are below levels that might cause adverse health effects in the people most sensitive to
such chemical-induced effects. MRLs are derived for acute (1–14 days), intermediate (15–364 days), and
chronic (365 days and longer) durations and for the oral and inhalation routes of exposure. Currently,
MRLs for the dermal route of exposure are not derived because ATSDR has not yet identified a method
suitable for this route of exposure. MRLs are generally based on the most sensitive chemical-induced end
point considered to be of relevance to humans. Serious health effects (such as irreparable damage to the
liver or kidneys, or birth defects) are not used as a basis for establishing MRLs. Exposure to a level
above the MRL does not mean that adverse health effects will occur.
MRLs are intended only to serve as a screening tool to help public health professionals decide where to
look more closely. They may also be viewed as a mechanism to identify those hazardous waste sites that
A-2 MANGANESE
APPENDIX A
are not expected to cause adverse health effects. Most MRLs contain a degree of uncertainty because of
the lack of precise toxicological information on the people who might be most sensitive (e.g., infants,
elderly, nutritionally or immunologically compromised) to the effects of hazardous substances. ATSDR
uses a conservative (i.e., protective) approach to address this uncertainty consistent with the public health
principle of prevention. Although human data are preferred, MRLs often must be based on animal studies
because relevant human studies are lacking. In the absence of evidence to the contrary, ATSDR assumes
that humans are more sensitive to the effects of hazardous substance than animals and that certain persons
may be particularly sensitive. Thus, the resulting MRL may be as much as 100-fold below levels that
have been shown to be nontoxic in laboratory animals.
Proposed MRLs undergo a rigorous review process: Health Effects/MRL Workgroup reviews within the
Division of Toxicology and Human Health Sciences (proposed), expert panel peer reviews, and agency-
wide MRL Workgroup reviews, with participation from other federal agencies and comments from the
public. They are subject to change as new information becomes available concomitant with updating the
toxicological profiles. Thus, MRLs in the most recent toxicological profiles supersede previously
published levels. For additional information regarding MRLs, please contact the Division of Toxicology
and Human Health Sciences (proposed), Agency for Toxic Substances and Disease Registry, 1600 Clifton
Road NE, Mailstop F-62, Atlanta, Georgia 30333.

A-3 MANGANESE
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Manganese (inorganic manganese compounds)
CAS Number: 7439-96-5
Date: September, 2012
Profile Status: Final Post-Public Comment Draft
Route: [X] Inhalation [ ] Oral
Duration: [ ] Acute [ ] Intermediate [X] Chronic
Graph Key: 61
Species: Human
Minimal Risk Level: 0.0003 mg respirable manganese/m
3
(0.3 µg/m
3
)
Reference: Roels HA, Ghyselen P, Buchet JP, et al. 1992. Assessment of the permissible exposure level
to manganese in workers exposed to manganese dioxide dust. Br J Ind Med 49:25-34.
Experimental design: Neurological effects of manganese exposure were evaluated in 92 male workers in a
dry alkaline battery factory. The control group was 101 age- and area-matched workers not
occupationally exposed to manganese but with similar work schedules and workloads. Total and
respirable manganese dust concentrations were measured using personal air sampling in different
occupational areas within the factory. Each worker’s personal exposure was determined by the measured
concentration characteristic for their particular job and the number of years employed. Workers were
exposed for an average duration of 5.3 years (range 0.2–17.7 years) to average (geometric mean)
concentrations of 0.215 and 0.948 mg manganese/m
3
in respirable and total dust, respectively. The
authors noted that the work processes had not changed significantly in the last 15 years, indicating that
past exposures should be comparable to those measured in the study. Neurological function was
measured using an audioverbal short term memory test, a simple visual reaction time test using a
chronoscope, and three manual tests of hand steadiness, coordination, and dexterity. This report provided
good documentation of individual exposure data and characterization of the population studied.
Effects noted in study and corresponding doses: Manganese-exposed workers performed significantly
worse than the controls on the neurobehavioral tests, with particular differences in simple reaction time,
eye-hand coordination, and hand steadiness. Dr. Harry Roels provided the data on the manganese-
exposed group evaluated in this study. These data included individual exposure levels and whether the
individual had an abnormal performance in the neurobehavioral tests (scores below the 5
th
percentile
score of the control group). Actual scores on the tests for each individual were not provided by Dr. Roels.
Percent precision score in the eye-hand coordination test was the most sensitive end point among the end
points showing statistically significantly elevated incidences of abnormal scores and was selected as the
basis of the MRL. Average exposure concentration for each worker was calculated by dividing the
individual lifetime integrated respirable concentration (LIRD; calculated by Dr. Roels from occupational
histories and measurements of workplace air manganese concentrations) by the individual’s total number
of years working in the factory. Individuals were grouped into six exposed groups and the control group,
and the average of the range in each group was used in BMD modeling of the incidence data for number
of workers with abnormal percent precision eye-hand coordination scores (Table A-1).

A-4 MANGANESE
APPENDIX A
Table A-1. Incidence Data for Abnormal Eye-Hand Coordination Scores in
Workers Exposed to Respirable Manganese
a
Range of
Average
Number of
manganese manganese workers with
(respirable) (respirable) abnormal eye-
exposure exposure hand Total
concentrations
c
concentration coordination number of %
Group
b
(µg/m
3
)
(µg/m
3
)
score
d
workers
affected
1
Control
0
5
101
5
2
1.0–99
33
1
7
14
3
100–199
174
6
39
15
4
200–299
224
4
28
11
5
300–399
307
2
3
67
6
400–499
451
4
9
44
7
>500 (523–650)
565
4
6
67
a
Based on individual exposure and dichotomized response data collected by Roels et al. (1992).
b
Individuals were sorted into 7 groups, based on manganese exposure, for use in BMD modeling
c
For each individual, the time-weighted average exposure concentration (respirable manganese) was calculated by
dividing the individual lifetime integrated respirable concentrations (LIRD) by the individual’s respective total number
of years exposed.
d
An abnormal eye-hand coordination score was defined by Roels as a score below the 5
th
percentile score in the
control group for percent precision (52.4) in the eye-hand coordination test.
Available dichotomous models in the EPA Benchmark Dose Software (version 1.4.1c) were fit to the
incidence data for abnormal eye-hand coordination scores in workers exposed to respirable manganese
(Roels et al. 1992, Table A-1). Results from the modeling are shown in Table A-2, including: (1) the
BMC
10
and the 95% lower confidence limit (BMCL
10
) calculated as an estimate of the concentration
associated with a 10% extra risk for an abnormal score; (2) BMC
05
and BMCL
05
values; (3) the p-value
for the chi-square goodness of fit statistic (adequate fit, p>0.1); and (4) AIC (lower AIC indicates better
fit when comparing models, EPA [2000]). Based on the chi-square and AIC measures of fit, all of the
models provided adequate and comparable fits to the data (the quantal linear and one-stage multistage
models had the same parameter values
). BMCL
10
estimates from the different models showed an
approximate 2-fold range from 73 µg/m
3
from a one-stage multistage model to 142 µg/m
3
from the
logistic model. The logistic model was indicated as the best fitting model by the AIC measure
(Table A-2) and was used to provide the POD for the MRL. Figure A-1 plots predicted risks for
abnormal scores from the multistage model and observed incidence values calculated from data in
Table A-1.

Figure A-1. Predicted (Logistic Model) and Observed Incidence of Abnormal Eye-
Hand Coordination Scores in Workers Exposed to Respirable Manganese
(Roels et al. 1992)*
Logistic Model with 0.95 Confidence Level
Fraction Affected
1
0.8
0.6
0.4
0.2
0
B MDL B MD
Logistic
0 100 200 300 400 500
dose
09:27 06/13 2008
A-5 MANGANESE
APPENDIX A
Table A-2. Modeling Results for Incidences of Abnormal Eye-Hand Coordination
Scores in Workers Exposed to Respirable Manganese
BMC
10
BMCL
10
BMC
05
BMCL
05
Model
(µg/m
3
)
(µg/m
3
)
(µg/m
3
)
(µg/m
3
)
x
2
p-value
AIC
Gamma
a
185.46
90.53
134.95
44.07
0.46
134.99
Logistic
179.03
142.14
109.00
83.96
0.64
132.81
Log-logistic
b
186.37
98.40
136.04
46.67
0.47
134.98
Multi-stage
c
110.42
73.21
53.75
35.64
0.36
135.13
Probit
165.97
131.31
98.50
76.01
0.64
132.85
Log-probit
b
188.64
124.37
145.64
86.48
0.46
135.05
Weibull
a
182.58
91.23
126.65
44.41
0.47
134.94
a
Restrict power ≥1
b
Slope restricted to >1
c
Restrict betas ≥0; lowest degree polynomial with an adequate fit is reported; degree of polynomial=1
Source: Roels et al. 1992
*BMD=BMC, BMDL=BMCL; BMDs and BMDLs indicated are associated with a 10% extra risk change from the
control, and are in units of µg/m
3
.

A-6 MANGANESE
APPENDIX A
Dose and end point used for MRL derivation:
[ ] NOAEL [ ] LOAEL [X] Other BMCL
10
for incidence of workers with abnormal scores on an
eye-hand coordination test (142 µg/m
3
from the Logistic Model)
Uncertainty and modifying factors used in MRL derivation:
[ ] 10 for the use of a LOAEL
[ ] 10 for extrapolation from animals to humans
[X] 10 for human variability including possibly enhanced susceptibility of the elderly, infants, and
children; individuals with chronic liver disease or parenteral nutrition; and females and individuals with
iron deficiency. The current assessment does not use an additional modifying factor of 5 for potentially
increased susceptibility in children based on differential kinetics in the young (which was used in the
Agency for Toxic Substances and Disease Registry [2000] a ssessment), because recent toxicokinetic
studies in lactating rats and their offspring exposed to manganese by the oral or inhalation routes suggest
that the human variability factor of 10 provides sufficient protection for differential kinetics in children
and adults. For example, in neonatal rats orally exposed to 25 or 50 mg m anganese/kg/day m anganese
chloride from PND 1 through 21, manganese concentrations in various brain regions were about 2-fold
higher than brain manganese concentrations in adult rats exposed to the same oral dose levels for 21 days
(Dorman et al. 2000). Similarly, 18-day-old neonatal rats exposed from birth to aerosols of manganese
sulfate at 1 mg manganese/m
3
, 6 hours/day showed a 2.6-fold increase in
striatum
manganese
concentrations, compared with controls, while
lactating adults
exposed to the same aerosol concentration
showed a
1.7-fold increase
compared with controls (Dorman et al. 2005a). Likewise, simulations with
PBPK models
for
inhaled manganese in
lactating
rat
dams and
offspring
indicate
that
manganese
concentrations in the
striatum and olfactory bulb of the
brains of PND 19 offspring begin to increase
when
air co
ncentrations exceed
50–100 µg
manganese/m
3
,
whereas maternal
concentrations
begin to
increase at air concentrations between 100 and 300 µg manganese/m
3
(Yoon et al. 2009b).
These results
indicate that at
air concentrations above about 0.05–0.1
mg/m
3
, brain concentrations in neonates may be
elevated, compared with controls, to a greater d
egree than
in
lactating
dams,
but
the age-specific
difference in the
tested air concentration range does
not appear to be
large. Simulations from a human
PBPK
model
for i
nhaled
manganese in
lactating
mothers and
their o
ffspring
indicate that
average
daily
AUCs for manganese concentrations in the globus pallidus of
the
fetus, suckling neonate, and 3-year-old
child from
manganese air concentrations
increased beyond 10% of background concentrations
in fetuses
and 3-year-old children when air concentrations
exceeded 0.01 mg/m
3
(10 µg/m
3
) and in suckling
neonates when air
concentrations exceeded 0.001 mg/m
3
(1 µg/m
3
) (Yoon et al. 2011). Thus, the
inhalation MRL derived herein, 0.3 µg/m
3
, is below air concentrations at which brain concentrations in
human fetuses
(10 µg/m
3
) and nursing infants
(1 µg/m
3
) ar
e predicted
to
begin
to
rise under n
ormal
dietary manganese exposure conditions.
[X]
10 for
limitations/uncertainties
in
the
database
including
the
lack
of
epidemiological data
for
humans chronically exposed to soluble
forms of manganese and the
concern that the general
population may be exposed to more soluble forms of
manganese than most of t
he
manganese-
exposed workers
in the principal and supporting studies. Evidence from several rat studies
indicate that
inhalation
of
more soluble forms of m
anganese (e.g.,
manganese sulfate and
manganese chloride) r
esults
in
higher m
anganese concentrations
in brains
than inhalation of
less
soluble
forms, such as manganese phosphate, manganese tetroxide, or
manganese dioxide
(Dorman et al. 2001a, 2004a; Roels et al. 1997). In addition, data on developmental toxicity for
this route
and duration of
exposure are
lacking. There
is limited information on reproductive
effects in
females (one study
in
rat
dams) an
d
reported
effects on
male reproductive organs have
not been clearly associated with decreased reproductive function. Though it
is clear that
the
A-7 MANGANESE
APPENDIX A
neurological system is the most sensitive identified target organ for effects from subchronic- to
chronic-duration inhalation exposure to manganese, data are lacking to fully characterize the
potential risk for all organ systems from chronic inhalation exposure.
Was a conversion used from ppm in food or water to a mg/body weight dose? No.
If an inhalation study in animals, list the conversion factors used in determining human equivalent dose:
Not applicable.
Was a conversion used from intermittent to continuous exposure?
[X] 5/7 to account for intermittent exposure (5 days/week)
[X] 8/24 to account for intermittent exposure (8 hours/day)
MRL = 142 µg respirable manganese/m
3
x 5d/7d x 8h/24h x 1/100 = 0.3 µg respirable manganese/m
3
.
Other additional studies or pertinent information that lend support to this MRL:
Previous BMD analyses of exposure data and incidence data for abnormal eye-hand coordination test
scores from the Roels et al. (1992) study used a quantal linear model to arrive at a BMCL
10
value of about
74 µg respirable manganese/m
3
(Agency for Toxic Substances and Disease Registry 2000; EPA 1994c;
WHO 2001). This value is virtually the same as the BMCL
10
of 73.2 µg manganese/m
3
obtained from a
one-stage multistage model in the current analysis (Table A-2).
Several BMD analyses of results from other epidemiological studies of neurobehavioral end points in
manganese-exposed workers provide support for the MRL (Clewell and Crump 1999; Clewell et al. 2003;
Health Canada 2010). Estimated BMCL
10
values in these analyses were within 2–3-fold of the POD
(142 µg manganese/m
3
) selected for the chronic inhalation MRL herein.
Dr. Anders Iregren provided ATSDR with individual worker data on total dust manganese exposure and
performance on neurobehavioral tests for the occupational cohort that participated in his study (Iregren
1990; Wennberg et al. 1991). A BMD analysis was performed with these data under contract with
ATSDR (Clewell and Crump 1999) and the lowest BMCL
10
value among the end points analyzed was
0.07 mg respirable manganese/m
3
for a 10% change in simple reaction time. The BMD analysis applied
K-power and Weibull models to continuous variable data (from 11 different test scores collected by Dr.
Iregren) using current respirable manganese exposure estimates, age, and vocabulary test results as
explanatory variables, an assumption that 5% of unexposed subjects had adverse responses, and a
benchmark response of 10% change from unexposed mean scores. For each dataset, BMCL
10
values from
the Weibull model were lower (by 2–3-fold at the most) than BMCL
10
values from the K-Power model.
Weibull BMCL
10
values for the different test score datasets ranged from 0.07 to 0.67 mg respirable
manganese//m
3
. Thus, the lowest BMCL
10
value from this analysis of test score data from manganese-
exposed workers collected by Iregren (1990; Wennberg et al. 1991) is within 2-fold of the POD of 142 µg
manganese/m
3
for the MRL.
Clewell et al. (2003) conducted BMD analyses on data from three neuromotor tests in the Roels et al.
(1992) study (visual reaction time, eye-hand coordination, and hand steadiness) and from five neuromotor
tests in the Gibbs et al. (1999) study (hole 6 of the hand steadiness test, percent precision of the eye-hand
coordination test, reaction time in the complex reaction test, root mean square amplitude in the steady
test, and tap time). Exposure measures in these analyses were recent measures of manganese
concentrations in respirable dust. BMCL
10
values were 0.257, 0.099, and 0.202 mg manganese/m
3
for the
visual reaction time, eye-hand coordination, and hand steadiness data from the Roels et al. (1992) study;
A-8 MANGANESE
APPENDIX A
these results were obtained after fitting incidence data for abnormal scores in these tests to a Weibull
model for dichotomous data. The reported BMCL
10
value of 0.099 mg manganese/m
3
for the eye-hand
coordination test is similar to the BMCL
10
value of 0.091 mg manganese/m
3
obtained with the Weibull
model in the current ATSDR analysis (Table A-2). BMCL
10
values from the analyses of outcomes from
the Gibbs et al. (1999) study ranged from 0.09 to 0.27 mg manganese/m
3
(averaging the BMCLs within
end points across different BMD models applied to the data). Clewell et al. (2003) did not have
individual worker data from the Iregren (1990) or Mergler et al. (1994) studies, but, based on some
assumptions about exposures (e.g., all exposed workers were exposed to average concentrations for the
facilities and respirable manganese concentrations were calculated for the Iregren workers based on an
assumption that 50% of total dust manganese was respirable), they calculated BMCL
10
values for six end
points from the Mergler et al. (1994) study and the simple reaction time end point in the Iregren (1990)
study. BMCL
10
values ranged from about 0.1 to 0.3 mg manganese/m
3
from the Mergler et al. (1994)
study end points to 0.1 mg manganese/m
3
for the reaction time end point in the Iregren (1990) study.
Health Canada (2010) published a human health risk assessment for inhaled manganese in which BMD
analyses were conducted on data for neurobehavioral end points from the study of manganese alloy
workers by Lucchini et al. (1999). Dose-response data for six tests of fine motor control, two aspects of
memory tests, one test of mental arithmetic, and measured serum prolactin levels were fit to linear
models, using exposure metrics based on an average over all occupational history (ARE) or an average
over the latest five years of occupation (ARE5). Using a linear model, BMCL
10
values for the various
end points were 32–59 and 85–98 µg manganese/m
3
for the ARE5 and ARE exposure metrics,
respectively. Regardless of exposure metric, the values are within a 2–4-fold range of the selected POD
of 142 µg manganese/m
3
, based on eye-hand coordination test scores in workers in the Roels et al. (1992)
study.
Neurological effects from repeated inhalation exposure to manganese are well recognized as effects of
high concern based on case reports and epidemiological studies of groups of occupationally exposed
workers. A number of epidemiological studies have used batteries of neurobehavioral tests of
neuromotor, cognition, and mood states to study the psychological or neurological effects of exposure to
low levels of manganese in the workplace (Bast-Pettersen et al. 2004; Beuter et al. 1999; Blond and
Netterstrom 2007; Blond et al. 2007; Bouchard et al. 2003, 2005, 2007a, 2007b; Chia et al. 1993a, 1995;
Crump and Rousseau 1999; Deschamps et al. 2001; Gibbs et al. 1999; Iregren 1990; Lucchini et al. 1995,
1999; Mergler et al. 1994; Myers et al. 2003a, 2003b; Roels et al. 1987a, 1992, 1999; Summers et al.
2011; Wennberg et al. 1991). Some of these studies have found statistically significant differences
between exposed and non-exposed groups or significant associations between exposure indices and
neurological effects (Bast-Pettersen et al. 2004; Chia et al. 1993a; Iregren 1990; Lucchini et al. 1995,
1999; Mergler et al. 1994; Roels et al. 1987a, 1992; Wennberg et al. 1991), whereas others have not
found significant associations (Deschamps et al. 2001; Gibbs et al. 1999; Myers et al. 2003a, 2003b;
Summers et al. 2011; Young et al. 2005). Table A-3 summarizes results from these studies. Comparison
of the effect levels in these studies provides support for selection of the Roels et al. (1992) as the basis of
the MRL. The advantage of the Roels et al. (1992) study is that individual worker data were available to
support a BMD analysis, but, as discussed above, BMD analyses of other epidemiological data for
performance on tests of neurobehavior provided potential PODs within 2–4-fold of the POD selected as
the basis of the MRL.

Table A-3. Epidemiological Studies of Neurological End Points in Workers
Exposed to Low Levels of Inorganic Manganese in Workplace Air
Estimated
exposure
(mg Number Number
Place of manganese/ Years of of
3
)
a
Reference work m worked
b
exposed control Effects
Chia et al.
1993a
Roels et al.
1987a
Roels et al.
1992, 1999
Iregren
1990;
Wennberg et
al. 1991
Lucchini et
al. 1995
Lucchini et
al. 1999
Mergler et al.
1994
Gibbs et al.
1999
Deschamps
et al. 2001
Myers et al.
2003a
Myers et al.
2003b;
Young et al.
2005
Mn ore
process
Mn salt and
oxide plant
Dry alkaline
battery plant
Mn foundry
Mn alloy
plant
Mn alloy
plant
Mn alloy
plant
Mn process
plant
Enamels
production
plant
Mn mines
Mn smelter
1.59
0.97
0.948
(0.215)
0.14
0.149
0.097
(0.038)
0.23
(0.04)
0.18
(0.051)
2.05
(0.035)
0.21
0.85
(0.58)
7.4
7.1
5.3
9.9
13
11.5
16.7
12.7
19.7
10.8
18.2
17
141
92
30
58
61
115
75
134
489
509
17
104
37
60
None
87
115
75
137
None
67
↓ finger tapping, digit symbol,
pursuit aiming
↓ reaction time, short-term
memory, eye-hand
coordination, hand steadiness
↓ reaction time, short-term
memory, eye-hand
coordination, hand steadiness
↓ finger tapping, reaction time
↓ finger tapping, short-term
memory with increasing
exposure indices
↓ hand movements, finger
tapping, short-term memory
↓ rapid hand movements,
cognitive flexibility; ↑ indices for
tension, anger, fatigue,
confusion
No effects on neuromotor tests
or self-reported symptoms
No effects on self-reported
symptoms or several cognitive
tests; no neuromotor tests
given.
No associations between
indices of exposure and
outcomes from tests of
neuromotor and cognitive
functions or self-reported
symptoms
Neurobehavioral test batteries
showed significant effects in
only a few of the many end
points evaluated
A-9 MANGANESE
APPENDIX A

Table A-3. Epidemiological Studies of Neurological End Points in Workers
Exposed to Low Levels of Inorganic Manganese in Workplace Air
Estimated
exposure
(mg Number Number
Place of manganese/ Years of of
3
)
a
Reference work m worked
b
exposed control Effects
Summers et Mn smelter 0.384 10.6 143 None Associations between
al. 2011 (0.123) decreasing deficits on tests of
attention and executive function
(but not tests of short-term
memory span or information-
processing speed) and
increasing exposure. The
magnitude of deficits were not
expected by the study authors
to be of clinical significance.
Bast- Mn alloy 0.753 20.2 100 100 ↑ scores for hand tremor, but no
Pettersen et plant (0.049) effect on other neuromotor or
al. 2004 cognitive tests or symptoms
Blond and Steel works 0.07 24 60–92 14–19 ↓ fast hand and finger
Netterstrom movement, but no effects on
2007; Blond slow movements, reaction time,
et al. 2007 or cognitive end points
a
Mean, median, or midpoint of reported ranges of manganese concentration in total dust. Values for respirable dust
are noted in parentheses when they were available.
b
Mean, median, or midpoint of reported ranges of years employed at the facility.
MANGANESE A-10
APPENDIX A
The neurological effects associated with prolonged low-level manganese exposure generally have been
subtle changes including deficits in tests of neuromotor or cognitive functions and altered mood states;
they have been referred to by various authors as preclinical or subclinical neurological effects.
Manganese air concentrations associated with these effects in chronically exposed workers range from
about 0.07 to 1.59 mg manganese/m
3
(manganese in total or inhalable dust measurements; values for
manganese in respirable dust are noted in parentheses in Table A-3). For several of these work
environments, values of concentrations of manganese in respirable dust (generally particulate diameters
<10 µm) represented <20–80% of the total dust values.
Studies in communities surrounding manganese industries also have reported similar subclinical
neurological effects in adults and children. In a study of men and women living close to a manganese
alloy production plant, a blood manganese level-age interaction was observed, with the poorest
performance on neurological tests occurring among those >50 years old who had the highest blood
manganese levels (Baldwin et al. 1999; Beuter et al. 1999; Bowler et al. 1999; Mergler et al. 1999).
Additional studies of environmentally exposed adults reported attention impairments, poorer postural
stability, and subclinical motor impairments at environmental air exposures >0.1 μg manganese/m
3
;
however, other potential sources of environmental exposure were not accounted for (Kim et al. 2011;
Rodríguez-Agudelo et al. 2006; Solís-Vivano et al. 2009; Standridge et al. 2008). In children living in a
manganese mining area or close to a ferromanganese alloy plant, associations were found between
manganese concentrations in blood or hair and deficits in intellectual functions or motor impairments, but
the reported data are not useful for deriving an inhalation MRL for manganese (Hernández-Bonilla et al.
2011; Menezes-Filho et al. 2011; Riojas-Rodríguez et al. 2010).

MANGANESE A-11
APPENDIX A
The 2000 ATSDR Toxicological Profile for Manganese derived a chronic MRL for inorganic manganese
of 0.00004 mg manganese/m
3
(manganese in respirable dust, 0.04 μg manganese/m
3
), based on a BMCL
10
of 0.074 mg manganese/m
3
(manganese in respirable dust) for abnormal performance in tests of hand
steadiness, eye-hand coordination, or reaction time in the same study of 92 male workers in a dry alkaline
battery plant (Roels et al. 1992) used in the current assessment. The MRL was derived by adjustment of
the BMCL
10
to a continuous exposure basis and division by an uncertainty factor of 500 (10 for human
variability, 10 for database deficiencies and limitations, and a modifying factor of 5 for potentially
increased susceptibility in children based on differential kinetics in the young). The current MRL of
0.3 μg manganese/m
3
replaces the old MRL.
Agency Contact (Chemical Manager): Malcolm Williams, DVM, Ph.D.
B-1 MANGANESE
APPENDIX B. USER'S GUIDE
Chapter 1
Public Health Statement
This chapter of the profile is a health effects summary written in non-technical language. Its intended
audience is the general public, especially people living in the vicinity of a hazardous waste site or
chemical release. If the Public Health Statement were removed from the rest of the document, it would
still communicate to the lay public essential information about the chemical.
The major headings in the Public Health Statement are useful to find specific topics of concern. The
topics are written in a question and answer format. The answer to each question includes a sentence that
will direct the reader to chapters in the profile that will provide more information on the given topic.
Chapter 2
Relevance to Public Health
This chapter provides a health effects summary based on evaluations of existing toxicologic,
epidemiologic, and toxicokinetic information. This summary is designed to present interpretive, weight-
of-evidence discussions for human health end points by addressing the following questions:
1. What effects are known to occur in humans?
2. What effects observed in animals are likely to be of concern to humans?
3. What exposure conditions are likely to be of concern to humans, especially around hazardous
waste sites?
The chapter covers end points in the same order that they appear within the Discussion of Health Effects
by Route of Exposure section, by route (inhalation, oral, and dermal) and within route by effect. Human
data are presented first, then animal data. Both are organized by duration (acute, intermediate, chronic).
In vitro data and data from parenteral routes (intramuscular, intravenous, subcutaneous, etc.) are also
considered in this chapter.
The carcinogenic potential of the profiled substance is qualitatively evaluated, when appropriate, using
existing toxicokinetic, genotoxic, and carcinogenic data. ATSDR does not currently assess cancer
potency or perform cancer risk assessments. Minimal Risk Levels (MRLs) for noncancer end points (if
derived) and the end points from which they were derived are indicated and discussed.
Limitations to existing scientific literature that prevent a satisfactory evaluation of the relevance to public
health are identified in the Chapter 3 Data Needs section.
Interpretation of Minimal Risk Levels
Where sufficient toxicologic information is available, ATSDR has derived MRLs for inhalation and oral
routes of entry at each duration of exposure (acute, intermediate, and chronic). These MRLs are not
meant to support regulatory action, but to acquaint health professionals with exposure levels at which
adverse health effects are not expected to occur in humans.
B-2 MANGANESE
APPENDIX B
MRLs should help physicians and public health officials determine the safety of a community living near
a chemical emission, given the concentration of a contaminant in air or the estimated daily dose in water.
MRLs are based largely on toxicological studies in animals and on reports of human occupational
exposure.
MRL users should be familiar with the toxicologic information on which the number is based. Chapter 2,
"Relevance to Public Health," contains basic information known about the substance. Other sections such
as Chapter 3 Section 3.9, "Interactions with Other Substances,” and Section 3.10, "Populations that are
Unusually Susceptible" provide important supplemental information.
MRL users should also understand the MRL derivation methodology. MRLs are derived using a
modified version of the risk assessment methodology that the Environmental Protection Agency (EPA)
provides (Barnes and Dourson 1988) to determine reference doses (RfDs) for lifetime exposure.
To derive an MRL, ATSDR generally selects the most sensitive end point which, in its best judgement,
represents the most sensitive human health effect for a given exposure route and duration. ATSDR
cannot make this judgement or derive an MRL unless information (quantitative or qualitative) is available
for all potential systemic, neurological, and developmental effects. If this information and reliable
quantitative data on the chosen end point are available, ATSDR derives an MRL using the most sensitive
species (when information from multiple species is available) with the highest no-observed-adverse-effect
level (NOAEL) that does not exceed any adverse effect levels. When a NOAEL is not available, a
lowest-observed-adverse-effect level (LOAEL) can be used to derive an MRL, and an uncertainty factor
(UF) of 10 must be employed. Additional uncertainty factors of 10 must be used both for human
variability to protect sensitive subpopulations (people who are most susceptible to the health effects
caused by the substance) and for interspecies variability (extrapolation from animals to humans). In
deriving an MRL, these individual uncertainty factors are multiplied together. The product is then
divided into the inhalation concentration or oral dosage selected from the study. Uncertainty factors used
in developing a substance-specific MRL are provided in the footnotes of the levels of significant exposure
(LSE) tables.
Chapter 3
Health Effects
Tables and Figures for Levels of Significant Exposure (LSE)
Tables and figures are used to summarize health effects and illustrate graphically levels of exposure
associated with those effects. These levels cover health effects observed at increasing dose
concentrations and durations, differences in response by species, MRLs to humans for noncancer end
points, and EPA's estimated range associated with an upper- bound individual lifetime cancer risk of 1 in
10,000 to 1 in 10,000,000. Use the LSE tables and figures for a quick review of the health effects and to
locate data for a specific exposure scenario. The LSE tables and figures should always be used in
conjunction with the text. All entries in these tables and figures represent studies that provide reliable,
quantitative estimates of NOAELs, LOAELs, or Cancer Effect Levels (CELs).
The legends presented below demonstrate the application of these tables and figures. Representative
examples of LSE Table 3-1 and Figure 3-1 are shown. The numbers in the left column of the legends
correspond to the numbers in the example table and figure.

B-3 MANGANESE
APPENDIX B
LEGEND
See Sample LSE Table 3-1 (page B-6)
(1) Route of Exposure. One of the first considerations when reviewing the toxicity of a substance
using these tables and figures should be the relevant and appropriate route of exposure. Typically
when sufficient data exist, three LSE tables and two LSE figures are presented in the document.
The three LSE tables present data on the three principal routes of exposure, i.e., inhalation, oral,
and dermal (LSE Tables 3-1, 3-2, and 3-3, respectively). LSE figures are limited to the inhalation
(LSE Figure 3-1) and oral (LSE Figure 3-2) routes. Not all substances will have data on each
route of exposure and will not, therefore, have all five of the tables and figures.
(2) Exposure Period. Three exposure periods—acute (less than 15 days), intermediate (15–
364 days), and chronic (365 days or more)—are presented within each relevant route of exposure.
In this example, an inhalation study of intermediate exposure duration is reported. For quick
reference to health effects occurring from a known length of exposure, locate the applicable
exposure period within the LSE table and figure.
(3) Health Effect. The major categories of health effects included in LSE tables and figures are
death, systemic, immunological, neurological, developmental, reproductive, and cancer.
NOAELs and LOAELs can be reported in the tables and figures for all effects but cancer.
Systemic effects are further defined in the "System" column of the LSE table (see key number
18).
(4) Key to Figure. Each key number in the LSE table links study information to one or more data
points using the same key number in the corresponding LSE figure. In this example, the study
represented by key number 18 has been used to derive a NOAEL and a Less Serious LOAEL
(also see the two "18r" data points in sample Figure 3-1).
(5) Species. The test species, whether animal or human, are identified in this column. Chapter 2,
"Relevance to Public Health," covers the relevance of animal data to human toxicity and
Section 3.4, "Toxicokinetics," contains any available information on comparative toxicokinetics.
Although NOAELs and LOAELs are species specific, the levels are extrapolated to equivalent
human doses to derive an MRL.
(6) Exposure Frequency/Duration. The duration of the study and the weekly and daily exposure
regimens are provided in this column. This permits comparison of NOAELs and LOAELs from
different studies. In this case (key number 18), rats were exposed to “Chemical x” via inhalation
for 6 hours/day, 5 days/week, for 13 weeks. For a more complete review of the dosing regimen,
refer to the appropriate sections of the text or the original reference paper (i.e., Nitschke et al.
1981).
(7) System. This column further defines the systemic effects. These systems include respiratory,
cardiovascular, gastrointestinal, hematological, musculoskeletal, hepatic, renal, and
dermal/ocular. "Other" refers to any systemic effect (e.g., a decrease in body weight) not covered
in these systems. In the example of key number 18, one systemic effect (respiratory) was
investigated.
(8) NOAEL. A NOAEL is the highest exposure level at which no harmful effects were seen in the
organ system studied. Key number 18 reports a NOAEL of 3 ppm for the respiratory system,
which was used to derive an intermediate exposure, inhalation MRL of 0.005 ppm (see
footnote "b").

B-4 MANGANESE
APPENDIX B
(9) LOAEL. A LOAEL is the lowest dose used in the study that caused a harmful health effect.
LOAELs have been classified into "Less Serious" and "Serious" effects. These distinctions help
readers identify the levels of exposure at which adverse health effects first appear and the
gradation of effects with increasing dose. A brief description of the specific end point used to
quantify the adverse effect accompanies the LOAEL. The respiratory effect reported in key
number 18 (hyperplasia) is a Less Serious LOAEL of 10 ppm. MRLs are not derived from
Serious LOAELs.
(10) Reference. The complete reference citation is given in Chapter 9 of the profile.
(11) CEL. A CEL is the lowest exposure level associated with the onset of carcinogenesis in
experimental or epidemiologic studies. CELs are always considered serious effects. The LSE
tables and figures do not contain NOAELs for cancer, but the text may report doses not causing
measurable cancer increases.
(12) Footnotes. Explanations of abbreviations or reference notes for data in the LSE tables are found
in the footnotes. Footnote "b" indicates that the NOAEL of 3 ppm in key number 18 was used to
derive an MRL of 0.005 ppm.
LEGEND
See Sample Figure 3-1 (page B-7)
LSE figures graphically illustrate the data presented in the corresponding LSE tables. Figures help the
reader quickly compare health effects according to exposure concentrations for particular exposure
periods.
(13) Exposure Period. The same exposure periods appear as in the LSE table. In this example, health
effects observed within the acute and intermediate exposure periods are illustrated.
(14) Health Effect. These are the categories of health effects for which reliable quantitative data
exists. The same health effects appear in the LSE table.
(15) Levels of Exposure. Concentrations or doses for each health effect in the LSE tables are
graphically displayed in the LSE figures. Exposure concentration or dose is measured on the log
scale "y" axis. Inhalation exposure is reported in mg/m
3
or ppm and oral exposure is reported in
mg/kg/day.
(16) NOAEL. In this example, the open circle designated 18r identifies a NOAEL critical end point in
the rat upon which an intermediate inhalation exposure MRL is based. The key number 18
corresponds to the entry in the LSE table. The dashed descending arrow indicates the
extrapolation from the exposure level of 3 ppm (see entry 18 in the table) to the MRL of
0.005 ppm (see footnote "b" in the LSE table).
(17) CEL. Key number 38m is one of three studies for which CELs were derived. The diamond
symbol refers to a CEL for the test species-mouse. The number 38 corresponds to the entry in the
LSE table.

B-5 MANGANESE
APPENDIX B
(18) Estimated Upper-Bound Human Cancer Risk Levels. This is the range associated with the upper-
bound for lifetime cancer risk of 1 in 10,000 to 1 in 10,000,000. These risk levels are derived
from the EPA's Human Health Assessment Group's upper-bound estimates of the slope of the
cancer dose response curve at low dose levels (q
1
*).
(19) Key to LSE Figure. The Key explains the abbreviations and symbols used in the figure.

1
2
3
4
12
→
SAMPLE
Table 3-1. Levels of Significant Exposure to [Chemical x] – Inhalation
→
→
Exposure
Key to frequency/
figure
a
Species duration
INTERMEDIATE EXPOSURE
5 6
Systemic
↓ ↓
NOAEL
System (ppm)
7 8
↓ ↓
LOAEL (effect)
Less serious
(ppm)
9
↓
Serious (ppm)
Reference
10
↓
→
→
18 Rat 13 wk Resp 3
b
10 (hyperplasia)
5 d/wk Nitschke et al. 1981
6 hr/d
CHRONIC EXPOSURE
Cancer 11
↓
38 Rat 18 mo 20 (CEL, multiple Wong et al. 1982
5 d/wk organs)
7 hr/d
39 Rat 89–104 wk 10 (CEL, lung tumors, NTP 1982
5 d/wk nasal tumors)
6 hr/d
40 Mouse 79–103 wk 10 (CEL, lung tumors, NTP 1982
5 d/wk hemangiosarcomas)
6 hr/d
a
The number corresponds to entries in Figure 3-1.
b
Used to derive an intermediate inhalation Minimal Risk Level (MRL) of 5x10
-3
ppm; dose adjusted for intermittent exposure and divided
by an uncertainty factor of 100 (10 for extrapolation from animal to humans, 10 for human variability).
B-6 MANGANESE
APPENDIX B
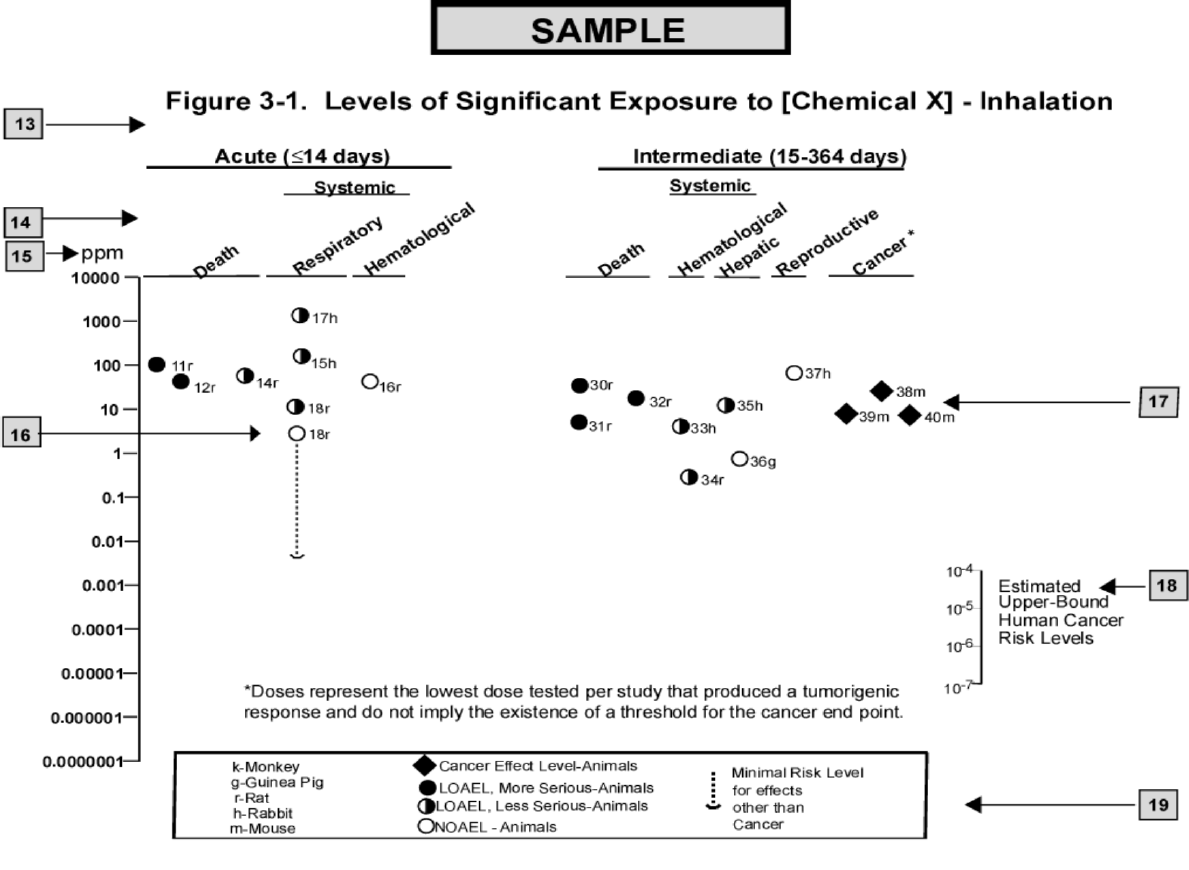
B-7 MANGANESE
APPENDIX B
B-8 MANGANESE
APPENDIX B
This page is intentionally blank.
ACGIH
ACOEM
ADI
ADME
AED
American Conference of Governmental Industrial Hygienists
American College of Occupational and Environmental Medicine
acceptable daily intake
absorption, distribution, metabolism, and excretion
atomic emission detection
AFID alkali flame ionization detector
AFOSH
ALT
Air Force Office of Safety and Health
alanine aminotransferase
AML
AOAC
AOEC
AP
APHA
acute myeloid leukemia
Association of Official Analytical Chemists
Association of Occupational and Environmental Clinics
alkaline phosphatase
American Public Health Association
AST
atm
ATSDR
AWQC
BAT
BCF
aspartate aminotransferase
atmosphere
Agency for Toxic Substances and Disease Registry
Ambient Water Quality Criteria
best available technology
bioconcentration factor
BEI
BMD/C
BMD
X
BMDL
X
Biological Exposure Index
benchmark dose or benchmark concentration
dose that produces a X% change in response rate of an adverse effect
95% lower confidence limit on the BMD
X
BMDS Benchmark Dose Software
BMR
BSC
benchmark response
Board of Scientific Counselors
C
CAA
centigrade
Clean Air Act
CAG
CAS
Cancer Assessment Group of the U.S. Environmental Protection Agency
Chemical Abstract Services
CDC Centers for Disease Control and Prevention
CEL cancer effect level
CELDS
CERCLA
CFR
Ci
Computer-Environmental Legislative Data System
Comprehensive Environmental Response, Compensation, and Liability Act
Code of Federal Regulations
curie
CI confidence interval
CL
CLP
cm
ceiling limit value
Contract Laboratory Program
centimeter
CML
CPSC
CWA
chronic myeloid leukemia
Consumer Products Safety Commission
Clean Water Act
DHEW
DHHS
DNA
DOD
DOE
DOL
Department of Health, Education, and Welfare
Department of Health and Human Services
deoxyribonucleic acid
Department of Defense
Department of Energy
Department of Labor
MANGANESE C-1
APPENDIX C. ACRONYMS, ABBREVIATIONS, AND SYMBOLS
MANGANESE C-2
APPENDIX C
DOT Department of Transportation
DOT/UN/ Department of Transportation/United Nations/
NA/IMDG North America/Intergovernmental Maritime Dangerous Goods Code
DWEL drinking water exposure level
ECD electron capture detection
ECG/EKG electrocardiogram
EEG electroencephalogram
EEGL Emergency Exposure Guidance Level
EPA Environmental Protection Agency
F Fahrenheit
F
1
first-filial generation
FAO Food and Agricultural Organization of the United Nations
FDA Food and Drug Administration
FEMA Federal Emergency Management Agency
FIFRA Federal Insecticide, Fungicide, and Rodenticide Act
FPD flame photometric detection
fpm feet per minute
FR Federal Register
FSH follicle stimulating hormone
g gram
GC gas chromatography
gd gestational day
GLC gas liquid chromatography
GPC gel permeation chromatography
HPLC high-performance liquid chromatography
HRGC high resolution gas chromatography
HSDB Hazardous Substance Data Bank
IARC International Agency for Research on Cancer
IDLH immediately dangerous to life and health
ILO International Labor Organization
IRIS Integrated Risk Information System
Kd adsorption ratio
kg kilogram
kkg metric ton
K
oc
organic carbon partition coefficient
K
ow
octanol-water partition coefficient
L liter
LC liquid chromatography
LC
50
lethal concentration, 50% kill
LC
Lo
lethal concentration, low
LD
50
lethal dose, 50% kill
LD
Lo
lethal dose, low
LDH lactic dehydrogenase
LH luteinizing hormone
LOAEL lowest-observed-adverse-effect level
LSE Levels of Significant Exposure
LT
50
lethal time, 50% kill
m meter
MA trans,trans-muconic acid
MAL maximum allowable level
mCi millicurie
MANGANESE C-3
APPENDIX C
MCL maximum contaminant level
MCLG maximum contaminant level goal
MF modifying factor
MFO mixed function oxidase
mg milligram
mL milliliter
mm millimeter
mmHg millimeters of mercury
mmol millimole
mppcf millions of particles per cubic foot
MRL Minimal Risk Level
MS mass spectrometry
NAAQS National Ambient Air Quality Standard
NAS National Academy of Science
NATICH National Air Toxics Information Clearinghouse
NATO North Atlantic Treaty Organization
NCE normochromatic erythrocytes
NCEH National Center for Environmental Health
NCI National Cancer Institute
ND not detected
NFPA National Fire Protection Association
ng nanogram
NHANES National Health and Nutrition Examination Survey
NIEHS National Institute of Environmental Health Sciences
NIOSH National Institute for Occupational Safety and Health
NIOSHTIC NIOSH's Computerized Information Retrieval System
NLM National Library of Medicine
nm nanometer
nmol nanomole
NOAEL no-observed-adverse-effect level
NOES National Occupational Exposure Survey
NOHS National Occupational Hazard Survey
NPD nitrogen phosphorus detection
NPDES National Pollutant Discharge Elimination System
NPL National Priorities List
NR not reported
NRC National Research Council
NS not specified
NSPS New Source Performance Standards
NTIS National Technical Information Service
NTP National Toxicology Program
ODW Office of Drinking Water, EPA
OERR Office of Emergency and Remedial Response, EPA
OHM/TADS Oil and Hazardous Materials/Technical Assistance Data System
OPP Office of Pesticide Programs, EPA
OPPT Office of Pollution Prevention and Toxics, EPA
OPPTS Office of Prevention, Pesticides and Toxic Substances, EPA
OR odds ratio
OSHA Occupational Safety and Health Administration
OSW Office of Solid Waste, EPA
OTS Office of Toxic Substances
MANGANESE C-4
APPENDIX C
OW Office of Water
OWRS Office of Water Regulations and Standards, EPA
PAH polycyclic aromatic hydrocarbon
PBPD physiologically based pharmacodynamic
PBPK physiologically based pharmacokinetic
PCE polychromatic erythrocytes
PEL permissible exposure limit
pg picogram
PHS Public Health Service
PID photo ionization detector
pmol picomole
PMR proportionate mortality ratio
ppb parts per billion
ppm parts per million
ppt parts per trillion
PSNS pretreatment standards for new sources
RBC red blood cell
REL recommended exposure level/limit
RfC reference concentration
RfD reference dose
RNA ribonucleic acid
RQ reportable quantity
RTECS Registry of Toxic Effects of Chemical Substances
SARA Superfund Amendments and Reauthorization Act
SCE sister chromatid exchange
SGOT serum glutamic oxaloacetic transaminase
SGPT serum glutamic pyruvic transaminase
SIC standard industrial classification
SIM selected ion monitoring
SMCL secondary maximum contaminant level
SMR standardized mortality ratio
SNARL suggested no adverse response level
SPEGL Short-Term Public Emergency Guidance Level
STEL short term exposure limit
STORET Storage and Retrieval
TD
50
toxic dose, 50% specific toxic effect
TLV threshold limit value
TOC total organic carbon
TPQ threshold planning quantity
TRI Toxics Release Inventory
TSCA Toxic Substances Control Act
TWA time-weighted average
UF uncertainty factor
U.S. United States
USDA United States Department of Agriculture
USGS United States Geological Survey
VOC volatile organic compound
WBC white blood cell
WHO World Health Organization
MANGANESE C-5
APPENDIX C
> greater than
≥ greater than or equal to
= equal to
< less than
≤ less than or equal to
% percent
α alpha
β beta
γ gamma
δ delta
μm micrometer
μg microgram
q
1
*
cancer slope factor
– negative
+ positive
(+) weakly positive result
(–) weakly negative result
MANGANESE D-1
APPENDIX D. INDEX
absorbed dose.................................................................................................................... 322, 326, 408, 412
acetylcholine ............................................................................................................................................. 183
acetylcholinesterase .................................................................................................................. 183, 201, 316
active transport.................................................................................................................. 239, 263, 286, 296
adrenal gland..................................................................................................................................... 154, 208
adsorbed ............................................................................................................................ 385, 394, 396, 402
adsorption.................................................................................................................................................. 396
alanine aminotransferase................................................................................................... 188, 189, 211, 212
ambient air .................................................................................................................. 11, 391, 398, 409, 411
anaerobic................................................................................................................................................... 397
anemia....................................................................................................................................... 230, 333, 417
bioaccumulation........................................................................................................................................ 395
bioavailability ..................................................................... 40, 229, 231, 324, 332, 402, 414, 417, 421, 422
bioconcentration factor ............................................................................................................................. 395
biomarker ..... 27, 230, 321, 322, 324, 325, 326, 327, 329, 352, 354, 355, 360, 406, 414, 416, 423, 425, 433
blood cell count........................................................................................................................... 30, 151, 157
body weight effects ..................................................................................................... 66, 155, 156, 206, 213
breast milk................................................................. 197, 229, 231, 235, 259, 320, 332, 359, 406, 413, 414
cancer .............................................................................................................. 5, 15, 208, 252, 309, 348, 441
carcinogenic ...................................................................................................... 15, 19, 41, 98, 204, 208, 348
carcinogenicity.............................................................................................................................. 15, 38, 441
cardiovascular ......................................................................................... 30, 63, 64, 148, 149, 205, 210, 361
cardiovascular effects.............................................................................................. 30, 63, 64, 148, 205, 210
chromosomal aberrations .......................................................................................... 218, 219, 223, 235, 348
clearance .................................................................... 42, 224, 225, 246, 256, 262, 263, 269, 275, 276, 278,
280, 281, 282, 286, 291, 301, 324, 338, 339
cognitive function ....................................................................... 17, 27, 68, 83, 88, 168, 173, 182, 302, 313
death.......................................................... 41, 60, 63, 98, 146, 147, 153, 158, 162, 174, 193, 205, 209, 342
deoxyribonucleic acid (see DNA)............................................................................................................. 221
dermal effects...................................................................................................... 66, 154, 155, 206, 213, 342
developmental effects .................................................... 18, 97, 98, 156, 182, 197, 198, 199, 202, 204, 207,
208, 216, 309, 317, 318, 335, 345, 349, 358
DNA (see deoxyribonucleic acid)............................................................................................. 219, 221, 322
dopamine.................................. 28, 29, 31, 92, 176, 177, 178, 181, 182, 184, 185, 186, 188, 192, 201, 256,
297, 298, 299, 301, 302, 303, 315, 316, 317, 324, 328, 331, 339, 346, 362
elimination half-time......................................................................................................................... 260, 262
elimination rate ................................................................................................................................. 275, 283
endocrine............................................................................................... 65, 66, 154, 206, 213, 305, 306, 307
endocrine effects ........................................................................................... 65, 66, 154, 206, 213, 306, 307
erythema.................................................................................................................................................... 206
fetal tissue ......................................................................................................... 232, 244, 286, 291, 293, 314
fetus......................................... 5, 24, 202, 234, 244, 246, 286, 287, 293, 308, 315, 320, 351, 359, 413, 436
follicle stimulating hormone (see FSH)...................................................................................................... 65
FSH (see follicle stimulating hormone) .............................................................. 65, 193, 194, 200, 306, 307
gastrointestinal effects ................................................................................................ 64, 149, 150, 205, 211
general population................. 12, 25, 168, 234, 318, 333, 342, 345, 383, 398, 407, 414, 415, 418, 422, 436
genotoxic..................................................................................................................................... 41, 223, 348
genotoxicity....................................................................................................................................... 223, 349
groundwater ...................................................................... 3, 33, 89, 198, 309, 333, 350, 383, 393, 395, 402
MANGANESE D-2
APPENDIX D
growth retardation..................................................................................................................................... 193
half-life................................................................................................ 60, 228, 254, 321, 338, 394, 396, 397
hematological effects .................................................................................................. 64, 150, 151, 205, 211
hepatic effects ............................................................................................... 64, 65, 152, 153, 206, 211, 329
hydroxyl radical ........................................................................................................................................ 302
immune system ........................................................................................................................... 67, 157, 351
immunological ...................................................................................................... 41, 67, 157, 158, 207, 214
immunological effects......................................................................................................................... 67, 157
K
ow
............................................................................................................................................ 369, 370, 371
LD
50
............................................................................................................................. 60, 146, 147, 205, 209
lymphatic .................................................................................................................................................. 228
lymphoreticular........................................................................................................... 67, 157, 158, 207, 214
menstrual................................................................................................................................................... 306
metabolic effects ........................................................................................................... 66, 67, 157, 207, 214
micronuclei ....................................................................................................................................... 219, 223
milk .............................................................. 14, 31, 170, 181, 202, 203, 229, 231, 234, 235, 240, 259, 286,
288, 291, 293, 319, 320, 332, 351, 406, 413, 414, 421
mucociliary ................................................................................................. 71, 224, 225, 275, 293, 337, 355
musculoskeletal effects ............................................................................................... 65, 151, 152, 205, 211
neonatal................................ 24, 29, 31, 32, 34, 93, 173, 182, 183, 184, 190, 200, 201, 244, 249, 260, 265,
291, 299, 300, 305, 313, 314, 315, 316, 319, 332, 350, 357, 359, 424, 435
neoplastic .................................................................................................................................................... 41
neurobehavioral.............................. 16, 17, 21, 22, 23, 25, 26, 27, 28, 29, 31, 32, 34, 37, 72, 75, 78, 79, 80,
81, 82, 83, 84, 85, 86, 87, 92, 163, 178, 186, 198, 303, 304, 306, 311,
320, 325, 326, 333, 344, 346, 347, 350, 351, 352, 357, 424
neurochemical..................... 30, 176, 182, 183, 184, 185, 186, 297, 300, 304, 314, 316, 320, 332, 350, 357
neurodevelopmental.................................................................................................................... 30, 313, 349
neurological effects .................. 14, 15, 16, 18, 20, 21, 27, 28, 36, 40, 67, 68, 69, 72, 75, 80, 84, 87, 92, 94,
97, 158, 159, 161, 163, 164, 170, 180, 189, 190, 191, 201, 207, 214,
215, 237, 310, 311, 317, 321, 326, 327, 329, 331, 333, 335, 342,
343, 344, 345, 346, 347, 352, 353, 355, 358, 359, 418
neurophysiological.................................................................................................................................... 332
neurotransmitter ........................................................................................................ 192, 203, 297, 332, 355
norepinephrine .......................................................................................................................... 183, 192, 315
nuclear....................................................................................................................................................... 154
ocular effects....................................................................................................................... 66, 155, 206, 213
odds ratio............................................................................................................................................... 73, 87
orofacial .............................................................................................................................................. 33, 178
oxidative phosphorylation......................................................................................................................... 300
partition coefficients ................................................................................................................. 269, 275, 285
pharmacodynamic ..................................................................................................................................... 264
pharmacokinetic...........24, 190, 203, 227, 264, 265, 266, 267, 278, 283, 289, 308, 319, 321, 356, 357, 358
photolysis.................................................................................................................................................. 396
placenta ......................................................................................................... 5, 244, 285, 287, 314, 320, 413
rate constant ...................... 269, 274, 275, 277, 278, 280, 281, 282, 284, 285, 286, 287, 288, 289, 290, 291
renal effects................................................................................................................. 65, 153, 154, 206, 212
reproductive effects............................... 18, 20, 25, 33, 94, 96, 154, 192, 195, 196, 207, 215, 216, 349, 436
respiratory effects...................................................................... 17, 20, 61, 63, 148, 205, 210, 310, 327, 335
retention ................................................................. 14, 64, 83, 164, 228, 229, 230, 231, 249, 254, 256, 260,
262, 291, 319, 321, 332, 339, 357, 358, 359, 394
sequestered................................................................................................................................................ 295
MANGANESE D-3
APPENDIX D
solubility ............................................................................................................. 40, 293, 356, 395, 420, 434
spermatogonia........................................................................................................................................... 219
systemic effects............................................................................................................. 30, 61, 147, 205, 210
T3.......................................................................................................................................................... 43, 99
T4.............................................................................................................................................................. 220
thyroid............................................................................................................................................... 154, 204
toxicokinetic.................................................. 24, 39, 215, 251, 256, 263, 321, 332, 335, 355, 357, 433, 435
tremors .................................................................................................................... 14, 16, 93, 158, 172, 207
tumors ......................................................................................................................... 41, 208, 211, 252, 318
vapor pressure ........................................................................................................................................... 394
weanling.................................................................... 149, 175, 186, 191, 193, 194, 199, 200, 225, 249, 316
